药物Latanoprostene bunod(拉坦前列素硝酸酯)合成检索总结报告
- 格式:pdf
- 大小:664.34 KB
- 文档页数:12

关于哒嗪酮类中间体药物及其合成进展哒嗪酮类药物是一类重要的心脑血管疾病治疗药物,其具有抗凝血、抗血小板聚集和扩张冠状动脉等多种药理作用。
目前常用的哒嗪酮类药物主要包括普锐卡素(Prasugrel)、替格瑞洛(Ticagrelor)和克洛吉雷(Clopidogrel)等。
本文将介绍哒嗪酮类药物的合成进展以及一些重要的中间体药物。
哒嗪酮类药物的合成通常包括两个主要步骤:首先是哒嗪环的合成,然后是与其他部分的连接。
哒嗪环的合成通常通过氧化环化反应进行,常用的方法包括酮酸酯缩合、胺和酮的反应和酮醇合成等。
酮酸酯缩合方法是最常用的方法,通过酰氯化剂和胺反应生成酸,然后与醇缩合成酮酸酯,再通过蒸馏反应生成哒嗪环。
这种方法的优点是反应条件温和,中间体易于合成,且生成的产物纯度高。
由于哒嗪环的合成具有一定的难度,合成过程中需要保护羰基等功能团,因此需要选择合适的保护基。
在哒嗪环合成完成后,需要进行与其他部分的连接,一般是通过亲核取代反应进行。
常用的连接方法包括亲核取代反应和偶联反应。
亲核取代反应是将哒嗪环与亲核试剂反应,形成C-N键,连接其他部分。
常用的亲核试剂包括胺、醇和硫醇等。
这种方法的优点是反应条件温和,中间体易于合成,且反应产率较高。
偶联反应是将哒嗪环上的卤代烃与其他分子进行偶联反应,一般需要选择合适的偶联试剂和催化剂。
常用的偶联试剂包括叠氮化钠、硒酸钠和硝酸銨等,催化剂包括钯、铜和铜催化剂等。
这种方法的优点是反应产率较高,但需要较严格的反应条件。
在哒嗪酮类药物的合成过程中,一些重要的中间体药物也得到了广泛应用。
普锐卡素的合成中,一种重要的中间体药物是N-(2-噁唑基)胺基硫酸盐,它是普锐卡素的关键中间体,通过与其他化合物反应,最终生成普锐卡素。
替格瑞洛的合成中,一种重要的中间体药物是5-氯-2-甲基噻二唑,它是替格瑞洛的合成起始物。
克洛吉雷的合成中,一种重要的中间体药物是克洛吉雷磺酸盐,它是克洛吉雷的合成关键中间体。

上市新药Tenapanor(坦帕诺)合成检索总结报告一、Tenapanor(坦帕诺)简介Tenapanor(坦帕诺)是由Ardelyx公司研发,于2019年9月在美国上市,主要用于成人便秘性肠易激综合征的治疗。
Tenapanor(坦帕诺)是一种钠/氢交换蛋白-3(NHE3)抑制剂。
NHE3在小肠和结肠表面表达,主要负责吸收食物中的钠离子。
本品抑制NHE3,能够减少钠离子在小肠和结肠的吸收,导致水向肠道内分泌,从而加快肠道蠕动并导致粪便疏松。
Tenapanor(坦帕诺)不良反应:严重腹泻。
Tenapanor(坦帕诺)分子结构式如下:CAS:1234423-95-0英文名称:Tenapanor中文名称:坦帕诺二、Tenapanor(坦帕诺)合成路线三、Tenapanor (坦帕诺)合成检索总结报告(一)Tenapanor (坦帕诺)中间体3的合成合成方法实验步骤参考文献合成方法一Into aIL 3-necked round-bottom flask purged and maintained with an inert atmosphere of nitrogen,was placed a solution of 2-bromo l-(3-bromophenyl)ethanone 1(55g,199.28mmol,1.00equiv),1,4-dioxane (300mL),TEA (40g,396.04mmol,1.99eqmv),and (2,4-dichlorophenyl)-N-methylmethanamme 2(38g,201.06mmol,1.01equiv).The resulting solution was stirred for 2h at 25o C.The solids 3were filtered out and the filtrate was used without any further purification.WO2010/78449;(2010);(A2)English (二)Tenapanor (坦帕诺)中间体4的合成合成方法实验步骤参考文献合成方法一Into a IL 3-necked round-bottom flaskpurged and maintained with an inert atmosphere of nitrogen,was placed a solution of 2-((2,4-dichlorobenzyl)(methyl)ammo)-l-(3-bromophenyl)ethanone 3(77g,198.97mmol,1.00equiv,the oretical yield)in methanol (300mL)This was followed by the addition of NaBH 4(15g,394.74mmol,1.98equiv)in several batches at 0o C.The resulting solution was stirred for 30min at 0o C m a water/ice bath.The reaction was then quenched by the addition of 100mL of acetone The resulting mixture was concentrated under vacuum.The resulting solution was extracted with 3x100mL of ethyl acetate and the organic layers combined and dϖed over anhydrous sodium sulfate.The residue was applied onto a silica gel column with ethyl acetate/petroleum ether (1:100).This resulted in 50g (65%)of 2-((2,4-dichlorobenzyi)(methyl)amino)-l-(3-bromophenyl)ethanol 4as a yellow oil.WO2010/78449;(2010);(A2)English (三)Tenapanor (坦帕诺)中间体5的合成合成方法实验步骤参考文献合成方法一Into a 500-mL 3-necked round-bottom flask,was placed a solution of 2-((2,4dichIorobenzyl)(methyl)ammo)-l-(3-bromophenyl)ethanol 4(25g,64.27mmol,1.00equiv)in dichloromethane (100mL).This was followed by the addition of sulfuric acid (100mL)dropwise with stirring at 0-5o C.The resulting solution was stirred for 4h at room temperature.The resulting solution was diluted with of ice water.The pH value of the solution was adjusted to 8with sodium hydroxide.The resulting solution was extracted with 3x300mL of dichloromethane and the organic layers combined and dried over anhydrous sodium sulfate and concentrated under vacuum.The crude product was re-crystallized from petroleum ether ethyl acetate in the ratio of 8:1.This resulted in 15g (63%)of 4-(3-bromophenyl)-6,8-di ‐chloro-2-methyl-l,2,3,4-tetrahydroisoquinoline 5as a white solid.WO2010/78449;(2010);(A2)English (四)Tenapanor (坦帕诺)中间体6的合成。

糖皮质激素类药物的作用是什么?美国FDA批准了9项新药、生物制剂和第一个基因治疗药物的申请,糖皮质激素类药物的作用是什么?接下来,就带你了解一下吧!获批产品降眼压药物Vyzulta(latanoprostene bunod 0.024%)适用于开角型青光眼和高眼压症患者的降眼压治疗,它是一种前列腺素类似物,每日一次,这是第一种以一氧化氮作为代谢物的前列腺素类似物。
一氧化氮可以增加小梁网和Schlemm管的流出量,从而促进房水排出。
三期研究对比了Vyzulta和噻吗洛尔有效性,Vyzulta 展现出了非劣效与优效的结果;二期研究结果显示,相较于拉坦前列腺素,Vyzulta具有更显著的降压效果。
Rhopressa(Netarsudil 0.02%,Aerie公司)是一种Rho激酶抑制剂,用于降低开角型青光眼或高眼压患者的眼压。
这是第一个被批准的Rho激酶抑制剂。
Rhopressa每日使用一次,它通过增加小梁网房水流出量来降低眼压。
Aerie Pharmaceuticals提交了一份NDA申请,将0.02%的Netarsudil和0.005%的Latanoprost(Roclatan)滴眼液固定剂量联合使用。
在MERCURY 1临床试验的第12个月,接受两种滴眼液联合使用的患者中,82%患者IOP≤18 mm Hg, 66%接受Roclatan治疗的患者IOP≤18 mm Hg,57%接受Rhopressa治疗的患者IOP≤18 mm Hg。
Xelpros(拉坦诺前列素眼用乳液,0.005%)是首个也是唯一一个无苯扎氯铵的拉坦前列素制剂。
用于开角型青光眼或高眼压症患者降眼压治疗。
糖皮质激素类药物Inveltys(LOTEPREDNOL etabonate 1% , Kala 公司)用于治疗眼科术后炎症和疼痛。
该产品利用其专有的基于纳米颗粒的黏液渗透颗粒(MPP)技术,增强药物通过黏液屏障的能力,可以每日2次给药。

药物Latanoprostenebunod(拉坦前列素硝酸酯)合成检索总结报告药物Latanoprostene bunod(拉坦前列素硝酸酯)合成检索总结报告一、Latanoprostene bunod(拉坦前列素硝酸酯)简介Latanoprostene bunod(拉坦前列素硝酸酯)于2017年11月在美国上市,主要用于降低伴有开角型青光眼或高眼压患者的眼内压。
Latanoprostene bunod(拉坦前列素硝酸酯)不良反应有结膜充血、眼睛刺激、眼痛和滴注部位疼痛等等。
Latanoprostene bunod(拉坦前列素硝酸酯)分子结构式如下:CAS:860005-21-6英文名称:Latanoprostene bunod中文名称:拉坦前列素硝酸酯二、Latanoprostene bunod(拉坦前列素硝酸酯)合成路线三、Latanoprostene bunod(拉坦前列素硝酸酯)合成检索总结报告(一)Latanoprostene bunod(拉坦前列素硝酸酯)中间体2合成合成方法实验步骤参考文献操作方法一Into a10L three-necked bottle equipped with a thermometerand mechanical stirring,add L-benzoylcolide1(500g,1.81mol)and methylene chloride(5L),Then use the ice-saltbath for the above mixing system,cool to an internaltemperature not higher than0°C,and stir at constanttemperature for10min;Next Dess Martin reagent(2.17mol)was slowly added to the reaction system,and the reactiontemperature was controlled to be not higher than10°C.After the addition of Dess Martin reagent was completed,themixture was stirred at constant temperature for2h.After the reaction was completed,saturated Na2S2O3(1000mL)wasadded to quench the reaction,and the aqueous phase was extracted with dichlorometha ne(0.5L×2).The combinedorganic phases were washed with a saturated sodiumchloride solution,dried over anhydrous sodium sulfate,filtered,and concentrated to obtain a pale yellow compound 2(480g,1.75mol),which was directly applied to the next reaction with a yield of96.7%CN110483360;(2019);(A)操作方法二A solution of1,3-diisopropylcarbodiimide(13.7g)in dimethyl sulfoxide(38mL)contained in anitrogen-substituted flask was added dropwise with a solution of[3aR-(3aα,4α,5α,6aα)]-5-(benzoyloxy)- hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one 1(14.9g)and orthophosphoric acid(1.1g)in dimethyl sulfoxide.The mixture was stirred at about30°C for2hours, and then added with methylene chloride,and the deposited white solid was removed by filtration.The filtrate was washed with water,dried over magnesium sulfate,then filtered,and concentrated to obtain23.2g of an aldehyde compound2.EP1886992;(2008);(A1)English;US2008/33176;(2008);(A1)EnglishA suspension of anhydrous chromium trioxide(20.8g,208 mmol)in methylene chloride(400mL)was stirred and操作方法三cooled in an ice bath asanhydrous pyridine (32.7mL,406mmol)was added.After 15min at 0°C.,the mixture was allowed to warm to ambient temperature for 2h.The reaction mixture was cooled to 0°C.and treated with a pre-cooled solution of (3aR,4S,5R,6aS)-5-(benzoyloxy)-hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one 1(Corey lactone,9.4g,34mmol)in methylene chloride (400mL)After 5min,the reaction was diluted with toluene (240mL)and filtered.The solid was washed with toluene.The combined filtrate was concentrated to give (3aR,4R,5R,6aS)-5-(benzoyloxy)hexahydro-2-oxo-2H-cyclopenta[b]f uran-4-carboxaldehyde 2.Toluene was added to give about 300mL of 2008/242713;(2008);(A1)English;EP1975163;(2008);(A 1)English;US2008/242714;(2008);(A1)English 操作方法四Dess-Martin periodinane (27.63g)and dichloromethane (120mL)are charged into a round bottom flask under nitrogen atmosphere and stirred for 5-10minutes.The reaction mixture is cooled to 0-5°C.A solution of (3aR,4S,5R,6aS)-4-(hydroxymethyl)-2-oxohexahydro-2H-cyclopenta [b]furan-5-yl benzoate 1(15g)in dichloromethane (90mL)is added to the reaction mixture at 0°C.The reaction mixture is maintained for 5hours.A solution of sodium thiosulphate pentahydrate (45g)and sodium bicarbonate (15g)in water (120mL)is added to the reaction mixture at 2°C.and maintained for 30minutes.The reaction mixture is heated to 16°C.and maintained for 30minutes.Both organic and aqueous layers are separated.Theaqueous layer is extracted with dichloromethane (75mL).The combined organic layer is washed with 10%sodium chloride solution (75mL).The solvent from the organic layer is evaporated to 10volumes at 30-35°C.to afford 260mL of (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate2013/184476;(2013);(A1)English(二)Latanoprostene bunod (拉坦前列素硝酸酯)中间体4的合成合成方法实验步骤参考文献To a suspension of 21g of LiCl (MW =42.39;4.4eq.)in 280mL of acetonitrile was added 37g of compound 3(MW =256.25;1.3eq.).The resulting mixture is cooled to -15°C。

新药Aprocitentan(阿普昔腾坦)合成检索总结报告一、Aprocitentan(阿普昔腾坦)简介Aprocitentan(阿普昔腾坦)适应于治疗高血压。
2017年12月Janssen宣布与Idorsia达成合作协议,共同开发和推广在研高血压药物Aprocitentan及其衍生化合物。
2020年1月,Idorsia Pharmaceuticals 和Janssen开启高血压肾功能不全,慢性三期临床研究。
Aprocitentan(阿普昔腾坦)分子结构式如下:英文名称:Aprocitentan中文名称:阿普昔腾坦本文主要对Aprocitentan(阿普昔腾坦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Aprocitentan(阿普昔腾坦)合成路线路线一三、Aprocitentan(阿普昔腾坦)合成路线路线二四、Aprocitentan (阿普昔腾坦)合成路线一检索总结报告(一)Aprocitentan (阿普昔腾坦)中间体2的合成(路线一)合成方法实验步骤参考文献操作方法一A mixtureof 5-bromo-2-chloro-pyrimidine 1(100g,0.51mol),THF (1.5L),and K 2CO 3(286g,24mol)was heated to 45o C,then ethylene glycol (43ml,0.7mol)was added to thereaction mixture and maintained at 45o C for 8h.The reaction mixture was cooled to room temperature,filtered,and the residue was washed with THF (400ml).The solvent of The filtratewas replaced with ethyl acetate using Dean-Stark apparatus,and the solution was refluxed for 1h,then cooled to 15o C,filtered and washed with ethyl acetate (200ml)and dried in theoven at 60o C for 6h to obtain 110g (98%)of 2as a white solid,mp 68o C-70o anic Preparations and Procedures International ;vol.49;nb.3;(2017);p.258-264.(二)Aprocitentan (阿普昔腾坦)中间体4的合成(路线一)合成方法实验步骤参考文献操作方法To a stirred solution of 100g dimethyl(4-bromophenyl)-malonate 3(10,0.348mol)in 400cm 3methanol,30g formamide (0.66mol)and 30g sodium methoxide (0.555mol)were added at 20-25o C.The reaction mass was heated to 70o C and maintained until completion of reaction (monitored by HPLC).After completion of the reaction,methanol was distilled off from the reaction mass under reduced pressure at 70o C to obtain the syrup.The syrup was cooled to 25-30o C and diluted with 2dm 3water.The pH of the solution was adjusted to 2-2.5using conc.hydrochloricMonatshefte fur Chemie ;vol.149;nb.3;(2018);p.一acid and maintained for 45min.The obtained solid was filtered and washed with water until the pH of the filtrate became 7-7.5.The product was suck dried and dried under reduced pressure at 100C to obtain crude 4.The crude solid was dissolved in 500cm3methanol at 60-65o C and maintained for 1h.The reaction mass was cooled to 25-30o C and maintained for 30min.The obtained solid was filtered,washed with methanol,anddried at 50-55o C under reduced pressure to offer 4.Yield:70g (75.25%);purity by HPLC:99.5%;m.p.:176-180C 653-661.操作方法二To the mixture containing the intermediate 3,100g of formazan hydrochloride was added,and the mixture was stirred and heated to 25°C for 16h.Adding water to the reaction solution,stir at 25°C until clarified,stand still,take the water phase,adjust the pH of the aqueous phase to 5with hydrochloric acid solution and stir for 1h.After suction filtration,the obtained filter cake was washed with a methanol aqueous solution having a mass fraction of 80%.Drying gave 270.2g of intermediate 4,The yield was 92.6%.CN108997223;(2018);(A)Chinese 操作方法三A solution of intermediate 3(11.73g)in MeOH (100mL)was added at 0o C to a solution of sodium (2.83g)in MeOH (100mL).The mixture was stirred for 18h at rt before formamidine hydrochloride (4.10g)was added.The suspension was stirred at rt for 4h.The solvent was removed and the residue was suspended in 10%aq.citric acid (100mL)and stirred for 10min.The white precipitate was collected,washed with 10%aq.citric acid,water,evaporated three times from cyclohexane and dried under HV at 40o C to give 5-(4-bromophenyl)-pyrimidine-4,6-diol 4.WO2009/24906;(2009);(A1)English;WO2006/51502;(2006);(A2)English;US2012/142716;(2012);(A1)English(三)Aprocitentan (阿普昔腾坦)中间体5的合成(路线一)合成方法实验步骤参考文献操作方法200g of the intermediate 4was taken in a 3L three-necked flask.Add 300g of toluene and 180g of N,N-dimethylaniline,mechanically stirred,230g of phosphorus oxychloride was added dropwise at 30°C,and the temperature was raised to 55°C after the addition.After the solid is completely dissolved,the temperature is raised to 100°C,the reaction is carried out for 4h,and then cooled to 25°C for use.450g of water was mixed with 500g of toluene,and cooled to 25°C CN108997223;(2018);(A)。
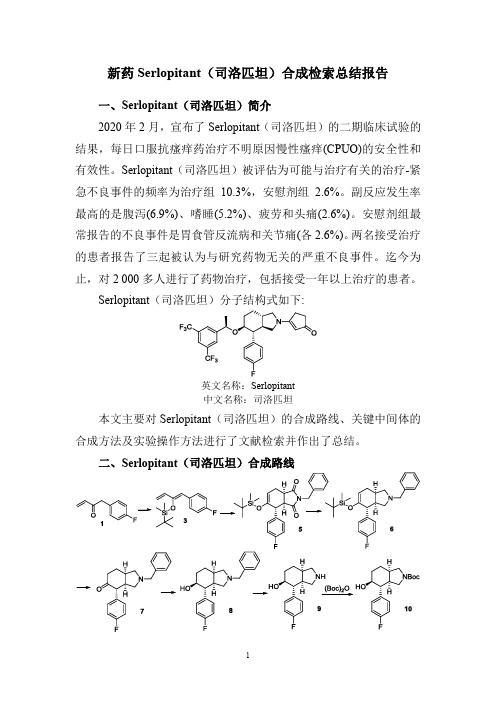
新药Serlopitant(司洛匹坦)合成检索总结报告
一、Serlopitant(司洛匹坦)简介
2020年2月,宣布了Serlopitant(司洛匹坦)的二期临床试验的结果,每日口服抗瘙痒药治疗不明原因慢性瘙痒(CPUO)的安全性和有效性。
Serlopitant(司洛匹坦)被评估为可能与治疗有关的治疗-紧急不良事件的频率为治疗组10.3%,安慰剂组2.6%。
副反应发生率最高的是腹泻(6.9%)、嗜睡(5.2%)、疲劳和头痛(2.6%)。
安慰剂组最常报告的不良事件是胃食管反流病和关节痛(各2.6%)。
两名接受治疗的患者报告了三起被认为与研究药物无关的严重不良事件。
迄今为止,对2 000多人进行了药物治疗,包括接受一年以上治疗的患者。
Serlopitant(司洛匹坦)分子结构式如下:
英文名称:Serlopitant
中文名称:司洛匹坦
本文主要对Serlopitant(司洛匹坦)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Serlopitant(司洛匹坦)合成路线
三、Serlopitant(司洛匹坦)合成检索总结报告(一) Serlopitant(司洛匹坦)中间体3的合成
(二) Serlopitant(司洛匹坦)中间体5的合成
(三) Serlopitant(司洛匹坦)中间体6的合成
(四) Serlopitant(司洛匹坦)中间体7的合成
(五) Serlopitant(司洛匹坦)中间体8的合成
(六) Serlopitant(司洛匹坦)中间体9的合成
(七) Serlopitant(司洛匹坦)中间体10的合成方法一。
专业<杂质对照品>解决方案,代理中检所/EP/BP/USP/TRC/TLC/MC/STD等品牌。
以下优势品种杂质大量现货供应:
青霉素V、硫酸庆大霉素、紫苏霉素、强力霉素、阿霉素、卡那霉素碱、地红霉素、柔红霉素、非达霉素、达托霉素、
土霉素、表阿霉素、格尔德霉素、两性霉素B、硫酸新霉素、雷帕霉素、利福霉素、盐酸壮观霉素(盐酸壮大霉素)等;q:300-头孢系列:头孢丙烯,头孢西丁 ,头孢唑林,头孢呋辛,头孢克肟,氟氧头孢,头孢特仑新戊酯,头孢哌酮,头孢硫脒,-3438-头孢地尼,头孢克洛,头孢地嗪,头孢拉宗,头孢唑肟,头孢噻肟酸,头孢罗林酯,头孢吡肟,头孢拉定,头孢他美酯,
-021
杂质品种齐全未能一一陈述——欢迎咨询!!!。
他喹莫德和奥贝胆酸的合成研究本论文由两个部分组成,分别为新型血管生成抑制剂他喹莫德的合成研究,以及法尼醇X受体激动剂奥贝胆酸的合成研究。
第一部分为他喹莫德的合成研究。
他喹莫德是由法国生物制药公司益普生(Ipsen)和瑞典生物技术公司活跃生物(Active Biotech)共同开发的具口服活性的抗前列腺癌新药。
目前已经完成了Ⅲ期临床实验。
本文通过对现有的合成路线进行比较和分析,确定了以2,6-二氟苯腈(1)作为起始原料,经亲核取代、水解、缩合成环、酰胺化反应等五步反应最终制得他喹莫德,总收率50.8%。
本文对2-甲氧基-6-甲氨基苄腈(2)的纯化方法进行研究,最后确定以与浓盐酸成盐的方法进行纯化,得到较高的纯度(99%)。
本文对水解步骤中碱和水的投料量进行优化,使产率提高到71.7%(文献为60%)。
此外,在关键中间体4-羟基-5-甲氧基-1-甲基-2-氧代-1,2-二氢喹啉-3-甲酸乙酯(5)的合成中筛选了反应中所需的丙二酸酯和碱,发现以丙二酸二乙酯和乙醇钠参与反应得到的收率最高,且通过减压蒸馏除去生成的乙醇能有效减少副反应的发生。
最后合成中间体5的总收率为53.8%(以1计),高于文献收率48%。
通过本文的工艺合成出来的他喹莫德杂质较少,通过检测分析只有一个脱羧杂质以及残留的原料酯(5)和原料N-甲基-4-三氟甲基苯胺(7),本文定向合成了这个脱羧杂质,经MS和1H-NMR结构确证,为终产品的质量控制奠定了基础。
经本工艺所制备的他喹莫德,其结构经MS、1H-NMR、13C-NMR确证,其纯度经HPLC测定达99.9%,单杂小于0.1%。
第二部分为奥贝胆酸的合成研究。
奥贝胆酸是由Intercept制药公司开发的口服性FXR激动剂,用于治疗对熊去氧胆酸(UDCA)反应不充分或不耐受的原发性胆汁性肝硬化(PBC)患者。
目前Ⅲ期临床已经结束,已获得美国快速审核资格,以及美国和欧洲的孤儿药资格认证。
奥贝胆酸可能会成为未来超过20年治疗原发性胆汁性肝硬化的首选新方法。
药物Venetoclax(维奈妥拉)合成检索总结报告一、Venetoclax(维奈妥拉)简介Venetoclax(维奈妥拉)于2016年4月在美国上市,主要用于治疗慢性淋巴细胞白血病患者。
Venetoclax(维奈妥拉)常见的不良反应有腹泻、恶心、贫血、血小板减少和疲劳等等。
Venetoclax(维奈妥拉)分子结构式如下:CAS:251980-67-3英文名称:Venetoclax中文名称:维奈妥拉本文主要对Venetoclax(维奈妥拉)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、布瓦西坦Venetoclax(维奈妥拉)合成路线三、Venetoclax (维奈妥拉)合成检索总结报告(一)Venetoclax (维奈妥拉)中间体3的合成合成方法实验步骤参考文献操作方法一To a stirred solution of 5-bromo-1H-pyrrolo[2,3-b]pyridine (1)(1.5g,7.5mmole)in 15ml of dioxane and 5ml of DMF,sodium hydride (345mg,9mmole)was added portionwise and,after 0.5hrs.,TIPSCl (2)(2.5ml,12.3mmole);the reaction was stirred at r.t.overnight,then diluted with water and extracted with methylene chloride;a fast purification by silica gel chromatography with dichloromethane gave 2.6g of 5-bromo-1-triisopropylsilanyl-1H-pyrrolo[2,3-b]pyridine (3)(97%yield).This product was used for the following reaction without any further purification.EP2070928(2009);(A1)English 操作方法二A Schlenk tube was charged with sodium hydride (95%pure,282mg,11.15mmol)and dry THF (12mL)under argon.The suspension was cooled to 0°C in an ice bath and 5-bromo-1H-pyrrolo[2,3-b]pyridine (1)(96%pure,2.00g,9.74mmol)was slowly added in dry THF (20mL).After 15min,chloro-triisopropylsilane (2)(95%pure,1.96g,10.15mmol)was added as a solution in dry THF (6mL)at 0°C and the ice bath was removed.At room temperature saturated aqueous solution of ammonium chloride (30mL)was added and the resulting mixture was extracted with cyclohexane (2×100mL).Thecombined organic phases were dried over MgSO 4,and evaporated.Column chromatographicpurification (SiO 2,CH/EtOAc,19:1)yielded a colorless,crystalline solid 3[3.25g,94%yield].MP:59-60°C Bioorganic and Medicinal Chemistry ;vol.23;nb.17;(2015);p.5734-5739操作方法三At room temperature,400ml of N,N-dimethylformamide was added to a 1L three-necked flask to start stirring.Add 5-bromo-7-azaindole 1(30g,0.15mol)and potassium tert-butoxide (25.6g,0.23mol)to the reaction flask.After stirring and clearing,cool the ice bath to wait for the internal temperature.At 0°C,triisopropylsilyl chloride 2(43.3g,0.225mol)diluted with 80ml of tetrahydrofuran was initially added dropwise.After the addition is completed,the reaction is maintained at 0-5°C for 10-20minutes.TLC control,the reaction is over.300ml of water was added toCN107434807;(2017);(A)Chinesethe reaction flask,and the mixture was stirred for15 minutes.The aqueous phase was extracted with methyltert-butyl ether(200ml×2).The organic phase was combined and the organic phase was washed with500ml of saturated aqueous sodium chloride solution once.After drying over anhydrous sodium sulfate,the solvent is concentrated to obtain an oil,and50ml of methanol is added to precipitate a solid.The solid is vacuum filtered and dried to obtain a compound of the formula3.Yield:50.5g,yield:93.8%.操作方法四5-Bromo-7-azaindole1(100.0g,0.51mol)was dissolved in200mL of tetrahydrofuran.Potassium tert-butoxide(68.5g,0.61mol)was added at room temperature and thetemperature was raised to60-65°C.Stir for2hours and coolto10-20°C.Add107.5g of triisopropylchlorosilane,0.56mol),After the completion of the dropwise addition,thereaction was continued at10to20°C for4hours.The TLCtest showed that the reaction of the starting material wascompleted.After the reaction was completed,500mL ofwater was added to quench it.Extract once with1000mL ofethyl acetate.The extract was washed once with1000mL ofsaturated brine and dried over anhydrous sodium sulfate.Filtration,spin dry,recrystallized from1000mL methanol,filtered,Drying pale yellow powder156.7g,The yield was87.5%.CN109320516;(2019);(A)Chinese操作方法五To5-bromo-7-azaindole1(1.5g,7.6mmol)inN,N-dimethylformamide(20mL)were added sodiumhydride(60%in mineral oil,0.27g,11.0mmol)andtrriisopropylsilyl chloride2(2.6mL,12.0mmol),under anatmosphere of nitrogen.The reaction was stirred for2hoursat room temperature.The reaction was poured into water andextracted with ethyl acetate.The organic layer was washedwith brine,dried over anhydrous sodium sulfate and filtered.The filtrate was concentrated and purified by silica gelcolumn chromatography eluting with10%ethyl acetate inhexane to give the compound3(1.6g,59%).US2008/167338;(2008);(A1)English;WO2007/2325;(2007);(A1)English操作方法六to a mixture of5-bromo-1H-pyrrolo[2,3-b]pyridine1(15.4g)in tetrahydrofuran(250ml)was added1M lithium hexamethyldisilazide in tetrahydrofuran(86ml),and after10minutes,TIPS-Cl2(triisopropylchlorosilane)(18.2ml)wasadded.The mixture was stirred at room temperature for24hours.The reaction was diluted with ether,and the resultingsolution was washed twice with water.The extracts weredried(Na2SO4),filtered,and concentrated.The crude product3was chromatographed on silica gel with10%ethylUS10213433(2019);(B2)English。
相关杂质整理列表中文名英文名CAS号规格纯度结构式拉坦前列素杂质1(15(S)-拉坦前列素)LatanoprostImpurity 1(15(S)-Latanoprost)145773-22-410mg-25mg-50mg-100mg ≥99%拉坦前列素杂质2(5-反式拉坦前列素)LatanoprostImpurity 2(5-Trans-Latanoprost)913258-34-110mg-25mg-50mg-100mg ≥99%拉坦前列素杂质3(反式-(15S)-拉坦前列素)LatanoprostImpurity 3(Trans-(15S)-Latanoprost)1235141-39-510mg-25mg-50mg-100mg ≥99%拉坦前列素杂质4 LatanoprostImpurity 4369585-22-810mg-25mg-50mg-100mg ≥99%拉坦前列素杂质5 LatanoprostImpurity 5135646-98-910mg-25mg-50mg-100mg ≥99%湖北扬信医药科技有限公司经营上万种杂质对照品(优势供应硫酸羟氯喹杂质、硝苯地平杂质、沙丁胺醇杂质、达格列净杂质、厄贝沙坦杂质、阿莫西林克拉维酸钾杂质、利伐沙班杂质、阿托伐他汀钙杂质、西格列汀杂质、利格列汀杂质等),并代理销售中检所、STD、LGC、TLC、EP、USP、TRC等多个品牌产品,提供上万种标准品对照品,真诚为您服务。
拉坦前列素杂质6 LatanoprostImpurity 6N/A 10mg-25mg-50mg-100mg ≥99%拉坦前列素杂质7 LatanoprostImpurity 7N/A 10mg-25mg-50mg-100mg ≥99%拉坦前列素杂质8(拉坦前列素酸)LatanoprostImpurity 8(Latanoprost Acid)41639-83-2 10mg-25mg-50mg-100mg ≥99%。
药物Latanoprostene bunod(拉坦前列素硝酸酯)合成检索总结报告一、Latanoprostene bunod(拉坦前列素硝酸酯)简介Latanoprostene bunod(拉坦前列素硝酸酯)于2017年11月在美国上市,主要用于降低伴有开角型青光眼或高眼压患者的眼内压。
Latanoprostene bunod(拉坦前列素硝酸酯)不良反应有结膜充血、眼睛刺激、眼痛和滴注部位疼痛等等。
Latanoprostene bunod(拉坦前列素硝酸酯)分子结构式如下:CAS:860005-21-6英文名称:Latanoprostene bunod中文名称:拉坦前列素硝酸酯二、Latanoprostene bunod(拉坦前列素硝酸酯)合成路线三、Latanoprostene bunod(拉坦前列素硝酸酯)合成检索总结报告(一)Latanoprostene bunod(拉坦前列素硝酸酯)中间体2合成合成方法实验步骤参考文献操作方法一Into a10L three-necked bottle equipped with a thermometerand mechanical stirring,add L-benzoylcolide1(500g,1.81mol)and methylene chloride(5L),Then use the ice-saltbath for the above mixing system,cool to an internaltemperature not higher than0°C,and stir at constanttemperature for10min;Next Dess Martin reagent(2.17mol)was slowly added to the reaction system,and the reactiontemperature was controlled to be not higher than10°C.Afterthe addition of Dess Martin reagent was completed,themixture was stirred at constant temperature for2h.After thereaction was completed,saturated Na2S2O3(1000mL)wasadded to quench the reaction,and the aqueous phase wasextracted with dichloromethane(0.5L×2).The combinedorganic phases were washed with a saturated sodiumchloride solution,dried over anhydrous sodium sulfate,filtered,and concentrated to obtain a pale yellow compound2(480g,1.75mol),which was directly applied to the nextreaction with a yield of96.7%CN110483360;(2019);(A)操作方法二A solution of1,3-diisopropylcarbodiimide(13.7g)indimethyl sulfoxide(38mL)contained in anitrogen-substituted flask was added dropwise with asolution of[3aR-(3aα,4α,5α,6aα)]-5-(benzoyloxy)-hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one1(14.9g)and orthophosphoric acid(1.1g)in dimethylsulfoxide.The mixture was stirred at about30°C for2hours,and then added with methylene chloride,and the depositedwhite solid was removed by filtration.The filtrate waswashed with water,dried over magnesium sulfate,thenfiltered,and concentrated to obtain23.2g of an aldehydecompound2.EP1886992;(2008);(A1)English;US2008/33176;(2008);(A1)EnglishA suspension of anhydrous chromium trioxide(20.8g,208mmol)in methylene chloride(400mL)was stirred and操作方法三cooled in an ice bath asanhydrous pyridine (32.7mL,406mmol)was added.After 15min at 0°C.,the mixture was allowed to warm to ambient temperature for 2h.The reaction mixture was cooled to 0°C.and treated with a pre-cooled solution of (3aR,4S,5R,6aS)-5-(benzoyloxy)-hexahydro-4-(hydroxymethyl)-2H-cyclopenta[b]furan-2-one 1(Corey lactone,9.4g,34mmol)in methylene chloride (400mL)After 5min,the reaction was diluted with toluene (240mL)and filtered.The solid was washed with toluene.The combined filtrate was concentrated to give (3aR,4R,5R,6aS)-5-(benzoyloxy)hexahydro-2-oxo-2H-cyclopenta[b]f uran-4-carboxaldehyde 2.Toluene was added to give about 300mL of 2008/242713;(2008);(A1)English;EP1975163;(2008);(A1)English;US2008/242714;(2008);(A1)English 操作方法四Dess-Martin periodinane (27.63g)and dichloromethane (120mL)are charged into a round bottom flask under nitrogen atmosphere and stirred for 5-10minutes.The reaction mixture is cooled to 0-5°C.A solution of (3aR,4S,5R,6aS)-4-(hydroxymethyl)-2-oxohexahydro-2H-cyclopenta [b]furan-5-yl benzoate 1(15g)in dichloromethane (90mL)is added to the reaction mixture at 0°C.The reaction mixture is maintained for 5hours.A solution of sodium thiosulphate pentahydrate (45g)and sodium bicarbonate (15g)in water (120mL)is added to the reaction mixture at 2°C.and maintained for 30minutes.The reaction mixture is heated to 16°C.and maintained for 30minutes.Both organic and aqueous layers are separated.The aqueous layer is extracted with dichloromethane (75mL).The combined organic layer is washed with 10%sodium chloride solution (75mL).The solvent from the organic layer is evaporated to 10volumes at 30-35°C.to afford 260mL of (3aR,4R,5R,6aS)-4-formyl-2-oxohexahydro-2H-cyclopenta[b]furan-5-yl benzoate 2013/184476;(2013);(A1)English(二)Latanoprostene bunod (拉坦前列素硝酸酯)中间体4的合成合成方法实验步骤参考文献To a suspension of 21g of LiCl (MW =42.39;4.4eq.)in 280mL of acetonitrile was added 37g of compound 3(MW =256.25;1.3eq.).The resulting mixture is cooled to -15°C。