普特彼(他克莫司软膏)简要说明书
- 格式:docx
- 大小:24.72 KB
- 文档页数:7

普特彼(他克莫司软膏)说明书【普特彼药品名称】通用名:他克莫司软膏商品名:普特彼英文名:TacrolimusOintment汉语拼音:TaKeMoSiRuanGao【普特彼成份】普特彼主要成份为:他克莫司。
【普特彼性状】普特彼为白色至淡黄色软膏。
【普特彼处方组成】每克普特彼含他克莫司0.03或0.1(w/w),软膏基质为矿物油、石蜡、碳酸丙烯酯、白凡士林和白蜡。
【普特彼药代动力学】综合对49例成年特应性皮炎患者进行的两项药代动力学研究的结果表明,局部应用0.1浓度的普特彼后,他克莫司会被吸收。
单次或多次应用0.1浓度的普特彼后,血中他克莫司峰浓度介于检测不出至20ng/ml之间,49例患者中有45例血药峰浓度值低于5ng/ml。
对20例儿童特应性皮炎患者(年龄6-13岁)进行的药代动力学研究结果表明,应用0.1浓度的普特彼后,所有患者血中他克莫司峰浓度均低于1.6ng/ml。
从血药浓度来看,间歇性局部应用普特彼长达一年也不会导致他克莫司在全身蓄积。
局部应用他克莫司的生物利用度尚不清楚。
以静脉注射他克莫司的历史数据作对比,特应性皮炎患者局部应用普特彼的相对生物利用度低于0.5。
在平均治疗体表面积(BSA)达53的成人中,局部应用普特彼后的吸收量(即AUC)约比肾或肝移植患者将他克莫司作为免疫抑制剂口服的吸收量低30倍。
能引起全身性作用的他克莫司血药浓度目前尚不清楚。
【普特彼适应症】普特彼适用于因潜在危险而不宜使用传统疗法、或对传统疗法反应不充分、或无法耐受传统疗法的中到重度特应性皮炎患者,作为短期或间歇性长期治疗。
0.03和0.1浓度的普特彼均可用于成人,但只有0.03浓度的普特彼可用于2岁及以上的儿童。
【普特彼用法用量】成人0.03和0.1他克莫司软膏在患处皮肤涂上一薄层普特彼,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。

他克莫司用法(一)他克莫司1. 介绍他克莫司(英文名称:Tacrolimus),是一种免疫抑制剂,广泛应用于器官移植手术后预防和治疗排斥反应。
2. 适应症•器官移植术后的免疫抑制:他克莫司可以用于肾脏、心脏、肺、肝脏、胰腺等器官移植手术后免疫抑制的预防和治疗。
3. 用法用量•肾移植患者:术后第1天开始,根据患者情况,每日口服他克莫司/kg分2次服用,调整剂量以维持血药浓度在正确的范围内。
4. 注意事项•高血压:长期使用他克莫司可能导致高血压,需要定期监测血压情况。
•肾功能不全:由于他克莫司主要通过肾脏代谢,肾功能不全的患者需要调整剂量,避免药物积累导致毒副作用。
•多种药物相互作用:他克莫司与某些药物(如氯霉素、酮康唑等)相互作用,可能影响药物的疗效或增加毒副作用,务必告知医生正在使用的其他药物。
5. 不良反应•肾功能损害:部分患者在使用他克莫司期间可能出现肾功能不全,需要定期监测肾功•皮肤反应:少数患者可能出现皮肤病变、瘙痒等不良反应,应立即停药并就医。
•高血糖:他克莫司使用时间较长的患者可能出现高血糖,需要监测血糖情况。
6. 禁忌症•对他克莫司过敏者禁用。
•孕妇和哺乳期妇女慎用。
7. 药物储存•存放在阴凉干燥处,避免阳光直射和高温。
以上就是关于他克莫司的一些用法和注意事项的简要介绍,希望可以对大家有所帮助。
请在使用药物前务必咨询医生,并仔细阅读药品说明书。
1. 药物副作用•恶心和呕吐:他克莫司治疗期间,部分患者可能出现恶心和呕吐的副作用,可适量进食并与医生沟通寻求缓解措施。
•腹泻:有些患者可能出现腹泻症状,应保持充足的水分摄入并避免高脂高纤维食物。
•高血压:他克莫司可能导致一些患者的血压升高,需要定期监测,并根据医生建议进行控制。
•感染:免疫抑制作用可能增加患者感染的风险,特别是真菌感染,应避免接触病原体,并及时发现和治疗感染。
2. 药物相互作用•他克莫司可能与一些药物相互作用,包括其他免疫抑制剂、抗生素、抗真菌药物等,应在使用其他药物前告知医生,避免药物相互作用导致不良反应。

他克莫司软膏注意事项他克莫司软膏是一种常用的外用药物,主要用于治疗皮肤病和炎症。
在使用他克莫司软膏之前,我们需要了解一些注意事项和禁忌症。
以下是与他克莫司软膏相关的一些重要注意事项:1. 注意病情:在使用他克莫司软膏之前,应先确诊病情,并确保其适用于外用药物治疗。
某些皮肤疾病或局部感染可能需要其他治疗方法。
因此,最重要的是在使用前咨询医生,并根据专业的指导使用。
2. 避免接触眼睛和黏膜:他克莫司软膏只适用于外用,禁止接触眼睛和黏膜。
使用药物后应用清水冲洗双手,避免将药物传到其他部位。
3. 保持干燥和清洁:在使用他克莫司软膏期间,应保持患处干燥和清洁。
避免过度搔抓或磨擦。
最好药物吸收后避免受潮,以免影响药效。
4. 遵循剂量和使用周期:根据医生的推荐使用他克莫司软膏的剂量和使用周期。
不要随意增加或减少药物剂量,以免出现药物残留或缺乏治疗效果的问题。
5. 不要与其他药物混合使用:在使用他克莫司软膏时,应避免与其他药物混合使用,特别是其他外用药物。
这可能导致不良反应或药物相互作用。
6. 孕妇和哺乳期妇女:如果您是孕妇或正在哺乳,使用他克莫司软膏前请先咨询医生。
有效成分可能对胎儿或婴儿有不利影响,需谨慎使用。
7. 不适合过敏体质者:他克莫司软膏可能引起过敏反应,因此对其中任何一种成分过敏的人不适合使用。
在使用药物前,最好做皮肤敏感性测试。
8. 不适合长期使用:他克莫司软膏通常用于短期治疗,长时间使用有可能出现皮肤萎缩、皮肤变薄、毛细血管扩张等不良反应。
如果症状没有改善,请咨询医生进行进一步的治疗方案。
9. 孩童使用:如果您为儿童使用他克莫司软膏,请先咨询医生。
儿童可能对药物成分更敏感,因此医生会根据儿童的年龄和体重来确定剂量和使用频率。
10. 药物保存:保持他克莫司软膏在室温下保存,避免阳光直射。
确保药物存放在儿童无法接触的地方。
总之,在使用他克莫司软膏前,请仔细阅读药物说明书,并咨询医生的指导。
遵循医嘱使用药物,注意患处的干燥和清洁,避免药物接触眼睛和黏膜。

他克莫司软膏作用和用途他克莫司软膏是一种常用的局部药物,主要成分为他克莫司。
他克莫司是一种免疫抑制剂,通过抑制T细胞的活性来达到其药理作用。
他克莫司软膏主要用于治疗多种皮肤炎症和过敏反应。
他克莫司软膏的主要作用机制是通过抑制免疫反应来减轻炎症和过敏反应。
他克莫司可以抑制细胞因子IL-2和IL-4的产生,这些细胞因子在免疫反应中起到关键作用。
通过抑制这些细胞因子的产生,他克莫司可以抑制T细胞的活性,从而减轻炎症和过敏反应。
他克莫司软膏主要用于治疗一些皮肤炎症和过敏反应,如湿疹、皮肤瘙痒、接触性皮炎、过敏性皮炎等。
这些疾病多数是由于免疫系统异常引起的,使用他克莫司软膏可以通过抑制免疫反应来减轻炎症和症状。
具体来说,他克莫司软膏可以减轻炎症反应,缓解皮肤红肿、瘙痒、热痛等症状。
同时,他克莫司软膏还具有减少皮肤免疫细胞数量和抑制炎症细胞活性的作用,从而阻断炎症反应的发展和恶化。
他克莫司软膏的使用方法很简单。
一般情况下,患者将软膏均匀涂抹在患处,每天使用2到3次。
在使用软膏前,需先将患处清洗干净,并确保患处干燥。
在涂抹软膏后,请轻轻按摩软膏,以帮助其更好地渗透。
然而,尽管他克莫司软膏具有显著的疗效,但仍然有一些需要注意的事项。
首先,患者在使用他克莫司软膏时应避免接触到眼睛和口腔黏膜,以免引起不必要的炎症或刺激。
另外,长期、过量或不当使用他克莫司软膏可能会导致皮肤萎缩、皮肤易受伤和感染等副作用,因此患者在使用时要按医生的建议进行合理用药。
总的来说,他克莫司软膏是一种常用的局部药物,主要用于治疗多种皮肤炎症和过敏反应。
该软膏通过抑制T细胞的活性来减轻炎症和过敏反应,从而缓解相关症状。
患者在使用该软膏时应注意遵循医生的建议,避免不必要的副作用和并发症的发生。

他克莫司胶囊以下内容仅供参考,请以药品包装盒中的说明书为准。
妊娠:慎用哺乳:服用本药后应停止哺乳核准日期:2009年09月30日修改日期:2009年10月23日2016年05月31日2016年08月29日2017年04月21日他克莫司胶囊说明书请仔细阅读说明书并在医师指导下使用警示语:由于免疫抑制,发生淋巴瘤和其他恶性肿瘤,尤其是皮肤癌的风险增加;对细菌、病毒、真菌和原虫感染包括机会感染在内的易感性增加。
本品应由有免疫抑制治疗和器官移植病人管理经验的医师处方。
服用本品的患者应由配备足够实验室设备和医护人员的医疗机构进行随访。
负责维持治疗的医师应掌握进行随访所需的全部信息。
【药品名称】通用名称:他克莫司胶囊英文名称:Tacrolimus Capsules汉语拼音:Takemosi Jiaonang【成份】本品主要成份为他克莫司。
【性状】本品为硬胶囊,内容物为白色或类白色粉末。
【适应症】预防肝脏或肾脏移植术后的移植物排斥反应。
治疗肝脏或肾脏移植术后应用其他免疫抑制药物无法控制的移植物排斥反应。
【规格】0.5mg(以他克莫司计)【用法用量】他克莫司的治疗需要在配备有充足实验设备和人员的条件下密切监测。
只有在免疫抑制治疗和移植患者管理方面有经验的医师才可处方他克莫司和改变免疫抑制治疗方案。
不慎、无意或在无监督下的他克莫司胶囊和他克莫司缓释胶囊之间的转换是不安全的。
这可能导致移植物排斥或增加不良反应发生,包括由于他克莫司全身暴露的临床相关差异而导致的免疫抑制不足或过度。
患者应维持他克莫司单一剂型及相应的日给药方案进行治疗。
改变剂型或调整剂量只能在移植专家严密的监督下进行。
任何剂型转换后,都需要监测治疗药物,并调整剂量以保证他克莫司的全身暴露前后一致。
以下推荐起始剂量仅作一般指导。
给药剂量主要基于对个体患者排斥反应和耐受性的临床评价辅以血药浓度监测(参见以下推荐目标全血谷浓度)。
如果排斥反应临床症状明显,则应考虑改变免疫抑制治疗方案。

他克莫司软膏的功能主治1. 介绍他克莫司软膏是一种常用的外用药物,主要用于治疗各种皮肤炎症和过敏症。
它以他克莫司为主要成分,具有抗炎、抗过敏的作用,能够缓解皮肤的痒、红、肿等症状,有效改善皮肤炎症的状态。
2. 功能主治使用他克莫司软膏可以对多种疾病进行治疗和缓解,以下为他克莫司软膏的功能主治:•皮肤炎症:他克莫司软膏可用于治疗各种原因引起的皮肤炎症,如湿疹、过敏性皮炎、接触性皮炎等。
它能够减轻皮肤发痒、烧灼感、红肿等症状,同时减少炎症反应,促进皮肤的修复和恢复。
•肛门瘙痒:他克莫司软膏在治疗肛门瘙痒方面也具有一定的效果。
肛门瘙痒是一种常见的症状,往往给患者带来不适和困扰。
使用他克莫司软膏可以减轻瘙痒感,缓解肛门不适,改善患者的生活质量。
•异位性皮炎:异位性皮炎是一种常见的慢性皮肤病,常表现为皮肤瘙痒、红斑、脱屑等症状。
他克莫司软膏可以帮助减轻异位性皮炎的症状,改善皮肤的炎症状态,缓解病情进展。
•过敏性鼻炎:除了治疗皮肤炎症外,他克莫司软膏还可用于治疗过敏性鼻炎。
过敏性鼻炎是一种常见的过敏疾病,主要表现为鼻塞、流涕、打喷嚏等症状。
通过鼻腔内使用他克莫司软膏,可以减轻鼻腔炎症反应,缓解过敏性鼻炎的症状。
•其他炎症性皮肤病:他克莫司软膏还可用于治疗其他炎症性皮肤病,如银屑病、牛皮癣等。
它能够减轻皮肤的炎症反应,改善病情,促进皮肤的康复。
3. 注意事项在使用他克莫司软膏时,需要注意以下事项:•使用方法:按照医生或药剂师的指示正确使用软膏,可外用于患处,少量涂抹并轻轻按摩。
避免接触到眼睛和口腔等敏感部位。
•副作用:他克莫司软膏可能会引起皮肤刺激、刺痛、烧灼感等副作用,如果出现严重过敏反应,请立即停止使用并咨询医生。
•孕妇和哺乳期妇女:孕妇和哺乳期妇女需在医生指导下使用他克莫司软膏。
•与其他药物的相互作用:在使用他克莫司软膏时,需告知医生正在使用的其他药物情况,以避免不良药物相互作用。
•注意保湿:在使用他克莫司软膏的同时,也需要注意保湿护理,可选择适合自己的保湿产品。


普特彼(他克莫司软膏)【用法用量】成人0.03%和0.1%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
本品不应采用封包敷料外用。
儿童0.03%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
本品不应采用封包敷料外用。
【注意事项】1.肝肾功能不全、糖尿病、高钾血症、心室肥大、有神经性毒性表现者,如震颤、头痛、共济失调、精神状态改变等慎用。
2.对老年患者用药的临床数据较少,但均提示应与其它成人剂量相同。
3.肝肾移植的维持治疗阶段,必须持续使用本品来维持移植物功能。
推荐需根据患者个体差异来定。
在维持治疗期间有本品用量逐渐减少的趋势。
剂量调整主要根据对排斥反应的临床治疗效果和患者的耐受性判断。
4.妊娠时禁用本品,动物实验(小鼠及兔子)表明,本品具有致畸作用,并且某些剂量还显示出对母体具有毒性。
临床前及临床数据表明,该药能透过胎盘。
因此在应用本品前应排除妊娠的可能性。
本品能干扰口服避孕药的代谢,应改用其它方式避孕。
临床前兔身上的试验表明,本品分泌进乳汁。
5.哺乳期使用本品的经验有限。
不能排除对新生儿的有害影响,妇女患者在使用本品时不应哺乳。
6.本品用量应根据临床诊断辅以全血药物浓度相应调整,其全血药物浓度在20ng/ml均能取得较好效果。
由于其半衰期长,调整剂量需要几天时间才能真正反映其血液中药物浓度的变化。
剂量和血药浓度的调节必须是在负责管理患者的移植中心。
【不良反应】在服用本品期间,如果感到不适要尽快告诉医师或药师。
情况紧急可先停止服药。
详情请看说明书。
【禁忌】1.妊娠禁用。
2.本品化学结构属于大环内酯类,对他克莫司或其它大环内酯类药物过敏者、对胶囊中其它成份过敏者禁用。
【适应症】本品适用于因潜在危险而不宜使用传统疗法、或对传统疗法反应不充分、或无法耐受传统疗法的中到重度特应性皮炎患者,作为短期或间歇性长期治疗。
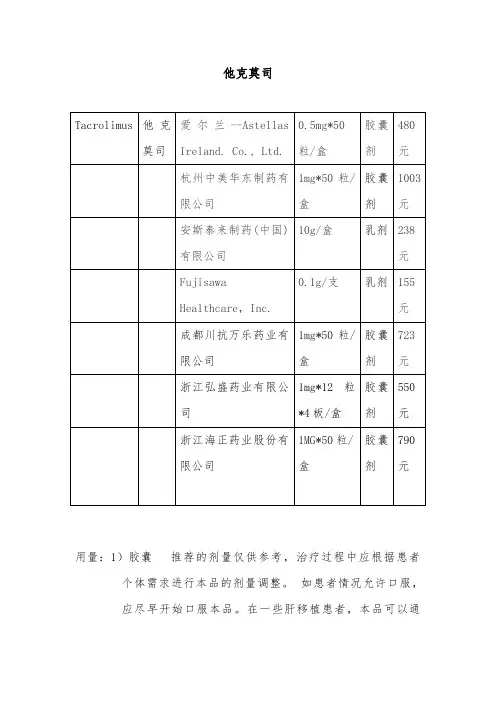
他克莫司用量:1)胶囊推荐的剂量仅供参考,治疗过程中应根据患者个体需求进行本品的剂量调整。
如患者情况允许口服,应尽早开始口服本品。
在一些肝移植患者,本品可以通过鼻饲来口服给药。
本品通常与其他免疫抑制药物一起使用,亦出现有单独使用本品的个例报道。
本品不能与环孢素并用。
如出现排斥反应或不良事件发生,需考虑更改免疫抑制治疗方案。
在维持治疗阶段,建议持续使用本品来维持移植物的存活。
如患者病情恶化(如出现急性排斥反应的征兆),应考虑改变免疫抑制剂用药方案。
多种方案均可用于控制排斥反应,如增加类固醇激素用量、加用短期的单克隆或多克隆抗体、增加本品的用量。
如出现中毒征兆(如明显的不良事件),应减少本品的用量。
并应告诉患者,在未经主管医师同意的情况下,不应擅自减量。
在移植术后患者的情况改善期内,本品的药代动力学可能会发生改变,需要调整本品的剂量。
每日服药两次(早晨和晚上),最好用水送服。
建议空腹,或者至少在餐前1小时或餐后2-3小时服用。
如必要可将胶囊内容物悬浮于水,经鼻饲管给药。
若患者临床状况不能口服,首剂须静脉给药。
2)乳膏成人0.03%和0.1%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
本品不应采用封包敷料外用。
儿童0.03%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
普特彼(他克莫司软膏) 不应采用封包敷料外用。
市场他克莫司在中国上市的短时间内,市场份额迅速上升,已成为肝脏及肾脏移植后排斥反应的临床一线药物。
目前我国免疫抑制剂市场规模为50~60亿,其中国外公司将的他克莫司免疫抑制剂市场份额占80%,开发我国内他克莫司具有自主知识产权的生产技术及开发技术迫在眉急。
PDR? Electronic Library(TM)This report is based solely on product labeling as published by Physicians?Desk Reference ? Copyright ?2003 Thomson Medical Economics. All rights reserved.Report generated 09-06-2004 at 08:02 pmProtopic Ointment(Fujisawa)FOR DERMATOLOGIC USE ONLYNOT FOR OPHTHALMIC USEDESCRIPTIONPROTOPIC (tacrolimus) Ointment contains tacrolimus, a macrolide immunosuppressant produced by Streptomyces tsukubaensis . It is for topical dermatologic use only. Chemically, tacrolimus is designated as [3 S -[3 R *[ E (1 S *,3 S *,4 S *)],4 S *,5 R *,8 S *,9 E ,12 R *,14 R *, 15 S *, 16 R *,18 S *,19 S *,26a R *]] - 5,6,8,11,12,13,14,15,16,17,18, 19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(2-propenyl)-15, 19-epoxy-3H-pyrido[2,1- c ][1,4]oxaazacyclotricosine-1,7,20,21(4H,23H)-tetrone, monohydrate. It has the following structural formula:Tacrolimus has an empirical formula of C44 H69NO12·H2O and a formula weight of 822.05. Each gram of PROTOPIC Ointmentcontains (w/w) either 0.03% or 0.1% of tacrolimus in a base of mineral oil, paraffin, propylene carbonate, white petrolatum and white wax.CLINICAL PHARMACOLOGYMechanism of ActionThe mechanism of action of tacrolimus in atopic dermatitis is not known. While the following have been observed, the clinicalsignificance of these observations in atopic dermatitis is not known. It has been demonstrated that tacrolimus inhibits T-lymphocyte activation by first binding to an intracellular protein, FKBP-12. A complex of tacrolimus-FKBP-12, calcium, calmodulin, and calcineurin is then formed and the phosphatase activity of calcineurin is inhibited. This effect has been shown to prevent the dephosphorylation and translocation of nuclear factor of activated T-cells (NF-AT), a nuclear component thought to initiate genetranscription for the formation of lymphokines (such as interleukin-2, gamma interferon). Tacrolimus also inhibits the transcription for genes which encode IL-3, IL-4, IL-5, GM-CSF, and TNF-(alpha), all of which are involved in the early stages of T-cell activation. Additionally, tacrolimus has been shown to inhibit the release of pre-formed mediators from skin mast cells and basophils, and to downregulate the expression of Fc[egr ]Rl on Langerhans cells. PharmacokineticsThe pooled results from two pharmacokinetic studies in 49 adult atopic dermatitis patients indicate that tacrolimus is absorbed after the topical application of 0.1% PROTOPIC Ointment. Peak tacrolimus blood concentrations ranged from undetectable to 20 ng/mL after single or multiple doses of 0.1% PROTOPIC Ointment, with 45 of the 49 patients having peak blood concentrations less than 5ng/mL. The results from a pharmacokinetic study of 0.1% PROTOPIC Ointment in 20 pediatric atopic dermatitis patients (ages 6-13 years), show peak tacrolimus blood concentrations below 1.6 ng/mL in all patients.There was no evidence based on blood concentrations that tacrolimus accumulates systemically upon intermittent topical application for periods of up to 1 year. The absolute bioavailability of topical tacrolimus is unknown. Using IV historical data for comparison, the bioavailability of tacrolimus from PROTOPIC in atopic dermatitis patients is less than 0.5%. In adults with an average of 53% BSA treated, exposure (i.e., AUC) of tacrolimus from PROTOPIC is approximately 30-fold less than that seen with oralimmunosuppressive doses in kidney and liver transplant patients. The lowest tacrolimus blood level at which systemic effects can be observed is not known. CLINICAL STUDIESThree randomized, double-blind, vehicle-controlled, multi-center, phase 3 studies were conducted to evaluate PROTOPIC Ointment for the treatment of patients with moderate to severe atopic dermatitis. One (Pediatric) study included 351 patients 2-15 years of age, and the other two (Adult) studies included a total of 632 patients 15-79 years of age. Fifty-five percent (55%) of the patients were women and 27% were black. At baseline, 58% of the patients had severe disease and the mean body surface area (BSA) affected was 46%. Over 80% of patients had atopic dermatitis affecting the face and/or neck region. In these studies, patients applied eitherPROTOPIC Ointment 0.03%, PROTOPIC Ointment 0.1%, or vehicle ointment twice daily to 10%-100% of their BSA for up to 12 weeks.In the pediatric study, a significantly greater (p < 0.001) percentage of patients achieved at least 90% improvement based on the physician's global evaluation of clinical response (the pre-defined primary efficacy end point) in the PROTOPIC Ointment 0.03% treatment group compared to the vehicle treatment group, but there was insufficient evidence that PROTOPIC Ointment 0.1% provided more efficacy than PROTOPIC Ointment 0.03%.In both adult studies, a significantly greater (p < 0.001) percentage of patients achieved at least 90% improvement based on the physician's global evaluation of clinical response in the PROTOPIC Ointment 0.03% and PROTOPIC Ointment 0.1% treatmentgroups compared to the vehicle treatment group. There was evidence that PROTOPIC Ointment 0.1% may provide more efficacy than PROTOPIC Ointment 0.03%. The difference in efficacy between PROTOPIC Ointment 0.1% and 0.03% was particularly evident in adult patients with severe disease at baseline, adults with extensive BSA involvement, and black adults. Response rates for each treatment group are shown below by age groups. Because the two adult studies were identically designed, the results from these studies were pooled in this table.A statistically significant difference in the percentage of adult patients with >/= 90% improvement was achieved by week 1 for those treated with PROTOPIC Ointment 0.1%, and by week 3 for those treated with PROTOPIC Ointment 0.03%. A statistically significant difference in the percentage of pediatric patients with >/= 90% improvement was achieved by week 2 for those treated with PROTOPIC Ointment 0.03%.In adult patients who had achieved >/= 90% improvement at the end of treatment, 35% of those treated with PROTOPIC OintmentGlobal Improvement over Baseline at the End-of-Treatment in Three Phase 3 Studies Physician's Global Evaluation of Clinical Response (% Improvement)Pediatric Study (2-15 Years of Age) Adult StudiesVehicle Ointment N = 116 PROTOPIC Ointment 0.03% N = 117VehicleOintment N = 212 PROTOPICOintment 0.03% N = 211PROTOPIC Ointment 0.1% N = 209100% 4 (3%) 14 (12%) 2 (1%) 21 (10%) 20 (10%) >/=90% 8 (7%) 42 (36%) 14 (7%) 58 (28%) 77 (37%) >/=75% 18 (16%) 65 (56%) 30 (14%) 97 (46%) 117 (56%) >/=50%31 (27%)85 (73%)42 (20%)130 (62%)152 (73%)0.03% and 41% of those treated with PROTOPIC Ointment 0.1%, regressed from this state of improvement at 2 weeks after end-of-treatment. In pediatric patients who had achieved >/= 90% improvement, 54% of those treated with PROTOPIC Ointment 0.03% regressed from this state of improvement at 2 weeks after end-of-treatment. Because patients were not followed for longer than 2 weeks after end-of-treatment, it is not known how many additional patients regressed at periods longer than 2 weeks after cessation of therapy.In both PROTOPIC Ointment treatment groups in adults and in the PROTOPIC Ointment 0.03% treatment group in pediatric patients, a significantly greater improvement compared to vehicle (p < 0.001) was observed in the secondary efficacy endpoints of percent body surface area involved, patient evaluation of pruritus, erythema, edema, excoriation, oozing, scaling, and lichenification. The following two graphs depict the time course of improvement in the percent body surface area affected in adult and in pediatric patients as a result of treatment.The following two graphs depict the time course of improvement in erythema in adult and in pediatric patients as a result of treatment.The time course of improvement in the remaining secondary efficacy variables was similar to that of erythema, with improvement in lichenification slightly slower.A total of 571 patients applied PROTOPIC Ointment 0.1% in long-term adult and pediatric safety studies for up to one year. In the adult study, 246 patients were evaluated for at least 6 months and 68 patients for 12 months. In the pediatric study, 219 patients were evaluated for at least 6 months and 180 patients for 12 months. On average, patients received treatment for 87% of study days.INDICATIONS AND USAGEPROTOPIC Ointment, both 0.03% and 0.1% for adults, and only 0.03% for children aged 2 to 15 years, is indicated for short-term and intermittent long-term therapy in the treatment of patients with moderate to severe atopic dermatitis in whom the use of alternative, conventional therapies are deemed inadvisable because of potential risks, or in the treatment of patients who are not adequately responsive to or are intolerant of alternative, conventional therapies.CONTRAINDICATIONSPROTOPIC Ointment is contraindicated in patients with a history of hypersensitivity to tacrolimus or any other component of the preparation.PRECAUTIONSGeneralStudies have not evaluated the safety and efficacy of PROTOPIC Ointment in the treatment of clinically infected atopic dermatitis. Before commencing treatment with PROTOPIC Ointment, clinical infections at treatment sites should be cleared.While patients with atopic dermatitis are predisposed to superficial skin infections including eczema herpeticum (Kaposi's varicelliform eruption), treatment with PROTOPIC Ointment may be associated with an increased risk of varicella zoster virus infection (chicken pox or shingles), herpes simplex virus infection, or eczema herpeticum. In the presence of these infections, the balance of risks and benefits associated with PROTOPIC Ointment use should be evaluated.In clinical studies, 33 cases of lymphadenopathy (0.8%) were reported and were usually related to infections (particularly of the skin) and noted to resolve upon appropriate antibiotic therapy. Of these 33 cases, the majority had either a clear etiology or were known to resolve. Transplant patients receiving immunosuppressive regimens (e.g., systemic tacrolimus) are at increased risk for developing lymphoma; therefore, patients who receive PROTOPIC Ointment and who develop lymphadenopathy should have the etiology of their lymphadenopathy investigated. In the absence of a clear etiology for the lymphadenopathy, or in the presence of acute infectious mononucleosis, discontinuation of PROTOPIC Ointment should be considered. Patients who develop lymphadenopathy should be monitored to ensure that the lymphadenopathy resolves.The enhancement of ultraviolet carcinogenicity is not necessarily dependent on phototoxic mechanisms. Despite the absence of observed phototoxicity in humans (see ADVERSE REACTIONS ), PROTOPIC Ointment shortened the time to skin tumor formation in an animal photocarcinogenicity study (see Carcinogenesis, Mutagenesis, Impairment of Fertility ). Therefore, it is prudent for patients to minimize or avoid natural or artificial sunlight exposure.The use of PROTOPIC Ointment may cause local symptoms such as skin burning (burning sensation, stinging, soreness) or pruritus. Localized symptoms are most common during the first few days of PROTOPIC Ointment application and typically improve as the lesions of atopic dermatitis heal. With PROTOPIC Ointment 0.1%, 90% of the skin burning events had a duration between 2 minutes and 3 hours (median 15 minutes). Ninety percent of the pruritus events had a duration between 3 minutes and 10 hours (median 20 minutes).The use of PROTOPIC Ointment in patients with Netherton's Syndrome is not recommended due to the potential for increased systemic absorption of tacrolimus. The safety of PROTOPIC Ointment has not been established in patients with generalized erythroderma.Information for Patients(See patient package insert)Patients using PROTOPIC Ointment should receive the following information and instructions:1.Patients should use PROTOPIC Ointment as directed by the physician. PROTOPIC Ointment is for external use only. As withany topical medication, patients or caregivers should wash hands after application if hands are not an area for treatment.2.Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while usingPROTOPIC Ointment.3.Patients should not use this medication for any disorder other than that for which it was prescribed.4.Patients should report any signs of adverse reactions to their physician.5.Before applying PROTOPIC Ointment after a bath or shower, be sure your skin is completely dry.Drug InteractionsFormal topical drug interaction studies with PROTOPIC Ointment have not been conducted. Based on its minimal extent of absorption, interactions of PROTOPIC Ointment with systemically administered drugs are unlikely to occur but cannot be ruled out. The concomitant administration of known CYP3A4 inhibitors in patients with widespread and/or erythrodermic disease should be done with caution. Some examples of such drugs are erythromycin, itraconazole, ketoconazole, fluconazole, calcium channel blockers and cimetidine.Carcinogenesis, Mutagenesis, Impairment of FertilityNo evidence of genotoxicity was seen in bacterial ( Salmonella and E. coli ) or mammalian (Chinese hamster lung-derived cells) in vitro assays of mutagenicity, the in vitro CHO/HGPRT assay of mutagenicity, or in vivo clastogenicity assays performed in mice. Tacrolimus did not cause unscheduled DNA synthesis in rodent hepatocytes.Oral (feed) carcinogenicity studies have been carried out with systemically administered tacrolimus in male and female rats and mice. In the 80-week mouse study and in the 104-week rat study no relationship of tumor incidence to tacrolimus dosage was found at daily doses up to 3 mg/kg [9X the Maximum Recommended Human Dose (MRHD) based on AUC comparisons] and 5 mg/kg (3X the MRHD based on AUC comparisons), respectively.A 104-week dermal carcinogenicity study was performed in mice with tacrolimus ointment (0.03%-3%), equivalent to tacrolimus doses of 1.1-118 mg/kg/day or 3.3-354 mg/m 2 /day. In the study, the incidence of skin tumors was minimal and the topical application of tacrolimus was not associated with skin tumor formation under ambient room lighting. However, a statistically significant elevation in the incidence of pleomorphic lymphoma in high dose male (25/50) and female animals (27/50) and in the incidence of undifferentiated lymphoma in high dose female animals (13/50) was noted in the mouse dermal carcinogenicity study. Lymphomas were noted in the mouse dermal carcinogenicity study at a daily dose of 3.5 mg/kg (0.1% tacrolimus ointment) (26X MRHD based on AUC comparisons). No drug-related tumors were noted in the mouse dermal carcinogenicity study at a daily dose of 1.1 mg/kg (0.03% tacrolimus ointment) (10X MRHD based on AUC comparisons).In a 52-week photocarcinogenicity study, the median time to onset of skin tumor formation was decreased in hairless mice following chronic topical dosing with concurrent exposure to UV radiation (40 weeks of treatment followed by 12 weeks of observation) with tacrolimus ointment at >/=0.1% tacrolimus.Reproductive toxicology studies were not performed with topical tacrolimus. In studies of oral tacrolimus no impairment of fertility was seen in male and female rats. Tacrolimus, given orally at 1.0 mg/kg (0.12X MRHD based on body surface area [BSA]) to male and female rats, prior to and during mating, as well as to dams during gestation and lactation, was associated with embryolethality and with adverse effects on female reproduction. Effects on female reproductive function (parturition) and embryolethal effects were indicated by a higher rate of pre-implantation loss and increased numbers of undelivered and nonviable pups. When given at 3.2mg/kg (0.43X MRHD based on BSA), tacrolimus was associated with maternal and paternal toxicity as well as reproductive toxicity including marked adverse effects on estrus cycles, parturition, pup viability, and pup malformations.PregnancyTeratogenic Effects: Pregnancy Category CThere are no adequate and well-controlled studies of topically administered tacrolimus in pregnant women. The experience with PROTOPIC Ointment when used by pregnant women is too limited to permit assessment of the safety of its use during pregnancy. Reproduction studies were carried out with systemically administered tacrolimus in rats and rabbits. Adverse effects on the fetus were observed mainly at oral dose levels that were toxic to dams. Tacrolimus at oral doses of 0.32 and 1.0 mg/kg (0.04X-0.12X MRHD based on BSA) during organogenesis in rabbits was associated with maternal toxicity as well as an increase in incidence of abortions. At the higher dose only, an increased incidence of malformations and developmental variations was also seen. Tacrolimus, at oral doses of 3.2 mg/kg during organogenesis in rats, was associated with maternal toxicity and caused an increase in late resorptions, decreased numbers of live births, and decreased pup weight and viability. Tacrolimus, given orally at 1.0 and 3.2 mg/kg (0.04X-0.12X MRHD based on BSA) to pregnant rats after organogenesis and during lactation, was associated with reduced pup weights.No reduction in male or female fertility was evident.There are no adequate and well-controlled studies of systemically administered tacrolimus in pregnant women. Tacrolimus is transferred across the placenta. The use of systemically administered tacrolimus during pregnancy has been associated with neonatal hyperkalemia and renal dysfunction. PROTOPIC Ointment should be used during pregnancy only if the potential benefit to the mother justifies a potential risk to the fetus.Nursing MothersAlthough systemic absorption of tacrolimus following topical applications of PROTOPIC Ointment is minimal relative to systemic administration, it is known that tacrolimus is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from tacrolimus, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.Pediatric UsePROTOPIC Ointment 0.03% may be used in pediatric patients 2 years of age and older. Two phase 3 pediatric studies were conducted involving 606 patients 2-15 years of age: one 12-week randomized vehicle-controlled study and one open-label, 1 year, long-term safety study. Three hundred and thirty (330) of these patients were 2 to 6 years of age.The most common adverse events associated with PROTOPIC Ointment application in pediatric patients were skin burning and pruritus (see ADVERSE REACTIONS). In addition to skin burning and pruritus, the less common events (<5%) of varicella zoster (mostly children pox), and vesiculobullous rash were more frequent in patients treated with PROTOPIC Ointment 0.03% compared to vehicle. In the long-term 1 year safety study involving 255 pediatric patients using PROTOPIC Ointment, the incidence of adverse events, including infections, did not increase with increased duration of study drug exposure or amount of ointment used. In 491 pediatric patients treated with PROTOPIC Ointment, 3(0.6%) developed eczema herpeticum. Since the safety and efficacy of PROTOPIC Ointment have not been established in pediatric patients below 2 years of age, its use in this age group is not recommended.Geriatric UseTwenty-five (25) patients >/= 65 years old received PROTOPIC Ointment in phase 3 studies. The adverse event profile for these patients was consistent with that for other adult patients.ADVERSE REACTIONSNo phototoxicity and no photoallergenicity was detected in clinical studies of 12 and 216 normal volunteers, respectively. One out of 198 normal volunteers showed evidence of sensitization in a contact sensitization study.In three randomized vehicle-controlled studies and two long-term safety studies, 655 and 571 patients respectively, were treated with PROTOPIC Ointment.The following table depicts the adjusted incidence of adverse events pooled across the 3 identically designed 12 week studies for patients in vehicle, PROTOPIC Ointment 0.03%, and PROTOPIC Ointment 0.1% treatment groups, and the unadjusted incidence of adverse events in two one year long-term safety studies, regardless of relationship to study drug.Phase 3 Studies12-Week Adjusted Incidence Rate (%) Tacrolimius Ointment Incidence(%)Adult Pediatric Adult PediatricVehicle n=2120.03%TacrolimusOintmentn=2100.1%TacrolimusOintmentn=209Vehiclen=1160.03%TacrolimusOintmentn=118 n=316 n=255Skin Burning †26 46 58 29 43 47 26 Pruritus †37 46 46 27 41 25 25 Flu-like symptoms †19 23 31 25 28 22 35 Allergic Reaction 8 12 6 8 4 22 15 Skin Erythema 20 25 28 13 12 12 9 Headache †11 20 19 8 5 10 18 Skin Infection 11 12 5 14 10 11 11 Fever 4 4 1 13 21 2 18 Infection 1 1 2 9 7 14 8 Cough Increased 2 1 1 14 18 3 15 Asthma 4 6 4 6 6 5 16 Herpes Simplex 4 4 4 2 0 12 5 Eczema Herpeticum 0 1 1 0 2 2 0 Pharyngitis 3 3 4 11 6 5 10 Accidental Injury 4 3 6 3 6 4 12 Pustular Rash 2 3 4 3 2 6 8 Folliculitis † 1 6 4 0 2 11 2 Rhinitis 4 3 2 2 6 5 5 Otitis Media 4 0 1 6 12 1 7 Sinusitis † 1 4 2 8 3 3 7 Diarrhea 3 3 4 2 5 4 6 Urticaria 3 3 6 1 1 5 5 Lack of Drug Effect 1 1 0 1 1 10 2 Bronchitis 0 2 2 3 3 3 6 Vomiting 0 1 1 7 6 1 5 Maculopapular Rash 2 2 2 3 0 4 3 Rash † 1 5 2 4 2 2 5 Abdominal Pain 3 1 1 2 3 1 5 Fungal Dermatitis 0 2 1 3 0 2 6 Gastroenteritis 1 2 2 3 0 4 2 Alcohol Intolerance †0 3 7 0 0 6 0 Acne † 2 4 7 1 0 2 4 Sunburn 1 2 1 0 0 4 4 Skin Disorder 2 2 1 1 4 1 4 Conjunctivitis 0 2 2 2 1 4 2 Pain 1 2 1 0 1 4 3 Vesiculobullous Rash† 3 3 2 0 4 2 2 Lymphadenopathy 2 2 1 0 3 2 3 Nausea 4 3 2 0 1 1 2 Skin Tingling † 2 3 8 1 2 2 1 Face Edema 2 2 1 2 1 3 1 Dyspepsia † 1 1 4 0 0 1 4Other adverse events which occurred at an incidence greater than or equal to 1% in any clinical study include: alopecia, ALT or AST increased, anaphylactoid reaction, angina pectoris, angioedema, anorexia, anxiety, arrhythmia, arthralgia, arthritis, bilirubinemia, breast pain, cellulitis, cerebrovascular accident, cheilitis, chills, constipation, creatinine increased, dehydration, depression, dizziness, dyspnea, ear pain, ecchymosis, edema, epistaxis, exacerbation of untreated area, eye disorder, eye pain, furunculosis, gastritis, hemia, hyperglycemia, hypertension, hypoglycemia, hypoxia, laryngitis, leukocytosis, leukopenia, liver function tests abnormal, lung disorder, malaise, migraine, neck pain, neuritis, palpitations, paresthesia, peripheral vascular disorder, photosensitivity reaction, procedural complication, routine procedure, skin discoloration, sweating, taste perversion, tooth disorder, unintended pregnancy, vaginal moniliasis, vasodilatation, and vertigo. OVERDOSAGEPROTOPIC Ointment is not for oral use. Oral ingestion of PROTOPIC Ointment may lead to adverse effects associated with systemic administration of tacrolimus. If oral ingestion occurs, medical advice should be sought. DOSAGE AND ADMINISTRATION ADULTPROTOPIC Ointment 0.03% and 0.1%Apply a thin layer of PROTOPIC Ointment 0.03% or 0.1% to the affected skin areas twice daily and rub in gently and completely. Treatment should be continued for one week after clearing of signs and symptoms of atopic dermatitis.The safety of PROTOPIC Ointment under occlusion which may promote systemic exposure, has not been evaluated. PROTOPIC Ointment 0.03% and 0.1% should not be used with occlusive dressings. PEDIATRICPROTOPIC Ointment 0.03%Apply a thin layer of PROTOPIC Ointment 0.03% to the affected skin areas twice daily and rub in gently and completely. Treatment should be continued for one week after clearing of signs and symptoms of atopic dermatitis. The safety of PROTOPIC Ointment under occlusion, which may promote systemic exposure, has not been evaluated. PROTOPIC Ointment 0.03% should not be used with occlusive dressings.Dry Skin 7 3 3 0 1 0 1 Hyperesthesia † 1 3 7 0 0 3 0 Skin Neoplasm Benign ‡‡ 1 1 1 0 0 2 3 Back Pain † 0 2 2 1 1 3 1 Peripheral Edema 2 4 3 0 0 2 1 Varicella Zoster/ Herpes Zoster ‡ 0 1 0 0 5 1 3 Contact Dermatitis 1 3 3 3 4 1 1 Asthenia 1 2 3 0 0 2 1 Pneumonia 0 1 1 2 0 1 2 Eczema 2 2 2 0 0 3 0 Insomnia 3 4 3 1 1 1 0 Exfoliative Dermatitis 3 3 1 0 0 0 2 Dysmenorrhea 2 4 4 0 0 0 2 Periodontal Abscess 1 0 1 0 0 3 0 Myalgia † 0 3 2 0 0 1 0 Cyst †13† May be reasonably associated with the use of this drug product.‡ Four cases of chicken pox in the pediatric 12-week study, 1 case of "zoster of the lip" in the adult 12-week study; 7 cases ofchicken pox and 1 case of shingles in the open-label pediatric study; 2 cases of herpes zoster in the open-label adult study.‡‡ Generally "warts".Patient Information AboutProtopic® (tacrolimus) OintmentRead this important information before you start using PROTOPIC [pro-TOP-ik] Ointment and each time you refill your prescription. There may be new information. This summary is not meant to take the place of your doctor's advice.What is PROTOPIC?PROTOPIC Ointment is a prescription medicine that is used to treat eczema (atopic dermatitis). It is for adults and children age 2 years and older. You can use PROTOPIC for short or intermittent long periods of treatment. Intermittent means starting and stopping repeatedly, as directed by your doctor. You can use it on all affected areas of your skin, including your face and neck.Who should not use PROTOPIC?Do not use PROTOPIC if you arel breastfeedingl allergic to PROTOPIC Ointment or any of its ingredients. The active ingredient is tacrolimus. Ask your doctor or pharmacist about the inactive ingredients.Before you start using PROTOPIC, tell your doctor if you are:l using any other prescription medicines, non-prescription (over-the-counter) medicines, or supplementsl receiving any form of light therapy (phototherapy, UVA or UVB) on your skinl using any other type of skin productl pregnant or planning to become pregnantHow do I use PROTOPIC?Use PROTOPIC only to treat eczema that has been diagnosed by a doctor.l Wash your hands before using PROTOPIC.l Apply a thin layer of PROTOPIC to all skin areas that your doctor has diagnosed as eczema. Try to cover the affected areas completely. Most people find that a pea-sized amount squeezed from the tube covers an area about the size of a two-inchcircle (approximately the size of a silver dollar).l Apply the ointment twice a day, about 12 hours apart.l Before applying PROTOPIC Ointment after a bath or shower, be sure your skin is completely dryl Do not cover the skin being treated with bandages, dressings or wraps. Unless otherwise instructed by your doctor, do not apply another type of skin product on top ofPROTOPIC Ointment. However, you can wear normal clothingl Do not bathe, shower or swim right after applyingPROTOPIC. This could wash off the ointment.l If you are a caregiver applying PROTOPIC Ointment to a patient, or if you are a patient who is not treating your hands, wash your hands with soap and water after applying PROTOPIC. This should remove any ointment left on the hands.l Use PROTOPIC only on your skin. Do not swallowPROTOPIC.Because 2 strengths of PROTOPIC are available for adult patients, your doctor will decide what strength ofPROTOPIC Ointment is best for you.Many people notice that their skin starts to improve after the first few weeks of treatment. Even though your skin looks and feels better, it is important to keep usingPROTOPIC as instructed by your doctor.If you do not notice an improvement in your eczema or if your eczema gets worse within the first few weeks of treatment, tell your doctor.What Should I Avoid While Using PROTOPIC?l Avoid sunlight and sun lamps, tanning beds, and treatment with UVA or UVB light. If you need to be outdoors after applying PROTOPIC, wear loose fitting clothing that protects the treated area from the sun. In addition, ask your doctor what other type of protection from the sun you should use.l Check with your doctor or pharmacist before you¡start taking any new medicines while using PROTOPIC¡start using any other ointment, lotions, or creams on you skinWhat Are The Possible Side Effects of PROTOPIC?。

他克莫司软膏说明书【药品名称】通用名称:他克莫司软膏商品名称:_____英文名称:Tacrolimus Ointment【成份】本品主要成份为他克莫司。
【性状】本品为白色至淡黄色软膏。
【适应症】本品适用于因潜在危险而不宜使用传统疗法、或对传统疗法反应不充分、或无法耐受传统疗法的中到重度特应性皮炎患者,作为短期或间歇性长期治疗。
【规格】_____【用法用量】1、成人在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次。
2、儿童儿童使用本品的安全性和有效性尚未确立。
如需使用,应在医生的指导下谨慎使用,并密切观察。
【不良反应】1、局部症状使用本品后,常见的局部症状包括皮肤烧灼感、瘙痒、刺痛、红斑等。
这些症状通常在使用初期较为明显,随着用药时间的延长,可能会逐渐减轻或消失。
如果局部症状持续加重或出现肿胀、渗出等异常情况,应及时停药并就医。
2、全身性反应极少数情况下,可能会出现全身性反应,如头痛、发热、关节痛等。
如果出现此类症状,也应及时告知医生。
【禁忌】对他克莫司或本品中任何其他成份有过敏反应的患者禁用。
【注意事项】1、用药部位仅将本品用于患处皮肤,避免接触到眼睛、口腔和鼻腔等黏膜部位。
2、感染如果患处有感染症状(如红肿、化脓等),应在使用本品前先进行抗感染治疗。
3、特殊人群(1)孕妇及哺乳期妇女:由于缺乏足够的安全性数据,孕妇和哺乳期妇女应在医生的评估和指导下权衡利弊后使用。
(2)老年人:一般无需调整剂量,但应密切观察用药后的反应。
(3)免疫受损患者:使用本品时应谨慎,因为免疫受损患者发生感染的风险可能增加。
4、药物相互作用目前尚未发现本品与其他药物有明确的相互作用,但在同时使用多种药物时,仍应告知医生,以便医生进行评估。
5、自我监测在使用本品期间,应密切观察皮肤症状的变化,如果症状没有改善或加重,应及时就医。
【孕妇及哺乳期妇女用药】孕妇:目前尚无足够的孕妇使用本品的临床数据。
动物实验显示,他克莫司可能对胚胎发育有一定影响。
[他克莫司软膏的作用]他克莫司软膏多久用一次篇一: 他克莫司软膏多久用一次他克莫司软膏多久用一次?他克莫司软膏是用于治疗皮炎、银屑病、湿疹等皮肤病的进口免疫调节剂,可作为短期或间歇性长期治疗药物,接下来我们就马上来看看他克莫司软膏多久用一次吧。
他克莫司软膏适用于因潜在危险而不宜使用传统疗法、或对传统疗法反应不充分、或无法耐受传统疗法的中到重度特应性皮炎患者,作为短期或间歇性长期治疗。
他克莫司软膏有0.03%*10g 和0.1%*10g 两种规格,0.03%和0.1%浓度的他克莫司软膏均可用于成人,但只有0.03%浓度的他克莫司软膏可用于2岁及以上的儿童。
那么,他克莫司软膏多久用一次呢?他克莫司软膏的使用方法介绍如下:如想了解更多他克莫司软膏的相关评论,欢迎点击-comment/1、成人:0.03%和0.1%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
2、儿童:0.03%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
3、他克莫司软膏是一种霜剂型的外用药,药膏为无色,油腻状,外形有点像一支牙膏,使用的时候先用刀具在胶袋的前端开一个小口,使用后应放在避光和温度适宜的地方。
4、他克莫司软膏每天使用两次,间隔为12小时一次,一般使用过的朋友都是在晚上睡觉前和早上起床后使用。
有的朋友会在使用前沐浴,以清洁皮肤。
当然,沐浴并不是必须的。
壹药网温馨提示:相信通过本文的阐述后,大家对他克莫司软膏多久用一次这个问题已经有答案了吧,建议大家在用药前先仔细阅读药品说明书或先咨询专业医师或药师的意见,以保证用药安全。
如需购买他克莫司软膏,可登录.html篇二: 他克莫司软膏使用注意事项他克莫司软膏是一种常见的用于治疗皮肤性炎症的药膏,治疗效果非常明显,适用性也非常不错。
对于常患有皮肤癣、湿疹的患者来说,非常值得推荐。
他克莫司软膏的功效与作用他克莫司软膏,是一种广谱抗真菌药物,主要用于治疗皮肤真菌感染引起的病症。
它具有抗菌、杀菌、消炎、止痒等多种功效,对于皮肤真菌感染具有显著的疗效。
下面将详细介绍他克莫司软膏的功效与作用。
他克莫司软膏的主要成分是克霉唑,属于类硝酸酯类抗真菌药物。
该药物经皮肤应用后,能够迅速渗透进入真菌细胞内,通过阻断酵母菌和真菌的细胞膜的合成,干扰其细胞呼吸作用,从而达到杀菌的目的。
同时,他克莫司软膏还具有抗炎和抗过敏作用,能够减轻皮肤炎症和止痒症状。
在临床应用中,他克莫司软膏主要用于治疗各种类型的皮肤真菌感染病症,如白色念珠菌感染、毛癣、体癣、脚癣、股癣等。
白色念珠菌感染是一种常见的真菌感染病症,常见于口腔、阴道、皮肤等部位,使用他克莫司软膏可以有效杀灭白色念珠菌,缓解症状。
毛癣是指身体各部位的脱屑性皮肤病,使用他克莫司软膏可以杀灭病原体,减轻病情。
脚癣主要发生于足部,使用他克莫司软膏可以迅速缓解病情,减少瘙痒和疼痛感。
股癣同样是一种常见的真菌感染,使用他克莫司软膏可以有效杀灭股癣的病原菌,减轻炎症症状。
他克莫司软膏的使用方法比较简单。
首先,清洗感染部位的皮肤,用清水洗净,然后将其涂抹在感染部位,并轻轻按摩,使药物均匀分布。
使用时要注意不要过度涂抹,尽量覆盖感染区域即可,避免过度使用引起药物的浪费。
一般情况下,每日使用2-3次,持续使用2-4周。
很多真菌感染病症常常伴有严重的瘙痒和疼痛感,此时患者可以搭配口服抗过敏药物和止痒药片,以加快症状的缓解。
除了治疗皮肤真菌感染病症,他克莫司软膏还可以用于预防皮肤真菌感染的复发。
皮肤真菌感染病症常常会反复发作,一旦感染复发,就可能对人体健康造成较大的影响。
在感染症状缓解后,患者可以继续使用他克莫司软膏进行预防治疗,减少真菌感染的概率,保持皮肤的清洁和健康。
尽管他克莫司软膏在治疗皮肤真菌感染方面具有显著的疗效,但也存在一些副作用和注意事项。
使用过程中,有可能引起皮肤过敏、刺激等不良反应,一旦出现不良反应,应立即停药,并咨询医生的意见。
快易捷医药网【通用名】他克莫司软膏【商品名】普特彼【英文名】英文名:Tacrolimus Ointment【汉语拼音】Ta Ke Mo Si Ruan Gao本品主要成分及其化学名称为:他克莫司,【性状】本品为白色至淡黄色软膏。
【处方组成】每克本品含他克莫司0.03%或0.1%(w/w),软膏基质为矿物油、石蜡、碳酸丙烯酯、白凡士林和白蜡。
【适应症】本品适用于因潜在危险而不宜使用传统疗法、或对传统疗法反应不充分、或无法耐受传统疗法的中到重度特应性皮炎患者,作为短期或间歇性长期治疗。
0.03%和0.1%浓度的本品均可用于成人,但只有0.03%浓度的本品可用于2岁及以上的儿童。
【用法与用量】成人0.03%和0.1%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
本品不应采用封包敷料外用。
儿童0.03%他克莫司软膏在患处皮肤涂上一薄层本品,轻轻擦匀,并完全覆盖,一天两次,持续至特应性皮炎症状和体征消失后一周。
封包疗法可能会促进全身性吸收,其安全性未进行过评价。
本品不应采用封包敷料外用。
【禁忌症】对他克莫司或制剂中任何其他成分有过敏史的患者禁用本品。
【注意事项】外用本品可能会引起局部症状,如皮肤烧灼感(灼热感、刺痛、疼痛)或瘙痒。
局部症状最常见于使用本品的最初几天,通常会随着特应性皮炎受累皮肤好转而消失。
应用0.1%浓度的本品治疗时,90%的皮肤烧灼感持续时间介于2分钟至3小时(中位时间为15分钟)之间,90%的瘙痒症状持续时间介于3分钟至10小时(中位时间为20分钟)之间。
不推荐使用本品治疗Netherton综合征患者,因为可能会增加他克莫司的全身性吸收。
本品对弥漫性红皮病患者治疗的安全性尚未建立。
【患者须知】使用本品的患者应接受下列信息和指导:1.患者应在医生的指导下使用本品。
本品仅供外用。
他克莫司软膏的作用与功效他克莫司软膏是一种常用的护肤药膏,其主要成分是他克莫司。
他克莫司是一种免疫抑制剂,具有抗炎、抗过敏、抗氧化以及免疫调节等多种作用。
使用他克莫司软膏,可以用于治疗多种皮肤问题,包括过敏性皮炎、银屑病、湿疹、牛皮癣等。
下面将详细介绍他克莫司软膏的作用与功效。
一、抗炎作用他克莫司软膏具有抗炎作用,能够抑制炎症反应,减轻红肿、瘙痒等炎症症状。
他克莫司通过抑制细胞因子的释放,降低炎症过程中的炎性介质水平,并抑制炎症细胞的活化,从而达到抗炎作用。
对于过敏性皮炎、湿疹等炎症性皮肤病的治疗是非常有效的。
二、抗过敏作用他克莫司软膏可通过抑制过敏反应,减轻过敏性皮炎等疾病的症状。
过敏反应是免疫系统对外界物质过度敏感所引起的一系列症状,包括发红、瘙痒、水肿等。
他克莫司能够抑制免疫反应的发生和发展,减少过敏介质的释放,从而缓解过敏症状。
三、抗氧化作用他克莫司具有抗氧化作用,能够减轻自由基对皮肤的损伤。
自由基是一种高活性氧化物质,它们能够与皮肤细胞中的脂质、蛋白质和DNA等结合,导致细胞损伤和老化。
他克莫司能够中和自由基,减轻其对皮肤细胞的损害,从而保护皮肤的健康。
四、免疫调节作用他克莫司软膏能够调节免疫系统的功能,增强皮肤的免疫力。
免疫系统是人体的防御系统,能够识别和消灭病原体。
但当免疫系统出现异常时,就会导致各种免疫性疾病的发生,如牛皮癣、红斑狼疮等。
他克莫司通过抑制免疫反应的发生,调整免疫系统的功能,从而减轻免疫性皮肤疾病的症状。
五、改善皮肤屏障他克莫司软膏能够改善皮肤屏障功能,提高皮肤的保湿能力。
皮肤屏障是皮肤表面的一层角质层,能够防止水分的流失,保护皮肤免受外界刺激。
当皮肤屏障功能受损时,皮肤就会变干燥、敏感。
他克莫司具有保湿作用,能够改善皮肤屏障功能,提高水分的保持能力,从而保持皮肤的健康和弹性。
六、减轻瘢痕形成他克莫司软膏对于减轻瘢痕形成也有一定的效果。
瘢痕是损伤后皮肤修复过程中形成的疤痕组织,常常会导致皮肤外观的不美观。
孚诺(复方多粘菌素B软膏),他克莫司(普特彼)说明书如下:【普特彼药品名称】通用名:他克莫司软膏商品名:普特彼英文名:Tacrolimus Ointment汉语拼音:TaKeMoSiRuanGao【普特彼成份】普特彼主要成份为:他克莫司。
【普特彼性状】普特彼为白色至淡黄色软膏。
【普特彼处方组成】每克普特彼含他克莫司0.03或0.1(w/w),软膏基质为矿物油、石蜡、碳酸丙烯酯、白凡士林和白蜡。
【普特彼药理毒理】药理作用他克莫司治疗特应性皮炎的作用机制还不清楚。
虽然对他克莫司的作用机制已有一定了解,但是这些发现与特应性皮炎的临床关系还不明确。
他克莫司已被证实可以抑制T淋巴细胞活化,首先与细胞内蛋白FKBP-12结合,形成由他克莫司-FKBP-12、钙、钙调蛋白和钙调磷酸酶构成的复合物,从而抑制钙调磷酸酶的磷酸酶活性,阻止活化T细胞核转录因子(NF-AT)的去磷酸化和易位,NF-AT这种核成分会启动基因转录形成淋巴因子(例如IL-2,γ干扰素)。
他克莫司还可以抑制编码IL-3、IL-4、IL-5、GM-CSF和TNF-?的基因的转录,所有这些因子都参与早期阶段的T细胞活化。
此外,他克莫司可以抑制皮肤肥大细胞和嗜碱性粒细胞内已合成介质的释放,下调朗格罕细胞表面FCεRI的表达。
毒理作用在为期26周的大鼠实验和为期28天的家兔实验中,每天外用他克莫司软膏(0.03-1)后,在显微镜下观察到皮肤变化(增生、表皮空泡形成、棘层肥厚、浅表炎症)。
由于这些皮肤变化与他克莫司浓度不相关,也见于赋形剂组,而空白对照组极少见,因而被认为与赋形剂有关而与他克莫司本身无关。
在大鼠中外用高浓度软膏(基本上≥0.3)观察到全身毒性反应,与经口服或静脉摄入后相似。
在为期52周的尤卡坦微型猪局部实验中,肉眼或显微镜下所见的改变均被认为与外用他克莫司(0.03?0.3)无关,因为在赋形剂对照组也观察到同样的改变。
在对豚鼠进行的实验中,他克莫司软膏(0.03?3)不诱发接触过敏或光敏化反应,对白化无毛小鼠也不诱发皮肤光毒性。