Rewriting on cyclic structures
- 格式:pdf
- 大小:356.58 KB
- 文档页数:19

自动化专业英语常用词汇acceleration transducer 加速度传感器accumulated error 累积误差AC-DC-AC frequency converter交-直-交变频器AC (alternating current) electric drive 交流电子传动active attitude stabilization 主动姿态稳定adjoint operator 伴随算子admissible error 容许误差amplifying element 放大环节analog-digital conversion 模数转换operational amplifiers运算放大器aperiodic decomposition 非周期分解approximate reasoning 近似推理a priori estimate 先验估计articulated robot 关节型机器人asymptotic stability 渐进稳定性attained pose drift 实际位姿漂移attitude acquisition 姿态捕获AOCS (attitude and orbit control system) 姿态轨道控制系统attitude angular velocity 姿态角速度attitude disturbance 姿态扰动automatic manual station 自动-手动操作器automaton 自动机base coordinate system 基座坐标系bellows pressure gauge 波纹管压力表 gauge测量仪器black box testing approach 黑箱测试法bottom-up development 自下而上开发boundary value analysis 边界值分析brainstorming method 头脑风暴法CAE (computer aided engineering) 计算机辅助工程CAM (computer aided manufacturing) 计算机辅助制造capacitive displacement transducer 电容式位移传感器capacity电容 displacement 位移capsule pressure gauge 膜盒压力表rectangular coordinate system直角坐标系cascade compensation 串联补偿using series or parallel capacitors用串联或者并联的电容chaos 混沌calrity 清晰性classical information pattern 经典信息模式classifier 分类器clinical control system 临床控制系统closed loop pole 闭环极点 open loop 开环closed loop transfer function 闭环传递函数c ombined pressure and vacuum gauge 压力真空表command pose 指令位姿companion matrix 相伴矩阵compatibility 相容性,兼容性compensating network 补偿网络Energy is conserved in all of its forms能量是守恒的compensation 补偿,矫正conditionally instability 条件不稳定性configuration 组态connectivity 连接性conservative system 守恒系统consistency 一致性constraint condition 约束条件control accuracy 控制精度Gyroscope陀螺仪control panel 控制屏,控制盘control system synthesis 控制系统综合corner frequency 转折频率coupling of orbit and attitude 轨道和姿态耦合critical damping 临界阻尼临界criticalDamper阻尼器critical stability 临界稳定性cross-over frequency 穿越频率,交越频率cut-off frequency 截止频率cybernetics 控制论cyclic remote control 循环遥控 cycle 循环 cycliccylindrical robot 圆柱坐标型机器人damped oscillation 阻尼振荡oscillation 振荡;振动;摆动damper 阻尼器damping ratio 阻尼比 ratio 比data acquisition 数据采集data preprocessing 数据预处理data processor 数据处理器D controller 微分控制器微分控制:Differential control 积分控制:integral control 比例控制:proportional controldescribing function 描述函数desired value 希望值真值:truth values 参考值:reference value destination 目的站detector 检出器deviation 偏差deviation alarm 偏差报警器differential dynamical system 微differential pressure level meter 差压液位计 meter=gauge 仪表 differential 差别的微分的differential pressure transmitter 差压变送器differential transformer displacement transducer 差动变压器式位移传感器differentiation element 微分环节digital filer 数字滤波器 filter 滤波器digital signal processing 数字信号处理dimension transducer 尺度传感器discrete system simulation language 离散系统仿真语言 discrete离散的不连续的displacement vibration amplitude transducer 位移振幅传感器幅度:amplitudedistrubance 扰动disturbance compensation 扰动补偿diversity 多样性divisibility 可分性domain knowledge 领域知识dominant pole 主导极点零点zero调制:modulation ;modulate 解调:demodulationcountermodulationduty ratio负载比dynamic characteristics 动态特性dynamic deviation 动态偏差dynamic error coefficient 动态误差系数dynamic input-output model 动态投入产出模型Index指数eddy current thickness meter 电涡流厚度计 meter 翻译成计 gauge 翻译成表electric conductance level meter 电导液位计electromagnetic flow transducer 电磁流量传感器electronic batching scale 电子配料秤 scale 秤electronic belt conveyor scale 电子皮带秤electronic hopper scale 电子料斗秤elevation 仰角 depression 俯角equilibrium point 平衡点error 误差estimate 估计量estimation theory 估计理论expected characteristics 希望特性failure diagnosis 故障诊断feasibility study 可行性研究feasible 可行的feasible region 可行域feature detection 特征检测feature extraction 特征抽取feedback compensation 反馈补偿Feed forward path 前馈通路前馈:feed forward 反馈feedbackFMS (flexible manufacturing system) 柔性制造系统柔性:flexible 刚性:rigidity bending deflection 弯曲挠度 deflect 偏向偏离flow sensor/transducer 流量传感器flow transmitter 流量变送器forward path 正向通路frequency converter 变频器frequency domain model reduction me thod 频域模型降阶法频域frequency response 频域响应functional decomposition 功能分解FES (functional electrical stimulation) 功能电刺激stimulate 刺激functional simularity 功能相似fuzzy logic模糊逻辑generalized least squares estimation 广义最小二乘估计geometric similarity 几何相似global optimum 全局最优goal coordination method 目标协调法graphic search 图搜索guidance system 制导系统gyro drift rate 陀螺漂移率gyrostat 陀螺体Hall displacement transducer 霍尔式位移传感器horizontal decomposition横向分解hydraulic step motor 液压步进马达I controller 积分控制器 integral 积分identifiability 可辨识性image recognition 图像识别impulse 冲量impulse function 冲击函数,脉冲函数index of merit 品质因数 index 指数inductive force transducer 电感式位移传感器感应的inductive 电感:inductance industrial automation 工业自动化inertial attitude sensor 惯性姿态敏感器inertial coordinate system 惯性坐标系information acquisition 信息采集infrared gas analyzer 红外线气体分析器 infrared 红外线红外线的ultraviolet ray紫外线的 visible light可见光inherent nonlinearity 固有非线性inherent regulation 固有调节initial deviation 初始偏差input-output model 投入产出模型instability 不稳定性integrity 整体性intelligent terminal 智能终端internal disturbance 内扰invariant embedding principle 不变嵌入原理inverse Nyquist diagram 逆奈奎斯特图investment decision 投资决策joint 关节knowledge acquisition 知识获取knowledge assimilation 知识同化knowledge representation 知识表达lag-lead compensation 滞后超前补偿Laplace transform 拉普拉斯变换large scale system 大系统least squares criterion 最小二乘准则 criterion 准则linearization technique 线性化方法linear motion electric drive 直线运动电气传动linear motion valve 直行程阀linear programming 线性规划load cell 称重传感器local optimum 局部最优local 局部log magnitude-phase diagram 对数幅相图magnitude大小的程度amplitude振幅long term memory 长期记忆Lyapunov theorem of asymptotic stability 李雅普诺夫渐近稳定性定理magnetoelastic weighing cell 磁致弹性称重传感器magnitude-frequency characteristic 幅频特性magnitude margin 幅值裕度 margin 边缘magnitude scale factor 幅值比例尺manipulator 机械手man-machine coordination 人机协调MAP (manufacturing automation protocol) 制造自动化协议 protocol 协议marginal effectiveness 边际效益Mason‘‘s gain formula 梅森增益公式matching criterion 匹配准则maximum likelihood estimation 最大似然估计maximum overshoot 最大超调量maximum principle 极大值原理mean-square error criterion 均方误差准则minimal realization 最小实现minimum phase system 最小相位系统minimum variance estimation 最小方差估计model reference adaptive control system 模型参考适应控制系统model verification 模型验证modularization 模块化MTBF (mean time between failures) 平均故障间隔时间 mean 平均MTTF (mean time to failures) 平均无故障时间multiloop control 多回路控制multi-objective decision 多目标决策Nash optimality 纳什最优性nearest-neighbor 最近邻necessity measure 必然性侧度negative feedback 负反馈neural assembly 神经集合neural network computer 神经网络计算机Nichols chart 尼科尔斯图Nyquist stability criterion 奈奎斯特稳定判据objective function 目标函数on-line assistance 在线帮助on-off control 通断控制optic fiber tachometer 光纤式转速表optimal trajectory 最优轨迹optimization technique 最优化技术order parameter 序参数orientation control 定向控制oscillating period 振荡周期周期:period cycleoutput prediction method 输出预估法oval wheel flowmeter 椭圆齿轮流量计Over damping 过阻尼underdamping 欠阻尼PR (pattern recognition) 模式识别P control 比例控制器peak time 峰值时间penalty function method 罚函数法perceptron 感知器phase lead 相位超前 phase lag相位滞后Photoelectri c光电 tachometric transducer 光电式转速传感器piezoelectric force transducer 压电式力传感器PLC (programmable logic controller) 可编程序逻辑控制器plug braking 反接制动pole assignment 极点配置pole-zero cancellation 零极点相消polynomial input 多项式输入portfolio theory 投资搭配理论pose overshoot 位姿过调量position measuring instrument 位置测量仪posentiometric displacement transducer 电位器式位移传感器positive feedback 正反馈power system automation 电力系统自动化pressure transmitter 压力变送器primary frequency zone 主频区priority 优先级process-oriented simulation 面向过程的仿真proportional control 比例控制proportional plus derivative controller 比例微分控制器pulse duration 脉冲持续时间pulse frequency modulation control system 脉冲调频控制系统:frequency modulation 频率调制调频pulse width modulation control system 脉冲调宽控制系统PWM inverter 脉宽调制逆变器QC (quality control) 质量管理quantized noise 量化噪声ramp function 斜坡函数random disturbance 随机扰动random process 随机过程rate integrating gyro 速率积分陀螺real time telemetry 实时遥测receptive field 感受野rectangular robot 直角坐标型机器人redundant information 冗余信息regional planning model 区域规划模型regulating device 调节装载regulation 调节relational algebra 关系代数remote regulating 遥调reproducibility 再现性resistance thermometer sensor 热电阻电阻温度计传感器response curve 响应曲线return difference matrix 回差矩阵return ratio matrix 回比矩阵revolute robot 关节型机器人revolution speed transducer 转速传感器rewriting rule 重写规则rigid spacecraft dynamics 刚性航天动力学 dynamics 动力学robotics 机器人学robot programming language 机器人编程语言robust control 鲁棒控制robustness 鲁棒性root locus 根轨迹roots flowmeter 腰轮流量计rotameter 浮子流量计,转子流量计sampled-data control system 采样控制系统sampling control system 采样控制系统saturation characteristics 饱和特性scalar Lyapunov function 标量李雅普诺夫函数s-domain s域self-operated controller 自力式控制器self-organizing system 自组织系统self-reproducing system 自繁殖系统self-tuning control 自校正控制sensing element 敏感元件sensitivity analysis 灵敏度分析sensory control 感觉控制sequential decomposition 顺序分解sequential least squares estimation 序贯最小二乘估计servo control 伺服控制,随动控制servomotor 伺服马达settling time 过渡时间sextant 六分仪short term planning 短期计划short time horizon coordination 短时程协调signal detection and estimation 信号检测和估计signal reconstruction 信号重构similarity 相似性simulated interrupt 仿真中断simulation block diagram 仿真框图simulation experiment 仿真实验simulation velocity 仿真速度simulator 仿真器single axle table 单轴转台single degree of freedom gyro 单自由度陀螺翻译顺序呵呵spin axis 自旋轴spinner 自旋体stability criterion 稳定性判据stability limit 稳定极限stabilization 镇定,稳定state equation model 状态方程模型state space description 状态空间描述static characteristics curve 静态特性曲线station accuracy 定点精度stationary random process 平稳随机过程statistical analysis 统计分析statistic pattern recognition 统计模式识别steady state deviation 稳态偏差顺序翻译即可steady state error coefficient 稳态误差系数step-by-step control 步进控制step function 阶跃函数strain gauge load cell 应变式称重传感器subjective probability 主观频率supervisory computer control system 计算机监控系统sustained oscillation 自持振荡swirlmeter 旋进流量计switching point 切换点systematology 系统学system homomorphism 系统同态system isomorphism 系统同构system engineering 系统工程tachometer 转速表target flow transmitter 靶式流量变送器task cycle 作业周期temperature transducer 温度传感器tensiometer 张力计texture 纹理theorem proving 定理证明therapy model 治疗模型thermocouple 热电偶thermometer 温度计thickness meter 厚度计three-axis attitude stabilization 三轴姿态稳定three state controller 三位控制器thrust vector control system 推力矢量控制系统thruster 推力器time constant 时间常数time-invariant system 定常系统,非时变系统 invariant不变的time schedule controller 时序控制器time-sharing control 分时控制time-varying parameter 时变参数top-down testing 自上而下测试TQC (total quality control) 全面质量管理tracking error 跟踪误差trade-off analysis 权衡分析transfer function matrix 传递函数矩阵transformation grammar 转换文法transient deviation 瞬态偏差短暂的瞬间的transient process 过渡过程transition diagram 转移图transmissible pressure gauge 电远传压力表transmitter 变送器trend analysis 趋势分析triple modulation telemetering system 三重调制遥测系统turbine flowmeter 涡轮流量计Turing machine 图灵机two-time scale system 双时标系统ultrasonic levelmeter 超声物位计unadjustable speed electric drive 非调速电气传动unbiased estimation 无偏估计underdamping 欠阻尼uniformly asymptotic stability 一致渐近稳定性uninterrupted duty 不间断工作制,长期工作制unit circle 单位圆unit testing 单元测试unsupervised learing 非监督学习upper level problem 上级问题urban planning 城市规划value engineering 价值工程variable gain 可变增益,可变放大系数variable structure control system 变结构控制vector Lyapunov function 向量李雅普诺夫函数function 函数velocity error coefficient 速度误差系数velocity transducer 速度传感器vertical decomposition 纵向分解vibrating wire force transducer 振弦式力传感器vibrometer 振动计 vibrationVibrate振动viscous damping 粘性阻尼voltage source inverter 电压源型逆变器vortex precession flowmeter 旋进流量计vortex shedding flowmeter 涡街流量计WB (way base) 方法库weighing cell 称重传感器weighting factor 权因子weighting method 加权法Whittaker-Shannon sampling theorem 惠特克-香农采样定理Wiener filtering 维纳滤波w-plane w平面zero-based budget 零基预算zero-input response 零输入响应zero-state response 零状态响应z-transform z变换《信号与系统》专业术语中英文对照表第 1 章绪论信号(signal)系统(system)电压(voltage)电流(current)信息(information)电路(circuit)确定性信号(determinate signal)随机信号(random signal)一维信号(one–dimensional signal)多维信号(multi–dimensional signal)连续时间信号(continuous time signal)离散时间信号(discrete time signal)取样信号(sampling signal)数字信号(digital signal)周期信号(periodic signal)非周期信号(nonperiodic(aperiodic) signal)能量(energy)功率(power)能量信号(energy signal)功率信号(power signal)平均功率(average power)平均能量(average energy)指数信号(exponential signal)时间常数(time constant)正弦信号(sine signal)余弦信号(cosine signal)振幅(amplitude)角频率(angular frequency)初相位(initial phase)频率(frequency)欧拉公式(Euler’s formula)复指数信号(complex exponential signal)复频率(complex frequency)实部(real part)虚部(imaginary part)抽样函数 Sa(t)(sampling(Sa) function)偶函数(even function)奇异函数(singularity function)奇异信号(singularity signal)单位斜变信号(unit ramp signal)斜率(slope)单位阶跃信号(unit step signal)符号函数(signum function)单位冲激信号(unit impulse signal)广义函数(generalized function)取样特性(sampling property)冲激偶信号(impulse doublet signal)奇函数(odd function)偶分量(even component)偶数 even 奇数 odd 奇分量(odd component)正交函数(orthogonal function)正交函数集(set of orthogonal function)数学模型(mathematics model)电压源(voltage source)基尔霍夫电压定律(Kirchhoff’s voltage law(KVL))电流源(current source)连续时间系统(continuous time system)离散时间系统(discrete time system)微分方程(differential function)差分方程(difference function)线性系统(linear system)非线性系统(nonlinear system)时变系统(time–varying system)时不变系统(time–invariant system)集总参数系统(lumped–parameter system)分布参数系统(distributed–parameter system)偏微分方程(partial differential function)因果系统(causal system)非因果系统(noncausal system)因果信号(causal signal)叠加性(superposition property)均匀性(homogeneity)积分(integral)输入–输出描述法(input–output analysis)状态变量描述法(state variable analysis)单输入单输出系统(single–input and single–output system)状态方程(state equation)输出方程(output equation)多输入多输出系统(multi–input and multi–output system)时域分析法(time domain method)变换域分析法(transform domain method)卷积(convolution)傅里叶变换(Fourier transform)拉普拉斯变换(Laplace transform)第 2 章连续时间系统的时域分析齐次解(homogeneous solution)特解(particular solution)特征方程(characteristic function)特征根(characteristic root)固有(自由)解(natural solution)强迫解(forced solution)起始条件(original condition)初始条件(initial condition)自由响应(natural response)强迫响应(forced response)零输入响应(zero-input response)零状态响应(zero-state response)冲激响应(impulse response)阶跃响应(step response)卷积积分(convolution integral)交换律(exchange law)分配律(distribute law)结合律(combine law)第3 章傅里叶变换频谱(frequency spectrum)频域(frequency domain)三角形式的傅里叶级数(trigonomitric Fourier series)指数形式的傅里叶级数(exponential Fourier series)傅里叶系数(Fourier coefficient)直流分量(direct component)基波分量(fundamental component) component 分量n 次谐波分量(n th harmonic component)复振幅(complex amplitude)频谱图(spectrum plot(diagram))幅度谱(amplitude spectrum)相位谱(phase spectrum)包络(envelop)离散性(discrete property)谐波性(harmonic property)收敛性(convergence property)奇谐函数(odd harmonic function)吉伯斯现象(Gibbs phenomenon)周期矩形脉冲信号(periodic rectangular pulse signal)直角的周期锯齿脉冲信号(periodic sawtooth pulse signal)周期三角脉冲信号(periodic triangular pulse signal)三角的周期半波余弦信号(periodic half–cosine signal)周期全波余弦信号(periodic full–cosine signal)傅里叶逆变换(inverse Fourier transform)inverse 相反的频谱密度函数(spectrum density function)单边指数信号(single–sided exponential signal)双边指数信号(two–sided exponential signal)对称矩形脉冲信号(symmetry rectangular pulse signal)线性(linearity)对称性(symmetry)对偶性(duality)位移特性(shifting)时移特性(time–shifting)频移特性(frequency–shifting)调制定理(modulation theorem)调制(modulation)解调(demodulation)变频(frequency conversion)尺度变换特性(scaling)微分与积分特性(differentiation and integration)时域微分特性(differentiation in the time domain)时域积分特性(integration in the time domain)频域微分特性(differentiation in the frequency domain)频域积分特性(integration in the frequency domain)卷积定理(convolution theorem)时域卷积定理(convolution theorem in the time domain)频域卷积定理(convolution theorem in the frequency domain)取样信号(sampling signal)矩形脉冲取样(rectangular pulse sampling)自然取样(nature sampling)冲激取样(impulse sampling)理想取样(ideal sampling)取样定理(sampling theorem)调制信号(modulation signal)载波信号(carrier signal)已调制信号(modulated signal)模拟调制(analog modulation)数字调制(digital modulation)连续波调制(continuous wave modulation)脉冲调制(pulse modulation)幅度调制(amplitude modulation)频率调制(frequency modulation)相位调制(phase modulation)角度调制(angle modulation)频分多路复用(frequency–division multiplex(FDM))时分多路复用(time–division multiplex(TDM))相干(同步)解调(synchronous detection)本地载波(local carrier)载波系统函数(system function)网络函数(network function)频响特性(frequency response)幅频特性(amplitude frequency response)幅频响应相频特性(phase frequency response)无失真传输(distortionless transmission)理想低通滤波器(ideal low–pass filter)截止频率(cutoff frequency)正弦积分(sine integral)上升时间(rise time)窗函数(window function)理想带通滤波器(ideal band–pass filter)太直译了第 4 章拉普拉斯变换代数方程(algebraic equation)双边拉普拉斯变换(two-sided Laplace transform)双边拉普拉斯逆变换(inverse two-sided Laplace transform)单边拉普拉斯变换(single-sided Laplace transform)拉普拉斯逆变换(inverse Laplace transform)收敛域(region of convergence(ROC))延时特性(time delay)s 域平移特性(shifting in the s-domain)s 域微分特性(differentiation in the s-domain)s 域积分特性(integration in the s-domain)初值定理(initial-value theorem)终值定理(expiration-value)复频域卷积定理(convolution theorem in the complex frequency domain)部分分式展开法(partial fraction expansion)留数法(residue method)第 5 章策动点函数(driving function)转移函数(transfer function)极点(pole)零点(zero)零极点图(zero-pole plot)暂态响应(transient response)稳态响应(stable response)稳定系统(stable system)一阶系统(first order system)高通滤波网络(high-pass filter)低通滤波网络(low-pass filter)二阶系统(second order system)最小相位系统(minimum-phase system)高通(high-pass)带通(band-pass)带阻(band-stop)有源(active)无源(passive)模拟(analog)数字(digital)通带(pass-band)阻带(stop-band)佩利-维纳准则(Paley-Winner criterion)最佳逼近(optimum approximation)过渡带(transition-band)通带公差带(tolerance band)巴特沃兹滤波器(Butterworth filter)切比雪夫滤波器(Chebyshew filter)方框图(block diagram)信号流图(signal flow graph)节点(node)支路(branch)输入节点(source node)输出节点(sink node)混合节点(mix node)通路(path)开通路(open path)闭通路(close path)环路(loop)自环路(self-loop)环路增益(loop gain)不接触环路(disconnect loop)前向通路(forward path)前向通路增益(forward path gain)梅森公式(Mason formula)劳斯准则(Routh criterion)第 6 章数字系统(digital system)数字信号处理(digital signal processing)差分方程(difference equation)单位样值响应(unit sample response)卷积和(convolution sum)Z 变换(Z transform)序列(sequence)样值(sample)单位样值信号(unit sample signal)单位阶跃序列(unit step sequence)矩形序列 (rectangular sequence)单边实指数序列(single sided real exponential sequence)单边正弦序列(single sided exponential sequence)斜边序列(ramp sequence)复指数序列(complex exponential sequence)线性时不变离散系统(linear time-invariant discrete-time system)常系数线性差分方程(linear constant-coefficient difference equation)后向差分方程(backward difference equation)前向差分方程(forward difference equation)海诺塔(Tower of Hanoi)菲波纳西(Fibonacci)冲激函数串(impulse train)第 7 章数字滤波器(digital filter)单边 Z 变换(single-sided Z transform)双边 Z 变换(two-sided (bilateral) Z transform)幂级数(power series)收敛(convergence)有界序列(limitary-amplitude sequence)正项级数(positive series)有限长序列(limitary-duration sequence)右边序列(right-sided sequence)左边序列(left-sided sequence)双边序列(two-sided sequence)Z 逆变换(inverse Z transform)围线积分法(contour integral method)幂级数展开法(power series expansion)z 域微分(differentiation in the z-domain)序列指数加权(multiplication by an exponential sequence)z 域卷积定理(z-domain convolution theorem)帕斯瓦尔定理(Parseval theorem)传输函数(transfer function)序列的傅里叶变换(discrete-time Fourier transform:DTFT)序列的傅里叶逆变换(inverse discrete-time Fourier transform:IDTFT)幅度响应(magnitude response)相位响应(phase response)量化(quantization)编码(coding)模数变换(A/D 变换:analog-to-digital conversion)数模变换(D/A 变换:digital-to- analog conversion)第 8 章端口分析法(port analysis)状态变量(state variable)无记忆系统(memoryless system)有记忆系统(memory system)矢量矩阵(vector-matrix )常量矩阵(constant matrix )输入矢量(input vector)输出矢量(output vector)直接法(direct method)间接法(indirect method)状态转移矩阵(state transition matrix)系统函数矩阵(system function matrix)冲激响应矩阵(impulse response matrix)光学专业词汇大全Accelaration 加速度Myopia-near-sighted近视Sensitivity to Light感光灵敏度boost推进lag behind落后于Hyperopic-far-sighted远视visual sensation视觉ar Pattern条状图形approximate近似adjacent邻近的normal法线Color Difference色差V Signal Processing电视信号处理back and forth前后vibrant震动quantum leap量子越迁derive from起源自inhibit抑制,约束stride大幅前进obstruction障碍物substance物质实质主旨residue杂质criteria标准parameter参数parallax视差凸面镜 convex mirror凹面镜 concave mirror分光镜spectroscope入射角 angle of incidence出射角emergent angle平面镜 plane mirror放大率角度放大率angular magnification 放大率:magnification 折射 refraction反射 reflect干涉 interfere衍射 diffraction干涉条纹interference fringe衍射图像 diffraction fringe衍射条纹偏振polarize polarization透射transmission透射光 transmission light光强度] light intensity电磁波 electromagnetic wave振动杨氏干涉夫琅和费衍射焦距brewster Angle布鲁斯特角quarter Waveplates四分之一波片ripple波纹capacitor电容器vertical垂直的horizontal 水平的airy disk艾里斑exit pupil出[射光]瞳Entrance pupil 入瞳optical path difference光称差radius of curvature曲率半径spherical mirror球面镜reflected beam反射束YI= or your information供参考phase difference相差interferometer干涉仪ye lens物镜/目镜spherical球的field information场信息standard Lens标准透镜refracting Surface折射面principal plane主平面vertex顶点,最高点fuzzy失真,模糊light source 光源wavelength波长angle角度spectrum光谱diffraction grating衍射光栅sphere半球的DE= ens data editor Surface radius of curvature表面曲率半径surface thickness表面厚度semi-diameter半径focal length焦距field of view视场stop 光阑refractive折射reflective反射金属切削 metal cutting机床 machine tool tool 机床金属工艺学 technology of metals刀具 cutter摩擦 friction传动 drive/transmission轴 shaft弹性 elasticity频率特性 frequency characteristic误差 error响应 response定位 allocation动力学 dynamic运动学 kinematic静力学 static分析力学 analyse mechanics 力学拉伸 pulling压缩 hitting compress剪切 shear扭转 twist弯曲应力 bending stress强度 intensity几何形状 geometricalUltrasonic超声波精度 precision交流电路 AC circuit机械加工余量 machining allowance变形力 deforming force变形 deformation应力 stress硬度 rigidity热处理 heat treatment电路 circuit半导体元件 semiconductor element反馈 feedback发生器 generator直流电源 DC electrical source门电路 gate circuit逻辑代数 logic algebra磨削 grinding螺钉 screw铣削 mill铣刀 milling cutter功率 power装配 assembling流体动力学 fluid dynamics流体力学 fluid mechanics加工 machining稳定性 stability介质 medium强度 intensity载荷 load应力 stress可靠性 reliability精加工 finish machining粗加工 rough machining腐蚀 rust氧化 oxidation磨损 wear耐用度 durability随机信号 random signal离散信号 discrete signal超声传感器 ultrasonic sensor摄像头 CCD cameraLead rail 导轨合成纤维 synthetic fibre电化学腐蚀 electrochemical corrosion 车架 automotive chassis悬架 suspension转向器 redirector变速器 speed changer车间 workshop工程技术人员 engineer数学模型 mathematical model标准件 standard component零件图 part drawing装配图 assembly drawing刚度 rigidity内力 internal force位移 displacement截面 section疲劳极限 fatigue limit断裂 fracture 破裂塑性变形 plastic distortionelastic deformation 弹性变形脆性材料 brittleness material刚度准则 rigidity criterion齿轮 gearGrain 磨粒转折频率 corner frequency =break frequencyConvolution 卷积Convolution integral 卷积积分Convolution property 卷积性质Convolution sum 卷积和Correlation function 相关函数Critically damped systems 临界阻尼系统Crosss-correlation functions 互相关函数Cutoff frequencies 截至频率transistor n 晶体管diode n 二极管semiconductor n 半导体resistor n 电阻器capacitor n 电容器alternating adj 交互的amplifier n 扩音器,放大器integrated circuit 集成电路linear time invariant systems 线性时不变系统voltage n 电压,伏特数Condenser=capacitor n 电容器dielectric n 绝缘体;电解质electromagnetic adj 电磁的adj 非传导性的deflection n偏斜;偏转;偏差linear device 线性器件the insulation resistance 绝缘电阻anode n 阳极,正极cathode n 阴极breakdown n 故障;崩溃terminal n 终点站;终端,接线端emitter n 发射器collect v 收集,集聚,集中insulator n 绝缘体,绝热器oscilloscope n 示波镜;示波器gain n 增益,放大倍数forward biased 正向偏置reverse biased 反向偏置P-N junction PN结MOS(metal-oxide semiconductor)金属氧化物半导体enhancement and exhausted 增强型和耗尽型integrated circuits 集成电路analog n 模拟digital adj 数字的,数位的horizontal adj, 水平的,地平线的vertical adj 垂直的,顶点的amplitude n 振幅,广阔,丰富multimeter n 万用表frequency n 频率,周率the cathode-ray tube 阴极射线管dual-trace oscilloscope 双踪示波器signal generating device 信号发生器peak-to-peak output voltage 输出电压峰峰值sine wave 正弦波triangle wave 三角波square wave 方波amplifier 放大器,扩音器oscillator n 振荡器feedback n 反馈,回应phase n 相,阶段,状态filter n 滤波器,过滤器rectifier n整流器;纠正者band-stop filter 带阻滤波器band-pass filter 带通滤波器decimal adj 十进制的,小数的hexadecimal adj/n十六进制的binary adj 二进制的;二元的octal adj 八进制的domain n 域;领域code n代码,密码,编码v编码the Fourier transform 傅里叶变换Fast Fourier Transform 快速傅里叶变换microcontroller n 微处理器;微控制器assembly language instrucions n 汇编语言指令chip n 芯片,碎片modular adj 模块化的;模数的sensor n 传感器plug vt堵,塞,插上n塞子,插头,插销coaxial adj 同轴的,共轴的fiber n 光纤relay contact 继电接触器Artificial Intelligence 人工智能Perceptive Systems 感知系统neural network 神经网络fuzzy logic 模糊逻辑intelligent agent 智能代理electromagnetic adj 电磁的coaxial adj 同轴的,共轴的microwave n 微波charge v充电,使充电insulator n 绝缘体,绝缘物nonconductive adj非导体的,绝缘的simulation n 仿真;模拟prototype n 原型array n 排队,编队vector n 向量,矢量inverse adj倒转的,反转的n反面;相反v倒转high-performance 高精确性,高性能two-dimensional 二维的;缺乏深度的three-dimensional 三维的;立体的;真实的object-oriented programming面向对象的程序设计spectral adj 光谱的distortion n 失真,扭曲,变形wavelength n 波长refractive adj 折射的ivision Multiplexing单工传输simplex transmission半双工传输half-duplex transmission全双工传输full-duplex transmission电路交换 circuit switching数字传输技术Digital transmission technology灰度图像Grey scale images灰度级Grey scale level幅度谱Magnitude spectrum相位谱Phase spectrum频谱frequency spectrum相干解调coherent demodulation coherent相干的数字图像压缩digital image compression图像编码image encoding量化quantization人机交互man machine interface交互式会话Conversational interaction路由算法Routing Algorithm目标识别Object recognition话音变换Voice transform中继线trunk line传输时延transmission delay远程监控remote monitoring光链路optical linkhalf-duplex transmission 半双工传输accompaniment 伴随物,附属物reservation 保留,预定quotation 报价单,行情报告,引语memorandum 备忘录redundancy 备用be viewed as 被看作…be regards as 被认为是as such 本身;照此;以这种资格textual 本文的,正文的variation 变化,变量conversion 变化,转化。


The modification of glassy carbon and gold electrodes with aryl diazonium salt:The impact of the electrode materialson the rate of heterogeneous electron transferGuozhen Liu,Jingquan Liu,Till Bo ¨cking,Paul K.Eggers,J.Justin Gooding*School of Chemistry,The University of New South Wales,Sydney,NSW 2052,AustraliaReceived 1December 2004;accepted 23March 2005Available online 23May 2005AbstractThe heterogeneous electron-transfer properties of ferrocenemethylamine coupled to a series of mixed 4-carboxyphenyl/phenyl monolayers on glassy carbon (GC)and gold electrodes were investigated,by cyclic voltammetry,in aqueous buffer solutions.The electrodes were derivatized in a step-wise process.Electrochemical reduction of mixtures of 4-carboxyphenyl and phenyl dia-zonium salts on the electrode surfaces yielded stable monolayers.The introduction of carboxylic acid moieties onto the surfaces was verified by X-ray photoelectron spectroscopy.Subsequently the 4-carboxyphenyl moieties were activated using water-soluble carbo-diimide and N -hydroxysuccinimide and reacted with ferrocenemethylamine.The rate constants of electron transfer through the monolayer systems were determined from cyclic voltammograms using the Marcus theory for electron transfer and were found to be an order of magnitude higher for the ferrocene-modified monolayer systems on gold than those on GC electrodes.The results suggest the electrode material has an important influence on the rate of electron transfer.Ó2005Elsevier B.V.All rights reserved.Keywords:Self-assembled monolayers;Electron transfer;Carbon;Gold;Diazonium salts1.IntroductionThe modification of conducting surfaces with mono-layers has received extensive research interest of late be-cause of their utility as model systems for understanding electron transfer [1,2],molecular electronics [3,4],bio-electronics [5,6]and sensors [7]amongst other applica-tions.The most popular chemistry for forming monolayers on electrode surfaces is alkanethiol self-assembly onto coinage metals,in particular gold [8],although other systems have also attracted interest such as silanes on metal oxide electrodes [9]and alkenes on highly doped silicon [10].The attractiveness of gold–thiol chemistry is that well ordered monolayers can be formed relatively easily,with a reasonably strong bond formed between the organic molecule and the electrode,and that a diverse range of molecules can be synthesized with which to modify an electrode.The advantages of gold–thiol chemistry are somewhat offset by a number of disadvantages,including alkanethiols being oxida-tively or reductively desorbed at potentials typically out-side the window defined by À800to +800mV versus Ag/AgCl.Other disadvantages include:alkanethiols being desorbed at temperatures over 100°C,gold being a highly mobile surface which results in the monolayers moving across the electrode surface,the gold–thiolate bond being prone to oxidation and the gold/thiol junc-tion creating a rather large tunneling barrier ($2eV)[11].The last point regarding a large tunneling barrier0301-0104/$-see front matter Ó2005Elsevier B.V.All rights reserved.doi:10.1016/j.chemphys.2005.03.033*Corresponding author.E-mail address:justin.gooding@.au (J.J.Gooding)./locate/chemphysChemical Physics 319(2005)136–146has implications for the rate of electron transfer from the organic monolayer to the electrode which is impor-tant for all molecular scale devices where communica-tion with the macroscopic world is achieved through electron transport.We are interested in alternative monolayer systems to gold–thiol chemistry which overcome some of the disad-vantages but do not severely compromise the advanta-ges of gold/thiol chemistry.The electrochemical reduction of aryl diazonium salts is one possible alterna-tive which has most frequently been used as a method for the covalent derivatization of glassy carbon(GC) surfaces[12–14].The reduction reaction results in the loss of the N2and the formation of a carbon–carbon covalent bond which is strong,stable over both time and temperature,non-polar and conjugated[11].Thus, the conjugated carbon network in the GC electrode can be thought of as continuing into the monolayer sys-tem rather than the abrupt change from electrons being in a metallic environment to an organic environment. The continuity of the electrode material into the mono-layer has resulted in the suggestion that GC electrodes modified by aryl diazonium salts have the potential to reduce the barrier towards electron transfer from the carbon electrode into the monolayer[11].McCreery and coworkers[15–18]have extensively studied the elec-tron transfer kinetics of GC surfaces in different redox probe solutions.However,to the best of our knowledge heterogeneous electron transfer between redox active molecules and GC electrodes through aryl diazonium salt derived monolayers has yet to be investigated.Nor has the notion that the C–C bond will allow efficient electron transfer.The attractiveness of aryl diazonium salts are en-hanced further by recent studies showing they can also be grafted to a variety of metal[19,20]and semicon-ductor[21]surfaces as well as carbon nanotubes[22]. This feature raises the exciting possibility of one monolayer forming system being suitable for a large range of electrode materials for a diverse range of applications.This possibility is helped by a rich array of different diazonium salts which have now been pre-pared including molecular wires[23]and polyethylene glycol terminated molecules designed to resist protein adsorption[24,25].The purpose of this study is to modify GC and gold substrates using mixtures of aryl diazonium salt molecules(introducing phenyl and4-carboxyphenyl groups onto the surface)and to com-pare the kinetics of electron transfer to GC and gold surfaces from the same ferrocene-based monolayer sys-tem.A similar ferrocene-based system prepared by a mixed self-assembled monolayers(SAMs)of4-merca-ptobenzoic acid(MBA)and1-propanethiol(PT)has also been prepared on gold surfaces and the rates of electron transfer have been studied for further comparison.2.Experimental2.1.Reagents and materialsTetrabutylammonium tetrafluoroborate(NBu4BF4), sodium tetrafluoroborate(NaBF4),p-aminobenzoic acid,aniline,4-mercaptobenzoic acid(MBA),1-propa-nethiol(PT),ferricyanide(K4Fe(CN)6),1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC),N-hydroxysuccinimide(NHS),N-[2-hydroxy-ethyl]piperazine-N0-[2-ethanesulfonic acid](HEPES), ferrocenecarboxaldehyde,sodium cyanoborohydride and acetonitrile(CH3CN,HPLC grade)were obtained from Sigma(Sydney,Australia).Benzoic acid diazo-nium tetrafluoroborate and benzene diazonium tetra-fluoroborate were synthesized according to the method by Saby et al.[26].Ferrocenemethylamine was synthe-sized using the procedure from Kraatz[27].Reagent grade dipotassium orthophosphate,potassium dihydro-gen orthophosphate,potassium chloride,sodium hydroxide,sodium chloride,sodium nitrite,ammonium acetate,sulphuric acid,hydrochloric acid,methanol and diethyl ether were purchased from Ajax Chemicals Pty. Ltd.(Sydney,Australia).All reagents were used as re-ceived,and aqueous solutions were prepared with puri-fied water(18M X cmÀ1,Millipore,Sydney,Australia). Phosphate buffer solutions used in this work contained 0.05M KCl and0.05M K2HPO4/KH2PO4and were ad-justed with NaOH or HCl solution.2.2.Modification of electrodesThe GC and gold electrodes were modified with dia-zonium salts followed by attachment of ferrocenemeth-ylamine as depicted in Scheme1.The GC electrodes were purchased commercially(Bioanalytical System Inc.,USA)as3-mm-diameter rods.The electrodes were polished successively in1.0,0.3,and0.05l m alumina slurries made from dry Buehler alumina and Milli-Q water on microcloth pads(Buehler,Lake Bluff,IL, USA).The electrodes were thoroughly rinsed with Milli-Q water and sonicated in Milli-Q water for 5min between polishing steps.Before derivatization, the electrodes were dried with an argon gas stream. The bare GC electrodes had an electrochemical rough-ness factor(the ratio of the electrochemical area to geo-metric area)of 1.43.Surface derivatization of GC electrodes was performed in a solution of1mM aryl diazonium salt and0.1M NaBu4BF4in acetonitrile using cyclic voltammetry(CV)with a scan rate of 100mV sÀ1for two cycles between+1.0andÀ1.0V. The diazonium salt solution was deaerated with argon for at least15min prior to derivatization.The elec-trodes were rinsed with copious amounts of acetonitrile and then water and dried under a stream of argon prior to the next step.G.Liu et al./Chemical Physics319(2005)136–146137Poly-crystalline gold electrodes,prepared as de-scribed previously[28],were polished to a mirror-like finish with 1.0l m alumina,followed by0.3and 0.05l m alumina slurry on microcloth pad.After re-moval of trace alumina from the surface,by rinsing with water and brief cleaning in an ultrasonic bath with eth-anol and then water,electrochemical cleaning in0.05M H2SO4by cycling the electrodes betweenÀ0.3and1.5V was carried out until a reproducible CV was obtained. Before derivatization,the cleaned electrodes were rinsed with water and dried under a stream of argon.The derivatization of gold electrodes with a mixture of dia-zonium salts was conducted in exactly the same manner as described for the carbon electrodes.The alkanethiol modified gold electrodes were prepared by immersing the gold electrodes in a1mM mixed thiol solution(mer-captobenzoic acid and propanethiol with different dilu-tion ratios)in ethanol overnight(see Scheme2).The electrode was rinsed with copious amounts of ethanol, then water andfinally dried under a stream of argon prior to the next step.Covalent attachment of ferrocenemethylamine to car-boxylic acid terminated monolayers followed the proce-dures described by Liu et al.[29].The modified surfaces were incubated in an aqueous solution of10mM N-hydroxysuccinimide(NHS)and40mM1-ethyl-3-(3-di-methyl aminopropyl)carbodiimide hydrochloride (EDC)for1h.After the activation,the electrodes were rinsed with water and incubated in a5mM ferrocenem-ethylamine solution in HEPES buffer pH7.3for24h.2.3.Electrochemical measurementsAll electrochemical measurements were performed with a BAS-100B electrochemical analyser(Bioanalyti-cal System fayette,IL)and a conventional three-electrode system,comprising a GC or a gold work-ing electrode,a platinum foil as the auxiliary electrode, and a Ag/AgCl3.0M NaCl electrode(from BAS)as ref-erence.All potentials were reported versus the Ag/AgCl reference electrode at room temperature.All CV mea-surements were conducted in pH7.0phosphate buffer. The area under the Faradaic peaks in the CVs of the fer-rocene modified electrodes were used to determine the surface coverage of ferrocene.The rate constants for electron transfer were calculated from the variation in peak potential over a wide range of scan rates.For elec-trodes with prominent redox peaks the rate constants were determined byfitting the variation in peak poten-tial with scan rate using the Marcus theory for electron transfer as described previously[30–32]whilst at low surface coverages of ferrocene the rates of electronScheme2.Schematic of ferrocenemethylamine immobilized covalently on mixed monolayers of MBA and PT on gold surfaces. 138G.Liu et al./Chemical Physics319(2005)136–146transfer were determined using the Laviron[33]formal-ism.This was because peak shape and position was very sensitive background subtraction at with small redox peaks and thereforefitting the entire background sub-tracted CV peak as required for our Marcus simulation became unreliable.When both methods were employed on the same data very similar rate constants for electron transfer were obtained.2.4.XPS measurementsXP spectra were obtained using an EscaLab220-IXL spectrometer with a monochromated Al K a source (1486.6eV),hemispherical analyzer and multichannel detector.The spectra were accumulated at a take-offan-gle of90°with a0.79mm2spot size at a pressure of less than10À8mbar.3.Results3.1.Aryl diazonium salt modified glassy carbon electrodesGlassy carbon electrodes were modified with diazo-nium salts via electrochemical reduction of an aryl dia-zonium salt(1mM in acetonitrile)with0.1M tetrabutylammonium tetrafluoroborate as background electrolyte.Thefirst sweep showed the characteristic reduction peak atÀ0.16V versus Ag/AgCl with no associated oxidation peak indicative of the loss of N2 and the formation of a4-carboxyphenyl radical fol-lowed by covalent binding to the carbon surface[34]. Subsequent scans showed no electrochemistry indicative of a passivated electrode.The passivation of the GC sur-faces after the modification with aryl diazonium salts was confirmed using potassium ferricyanide as a redox probe.Fig.1shows a cyclic voltammogram before and after modification with(4-carboxyphenyl)diazonium tetrafluoroborate in1mM ferricyanide in a0.05M phosphate buffer(0.05M KCl,pH7.0)at a scan rate of100mV sÀ1.After the modification of the surface with the aryl diazonium salts,the redox peaks of ferricy-anide observed with bare GC electrodes were almost completely suppressed.This gave strong evidence that a uniform monolayer which blocked access of ferricya-nide to the electrode had formed on the GC surfaces. Based on the area of the reduction peak during the mod-ification of the GC electrode surface with the aryl diazo-nium salt,the coverage of the4-carboxyphenyl moieties was calculated to be7.4·10À10mol cmÀ2.The reported surface coverage on GC substrates varies in the range of 4–30·10À10mol cmÀ2[35]with the theoretical maxi-mum surface coverage[29]for a monolayer on GC sur-faces being12·10À10mol cmÀ2.The surface coverage of7.4·10À10mol cmÀ2indicates the GC electrode was modified with a monolayer rather than multilayers of aryl groups as has been reported by some workers[36–38].The modification of the GC electrode by electro-chemical reduction of(4-carboxyphenyl)diazonium tet-rafluoroborate was confirmed by X-ray photoelectron spectroscopy(XPS).Survey spectra showed the expected carbon1s and oxygen1s peaks at$284and$532eV, respectively,and also a small nitrogen1s peak at $400eV(Fig.2).The level of oxygen was increased in comparison to the bare GC surface as expected for the introduction of4-carboxyphenyl groups onto the sur-face.The presence of the small nitrogen1s peak in the survey spectra was partially due to nitrogen containing species already detectable on the unmodified GC elec-trodes,which has been observed previously[13,34].Fur-thermore,nitrogen species with a binding energy of $400eV can be introduced onto the surface during the modification reaction.It has been proposed that these are due to a hydrazine generated by reaction of the dia-zonium salt with phenol groups on the GC surface[26].Fig.2.XP survey spectrum and carbon1s narrow scan(inset)of a GCelectrode modified by electrochemical reduction of(4-carboxyphenyl)diazonium tetrafluoroborate.G.Liu et al./Chemical Physics319(2005)136–146139Nitrogen1s narrow scans(not shown)were consistent with the formation of low levels of the hydrazine,which exhibited a slightly different binding energy to that of the nitrogen species already present on the bare GC electrode.The carbon1s narrow scan(Fig.2,inset)showed a peak centred at288.8eV,which was typical of the car-bon of the carboxylic acid group[13].This peak was ab-sent from the carbon1s narrow scan of unmodified GC surfaces.The carbon1s peak at284.4eV was slightly broadened compared to the graphitic peak of an unmodified GC surface and was assigned to the gra-phitic carbon of the underlying GC electrode and the aromatic carbons of the monolayer.The pronounced asymmetry of this peak with a broad shoulder on the high binding energy side($286.2eV)was attributed to an oxidized species present on the GC surface and or-ganic contaminants adsorbed on the monolayer.After modification of the GC electrode surface with the aryl diazonium salt the next step in the fabrication of the modified electrodes was the attachment of ferro-cene.CVs measured in an aqueous solution of0.05M phosphate buffer(0.05M KCl,pH7.0)at a scan rate of100mV sÀ1before and after the immobilization of ferrocene on the4-carboxyphenyl modified GC elec-trode are shown in Fig.3.The strong redox peaks after the attachment of ferrocene showed linear variation in peak current with scan rate indicating that the ferrocene was surface bound.In the absence of EDC and NHS such that no covalent coupling of the ferrocene could occur,only very weak redox peaks due to physisorption were observed.The CVs of the ferrocene coupled to the 4-carboxyphenyl monolayers show non-ideal behaviour[1]with regards to peak separation at slow scan rates(D E p=79mV rather than the ideal D E p=0mV)and the full width half maximum(greater than200mV rather than the ideal E FWHM=90.6mV/n where in this case n=1).With regards to both peak separation and the E FWHM the non-ideal behaviour has been attributed to the ferrocene molecules being located in a range of environments with a range of formal electrode potentials (E0)[39,40].We[29,41]and others[42]have noted pre-viously that fabricating redox active SAMs by assem-bling the SAM and then attaching the redox molecule, results in broader FWHM than observed with electrodes where a redox active alkanethiol was attached directly to the electrode.The reason for modifying electrodes in this step-wise manner,where the monolayer is formed and then the redox active molecule attached,rather than synthesizing a pure redox active self-assembling mole-cule followed by assembly on the electrode,is because in applications of our interest,bioelectronics,the step-wise strategy is the only viable approach.With a monolayer containing only4-carboxyphenyl moieties the number of redox active molecules attached to the surface,as determined from the charge passed under the Faradaic peaks in the ferrocene modified electrode,is approximately(0.073±0.012)·10À10 mol cmÀ2with a close to unity ratio of anodic to cathodic peak areas(see Table1).Comparing the surface coverage of4-carboxyphenyl groups of7.4·10À10mol cmÀ2,to that of the number of redox centres attached indicates that only approximately10%of the 4-carboxyphenyl monolayers had a ferrocene attached. At this surface coverage the average area per ferrocene molecule,assuming homogeneous distribution,is 2.2nm2which suggests there is a high possibility of interaction between redox active centres[29,42].Interaction between redox active centres has been re-ported to decrease the reorganization energy and in-crease the electron transmission efficiency[4,43,44], hence providing an anomalously high measure of the rate constant for electron transfer.As a consequence, the number of coupling points within the monolayer that the ferrocene could couple was reduced by forming mixed monolayers composed of the4-carboxyphenyl diazonium salt and the phenyl diazonium salt(Table 1).Table1shows that the surface coverage initially in-creased with the spacing of the coupling points followed by the more expected decrease as the number of cou-pling points decreased.The reason for the initial in-crease in surface coverage of ferrocene as the solution composition from which the monolayer forms changes from entirely4-carboxyphenyl diazonium salt to a1:1 ratio of4-carboxyphenyl to phenyl diazonium salt is un-clear.The percentage of carboxyl groups to be activated using EDC/NHS,as used here,in a SAM composed of entirely carboxylic acids has been shown to be approxi-mately50%[45]which is equivalent to all the4-carboxy-phenyl groups being activated in a1:1monolayer. Furthermore,the relative surface coverages of the4-carboxyphenyl to ferrocene is10:1in the entirely4-carb-oxyphenyl monolayer so there should be excess coupling points for the ferrocene to attach.Therefore,it is sug-gested that the introduction of a second component into the monolayer(the phenyl diluent)in effect introduces a hydrophobic component into the monolayer.As ferro-cene has been shown previously to adsorb onto the sur-face of hydrophobic self-assembled monolayers[41,46]. Therefore,it is proposed that more ferrocene is attached when the phenyl component is introduced into the monolayer because the surface is more energetically favourable location for the ferrocene compared with an entirely carboxyphenyl monolayer.The rate constant for electron transfer was deter-mined from the variation in peak position between the anodic and cathodic scans as a function of scan rate. In this study,the variation in peak potential over a wide range of scan rates wasfitted using the Marcus theory for electron transfer as described previously[30–32] rather than the Laviron[33]formalism which relies on simple Butler–Volmer kinetics and gives rate constants for electron transfer which are sensitive to the choice of sweep rates investigated.Table1shows that across the spectrum of dilution ratios investigated the rate con-stant for electron transfer(k app)is approximately15–20sÀ1.3.2.Aryl diazonium salt modified gold electrodesGold electrodes were modified with aryl diazonium salts via electrochemical reduction in exactly the same manner to the GC electrodes.The reduction peak for the attachment of the aryl diazonium salt onto the gold electrode,observed in thefirst sweep,was shifted anod-ically230mV relative to carbon being at+70mV versus Ag/AgCl.Subsequent sweeps showed no electrochemis-try indicating a monolayer coverage of4-carboxyphenyl moieties on the electrode surface.The4-carboxyphenyl monolayer blocked access of potassium ferricyanide to the electrode in a similar manner to that depicted in Fig.1for the carbon electrode but to a lesser extent. The coverage of the4-carboxyphenyl moieties on the electrode surface was6.4·10À10mol cmÀ2which was lower than the7.4·10À10mol cmÀ2observed on GC electrodes and hence lower than the theoretical maxi-mum surface coverage[34]for a monolayer of 12·10À10mol cmÀ2.The lower surface coverage could be a reflection of the aryl diazonium salt not being nor-mal to the surface of the gold,as suggested by infra-red spectroscopy[20].Again,the presence of a monolayer or submonolayer of aryl diazonium salt on the gold elec-trode is important due to the possibilities of obtaining multilayers with aryl diazonium salts as shown for both carbon[36–38]and metal surfaces[20].An XP survey spectrum of gold modified with(4-carboxyphenyl)diazonium tetrafluoroborate showed the expected1s peaks of carbon and oxygen at$285 and$532eV,respectively,but no significant evidence of a nitrogen1s peak(Fig.4).The carbon1s envelope (Fig.4,inset)wasfitted with four peaks at288.7, 286.2,284.6and283.9eV assigned to the carboxylic acid moieties,C–O species,the aromatic carbons of the monolayer and the metal-bonded carbon,respectively. The binding energy observed for the carboxylic acid group on gold was consistent with that observed on the GC surface.The inclusion of the metal carbide peak is exceedingly tentative as a goodfit to the spectra could be obtained without the presence of this peak.The assignment of the metal carbide peak is based on theTable1Some parameters of ferrocenemethylamine immobilized on GC electrodes modified with mixed monolayers of4-carboxyphenyl and phenyl moieties.D E p is recorded at a scan rate of100mV sÀ1[Benzyl]/[benzoic acid]E0(mV)D E(mV)E FWHM(mV)C(pmol cmÀ2)C a/C c k app(sÀ1) 0264±1579±10241±1072.8±11.60.89±0.0717±10 1279±1378±14213±19100.3±10.4 1.03±0.0428±10 5292±989±25227±2567.4±10.70.94±0.0515±5 10298±793±10262±3848.1±6.90.88±0.1116±2 20304±17101±21220±2929.4±3.40.71±0.1615±10 40317±19107±10289±1413.3±2.00.77±0.1310±2Fig.4.XP survey spectrum and carbon1s narrow scan(inset)of agold electrode modified by electrochemical reduction of(4-carboxy-phenyl)diazonium tetrafluoroborate.G.Liu et al./Chemical Physics319(2005)136–146141precedence of Pinson and co-workers[19,20,47]who have previously proposed the existence of such a peak for the electroreduction of diazonium salts onto metal surfaces.On iron surfaces the case for a metal–carbide peak is compelling with a very pronounced shoulder when a high resolution instrument is used[47]with the intensity of this shoulder sensitive to take-offangle indi-cating it is a surface bound species.However,on copper electrodes[20]and other examples on iron[19]the shoulder on the carbon1s spectra is less pronounced similar to the observations on gold here.The electrochemical parameters after the attachment of ferrocene to the4-carboxyphenyl modified gold elec-trodes are shown in Table2.The trends were very sim-ilar to the GC modified electrodes with broader than ideal E FWHM and non-ideal D E p at slow scan rates. The surface coverage of ferrocene with different ratios of diluent to4-carboxyphenyl were slightly lower than those on GC in common with the lower coverage in gen-eral of the aryl diazonium salts on gold compared with GC.Most importantly,the rates of electron transfer measured on the gold modified surface were significantly greater than that observed on carbon.Typically rates of more than a100sÀ1were observed,which was approx-imately one order of magnitude higher than for the same monolayer system on GC electrodes.The values of the rate constants at the low surface coverage of ferrocene (last three entries in the table)were particularly difficult to determine because with small redox peaks back-ground subtraction can have a large impact on the peak positions.As a consequence the rate constants quoted represent the lower limits and therefore we expect the true rate constant is closer to that observed at the1:5 monolayer.3.3.Aryl thiol modified gold electrodesFor comparison with the monolayers formed by elec-trochemical reduction of aryl diazonium salts we also prepared mixed monolayers of aryl thiol self-assembled monolayers on gold electrodes with attached ferrocene moieties as shown in Scheme2.The rate constants deter-mined for this equivalent aryl thiol system were in the order of103sÀ1(at the limits of what can be measured electrochemically)which was approximately5–10times the values observed for the aryl diazonium salt–gold sys-tem but two orders of magnitude higher than those ob-served for the aryl diazonium salt–GC system.These observations indicate that the metal surface has a signif-icant effect on the rate of electron transfer.4.DiscussionThe rate constants for electron transfer are remark-ably slower for the carbon electrodes relative to the gold electrodes.This is contrary to the suggestion that with diazonium salt modified carbon electrodes the continu-ity of conjugated carbon network from the electrode into the monolayer will result in a lower barrier for elec-tron transfer than with organic monolayers on metallic electrodes[11].The question that arises is why there is a difference in rate constants of around one order of magnitude for the same redox active molecule connected to electrodes by the same bridge molecule?The Marcus–Hush expression for electron transfer between a donor and acceptor through an organic bridge in solution includes terms for electronic coupling between the donor and acceptor,the Gibbs free energy for electron transfer(the driving force,D G ET)and the nuclear reorganization energy(k)of the redox molecule as a consequence of its change in oxidation state[48]. For a given donor and acceptor pair the rate of electron transfer decays exponentially with distance according to a proportionality constant,the b value,sometimes called a damping factor.When the organic bridge is anchored to an electrode such that it can act as the donor and/or acceptor the situation is complicated somewhat as the electronic properties of the electrode can also play a role in the rate of electron transfer[2].Equations describing the rate constant for electron transfer now incorporate terms related to the Fermi levels of the electrode and the effective density of electronic states near the Fermi level.In this study,the only changes between the mono-layer systems studied relate to the electrode material and the bond to the electrode.Hence,the reorganization en-ergy and the driving force will remain unchanged.The electronic coupling may be influenced by the electrodeTable2Some parameters of ferrocenemethylamine immobilized on gold electrodes modified with mixed monolayers of4-carboxyphenyl and phenyl moieties.D E p is recorded at a scan rate of100mV sÀ1[Benzyl]/[benzoic acid]E0(mV)D E(mV)E FWHM(mV)C(pmol cmÀ2)C a/C c k app(sÀ1) 0268±1281±14209±1149.3±7.60.87±0.13257±41 1277±2585±9191±1780.6±5.90.86±0.09530±42 5280±1875±10227±853.7±5.40.72±0.14211±23 10282±1492±12260±1525.8±4.00.92±0.0783±50 20292±1989±8272±1413.3±2.40.76±0.0369±50 40317±2181±16308±107.1±1.00.93±0.0268±50 142G.Liu et al./Chemical Physics319(2005)136–146。
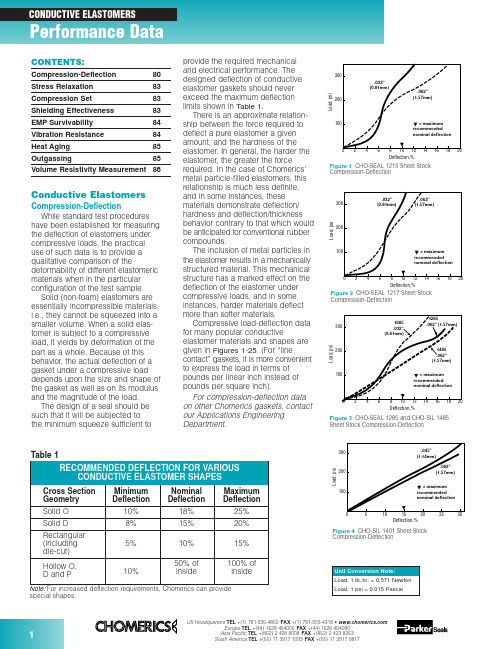
Figure 3CHO-SEAL 1285 and CHO-SIL 1485Sheet Stock Compression-DeflectionCompression-Deflectionspecial shapes.Conductive ElastomersCompression-DeflectionWhile standard test procedures have been established for measuring the deflection of elastomers under compressive loads, the practical use of such data is to provide a qualitative comparison of thedeformability of different elastomeric materials when in the particular configuration of the test sample.Solid (non-foam) elastomers are essentially incompressible materials;i.e., they cannot be squeezed into a smaller volume. When a solid elas-tomer is subject to a compressive load, it yields by deformation of the part as a whole. Because of this behavior, the actual deflection of a gasket under a compressive load depends upon the size and shape of the gasket as well as on its modulus and the magnitude of the load.The design of a seal should be such that it will be subjected to the minimum squeeze sufficient toprovide the required mechanical and electrical performance. The designed deflection of conductive elastomer gaskets should never exceed the maximum deflection limits shown in Table 1.There is an approximate relation-ship between the force required to deflect a pure elastomer a given amount, and the hardness of the elastomer. In general, the harder the elastomer, the greater the force required. In the case of Chomerics’metal particle-filled elastomers, this relationship is much less definite,and in some instances, these materials demonstrate deflection/hardness and deflection/thickness behavior contrary to that which would be anticipated for conventional rubber compounds.The inclusion of metal particles in the elastomer results in a mechanically structured material. This mechanical structure has a marked effect on the deflection of the elastomer under compressive loads, and in some instances, harder materials deflect more than softer materials.Compressive load-deflection data for many popular conductiveelastomer materials and shapes are given in Figures 1-25. (For “linecontact” gaskets, it is more convenient to express the load in terms of pounds per linear inch instead of pounds per square inch).For compression-deflection data on other Chomerics gaskets, contact our Applications Engineering Department.Compression-DeflectionCompression-DeflectionCONTENTS:Compression-Deflection 80Stress Relaxation 83Compression Set 83Shielding Effectiveness 83EMP Survivability 84Vibration Resistance 84Heat Aging 85Outgassing85Volume Resistivity Measurement86Figure 80.125 in. (3.18 mm) Dia. O-Strip Compression-DeflectionDeflection,%Compression-DeflectionCompression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 210.156 in. (3.96 mm) High Hollow D-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 220.312 in. (7.92 mm) High Hollow D-Strip Compression-DeflectionFigure 200.250 in. (6.35 mm) Dia. Hollow O-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %L o a d , l b ./i n c hDeflection, %Figure 230.250 in. (6.35 mm) Dia. Hollow P-Strip Compression-DeflectionFigure 240.360 in. (9.14 mm) Dia. Hollow P-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %L o a d , l b ./i n c hDeflection,%Figure 190.156 in. (3.96 mm) Dia. Hollow O-Strip Compression-DeflectionL o a d , l b ./i n c hDeflection, %Figure 170.250 in. (6.35 mm) Wide Rectangular Strip Compression-Deflection0.40.81.20.20.61.0C o m p r e s s i o n F o r c e (l b /i n )00.5 1.50.10.2Deflection (inch)1356P/N 10-09-W864-XXXXFigure 250.410 in. (10.41 mm) High V-Strip Compression-DeflectionStress RelaxationAs important as Compression Set and Compression-Deflection, is the Stress Relaxation characteristic of a gasket.If a rubber is subject to a com-pressive load, it will deflect. There is a stress/strain relationship, which for rubbers is generally non-linear except for very small deflections.After the load is applied, a stress decay occurs within the polymer resulting from the internal rearrange-ment of the molecular structure. An approximate rule is that the relaxed stress for cured silicone will finally settle at 70 to 75 percent of the initial stress.There are two ways in which a rubber gasket can be loaded to a desired value. One way is to load it to a point, let it relax, and reapply the load to restore the original stress. The next time it will relax, but not so much.If this is repeated a sufficient number of times, the correct static load on the gasket will reach equilibrium.A more practical way to reach the design value of stress is to load the gasket to 125 percent of its final design value, so that after the relax-ation process is completed the gasket will settle to 100 percent of the design load. This is very reproducible.Figure 26shows a typical stress relaxation curve for Chomerics’conductive elastomers.Compression SetWhen any rubber is deformedfor a period of time, some of the defor-mation is retained permanently even after the load is removed. The amount of permanent deformation, asmeasured by ASTM D395, is termed “Compression Set.” Compression set is measured under conditions of constant deflection (ASTM D395Method B) and is normally expressed as a percentage of the initialdeflection, not as a percentage of the initial height.For gaskets that are used once, or where the gasket/flange periphery relationship is constant (such as a door gasket), compression set is of minor significance if the original load condition and the service temperature are within the design limitations of the gasket material.For gaskets that are randomlyreseated one or more times in normal service life, it is important that the maximum change in gasket thickness does not exceed twice the maximum mismatch between the opposing mating surfaces.Shielding EffectivenessMost shielding effectiveness data given in Table 3 of the Conductive Elastomer section (pages 32-34) is based on a MIL-G-83528B testmethod, with a 24 in. x 24 in. aperture in a rigid enclosure wall and about 100 psi on the gasket. It is a valid and useful way of comparing variousgasket materials, but does not reflect the shielding effectiveness one can expect at seams of typical enclosures.CHO-TM-TP08 is a modified version of the MIL test that provides typical values achieved in actual applications.Since many factors will affect the actual shielding effectiveness of anenclosure seam (flange design,stiffness, flatness, surface resistivity,fastener spacing, enclosuredimensions, closure force, etc.), the only way to determine shielding effectiveness for real enclosures is to test them.Figures 28and 29provide dataon shielding effectiveness for actualFigure 27Formula for Calculation of Compression Setenclosures. The data in Figure 28shows the difference in attenuation between a shelter door closed with no gasket and the same door closed against a CHO-SEAL 1215 hollow D-strip gasket. Instead of single data points at each frequency tested, a range of data is shown for eachfrequency, representing the worst and best readings measured at many points around the door. Figure 29 shows the effects of closure force on shielding effectiveness of an enclosure tested at high frequencies (1-40 GHz) using CHO-SEAL 1215 solid D-strip gaskets.In order to establish reasonable upper limits on gasket resistivity, it is necessary to understand the rela-tionship between flange interface resistance and EMI leakage through the flange. Figure 30presents this relationship for an aluminum enclosure 3 in. x 3 in. x 4 in. deep, measured at 700 MHz. Die-cut gaskets 0.144 in.wide by 0.062 in. thick, in a wide range of resistivities, were clamped between the gold-plated flanges of thisenclosure. Simultaneous measure-ments of flange interface resistance (all attributable to the gaskets) versus RF leakage through the seamproduced a classic S-shaped curve.For the gasket configuration used in this test, the dramatic change in shielding effectiveness occursbetween gasket volume resistivities of 0.01 and 0.4 ohm-cm. Since real enclosures do not have gold-plated flanges, but rather have surfacefinishes (such as MIL-C-5541 Class 3chromate conversion coatings) which also increase in resistance over time, it is recommended that gasket volume resistivity be specified at 0.01 ohm-cm max. for the life of the equipment.Frequency, HzA t t e n u a t i o n (dB )Figure 28Shielding Effectiveness of a Shelter Door Gasket (14 kHz to 10 GHz)kA/inch of gasket (peak-to-peak).Pure silver (1224) and silver-plated-aluminum filled (1285) gaskets have less current carrying capability than silver-plated-copper materials, but are generally acceptable for EMP hardened systems (depending on specific EMP threat levels, gasket cross section dimensions, etc.).Vibration ResistanceCertain conductive elastomers are electrically stable during aircraft-level vibration environments, while others are not. The key factor which deter-mines vibration resistance is theshape and surface texture of the filler particles. Smooth, spherical fillers (such as those used in silver-plated-Figure 32Scanning Electron Microscopy Illustrates EMP Damage Mechanism for Silver/Glass ElastomersL e a k a g e (d B )Vibration (g)Figure 33Effects of Vibration on Shielding Effectiveness of Conductive Elastomer GasketsEMP SurvivabilityIn order for an enclosure to continue providing EMI isolationduring and after an EMP environment,the conductive gaskets at joints and seams must be capable of carrying EMP-induced current pulses without losing their conductivity. Figure 31shows the EMP current response of various types of conductive elastomer gaskets. Note that gaskets based on silver-plated-glass fillers (1350)become nonconductive at low levels of EMP current, and should therefore not be used when EMP is a design consideration. Figure 32is an electron microscope photo which clearly shows the damage mechanism.Silver-plated-copper filled (1215)gaskets have the highest resistance to EMP type currents, showing no loss of conductivity even at 2.50102030405060Shielding Degradation, dBIn t e r f a c e R e s i s t a n c e , m i l l i o h m sFigure 30Interface Resistance vs. Shielding Degradation at a Flange Jointglass materials) tend to move apart during vibration, leading to dramatic increases in resistance and loss of shielding effectiveness (although they normally recover their initial properties after the vibration has ended). Rough, less spherical particles resist vibration with very little electrical degradation. Figure 33shows the effects of vibration on three types of conductive gaskets.Although Chomerics’ silver-plated-copper filled 1215 gasket, with rough,irregular particle agglomerations,exhibits excellent stability during vibration, users of conductive elastomers should be aware that smooth, spherical silver-plated-copper fillers can be almost asunstable as silver-plated-glass fillers.Frequency, GHzA t t e n u a t i o n (dB )Figure 29Effect of Closure Force on Shielding Effectiveness (1 GHz to 40 GHz)Heat AgingThe primary aging mechanism which affects electrical stability of conductive elastomers is the oxidation of filler particles. Formaterials based on pure silver fillers,particle oxidation is not generally a problem because the oxide of silver is relatively soft and reasonably conductive. If the filler particles are non-noble (such as copper, nickel,aluminum, etc.) they will oxidize readily over time and become nonconductive. Even silver-plated base metal powders, such as silver-V o l u m e R e s i s t i v i t y (o h m -c m )Hours at 150°C (Solid Line)Hours at 125°C (Dotted Line)Figure 34Typical heat aging characteristics of Chomerics’ plated-powder-filled conductiveelastomers. Flanged 1000-hr test recommended for qualification. Unflanged 48-hr. test recommended for QC acceptance.plated-copper or silver-plated-aluminum will become non-conductive over time if the plating is not done properly (or if other processingvariables are not properly controlled).These are generally batch control problems, with each batch being potentially good or bad.The most reliable method of predicting whether a batch will be electrically stable is to promote the rate at which poorly plated or processed particles will oxidize, by heat aging in an air circulating oven.For qualification, 1000 hours (42 days)at maximum rated use temperature (with the gasket sample deflected 7-10% between flanges) is the recommended heat aging test for accelerating the effects of long-term aging at normal ambient tempera-tures. A quicker heat aging test,which correlates well with the 1000hour test and is useful for QC acceptance testing, involves a 48hour/150°C oven bake with thegasket sample on an open wire-grid tray (rather than being clamped between flanges). Figure 34shows typical data for volume resistivity versus time for each of these tests.Note:It is essential that no source of free sulfur be placed in the aging oven, as it will cause the material to degrade electrically and mask any oxidation aging tendencies. Common sources of sulfur are neoprenes,most cardboards and other paper products.OutgassingMany spacecraft specifications require that nonmetallic components be virtually free of volatile residues which might outgas in the hard vacuum environment of space. The standard test method for determining outgassing behavior is ASTM E595-93, which provides for measurement of total mass loss (TML) and collected volatile condensable materials (CVCM) in a vacuum environment. Data for a number of Chomerics conductive elastomers,based on ASTM E595-93 testing done by NASA Goddard SpaceflightCenter, is presented in Table 2. The normal specification limits or guide-lines on outgassing for NASA applications are 1% TML max.,and 0.1% CVCM max.。

heterogeneous interfacial structure英文版Heterogeneous Interfacial StructureHeterogeneous interfacial structure refers to the structural differences that exist at the boundary between two different materials or phases. This structure plays a crucial role in determining the physical and chemical properties of the interface, as well as its stability and reactivity.At the interface between two materials, the atomic arrangement, bonding configuration, and electronic structure can all differ significantly from the bulk materials on either side. This heterogeneity can lead to a range of unique properties, such as charge accumulation, bond formation, and catalytic activity. For example, in the field of materials science, heterogeneous interfaces are often exploited to enhance the performance of devices such as solar cells and fuel cells.The study of heterogeneous interfacial structure is challenging due to the complexity of the interactions involved. Experimental techniques such as scanning probe microscopy, spectroscopy, and diffraction methods can provide insights into the atomic-scale structure and electronic properties of interfaces. Computational modeling is also an important tool for understanding and predicting interfacial behavior.In recent years, there has been increasing interest in the use of heterogeneous interfacial structures in nanotechnology and materials science. This interest is driven by the potential for novel materials with enhanced properties, as well as the development of new technologies such as nanodevices and sensors.In conclusion, heterogeneous interfacial structure is a crucial aspect of materials science and nanotechnology. Its understanding and control offer the potential for the development of novel materials and devices with enhanced performance and functionality.中文版异质界面结构异质界面结构指的是两种不同材料或相之间的边界处存在的结构差异。

Low percolation threshold in single-walled carbon nanotube/high density polyethylene compositesprepared by melt processing techniqueQinghua Zhanga,*,Sanjay Rastogi b ,Dajun Chen a ,Dirk Lippits b ,Piet J.LemstrabaState Key Laboratory of Chemical Fibers and Polymer Materials,College of Materials Science and Engineering,Donghua University,Shanghai 200051,PR ChinabDepartment of Chemical Engineering and Chemistry,Eindhoven University of Technology,P.O.Box 513,5600MB Eindhoven,The NetherlandsReceived 6May 2005;accepted 7September 2005Available online 15November 2005AbstractA new method was developed to disperse carbon nanotubes (CNTs)in a matrix polymer and then to prepare composites by melt processing technique.Due to high surface energy and strong adsorptive states of nano-materials,single-walled carbon nanotubes (SWNTs)were adsorbed onto the surface of polymer powders by spraying SWNT aqueous suspected solution onto fine high density polyethylene (HDPE)powders.The dried SWNTs/powders were blended in a twin-screw mixture,and the resulting composites exhibited a uniformly dispersion of SWNTs in the matrix polymer.The electrical conductivity and the rheological behavior of these composites were investigated.At low frequencies,complex viscosities become almost independent of the frequency as nanotubes loading being more than 1.5wt%,suggesting an onset of solid-like behavior and hence a rheological percolation threshold at the loading level.However,the electrical percolation threshold is $4wt%of nanotube loading.This difference in the percolation thresholds is understood in terms of the smaller nanotube–nanotube distance required for electrical conductivity as compared to that required to impede polymer mobility.The measurements of mechanical properties indicate that this processing method can obviously improve the tensile strength and the modulus of the composites.Ó2005Elsevier Ltd.All rights reserved.Keywords:Carbon nanotubes;Electrical properties;Rheology1.IntroductionThe potential utility of carbon nanotubes (CNTs)[1,2]in a variety of technologically important application,such as molecular wires and electronics,sensor,high strength fibers,and field emission,is now well established [3].The conductivity and tensile strength properties of this new class of nanoscale materials have attracted a great deal of investigation,which has immensely expanded their scope of investigation.The first realized major commercial appli-cation of CNTs maybe is their potential use in the develop-ment of the next generation of composite materials and electrically conducting components in polymer composites [4].Hence,CNTs are expected to serve as mechanical rein-forcements for lightweight composite systems with the fur-ther promise of multifunctionality.For instance,SWNTs possess a tensile strength of 50–100GPa and a modulus of 1–2TPa and these values are 5and 10times greater than those for steel,respectively,at just a sixth the weight [1,2,5–7].However,the potential of using nanotubes as polymer composites has not been presently realized mainly because of the difficulties in the solubility and the processability.Several fundamental processing challenges must be over-come to enable the applicable composites by nanotubes.The dispersion property becomes more important when0008-6223/$-see front matter Ó2005Elsevier Ltd.All rights reserved.doi:10.1016/j.carbon.2005.09.039*Corresponding author.Tel.:+862162373727;fax:+862162193062.E-mail address:qhzhang@ (Q.Zhang)./locate/carbonCarbon 44(2006)778–785nanotubes are blended into a Ts tend to remain as entangled agglomerates and thus homogeneous dispersed state within a polymer is not easily carried out.Two main different approaches are employed to improve the dispersion of nanotubes in some solvents or polymers: One is chemical modification on carbon nanotube sur-face,i.e.,functionalization at defect sites.An active group such as carboxylic acid or a polymer is introduced onto the surface of CNTs[8–15].The solubilized carbon nanotubes, especially those from the polymer furnctionalization,are important to the preparation of polymeric carbon compos-ites[16–18].SWNTs and MWNTs functionalized with poly(vinyl alcohol)(PVA)in esterification reactions can be dissolved in highly polar solvents such as DMSO or water[19,20].Polystyrene composites with functionalized SWNTs demonstrated a percolated SWNTs network struc-ture at concentrations of1vol%SWNTs,which is only a half content of the unfunctionalized SWNTs-based composites[21].The mechanical properties of epoxy com-posites containing modified nanotubes exhibit a high mod-ulus and a high strength[22–24].Even though chemical functionalizaion of carbon nanotubes can improve the sol-ubility,the dispersion and thus the processability,the method is relatively complicated due to involving chemical reaction that may leads to the nanotube breakage.The dispersion of carbon nanotubes in solvents or poly-mers at the aid of a surfactants or a copolymer is another important method that does not contain chemical reaction.A single-step solubilization scheme has been developed in which nanotubes are mixed with surfactants in low-power, high-frequency sonicators,and the scheme enhances the disaggregation of bundles with dramatically reduced tube breakage.Carbon nanotubes can suspend in aqueous media as individuals or bundles surrounded by surfac-tants[25,26]such as sodium dodecylsulfate(SDS),sodium octylbenzene sulfonate,sodium dodecylbenzensulfate,or copolymers[27–33]such as poly(m-phenylenevinylene) (PmPV),poly(aryleneethynylene)s(PEEs),poly(vinyl pyr-rolidone)(PVP),Gum Arabic(a highly branched arabino-galactan polysaccharide),polyvinyliden difluorid and its copolymers,styrene maleic anhydride copolymer(SMA), Triton X-100(C8H17C6H4(OCH2CH2)n–OH,n%10),etc. Polymeric composites containing carbon nanotubes can be thus obtained by dissolving or dispersing the polymer into the suspended solution.Zhang et al.[34]reported that both the tensile yield strength and the tensile modulus of PVA/PVP/SDS/SWNT were doubled approximately,as compared to PVA.Regev et al.[35]prepared SWNT–SDS–PS composites using latex technology,which exhibits an excellent conductive property with a percolation thresh-old of0.28wt%.However,to prepare nanotube/polymer composites with a homogeneous dispersion of nanotubes and a low electrical percolation threshold,the polymers are requested to be water-soluble or emulsible.In other words,even though CNTs can be dispersed in water at the aid of surfactants or copolymer,it is still difficult to uni-formly disperse CNTs into a matrix polymer.In this report,we develop a new method to prepare com-posites—spraying carbon nanotubes suspended solution onto the surface of polymer powders.Nanotubes are pref-erably adsorbed on the surface of polymer powders.Once water was vaporized,the composites can be processed by melt or solution processing method.The method can reduce loading level of CNTs,comparing to directly adding CNTs into polymer by melt processing.2.Experimental2.1.MaterialsThe purified HiPCO SWNTs with an average diameter of0.8–2nm used in this work were made by Carbon Nano-technologies Inc.High density polyethylene(HDPE)with an average molecular weight of M w$14,000g/mol were used as a matrix.Sodium dodecylsulfate(SDS)was bought from Aldrich Company.2.2.Dispersion of SWNTs in waterSWNT aqueous solution was prepared at the aid of SDS,according to a reported method by Regev[35].In detail,20ml solution containing0.22g SWNTs and 0.5wt%SDS based on H2O was sonicated for15min and then centrifuged at3000rpm for20min.About90% SWNTs were suspended in the aqueous solution,and the others were deposits.2.3.Preparation of compositesThe aqueous solution with SWNT of0.2wt%was sprayed onto the surface of HDPE,as schemed in Fig.1. As spraying,the substrate with a thin layer of HDPE was being shaken to ensure that the SWNT solution was uniformly absorbed onto all round of the polymer particles.The HDPE powder adsorbed SWNTs was dried for1h at90°C in a vacuum oven.The sample was extrudedusing Fig.1.A spraying scheme:SWNTs suspended solution was uniformly spurted onto the surface of HDPE powders from sprinkler.Q.Zhang et al./Carbon44(2006)778–785779DACA Micro Compounder with a co-rotating twin-screw for a mixing time of about20min at a barrel temperature of160°C and a screw speed of50rpm.When the torque no longer changed with time for5min,the blend processing was stopped.2.4.Characterization techniquesHigh resolution optical microscope(HROM,Carl Zeiss Vision GmbH)was used to observe the distribution of SWNT in HDPE.The thin sample on a slide was prepared at170°C prior to observation.A scanning electron microscope(SEM)and a transmission electron microscope (TEM)were employed to study the dispersion of SWNT in water and on the surface of HDPE,respectively.The diameter of the bundles can be measured by the software of AxioVs40LE by Carl Zeiss Vission GmbH.Prior to electrically conductive measurements,the com-posites obtained above were further compressed into about 1mm thickness sheets by a hot press at160°C.The electri-cal conductivities of the conducting compositefilms were measured with a four-probe technique,while that of the samples with a low conductivity(<10À8S/cm)were per-formed on ZC43Ohmmeter with a two-probe.For each sample,five points were measured and averaged for the conductivity.Dynamic rheological properties were investigated on a Rheometric Scientific RMS-800.The measurements were carried out in an oscillatory shear mode using a parallel plate geometry with a diameter of25mm at180°C under nitrogen atmosphere.Frequency sweeps between0.1and 500rad/s were applied at a low strain(0.1–10%).Speci-mens were placed between the preheated plates and were allowed to equilibrate for approximately10min prior to each frequency sweep run.The measurement of the mechanical properties was con-ducted on a DXL2000tensile instrument made in Shang-hai,China.The composites were compressed into sheets with the thickness of$1mm.In order to avoid the influ-ence of the processing procedure on the mechanical proper-ties of the composites,the process of all samples was performed in the same way,and one specimen was mea-suredfive times and the data were averaged.3.Results3.1.MorphologyElectron microscopes were employed to observe the morphologies of dispersion of SWNTs.As shown in Fig.2,SWNTs can be uniformly dispersed in water at the aid of the SDS.SWNTs are in the form of the aggre-gated morphology—bundles.It should be note,the diame-ters of the most aggregated SWNTs are in the range of 2–5nm,and these SWNT bundles maybe consist of several individual tubes.However,the average diameter of SWNT bundles in2–5nm does not involve the contribution of a few very large bundles,such as a bundle at the right-up cor-ner in Fig.2.In this work,we utilize the property of high surface energy of polymer powders to prepare composites of car-bon nanotubes and HDPE.Fig.3shows the surface mor-phology of the HDPE powder adsorbing SWNTs. Apparently,SWNTs on small bundle form arefixed on the surface of the powder via spraying SWNT aqueous solution method.The nanotubes can be clearly distin-guished in the micrograph.The interaction between SWNT and HDPE is so strong that when the dried SWNT–HDPE powders are immerged into water,SWNTs cannot be sus-pended in water again and the water is still clear and transparent.Fig.4exhibits the high resolution optical micrographs of the composites with different weight fraction of SWNTs. There are no obvious agglomerations of the nanotubes in Fig.4a with1.5wt%SWNTs.However,a few ofblack Fig.2.TEM image of SWNT dispersed in water at the aid ofSDS.Fig.3.SEM image of the morphology of SWNTs on the surface of HDPE powder by spraying method.780Q.Zhang et al./Carbon44(2006)778–785spots,which we attribute to nanotube agglomerates,appear on the sample containing 4wt%SWNTs,shown in Fig.4c.More black spots appear on the film with con-taining 5wt%SWNTs,shown in Fig.4d.This contrastive result indicates that the nanotubes are uniformly distrib-uted within the matrix HDPE containing not more than 2.6wt%SWNTs.The composite films containing 4wt%or 5wt%SWNTs exhibit some black spots with a dimen-sion of $5l m,which is caused by the aggregation of SWNTs.The specific surface area of HDPE powder is greatly less than that of SWNTs.Therefore,we deduce the surface of the polymer can merely adsorb a certain amount of SWNTs.To spray more nanotube solution onto the HDPE surface necessarily leads to aggregation of SWNTs.In our experiment,SWNT aqueous suspected solution contains about 1wt%SWNTs.To prepare 10g SWNT–HDPE composite with a loading of 4%SWNTs,40g the suspected solution was sprayed onto the surface of HDPE powders.When spraying 40g solution into 10g HDPE,in fact,HDPE is immersed into the aqueous solution.After drying,some of SWNTs are absorbed onto the surface of HDPE powders and the others are aggregated into some particles,as shown in Fig.4c or d.Hence,when the loadinglevel of SWNTs in HDPE is more than 4.0wt%,the obvi-ous agglomerates appear in the composites.3.2.ConductivityFig.5shows the relationship between the conductivity and the weight fraction of SWNTs in the composites.Apparently,the conductive property was improved by increasing SWNTs.The addition of SDS has a slight influ-ence on conductivity of the materials by less than one order of magnitude,since the surfactant on the surface of HDPE can absorb a trace of water in air.At very low concentra-tion of SWNT (<3.4wt%),conductivity gradually increases as increasing nanotube content.However,conductivity of the composite containing 5wt%SWNTs increases to 10À3S/cm from that of 2.6wt%SWNTs of 10À10S/cm.This stepwise change in conductivity is a result of the for-mation of an interconnected structure of SWNTs and can be regarded as an electrical percolation threshold.In other words,at about 4wt%SWNT loading,a very high percent-age of electrons are permitted to flow through the sample at applied electric filed due to the creation of the intercon-necting conductive channels,which has been highlighted using an upright bar in Fig.5.Fig.4.High resolution optical micrographs of SWNT/HDPE composite films containing 1.5wt%(a),2.6wt%(b),4.0wt%and 5.0wt%(c),prepared by melt mixing process.Q.Zhang et al./Carbon 44(2006)778–7857813.3.Rheological propertiesThe complex viscosities,[g *],of the composites and neat HDPE are shown in Fig.6.It is apparent from the figure that nanotubes have a dramatic effect on the rheological behavior,even at loading as low as 0.5wt%.The composite containing more SWNTs exhibits a very strong shear thin-ning effect;whereas,the neat HDPE shows only small fre-quency dependence.The figure indicates that the addition of SDS in the matrix polymer has a very slight influence on the complex viscosity,so the effect of SDS on the rheo-logical properties will be not taken in account in the follow-ing discussion.As shown in Fig.6,the complex viscosity increases with increasing SWNT content.The effect of the nanotubes is most pronounced at low frequencies and the relative effect diminishes with increasing frequency due to shear thinning.This is in accordance with theoreti-cal expectations and experimental observations for fiberreinforced composites [36,37].The viscosity curve for 0.5wt%SWNTs reveals a Newtonian plateau at low fre-quencies,similar to the neat HDPE behavior.However,the viscosity curves for the samples containing more than 1.5wt%exhibit much steeper slope at the frequency region.At high frequencies,the fraction of SWNTs has a rela-tive minor influence on the complex viscosities of the composites.The rheological behavior for the samples containing various SWNTs at different frequencies is maybe caused by the following reason:If the platelets form a nanoscale network structure,then the rheological behavior of the composites should be solid-like with only minor influence of temperature at low shear rates.At high shear rates the rheology will be determined by the polymer matrix.Thus the composite will behave much like a liquid with the well-known strong influence of temperature on viscosity.Fig.6shows that complex viscosities increase sharply at 1.5wt%loading,indicating that there is a sudden change in the material structure.This sudden change in g *means that the composites have reached a rheological percolation at which the nanotubes impede the motion of polymers and form a nanoscale network structure.The change in complex viscosity with nanotube compo-sition is primarily caused by a dramatic change in the stor-age modulus G 0and the loss modulus G 00.Fig.7shows the function of the storage modulus and the loss modulus at 180°C as the addition of SWNTs into the matrix.At low frequencies,HDPE chains are fully relaxed and exhibit typ-ical homopolymer-like terminal behavior.However,at nanotube loadings higher than 1.5wt%,this terminal behavior disappears and the dependence of G 0and G 00on x at low frequency is weak.Thus,large-scale polymer relaxations in the composites are effectively restrained by the presence of SWNTs.As shown in Fig.7a,the G 0is almost independent of frequency at low frequencies when the nanotube loading is more than 1.5wt%,which is indic-ative of a transition from liquid-like to solid-like viscoelas-tic behavior.This non-terminal low frequency behavior can be attributed to a nanotube network,which restrains the long-range motion of polymer chains.As a result in Fig.7b,the low frequency dependence of G 00shows a similar trend.However,the corresponding increase in the loss modulus G 00is much lower than that in the storage modulus G 00at a fixed nanotube content,by comparing Fig.7a with Fig.7b.As the nanotube content increases in this composite sys-tem,nanotube–nanotube interactions begin to dominate,and eventually lead to percolation and the formation of an interconnected structure of nanotubes.Starting at about 1.5wt%nanotubes,G 0seems to reach such a plateau at low frequencies.Therefore,an interconnected structure is assumed to form.This critical composition is regarded as a rheological percolation composition.Once the nanotubes form a network structure,the rheological properties exhibit a similar behavior even if we further increase the nanotube loadings.The g *,G 0and G 00curves for the samples of782Q.Zhang et al./Carbon 44(2006)778–7852.6wt%and5.0wt%loadings exhibit nearly a similar rhe-ological behavior,as shown in Figs.6,7a and b, respectively.3.4.Mechanical propertiesIn order to avoid the influence of the processing proce-dure on the mechanical properties of the composites,the process of each sample was performed in the same way, and one specimen was measuredfive times and then the data were averaged.The effects of nanotube loading levels on tensile strength and modulus are shown in Fig.8.In the figure,the two star-symbols refer to comparable data of HDPE containing2wt%SDS,which is prepared via a sim-ilar method by spraying SDS aqueous solution onto the surface of HDPE powders and then preparing afilm using melt processing.As a result,the addition of2wt%SDS has a slight influence on the tensile and modulus of HDPE; whereas,thefigure indicates that SWNTs obviously rein-force the mechanical properties.The addition of0.5wt% SWNTs leads to the increase of tensile strength and initial modulus by$30%and$20%,respectively.Furthermore, the loading level of2.6wt%causes the increasingfigures of65%and50%,respectively.The high increases of the mechanical properties are attributed to the homogeneous dispersion of SWNTs in HDPE.As a comparison,Tang et al.[38]reported that composites based on polypropylene and MWNTs,prepared by directly melt processing method,possess the increase of the tensile by$12%at 5wt%MWNT loading level.4.Discussion4.1.Dependence of dispersion on processing methodsThe conducting properties of a binary compositefilled with a conducting component are mainly dominated by morphology and dispersion of thefiller.When conducting carbon black with a particle form is loaded into a matrix polymer,the electrical percolation threshold is usually up to15–25wt%,depending on the dimension and dispersion of the carbon black[39].Carbonfibers with a rod form arefilled into a thermoplastic,reported by Lozano and coworkers[40,41],and then the threshold is in the range of9–18wt%.However,when nanotubes are used as con-ductingfillers,the threshold is very low due to their high aspect ratio and thin diameter.The percolation threshold of nanotubesfilled compos-ites primarily is dependent on the processing methods, eventually on the dispersion of the SWNTs or MWNTs in a matrix.Direct blend of nanotubes and a thermoplas-tic polymer by melt processing results in a high threshold since the dispersion of the very thin tubes is not facile. Bhattacharyya et al.[42]directly blended HiPCo TM SWNT and polypropylene powder in a Haake mixer, and the resulting composite containing0.8wt%SWNTs exhibited some large nanotube agglomerates with a dimension of10–20l m.Meincke et al.[43]reported that a binary composite of MWNT and polyamide-6prepared by melt processing has a percolation of$6wt%with a conductivity of10À7S/cm at the loading level.In this work,we utilize the high surface energy of SWNTs andFig.7.Storage modulus G0(a)and loss modulus G00(b)of SWNT/HDPEcomposites with various nanotube loadings at180°C.Q.Zhang et al./Carbon44(2006)778–785783adsorptive property to improve the dispersion of nano-tubes in HDPE via spraying nanotube suspected solution onto polymer powders,and then produce the composites with a relatively uniform dispersion of the nanotubes by melt processing.Once SWNTs are adsorbed on the sur-face of the polymer,aggregation of the individual nano-tubes or the thin bundles is no longer paring to direct melt processing,the convenient spraying tech-nique can improve the dispersion,and thus reduce perco-lation threshold of the composite system.As another processing method,solution blending method can improve the dispersed state of nanotubes in a polymer.Carbon nanotubes arefirstly suspended in water or an organic solvent at the aid of a surfactant or copoly-mer,and then a matrix is blended with the suspend solu-tion to prepare the composites.Ramasubramaniam and coworkers[32]reported that SWNTs were solubilized in chloroform with poly(phenyleneethynylene)s(PPEs)along with vigorous shaking and/or short bath sonication,and the resulting PPE-functionalized SWNTs solution was then mixed with a host polymer(polycarbonate or polystyrene) solution in chloroform to produce a homogeneous nano-tube/polymer composite by casting the solution.The threshold percolation of PPE-SWNT/PS is merely $0.1wt%.Composites based on epoxy resin produced by curing of nanotube dispersions in the liquid precursor exhi-bit a percolation threshold below0.5wt%[44,45].How-ever,more organic solvent used in this solution processing method should be eliminated,so this is not con-venient on a large-scale production.4.2.Difference between electrical and rheological percolation thresholdsAs discussed above in Figs.6–8,there exists a difference between the electrical percolation threshold and the rheo-logical one:The former is$4wt%loading and the latter is$1.5wt%.This can be explained by the different tube–tube distances required for electrical or rheological percola-tion.Assuming that the electron hopping mechanism applies to the electrical conductivity of nanotube/polymer composites,the required tube–tube distance has to be less than$5nm for the composites to be electrically conduc-tive.However,as long as the tube–tube distance is compa-rable to the diameter of random coils of the polymer chains,which is estimatively more than10nm,the nano-tube network can effectively restrain polymer motion[46]. Therefore,electrical percolation threshold for a given nanotube/polymer system needs high loading level of nano-tube than rheological percolation threshold.On the other hand,there maybe exists an adsorbed polymer layer around individual nanotubes or bundles,which reduces the quality and quantity of electrical contacts between the nanotubes,whereas the adsorbed polymer layer may have a slight influence on the rheological properties[20].There-fore,the nanotube/polymer system exhibits a high electri-cal percolation threshold,comparing with the rheological percolation.5.ConclusionThe dispersion of carbon nanotubes is one of problems in the application of polymer composites.Even though chemical functionalization on nanotube surface can enhance the solubility in solvents and eventually improve the dispersion of the nanotubes in a matrix,these compli-cated functionalizing procedures impede the utilization of nanotubes on a large scale.In this report,relatively homo-geneous dispersion can be carried out via spraying nano-tube suspected solution onto surface of a polymer powder.The results indicate that SWNTs can uniformly be dispersed in HDPE,and consequently the composites exhibit a low electrical percolation threshold of4%SWNT loading and a low rheological one of1.5%.This difference in the electrical and rheological percolation thresholds can be understood in terms of the smaller nanotube–nanotube distance required for electrical conductivity as compared to that required to impede polymer mobility.Moreover,the measurements of mechanical properties indicate that SWNTs play a reinforcing role.Tensile strength and initial modulus greatly increase as increasing SWNTs in the matrix polymer,and values rise by65%and50%at a load-ing level of2.6wt%,respectively.AcknowledgementsWe thank Professor Oren Regev for valuable assistance in the dispersion of SWNTs in water.We are grateful to Dr.Xuejing Zheng and Dr.Xiaoniu Yang for their help in SEM and TEM observation.References[1]Ijima S,Ichihashi T.Single-shell carbon nanotubes of1-nm diameter.Nature1993;363:603–4.[2]Bethune DS,Kiang CH,de Vries MS,Gorman G,Savoy R,VazqueZ,et al.Cobalt-catalysed growth of carbon nanotubes with single-atomic-layer walls.Nature1993;363:605–6.[3]Ajayan PM.Nanotubes from carbon.Chem Rev1999;99:1787–800.[4]Baughman RH,Zakhidov AA,de Heer WA.Carbon nanotubes—theroute toward application.Science2002;297:787–92.[5]Kong J,Franklin N,Zhou C,Chapline A,Peng S,Cho K,et al.Nanotube molecular wires as chemical sensors.Science2000;287: 622–5.[6]Walters DA,Ericson LM,Casavant MJ,Liu J,Colbert DT,SmithKA,et al.Elastic strain of freely suspended single-wall carbon nanotube ropes.Appl Phys Lett1999;74:3803–5.[7]Jin L,Bower C,Zhou O.Alignment of carbon nanotubes in apolymer matrix by mechanical stretching.Appl Phys Lett1998;73:1197–9.[8]Liu M,Yang Y,Zhu T,Liu Z.Chemical modification of single-walledcarbon nanotubes with peroxytrifluoroacetic acid.Carbon2005;43:1470–8.[9]Eitan A,Jiang K,Dukes D,Andrews R,Schadler LS.Surfacemodification of multiwalled carbon nanotubes:toward the tailoring of the interface in polymer composites.Chem Mater2003;15: 3198–221.784Q.Zhang et al./Carbon44(2006)778–785[10]Hu H,Bhowmik P,Zhang B,Hamon MA,Itkis ME,Haddon RC.Determination of the acidic sites of purified single-walled carbon nanotubes by acid–base titration.Chem Phys Lett2001;345:25–8. [11]Viswanathan G,Chakrapani N,Yang H,Wei B,Chung H,Cho K,et al.Single-step in situ synthesis of polymer-grafted single-wall nanotube composites.J Am Chem Soc2003;125:9258–9.[12]An KH,Jeon KK,Moon JM,Eum SJ,Yang CW,Park GS,et al.Transformation of singlewalled carbon nanotubes to multiwalled carbon nanotubes and onion-like structures by nitric acid treatment.Synth Met2004;140:1–8.[13]Sreekumar TV,Liu T,Kumar S,Ericson LM,Hauge RH,SmalleyRE.Single-wall carbon nanotubefilms.Chem Mater2003;15:175–8.[14]Chen J,Hamon MA,Hu H,Chen Y,Rao AM,Eklund PC,et al.Solution properties of single-walled carbon nanotubes.Science 1998;282:95–8.[15]Star A,Stoddart JF,Steuerman D,Diehl M,Boukai A,Wang EW,et al.Preparation and properties of polymer-wrapped single-walled carbon nanotubes.Angew Chem Int Ed2001;40:1721–5.[16]Hill DE,Lin Y,Rao AM,Allard LF,Sun YP.Functionalization ofcarbon nanotubes with polystyrene.Macromolecules2002;35: 9466–71.[17]Grady BP,Pompeo F,Shambaugh RL,Resasco DE.Nucleation ofpolypropylene crystallization by single-walled carbon nanotubes.J Phys Chem B2002;106:5852–8.[18]Jin Z,Sun X,Xu G,Goh SH,Ji W.Nonlinear optical properties ofsome polymer/multi-walled carbon nanotube composites.Chem Phys Lett2000;318:505–10.[19]Lin Y,Zhou B,Fernando KAS,Liu P,Allard LF,Sun YP.Polymericcarbon nanocomposites from carbon nanotubes functionalized with matrix polymer.Macromolecules2003;36:7199–204.[20]Shaffer MSP,Windle AH.Fabrication and characterization of carbonnanotube/poly(vinyl alcohol)composites.Adv Mater1999;11:937–41.[21]Mitchell CA,Bahr JL,Arepalli S,Tour JM,Krishnamoorti R.Dispersion of functionalized carbon nanotubes in polystyrene.Macromolecules2002;35:8825–30.[22]Zhu J,Kim JD,Peng H,Margrave JL,Khabashesku VH,BarreraEV.Improving the dispersion and integration of single-walled carbon nanotubes in epoxy composites through functionalization.Nano Lett 2003;3:1107–13.[23]Wang Z,Liang Z,Wang B,Zhang C,Kramer L.Processing andproperty investigation of single-walled carbon nanotube(SWNT) buckypaper/epoxy resin matrix posites:A 2004;35:1225–32.[24]Barrau S,Demont P,Perez E,Peigney A,Laurent C,Lacabanne C.Effect of palmitic acid on the electrical conductivity of carbon nanotubes–epoxy resin composites.Macromolecules2003;36: 9678–80.[25]Islam MF,Eojas E,Bergey DM,Johnson AT,Yodh AG.Highweight fraction surfactant solubilization of single-wall carbon nano-tubes in water.Nano Lett2003;3:269–73.[26]Moore VC,Strano MS,Haroz EH,Hauge RH,Smalley RE.Individually suspended single-walled carbon nanotubes in various surfactants.Nano Lett2003;3:1379–82.[27]McQuade DT,Hegedus AH,Swager TM.Signal amplification of a‘‘turn-on’’Sensor:harvesting the light captured by a conjugated polymer.J Am Chem Soc2000;122:12389–90.[28]Levi N,Czerw R,Xing S,Iyer P,Carroll DL.Properties ofpolyvinylidene difluoride-carbon nanotube blends.Nano Lett 2004;4:1267–71.[29]Bandyopadhyaya R,Nativ-Roth E,Regev O,Yerushalmi-Rozen R.Stabilization of individual carbon nanotubes in aqueous solutions.Nano Lett2002;2:25–8.[30]OÕConnell MJ,Boul P,Ericson LM,Huffman C,Wang Y,Haroz E,et al.Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping.Chem Phys Lett2001;342: 265–71.[31]Chen J,Liu H,Weimer WA,Halls MD,Waldeck DH,Walker GC.Noncovalent engineering of carbon nanotube surfaces by rigid, functional conjugated polymers.J Am Chem Soc2002;124:9034–5.[32]Ramasubramaniam R,Chen J,Liu H.Homogeneous carbonnanotube polymer composites for electrical applications.Appl Phys Lett2003;83:2928–30.[33]Bhattacharyya AR,Potschke P,Abdel-Goad M,Fischer D.Effect ofencapsulated SWNT on the mechanical properties of melt mixed PA12/SWNT composites.Chem Phys Lett2004;392:28–33.[34]Zhang X,Liu T,Sreekumar TV,Kumar S,Moore VC,Hauge RH,et al.Poly(vinyl alcohol)/SWNT compositefilm.Nano Lett2003;3:1285–8.[35]Regev O,Elkati PNB,Loos J,Koning CE.Preparation of conductivenanotube–polymer composites using latex technology.Adv Mater 2004;16:248–51.[36]Kitano T,Kataoka T.The effect of the mixing methods on viscousproperties of polyethylene meltsfilled withfibers.Rheol Acta 1980;19:753–63.[37]Kitano T,Kataoka T,Nagatsuka Y.Shearflow rheological proper-ties of vinylon-and glass-fiber reinforced polyethylene melts.Rheol Acta1984;23:20–30.[38]Tang W,Santare MH,Advani SG.Melt processing and mechanicalproperty characterization of multi-walled carbon nanotube/high density polyethylene(MWNT/HDPE)compositefilms.Carbon 2003;41:2779–85.[39]Zhang QH,Chen QH.Conductive properties and morphology of thecomposites of conducting carbon black/polypropylene/EVA.J Mater Sci2004;39:1751–7.[40]Lozano K,Barrera EV.Nanofiber-reinforced thermoplastic compos-ites.I.Thermoanalytical and mechanical analyses.J Appl Polym Sci 2001;79:125–33.[41]Lozano K,Bonilla-Rios J,Barrera EV.A study on nanofiber-reinforced thermoplastic composites(II):investigation of the mixing rheology and conduction properties.J Appl Polym Sci2001;80:1162–72.[42]Bhattacharyya AR,Sreekumar TV,Liu T,Kumar S,Ericsonb LM,Hauge RH,et al.Crystallization and orientation studies in polypro-pylene/single wall carbon nanotube composite.Polymer2003;44: 2373–7.[43]Meincke O,Kaempfer D,Weickmann H,Friedrich C,Vathauer M,Warth H.Mechanical properties and electrical conductivity of carbon-nanotubefilled polyamide-6and its blends with acryloni-trile/butadiene/styrene.Polymer2004;45:739–48.[44]Song YS,Youn JR.Influence of dispersion states of carbonnanotubes on physical properties of epoxy nanocomposites.Carbon 2005;43:1378–85.[45]Kim YJ,Shin TS,Choi HD,Kwon JH,Chung YC,Yoon HG.Electrical conductivity of chemically modified multiwalled carbon nanotube/epoxy composites.Carbon2005;43:23–30.[46]Du F,Scogna RC,Zhou W,Brand S,Fischer JE,Winey KI.Nanotube networks in polymer nanocomposites:rheology and electrical conductivity.Macromolecules2004;37:9048–55.Q.Zhang et al./Carbon44(2006)778–785785。
EXPERIMENTAL RESEARCH OF REINFORCED CONCRETE COLUMNRETROFIT METHODSIntroductionAs the infrastructure of our country continues to age, the need for effective retrofittreatments has increased. Many building and bridge structural components no longerprovide capacity sufficient to meet the required code standards. Seismic upgrading andreinforcement protection are two of the major issues requiring retrofits. Additionally,many aging structural members no longer provide the load capacity of the original designbecause of concrete cracking, steel corrosion, or other damage. In this research, severalretrofit methods for increasing the axial load capacity of reinforced concrete columnswere tested and analyzed。
Several currently applied methods for retrofitting columns include concrete jacketing,steel jacketing, and fiber reinforced polymer (FRP) jacketing. All three methods havebeen shown to effectively in increase the axial load capacity of columns。
Creating value with digital art NFT席卷艺术界A new wave of creativity is taking the art world by virtual storm.In November, “NFT” 1 _________(choose) as 2021’s “Word of the Year” by Collins Dictionary. NFT is the abbreviation(缩略词)of Non-Fungible Token.According to the BBC, it is a “one-of-a-kind” asset in the digital world 2_______can be bought and sold like any other piece of property 3______has no tangible form”.Now, NFTs are becoming popular in the art world.Unlike the traditional artworks that are made by hand and have their own special technique, digital arts can be duplicated and 4_________(easy) obtained for free.But NFTs could provide a digital certificate, 5___________(show) ownership of a unique virtual or physical asset that someone has produced.“In the digital world, you wouldn’t know if something is a copy of something else primarily because the copy is almost perfect.The NFT was the first6 _________(prove) who owns th is property,” Along with protecting ownership, NFTs allow the creator to get a royalty payment each time the work is sold.As The Guardian noted, the artwork itself still 7__________(belong) to the creator,but the buyers can get limited rights to display the digital artwork.8______ whenever the NFTs are sold, each transaction can only be made with a corresponding transfer to the creator.“NFTs can ensure that creators, including artists or music composers, can have a portion of the revenue from every subsequent transfer, which could encourage their creations,”That’s why many analysts believe that “NFTs are a revolution for the art industry”, noted China Daily.According to the latest report by Reuters, in the first half of 2021, the sales of NFT digital artworks reached 2.5 billion dollars (about 16 billion yuan), 9______ increase from just 13.7 million dollars for the same period in 2020. US artist Mike Winkelmann said that with the help of NFT technology, the art field itself has entered uncharted territory.“I do see this 10 ______ the next chapter of art history,” Winkelmann told The Verge.The First 5000 Days, which is a collage of 5,000 images the artist captured.In March, it was sold for a record 69 million dollars.参考答案:1 was chosen 2 that 3 but 4 to prove 5 belongs 6 So 7 easily 8 showing 9 an 10 asAre these robots alive? 全球首个可繁殖活体机器人问世Living robots can reproduce on their own in a dish.This is not a science-fiction movie, 1 _____the result of a new research.The study 2 _____________(publish) in the Proceedings of the National Academy of Sciences in the US on Nov 29. Xenobots, 3 ______ type of tiny robot, were first created in 2020, 4_________(use) cells taken from the embryo of an African frog species.Under the right lab conditions, the cells formed small structures that could self-assemble, move in groups5 _______ react to their environment.Now, the researchers have found that xenobots can also self-replicate, according to the journal of New Scientist. Xenobots straddle an unusual line.Are they living organisms or robots? They are organisms 6 _________they are made of stem cells and can reproduce. But they are also robots because they can move on their own and perform physical labor, co-author Sam Kriegman told The Washington Post.“People have thought for quite a long time that we’ve worked out all the ways 7 _____ life can reproduce or replicate. But this is something that’s never been observed before,” co-author Douglas Blackiston, a senior scientist at Tufts University in the US, told Science Daily website. The ability to replicate 8 _______(add) a new layer of potential utility to the robots. Kriegman told The Washington Post that while xenobots are not yet 9 ___________( commercial) useful, they have the potential to provide a number of services, from cleaning up microplastics in the ocean to safely delivering drugs to a specific spot in a person’s body.However, the 10 _________(create) of xenobots comes with concerns. Some think more advanced future xenobots, could out-compete other species. The researchers think these risks are manageable.“If you change the amount of sodium in that water to be too high or too low, they’ll die,” Kriegman told The Washington Post. “If there’s a piece of copper in the dish, they’ll all die. It’s an extremely controllable and stoppable and safe system.”参考答案1 but 2 was published 3 a 4 using 5 and 6 because 7 that 8 adds 9 commercially 10 creationR estoring the past宁波“00后时光修复师”为烈士修复遗物“After taking off the tap e and glue and smoothing out the creases across the pages,the near hundred-year-old letters and martyr’s certificate were revitalized,”said Chen Hezhen, a 71-year-old grandmother from Ningbo, Zhejiang.On Nov 13, she received the _________(restore) memorial papers left by her father,who sacrificed his life on the battlefield when she was only 1 year old.She was grateful ____ a group of college students from Ningbo University of Finance & Economics (NUFE), who helped bring her precious memory to life.__________(repair) relics left by veterans and martyrs is a part of NUFE’s social practice project.Since June, these young people ___________(help) restore letters and other documents and returned them to families of revolutionary martyrs.“When we visited martyrs‘ families, we _________(hear) many inspiring stories and were touched by those late soldiers,”said Wang Yiqun, 21, the project’s leader.“Their family members have saved their letters in memory of them. _______we’ve found that those objects have different degrees of damage, so we want to do something to help them.”However, it isn’t easy to restore these relics.Pan Yi, 22, one young restorer, stared at a damaged page of a martyr’s letter with adhesive tape. For days, she worked to separate the tape and paper________(safe) . Her teacher helped her make a special degumming agent to spray on the tape, ________could then be peeled off by small tweezers little by little. After that, Pan carefully checked the holes and worn-out margins.She then stuck paper made of the same material over the damaged area with a brush full of special paste. As for the creases, Pan glued striped craft paper over them.“I need to be especially careful and patient because the process can take a few weeks just__________(repair) a single page,” said Pan.“What _____________(motivate) us is our faith – those old papers carrying the spirit of undaunted heroes and the feelings of mourning that their family members endure. We need to repair them as they were before.”Now, these students are preparing to hold an exhibition of restored relics, sharing their stories with more people.“I’m proud of our students because they can apply what they’ve learned to help familiesof martyrs and spread the heroic stories in a more vivid way.” said Cao Ming, their tutor.参考答案:1 restored 2 to 3 Repairing4 have helped5 heard6 But7 safely8 which9 to repair 10 motivates。
Management Studies, July-Aug. 2019, Vol. 7, No. 4, 354-360doi: 10.17265/2328-2185/2019.04.010Waste Cooking Oil as a Fuel Source for Diesel Engines*John PumwaThe Papua New Guinea University of Technology, Lae, Papua New GuineaAlmost all cities and towns in Papua New Guinea are producing tonnes of waste vegetable oils annually, mainlyfrom industrial deep fryers in potato processing plants, snack food factories, fast food restaurants, and institutionaldinning facilities. These waste vegetable oils are directed to waterways, rivers, and finally into the ocean whichdestroys the ocean shores and damages the environment. With increasing population, not only the demand forcooking oil will increase but also the environmental problems caused by the waste cooking oil. Most brands ofcooking oil that is used in Papua New Guinea are from locally produced palm oil. Palm oil consists mainly oftriglycerides made up of a range of fatty acids and contains other minor constituents, such as free fatty acids andnon-glyceride components. This composition determines the oil’s chemical and physical characteristics. This is anattempt to improve the waste vegetable oil’s chemical and physical characteristics that will allow the oil to be usedas an energy source and at the same time reduce the associated environmental problems. It has been observed thatthe waste cooking oil can be converted into a useful energy source using the transesterification process. Theconverted fuel has been tested and found its performance to be equivalent to petroleum diesel.All Rights Reserved.Keywords: vegetable oil, biofuel, biodiesel, renewable energy, triglycerides, transesterificationIntroductionIn many cities and towns in Papua New Guinea, there are tonnes of waste vegetable oil that is being produced annually from various commercial dinning facilities at the learning institutions, industrial deep fryers,and fast food restaurants etc. within the major cities and towns. With increasing population, not only thedemand for cooking oil will increase but also the environmental problems caused by the waste cooking oil. Inmany surveys conducted, restaurants may produce more than 1-liter of waste cooking oil per day and most ofthis oil is likely to be discharged into the sewers and water ways that may finally end up in the ocean that maydestroy the ocean shores and damage the environment. If discarded oil can be used as an energy source, it maynot only be an alternative energy source but also assist in reducing the cost of waste water treatments andgenerally assist in recycling of resources.When the fresh cooking oil is used for deep frying, the oils are generally heated to 160 °C -180 °C, and several changes occur such as oxidation and lowering of molecular weight due to hydrolytic release of fattyacids from the triglycerides and thermal decomposition, and polymerization with formation of cyclic*Acknowledgements: The author would like to express his sincere thanks and appreciation to the final year students especially,Albert Warkia, Ephraim Guan, Terence Sadua, and Rodney Donald for carrying out the experiment.John Pumwa, Ph.D., professor, head of department, Mechanical Engineering Department, the Papua New Guinea University ofTechnology, Lae, Papua New Guinea.Correspondence concerning this article should be addressed to John Pumwa, Mechanical Engineering Department, PNGUniversity of Technology, Private Mail Bag Service, MP 411, Lae, Papua New Guinea.WASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES 355compounds (Yusaf, 1998). Some characteristic changes occur such as bubble formation, coloration, andstability against oxidation. The viscosity and the density increases slightly and atomization characteristicsdeteriorate. However by filtering out suspended matter and taking appropriate cleaning action on the oil inorder to improve its quality, waste cooking oil may adequately be used as an energy source, and in this formthey may be very attractive, in regard to the cities and towns environmental maintenance and resource recyclingconsiderations. Waste cook ing oil’s properties can be improved to an equivalent of diesel fuel by using thetransesterification process and producing methyl esters.Like many other universities in the world, the Papua New Guinea University of Technology has more than 1,200 resident students who are fed from the university dinning facilities throughout their residential studyperiod. A large quantity of locally processed palm oil is used annually to prepare students meals, which end upas waste cooking oil. Papua New Guinea is one of the countries in the Asia Pacific region that produces palmoil as an export commodity as well as supplying the local market needs for cooking oil. Palm oil is derivedfrom the mesocarp of the oil palm fruit. Palm oil consists mainly of triglycerides made up of a range of fattyacids and also contains other minor constituents such as free fatty acids and non-glyceride components. Thiscomposition determines the oil’s chemical and physical characteristics.This paper reports an investigation that has been conducted to determine if the waste oil produced from the university student’s dinning facilities could be utilized as an energy source since the original oil used forcooking is locally produced palm oil by using the transesterification process and producing methyl esters.ExperimentationAll Rights Reserved.Materials and MethodsThe materials used for this project are mainly the waste cooking oil as the feedstock, which was supplied by the university’s dinning department. The waste cooking oil is produced as a waste after the university’sdinning department uses palm oil supplied by the local palm oil manufacturers for the preparation of the foodfor the students.The other secondary materials used in this project are small (1-2 mm) Pearl Caustic Soda are 99% + (Sodium Hydroxide-NaOH) or lye and 100% Methanol or Methyl Alcohol (CH3OH). Sodium hydroxide wasused as a catalyst in the transesterification process and it is hygroscopic—it absorbs water from the atmosphereand fresh sodium hydroxide or lye is essential and it has to be kept in a tightly sealed container. Similarly,methanol is required to convert triglycerides (fats and oils) into esters (biodiesel)—the “methyl”portion ofmethyl esters. Both of these chemicals are toxic and hazardous and it was handled and stored with great careand cautious.Test ApparatusThe biodiesel laboratory processor unit with a capacity of about 76-90-liters per batch consists of a single, multi-use tank system that is used to process, separate, wash, and filter the fuel. It is facilitated with a powerful,heavy-duty pump which serves as both as mixer and a transfer system as shown in Figure 1. The processor isalso facilitated with an electric heater that is used to heat the water before the hot water is circulated through theheating copper coil tubes in the processor to heat the waste cooking oil or the biodiesel to remove moisture.The biodiesel fuel produced from the waste cooking oil was tested for performance using a sisson single cylinder direct injection (DI), water-cooled diesel engine with a bore of 114-mm and a stroke length of 127-mmWASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES356 as shown in Figure 2. The engine is rated at a power capacity of 4.1-kW and a speed of 750-rpm with a Bryce Single piston injector pump and a pintle injector. The engine is coupled to a brake dynamometer for loading purposes with friction brake and graduated cylinder for measuring the fuel used.Figure 1. Biodiesel laboratory processor unit. Figure 2.Fuel performance testing apparatus. All Rights Reserved.WASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES 357Test ProceduresUnlike fresh oil, waste-cooking oil supplied from the u niversity’s restaurant contained a lot of dirt and solid waste materials that required a through cleaning and filtering. After vigorously cleaning and filtering thewaste cooking oil, the clean oil was then poured into the processor for processing using the transesterificationprocess.Because of the feedstock being waste cooking oil, it was important to determine the correct amount of catalyst to use to crack the fuel from the oil. In order to determine the accurate amount of catalyst required, atitration method was used. Using the detail titration procedures found in other literature (The Papua NewGuinea University of Technology, 2007), the required amount for sodium hydroxide (NaOH) was determinedto be about 5.5-grams per litre of waste cooking oil and about 0.23-liter of methanol (CH3OH) per litre of wastecooking oil. The required amount of methanol and sodium hydroxide was mixed in the polyethylene methoxidetank creating a sodium methoxide (Na+CH3O-) solution that was to be used to convert about 10-gallons ofwaste cooking oil. This is a strong chemical that is intended to crack the transfatty acid into glycerine and alsothe methyl ester chains or biodiesel fuel.The sodium methoxide was then injected into the processor with the waste cooking oil. Immediately after the injection of the methoxide, the circulation pump was used to vigorously mix the waste cooking oil with themethoxide for more than three hours before letting it settle overnight for the process to complete its reaction.After the reaction has completed, there was a clear distinct layers between the fuel and the glycerine as shownin Figure 3.All Rights Reserved.The glycerine was drained from the processor into other contains for further processing. The remaining fuel in the processor was washed using the fine water mist from the misting head installed in the centre of theprocessor lid. The water mist washing was necessary to remove soluble elements such as salts, soap, catalyst,and alcohols in the fuel. After washing, the fuel was left to settle into layers of water and fuel. After setting, thewater is then drained and the fuel is dried by heating the fuel to about 50 °C using the heating system of theWASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES358processor and holding it there for a period of time. This allows any excess water in the fuel to drop freely outand then drained out of the bottom of the processor. The final fuel is then pumped through a filter into a storagecontainer for further testing.Fuel Performance TestingThe sisson single cylinder engine was started using biodiesel fuel from the supply tank and allowed to run for at least 30 minutes to warm up the engine with no loading before testing the fuel with loading as per thelaboratory manual (The Papua New Guinea University of Technology, 2007). After the engine was warmed up,the engine was loaded by increasing the tension in the brake rope until the tension in the tight side reached themaximum loading of about 180-N.The governor was then adjusted in order to maintain the engine speed steady at 600-rpm. The biodiesel fuel supplied from the main tank was turned off and the time taken by the engine to use the 100-cc of biodieselfuel in the calibrated cylinder was measured. The valve was carefully turned back to open immediately after the100-cc of fuel was used, as the engine may cut out if the main fuel supply from the supply tank is notreconnected. The tension in the tight side of the rope was reduced with increments of about 20-N and repeatedthe procedure until the tension reached the minimum loading of 60-N. All necessary measurements were takenand recorded for further processing.Results and ObservationsThe biodiesel fuel produced from waste cooking oil appeared to be having some similarities with petroleum diesel fuel in terms of bubble formation. Moreover, when the fuel was tested in the engine, the All Rights Reserved.engine started without any difficulties and it continued running smoothly even after loading. Clean colourlessgases were observed to be coming out of the exhaust at loads less than 100-N. However, more light clean bluegases were observed to be coming out of the exhaust as the loads were increased.Using the data collected from the experiment, the brake power of the engine was determined for each loading. The results were plotted against the load as shown in Figure 4 for the three different types of fuel, 100%diesel fuel (DF), biodiesel blended with diesel fuel (B20), and 100% waste cooking oil biodiesel (B100) forcomparison. When the curves are compared, it appears from this linear relationship that there is a negligibledifference between the three fuels tested.Similarly, brake thermal efficiencies were determined for each loading and plotted against its loading as shown in Figure 5. Brake thermal efficiency is the ratio of the brake power over the rate of energy releasedfrom the fuel. When the curves are compared, it appears that the brake thermal efficiency for all fuels increasedas the loading increased to a specific loading before declining. The declining efficiency appears to be about100-N for the blends and the 100% waste oil biodiesel (B100) but for the 100% diesel fuel, the decliningefficiency appears to be about 125-N. Blended (B20) fuel has the highest brake thermal efficiency followed by100% diesel fuel and then 100% waste oil biodiesel.Also, the specific fuel consumption was determined for each loading and plotted against its loading for the three different fuel types for comparison as shown in Figure 6. Note that specific fuel consumption is the massof fuel consumed by the engine to produce one unit of power per hour (kg/kWh). The curves generally appearto be decreasing as the load increases until it reaches a loading of about 100-N when it begins to increase to itsmaximum load. When the curves are compared, it appears that the specific fuel consumption of the 100% wasteWASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES 359oil biodiesel (B100) is the lowest at maximum load with the 100% diesel fuel being the next lowest and blends (B20) having the highest.Figure 4. Brake power vs. load. Figure 5. Brake thermal efficiency vs. load. Figure 6.Specific fuel consumption vs. load. All Rights Reserved.WASTE COOKING OIL AS A FUEL SOURCE FOR DIESEL ENGINES360The results of the three different fuel types appear to indicate that there is a negligible difference at low and high loads but there is a slight difference at loads ranging from 80-N to about 145-N. This may be due tothe difference in the viscosity and heating value of the three different fuel types. Generally, it appears that thebiodiesel fuel produced from waste cooking oil is compatible with petroleum diesel and it can be used in adiesel engine without any modifications.ConclusionsA successful attempt has been made to make use of the waste cooking oil from the student’s dinningfacilities as an energy source. The results are summarized as follows:1. The waste cooking oil has been successfully converted as an energy source.2. The biodiesel fuel produced from waste cooking oil is comparable with petroleum diesel.3. Visual inspection of the exhaust gases indicates clean and environmentally friendly emissions beingreleased from the exhaust system without any thick smoke.ReferencesDepartment of Mechanical Engineering, The Papua New Guinea University of Technology, Lae, Papua New Guinea. (2007).Laboratory coordinator, laboratory manual.Kopial, T., Pumwa, J., & Turlom, S. (August 29-September 3, 2004). Effects of reducing coconut oil viscosity on engine performance. The Proceedings of the World Renewable Energy (WRE) Congress VIII and Expo, Denver, Colorado, USA.Pumwa, J. (December 10, 2004). The investigation of using coconut oil as a possible fuel substitute for diesel engines. A Research Report, Department of Engineering, School of Engineering & Computer Science, Baylor University, Waco, Texas, USA.All Rights Reserved.Pumwa, J. (August 19-25, 2006). Production of bio-diesel using virgin coconut oil as feedstock. The Proceedings of the World Renewable Energy Congress—IX (WREC), Florence, Itally.Yusaf, F. T. (1998). A report on the performance and emission from a commercial high speed diesel engine fuelled with waste cooking oil (palm oil) methyl ester. College of Engineering, University of Tenaga Nasional, Km 7 Jalan Kajang-Puchong,43009 Kajang, Selangor, Malaysia.。