【2013】信息-吡嗪酰胺片 美国 DAVA Pharmaceuticals , Inc
- 格式:pdf
- 大小:199.47 KB
- 文档页数:9

今年FDA批准的新药数量上少于去年,但质量上却高于去年,其中10个有重磅潜力。
1. Nesina:苯甲酸阿格列汀片阿格列汀(alogliptin)是Takeda研发的新型DPP-4抑制剂,用于治疗II型糖尿病,已获得FDA批准的同类药物还有西他列汀(sitagliptin)、沙格列汀(saxagliptin)、利拉利汀(Linagliptin),另外维格列汀(vildagliptin)已在欧洲上市。
此外,FDA还一同批准了两个含阿格列汀的复方制剂,即Oseni(阿格列汀/吡格列酮)和Kazano (阿格列汀/二甲双胍)。
在14项涉及8500名II型糖尿病患者的临床试验中,相比于安慰剂,Nesina能额外降低糖化血红蛋白(HbA1c)0.4%-0.6%。
在4项涉及2500名II型糖尿病患者的临床试验中,相比于二甲双胍,Kazano 能额外降低HbA1c 0.5%。
在4项涉及1500名II型糖尿病患者的临床试验中,相比于吡格列酮,Kazano能额外降低HbA1c 0.4%-0.6%。
2. Kynamro:米泊美生钠注射液米泊美生(mipomersen)是Genzyme研发的一种合成的硫代磷酸寡核苷酸,被FDA批准用于治疗纯合子型家族性高胆固醇血症(homozygous familial hypercholesterolemia,FoFH)。
作为反义核酸类药物,米泊美生通过与Apo B-100蛋白mRNA的编码区互补配对,抑制Apo B-100蛋白(LDL和VLDL的主要载脂蛋白)的翻译合成,降低FoFH患者的LDL-C、TC、Non-HDL-C水平。
在为期26周涉及56名FoFH的多国随机对照试验中,治疗组平均LDL-C、TC、Apo B、Non-HDL-C、TG水平分别降低25、21、27、25、18 mg/dL,平均HDL-C水平增加15,而安慰剂组各项指标变化均在5 mg/dL以内。
值得注意的是,该药说明书中有一黑框警告,须警惕肝毒性。
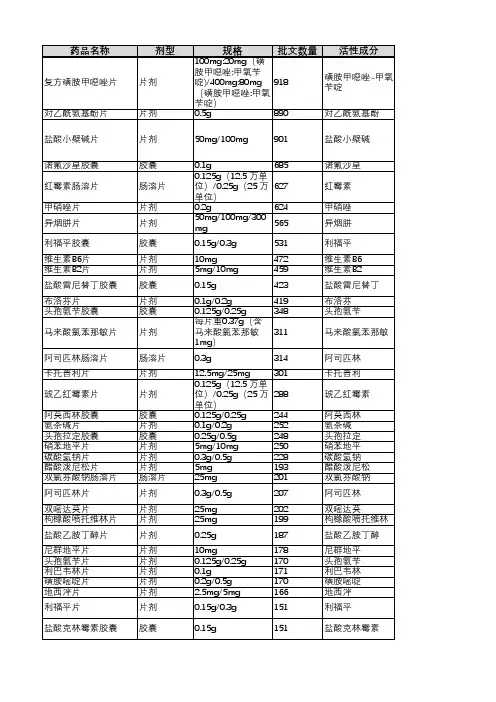
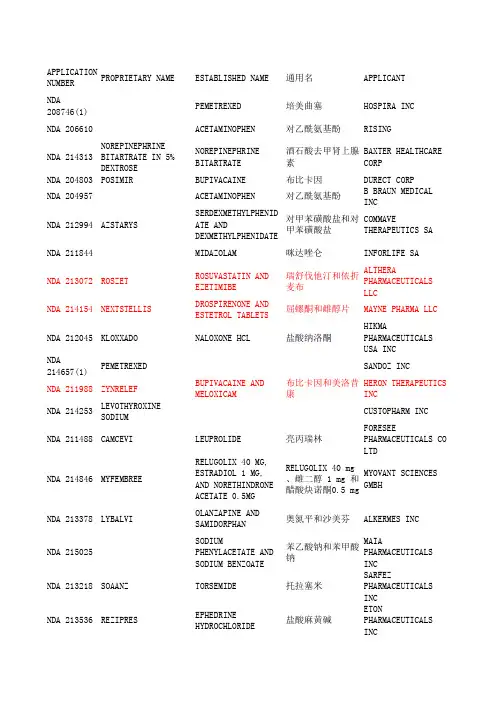
APPLICATIONNUMBERPROPRIETARY NAME ESTABLISHED NAME通用名APPLICANTNDA208746(1)PEMETREXED培美曲塞HOSPIRA INC NDA 206610ACETAMINOPHEN对乙酰氨基酚RISINGNDA 214313NOREPINEPHRINEBITARTRATE IN 5%DEXTROSENOREPINEPHRINEBITARTRATE酒石酸去甲肾上腺素BAXTER HEALTHCARECORPNDA 204803POSIMIR BUPIVACAINE布比卡因DURECT CORPNDA 204957ACETAMINOPHEN对乙酰氨基酚B BRAUN MEDICAL INCNDA 212994AZSTARYS SERDEXMETHYLPHENIDATE ANDDEXMETHYLPHENIDATE对甲苯磺酸盐和对甲苯磺酸盐COMMAVETHERAPEUTICS SANDA 211844MIDAZOLAM咪达唑仑INFORLIFE SANDA 213072ROSZET ROSUVASTATIN ANDEZETIMIBE瑞舒伐他汀和依折麦布ALTHERAPHARMACEUTICALSLLCNDA 214154NEXTSTELLIS DROSPIRENONE ANDESTETROL TABLETS屈螺酮和雌醇片MAYNE PHARMA LLCNDA 212045KLOXXADO NALOXONE HCL盐酸纳洛酮HIKMA PHARMACEUTICALS USA INCNDA214657(1)PEMETREXED SANDOZ INCNDA 211988ZYNRELEF BUPIVACAINE ANDMELOXICAM布比卡因和美洛昔康HERON THERAPEUTICSINCNDA 214253LEVOTHYROXINESODIUMCUSTOPHARM INCNDA 211488CAMCEVI LEUPROLIDE亮丙瑞林FORESEE PHARMACEUTICALS CO LTDNDA 214846MYFEMBREE RELUGOLIX 40 MG,ESTRADIOL 1 MG,AND NORETHINDRONEACETATE 0.5MGRELUGOLIX 40 mg、雌二醇 1 mg 和醋酸炔诺酮0.5 mgMYOVANT SCIENCESGMBHNDA 213378LYBALVI OLANZAPINE ANDSAMIDORPHAN奥氮平和沙美芬ALKERMES INCNDA 215025SODIUMPHENYLACETATE ANDSODIUM BENZOATE苯乙酸钠和苯甲酸钠MAIAPHARMACEUTICALSINCNDA 213218SOAANZ TORSEMIDE托拉塞米SARFEZ PHARMACEUTICALS INCNDA 213536REZIPRES EPHEDRINEHYDROCHLORIDE盐酸麻黄碱ETONPHARMACEUTICALSINCNDA 212156MICAFUNGIN米卡芬净PAR STERILE PRODUCTS LLCNDA 210282DAPTOMYCIN达托霉素HOSPIRA INC NDA 214965VERKAZIA CYCLOSPORINE环孢素SANTEN INCNDA 212303(1)DULUTEGRAVIR,LAMIVUDINE, ANDTENOFOVIRDISOPROXIL度替拉韦、拉米夫定和富马酸替诺福韦酯LUPIN LTDNDA 214902TWYNEO TRETINOIN ANDBENZOYL PEROXIDE维 A 酸和过氧化苯甲酰SOL-GELTECHNOLOGIES LTDNDA 215143SUCCINYLCHOLINECHLORIDE琥珀胆碱氯化物HIKMAPHARMACEUTICALSUSA INCNDA 210735CYCLOPHOSPHAMIDE环磷酰胺EUGIA PHARMA SPECIALITIES LTDNDA 213895VANCOMYCIN万古霉素XELLIA PHARMACEUTICALS APSNDA 214826LOREEV XR LORAZEPAM劳拉西泮ALMATICA PHARMA LLCNDA 211566(1)SITAGLIPTIN西他列汀ZYDUS WORLDWIDEDMCCNDA 213436TRUDHESA DIHYDROERGOTAMINEMESYLATE甲磺酸二氢麦角胺IMPEL NEUROPHARMANDA 215133SERTRALINEHYDROCHLORIDE盐酸舍曲林ALMATICA PHARMALLCNDA 212854ZIMHI NALOXONEHYDROCHLORIDE盐酸纳洛酮ADAMISPHARMACEUTICALSCORPNDA 213426SEGLENTIS CELECOXIB ANDTRAMADOLHYDROCHLORIDE塞来昔布和盐酸曲马多KOWAPHARMACEUTICALSAMERICA INCNDA 213978TYRVAYA VARENICLINESOLUTION伐尼克兰溶液OYSTER POINTPHARMA INCNDA 211950XIPERE TRIAMCINOLONEACETONIDE曲安奈德BAUSCH AND LOMBINCNDA 214028VUITY PILOCARPINEHYDROCHLORIDE盐酸匹洛卡品ABBVIE INCNDA 210526DYANAVEL XR AMPHETAMINE安非他明TRIS PHARMA INCNDA 213005(1)YUTREPIA TREPROSTINIL曲前列尼尔LIQUIDIATECHNOLOGIESNDA 214679EPRONTIA TOPIRAMATE托吡酯AZURITY PHARMACEUTICALS INCNDA 214869DHIVY CARBIDOPA ANDLEVODOPACARBIDOPA 和左旋多巴AVIONPHARMACEUTICALSLLCNDA 215668(1)BENDAMUSTINEHYDROCHLORIDE盐酸苯达莫司汀DR REDDYSLABORATORIES LTDNDA 213312FYARRO SIROLIMUS PROTEIN-BOUND PARTICLESSIROLIMUS 蛋白结合颗粒AADI BIOSCIENCEINCNDA 215422LYVISPAH BACLOFEN巴氯芬SAOL THERAPEUTICS RESEARCH LTDNDA 215650XACIATO CLINDAMYCINPHOSPHATE磷酸克林霉素DARE BIOSCIENCEINCNDA 215423ENTADFI TADALAFIL ANDFINASTERIDE他达拉非和非那雄胺VERU INCNDA 215935TARPEYO BUDESONIDE布地奈德CALLIDITAS THERAPEUTICS ABNDA 215019DARTISLA ODT GLYCOPYRROLATE甘氨酰吡咯酸EDENBRIDGE PHARMACEUTICALS LLCNDA 2I4032ILLUCCIX KIT FOR THEPREPARATION OF GA-68 PSMA-11GA-68 PSMA-11 制备试剂盒TELIXPHARMACEUTICALS USINCNDA 215395LANREOTIDE ACETATE醋酸来那度胺INVAGEN PHARMACEUTICALS INCNDA 214133RECORLEV LEVOKETOCONAZOLE左酮康唑STRONGBRIDGE DUBLIN LTDREVIEW CLASSIFI CATION 505(B)(2)APPROVALAPPROVAL DATE批准类型S Y1/8/2021Type 3 - New Dosage FormS Y1/15/2021Type 5 - New Formulation or New ManufacturerS Y1/15/2021Type 5 - New Formulation or New ManufacturerS Y2/1/2021Type 3 - New Dosage FormS Y2/18/2021Type 5 - New Formulation or New ManufacturerS Y3/2/2021Type 1 - New Molecular Entity and Type 4 - New CombinationS Y3/22/2021Type 5 - New Formulation or New ManufacturerS Y3/23/2021Type 4 - New CombinationS Y4/15/2021Type 1 - New Molecular EntityS Y4/29/2021Type 5 - New Formulation or New ManufacturerS Y5/6/2021Type 5 - New Formulation or New ManufacturerP Y5/12/2021Type 4 - New CombinationS Y5/17/2021Type 5 - New Formulation or New ManufacturerS Y5/25/2021Type 2 - New Active IngredientS Y5/26/2021Type 4 - New CombinationS Y5/28/2021Type 1 - New Molecular Entity and Type 4 - New CombinationS Y6/10/2021Type 5 - New Formulation or New ManufacturerS Y6/14/2021Type 5 - New Formulation or New ManufacturerS Y6/14/2021Type 5 - New Formulation or New ManufacturerNew ManufacturerS Y6/21/2021Type 5 - New Formulation or New ManufacturerS,O Y6/23/2021Type 5 - New Formulation or New ManufacturerS Y6/25/2021Type 4 - New Combination S Y7/26/2021Type 4 - New CombinationS Y8/20/2021Type 5 - New Formulation or New ManufacturerS Y8/25/2021Type 5 - New Formulation or New ManufacturerP Y8/26/2021Type 5 - New Formulation or New ManufacturerS Y8/27/2021Type 3 - New Dosage FormS Y9/2/2021Type 2 - New Active IngredientS Y9/2/2021Type 5 - New Formulation or New ManufacturerS Y10/4/2021Type 3 - New Dosage FormS Y10/15/2021Type 5 - New Formulation or New ManufacturerS Y10/15/2021Type 4 - New Combination S Y10/15/2021Type 3 - New Dosage Form S Y10/22/2021Type 3 - New Dosage FormS Y10/28/2021Type 5 - New Formulation or New ManufacturerS Y11/4/2021Type 3 - New Dosage Form S Y11/4/2021Type 3 - New Dosage FormS Y11/5/2021Type 3 - New Dosage FormS Y11/12/2021Type 5 - New Formulation or New ManufacturerNew ManufacturerP,O Y11/22/2021Type 5 - New Formulation or New ManufacturerS Y11/22/2021Type 3 - New Dosage FormP Y12/7/2021Type 5 - New Formulation or New ManufacturerS Y12/9/2021Type 4 - New CombinationP,O Y12/15/2021Type 5 - New Formulation or New ManufacturerS Y12/16/2021Type 3 - New Dosage FormS Y12/17/2021Type 3 - New Dosage Form and Type 4 - New CombinationS Y12/17/2021Type 5 - New Formulation or New ManufacturerS,O Y12/30/2021Type 2 - New Active Ingredient适应症NSCLC、Mesothelioma间皮瘤(注射剂)止痛、退烧(注射剂)升高低血压(注射剂)局部麻醉(注射剂)止痛、退烧CNS兴奋剂注射麻醉剂降低LDL-C预防妊娠阿片类拮抗剂,用于阿片类过量NSCLC、Mesothelioma间皮瘤术后止痛(注射剂)粘液性水肿昏迷myxedema coma晚期前列腺癌子宫平滑肌瘤月经量过多成人精神分裂症、成人双相 i 型障碍急性高血氨症成人心力衰竭、肾病相关水肿麻醉状态的低血压念珠菌血症、急性播散性念珠菌病、念珠菌腹膜炎和脓肿cSSSI、Bacteremia菌血症春季角结膜炎(Tentative Approval)痤疮局部治疗作为全身麻醉的辅助治疗、便于气管插管、在手术或机械通气期间提供骨骼肌松弛label not available败血症、感染性心内膜炎、皮肤和皮肤结构感染、骨感染、下呼吸道感染、艰难梭菌相关性腹泻、金黄色葡萄球菌引起的小肠结肠炎label not availablelabel not available偏头痛急性治疗MDD、OCD紧急治疗阿片类药物过量治疗需要阿片类镇痛剂且替代治疗效果不佳的成人急性疼痛。

盘点:近20年~那些化学结构“有意思”的42个上市药物(上篇)一个能够获批上市的药物分子,他的脚下往往铺垫着成千上万的分子基石,故每个成药分子都有他背后可圈可点且值得学习的故事。
今天,在我们绞尽脑汁探索“A口袋” 、“B 口袋”、“疏水作用”、“亲水作用”的同时,总是会有一些化学结构“出人意料”的药物分子脱颖而出,并成功上市,其成绩着实让人羡慕,其结构也着实让人感叹。
笔者通过查询近20年上市药物的分子结构,总结了42个化学结构“很有意思”的药物分子,附之结构、药物简介、以及部分合成信息,望各位同仁茶余饭后之际,进一步开拓药物开发设计的思路。
虽研发方向不同、靶点不同、设计思路不同,但毕竟这些分子都成药了,还是很值得推敲借鉴的。
以下总结的这42个药物,将按照上市时间进行排列,细述。
1. NalbuphineSebacate(那布扶林)入选理由:1)结构对称;2)“老结构”再用。
药物简介:那布扶林,由LumosaTherapeutics 研发,2017年年3月获台湾卫生福利部食品药物管理局批准上市,商品名为Naldebain?;那布扶林为μ-受体拮抗剂,同时也是κ-受体激动剂,用于缓解术后中、重度急性疼痛;纳疼解?活性成分已取得台、美、欧、韩、日、中等世界先进国与主要市场的专利保护;纳疼解?是一种长效剂型,肌肉注射后缓慢释出主要成分那布扶林。
合成简介:参考:J. Chromatogr. B , 2000, 746, 241-247;US6225321。
2. Lutetium(177Lu) oxodotreotide入选理由:1)引入元素Lu-177;2)引入二硫键;3)多个手性中心;4)裸露氨基、羟基、羧基等。
药物简介:Lutetium(177Lu) oxodotreotide,由Advanced AcceleratorApplications研发,2017年9月26日获欧洲EMA 批准上市,2018年1月26日获美国FDA批准上市,由Advanced Accelerator Applications在欧盟和美国上市销售,商品名为Lutathera?;Lutetium (177Lu)oxodotreotide是一种Lu-177标记的生长抑素类似物,可与生长抑素受体结合,尤其对生长抑素受体2(SSRT2)亲和力最高;Lutathera?获批用于治疗生长抑素受体阳性的胃肠胰腺神经内分泌肿瘤。

烟酰胺片Nicotinamide【警示语】当药品性状发生改变时禁止服用,请将此药品放在儿童不能接触的地方【成份】本品主要成份为烟酰胺,其化学名称为3-吡啶甲酰胺。
化学结构式:分子式:C6H6N2O;分子量:122.13【性状】本品为白色片。
【适应症】用于防治糙皮病等烟酸缺乏病。
【规格】50mg【用法用量】推荐膳食每日摄入量,初生-3岁为5-9mg,4岁-6岁为12mg,7岁-10岁为13mg,男性青少年及成人为15?20mg,女性青少年及成人为13?15mg,孕妇为17mg,乳母为20mg。
口服:防治糙皮病,每次50-200mg(1?4片),每日500mg(10片)。
【不良反应】个别有头昏、恶心、上腹不适、食欲不振等。
【禁忌】尚未明确。
【注意事项】烟酰胺无扩张血管作用,高血压患者需要时可用烟酰胺。
【孕妇及哺乳期妇女用药】妊娠初期过量服用有致畸的可能。
【儿童用药】尚未明确。
【老年患者用药】尚未明确。
【药物过量】用量超过需要量时排泄增多。
【药理毒理】本品在体内与核糖、磷酸、腺嘌呤形成烟酰胺腺嘌呤二核苷酸(辅酶I)和烟酰胺腺嘌呤二核苷酸磷酸(辅酶II),为脂质代谢、组织呼吸的氧化作用和糖原分解所必需,缺乏时可影响细胞的正常呼吸和代谢而引起糙皮病。
【药代动力学】胃肠道易吸收,吸收后分布到全身组织,半衰期(t1/2)约为45分钟。
经肝脏代谢,治疗量仅少量以原形自尿排出。
【药物相互作用】烟酰胺与异烟肼有拮抗作用,长期服用异烟肼时,应适当补充烟酰胺。
【妊娠分级】FDA妊娠分级:A;C-如剂量超过美国的每日推荐摄入量。

美国专利到期的药物精选一、精神、神经系统药类1、郁复伸中文通用名:文拉法辛;别名:维拉法辛。
英文通用名:venlafaxine ;英文商品名:Effexor。
药品简介:文拉法辛是中枢神经系统药物,用于治疗精神失常、躁狂抑郁症和抑郁症。
由惠氏公司开发并于1994 年4 月首次在美国上市,此后相继在加拿大、丹麦、英国、意大利、澳大利亚等国上市。
文拉法辛具有临床疗效好、安全性高和治疗成本较低等特点,其2002 年全球销售约为21 亿美元,位列药品销售400 强的第21 位。
美国专利名称:2-苯基-2-(1-羟基环炔基或1-羟基环烷基-2-烯基) 乙胺衍生物(专利号:US4535186)专利权人:American Home Prod注:该专利到期日:2007 年12 月13 日(该专利申请日:1983 年10 月26 日;原来到期日:2002 年12 月13日;后被批准延长5 年) 。
该专利因获得美国儿科药市场独占,到期日延至2008 年6 月13 日。
同族专利: US4535186[1985208213 ]2、罗匹尼罗英文通用名:Ropinirole ;英文商品名:ReQuip 。
药品简介:罗匹尼罗于1997 年首次被批准用于帕金森病,是一种类似多巴胺的多巴胺激动剂,与第一代多巴胺激动剂不同的是其没有麦角林结构。
因为多巴胺激动剂较少引起运动不良反应,2001 年7 月,新的帕金森病治疗指南建议用多巴胺激动剂如葛兰素- 史克公司的罗匹尼罗(ropinirole ,ReQuip ○R ) 代替左旋多巴作为疾病早期的初始一线治疗用药。
2005 年美国食品药品管理局(FDA) 批准罗匹尼罗用于治疗中度到重度的多动腿综合征(RLS) 。
美国专利名称:42氨烷基22 (3H)2吲哚酮类化合物(专利号:US4452808 )专利权人: Smithkline Beckman Corp该专利到期日:2007 年12 月7 日(该专利申请日:1982 年12 月7 日;原来到期日:2002 年12 月7 日;后被批准延长5 年) 。
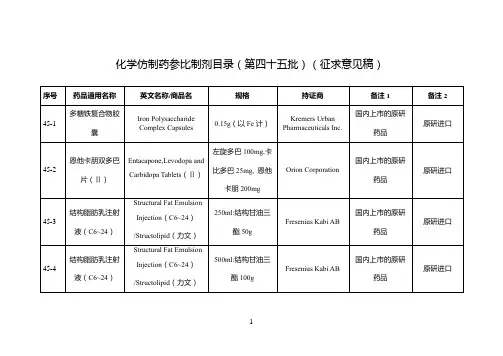
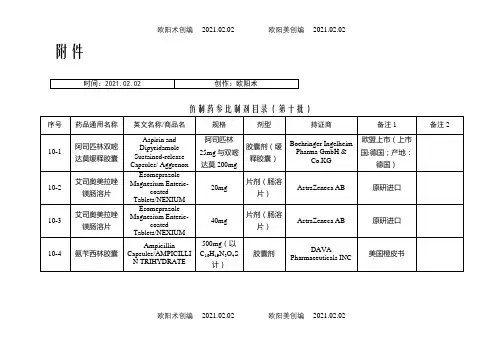
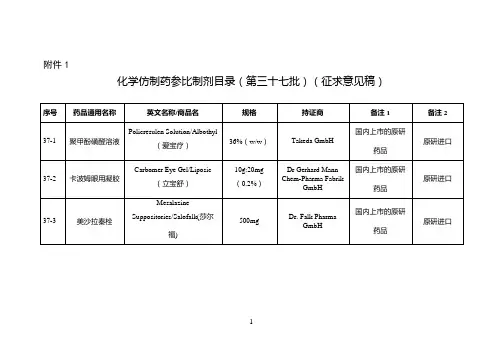
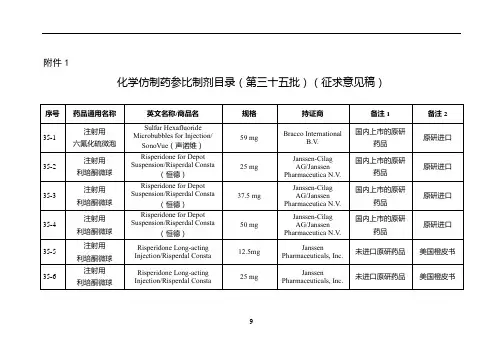
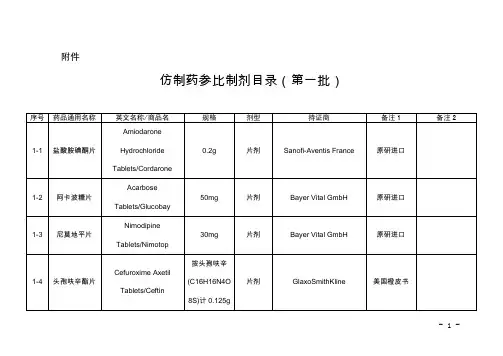
附件仿制药参比制剂目录(第十批)欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02序号药品通用名称英文名称/商品名规格剂型持证商备注1 备注210-145 头孢丙烯干混悬剂Cefprozil forSuspension/--0.125g/5ml(按C18H19N305S计)口服混悬剂(干混悬剂)Lupin LTD 美国橙皮书10-146 头孢丙烯片Cefprozil Tablets/--0.5g(按C18H19N305S计)片剂Lupin LTD 美国橙皮书10-147 头孢丙烯片Cefprozil Tablets/--0.25g(按C18H19N305S计)片剂Lupin LTD 美国橙皮书10-148 头孢泊肟酯颗粒Cefpodoxime ProxetilGranules/ORELOXENFANTS ETNOURRISSONS40mg/5ml 颗粒剂SANOFI-AVENTISFRANCE欧洲上市(上市国家:法国;产地:法国)法国上市名称:Cefpodoximeproxétilgranulés poursuspensionbuvable10-149 头孢泊肟酯片Cefpodoxime ProxetilTablets/BANAN100mg(按C15H17N5O6S2计)片剂Daiichi Sankyo Co.,Ltd.日本橙皮书10-150 头孢泊肟酯片Cefpodoxime ProxetilTablets/Orelox200mg(按C15H17N5O6S2计)片剂Daiichi SankyoEurope GmbH欧洲上市(上市国家:德国;产地:德国)商品名为Podomexef的产品亦可欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02欧阳术创编 2021.02.02 欧阳美创编 2021.02.02。
通用名生产企业紫杉醇注射液澳大利亚Hospira Australia Pty Ltd注射用盐酸吡柔比星(冻干)深圳万乐药业有限公司七叶皂苷钠片(薄膜衣)山东绿叶制药有限公司注射用七叶皂苷钠(冻干)山东绿叶制药有限公司注射用醋酸曲普瑞林(冻干)法国Ipsen Pharma Biotech马来酸桂哌齐特注射液北京四环制药有限公司头孢克洛胶囊礼来苏州制药有限公司头孢克洛干混悬剂礼来苏州制药有限公司头孢克洛缓释片(Ⅱ)礼来苏州制药有限公司阿德福韦酯片天津药物研究院药业有限责任公司注射用阿莫西林钠舒巴坦钠阿根廷Laboratorios Bago S.A盐酸西替利嗪片(薄膜衣)瑞士 UCB FARCHIM S.A.盐酸西替利嗪滴剂意大利UCB Pharma S.p.A.盐酸左西替利嗪片(薄膜衣)瑞士 UCB FARCHIM S.A.左乙拉西坦片(薄膜衣)比利时UCB S.A盐酸曲马多缓释片意大利Farmaceutici Formenti S.P.A.
非诺贝特片(Ⅲ)(薄膜衣)法国利博福尼制药公司(Laboratoires FournierSA)匹维溴铵片(薄膜衣)法国苏威(Solvay pharma)胰酶肠溶胶囊德国苏威制药马来酸氟伏沙明片(薄膜衣)荷兰苏威制药公司氯雷他定糖浆比利时先灵葆雅制药厂糠酸莫米松鼻喷雾剂比利时先灵葆雅制药厂去氧孕烯炔雌醇片荷兰欧加农公司N.V.Organon依折麦布片新加坡SCHERING-PLOUGH(SINGAPORE)PTE LTD氟他胺片加拿大先灵葆雅公司(Schering-Plough Canada)乳果糖口服溶液荷兰苏威制药公司
夫西地酸滴眼液丹麦利奥制药有限公司(Leo PharmaceuticalProducts)贝美前列素滴眼液美国Allergan,Inc注射用洛铂(冻干)海南长安国际制药有限公司
布林佐胺滴眼液美国爱尔康眼药厂比利时分厂s.a. ALCON-COUVREUR n.v.
PYRAZINAMIDE- pyrazinamide tablet DAVA Pharmaceuticals, Inc.----------PYRAZINAMIDE TABLETS, USP 500 mg Rx Only DESCRIPTIONPyrazinamide, the pyrazine analogue of nicotinamide, is an antituberculous agent. It is a white crystalline powder, stable at room temperature, and sparingly soluble in water. Pyrazinamide has the followingstructural formula:C H N O M.W. 123.11Each Pyrazinamide tablet for oral administration contains 500 mg of pyrazinamide and the following inactive ingredients: Corn Starch, Magnesium Stearate, Pregelatinized Starch and Stearic Acid.CLINICAL PHARMACOLOGYPyrazinamide is well absorbed from the GI tract and attains peak plasma concentrations within 2 hours.Plasma concentrations generally range from 30 to 50 mcg/mL with doses of 20 to 25 mg/kg. It is widely distributed in body tissues and fluids including the liver, lungs and cerebrospinal fluid (CSF). The CSF concentration is approximately equal to concurrent steady-state plasma concentrations in patients with inflamed meninges. Pyrazinamide is approximately 10% bound to plasma proteins.The half-life (t1/2) of pyrazinamide is 9 to 10 hours in patients with normal renal and hepatic function.The plasma half-life may be prolonged in patients with impaired renal or hepatic function. Pyrazinamide is hydrolyzed in the liver to its major active metabolite, pyrazinoic acid. Pyrazinoic acid is hydroxylated to the main excretory product, 5-hydroxypyrazinoic acid.Approximately 70% of an oral dose is excreted in urine, mainly by glomerular filtration within 24hours.Pyrazinamide may be bacteriostatic or bactericidal against Mycobacterium tuberculosis depending on the concentration of the drug attained at the site of infection. The mechanism of action is unknown. In vitro and in vivo the drug is active only at a slightly acidic pH.INDICATIONS AND USAGEPyrazinamide is indicated for the initial treatment of active tuberculosis in adults and children when combined with other antituberculous agents. (The current recommendation of the CDC for drug-susceptible disease is to use a six-month regimen for initial treatment of active tuberculosis, consisting of isoniazid, rifampin and pyrazinamide given for 2 months, followed by isoniazid and rifampin for 455312334months.*)(Patients with drug-resistant disease should be treated with regimens individualized to their situation.Pyrazinamide frequently will be an important component of such therapy.)(In patients with concomitant HIV infection, the physician should be aware of current recommendations of CDC. It is possible these patients may require a longer course of treatment.)It is also indicated after treatment failure with other primary drugs in any form of active tuberculosis.Pyrazinamide should only be used in conjunction with other effective antituberculous agents.*See recommendations of Center for Disease Control (CDC) and American Thoracic Society for complete regimen and dosage recommendations.CONTRAINDICATIONSPyrazinamide is contraindicated in persons:•••WARNINGSPatients started on pyrazinamide should have baseline serum uric acid and liver function determinations.Those patients with preexisting liver disease or those at increased risk for drug related hepatitis (e.g.,alcohol abusers) should be followed closely.Pyrazinamide should be discontinued and not be resumed if signs of hepatocellular damage or hyperuricemia accompanied by an acute gouty arthritis appear.PRECAUTIONS GeneralPyrazinamide inhibits renal excretion of urates, frequently resulting in hyperuricemia which is usually asymptomatic. If hyperuricemia is accompanied by acute gouty arthritis, pyrazinamide should be discontinued.Pyrazinamide should be used with caution in patients with a history of diabetes mellitus, as management may be more difficult.Primary resistance of M. tuberculosis to pyrazinamide is uncommon. In cases with known or suspected drug resistance, in vitro susceptibility tests with recent cultures of M. tuberculosis against pyrazinamide and the usual primary drugs should be performed. There are few reliable in vitro tests for pyrazinamide resistance. A reference laboratory capable of performing these studies must be rmation for PatientsPatients should be instructed to notify their physicians promptly if they experience any of the following:fever, loss of appetite, malaise, nausea and vomiting, darkened urine, yellowish discoloration of the skin and eyes, pain or swelling of the joints.Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be boratory Tests4 4with severe hepatic damage.who have shown hypersensitivity to it.with acute gout.Baseline liver function studies [especially ALT (SGPT), AST (SGOT) determinations] and uric acid levels should be determined prior to therapy. Appropriate laboratory testing should be performed at periodic intervals and if any clinical signs or symptoms occur during therapy.Drug/Laboratory Test InteractionsPyrazinamide has been reported to interfere with ACETEST and KETOSTIX urine tests to produce a pink-brown color.Carcinogenicity, Mutagenicity, Impairment of Fertility In lifetime bioassays in rats and mice, pyrazinamide was administered in the diet at concentrations of up to 10,000 ppm. This resulted in estimated daily doses for the mouse of 2 g/kg, or 40 times the maximum human dose, and for the rat of 0.5 g/kg, or 10 times the maximum human dose. Pyrazinamide was not carcinogenic in rats or male mice and no conclusion was possible for female mice due to insufficient numbers of surviving control mice.Pyrazinamide was not mutagenic in the Ames bacterial test, but induced chromosomal aberrations in human lymphocyte cell cultures.Pregnancy: Teratogenic Effects – Pregnancy Category CAnimal reproduction studies have not been conducted with pyrazinamide. It is also not known whether pyrazinamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pyrazinamide should be given to a pregnant woman only if clearly needed.Nursing MothersPyrazinamide has been found in small amounts in breast milk. Therefore, it is advised the pyrazinamide be used with caution in nursing mothers taking into account the risk-benefit of this age in ChildrenPyrazinamide regimens employed in adults are probably equally effective in children.Pyrazinamide appears to be well tolerated in children.Geriatric Use Clinical studies of pyrazinamide did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range,reflecting the greater frequency of decreased hepatic or renal function, and of concomitant disease or other drug therapy.It does not appear that patients with impaired renal function require a reduction in dose. It may be prudent to select doses at the low end of the dosing range, however.ADVERSE REACTIONS GeneralFever, porphyria and dysuria have rarely been reported. Gout (see PRECAUTIONS ).GastrointestinalThe principal adverse effect is a hepatic reaction (see WARNINGS). Hepatotoxicity appears to be dose related, and may appear at any time during therapy. GI disturbances including nausea, vomiting and anorexia have also been reported.®® 56,7,894, 10, 111213PO mg/kgPO2 gmg/kg50 to 70 mg/kgStreptomycin20 to 40 mg/kgIM 15 mg/kg**IM1 g**25 to 30mg/kgIM25 to 30 mg/kgIMEthambutol15 to 25 mg/kgPO 15 to 25mg/kgPO2.5 g50 mg/kg50 mg/kgDefinition of abbreviations: PO = perorally; IM = intramuscularly.* Doses based on weight should be adjusted as weight changes.**In persons older than 60 yrs of age the daily dose of streptomycin should be limited to 10 mg/kg with a maximal dose of 750 mg.HOW SUPPLIEDPyrazinamide Tablets, USP 500 mg are round, white, scored tablets, debossed S above the score, 660 below the score.NDC 67253-660-10 - Bottle of 100NDC 67253-660-50 - Bottle of 500Store at 20-25°C (68-77°F) [See USP Controlled Room Temperature].Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure. REFERENCES1. 2. 3. 4. 5. 6. 7. 8. 9.10.11.12.Drug Information, American Hospital Formulary Service. American Society of Hospital Pharmacists. Bethesda, MD 1991.USPDI, Drug Information for the Health Care Professional. United States Pharmacopeial Convention, Inc. Rockville, MD 1991:1B:2226-2227.Goodman-Gilman A, Rall TW, Nies AS, Taylor P. The Pharmacological Basis of Therapeutics, ed 8. New York, Pergamon Press. 1990;1154.Treatment of tuberculosis and tuberculosis infection in adults and children. Am Rev Respir Dis. 1986;134:363-368.Reynolds JEF, Parfitt K, Parsons AV, Sweetman-SC. Martindale The Extra Pharmacopoeia, ed 29. London, The Pharmaceutical Press. 1989;569-570.Bioassay of pyrazinamide for possible carcinogenicity. National Cancer Institute Carcinogenesis Technical Report Series No. 48, 1978.Zerger E, Anderson B, Haworth S, Lawlor T, Mortelmans K, Speck W. Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ Mutagen. 1987;9 (Suppl 9):1-109. Roman IC, Georgian L. Cytogenetic effects of some antituberculosis drugs in vitro. Mutation Research. 1977;48:215-224.Holdiness M. Antituberculosis drugs and breast-feeding. Arch Intern Med. 1984;144:1888. Turcios N, Evans H. Preventing and managing tuberculosis in children. J Resp Dis.1989;10(6)(Jun):23.Starke JR. Multidrug therapy for tuberculosis in children. Pediatr Infec Dis J. 1990;9:785-793. Specific requirements on content and format of labeling for human prescription drugs; proposed addi-tion of “geriatric use” subsection in the labeling. Federal Register. 1990;55(212)(Nov 1):46134-46137.13.Manufactured For:DAVA Pharmaceuticals, Inc.Fort Lee, NJ 07024 USA By:ULTRAtab Laboratories, Inc.Highland, NY 12528 USA Revised: 04/13PACKAGE/LABEL PRINCIPLE DISPLAY PANEL – 500 mg 100 tablets labelNDC 67253-660-10 PYRAZINAMIDE TABLETS, USP 500 mg100 tablets Rx onlyUSUAL ADULT DOSAGE:15-30 mg/kg once daily, not to exceed 2 g. Consult package literature for more complete information.This package not for household dispensing.Store at 20°-25°C (68°-77°F). [See USP Controlled Room Temperature.]Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.Stamathakis G, Montes C, Trouvin JH, et al. Pyrazinamide and pyrazinoic acid pharmacokinetics in patients with chronic renal failure. Clinical Nephrology . 1988;30:230-234.Manufactured for:DAVA Pharmaceuticals, Inc.Fort Lee, NJ 07024, USABy:ULTRAtab Laboratories, Inc.Highland, NY 12528 USARev. 04/13PACKAGE/LABEL PRINCIPLE DISPLAY PANEL – 500 mg 500 tablets labelNDC 67253-660-50PYRAZINAMIDETABLETS, USP500 mg500 tablets Rx onlyUSUAL ADULT DOSAGE:15-30 mg/kg once daily, not to exceed 2 g. Consultpackage literature for more complete information.This package not for household dispensing.Store at 20°-25°C (68°-77°F). [See USPControlled Room Temperature.]Dispense in a tight, light-resistant containeras defined in the USP using a child-resistantclosure.Manufactured for:DAVA Pharmaceuticals, Inc.Fort Lee, NJ 07024, USABy:ULTRAtab Laboratories, Inc.Highland, NY 12528 USARev. 04/13PYRAZINAMIDEpyrazinamide tabletProduct InformationProduct T ype HUMAN PRESCRIPTION DRUG Ite m Code (Source)NDC:67253-660 Route of Administration ORALActive Ingredient/Active MoietyIngredient Name Basis of Strength Strength PYRAZINAMIDE (UNII: 2KNI5N06TI) (PYRAZINAMIDE - UNII:2KNI5N06TI)PYRAZINAMIDE500 mgInactive IngredientsIngredient Name Strength STARCH, CO RN (UNII: O8232NY3SJ)MAGNESIUM STEARATE (UNII: 70097M6I30)STEARIC ACID (UNII: 4ELV7Z65AP)Product CharacteristicsColor WHITE Score 2 piecesShape ROUND Siz e13mmFlavor Imprint Code S;660ContainsPackaging#Item Code Package Description Marketing Start Date Marketing End Date1NDC:67253-660-10100 in 1 BOTTLE2NDC:67253-660-50500 in 1 BOTTLEMarketing InformationMarke ting Cate gory Application Numbe r or Monograph Citation Marke ting Start Date Marke ting End Date ANDA ANDA08015706/03/1971Labeler - DAVA Pharmaceuticals, Inc. (172202025)EstablishmentName Addre ss ID/FEI Busine ss Ope rationsULTRAtab Labo rato ries, Inc.151051757ANALYSIS(67253-660) , MANUFACTURE(67253-660) , LABEL(67253-660) , PACK(67253-660)EstablishmentName Addre ss ID/FEI Busine ss Ope rationsCalyx Chemicals & Pharmaceuticals Limited650427912API MANUFACTURE(67253-660)DAVA Pharmaceuticals, Inc.Revised: 6/2013。