Significant figures
- 格式:ppt
- 大小:1.61 MB
- 文档页数:13
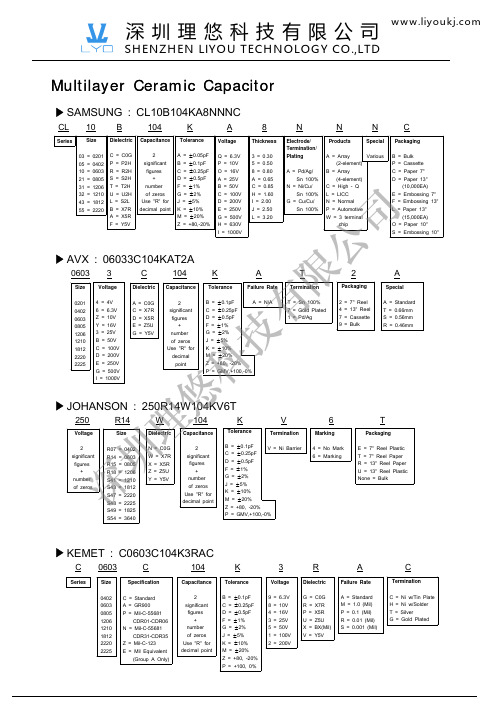

significant的用法归纳关键信息项:1、词性:形容词2、释义:重要的;有意义的;显著的;意味深长的3、常见搭配:significant improvement(显著的改进);significant effect(重大影响);significant change(重大变化);significant figure (有效数字)11 作为形容词的基本用法111 表示“重要的;有意义的”This is a significant event in our history(这是我们历史上的一个重要事件。
)The discovery was of significant importance(这一发现具有重要意义。
)112 表示“显著的;明显的”There has been a significant increase in population(人口有了显著的增长。
)The difference between the two groups was significant(两组之间的差异很显著。
)113 表示“意味深长的;别有深意的”He gave me a significant look(他意味深长地看了我一眼。
)Her words carried a significant message(她的话别有深意。
)12 常见搭配121 “significant improvement”The new policy has led to a significant improvement in the economy (新政策使经济有了显著的改善。
)We have seen a significant improvement in his performance(我们看到他的表现有了显著的提高。
)122 “significant effect”The medicine has a significant effect on the disease(这种药对这种疾病有显著的疗效。



有效数字是指用来表示一个量值的数字中,对所测得的数值给出准确度的数字。
在科学实验和工程设计中,确定一个测量值的有效数字是非常重要的,它直接影响到实验结果或工程设计的准确性和可靠性。
在本文中,我们将探讨有效数字的相关概念、规则和应用,并探讨在实际工作中如何正确地应用有效数字。
一、有效数字的概念有效数字是指一个数字中能够表达出一个量值的准确度的数字。
在一个测量值中,有效数字通常是从左到右第一个非零数字开始,一直到最后一个数字为止。
对于测量值45.678,有效数字为4、5、6、7、8,因为它们都对测量值的准确度有贡献。
二、有效数字的规则1. 非零数字都是有效数字。
测量值123.45中的1、2、3、4、5都是有效数字。
2. 在非零数字之间的零都是有效数字。
测量值305.007中的3、5、7都是有效数字。
3. 在小数点后的零都是有效数字。
测量值0.00340中的3、4、0都是有效数字。
4. 在科学计数法下,所有表示数量的数字都是有效数字。
科学计数法下的测量值2.34×10^3中的2、3、4都是有效数字。
5. 数字中除了有效数字以外的其他数字,都属于非有效数字。
测量值1.23中的1和2是有效数字,而3属于非有效数字。
三、有效数字的运算规则在进行有效数字的加减运算时,结果的有效数字取决于有效数字最少的测量值。
在进行有效数字的乘除运算时,结果的有效数字取决于有效数字最少的测量值中有效数字的个数。
对于测量值3.21和5.743的乘法运算,结果的有效数字为3个。
四、有效数字的应用在科学实验和工程设计中,正确理解和应用有效数字是非常重要的。
在实验测量中,要根据仪器精度和测量范围选择合适的有效数字,以确保测量结果的准确性。
在工程设计中,要根据设计要求和制造工艺选择合适的有效数字,以确保产品的性能和质量。
五、有效数字的误差分析在测量中,由于仪器精度和人为因素等原因,测量结果往往存在一定的误差。
正确理解和应用有效数字可以帮助我们分析和评估测量误差,从而更准确地判断测量结果的可靠性和真实性。
有效數字 普化教學組2007/9/26一、有效數字(significant figure )滴定,右圖中的滴定管,它的最小測量單位是mL。
其中2.1右方的數值「5」則為估計值(uncertain digit )mL 為3位有效數字。
稱量,得到1.10 g 及1.0745 g 。
記錄數字「1.10 g 」中的 0 很重要,不得略寫為。
(三位有效數字) 估計值 「0」這個數字的出現常會令有效位數有些困擾,其原則為:小數點後面的「0」均為有效數字,例如1.10為三位有效數字;夾在數字中間的「0」均為有效數字,例如1.0725為五位有效數字;所有非零數值前的「0」均不是有效數字,例如0.011 為二位有效數字。
然而在整數中的尾數若為「0」時會具有混淆性,例如「1500 mL 」可能是二位有效數字,但也可能為三位或四為有效數字,為正確表示「1500 mL 」測量值的有效數字,可根據最小測量單位,以科學記數法表示。
例如:1.5 × 103 mL(二位有效數字,表示 ±100 mL ) 1.50 × 103 mL(三位有效數字,表示 ±10 mL ) 1.500 × 103 mL(四位有效數字,表示 ±1 mL )二、有效數字的四則運算當多個數字要進行四則運算時,必須注意有效位數的決定。
舉例說明如下:(1) 752.46 − 21 = 731.46,答案應表示為731,雖然752.46的不準度在小數點後第二位,但是21的不準度卻在個位,這將導致其差731.46中自個位起均為不準位數,按規定只能保留一位有誤差的數值,因此在此例中不準位取在最前面的個位,經四捨五入取捨後為752.46 − 21 = 731.46 = 731(2) 若為乘除運算,所得答案須配合有效位數最少的數值,以下算式為例,最小有效位數為「0.011」的二位有效數字,所以最後答案也以二位有效數字表示。
Worksheet on Significant Figures有效数字练习题Take the guidance on the concept of Significant Figures by taking the help of Worksheet on Significant Figures. Practice all the problems present in the Significant Figures Worksheets and get a good grip on the topic. Test your subject knowledge by taking the practice tests available in the document. Also, you can easily enhance your problem-solving skills by solving Worksheet on Significant Figures on a daily basis. Assess your preparation standards and concentrate on the areas you are facing difficulty.借助有效数字练习题,对有效数字概念进行指导。
练习重要数字练习题中的所有问题,并掌握好这个主题。
通过文档里的提供的实践测试可以测试你的掌握程度。
此外,你可以轻松地提高你解决问题的能力,你可以每天练习。
评估你的标准,集中精力在你觉得困难的领域。
Significant Figures Examples with Answers重要数字题目示例(附答案)Check different problems impose on Significant Figures and get a grip on every concept available in the Significant Figures.检查对有效数字所问的不同问题,掌握有效数字中可用的每个概念。
Syllabus of Chemistry AnalysisApplicable subjects:major in pharmacy(Credit:2 class hour:36 )1. Course DescriptionAnalytical Chemistry is a required course in pharmacy. It is a measurement science, responsible for characterizing the composition of natural and artificial materials, both qualitatively and quantitatively. With the advancement of science, the current definition of analytical chemistry would be “analytical chemistry is the science of inventing and applying the concepts, principles, and strategies for measuring the characteristics of chemical systems and species”. The content is divided into two parts: chemical analysis and instrumental analysis. This course is major in chemical analysis. Chemical analysis mainly deals with the content of classical chemical analysis.2. Teaching contents, basic requirements and scheduleChapter 1 Introduction of Analytical ChemistryTo understand the nature, tasks, basic content, development trend of analytical chemistry and its function in pharmaceutical related specialties.To learn steps in the development of an Analytical Method, and classification of Quantitative Analytical Chemistry.Major contents1.1what is Analytical Chemistry1.2steps in the development of an Analytical Method1.3Classification of Quantitative Analytical Chemistry1.3.1Chemical Analysis1.3.2Instrumental AnalysisChapter 2 data analysisTo grasp the cause of error and the method of reduction, the method of expressing accuracy and precision; the distribution of random errors; significant figures and numerical rounding in calculaions.To be familiar with predicting the probability of random errors and confidence interval for population mean; the abandonment of the escape value, and statistical aids to hypothesis testing;To understand statistical data treatment; the influence of measurement error on the calculation result,Major contents2.1 error and classification2.1.1 accuracry and precision2.1.2 errors and deviation2.1.3 systematic and random errors2.1.4 improve the accuracy of the method2.2 distribution of random errors2.2.1 frequency distribution2.2.2 normal distribution2.2.3 predicting the probability of random errors2.3 statistical data treatment2.3.1 estimation of Population Mean and Population Standard Deviation2.3.2 Confidence interval for population mean2.3.3 statistical aids to hypothesis testing2.3.4 detection of Gross Errors2.4 propagation of error2.4.1 systematic error2.4.2 random errors2.4.3 maximum errors2.4.4 distribution of errors2.5 significant figure convention2.5.1 significant figures2.5.2 numerical rounding in calculationsKey points:1.basic knowledge of error2.causes of error and ways of reduction and avoiding3.distribution of random errors4.confidence interval for population mean5. rejection of doubtful data and statistical aids to hypothesis testing6. significant figures and numerical rounding in calculationsNodus:1. causes of error and ways of reduction and avoiding2. confidience level and confidence interval3. statistical aids to hypothesis testing4. numerical rounding in calculationsChapter 3 principles of volumetric titrationTo grasp the basic concepts of titrimetric analysis, application of titrimetric analysis and calculation methods of titrimetric analylsis and to learn about the classification of titrimetric analysis.Major Contens3.1. Basic term, includes titrimetry, titrand, stoichiometric point, indicator, primary standards and standard solution.3.2 requirements of titration reactions3.3 classification of titration process3.4 primary standards and standard solutions3.5 basic apparatus in chemical analyses3.6 Calculations in volumetric titration3.6.1 preparation of standard solutions3.6.2 titration results.Key points1.Basic concepts and calculation methods of titrimetric analysis2.requirements of titration reactions3.primary standards and the preparation of standard solutionsChapter 4-1 acid-base titrationTo master proton condition equotion, pH calculations.To be familiar with the basic concept of the theory of Bronsted-Lowry, magnitude of dissociating species at a given pH, acid-base reactions and equilibria,.To understand the concept of the activity; buffer capacity and the preparation of buffer solutions.Major Contens4.1 acid-base equilibrium4.1.1 equilibrium constants and effect of electrolytes4.1.2 acid-base reactions and equilibriums4.1.2.1 acid-base bronsted concept4.1.2.2 dissociation of acid or base and acid-base equilibria4.1.2.3 magnitude of dissociating species at a given pH4.1.3 solving equlibrim calculations using pH calculations as an example4.1.3.1 general approaches (systematic approaches)① mass balance equation② charge-balance equation③ proton condition equotion4.1.3.2 pH calculations① weak monoprotic acids and bases② polyprotic acids③ amphiprotic species④ mixture of strong acid and weak acid4.1.4 buffer solutions4.1.4.1 pH calculation of buffer solution4.1.4.2 buffer capacity4.1.4.3 preparation of buffersKey points1.acid-base bronsted concept2.acid-base reactions and equilibriums3.magnitude of dissociating species at a given pH4.proton condition equation5. pH calculation in solution of weak monoprotic acids/bases, polyprotic acids, amphiprotic species, mixture of strong acid and weak acid, or buffer solutions.Nodus:1.distribution factor and distribution curve, confirmation of proton balance equation2.magnitude of dissociating species at a given pH3.calculation of acidity of ampholyteric compound solution and buffer solutions.Chapter 4-2 acid-base titrationTo master the principle of acid/base indicatorsTo be familiar with the factors influencing performance of acid/base indicators.To master the principle of method of titration, titration curve, end point judge, end point error, the way of choosing the right indicator and the calculation of titration results.To be familiar with the law of pH changing in various processes of acid-base titration; the application of acid-base titration; and the calculation of titration results..To understand the titration of polyfunctional weak acids (bases); the preparation of standard acid/base solutions.Major Contens:4.2 acid-base titration4.2.1 acid-base indicators① principle② examples③ factors infulencing performance4.2.2 titration curves and selection of indicators4.2.2.1 strong acids (base)4.2.2.2 monoprotic acids (bases4.2.2.3 polyfunctional weak acids (bases)4.2.3 titration error calculations4.2.4 preparation of standard solutions4.2.4.1 standard acid solutions4.2.4.2 standard base solutions4.2.5 examples of acid-base titrationsKey points1.principle of acid/base indicators2. basic concepts of acid-base titration (titration, end point, stoichiometric point, indicator, standard solution, standard substance)3. principle of acid-base titration analysis, titrate curve, end point judgement, end point error4. application of acid-base titration and calculation of titration result.Nodus:1.the law of pH changing in various processes of acid-base titration and the way of choosing the rightindicator.Chapter 5 complexation reaction and complexometric titrationTo master side reaction coefficients and conditional formation constants in complexation reaction, principle of metallochromic indicator and color-transition point pM for metallochromic indicators; the titration curves and titration errors.To understand metal EDTA compleses and frequently used metallochromic indicators; selective titrations of metal ions in the presence of multiple metal ions; and the titration methods and applications.To learn preparation of standard solutionsMajor contents:5.1 complexes and formation constants5.1.1 formation constants5.1.2 concentration of ML n in complexation equilibria5.1.3 ethylenediaminetetraacetic acid (EDTA) and metal EDTA compleses5.1.4 side reaction coefficients and conditional formation constants in complexation reactions5.2 metallochromic indicators5.2.1 how a metallochromic indicator works5.2.2 color transition point pM((pM)t) for metallochromic indicators5.2.3 frequently used metallochromic indicators5.3 titration curves and titration errors5.3.1 titration curves5.3.2 titration errors5.3.3 pH control in complexometric titrations5.4 selective titrations of metal ions in the presence of multiple metal ions5.4.1 selective titration by regulating Ph5.4.2 selective titration using masking reagents5.5 applications of complexometric titrations5.5.1 buffer selection in complexometric titrations5.5.2 titration methods and applications5.5.3 preparation of standard solutionsKey points1.the principle of complexometry2.formation constants and the concentration of ML n in complexation equilibria3.side reaction coefficients and conditional formation constants in complexation reactions and theircalculation4.the principle of a metallochromic indicator works5 titration curves6. titration errors7. buffer selection in complexometric titrationsNodus:1.The concept of side reaction coefficients and conditional formation constants in complexation reactionsand their calculation.2.color transition point pM((pM)t) for metallochromic indicators3. selective titrations of metal ions in the presence of multiple metal ions.4. buffer selection in complexometric titrationsChapter 6 redox equlibrium and titrationTo master the concept of the Nernst equation, standard electrode potentials, formal potentials, the equilibrium constant of redox reaction and their calculation; basic concepts of redox titration analysis (titration, end point, stoichometric point, indicator, standard solution, standard substance); the principle and titration curves of potassium permanganate redox titrations; and the calculation of titration result.To be familiar with the way of choosing the right condition for redox titration.To be understand factors affecting the reaction rateTo learn the other redox titrations.Major contents:6.1 standard electrode potentials, formal potentials and redox equilibria6.1.1 standard electrode potentials6.1.2 the Nernst equation and formal potentials6.1.3 factors affecting the formal potential6.1.4 the equilibrium constant of redox reaction6.2 factors affecting the reaction rate6.2.1 concentration6.2.2 temperature6.2.3 catalysis and reaction rate6.2.4 induced reaction6.3 redox titration6.3.1 constructing redox titration curves6.3.2 indicators6.3.3 auxiliary oxidizing and reducing agents6.4 examples of redox titrations6.4.1 potassium permanganate6.4.2 potassium dichromate6.4.3 iodine: iodimetry and iodometry6.4.4 potassium bromate6.4.5 ceric sulfateKey points1.The concepts of the Nernst equation, standard electrode potentials, formal potentials, the equilibriumconstant of redox reaction and their calculation.2.basic concepts of redox titration analysis (titration, end point, stoichometric point, indicator, standardsolution, standard substance)3.principle of potassium permanganate redox titrations, and their titrate curve, end point judge, titrationerror4.application of redox titration analysis and calculation of titration result.Nodus:1.the concept of formal potentials and its calculation.2.basic concepts of redox titration analysis(titration, end point, stoichometric point, indicator, standardsolution, standard substance)3.potassium permanganate redox titrations, and their titrate curve, end point judge, titration errorChapter 7 precipitation equilibrium, titration, and gravimetryTo master the contents and relative calculations about precipitation equilibria and solubility, factors that affect the solubility of precipitates. ②the principle of precipitation titration analysis, end point judge.To be familiar with the feature of gravimetric method and its principle and process and how to obtaining high purity precipitation.To understand the precipitate formation and requirements for precipitation.Major contents:7.1 precipitation equilibria and solubility7.1.1 solubility of precipitation in pure water7.1.2 ionic strength and the solubility of precipitates7.1.3 common ion and the solubility of precipitates7.1.4 pH and the solubility of precipitates7.1.5 complexing agents and the solubility of precipitates7.2 precipitation titations7.2.1 titration curves7.2.2 examples of methods classified by endpoint indication7.2.3 preparation of standard solutions7.3 precipitation gravimetry7.3.1 classification of gravimetric methods of analysis7.3.2 general precedure and requirements for precipitation7.3.3 precipitate formation7.3.4 obtaining high purity precipitation7.3.5 experimental consideration7.3.6 examples of organic precipitating reagentsKey points1.precipitation-dissolution equilibrium, solubility product principle2.precipitation titration analysis and calculation of titration result3.gravimetric method and its principle and process4.how to obtaining high purity precipitation.Nodus1.precipitation equilibria and factors that affect the solubility of precipitates.2.principle of precipitation titration analysis and end point judge3.how to obtaining high purity precipitation.3. schedual4. recommended teaching materials1. analytical chemistry(seventh version),edited by Yufeng Cai, et al. ISBN 798-7-117-14378-3/R-14379, People’s Medical Publishing House2. analytical chemistry(fifth version),edited by Wuhan university, IBSN 978-7-04-019382-4, Higher Education Press.3. Quantitative chemical analysis (first version), edited by Li Na et al. IBSN 978-7-301-15659-9/O·0786, Peking university press.。