三环类抗抑郁药(专业知识值得参考借鉴)
- 格式:doc
- 大小:64.50 KB
- 文档页数:2

最好抗抑郁药有哪些抑郁症是一种心理疾病,严重的抑郁症,对患者的身心健康,以及身边的人都会造成不同程度的影响,所以对抑郁症当然也要了解有效的治疗方法,而抗抑郁症的,药物有很多种,比如常见的包括单胺氧化酶,还有三环类的抗抑郁药物,但是药物的使用一定要注意遵循医生的一种建议。
★一、单胺氧化酶异丙肼是上世纪50年代问世的第一个抗抑郁药物。
异丙肼原是一种抗结核药,因有多说、多动、失眠和欣快感等中枢兴奋作用,1957年试用于抑郁病人并获得成功。
动物实验证实其可逆转利血平引起的淡漠、少动,同时,脑单胺含量升高。
推测其中枢兴奋和抗抑郁作用是因为大脑单胺氧化酶受抑制单胺降解减少,使突解间隙单胺含量升高的缘故。
从而提示了动物行为和大脑单受类递质之间的相互关系,有着重要理论和实践意义,为精神药理和精神疾病病因学研究奠定的基础。
属于这一类的还有异卡波肼、苯乙肼、反苯环丙胺等。
这些药物曾一度广为应用,不久因陆续出现与某些食物和药物相互作用,引起高血压危象、急性黄色肝萎缩等严重不良反应而被淘汰。
★二、三环类是紧接单胺氧化酶抑制剂之后的另一类抗抑郁药,以丙咪嗪为代表。
它的化学结构与氯丙嗪相似,原以为可能是一种新的抗精神病药,但临床试验结果大出所料,该药对精神分裂症无效,却能改善抑郁心境。
以后又经大量,双盲安慰剂对照研究证实,从而取代单胺氧化酶抑制剂,一跃成为抑郁症治疗的首选药,垄断抗抑郁药市场长达30年之久。
三环类抗抑郁药共有产品10余种,我国除丙咪嗪外还有阿米替林、多虑平和氯丙咪嗪。
马普替林虽为四环结构,但药理作用与三环类抗抑郁药一致。
三环类抗抑郁药的适应证为各种类型抑郁症,有效率约70%-80%,起效时间1-2周,剂量范围50-250mg/d,缓慢加量,分次服。
因镇静作用较强,晚间剂量宜大些。
治疗范围血药浓度丙咪嗪和阿米替林为50-250ng/ml。
★三、新型药物选择性5-HT再摄取抑制药(SSRI)是新型抗抑郁药物。
本类药物从20世纪70年代开始研制,已开发有数十种,临床常用的有氟西汀、帕罗西汀、舍曲林、氟伏沙明、西酞普兰等。

三环类抗抑郁药物不良反应最重要的是什么三环类抗抑郁药物是抗抑郁药物的其中一种,也是临床使用时间较长的一种了,那么你知道三环类抗抑郁药物不良反应最重要的是什么吗?下面是店铺为你整理的三环类抗抑郁药物不良反应最重要的是什么的相关内容,希望对你有用!三环类抗抑郁药物的不良反应使人困倦、口干、视物模糊、便秘、心跳加快、排尿困难和体位性低血压,这类副作用一般不影响治疗,在治疗过程中可逐渐适应;严重的心血管副作用、尿潴留和肠麻痹少见。
过量可致急性中毒甚至死亡。
最新研究还证明可增加脑出血风险。
三环类抗抑郁药物的简介是紧接单胺氧化酶抑制剂之后的另一类抗抑郁药,以丙咪嗪为代表。
它的化学结构与氯丙嗪相似,原以为可能是一种新的抗精神病药,但临床试验结果大出所料,该药对精神分裂症无效,却能改善抑郁心境。
以后又经大量,双盲安慰剂对照研究证实,从而取代单胺氧化酶抑制剂,一跃成为抑郁症治疗的首选药,垄断抗抑郁药市场长达30年之久。
三环类抗抑郁药共有产品10余种,我国除丙咪嗪外还有阿米替林、多虑平和氯丙咪嗪。
马普替林虽为四环结构,但药理作用与三环类抗抑郁药一致。
三环类抗抑郁药的适应证为各种类型抑郁症,有效率约70%-80%,起效时间1-2周,剂量范围50-250mg/d,缓慢加量,分次服。
因镇静作用较强,晚间剂量宜大些。
治疗范围血药浓度丙咪嗪和阿米替林为50-250ng/ml。
三环类抗抑郁药临床应用时间最长,药理作用研究得也最多最充分,简言之,其主要药理作用为:1阻滞单胺递质(主要为肾上腺素和5-HT)再摄取,使突触间隙单受含量升高而产生抗抑郁作用。
2阻断多种递质受体,它与治疗作用无关,却是诸多不良反应的主要原因,如阻滞乙酰胆碱M受体,可能出现口干、视力模糊、窦性心动过速、便秘、尿潴留、青光眼加剧、记忆功能障碍;阻滞肾上腺素a1受体,可能出现加强哌唑嗪的降压作用、体位性低血压、头昏、反射性心动过速;阻滞组胺H1受体,可出现加强中枢抑制剂作用、镇静、嗜睡、增加体重、降低血压;阻滞多巴胺D2受体,可出现锥体外系症状、内分泌改变。

CPIC GuIdelInesnature publishing groupPolymorphisms in CYP2D6 and CYP2C19 affect the efficacy and safety of tricyclics, with some drugs being affected by CYP2D6 only, and others by both polymorphic enzymes. Amitriptyline, c lomipramine, doxepin, imipramine, and trimipramine are demethylated by CYP2C19 to pharmacologically active metabolites. These drugs and their metabolites, along with desipramine and nortriptyline, undergo hydroxylation by CYP2D6 to less active metabolites. Evidence from published literature is presented for CYP2D6 and CYP2C19 genotype–direc ted dosing of tric yc lic antidepressants.The use of tricyclics to treat psychological disorders has declined in part because of the occurrence of undesirable side effects. Although tricyclics are still used to treat depression,1 their main therapeutic use is often for pain management.2,3 Interindividual differences in side effects and treatment response have been associated with variability of tricyclic plasma concentrations.4,5 Because both enzymes influence plasma concentrations, the effectiveness and tolerability of tricyclics are affected by CYP2D6 metabolism and partially by CYP2C19 metabolism.4 The purpose of this guideline is to provide information regarding how to use existing CYP2D6 and/or CYP2C19 genotyping test results to guide dosing of tricyclics for psychological disorders and pain manage-ment, focusing particularly on amitriptyline and nortriptyline.Optimal therapeutic plasma concentrations for the tricyclics have been defined.6 Poor or ultrarapid metabolizers of CYP2D6and CYP2C19 may have tricyclic plasma concentrations outside the recommended therapeutic range, thereby increasing the risk of treatment failure or side effects.7–10 Therefore, this guideline takes into consideration both clinical outcomes and observed tricyclic plasma concentrations based on genotype/phenotype characteristics. Detailed guidelines for use of other laboratory tests including therapeutic drug monitoring of tricyclics are beyond the scope of this article. The Clinical Pharmacogenetics Implementation Consortium (CPIC) of the National Institutes of Health’s Pharmacogenomics Research Network develops peer-reviewed gene–drug guidelines that are published and updated periodically at based on new developments in the field.FOCUSED LITERATURE REVIEWA systematic literature review focused on CYP2D6 and CYP2C19 genotyping and its relevance to gene-based dosing of tricyclics was conducted (see Supplementary Data online). This guideline was developed based on interpretation of the literature by the authors and experts in the field.GENES: CYP2D6 AND CYP2C19CYP2D6 backgroundThe CYP2D6 gene is highly polymorphic.11 More than 100 known allelic variants and subvariants have been identified, and there are substantial ethnic differences in observed allele frequencies (Supplementary Data online). The most commonly reportedReceived 1 October 2012; accepted 27 December 2012; advance online publication 13 March 2013. doi:10.1038/clpt.2013.2Clinical Pharmacogenetics Implementation Consortium Guideline for CYP2D6 andCYP2C19 Genotypes and Dosing of Tricyclic AntidepressantsJK Hicks 1, JJ Swen 2, CF Thorn 3, K Sangkuhl 3, ED Kharasch 4, VL Ellingrod 5,6, TC Skaar 7, DJ Müller 8, A Gaedigk 9 and JC Stingl 101Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA; 2Department of Clinical Pharmacy and Toxicology, LeidenUniversity Medical Center, Leiden, The Netherlands; 3Department of Genetics, Stanford University, Stanford, California, USA; 4Division of Clinical and Translational Research, Department of Anesthesiology, Washington University in St. Louis, St. Louis, Missouri, USA; 5Department of Clinical, Social and Administrative Sciences,College of Pharmacy, Ann Arbor, Michigan, USA; 6Department of Psychiatry, School of Medicine, University of Michigan, Ann Arbor, Michigan, USA; 7Division of Clinical Pharmacology, Department of Medicine, Indiana University School of Medicine, Indianapolis, Indiana, USA; 8Neurogenetics Section, Centre for Addiction and Mental Health, and Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada; 9Division of Pediatric Pharmacology and Medical Toxicology, Children’s Mercy Hospital & Clinics, Kansas City, Missouri, USA; 10Division of Research, Federal Institute for Drugs and Medical Devices, University Bonn Medical Faculty, Bonn, Germany. Correspondence: JC Stingl (cpic@ )CPIC GuIdelInesalleles are categorized into functional groups as follows: functional (e.g., CYP2D6*1 and *2), reduced function (e.g., CYP2D6*9, *10, and *41), and nonfunctional (e.g., CYP2D6*3–*6).11,12 Because CYP2D6 is subject to deletions or duplications, most clinical labo-ratories also report copy number. Deletions are indicated by the CYP2D6*5 allele, and gene duplications are denoted by an “xN” following the allele (e.g., CYP2D6*1xN, where xN represents the number of CYPD6 gene copies).CYP2C19 backgroundSimilar to CYP2D6, the CYP2C19 gene is highly polymorphic; more than 30 known allelic variants and subvariants have been identified.13 Although there are ethnic differences in allele fre-quencies (Supplementary Data online), the majority of patients carry a CYP2C19*1, *2, or *17 allele.13CYP2C19*1 is the wild-type allele encoding a fully functional enzyme, and CYP2C19*2 is the most common loss-of-function allele. Multiple CYP2C19 loss-of-function alleles have been identified (e.g., CYP2C19*3–*8), but their allele frequencies are <1%, with the exception of CYP2C19*3, which has a frequency of 2–15% in Asians.13The CYP2C19*17 allele results in enhanced gene transcription purportedly leading to increased metabolic activity.14 However, the clinical importance of the CYP2C19*17 allele is a matter of debate. Some studies indicate that CYP2C19*17 enhances clopidogrel response and increases the probability of therapeu-tic failure for proton pump inhibitors or antidepressants due to altered drug plasma concentrations, but there are conflicting clinical data.13–19Genetic test interpretationClinical laboratories usually test for the more frequently observed CYP2D6 and CYP2C19 genetic variants and translate the results into star-allele (*) nomenclature. Each star-allele, or haplotype, is defined by a specific combination of single-nucleotide polymor-phisms and/or other genetic variants within the gene locus of either CYP2D6 or CYP2C19.11,13 Genetic test results are reported as the summary of inherited maternal and paternal star-alleles referred to as a diplotype (e.g., CYP2D6*1/*2 and CYP2C19*1/*1). See Supplementary Data online for the more frequently observed alleles and their functional status.Scoring systems have been developed in an attempt to provide a uniform approach to quantitate the predicted functional status of CYP2D6 alleles as follows: 1 for functional, 0.5 for reduced function, and 0 for nonfunctional alleles (Supplementary Data online).11,12 The activity value for each allele of the diplotype is totaled to provide a CYP2D6 activity score. If CYP2D6 gene duplications are detected, the activity value of the duplicated allele is multiplied by the number of duplications present before calculating the activity score. The CYP2D6 activity score is used to assign phenotype in this guideline as follows: patients with an activity score of 0 are classified as poor metabolizers, those with a score of 0.5 are intermediate metabolizers, those with a score from 1.0 to 2.0 are extensive metabolizers, and those with a score >2.0 are classified as ultrarapid metabolizers (Table 1, Supplementary Data online). Extensive metabolizers are con-sidered to have normal CYP2D6 enzyme activity.There is a lack of consensus with regard to whether patients with a CYP2D6 activity score of 1.0 should be assigned an exten-sive or intermediate phenotype.11 Pharmacokinetic data suggest that patients with an activity score of 1.0 have a higher CYP2D6 metabolic capacity as compared with patients with an activity score of 0.5 but less CYP2D6 enzyme activity as compared with patients with an activity score of 2.0.12 Patients with one functional and one nonfunctional CYP2D6 allele do not haveTable 1 Assignment of likely phenotypes based on diplotypesLikely phenotype Activity score a Genotypes Examples of diplotypes Assignment of CyP2D6 phenotypeUltrarapid metabolizer (~1–2% of patients)b>2.0An individual carrying duplications of functionalalleles (*1/*1)xN, (*1/*2)xN, (*2/*2)xN cExtensive metabolizer (~77–92% of patients) 1.0–2.0d An individual carrying two functional alleles ortwo reduced function alleles or one functional andnonfunctional allele or one functional and reducedfunction allele *1/*1, *1/*2, *2/*2, *1/*9, *1/*41, *41/*41, *1/*5,*1/*4Intermediate metabolizer (~2–11% of patients)0.5An individual carrying one reduced function andone nonfunctional allele*4/*41, *5/*9, *4/*10 Poor metabolizers (~5–10% of patients)0An individual carrying only nonfunctional alleles*4/*4, *3/*4, *5/*5, *5/*6 Assignment of CyP2C19 phenotypeUltrarapid metabolizer (~5–30% of patients)e An individual carrying two gain-of-function allelesor one functional allele and one gain-of-functionallele*17/*17, *1/*17Extensive metabolizer (~35–50% of patients)An individual carrying two functional alleles*1/*1 Intermediate metabolizer (~18–45% of patients)An individual carrying one functional allele and oneloss-of-function allele*1/*2, *1/*3Poor metabolizers (~2–15% of patients)An individual carrying two loss-of-function alleles*2/*2, *2/*3, *3/*3a See Supplementary Data for additional information about CyP2D6 activity score and its limitations.b CyP2D6 metabolizer status frequencies are based on data from Caucasians and may differ from other ethnicities.c xN represents the number of CYP2D6 gene copies.d Patients with an activity score of 1.0 may be classified as intermediate metabolizers by some reference laboratories. e CyP2C19 metabolizer status frequencies are based on average multiethnic frequency.CPIC GuIdelInessignificantly different nortriptyline plasma concentrations as compared with patients with two functional CYP2D6 alleles,20 although another study reported conflicting data.7 Herein, we classified patients with a CYP2D6 activity score of 1.0 as exten-sive metabolizers, which is analogous to the CPIC guideline for codeine.11The predicted CYP2C19 phenotype based on the diplotype (Table 1 and Supplementary Data online) is consistent with the CPIC guideline for clopidogrel.13 Patients with two func-tional alleles are categorized as extensive metabolizers, which is considered normal CYP2C19 enzyme activity. Individuals carrying one or two loss-of-function alleles are considered intermediate and poor metabolizers, respectively. Patients with two gain-of-function alleles are classified as ultrarapid metabolizers. The predicted phenotype for a patient carrying the CYP2C19*17 gain-of-function allele in combination with a loss-of-function allele is a matter of debate. Limited data sug-gest that CYP2C19*17 may not compensate for the CYP2C19*2 allele.15,21Reference laboratories use varying methods to assign pheno-types. Before pharmacotherapy modifications are made based on this guideline, it is advisable to determine a patient’s pheno-type as described above.Available genetic test optionsCYP2D6 and CYP2C19 genotyping is available from several ref-erence laboratories (see Supplementary Data online and http:// ).Incidental findingsIndependent of drug metabolism and response, there are cur-rently no diseases or conditions that have been convincingly linked to variants in the CYP2D6 or CYP2C19 genes.11,13 Reports describing an association between CYP2D6 ultrarapid metabolizers and suicidality, and CYP2C19 polymorphisms and depressive symptoms are available.22–24 These associations are poorly understood and may be explained by alterations in either drug or endogenous substrate metabolism.DRUGS: AMITRIPTYLINE AND NORTRIPTYLINE BackgroundTricyclics are mixed serotonin and norepinephrine reuptake inhibitors used to treat several disease states including depres-sion, obsessive–compulsive disorder, and neuropathic pain in addition to migraine prophylaxis. The tricyclics have simi-lar but distinct chemical structures referred to as tertiary and secondary amines. The pharmacological properties of the ter-tiary and secondary amines differ, with tertiary amines having a more pronounced serotonergic effect and secondary amines having a greater noradrenergic effect (Supplementary Data online).25,26 The tertiary amines (e.g., amitriptyline) are mainly metabolized by CYP2C19 to desmethyl metabolites (Figure 1), also referred to as secondary amines (e.g., nortriptyline). It should be noted that the desmethyl metabolites, nortriptyline as well as desipramine, are antidepressant drugs themselves with distinct clinical features that differ from the parent drugs amitriptyline and imipramine. Both the tertiary and second-ary amines are metabolized by CYP2D6 to less active hydroxy metabolites (Figure 1, Supplementary Data online). CYP2C19 impacts the ratio of tertiary to secondary amine plasma concen-trations but may have less influence on overall drug clearance than CYP2D6.27 However, CYP2C19 metabolism may modu-late antidepressant activity and side effects through the phar-macological actions of the tertiary amines. Serotonin reuptake inhibition is expected be more pronounced in CYP2C19 poor metabolizers due to the decreased conversion of tertiary to sec-ondary amines.26Patients may be predisposed to treatment failure or adverse effects due to polymorphisms in CYP2D6 and CYP2C19 alter-ing drug clearance or the ratio of parent drug to metabolites, respectively. Tricyclics are associated with multiple adverse effects, which can cause patients to fail therapy. Common adverse effects include anticholinergic, central nervous system, and cardiac effects. Tertiary and secondary amines along with their metabolites have unique side-effect profiles as detailed in Supplementary Data online.Both amitriptyline and nortriptyline are used as model drugs for this guideline because the majority of pharmacogenomic studies have focused on these two drugs. However, the results of these studies may apply to other tricyclics because these drugs have comparable pharmacokinetic properties.4,8 Tricyclics are well absorbed from the gastrointestinal tract, and the average extent of first-pass metabolism is ~50%, although the average first-pass metabolism of doxepin may be closer to 70%.4 The clearance of tricyclics is mostly a linear process, but satura-tion of the hydroxylation pathway may occur at higher plasma concentrations for certain tricyclics, including imipramine and desipramine.4,28 In addition, extrapolated dose adjustments based on metabolizer status are similar across the tricyclic class.8 Because some studies investigating the influence of CYP2D6 and/or CYP2C19 genotype/phenotype on the pharmacokinetics of tricyclics used a single dose, it should be noted that tricyclic metabolism is thought to be similar after single and multiple dosing.5Linking genetic variability to variability in drug-relatedp henotypesPsychiatric disorders such as depression have a 30–50% failure rate with initial treatment, which may, in part, be attributed to adverse effects or altered plasma concentrations.9,29 There is substantial evidence linking CYP2D6 and CYP2C19 genotypes to phenotypic variability in tricyclic side-effect and pharma-cokinetic profiles. Modifying pharmacotherapy for patientsFigure 1 Major metabolic pathway of amitriptyline and nortriptyline.demethylationNortriptylineAmitriptylineCYP2C19CPIC GuIdelIneswho have CYP2D6 or CYP2C19 genomic variants that affect drug efficacy and safety could potentially improve clinical outcomes and reduce the failure rate of initial treatment. The application of a grading system to the evidence linking CYP2D6 and CYP2C19 genotypic variations to phenotypic variability in response to amitriptyline or nortriptyline indicates a high qual-ity of evidence in the majority of cases (Supplementary Data online). This body of evidence, rather than randomized clinical trials, provides the basis for amitriptyline and nortriptyline dos-ing recommendations in Tables 2 and 3. Because the tricyclics have comparable pharmacokinetic properties, it may be reason-able to apply this guideline to other tricyclics, including clomi-pramine, desipramine, doxepin, imipramine, and trimipramine (Supplementary Data online), with the acknowledgment that there are fewer data supporting dose adjustments for these drugs than for amitriptyline or nortriptyline.Table 2 Dosing recommendations for amitriptyline and nortriptyline based on CYP2D6 phenotypephenotype implication Therapeutic recommendation Classification of recommendation aCyP2D6 ultrarapid metabolizer Increased metabolism of tricyclics to lessactive compounds as compared with extensivemetabolizersAvoid tricyclic use due to potential lack of efficacy.Consider alternative drug not metabolized byCyP2D6StrongLower plasma concentrations will increaseprobability of pharmacotherapy failureIf a tricyclic is warranted, consider increasing thestarting dose.b Use therapeutic drug monitoring toguide dose adjustmentsCyP2D6 extensivemetabolizerNormal metabolism of tricyclics Initiate therapy with recommended starting dose b StrongCyP2D6 intermediate metabolizer Reduced metabolism of tricyclics to less activecompounds as compared with extensive metabolizersConsider 25% reduction of recommended startingdose.b Use therapeutic drug monitoring to guidedose adjustmentsModerateHigher plasma concentrations will increase theprobability of side effectsCyP2D6 poor metabolizer Greatly reduced metabolism of tricyclics to less activecompounds as compared with extensive metabolizersAvoid tricyclic use due to potential for side effects.Consider alternative drug not metabolized byCyP2D6StrongHigher plasma concentrations will increase theprobability of side effectsIf a tricyclic is warranted, consider a 50% reductionof recommended starting dose.b Use therapeuticdrug monitoring to guide dose adjustmentsIf CYP2C19 genotyping results are also available, see Table 3 for CYP2C19-based dosing recommendations along with Supplementary Data online. Dosing recommendations apply only to higher initial doses of amitriptyline or nortriptyline for treatment of conditions such as depression. See “Other Considerations” for dosing recommendations for conditions in which lower initial doses are used, such as neuropathic pain. For the dosing guidelines for clomipramine, desipramine, doxepin, imipramine, and trimipramine, see Supplementary Data online.a The rating scheme for the recommendation classification is described in the Supplementary Data online.b Patients may receive an initial low dose of tricyclics, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose.Table 3 Dosing recommendations of amitriptyline based on CYP2C19 phenotypephenotype implication Therapeutic recommendation Classification of recommendation aCyP2C19 ultrarapid metabolizer Increased metabolism of amitriptyline as comparedwith extensive metabolizersConsider alternative drug not metabolized byCyP2C19OptionalIf a tricyclic is warranted, use therapeutic drugmonitoring to guide dose adjustmentsCyP2C19 extensive metabolizer Normal metabolism of amitriptyline Initiate therapy with recommended startingdose bStrongCyP2C19 intermediate metabolizer Reduced metabolism of amitriptyline as comparedwith extensive metabolizersInitiate therapy with recommended startingdose bStrongCyP2C19 poor metabolizer Greatly reduced metabolism of amitriptyline ascompared with extensive metabolizersConsider a 50% reduction of recommendedstarting dose.b Use therapeutic drug monitoringto guide dose adjustmentsModerateHigher plasma concentrations of amitriptyline willincrease the probability of side effectsIf CYP2D6 genotyping results are also available, see Table 2 for CYP2D6-based dosing recommendations along with Supplementary Data online. Dosing recommendations apply only to higher initial doses of amitriptyline for treatment of conditions such as depression. See “Other Considerations” for dosing recommendations for conditions at which lower initial doses are used, such as neuropathic pain. For dosing guidelines for clomipramine, doxepin, imipramine, and trimipramine, see Supplementary Data online.a The rating scheme for the recommendation classification is described in the Supplementary Data online.b Patients may receive an initial low dose of tricyclics, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose.CPIC GuIdelInesTherapeutic recommendationsCYP2D6 dosing recommendations. For neuropathic pain treat-ment, in which lower initial doses of tricyclics are used, gene-based dosing recommendations are found in the “Other Considerations” section. Table 2 summarizes the gene-based dosing recommendations for amitriptyline and nortriptyline based on CYP2D6 phenotype for situations requiring a higher initial dose, such as depression treatment. The recommended starting dose of amitriptyline or nortriptyline does not need adjustment based on genotype for CYP2D6 extensive metabo-lizers. A 25% reduction of the recommended dose may be considered for CYP2D6 intermediate metabolizers.30 Because patients with a CYP2D6 activity score of 1.0 are inconsistently categorized as intermediate or extensive metabolizers in the literature, these are difficult to evaluate, resulting in a moder-ate recommendation classification.CYP2D6 ultrarapid metabolizers have a higher probability of failing amitriptyline or nortriptyline pharmacotherapy due to subtherapeutic plasma concentrations, and therefore alternative agents are preferred. There are documented cases of CYP2D6 ultrarapid metabolizers receiving large doses of nortriptyline to achieve therapeutic concentrations.10 However, very high plasma concentrations of the nortriptyline hydroxy metabolite were present, which may increase the risk for cardiotoxicity. If a tricyclic is warranted, there are insufficient data in the literature to calculate a starting dose for a patient with CYP2D6 ultrarapid metabolizer status, and therapeutic drug monitoring is strongly recommended. Adverse effects are more likely in CYP2D6 poor metabolizers due to elevated tricyclic plasma concentrations,31 therefore, alternative agents are preferred. If a tricyclic is war-ranted, consider a 50% reduction of the usual dose; therapeutic drug monitoring is strongly recommended.CYP2C19 dosing recommendations. Dosing recommendations for neuropathic pain treatment with amitriptyline are discussed in the “Other Considerations” section. Table 3 summarizes the gene-based dosing recommendations for CYP2C19 and ami-triptyline when higher initial starting doses are warranted. The usual starting dose of amitriptyline may be used in CYP2C19 extensive and intermediate metabolizers. Although CYP2C19 intermediate metabolizers would be expected to have a modest increase in the ratio of amitriptyline to nortriptyline plasma concentrations, the evidence does not indicate that CYP2C19 intermediate metabolizers should receive an alternative dose. Patients taking amitriptyline who are CYP2C19 ultrarapid metabolizers may be at risk of having altered plasma concen-trations or adverse events. Although the CYP2C19*17 allele did not alter the sum of amitriptyline plus nortriptyline plasma con-centrations, it was associated with higher nortriptyline plasma concentrations, possibly increasing the risk of adverse events.15 For patients taking amitriptyline, extrapolated pharmacokinetic data suggest that CYP2C19 ultrarapid metabolizers may need a dose increase.8 Due to the need for further studies investi-gating the clinical importance of the CYP2C19*17 allele and the possibility of altered tricyclic concentrations, we recom-mend consideration of an alternative tricyclic or other drug not affected by CYP2C19. Because the clinical importance of CYP2C19*17 is currently poorly understood, this recommenda-tion is classified as optional. If amitriptyline is administered to a CYP2C19 ultrarapid metabolizer, therapeutic drug monitoring is recommended.CYP2C19 poor metabolizers are expected to have a greater ratio of amitriptyline to nortriptyline plasma concentrations.32 The elevated amitriptyline plasma concentrations may increase the chance of a patient experiencing side effects. Consider a 50% reduction of the usual amitriptyline starting dose along with therapeutic drug monitoring.8CYP2D6 and CYP2C19 combined dosing recommendations. Although specific combinations of CYP2D6 and CYP2C19 alleles are likely to result in additive effects on the pharmacokinetic properties of tricyclics, little information is available on how to adjust initial doses based on combined genotype information. Patients carrying at least one CYP2D6 nonfunctional allele and two CYP2C19 functional alleles had an increased risk of expe-riencing side effects when administered amitriptyline, whereas patients with at least one CYP2C19 loss-of-function allele and two CYP2D6 functional alleles had a low risk of experienc-ing side effects.10,33 Because there is only sparse clinical evi-dence for an additive effect of CYP2D6 and CYP2C19 on tri-cyclic dosing, the recommendations are classified as optional (Supplementary Data online).Other considerationsGene-based dosing recommendations for neuropathic pain treatment. Amitriptyline is often used at lower dosages (e.g., 0.1 mg/kg/ day in pediatric patients) for treatment of neuropathic pain than when used for depressive disorders.2,3 Because of the lower dosage, it is less likely that CYP2D6 or CYP2C19 poor or intermediate metabolizers will experience adverse effects due to supratherapeutic plasma concentrations of amitriptyline.34 Therefore, we recommend no dose modifications for poor or intermediate metabolizers when prescribed amitriptyline at a lower dose for treatment of neuropathic pain, but these patients should be monitored closely for side effects. If larger doses of amitriptyline are warranted, we recommend following the gene-based dosing guidelines presented in Tables 2 and 3. Providing dose recommendations for CYP2C19 ultrarapid metabolizers when amitriptyline is prescribed at lower doses for neuropathic pain treatment is difficult. On the basis of predicted and observed pharmacokinetic data, CYP2D6 ultrarapid metab-olizers are at risk of failing amitriptyline therapy for neuropathic pain, and thus alternative agents such as gabapentin should be considered.35 Although little information is available on how to adjust initial amitriptyline doses based on combined CYP2D6 and CYP2C19 genetic results when treating neuropathic pain, caution should be used when patients have a combination of poor or ultrarapid phenotypes (e.g., a CYP2D6 poor metabolizer also having CYP2C19 ultrarapid or poor metabolism).Consideration of drug interactions and patient characteristics. Patients treated for psychiatric disorders often require m ultipleCPIC GuIdelInesm edications, which can influence tricyclic plasma concen-trations, side effects, and therapeutic failure.4 For example, patients taking amitriptyline in combination with a potent CYP2D6 inhibitor, such as fluoxetine, may have dramatic increases in plasma concentrations.36 It has been suggested that patients taking strong CYP2D6 inhibitors should be treated similarly to CYP2D6 poor metabolizers.11 In addition, patients with increased age, liver disease, and reduced renal function may require reduced doses of tricyclics.4,37,38 Drug–drug interactions along with patient characteristics should be considered in addition to the gene-based dosing recommenda-tions presented herein.Minor metabolic pathw ays of tricyclics. Other cytochrome P450 enzymes, including CYP3A4 and CYP1A2, metabolize tri-cyclics to a lesser extent.4,34,39,40 There is currently no strong evidence supporting gene-based dosing recommendations for other cytochrome P450 enzymes that metabolize tricyclics.Potential benefits and risks for the patientFor patients who have existing CYP2D6 and/or CYP2C19 gen-otyping test results, the potential benefit is identifying those patients who are at an elevated risk of experiencing side effects or therapeutic failure. For those patients, dose adjustments can be made or an alternative agent selected. A limitation inherent to most commercially available genotyping tests is that rare or de novo variants are not detected. In addition, some alleles are not well characterized, resulting in uncertainty when predict-ing the phenotype for some genetic test results. Genotyping is reliable when performed in qualified reference laboratories, but, as with any laboratory test, an error can occur. Any errors in genotyping or phenotype prediction, along with the presence of a rare genomic variant not tested for, could potentially affect the patient lifelong.Caveats: appropriate use and/or potential misuse ofg enetic testsThe application of genotype-based dosing is most appropriate when initiating therapy with a tricyclic. Obtaining a pharma-cogenetic test after months of drug therapy may be less helpful in some instances, given that the drug dose may have already been adjusted based on plasma concentrations, response, or side effects. Similar to all diagnostic tests, genetic tests are one of several pieces of clinical information that should be considered before initiating drug therapy.SUPPLEMENTARY MATERIAL is linked to the online version of the paper at /cptDISCLAIMERCPIC guidelines reflect expert consensus based on clinical evidenceand peer-reviewed literature available at the time they are written; theyare intended only to assist clinicians in decision making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guideline is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to persons or property arising out of or related to any use of CPIC’s guidelines, or for any errors or omissions.ACKNOWLEDGMENTSWe acknowledge the critical input of M. Relling, T. Klein, K. Crews, and members of the Clinical Pharmacogenetics Implementation Consortiumof the Pharmacogenomics Research Network and funding by the National Institutes of Health (NIH). J.K.H. is supported by NIH/National Instituteof General Medical Sciences (NIGMS) Pharmacogenomics Research Network (U01 GM92666 and U01 HL105198) and by ALSAC. C.F.T. and K.S. are supported by NIH/NIGMS (R24 GM61374). E.D.K. is supported by R01 GM63674 and R01 DA14211. T.C.S. is supported by R01 GM088076 andthe Agency for Healthcare Research and Quality (R01 HS19818-01). V.L.E.is supported by the NIMH (R01 MH082784). D.J.M. is supported by a New Investigator Salary Award from the Canadian Institutes of Health Research, a New Investigator Fellowship Award from the Ontario Mental Health Foundation, and an Early Researcher Award by the Ministry of Research and Innovation of Ontario. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.AUTHOR CONTRIBUTIONSAll the authors contributed to the development of the gene-based dosing guidelines and writing of the manuscript and supplementary material. CONFLICT OF INTERESTThe authors declared no conflict of interest.© 2013 American Society for Clinical Pharmacology and Therapeutics1. American Psychiatric Association, A.P. Practice Guideline for the Treatmentof Patients With Major Depressive Disorder 3rd edn. (American PsychiatricPublishing, Arlington, VA, 2010).2. Watson, C.P. The treatment of neuropathic pain: antidepressants and opioids.Clin. J. Pain16, S49–S55 (2000).3. Laird, B., Colvin, L. & Fallon, M. Management of cancer pain: basic principlesand neuropathic cancer pain. Eur. J. Cancer44, 1078–1082 (2008).4. Rudorfer, M.V. & Potter, W.Z. Metabolism of tricyclic antidepressants. Cell. Mol.Neurobiol.19, 373–409 (1999).5. Potter, W.Z., Zavadil, A.P. 3rd, Kopin, I.J. & Goodwin, F.K. Single-dose kineticspredict steady-state concentrations on imipramine and desipramine. Arch.Gen. Psychiatry37, 314–320 (1980).6. Hiemke, C. et al. AGNP consensus guidelines for therapeutic drug monitoringin psychiatry: update 2011. Pharmacopsychiatry44, 195–235 (2011).7. Dalén, P., Dahl, M.L., Bernal Ruiz, M.L., Nordin, J. & Bertilsson, L.10-Hydroxylation of nortriptyline in white persons with 0, 1, 2, 3, and 13functional CyP2D6 genes. Clin. Pharmacol. Ther.63, 444–452 (1998).8. Stingl, J.C., Brockmöller, J. & Viviani, R. Genetic variability of drug-metabolizingenzymes: the dual impact on psychiatric therapy and regulation of brainfunction. Mol. Psychiatry (2012); e-pub ahead of print 8 May 2012.9. Kirchheiner, J. & Seeringer, A. Clinical implications of pharmacogenetics ofcytochrome P450 drug metabolizing enzymes. Biochim. Biophys. Acta1770, 489–494 (2007).10. Bertilsson, L., Aberg-Wistedt, A., Gustafsson, L.L. & Nordin, C. Extremely rapidhydroxylation of debrisoquine: a case report with implication for treatment with nortriptyline and other tricyclic antidepressants. Ther. Drug Monit.7,478–480 (1985).11. Crews, K.R. et al. Clinical Pharmacogenetics Implementation Consortium(CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CyP2D6) genotype. Clin. Pharmacol. Ther.91, 321–326 (2012).12. Gaedigk, A., Simon, S.D., Pearce, R.E., Bradford, L.D., Kennedy, M.J. & Leeder, J.S.The CyP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin. Pharmacol. Ther.83, 234–242 (2008).13. Scott, S.A. et al. Clinical Pharmacogenetics Implementation Consortiumguidelines for cytochrome P450-2C19 (CyP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther.90, 328–332 (2011).。

苯妥英钠(专业知识值得参考借鉴)一概述苯妥英钠(Phenytoinsodium)对大脑皮层运动区有高度选择性抑制作用,一般认为系通过稳定脑细胞膜的功能及增加脑内抑制性神经递质5-羟色胺(5-HT)和γ-氨基丁酸(GABA)的作用,来防止异常放电的传播而具有抗癫痫的作用。
抗神经痛的作用机制可能与本品作用与中枢神经系统,降低突触传递或降低引起神经元放电的短暂刺激有关。
还可对心房与心室的异位节律有抑制作用,也可加速房室的传导,降低心肌自律性,具有抗心律失常作用。
二适应证1.主要适用于治疗复杂部分性癫痫发作(颞叶癫痫、精神运动性发作)、单纯部分性发作(局限性发作)、全身强直阵挛性发作和癫痫持续状态。
本品在脑组织中达到有效浓度较慢,因此疗效出现缓慢,需要连续多次服药才能有效。
2.治疗三叉神经痛和坐骨神经痛、发作性舞蹈手足徐动症,发作性控制障碍、肌强直症及隐性营养不良性大疱性表皮松解。
3.用于治疗室上性或室性早搏,室性心动过速,尤适用于强心苷中毒时的室性心动过速,室上性心动过速也可用。
三临床应用口服:250~300mg/日,分2~3次服用,极量300mg/次,500mg/日;静注:100~250mg/次(不超过50mg/min),总量不超过500mg/日。
用于三环类抗抑郁药过量时心脏传导障碍和洋地黄中毒所致的室性及室上性心律失常,口服:100~300mg/日,分1~3次服用;静注:中止心律失常100mg/次,10~15分钟后可重复至心律失常中止或出现不良反应,总量不超过500mg/日。
小儿开始每日5mg/kg,分2~3次使用,最大量不超过250mg/日;维持量为每日4~8mg/kg,分2~3次使用。
用于胶原酶合成抑制剂,口服:开始每日2~3mg/kg,分2次服用,在2~3周内增加到患者能够耐受的用量,血药浓度至少达8μg/ml。
四不良反应较常见的不良反应有行为改变、笨拙或步态不稳,思维混乱,发音不清,手抖、神经质或烦躁易怒,这些反应往往是可逆的,一旦停药很快就消失。
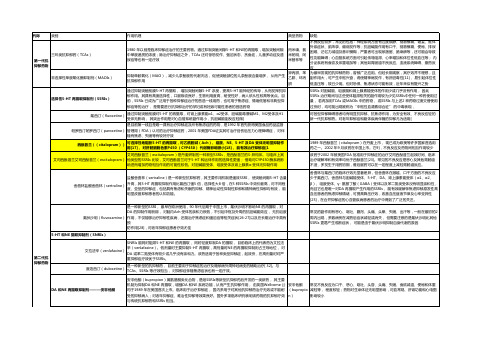

三环类抗抑郁药的药物分析摘要】三环类抗抑郁药主要通过抑制突触前膜对5-HT及NE的再摄取,增强中枢5-HT及NE能神经的功能而发挥抗抑郁作用。
在肝微粒体内被细胞色素 P450酶催化羟化和去甲基化代谢。
临床上发现抑郁症病人服用小剂量去甲替林即出现明显的药物蓄积和不良反应。
【关键词】三环类;抗抑郁药;中毒及处理【中图分类号】R453 【文献标识码】A 【文章编号】1007-8231(2016)27-0029-02三环类抗抑郁制药(TCAs),为经典抗抑郁药或第一代抗抑郁药,属于单胺回收抑制药(简称MARl)。
它们由两个苯环和一个丙咪嗪类杂环构成,故称为三环类抗抑郁药。
1.药理作用及机制TCAs主要的作用机理是抑制单胺类递质的摄取,使突触间隙的单胺类递质浓度增加而达到治疗作用。
而TCAs作用于NE比5-HT强。
TCAs在抑制NE和5-HT过程中,它们也有拮抗其他各种神经递质受体的作用[1]。
一般说来,引起的副作用也由于这些受体的被阻断,TCAs有奎尼丁样膜稳定作用。
这可以解释,在过度剂量时会出现损害心脏传导和引起高的毒性反应,如可以阻断M1、α1和H1等受体,而出现相应的副作用。
2.临床应用在治疗抑郁症时,三环类药物最易因骤然停药而出现撤药综合征。
三环类抗抑郁药对抑郁症的治疗效果肯定,但作用发挥较慢,抗焦虑作用多在1周内显现,而抗抑郁作用需要2~3周后才出现。
药物发挥作用后,需要维持治疗6个月甚至更长的时间,才能起到稳定病情和减少复发的效果。
但由于种种原因,许多人不能坚持服药,甚至骤然停药[2]。
人体对这类药物虽不会产生依赖,但骤然停药可引起撤药综合征。
轻者表现为躯体症状和胃肠道症状,重者伴有较为明显的运动障碍及精神障碍症状和特征。
躯体症状为全身不适、头痛、头晕、肌痛、疲乏、无力;胃肠道症状为恶心、呕吐、厌食、腹痛、腹泻等。
三环类抗抑郁药为胆碱能拮抗药,具有较强的抗胆碱作用,长期用药会使胆碱能受体被阻滞,一旦骤然停药,有可能引起胆碱能系统反弹,导致中枢神经系统和外周胆碱能出现亢进的症状和体征。
抑郁症治疗的常见药物有哪些在现代社会中,抑郁症已经成为一个备受关注的健康问题。
对于抑郁症患者来说,药物治疗是常见且重要的治疗方式之一。
那么,抑郁症治疗的常见药物都有哪些呢?首先,我们来了解一下选择性 5 羟色胺再摄取抑制剂(SSRIs)。
这类药物包括氟西汀、帕罗西汀、舍曲林、氟伏沙明和西酞普兰等。
它们通过抑制神经突触细胞对 5 羟色胺的再摄取,增加突触间隙中 5 羟色胺的浓度,从而改善抑郁症状。
这类药物通常具有较好的耐受性和安全性,副作用相对较小,常见的副作用可能包括恶心、呕吐、头痛、失眠等,但一般会在治疗初期出现,随着用药时间的延长逐渐减轻或消失。
5 羟色胺和去甲肾上腺素再摄取抑制剂(SNRIs)也是常用的抗抑郁药。
代表药物有文拉法辛和度洛西汀。
它们既能抑制 5 羟色胺的再摄取,又能抑制去甲肾上腺素的再摄取,从而发挥抗抑郁作用。
SNRIs 的疗效通常较为显著,但副作用可能相对稍大一些,比如可能会引起血压升高、口干、多汗等。
去甲肾上腺素和特异性 5 羟色胺能抗抑郁药(NaSSAs)中的代表药物为米氮平。
它通过增强去甲肾上腺素能和特异性 5 羟色胺能神经的传导,发挥抗抑郁作用。
米氮平对于伴有睡眠障碍和焦虑症状的抑郁症患者效果较好,常见的副作用包括嗜睡、体重增加等。
三环类抗抑郁药(TCAs)是较早应用于临床的一类抗抑郁药,如阿米替林、丙咪嗪等。
它们通过抑制去甲肾上腺素和 5 羟色胺的再摄取来发挥作用。
然而,由于这类药物副作用较多,如抗胆碱能副作用(口干、便秘、视物模糊等)、心血管副作用等,目前在临床上的使用相对较少,一般作为二线药物使用。
四环类抗抑郁药如马普替林,其作用机制与三环类抗抑郁药相似,但副作用相对较轻。
不过,由于其疗效和安全性不如新型抗抑郁药,使用也受到一定限制。
单胺氧化酶抑制剂(MAOIs)如苯乙肼、异卡波肼等,由于可能会引起严重的药物相互作用和不良反应,如高血压危象等,使用时需要严格控制饮食和避免与其他药物合用,因此在临床上也较少作为首选药物。
抗抑郁症药抗抑郁症药是一类用于治疗抑郁症的药物。
抑郁症是一种常见的心理疾病,患者常常体验到长期的低落情绪、失去兴趣和快乐感、精神疲惫等症状。
抗抑郁症药作为一种药物治疗方式,可以有效缓解抑郁症患者的症状,帮助他们恢复健康的心理状态。
一、常见的抗抑郁症药物目前,常见的抗抑郁症药物包括选择性5-羟色胺再摄取抑制剂(SSRI)、三环类抗抑郁药(TCA)、单胺氧化酶抑制剂(MAOI)和其他一些非特异性抗抑郁药物。
1. 选择性5-羟色胺再摄取抑制剂(SSRI)SSRI是当前抗抑郁症治疗的主要药物。
它们通过抑制脑内5-羟色胺再摄取,增加5-羟色胺的浓度,从而提高患者的情绪和精神状态。
常见的SSRI药物有舍曲林、帕罗西汀、氟西汀等。
这些药物具有副作用小、疗效稳定等优点,但也可能引发一些不良反应,如失眠、恶心、性功能障碍等。
2. 三环类抗抑郁药(TCA)三环类抗抑郁药是较早使用的一类抗抑郁药物,如阿米替林、丙米嗪等。
它们通过阻断去甲肾上腺素和/或5-羟色胺再摄取,起到抑制抑郁症状的作用。
然而,TCAs也存在明显的副作用,如口干、便秘、心律不齐等,且容易与其他药物发生药物相互作用。
3. 单胺氧化酶抑制剂(MAOI)MAOI是另一类抗抑郁药物,通过抑制单胺氧化酶的活性,增加脑内5-羟色胺、去甲肾上腺素和多巴胺的水平。
然而,MAOI药物在使用上需要严格控制饮食,避免与含有酪胺的食物一同摄入,否则容易导致危险的副作用,如高血压危机。
二、抗抑郁症药物的使用注意事项1. 个体化治疗:每个患者的症状和体质都不同,需要根据具体情况进行个体化治疗。
因此,在选择抗抑郁症药物时,应咨询专业医生,并根据医生的建议进行使用。
2. 药物副作用:抗抑郁症药物具有一定的副作用,如恶心、头痛、失眠等。
应注意监测患者的身体状况,若出现严重副作用应及时就医处理。
3. 用药持续时间:抗抑郁症药物治疗需要一定的时间,一般需要连续用药数周至数月才能见效。
患者在用药过程中要有足够的耐心,与医生保持良好的沟通,及时调整用药方案。
抑郁症的药物治疗常见的抗抑郁药物和副作用抑郁症是一种常见的心理健康问题,严重影响患者的生活质量。
药物治疗是常见的抑郁症治疗方式之一,其中抗抑郁药物是主要的治疗手段之一。
本文将介绍几种常见的抗抑郁药物和它们的副作用。
一、SSRI类抗抑郁药物SSRI(选择性5-羟色胺再摄取抑制剂)是抑郁症的一线治疗药物之一。
这类药物通过抑制大脑中5-羟色胺(一种神经递质)的再摄取来缓解抑郁症状。
1. 高效且少副作用的SSRI——氟伏沙明氟伏沙明是一种常用的SSRI抗抑郁药物,其疗效显著且副作用较少。
常见的副作用包括恶心、失眠和性功能减退等。
然而,这些副作用在使用一段时间后通常会逐渐减轻。
2. 副作用较多但效果显著的SSRI——帕罗西汀帕罗西汀是另一种常用的SSRI抗抑郁药物,疗效较好。
然而,与氟伏沙明相比,帕罗西汀的副作用相对更多。
常见的副作用包括头晕、口干、失眠等。
此外,帕罗西汀还可能导致食欲增加,引起体重的增加。
二、SNRI类抗抑郁药物SNRI(去甲肾上腺素和5-羟色胺再摄取抑制剂)是另一类常用的抗抑郁药物,与SSRI类药物相比,SNRI类药物还能提高去甲肾上腺素水平。
1. 常用的SNRI类抗抑郁药物——文拉法辛文拉法辛是一种常见的SNRI类抗抑郁药物,疗效广泛认可。
它主要通过抑制去甲肾上腺素和5-羟色胺的再摄取来发挥作用。
常见的副作用包括头痛、食欲不振和睡眠障碍等。
2. 需慎用的SNRI类抗抑郁药物——美拉法辛美拉法辛是另一种SNRI类抗抑郁药物,但该药物副作用较多,需要慎用。
常见的副作用包括心率增加、血压升高和口干等。
此外,使用美拉法辛的患者还应定期监测血压和心率等生理指标。
三、其他常见的抗抑郁药物除了SSRI和SNRI类抗抑郁药物,还有其他常见的抗抑郁药物,如三环类和四环类抗抑郁药物、MAOI(单胺氧化酶抑制剂)等。
1. 三环类和四环类抗抑郁药物这些药物以氯米帕明、阿米替林为代表,副作用相对更多。
常见的副作用包括体重增加、口干、便秘等。
三环类抗抑郁药(专业知识值得参考借鉴)
一概述三环类抗抑郁药(TcAs)是临床上治疗抑郁症最常用的药物之一,其核心结构是中间一个七元杂环两边连接一个苯环构成。
其中,丙米嗪是最早发现的具有抗抑郁作用的化合物,目前常用药物还有氯米帕明、阿米替林、多赛平等。
TCAs主要在肝脏代谢,参与的代谢酶有CYP1A2、CYP2D6、CYP3A4等。
主要代谢为三环核氧化,如2位或10位碳原子羟化(CYP2D6催化)、脂肪侧链氧化、氮原子的去甲基化(CYP3A4催化)。
由于CYP的活性受多种因素的影响,血浆中活性代谢产物的浓度与原药浓度的比率有明显的个体差异。
二药理作用TCAs阻断了去甲肾上腺素(NA)能和5羟色胺(5-HT)能神经末梢对NA和5-HT的再摄取,增加了突触间隙单胺类递质的浓度,临床上表现为抑郁症状的改善。
目前研究发现,抗抑郁药对递质再摄取的抑制作用是立即发生的,而长期用药后则可以降低受体的敏感性(下调作用),这与抗抑郁药的临床效应滞后(用药2~3周后起效)密切相关。
NA再摄取的阻断使神经突触间隙内源性NA浓度增加,进而可以降低突触前膜α2受体的敏感性,长期使用还可能减少中枢α2受体的数目。
5-HT再摄取的抑制首先也是增加胞体部位突触间隙内源性5-HT浓度,通过下调突触前胞体膜的5-HT1A受体,增加末梢释放5-HT,最终达到抗抑郁作用。
TCAs还有很强的阻断5-HT2A 受体作用。
三适用范围适用于治疗各类以抑郁症状为主的精神障碍,如内因性抑郁、恶劣心境障碍、反应性抑郁及器质性抑郁等。
还可用于治疗广泛性焦虑症、惊恐发作和恐怖症。
小剂量丙米嗪可米嗪可用于治疗儿童遗尿症,氯米帕明则常用于治疗强迫症。
对精神分裂症患者伴有的抑郁症状,治疗宜谨慎,TCAs可能使精神病性症状加重或明显化。
三环类抗抑郁药曾经是首选的抗抑郁药,但是由于该类药物抗胆碱能和心血管不良反应较大,禁忌证和药物相互作用较多,安全范围较窄,使其临床应用受限。
TCAs服用超过一天剂量的10倍时就有致命性危险,心律失常是最常见的致死原因。
四不良反应1.抗胆碱能不良反应
三环类抗抑郁药常见药物不良反应有口干、便秘、视物模糊、尿潴留、嗜睡、体重增加等,是其抗胆碱能作用和抗组织胺作用的结果,与血药浓度相关,在治疗浓度范围内即可出现,存在着较大的个体差异,并不是中毒的表现。
轻度的抗胆碱能药物不良反应一般不需做特殊处理,必要时可加用
少量拟胆碱药。
2.中枢神经系统毒性
TCAs引起的中枢神经系统毒性反应有情绪降低、注意力不集中、社会行为异常、震颤、运动失调、癫痫发作、思维障碍、幻觉妄想、定向力障碍、焦虑不安、谵妄、意识模糊、昏迷等。
3.心血管系统毒性
TCAs引起的心血管毒性反应包括体位性低血压、心动过速、传导阻滞、心律失常及心跳骤停等。
TCAs所致的体位性低血压,主要与受体的敏感性有关,在较低血药浓度时就可发生,更多见于老年人和患有充血性心力衰竭的病人。
五注意事项严重心、肝、肾疾病患者,以及粒细胞减少、青光眼、前列腺肥大患者禁用,妊娠头3个月禁用。
癫痫患者和老年人慎用。
(说明:上述内容仅作为介绍,药物使用必须经正规医院在医生指导下进行。
)
寄语:“身体是革命的本钱”。
身体健康是人最基本的,也是很难达到的目标。
今天,你能开口说话,能用眼睛、耳朵、鼻子去感知身边的一切事物,能正常地用双腿行走,无病无痛……这些看起来是很轻而易举的,但是你是否想过这些却是极度重要且来之不易的,如果某一天你失去了,怎么办?看到街上那些失明失聪、断手少腿的残疾人,你是否在想:幸好我没有像他们那样,你错了,生命充满意外,谁能保证你明天不会成为他们中的一员呢?那你又是否因此更加懂得珍惜健康呢?那就请不要透支自己的身体健康,赶快行动起来,锻炼身体,让身心健康吧!要清楚意识到自己目前的健康状况是稍纵即逝的,明确健康是我们做任何事情的本钱,要懂得珍惜健康!。