磺胺嘧啶银-说明书 by FDA
- 格式:pdf
- 大小:154.94 KB
- 文档页数:6


磺胺嘧啶银乳膏的作用和功效
磺胺嘧啶银乳膏是一种常见的外用药物,主要成分为磺胺嘧啶银。
下面将介绍其作用和功效:
1. 抗菌作用:磺胺嘧啶银乳膏具有广谱的抗菌作用,可有效抑制多种细菌的生长和繁殖。
它对于常见的引起皮肤感染的金黄色葡萄球菌和链球菌等具有很强的杀菌作用。
2. 消炎作用:该乳膏还具有一定的消炎作用,可以减轻皮肤红肿、热痛等炎症症状。
它能抑制炎症反应的发生,减少组织的炎性渗出和纤维素渗出,从而改善受损组织的炎症状态。
3. 促进伤口愈合:磺胺嘧啶银乳膏还具备促进伤口愈合的功效。
它能够阻断感染源,提供良好的外部环境,促进创面的修复和再生。
同时,它还能够减少疤痕形成,避免伤口愈合后留下明显的疤痕。
4. 防止感染扩散:当皮肤出现划伤、擦伤或破溃时,容易导致细菌进入体内造成感染。
磺胺嘧啶银乳膏能够迅速杀灭细菌,阻止感染的进一步扩散。
它可直接应用于受伤的皮肤表面,形成一层保护膜,减少外界细菌的侵袭。
需要注意的是,磺胺嘧啶银乳膏仅适用于外用,不宜内服。
在使用过程中,应遵医嘱使用适量的乳膏,涂抹于受伤处,并保持创面清洁和干燥。
如果出现过敏或其他不适症状,应立即停止使用并咨询医生。
此外,乳膏的使用期限有限,应严格遵守相关的保存和使用规定。
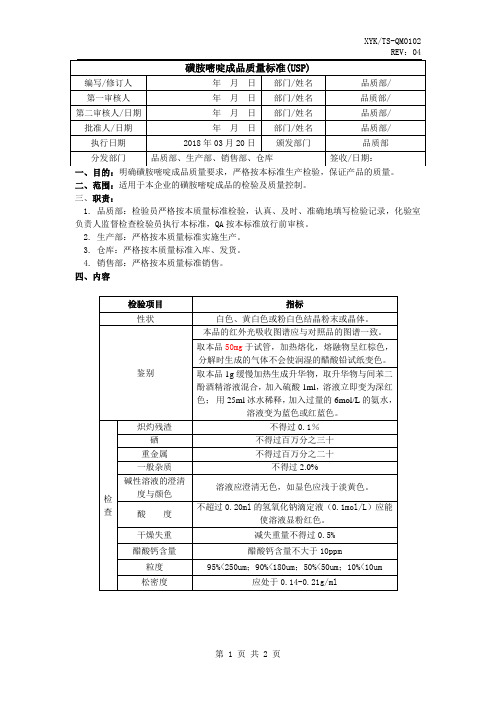
一、目的:明确磺胺嘧啶成品质量要求,严格按本标准生产检验,保证产品的质量。
二、范围:适用于本企业的磺胺嘧啶成品的检验及质量控制。
三、职责:
1. 品质部:检验员严格按本质量标准检验,认真、及时、准确地填写检验记录,化验室负责人监督检查检验员执行本标准,QA按本标准放行前审核。
2. 生产部:严格按本质量标准实施生产。
3. 仓库:严格按本质量标准入库、发货。
4. 销售部:严格按本质量标准销售。
四、内容
或一年检测一次,另有客户Pegasus要求残留DMF不得过0.03%。
【代码】CY001
【规格】25kg/袋(内袋:药用低密度聚乙烯袋);25kg/桶(内袋:药用低密度聚乙烯袋)【类别】磺胺类抗菌药
【贮藏】遮光,密封保存
【复验期】一年
【注意事项】N/A
五、参考文献:
USP
EP
六、相关文件:
XYK/SMP-QC0005 取样管理规程
XYK/SOP-QC0102 磺胺嘧啶成品检验操作规程
XYK/SOP-QC010201磺胺嘧啶成品检验记录
七、附录:
N/A
八、变更记录及原因:。

磺胺嘧啶银(SD—Ag)碘伏悬液治疗烧伤100例临床观察黎世纯
【期刊名称】《华南国防医学杂志》
【年(卷),期】1997(0)1
【摘要】我院于1994年始应用SD—Ag碘伏悬液治疗烧伤,临床观察100例,效果甚佳。
取SD—Ag 10~20g,碘伏100ml,均匀混合成10%~20%SD—Ag碘伏悬液。
生理盐水清洗创面,剪除皱缩腐皮,抽吸尽水泡液,沾干创面后将药液涂于创面,或浸透一层纱布紧贴创面敷盖,行暴露或半暴露烤灯照射。
浅Ⅱ°及四肢、面颈、腋窝、会阴行暴露治疗;深Ⅱ°及躯干行半暴露治疗。
以后每次换药时仅更换潮湿创面药液。
【总页数】1页(P89-89)
【关键词】磺胺嘧啶银;临床观察;治疗烧伤;碘伏;半暴露;烤灯照射;悬液;水泡液;水清洗;均匀混合
【作者】黎世纯
【作者单位】169医院
【正文语种】中文
【中图分类】R644
【相关文献】
1.莫匹罗星红氟洗剂碘伏SD-AG霜治疗烧伤晚期顽固小创面的比较 [J], 王雪;张艳;罗家旭
2.磺胺嘧啶银悬液治疗大面积烧伤创面的护理体会 [J], 王磊
3.磺胺嘧啶银联合碘伏治疗烧伤的疗效观察 [J], 吴祖馨
4.磺胺嘧啶银悬液治疗大面积烧伤创面的护理体会 [J], 王磊
5.磺胺嘧啶银联合碘伏治疗烧伤49例效果观察及护理 [J], 李成香;何忠军;孙友竹因版权原因,仅展示原文概要,查看原文内容请购买。
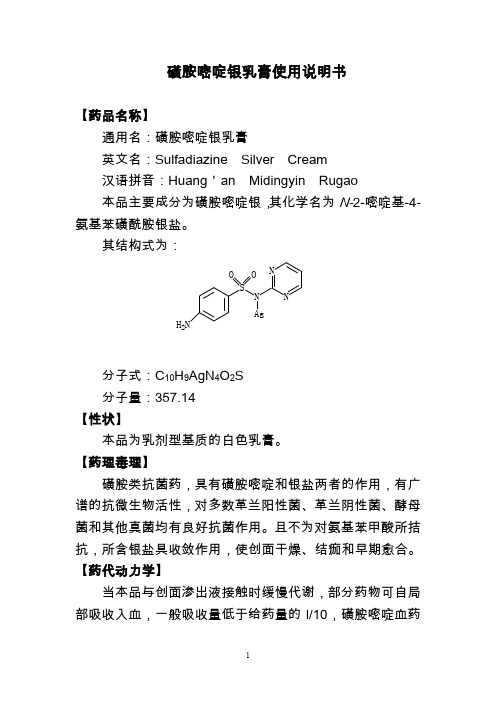
磺胺嘧啶银乳膏使用说明书【药品名称】通用名:磺胺嘧啶银乳膏英文名:Sulfadiazine Silver Cream汉语拼音:Huang 'an Midingyin Rugao本品主要成分为磺胺嘧啶银,其化学名为N-2-嘧啶基-4-氨基苯磺酰胺银盐。
其结构式为:分子式:C 10H 9AgN 4O 2S分子量:357.14【性状】本品为乳剂型基质的白色乳膏。
【药理毒理】磺胺类抗菌药,具有磺胺嘧啶和银盐两者的作用,有广谱的抗微生物活性,对多数革兰阳性菌、革兰阴性菌、酵母菌和其他真菌均有良好抗菌作用。
且不为对氨基苯甲酸所拮抗,所含银盐具收敛作用,使创面干燥、结痂和早期愈合。
【药代动力学】当本品与创面渗出液接触时缓慢代谢,部分药物可自局部吸收入血,一般吸收量低于给药量的l/10,磺胺嘧啶血药H 2NN N N S O O浓度约可达10~20mg/L,当创面广泛,用药量大时,吸收增加,血药浓度可更高。
一般情况下本品中银的吸收量不超过其含量的1%。
本品对坏死组织的穿透性较差。
【适应症】本品用于预防或治疗Ⅱ、Ⅲ度烧伤继发创面感染,包括对该药呈现敏感的肠杆菌科细菌、铜绿假单胞菌、金黄色葡萄球菌、肠球菌属,念珠菌等真菌所致者。
【用法用量】本品可直接以乳膏涂敷创面,约1.5mm厚度,也可以混悬剂制成油纱布敷用,l~2天换药1次。
【不良反应】局部有轻微刺激性,偶可发生短暂性疼痛。
本品自局部吸收后可发生各种不良反应,与磺胺药全身应用时相同,包括:1.过敏反应较为常见,可表现为药疹,严重者可发生渗出性多形红斑、剥脱性皮炎和大疱表皮松解萎缩性皮炎等;也有表现为光敏反应、药物热、关节及肌肉疼痛、发热等血清病样反应。
2.中性粒细胞减少或缺乏症、血小板减少症及再生障碍性贫血。
患者可表现为咽痛、发热、苍白和出血倾向。
3.溶血性贫血及血红蛋白尿。
缺乏葡萄糖-6-磷酸脱氢酶患者应用磺胺药后易发生,在新生儿和小儿中较成人为多见。
4.高胆红素血症和新生儿核黄疸。

磺胺嘧啶软膏磺胺嘧啶软膏使用说明书•【药品名称】通用名称:磺胺嘧啶软膏汉语拼音:Huang’an Miding Ruangao•【成份】磺胺嘧啶。
化学名称:N-2-嘧啶基-4-氨基苯磺酰胺。
•【性状】本品为淡黄色或黄色软膏。
•【适应症】适用于葡萄球菌感染、疮疖。
•【用法用量】涂于洗净的患处。
一日1~2次。
大面积感染在医生指导下使用。
•【不良反应】本品自局部吸收后可发生各种不良反应。
•【注意事项】由于本品可自局部部分吸收,其注意事项同磺胺嘧啶全身应用。
本品不可用于对磺胺药过敏的患者。
肝、肾功能损害者宜避免使用或慎用本品。
新生儿不宜用本品,因其吸收后也有发生新生儿核黄疸的可能。
•【孕妇及哺乳期妇女用药】请遵医嘱。
•【儿童用药】新生儿不宜使用。
•【药理毒理】磺胺嘧啶为广谱抗菌药,在结构上类似对氨基苯甲酸(PABA),可与PABA竞争性作用于细菌体内的二氢叶酸合成酶,从而阻止PABA作为原料合成细菌所需要叶酸的过程,减少了具有代谢活性的四氢叶酸的量,而后者则是细菌合成嘌呤、胸腺嘧啶核苷和脱氧核糖核酸(DNA)的必须物质,因此抑制了细菌的生长繁殖。
•【贮藏】密闭,在凉暗处保存。
•【有效期】60个月【注意】药物说明书里面有三种标识,一般要注意一下:1.第一种就是禁用,就是绝对禁止使用。
2.第二种就是慎用,就是药物可以使用,但是要密切关注患者口服药以后的情况,一旦有不良反应发生,需要马上停止使用。
3.第三种就是忌用,就是说明药物在此类人群中有明确的不良反应,应该是由医生根据病情给出用药建议。
如果一定需要这种药物,就可以联合其他的能减轻不良反应的药物一起服用。
大家以后在服用药物的时候,多留意说明书,留意注意事项,避免不良反应的发生。
本文到此结束,谢谢大家!。
磺胺嘧啶银成分
磺胺嘧啶银的成分
磺胺嘧啶银,化学名称为4-氨基-N-(2-嘧啶基)苯磺酰胺银盐,是一种有机化合物,化学式为CH₈N₆O₂S·Ag,为白色或略带浅绿色的结晶性粉末,无臭,有光泽,遇光色渐变深,在水中微溶,在乙醇中极微溶解,在氯仿或乙醚中不溶,在稀无机酸中易溶,在氢氧化钠试液中溶解。
磺胺嘧啶银为预防和治疗Ⅱ、Ⅲ度烧伤继发创面感染,包括该创面感染所致的全身性败血症与脓毒症。
其抗菌作用机制为在结构上类似对氨基苯甲酸(PABA),可与PABA竞争二氢叶酸合成酶,从而阻止二氢叶酸的合成,影响细菌叶酸代谢而抑制细菌的生长繁殖。
请注意,使用磺胺嘧啶银时应遵循医生的建议和指导,确保安全有效地使用。
同时,对其成分过敏的人应避免使用。
以上信息仅供参考,如有需要,建议查阅相关文献或咨询专业医生。
1例磺胺嘧啶银敷料治疗奴卡菌感染伤口的护理体会标签:磺胺嘧啶银;奴卡菌;伤口换药;护理奴卡菌病(Nocardiosis)是由奴卡菌引起的少见而严重的感染。
奴卡菌为需氧菌,包括星形奴卡氏菌、巴西奴卡菌和豚鼠奴卡菌,病菌可通过带菌的灰尘、土壤或食物经呼吸道、皮肤和消化道进入人体,然后局限于某一器官或组织,或经血循环播散至脑、肾或其他器官。
本病的发生和传播途径与机体的抵抗力有密切关系。
从皮肤侵入者,常有局限性,可表现为足菌肿型或皮肤脓肿型,很少呈血源性扩散。
研究表明磺胺哒嗪对本病具有特效[]。
2015年1月1日我科监护病房收治1例右大腿内侧及后侧奴卡菌感染患者,经过30天的精心治疗和护理,患者创面愈合达到90%以上,顺利出院。
现报告如下:1 临床资料1.1病例简介患者老年女性,主因“皮肤红肿伴破溃半年余,间断发热、黄疸及肝功能异常40余天,加重1周”收入急诊监护病房。
既往史:特发性血小板减少性紫癜,甲状腺机能亢进,Grave,s病病史11年;脾切除术后9年。
现诊断为“肺部感染,I型呼吸衰竭,奴卡菌感染,肝功能异常”。
查体:神清,满月脸,全身皮肤黏膜、巩膜黄染,口周胡须明显;腹部膨隆,右上腹轻压痛,无反跳痛,肝脏肋下未触及,Murphy征阴性,肠鸣音3-4次/分;双下肢凹陷性水肿,双侧足背动脉可触及。
体温39℃,脉搏110次/分,呼吸25次/分,血压115/80mmHg。
1.2皮肤情况双上肢散在数个结节性红斑,色暗红,有触痛,最大者位于右上臂外侧,约1.5cm*2cm,表面结痂。
右足外踝可见一陈旧6mm*6mm暗红色结节,伴结痂,无触痛。
左下肢皮肤暗红,水肿明显。
右季肋区外侧可触及一6cm*4cm 包块,质软,边界清,有触痛,无波动感。
患者右大腿内侧及后侧皮肤炎症伴多个红疹,无触痛,中央破溃,表面可见黄白色脓性分泌物。
1.3辅助检查①血气:pH 7.55,pC02 26mmHg,pO2 57mmHg,SpO2 93%。
SILV ADENE® CREAM 1%(silver sulfadiazine)DESCRIPTIONSILVADENE Cream 1% is a soft, white, water-miscible cream containing the antimicrobial agent silver sulfadiazine in micronized form, which has the following structural formula:Each gram of SILVADENE Cream 1% contains 10 mg of micronized silver sulfadiazine. The cream vehicle consists of white petrolatum, stearyl alcohol, isopropyl myristate, sorbitan monooleate, polyoxyl 40 stearate, propylene glycol, and water, with methylparaben 0.3% as a preservative. SILVADENE Cream 1% (silver sulfadiazine) spreads easily and can be washed off readily with water.CLINICAL PHARMACOLOGYSilver sulfadiazine has broad antimicrobial activity. It is bactericidal for many gram-negative and gram-positive bacteria as well as being effective against yeast. Results from in vitro testing are listed below.Sufficient data have been obtained to demonstrate that silver sulfadiazine will inhibit bacteria that are resistant to other antimicrobial agents and that the compound is superior to sulfadiazine. Studies utilizing radioactive micronized silver sulfadiazine, electron microscopy, and biochemical techniques have revealed that the mechanism of action of silver sulfadiazine on bacteria differs from silver nitrate and sodium sulfadiazine. Silver sulfadiazine acts only on the cell membrane and cell wall to produce its bactericidal effect.Results of In Vitro Testing with SILVADENE® Cream 1% (silver sulfadiazine) Concentration of Silver Sulfadiazine Number of Sensitive Strains/Total Number of Strains TestedGenus & Species 50 μg/mL 100 μg/mL Pseudomonasaeruginosa 130/130 130/130 Xanthomonas (Pseudomonas)maltophilia 7/7 7/7 Enterobacter species 48/50 50/50 Enterobacter cloacae 24/24 24/24 Klebsiella species 53/54 54/54 Escherichia coli 63/63 63/63 Serratia species 27/28 28/28Genus & Species 50 μg/mL 100 μg/mL Proteus mirabilis 53/53 53/53 Morganella morganii 10/10 10/10 Providencia rettgeri 2/2 2/2 Providencia species 1/1 1/1 Proteus vulgaris 2/2 2/2 Citrobacter species 10/10 10/10 Acinetobactercalcoaceticus 10/11 11/11 Staphylococcus aureus 100/101 100/101 Staphylococcusepidermidis 51/51 51/51β-HemolyticStreptococcus 4/4 4/4 Enterococcus species 52/53 53/53 Corynebacterium-diphtheriae 2/2 2/2 Clostridium perfringens 0/2 2/2 Candida albicans 43/50 50/50 Silver sulfadiazine is not a carbonic anhydrase inhibitor and may be useful in situations where such agents are contraindicated.INDICATIONS AND USAGESILVADENE Cream 1% (silver sulfadiazine) is a topical antimicrobial drug indicated as an adjunct for the prevention and treatment of wound sepsis in patients with second-and third-degree burns.CONTRAINDICATIONSSILVADENE Cream 1% (silver sulfadiazine) is contraindicated in patients who are hypersensitive to silver sulfadiazine or any of the other ingredients in the preparation. Because sulfonamide therapy is known to increase the possibility of kernicterus, SILVADENE Cream 1% should not be used on pregnant women approaching or at term, on premature infants, or on newborn infants during the first 2 months of life.WARNINGSAbsorption of silver sulfadiazine varies depending upon the percent of body surface area and the extent of the tissue damage. Although few have been reported, it is possible that any adverse reaction associated with sulfonamides may occur. Some of the reactions, which have been associated with sulfonamides, are as follows: blood dyscrasias including agranulocytosis, aplastic anemia, thrombocytopenia, leukopenia, and hemolytic anemia; dermatologic and allergic reactions, including life-threatening cutaneous reactions [Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN) and exfoliative dermatitis]; gastrointestinal reactions; hepatitis and hepatocellular necrosis; CNS reactions; and toxic nephrosis.There is potential cross-sensitivity between silver sulfadiazine and other sulfonamides. If allergic reactions attributable to treatment with silver sulfadiazine occur, continuation of therapy must be weighed against the potential hazards of the particular allergic reaction.Fungal proliferation in and below the eschar may occur. However, the incidence of clinically reported fungal superinfection is low.The use of SILVADENE Cream 1% (silver sulfadiazine) in some cases of glucose-6-phosphate dehydrogenase-deficient individuals may be hazardous, as hemolysis may occur. PRECAUTIONSGeneralIf hepatic and renal functions become impaired and elimination of drug decreases, accumulation may occur and discontinuation of SILVADENE Cream 1% (silver sulfadiazine) should be weighed against the therapeutic benefit being achieved.In considering the use of topical proteolytic enzymes in conjunction with SILVADENE Cream 1%, the possibility should be noted that silver may inactivate such enzymes.Laboratory TestsIn the treatment of burn wounds involving extensive areas of the body, the serum sulfa concentrations may approach adult therapeutic levels (8 mg% to 12 mg%). Therefore, in these patients it would be advisable to monitor serum sulfa concentrations. Renal function should be carefully monitored and the urine should be checked for sulfa crystals. Absorption of the propylene glycol vehicle has been reported to affect serum osmolality, which may affect the interpretation of laboratory tests.Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term dermal toxicity studies of 24 months' duration in rats and 18 months' in mice with concentrations of silver sulfadiazine three to ten times the concentration in SILVADENE Cream 1% revealed no evidence of carcinogenicity.PregnancyTeratogenic Effects.Pregnancy Category B. A reproductive study has been performed in rabbits at doses up to three to ten times the concentration of silver sulfadiazine in SILVADENE Cream 1% and has revealed no evidence of harm to the fetus due to silver sulfadiazine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly justified, especially in pregnant women approaching or at term. (See CONTRAINDICATIONS .)Nursing MothersIt is not known whether silver sulfadiazine is excreted in human milk. However, sulfonamides are known to be excreted in human milk, and all sulfonamide derivatives are known to increase the possibility of kernicterus. Because of the possibility for serious adverse reactions in nursing infants from sulfonamides, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Geriatric UseOf the total number of subjects in clinical studies of Silvadene Cream 1%, seven percent were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.Pediatric UseSafety and effectiveness in pediatric patients have not been established. (See CONTRAINDICATIONS .)ADVERSE REACTIONSSeveral cases of transient leukopenia have been reported in patients receiving silver sulfadiazine therapy.1,2,3 Leukopenia associated with silver sulfadiazine administration is primarily characterized by decreased neutrophil count. Maximal white blood cell depression occurs within2 to 4 days of initiation of therapy. Rebound to normal leukocyte levels follows onset within 2 to3 days. Recovery is not influenced by continuation of silver sulfadiazine therapy. An increased incidence of leukopenia has been reported in patients treated concurrently with cimetidine. Other infrequently occurring events include skin necrosis, erythema multiforme, skin discoloration, burning sensation, rashes, and interstitial nephritis.Reduction in bacterial growth after application of topical antibacterial agents has been reported to permit spontaneous healing of deep partial-thickness burns by preventing conversion of the partial thickness to full thickness by sepsis. However, reduction in bacterial colonization has caused delayed separation, in some cases necessitating escharotomy in order to prevent contracture.DOSAGE AND ADMINISTRATIONPrompt institution of appropriate regimens for care of the burned patient is of prime importance and includes the control of shock and pain. The burn wounds are then cleansed and debrided, and SILVADENE Cream 1% (silver sulfadiazine) is applied under sterile conditions. The burn areas should be covered with SILVADENE Cream 1% at all times. The cream should be applied once to twice daily to a thickness of approximately 1/16 inch. Whenever necessary, the cream should be reapplied to any areas from which it has been removed by patient activity. Administration may be accomplished in minimal time because dressings are not required. However, if individual patient requirements make dressings necessary, they may be used.Reapply immediately after hydrotherapy.Treatment with SILVADENE Cream 1% should be continued until satisfactory healing has occurred, or until the burn site is ready for grafting. The drug should not be withdrawn from the therapeutic regimen while there remains the possibility of infection except if a significant adverse reaction occurs.HOW SUPPLIEDSILVADENE Cream 1% (silver sulfadiazine) is available in jars containing 50 g (NDC 61570-131-50), 400 g (NDC 61570-131-40), and 1000 g (NDC 61570-131-98) and tubes containing 20 g (NDC 61570-131-20) and 85 g (NDC 61570-131-85).REFERENCES1. Caffee F, Bingham H. Leukopenia and silver sulfadiazine. J Trauma. 1982;22: 586–587.2. Jarret F, Ellerbe S, Demling R. Acute leukopenia during topical burn therapy with silversulfadiazine. Amer J Surg. 1978;135:818–819.3. Kiker RG, Carvajal HF, Micak RP, Larson DL. A controlled study of the effects of silversulfadiazine on white blood cell counts in burned children. J Trauma. 1977; 17:835–836. Prescribing Information as of July 2003.Distributed by:Monarch Pharmaceuticals, Inc.Bristol, TN 37620(A wholly owned subsidiary of King Pharmaceuticals, Inc.)Manufactured by:King Pharmaceuticals, Inc.Bristol, TN 37620LAB-0636-1.0Revised September 2012。