Proton polarizability contribution to hydrogen Lamb shift
- 格式:pdf
- 大小:53.53 KB
- 文档页数:3

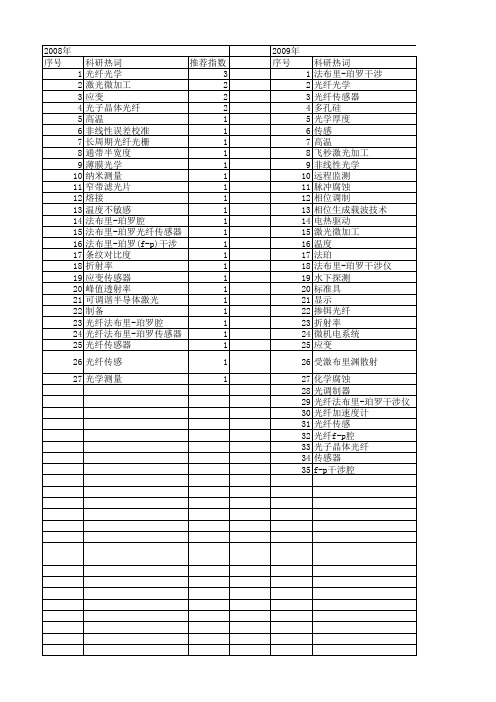
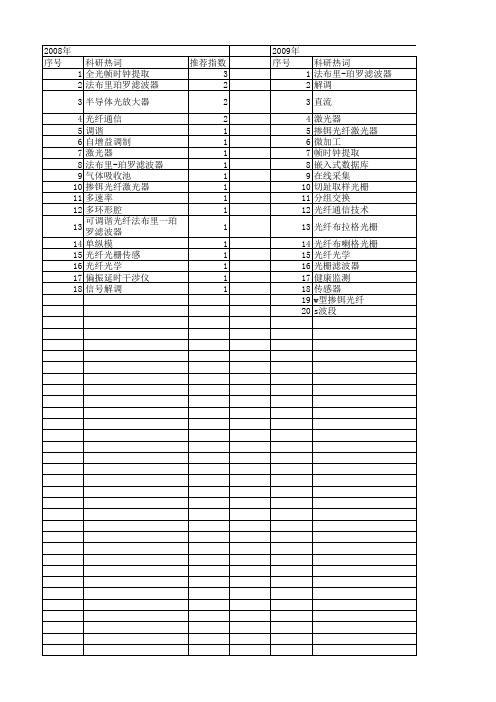
- disruption ,: Global convergence vs nationalSustainable - ,practices and dynamic capabilities in the food industry: A critical analysis of the literature5 Mesoscopic - simulation6 Firm size and sustainable performance in food -s: Insights from Greek SMEs7 An analytical method for cost analysis in multi-stage -s: A stochastic / model approach8 A Roadmap to Green - System through Enterprise Resource Planning (ERP) Implementation9 Unidirectional transshipment policies in a dual-channel -10 Decentralized and centralized model predictive control to reduce the bullwhip effect in - ,11 An agent-based distributed computational experiment framework for virtual - / development12 Biomass-to-bioenergy and biofuel - optimization: Overview, key issues and challenges13 The benefits of - visibility: A value assessment model14 An Institutional Theory perspective on sustainable practices across the dairy -15 Two-stage stochastic programming - model for biodiesel production via wastewater treatment16 Technology scale and -s in a secure, affordable and low carbon energy transition17 Multi-period design and planning of closed-loop -s with uncertain supply and demand18 Quality control in food - ,: An analytical model and case study of the adulterated milk incident in China19 - information capabilities and performance outcomes: An empirical study of Korean steel suppliers20 A game-based approach towards facilitating decision making for perishable products: An example of blood -21 - design under quality disruptions and tainted materials delivery22 A two-level replenishment frequency model for TOC - replenishment systems under capacity constraint23 - dynamics and the ―cross-border effect‖: The U.S.–Mexican border’s case24 Designing a new - for competition against an existing -25 Universal supplier selection via multi-dimensional auction mechanisms for two-way competition in oligopoly market of -26 Using TODIM to evaluate green - practices under uncertainty27 - downsizing under bankruptcy: A robust optimization approach28 Coordination mechanism for a deteriorating item in a two-level - system29 An accelerated Benders decomposition algorithm for sustainable - / design under uncertainty: A case study of medical needle and syringe -30 Bullwhip Effect Study in a Constrained -31 Two-echelon multiple-vehicle location–routing problem with time windows for optimization of sustainable - / of perishable food32 Research on pricing and coordination strategy of green - under hybrid production mode33 Agent-system co-development in - research: Propositions and demonstrative findings34 Tactical ,for coordinated -s35 Photovoltaic - coordination with strategic consumers in China36 Coordinating supplier׳s reorder point: A coordination mechanism for -s with long supplier lead time37 Assessment and optimization of forest biomass -s from economic, social and environmental perspectives – A review of literature38 The effects of a trust mechanism on a dynamic - /39 Economic and environmental assessment of reusable plastic containers: A food catering - case study40 Competitive pricing and ordering decisions in a multiple-channel -41 Pricing in a - for auction bidding under information asymmetry42 Dynamic analysis of feasibility in ethanol - for biofuel production in Mexico43 The impact of partial information sharing in a two-echelon -44 Choice of - governance: Self-managing or outsourcing?45 Joint production and delivery lot sizing for a make-to-order producer–buyer - with transportation cost46 Hybrid algorithm for a vendor managed inventory system in a two-echelon -47 Traceability in a food -: Safety and quality perspectives48 Transferring and sharing exchange-rate risk in a risk-averse - of a multinational firm49 Analyzing the impacts of carbon regulatory mechanisms on supplier and mode selection decisions: An application to a biofuel -50 Product quality and return policy in a - under risk aversion of a supplier51 Mining logistics data to assure the quality in a sustainable food -: A case in the red wine industry52 Biomass - optimisation for Organosolv-based biorefineries53 Exact solutions to the - equations for arbitrary, time-dependent demands54 Designing a sustainable closed-loop - / based on triple bottom line approach: A comparison of metaheuristics hybridization techniques55 A study of the LCA based biofuel - multi-objective optimization model with multi-conversion paths in China56 A hybrid two-stock inventory control model for a reverse -57 Dynamics of judicial service -s58 Optimizing an integrated vendor-managed inventory system for a single-vendor two-buyer - with determining weighting factor for vendor׳s ordering59 Measuring - Resilience Using a Deterministic Modeling Approach60 A LCA Based Biofuel - Analysis Framework61 A neo-institutional perspective of -s and energy security: Bioenergy in the UK62 Modified penalty function method for optimal social welfare of electric power - with transmission constraints63 Optimization of blood - with shortened shelf lives and ABO compatibility64 Diversified firms on dynamical - cope with financial crisis better65 Securitization of energy -s in China66 Optimal design of the auto parts - for JIT operations: Sequential bifurcation factor screening and multi-response surface methodology67 Achieving sustainable -s through energy justice68 - agility: Securing performance for Chinese manufacturers69 Energy price risk and the sustainability of demand side -s70 Strategic and tactical mathematical programming models within the crude oil - context - A review71 An analysis of the structural complexity of - /s72 Business process re-design methodology to support - integration73 Could - technology improve food operators’ innovativeness? A developing country’s perspective74 RFID-enabled process reengineering of closed-loop -s in the healthcare industry of Singapore75 Order-Up-To policies in Information Exchange -s76 Robust design and operations of hydrocarbon biofuel - integrating with existing petroleum refineries considering unit cost objective77 Trade-offs in - transparency: the case of Nudie Jeans78 Healthcare - operations: Why are doctors reluctant to consolidate?79 Impact on the optimal design of bioethanol -s by a new European Commission proposal80 Managerial research on the pharmaceutical - – A critical review and some insights for future directions81 - performance evaluation with data envelopment analysis and balanced scorecard approach82 Integrated - design for commodity chemicals production via woody biomass fast pyrolysis and upgrading83 Governance of sustainable -s in the fast fashion industry84 Temperature ,for the quality assurance of a perishable food -85 Modeling of biomass-to-energy - operations: Applications, challenges and research directions86 Assessing Risk Factors in Collaborative - with the Analytic Hierarchy Process (AHP)87 Random / models and sensitivity algorithms for the analysis of ordering time and inventory state in multi-stage -s88 Information sharing and collaborative behaviors in enabling - performance: A social exchange perspective89 The coordinating contracts for a fuzzy - with effort and price dependent demand90 Criticality analysis and the -: Leveraging representational assurance91 Economic model predictive control for inventory ,in -s92 - ,ontology from an ontology engineering perspective93 Surplus division and investment incentives in -s: A biform-game analysis94 Biofuels for road transport: Analysing evolving -s in Sweden from an energy security perspective95 - ,executives in corporate upper echelons Original Research Article96 Sustainable - ,in the fast fashion industry: An analysis of corporate reports97 An improved method for managing catastrophic - disruptions98 The equilibrium of closed-loop - super/ with time-dependent parameters99 A bi-objective stochastic programming model for a centralized green - with deteriorating products100 Simultaneous control of vehicle routing and inventory for dynamic inbound -101 Environmental impacts of roundwood - options in Michigan: life-cycle assessment of harvest and transport stages102 A recovery mechanism for a two echelon - system under supply disruption103 Challenges and Competitiveness Indicators for the Sustainable Development of the - in Food Industry104 Is doing more doing better? The relationship between responsible - ,and corporate reputation105 Connecting product design, process and - decisions to strengthen global - capabilities106 A computational study for common / design in multi-commodity -s107 Optimal production and procurement decisions in a - with an option contract and partial backordering under uncertainties108 Methods to optimise the design and ,of biomass-for-bioenergy -s: A review109 Reverse - coordination by revenue sharing contract: A case for the personal computers industry110 SCOlog: A logic-based approach to analysing - operation dynamics111 Removing the blinders: A literature review on the potential of nanoscale technologies for the ,of -s112 Transition inertia due to competition in -s with remanufacturing and recycling: A systems dynamics mode113 Optimal design of advanced drop-in hydrocarbon biofuel - integrating with existing petroleum refineries under uncertainty114 Revenue-sharing contracts across an extended -115 An integrated revenue sharing and quantity discounts contract for coordinating a - dealing with short life-cycle products116 Total JIT (T-JIT) and its impact on - competency and organizational performance117 Logistical - design for bioeconomy applications118 A note on ―Quality investment and inspection policy in a supplier-manufacturer -‖119 Developing a Resilient -120 Cyber - risk ,: Revolutionizing the strategic control of critical IT systems121 Defining value chain architectures: Linking strategic value creation to operational - design122 Aligning the sustainable - to green marketing needs: A case study123 Decision support and intelligent systems in the textile and apparel -: An academic review of research articles124 - ,capability of small and medium sized family businesses in India: A multiple case study approach125 - collaboration: Impact of success in long-term partnerships126 Collaboration capacity for sustainable - ,: small and medium-sized enterprises in Mexico127 Advanced traceability system in aquaculture -128 - information systems strategy: Impacts on - performance and firm performance129 Performance of - collaboration – A simulation study130 Coordinating a three-level - with delay in payments and a discounted interest rate131 An integrated framework for agent basedinventory–production–transportation modeling and distributed simulation of -s132 Optimal - design and ,over a multi-period horizon under demand uncertainty. Part I: MINLP and MILP models133 The impact of knowledge transfer and complexity on - flexibility: A knowledge-based view134 An innovative - performance measurement system incorporating Research and Development (R&D) and marketing policy135 Robust decision making for hybrid process - systems via model predictive control136 Combined pricing and - operations under price-dependent stochastic demand137 Balancing - competitiveness and robustness through ―virtual dual sourcing‖: Lessons from the Great East Japan Earthquake138 Solving a tri-objective - problem with modified NSGA-II algorithm 139 Sustaining long-term - partnerships using price-only contracts 140 On the impact of advertising initiatives in -s141 A typology of the situations of cooperation in -s142 A structured analysis of operations and - ,research in healthcare (1982–2011143 - practice and information quality: A - strategy study144 Manufacturer's pricing strategy in a two-level - with competing retailers and advertising cost dependent demand145 Closed-loop - / design under a fuzzy environment146 Timing and eco(nomic) efficiency of climate-friendly investments in -s147 Post-seismic - risk ,: A system dynamics disruption analysis approach for inventory and logistics planning148 The relationship between legitimacy, reputation, sustainability and branding for companies and their -s149 Linking - configuration to - perfrmance: A discrete event simulation model150 An integrated multi-objective model for allocating the limited sources in a multiple multi-stage lean -151 Price and leadtime competition, and coordination for make-to-order -s152 A model of resilient - / design: A two-stage programming with fuzzy shortest path153 Lead time variation control using reliable shipment equipment: An incentive scheme for - coordination154 Interpreting - dynamics: A quasi-chaos perspective155 A production-inventory model for a two-echelon - when demand is dependent on sales teams׳ initiatives156 Coordinating a dual-channel - with risk-averse under a two-way revenue sharing contract157 Energy supply planning and - optimization under uncertainty158 A hierarchical model of the impact of RFID practices on retail - performance159 An optimal solution to a three echelon - / with multi-product and multi-period160 A multi-echelon - model for municipal solid waste ,system 161 A multi-objective approach to - visibility and risk162 An integrated - model with errors in quality inspection and learning in production163 A fuzzy AHP-TOPSIS framework for ranking the solutions of Knowledge ,adoption in - to overcome its barriers164 A relational study of - agility, competitiveness and business performance in the oil and gas industry165 Cyber - security practices DNA – Filling in the puzzle using a diverse set of disciplines166 A three layer - model with multiple suppliers, manufacturers and retailers for multiple items167 Innovations in low input and organic dairy -s—What is acceptable in Europe168 Risk Variables in Wind Power -169 An analysis of - strategies in the regenerative medicine industry—Implications for future development170 A note on - coordination for joint determination of order quantity and reorder point using a credit option171 Implementation of a responsive - strategy in global complexity: The case of manufacturing firms172 - scheduling at the manufacturer to minimize inventory holding and delivery costs173 GBOM-oriented ,of production disruption risk and optimization of - construction175 Alliance or no alliance—Bargaining power in competing reverse -s174 Climate change risks and adaptation options across Australian seafood -s – A preliminary assessment176 Designing contracts for a closed-loop - under information asymmetry 177 Chemical - modeling for analysis of homeland security178 Chain liability in multitier -s? Responsibility attributions for unsustainable supplier behavior179 Quantifying the efficiency of price-only contracts in push -s over demand distributions of known supports180 Closed-loop - / design: A financial approach181 An integrated - / design problem for bidirectional flows182 Integrating multimodal transport into cellulosic biofuel - design under feedstock seasonality with a case study based on California183 - dynamic configuration as a result of new product development184 A genetic algorithm for optimizing defective goods - costs using JIT logistics and each-cycle lengths185 A - / design model for biomass co-firing in coal-fired power plants 186 Finance sourcing in a -187 Data quality for data science, predictive analytics, and big data in - ,: An introduction to the problem and suggestions for research and applications188 Consumer returns in a decentralized -189 Cost-based pricing model with value-added tax and corporate income tax for a - /190 A hard nut to crack! Implementing - sustainability in an emerging economy191 Optimal location of spelling yards for the northern Australian beef -192 Coordination of a socially responsible - using revenue sharing contract193 Multi-criteria decision making based on trust and reputation in -194 Hydrogen - architecture for bottom-up energy systems models. Part 1: Developing pathways195 Financialization across the Pacific: Manufacturing cost ratios, -s and power196 Integrating deterioration and lifetime constraints in production and - planning: A survey197 Joint economic lot sizing problem for a three—Layer - with stochastic demand198 Mean-risk analysis of radio frequency identification technology in - with inventory misplacement: Risk-sharing and coordination199 Dynamic impact on global -s performance of disruptions propagation produced by terrorist acts。


°ª¶¯Âå¾Ç¤j¾Ç¤K¤Q¤E¾Ç¦~«×¾Ç¤h«áÂå¾Ç¨t©Û¥Í¦Ò¸Õ¸ÕÃD¬ì¥Ø¡G¤Æ¾Ç ¦Ò¸Õ®É¶¡¡G¤K¤Q¤ÀÄÁI. Choose the correct answer from the following questions (each2%, total80%).(D) 1. Which element has the highest electron affinity?(A)oxygen (B)nitrogen (C)fluorine (D)chlorine(A) 2. Predict which of the following sets of ions would have the greatest coulombic attraction ina solid compound:(A)Na+, F- (B)Na+, Cl- (C)Na+, Br- (D)Na+, I-(B) 3. Identify the following species as Lewis acids:(A)NH3 (B)BF3 (C)CN- (D)F-(B) 4. On the basis of configuration for the neutral molecule O2, write the molecular orbitalO, give the expected bond order.configuration of the valence molecular orbital for -2(A)0.5 (B)1.0 (C)1.5 (D)2.0 (E)2.5(A) 5. Rank the following molecules, ions and radicals in order of increasing H¡ÐC¡ÐH bondangles:CH3+; CH3-; CH2; CH22+.(A)CH22+>CH3+>CH2>CH3- (B)CH3->CH2>CH3+>CH22+ (C)CH2>CH3+>CH3->CH22+(D)CH3+>CH22+CH2>CH3- (E)CH3+=CH22+=CH2=CH3-(B) 6. Identify which kind of intermolecular forces might arise between molecules ofthe chloromethane:(A)ion-ion (B)ion-dipole (C)dipole-dipole (D)hydrogen bonding(E)dispersion(A) 7. A gas sample is heated in a cylinder, using 375KJ of heat. At the same time a pistoncompresses the gas, using 645KJ of work. What is the change in internal energy of the gasduring this process?(A)1020KJ (B)270KJ (C)1.72KJ (D)500KJ (E)30KJ(B) 8. Calculate the enthalpy of the reaction P4(s) + 10Cl2(g)¡÷ 4PCl5(s) from the reactions:P4(s) + 6Cl2(g)¡÷ 4PCl3(l) ¡µH= -1278.8KJPCl3(l) + Cl2(g)¡÷ PCl5(s) ¡µH= -124KJ(A)1.154 (B)-1.775 (C)-1.154 (D)2.433 (E)1.503MJ¡Emol-1«Ø °ê «á ¦è Âå ¦Ò ÃD(A) 9. List the following substances in order of increasing molar entropy at 298K: (1)H2O(l),(2)H2O(g), (3)H2O(s), (4)C(s).(A)(2) > (1) > (3) > (4) (B)(4) > (3) > (2) > (1) (C)(1) = (2) = (3) = (4)(D)(1) > (2) > (3) > (4) (E)(3) > (1) > (2) > (4)(A) 10. Which of the following compounds become less stable with respect to the elements as thetemperature is raised? (1)PCl5(g), (2)HCN(g), (3)NO(g), (4)SO2(g)(A)(1) (B)(3) (C)(1), (2) (D)(3), (4) (E)(2)(E) 11. The following groups are found in some organic molecules. Which are hydrophilic?(1)¡ÐNH2; (2)CH3; (3)¡ÐBr; (4)¡ÐCOOH.(A)(1), (3) (B)(2), (4) (C)(1), (2) (D)(2), (3) (E)(1), (4)(C) 12. Estimate the boiling-point elevation of saturated solution of LiF at 100¢J (the solubility ofLiF is 230 mg / 1.00×102g of water at 100¢J). Assume that the ionic compounds undergo complete dissociation.(A)0.051¢J (B)0.22¢J (C)0.09¢J (D)0.030¢J (E)0.11¢J(D) 13. Arrange the following bases in order of increasing basicity strength: (1)ammonia,(2)ethylamine, (3)aniline.(A)(2) < (1) < (3) (B)(1) < (2) < (3) (C)(1) <(3) < (2) (D)(3) < (1) < (2)(E)(3) < (2) < (1)(B) 14. Calculating the pH of the solution resulting when 5.00mL of 0.150M NaOH(aq) is added to25.00mL of 0.100M HCOOH(aq). Use K a = 1.8×10-4 for HCOOH.(A)3.75 (B)3.38 (C)4.20 (D)7.5 (E)5.30(B) 15. Arrange the following metals in order of increasing strength as reducing agents: Li, Na, K,Mg.(A)Na > K > Li > Mg (B)K > Mg > Na > Li (C)Li > K > Na > Mg(D)Li > Na > K > Mg (E)Mg > K > Na > Li(E) 16. Which of the following atoms has the greatest polarizability?(A)manganese (B)iron (C)nitrogen (D)oxygen (E)tellurfium(C) 17. Xenon hexafluoride exists as the ionic solid XeF5+F-. Based on the Lewis structure forXeF5+ from VSEPR theory, predict XeF5+ shape.(A)octahedral (B)trigonal bipyramidal (C)square pyramidal (D)tetrahedral(E)distorted octahedral«Ø °ê «á ¦è Âå ¦Ò ÃD(B) 18. What can a tetrahedral complex show?(1)stereoisomerism (2)geometrical isomerism(3)optical isomerism(A)(1), (2) (B)(1), (3) (C)(2), (3) (D)(1) (E)(3)(E) 19 .Determine the type of structural isomerism that exists in the following pairs of compounds:[Co(NO2)(NH3)5]Br2 and [Co(ONO)(NH3)5]Br2.(A)ionization isomers (B)geometrical isomers (C)optical isomers(D)coordination isomers (E)linkage isomers(A) 20. Indicate which molecules display optical inactive: (1)CH3CHBrCH2CH3, (2)CH3CH2CHCl2,(3)1-bromo-2chloropropane, (4)1,2-dichloropentane(A)(2) (B)(1), (2) (C)(3) (D)(1) (E)(4)(A) 21.What mass of AgNO3 (169.9g / mol) is needed to convert 2.33g of Na2CO3 (106.0g / mol)to Ag2CO3?(A)7.47g (B)0.044g (C)0.022g (D)3.73g (E)1.57g(D) 22. Calculate the molar concentration of HNO3 (63.0 g / mol) in the solution that has a specificgravity of 1.42 and is 70% HNO3 (w / w).(A)8M (B)4M (C)13M (D)16M (E)12M(E) 23. Exactly 50.00mL of an HCl solution requires 29.71mL of 0.01963 M Ba(OH)2 to reach anend point with bromocresol green indicator. Calculate the molarity of the HCl.(A)0.0133M (B)0.0196M (C)0.029M (D)0.0046M (E)0.0233M(C) 24.Calculate the hydronium ion concentration of pure water at 25¢J.(A)1.0×10-6M (B)1.0×10-4M (C)1.0×10-7M (D)1.0×10-14M (E)1.0×10-8M(C) 25. Calculate the hydronium ion concentration in 0.200M aqueous NaOH(A)0.020M (B)1.0 ×10-14M (C)5.0 ×10-14M (D)0.1M (E)1.0 ×10-7M(E) 26. Which of the followings are Lewis acids?¡L ¡L(1)CH3CH2¡ÐO¡ÐH (B)CH3¡ÐNH¡ÐCH3 (3)MgBr2 (4)CH3¡ÐB¡ÐCH3¡L ¡UCH3+ ¡L(5)H¡ÐC¡ÐH (6)CH3¡ÐP¡ÐCH3¡U ¡UH CH3(A)(1), (2) (B)(2), (4), (5) (C)(1), (4), (5) (D)(4), (5), (6) (E)(3), (4), (5)«Ø °ê «á ¦è Âå ¦Ò ÃD«Ø °ê «á ¦è Âå ¦Ò ÃD(A) 27. Which of the follwing compounds can exist as pairs of cis-trans isomers?(1)CH 3CH¡×CH 2(2)(CH 3)2C¡×CHCH 3 (3)CH 3CH 2CH¡×CHCH 3 (4)(CH 3)2C¡×C(CH 3)CH 2CH 3(5)ClCH¡×CHCl (6)BrCH¡×CHCl(A)3, 5, 6 (B)2, 3, 6 (C)3, 4, 5 (D)1, 2, 4 (E)2, 5, 6(B) 28. Assign E or Z configuration to these alkenes, respectively:(1) H 3C CH 2OH (2) Cl CH 2CH 3 ¢@ ¡þ ¢@ ¡þ C¡×C C¡×C ¡þ ¢@ ¡þ ¢@ H 3CH 2C Cl H 3CO CH 3CH 2CH 3(3)(A)E, Z, E (B)Z, E, Z (C)Z, Z, E (D)E, E, Z (E)Z, Z, Z(B) 29. How many alkene products, including E,Z isomers, might be obtained by dehydration of3-methyl-3-hexanol with aqueous sulfuric acid?OH ¡UCH 3CH 2CCH 2CH 2CH 3 3-Methyl-3-hexanol ¡U CH 3(A)6 (B)5 (C)4 (D)3 (E)2(B) 30. What product would you obtain from catalytic hydrogenation of this alkene,(CH 3)2C¡×CHCH 2CH 3?(A)1-methylpentane (B)2-methylpentane (C)4-methylpentane(D)1,2-dimethylpentane (E)3-methylpentane(A) 31. Which of these compounds are chiral? (1) (2) (3) (A)(2) (B)(1) (C)3 (D)1, 2 (E)1, 3N N ¢@¡þO H 3CH 2C O H H NCH 2CH 2CH 3¢@H CH 3C ¡×C H 3C ¢@¡þCOOH CH 2OH«Ø °ê «á ¦è Âå ¦Ò ÃD(E) 32. Assign R, S configurations to these molecules, respectively:(1)(A)R, S, S (B)S, S, S (C)R, R, R (D)R, R, S (E)S, S, R(B)33. Which of these substances have a meso form, respectively? (1)2, 3-Dibromobutane,(2)2, 3-Dibromopentane, (3)2, 4-Dibromopentane.(A)1, 2 (B)1, 3 (C)2 (D)2, 3(A)34. Which reagent in the following compounds is more nucleophilic?(A)(CH 3)2N - (B)(CH 3)2NH (C)(CH 3)3B (D)(CH 3)3N (D)CH 3NH 2(E)35. Tell whether these reactions are S N l:(1)1-Bromobutane + NaN 3 ¡÷ 1-AzidobutaneCl ¡U (2)CH 3CH 2CHCH 3CH 3 + KOH ¡÷ CH 3CH 2CH¡×CHCH 3(3) + CH 3COOH ¡÷ + HCl(A)1, 2 (B)1, 3 (C)2, 3 (D)2 (E)3(E)36. How many nonequivalent kinds of protons are present in this compound, 2-methyl-1-butene?(A)1 (B)2 (C)3 (D)4 (E)5(E)37. Which of the following has the greatest Van der Waal’s attraction for other molecules of thesame kind?(A)CH 3CHCH 2CH 3 (B)CH 3CH 2CH 2CH 3 ¡U CH 3 CH 3¡U (C)H 3C¡ÐC¡ÐCH 3 (D)CH 3CH 2CH 2CH 2CH 3 ¡U CH 3Cl CH 3OCCH 3CH 3O ¡üH 3H 3Br CH 3°ê «á ¦è Âå ¦Ò ÃD«Ø °ê «á ¦è Âå ¦Ò ÃD CH 3¡U (E)CH 3CHCHCH 3 ¡U CH 3(A)38. Which of the following alkenes reacts with HCl at the slowest rate? (A)CH 3¡ÐCH¡ÐCH¡×CH 2 (B)CH 3¡ÐCH 2¡ÐC¡×CH 2 ¡U ¡U CH 3 CH 3 H CH 3 ¢@ ¡þ (C)CH 3¡ÐC¡×CH 2 (D) C¡×C ¡U ¡þ ¢@ CH 3 H 3C CH 3 (E)CH 3CH 2 CH 3 ¢@ ¡þ C¡×C ¡þ ¢@ CH 3 CH 3(E)39.What are the reagents needed to accomplish the following transformation?¡÷(A)H 2O / H + (B)H 2O / Peroxide (C)Hg(OAc)2, H 2O / NaBH 4 (D)BH 3 (E)BH 3 / OH -, H 2O 2, H 2O(A)40. Which of the following compounds shown below is/are the product(s) of this reaction: heat - ¢¹ ¢º(A)¢¹only (B)¢ºonly (C)¢¹and ¢ºof equal yield (D)¢¹is major, ¢ºis minor (E)¢¹is minor, ¢ºis major¢º. What major products (give the name of product)would you expect from eliminationreactions of these alkyl halides?(6%) Br CH 3 CH 3 Cl CH 3 ¡U ¡U ¡U ¡U ¡U (A)CH 3CH 2CHCHCH 3 (B)CH 3CHCH 2¡ÐC¡ÐCHCH 3 (C) ¡U CH 3CHCH 3¡U Br 3CH 3CH 3OH CH 3CH 3«Ø °ê «á ¦è Âå ¦Ò ÃD ¸Ñ¡G CH 3¡U3CH 2CH¡×CCH 3 (2-Methyl-2-pentene) 3¡U CH 3CH¡×CHCHCH 3 (4-Methyl-2-pentene) CH 3 CH 3 ¡U ¡U CH 3CHCH 2C¡×CCH 3 (2, 3, 5-Trimethyl-2-hexene) CH 3 3 CH 3 ¡U CH 3CH¡ÐCH¡×CCHCH 3(2, 3, 5-Trimethyl-3-hexene) CH 33(major)(Ethylidene Cyclohexane) 2(major)(Vinyl Cyclohexane)¢». An unknown compound (A), C 4H 7ClO 2, gave the following proton NMR data:(4%) (a)triplet, at 1.31 ppm (3H)(b)singlet, at 3.95 ppm (2H)(c)quartet, at 4.20 ppm (2H)Propose a structure for A.¸Ñ¡G O ¡ü ClCH 2COCH 2CH 3«Ø °ê «á ¦è Âå ¦Ò ÃD¢¼. Give the IUPAC name for each of the following compounds.(2%each, total 10%) (a) (3CH 2CH 2¡ÐCOOH(e)¸Ñ¡G (a)5-Chloro-2-methyl Cyclohexanol(b)3-Bromotoluene(c)Bicyclo [2.2.2.] Octane(d)Butanoic acid(e)4-(N.N-dimethylamino)pyridineCH 3OHClCH 3BrNN ¡þ¢@H 3C CH 3。

1、Direct Evidence for Methyl Group Coordination by Carbon-Oxygen Hydrogen Bonds in theLysine Methyltransferase SET7/9SET domain lysine methyltransferases (KMTs) are S-adenosylmethionine (AdoMet)-dependent enzymes that catalyze the site-specific methylation of lysyl residues in histone and nonhistone proteins. Based on crystallographic and cofactor binding studies, carbon-oxygen (CH center dot center dot center dot O) hydrogen bonds have been proposed to coordinate the methyl groups of AdoMet and methyllysine within the SET domain active site. However, the presence of these hydrogen bonds has only been inferred due to the uncertainty of hydrogen atom positions in x-ray crystal structures. To experimentally resolve the positions of the methyl hydrogen atoms, we used NMR (1)H chemical shift coupled with quantum mechanics calculations to examine the interactions of the AdoMet methyl group in the active site of the human KMT SET7/9. Our results indicated that at least two of the three hydrogens in the AdoMet methyl group engage in CH center dot center dot center dot O hydrogen bonding. These findings represent direct, quantitative evidence of CH center dot center dot center dot O hydrogen bond formation in the SET domain active site and suggest a role for these interactions in catalysis. Furthermore, thermodynamic analysis of AdoMet binding indicated that these interactions are important for cofactor binding across SET domain enzymes.2、The role of the methyl group in stabilising the weak N-H ... pi hydrogen bond in the4-fluorotoluene-ammonia complexThe 4-fluorotoluene-ammonia van der Waals complex has been studied using a combination of resonant two-photon ionisation (R2PI) spectroscopy, ab initio molecular orbital calculations and multidimensional Franck-Condon analysis. The R2PI spectrum shows two sets of features assignable to two distinct conformers: one in which the ammonia binds between the hydrogen meta to the methyl group and the fluorine atom in a planar configuration and the other a pi-bound structure involving one bond between an ammonia hydrogen and the pi-system and another between the ammonia lone pair and the slightly acidic hydrogens on the methyl group. Ground state estimated CCSD(T) interaction energies were computed at the basis-set limit: these calculations yielded very similar interaction energies for the two conformers, whilst zero point energy correction yielded a zero point binding energy for the pi-complex about 10% larger than that of the in-plane, sigma-complex. The results of multidimensional Franck-Condon simulations based on ab initio ground and excited state geometry optimisations and vibrational frequency calculations showed good agreement with experiment, with further improvements achieved using a fitting procedure. The observation of a pi-complex in addition to a pi-complex supports the intuitive expectation that electron-donating groups should help to increase pi-density and hence stabilise pi-proton acceptor complex formation. In this case, this occurs in spite of the presence ofa strongly electron-withdrawing fluorine atom.3、Hydrogen-Bonded Complexes of Phenylacetylene with Water, Methanol, Ammonia, andMethylamine. The Origin of Methyl Group-Induced Hydrogen Bond SwitchingThe infrared spectra in the acetylenic C-H stretching region for the complexes of phenylacetylene with water, methanol, ammonia, and methylamine are indicative of change in the intermolecular structure upon substitution with a methyl group. High-level ab initio calculations at CCSD(T)/aug-cc-pVDZ level indicate that the observed complexes of water and ammonia are energetically the most favored structures, and electrostatics play a dominant role in stabilizing these structures. The ability of the pi electron density of the benzene ring to offer a largercross-section for the interaction and the increased polarizability of the O-H and N-H groups in methanol and methylamine favor the formation of pi hydrogen-bonded complexes, in which dispersion is the dominant force. Further, the observed phenylacetylene-methylamine complex can be tentatively assigned to a kinetically trapped higher energy structure. The observed methyl group-induced hydrogen bond switching in the phenylacetylene complexes can be attributed to the switching of the dominant interaction from electrostatic to dispersion.4、Non-additivity of Methyl Group in the Single-electron Lithium Bond of H(3)C center dotcenter dot center dot Li-H ComplexThe non-additivity of the methyl groups in the single-electron lithium bond was investigated using ab initio calculations at the B3L YP/6-311++G** and UMP2/6-311++G** levels. The strength of the interaction in the H(3)C center dot center dot center dot LiH, H(3)CH(2)C center dot center dot center dot LiH, (H(3)C)(2)HC center dot center dot center dot LiH,and (H(3)C)(3)C center dot center dot center dot LiH complexes was analyzed in term of the geometries, energies, frequency shifts, stabilization energies, charges, and topological parameters. It is shown that (H(3)C)(3)C radical with LiH forms the strongest single-electron lithium bond, followed by (H(3)C)(2)HC radical, then H(3)CH(2)C radical, and H(3)C radical forms the weakest single-electron lithium bond. A positive non-additivity is present among methyl groups. Natural bond orbital and atoms in molecules analyses were used to estimate such conclusions. Furthermore, there are few linear/nonlinear relationships in the system and the interaction mode of single-electron Li-bond is different from the single-electron H-bond and single-electron halogen bond.5、Hydrogen-bonding ability of a methyl groupHydrogen bonds involving the methyl group have been studied by topological analysis of the electron density derived from quantum mechanical geometry-optimized structures of selected molecules/ions. The results indicate that hydrogen-bond formation not only depends on the distance from the methyl group to the proton acceptor, X, but also on the angle CH...X. The species investigated suggest the angle should be bigger than about 100degrees for hydrogen-bond formation.6、Intramolecular hydrogen bonds in ortho-substituted hydroxybenzenes and in 8-susbtituted 1-hydroxynaphthalenes: Can a methyl group be an acceptor of hydrogen bonds? Considering the findings of Fujii et al. showing that the cis isomer of the o-cresol radical cation shows a low-frequency shift of the OH stretching attributed to an intramolecular hydrogen bond with the CH3 group and considering the studies of Knak Jensen et al. concluding that such an O-(HC)-C-... interaction was not possible, the work presented in this article tries to understand if this is a consequence of the nature of the hydrogen bond acceptor (a CH3 group) or of the five-member ring that would be formed as a result of the intramolecular interaction. Thus, we have studied o-cresol, 8-methyl-1-hydroxynaphthalene, 1-hydroxy-1-propene, 1-hydroxy-3-methyl-1,3-butadiene, and their derivatives in which the -CH3 group has been substituted by a -F atom or by an -OH group. Taking into account interaction distances and angles, interaction energies (from isodesmic reactions), and electron density characteristics, we can conclude that, in general, a methyl group cannot behave as a hydrogen bond acceptor. In addition, we found that the formation of intramolecular hydrogen bonds driving to the formation of five-member rings is not favored even in the presence of a good acceptor. Moreover, different methods of evaluating intramolecular interaction energies have been analyzed.6、Hydrogen-bonding interaction of methyl-substituted pyridines with thioacetamide: sterichindrance of methyl groupThe hydrogen-bonding interaction between a series of methyl-substituted pyridines as proton acceptors and thioacetamide as a proton donor in CCl4 has been investigated using near-infrared absorption spectroscopy. The stability of the 1:1 hydrogen-bonded complex increases with the number of methyl groups and depends on the position of methyl groups. The steric hindrance of ortho-methyl groups particularly reduces the stability of complex. The relative stability agrees with the ease of miscibility of pyridines with water for methyl and dimethyl homologs. The calculated proton affinities and the DFT association energies using 6-31+G(d, p) and 6-311 ++G(2d, 2p) basis sets reveal the steric hindrance of ortho-methyl groups. (C) 2001 Elsevier Science B.V. All rights reserved.7、Hydrogen atoms in acetylsalicylic acid (Aspirin): the librating methyl group and probing thepotential well in the hydrogen-bonded dimerThe structure of acetylsalicylic acid (2-(acetoyloxy)benzoic acid; Aspirin) has been studied by variable temperature single crystal neutron diffraction. The usual large torsional librational motion of the terminal methyl group is observed and its temperature dependence analysed using a simple model for the potential. yielding the force constant and barrier height for this motion. In addition, asymmetry of the scattering density of the proton involved in the hydrogen bond forming the carboxylic acid dimer motif is observed at temperatures above 200 K. This asymmetry is discussed in terms of its possible implications for the shape of the hydrogen bonding potential well. (C) 2001 Elsevier Science B.V. All rights reserved. 8、。
附录三名词总结第二章正电荷(positive charge)原子核(atomic nucleus)负电荷(negative charge)质子(proton)中子(neutron)道尔顿模型(Dalton Model)汤姆逊模型(Thomason Model)库伦(coulomb)卢瑟福模型(Rutherford Model)波尔模型(N.Bohr Model)原子轨道学说(Atomic Orbital Hypothesis) 基态(ground state)激发态(excited state)电离(ionization)普朗克常数(Planck’s constant)频率(frequency)波长(wavelength)物质波假设(The L.de Broglie Hypothesis) 电子衍射实验(electron diffraction experiment)海森堡测不准原则(Heisenberg Uncertainty Principle)位置(position)动量(momentum)量子数(quantum number)电子层(shells)亚层(subshells)轨道(orbit)主量子数(Principal Quantum Number)角量子数(Azimuthal Quantum Number)磁量子数(Magnetic Quantum Number)自旋量子数(Spin Quantum Number)电子排布(Electron Configuration)保利不相容原理(Pauli’s Exclusion Principle)能量最低原理(Lowest Energy Principle, Aufbau Principle) 价电子(valence electron)洪特规则(Hund's Rules)能级交错原理(energy overlay)元素周期表(the Periodic Table)原子序数(Atomic Number)同位素(Isotope)质量数(Mass Number)原子质量(Atomic Masses)周期(period)族(group)主族(main group)碱金属(alkali metal)碱土金属(alkaline earth metal)卤素(halogen)惰性气体(noble gas)硼族元素(boron group element)碳族元素(carbon group element)氮族元素(nitrogen group element)氧族元素(oxygen group element)。
Part One Fundamentals of ChemistryAtoms and Atomic StructureAtoms‐‐‐‐The Atomic TheoryBy the end of the eighteenth century,experimenters had well established that each pure substance had its own characteristic set of properties such as density,specific heat,melting point, and boiling point.Also established was the fact that certain quantitative relationships,such as the Law of Conservation of Mass,governed all chemical changes.But there was still no understanding of the nature of matter itself.Was matter continuous,like a ribbon from which varying amounts could be snipped,or was it granular,like a string of beads from which only whole units or groups of units could be removed?Some scientists believed strongly in the continuity of matter,whereas others believed equally strongly in granular matter;both reasonings were based solely on speculation.In1803,an English schoolmaster named John Dalton(1766‐1844)summarized and extended the then‐current theory of matter.The postulates of his theory,changed only slightly from their original statement,form the basis of modern atomic theory.Today,we express these four postulates as:Matter is made up of tiny particles called atoms.(A typical atom has a mass of approximately10‐23g and a radius of approximately10‐10m.)Over100different kinds of atoms are known;each kind is an element.All the atoms of a particular element are alike chemically but can vary slightly in mass and other physical properties. Atoms of different elements have different masses.Atoms of different elements combine in small,whole‐number ratios to form compounds.For example,hydrogen and oxygen atoms combine in a ratio of2:1to form the compound water, H2O.Carbon and oxygen atoms combine in a ratio of1:2to form the compound carbon dioxide, CO2.Iron and oxygen atoms combine in a ratio of2:3to form the familiar substance rust,Fe203.Atomic StructureMatter has mass and takes up space.Atoms are basic building blocks of matter,and cannot be chemically subdivided by ordinary means.Atoms are composed of three types of particles:protons,neutrons,and electrons.Protons and neutrons are responsible for most of the atomic mass.The mass of an electron is very small.Both the protons and neutrons reside in the nucleus.Protons have a positive charge(+), neutrons have no charge‐‐‐‐they are neutral.Electrons reside in orbitals around the nucleus.They have a negative charge(‐).It is the number of protons that determines the atomic number.The number of protons in an element is constant but neutron number may vary,so mass number(protons+neutrons)may vary.The same element may contain varying numbers of neutrons;these forms of an element are called isotopes.The chemical properties of isotopes are the same,although the physical properties of some isotopes may be different.Some isotopes are radioactive‐‐‐‐meaning they "radiate"energy as they decay to a more stable form,perhaps another element half‐life:time required for half of the atoms of an element to decay into stable form.Another example is oxygen,with atomic number of8can have8,9,or10neutrons.This is a list of the basic characteristics of atoms:●Atoms cannot be divided using chemicals.They do consist of parts,which include protons, neutrons,and electrons,but an atom is a basic chemical building block of matter.●Each electron has a negative electrical charge.●Each proton has a positive electrical charge.The charge of a proton and an electron are equal in magnitude,yet opposite in sign.Electrons and protons are electrically attracted to each other.●Each neutron is electrically neutral.In other words,neutrons do not have a charge and are not electrically attracted to either electrons or protons.●Protons and neutrons are about the same size as each other and are much larger than electrons.The mass of a proton is essentially the same as that of a neutron.The mass of a proton is1840times greater than the mass of an electron.●The nucleus of an atom contains protons and neutrons.The nucleus carries a positive electrical charge.●Electrons move around outside the nucleus.●Almost all of the mass of an atom is in its nucleus;almost all of the volume of an atom is occupied by electrons.●The number of protons(also known as its atomic number)determines the element. Varying the number of neutrons results in isotopes.Varying the number of electrons results in ions.Isotopes and ions of an atom with a constant number of protons are all variations of a single element.●The particles within an atom are bound together by powerful forces.In general,electrons are easier to add or remove from an atom than a proton or neutron.Chemical reactions largely involve atoms or groups of atoms and the interactions between their electrons.New words and expressionscompound化合物,复合物,复合词electron电子granular由小粒而成的,粒状的ion离子isotope同位素Law of Conservation of Mass物质守恒定律,物质不灭定律neutron中子proton质子ratio比率,比例Part Two Inorganic ChemistryWhat is Inorganic ChemistryInorganic chemistry is the branch of chemistry concerned with the properties and behavior of inorganic compounds.This field covers all chemical compounds except the myriad organic compounds(carbon based compounds,usually containing C‐H bonds),which are the subjects of organic chemistry.The distinction between the two disciplines is far from absolute,and there is much overlap,most importantly in the sub‐discipline of organometallic chemistry.Many inorganic compounds are ionic compounds,consisting of canons and anions joined by ionic bonding.Examples of salts(which are ionic compounds)are magnesium chloride MgCl2, which consists of magnesium cations Mg2+and chloride anions Cl‐;or sodium oxide Na2O, which consists of sodium canons Na+and oxide anions O2‐.In any salt,the proportions of the ions are such that the electric charges cancel out,so that the bulk compound is electrically neutral. The ions are described by their oxidation state and their ease of formation can be inferred from the ionization potential(for cations)or from the electron affinity(anions)of the parent elements.Important classes of inorganic salts are the oxides,the carbonates,the sulfates and the halides.Many inorganic compounds are characterized by high melting points.Inorganic salts typically are poor conductors in the solid state.Another important feature is their solubility in water e.g.(see:solubility chart),and ease of crystallization.Where some salts(e.g.NaCI)are very soluble in water,others(e.g.SiO2)are not.The simplest inorganic reaction is double displacement when in mixing of two salts the ions are swapped without a change in oxidation state.In redox reactions one reactant,the oxidant, lowers its oxidation state anti another reactant,the reluctant,has its oxidation state increased. The net result is an exchange of electrons.Electron exchange can occur indirectly as well,e.g.in batteries,a key concept in electrochemistry.When one reactant contains hydrogen atoms,a reaction can take place by exchanging protons in acid‐base chemistry.In a more general definition,an acid can be any chemical species capable of binding to electron pairs is called a Lewis acid;conversely any molecule that tends to donate an electron pair is referred to as a Lewis base.As a refinement of acid‐base interactions, the HSAB theory takes into account polarizability and size of ions.Inorganic compounds are found in nature as minerals.Soil may contain iron sulfide as pyrite or calcium sulfate as gypsum.Inorganic compounds are also found multitasking as biomolecules: as electrolytes(sodium chloride),in energy storage(ATP)or in construction(the polyphosphate backbone in DNA).The first important man‐made inorganic compound was ammonium nitrate for soil fertilization through the Haber process.Inorganic compounds are synthesized for use as catalysts such as vanadium(V)oxide and titanium(111)chloride,or as reagents in organic chemistry such as lithium aluminium hydride.Subdivisions of inorganic chemistry are organometallic chemistry,cluster chemistry and bioinorganic chemistry.These fields are active areas of research in inorganic chemistry,aimed toward new catalysts,superconductors,and therapies.Inorganic chemistry is a highly practical area of science.Traditionally,the scale of a nation's economy could be evaluated by their productivity of sulfuric acid.The top20inorganic chemicalsmanufactured in Canada,China,Europe,Japan,and the US(2005data):aluminium sulfate, ammonia,ammonium nitrate,ammonium sulfate,carbon black,chlorine,hydrochloric acid, hydrogen,hydrogen peroxide,nitric acid,nitrogen,oxygen,phosphoric acid,sodium carbonate, sodium chlorate,sodium hydroxide,sodium silicate,sodium sulfate,sulfuric acid,and titanium dioxide.The manufacturing of fertilizers is another practical application of industrial inorganic chemistry.Inorganic Chemistry is a Creative FieldThe field of inorganic chemistry has traditionally been characterized by scientists with an artistic or creative flair.Many inorganic chemists say that they were drawn to the field in part by the pretty colors of the metals in the lab and by the interesting things that could be done in the lab.They often say the opportunities for creativity and inferential thinking are what they like best about their work.Describing themselves as thinkers,inorganic chemists like putting things together and solving problems and stress the importance of being detail oriented,precise,and persistent.Inorganic chemists describe their work as a constant challenge."The job changes all the time,”says Steve Caldwell,an inorganic chemist working at Dow Chemical."Everyday there are a new set of issues and I have to determine which are the most important ones to work on first.It's definitely not a nine to five job.”Inorganic Chemistry Integrates Many DisciplinesInorganic chemistry,like many scientific fields,is becoming more interdisciplinary. Breakthroughs are anticipated in the interface between fields rather than in the more traditional area.“In the future,jobs will not be filled by super specialists,”says Sauer,"but by scientists with a broad base of knowledge.”Even though a course of study like materials science or polymer science may appear to better position an individual for this interdisciplinary future,chemists in the field still strongly recommend getting a degree in inorganic chemistry.A degree in the basic discipline,will give a better understanding of bonding,valence,and orbital theory.In addition, students are advised to take courses outside inorganic chemistry both to prepare themselves to integrate knowledge towards problem solving as well as be flexible in today's tough job market. "Don't just stick to inorganic chemistry,”Sauer says."Learn inorganic chemistry and see how it applies in other areas.”Caldwell adds,"Starting out in inorganic chemistry doesn't mean that's what you'll always do.I spent a few years doing environmental research;there are always applications in related fields.”New words and expressionsaffinity姻亲,密切管理,吸引力aluminium sulfate硫酸铝ammonia氨ammonium nitrate硝酸铵ammonium sulfate硫酸铵anion阴离子,负离子carbon black炭黑,黑烟末carbonate碳酸盐,黑金刚石,使碳化cation阳离子chloride anion氯离子chlorine氯halide卤化物hydrochloric acid盐酸hydrogen peroxide过氧化氢inorganic chemistry无机化学interdisciplinary各学科间的,跨学科的ionization离子化,电离magnesium chloride氯化镁nitric acid硝酸organometallic有机金属的oxidant氧化剂oxide anion氧离子phosphoric acid磷酸pigment色素,颜料sodium carbonate碳酸钠sodium cation钠离子sodium chlorate碳酸钠sodium hydroxide氢氧化钠sodium silicate硅酸钠sodium sulfate硫酸钠surfactant表面活性剂sulfate硫酸盐sulfuric acid硫酸titanium dioxide二氧化钛Nomenclature of Inorganic CompoundsMastering chemical nomenclature is little different from learning a new language,such as German.In order to understand the German scientific literature,you must,e.g.learn that the compound H2is called Wasserstoff.English‐speaking chemists call it hydrogen.Your task now is to memorize the names of enough compounds and become sufficiently familiar with the several systems of naming compounds that chemistry ceases to be a"foreign language".The first thing to learn about naming chemical compounds is that there is usually more than one way to do it.We begin with the simplest system,in which a trivial name,i.e.one that has no sensible origin,is assigned to a compound.Some examples areH2O WaterNH3AmmoniaHg2Cl2CalomelSome names,such as quicklime for CaO,derive from the origin of the compound‐in this case, limestone,CaCO3.Such word origins are often remembered only by ety‐mologists,but the names have persisted for so long that they are an established part of the language.Can you imagine anyone seriously asking for a drink of dihydrogen oxide?The word water serves the purpose much better.As we come to less common and more complex compounds,the use of trivial names gives way to a more systematic approach.If there are only two elements in the compound it is customary to name the more metallic element first and the less metallic,or more electronegative element second,with the suffix“‐ide.”Some examples areKCl Potassium chlorideNaBr Sodium bromideCaO Calcium oxideHl Hydrogen iodideBaS Barium sulfideFor compounds containing still only two elements but more than two atoms,the prefixes “mono‐,””di‐,”“tri‐,”etc,become necessary.Some examples of such compounds are the oxides of nitrogen.Another such series is that of the oxides of chlorine.Because chlorine,like nitrogen, is slightly less electronegative than oxygen,the word chlorine comes first:Cl2O Dichlorine monoxideClO Chlorine monoxideClO2Chlorine dioxideClO3Chlorine trioxideCl2O7Dichlorine heptoxideClO4Chlorine tetroxideIf no confusion can result,the prefixes“mono‐"and"di‐“are sometimes dropped.A class of compounds in which such prefixes are seldom used is that in which the metal atom usually exhibits only one oxidation state.Depending on the oxidation state of the other element,the number of anions per cation is then fixed.Some examples areZnBr2Zinc bromideCaH2Calcium hydrideNa2O Sodium oxideAl2S3Aluminum sulfideThe next level of complexity in naming inorganic compounds arises when there are three elements present.Very often,one of these elements is oxygen.Such compounds are named by combining the suffix“‐ate”with the name of the less electronegative of the two non‐metallic elements.For example,NaNO3is sodium nitrate.The problem with this is that there is a similar compound with nitrogen in the+3oxidation state,NaNO2.Such compounds with the element in a lower oxidation state use the suffix“‐ite”,so NaNO2is sodium nitrite.But the number of chemical compounds is not bounded by the chemists’vocabulary,and there are several such examples entailing more than two oxidation states.To solve this problem,the prefix“hypo‐“(meaning “below”)is used in the name of the compound in which the less electronegative element is in the lowest oxidation state,and the prefix“per‐“(meaning“highest”)is used when it is in the highest oxidation state.Some examples of the use of this system are shown in the following table.Formula Atom Oxidation State Name of Salt Formula and Name of Corresponding Acid KNO2+3Potassium nitrite HNO2Nitrous acidKNO3+5Potassium nitrate HNO3Nitric acidRb2SO3+4Rubidium sulfite H2SO3Sulfurous acidRb2SO4+6Rubidium sulfate H2SO4Sulfuric acidCsClO+1Cesium Hypochorite HClO Hypochloroue acidCsClO2+3Cesium chlorite HClO2Chlorous acidCsClO3+5Cesium chlorate HClO3Chloric acidCsClO4+7Cesium Perchlorate HClO4Perchloric acidIn the inorganic acids,the suffixes“‐ous”and“‐ic”are used to denote the lower and higher oxidation states,respectively.These same suffixes are also used with the names of a number of metals,namely,those that usually exhibit more than one oxidation state.Some examples are cobaltous and cobaltic,and mer‐curous and mercuric.The nomenclature is complicated slightlyby the fact that,for a few such metals,these terms are derived from the Latin name of the element rather than the English name.All but eleven of the elements are given a symbol corresponding to one or two letters in the English name of the compound.(The first letter is always capitalized and the second letter is never capitalized.)One of these exceptions is tungsten,whose symbol(W)is derived from the German name of the element,Wolfram.The other ten have symbols derived from their Latin names.These are:stibium(Sb)for antimony,cuprum(Cu)for copper,aurum(Au)for gold, ferrum(Fe)for iron,plumbum(Pb)for lead,hydrargyrum(Hg)for mercury,kalium(K)for potassium,argentums(Ag)for silver,natrium(Na)for sodium,and stannum(Sn)for tin.The useof the suffixes“‐ous”and“‐ic”with three of these metals is illustrated below.Cul Cuprous iodideCul2Cupric iodideFeBr2Ferrous bromideFeBr3Ferric bromideSnCl2Stannous chlorideSnCI4Stannic chlorideThe system works well as long as there are only two major oxidation states of the metalatom,as in these examples.The most rational and self‐consistent system of nomenclature of inorganic compounds is that adopted in1957by the ultimate authority such matters,the International Union of Pure and Applied Chemistry.These rules,popularly called the IUPAC Rules,are the model for chemists throughout the world to follow,and are becoming ever more dominant in the chemical literature. Note that the oxidation state of the metal atom is specified by a Roman numeral whenever there could be some doubt about it,but not otherwise.Let us see how the examples shown above are named according to this system.Cul Copper(Ⅰ)iodideCul2Copper(Ⅱ)iodideFeBr2Iron(Ⅱ)bromideFeBr3Iron(Ⅲ)bromideSnCI2Tin(Ⅱ)chlorideSnCl4Tin(IV)chlorideThe best way for you to become familiar with the various systems of nomenclature is to practice.Each time you see the name of a compound,try to envision the formula and structure that it designates.Each time you see a new chemical formula,try to name it,using one or more of the principles described above.New words and expressionsammonia氨;氨水calomel甘汞;氯化亚汞quicklime生石灰etymologist词源学者bromide溴化物iodide碘化物barium钡prefix前缀series系列exhibit表示;显示anion阴离子;负离子cation阳离子;正离子zinc锌aluminum铝denote指示;表示namely即;也就是cobaltous钴的;二价钴的cobaltic钴的;二价钴的mercurous亚汞的mercuric汞的symbol符号capitalize大写antimony锑lead铅mercury汞tin锡stannous chloride氯化亚锡stannic chloride氯化锡complexity复杂性potasium nitrite亚硝酸钾potasium nitrate硝酸钾rubidium sulfite亚硫酸铷rubidium sulfate硫酸铷cesium hypochlorite次氯酸铯cesium chlorite亚氯酸铯cesium chlorate氯酸铯cesium perchlorate过氯酸铯nitrous acid亚硝酸nitric acid硝酸sulfurous acid亚硫酸sulfuric acid硫酸hypochlorous acid次氯酸chlorous acid亚氯酸chloric acid氯酸perchloric acid高氯酸rational合理的self‐consistent自相一致的ultimate最后的dominant支配的;统治的specify指定;详细说明Part Three Analytical ChemistryAnalytical chemistryPerhaps the most functional definition of analytical chemistry is that it is“the qualitative and quantitative characterization of matter".The word"characterization"is used in a very broad sense.It may mean the identification of the chemical compounds or elements present in a sample to answer questions such as"Is there any vitamin E in this shampoo as indicated on the label?”or"Is white tablet an aspirin tablet?"or“Is this piece of metal iron or nickel?”This type of characterization,to tell us what is'present is called qualitative analysis.Qualitative analysis is the identification of one or more chemical species present in a material.Characterization may also mean the determination of how much of a particular compound or element is present in a sample,to answer questions such as"How much acetylsalicylic acid is in this aspirin tablet?"or "How much nickel is in this steel?"This determination of how much of a species is present in a sample is called quantitative analysis.Quantitative analysis is the determination of the exact amount of a chemical species present in a sample.The chemical species may be an element, compound,or ion.The compound may be organic or inorganic.Characterization can refer to the entire sample(bulk analysis),such as the elemental composition of a piece of steel,or to the surface of a sample(surface analysis),such as the identification of the composition and thickness of the oxide layer that forms on the surface of most metals exposed to air and water.The characterization of a materials,the measurements like reaction kinetics.Examples of such measurements are the degree to which a polymer is crystalline as opposed to amorphous,the temperature at which a material loses its water of hydration,how long it takes or antacid"Brand A"to neutralize stomach acid,and how fast a pesticide degrades in sunlight.These diverse applications make analytical chemistry one of the broadest in scope of all scientific disciplines. Analytical chemistry is critical to our understanding of biochemistry,medicinal chemistry, geochemistry,environmental science,atmospheric chemistry,the behavior of material such as polymers,metal alloys,and ceramics,and many other scientific disciplines.For many years,analytical chemistry relied on chemical reactions to identify and determine the components present in a sample.These types of classics methods,often called“wet chemical methods",usually required that a part of the sample be taken,dissolved in a suitable solvent if necessary and the desired reaction carried out.The most important analytical fields based on this approach were volumetric and gravimetric analysis.Acid‐base titrations,oxidation‐reduction titrations,and gravimetric determinations,such as the determination of silver by precipitation as silver as silver chloride are all example of wet chemical analyses.These types of analyses require a high degree of skill and attention to detail on the part of the analyst if accurate and precise results are to be obtained.They are also time consuming and the demand of today's high‐throughput pharmaceutical development labs and industrial quality control labs often do not permit the use of such time‐consuming methods for routine analysis.In addition,it may be necessary to analyze samples without destroying them.Examples include evaluation of valuable artwork to determine if a painting is really by a famous"Old Master"or is a modern forgery,as well as in forensic analysis,where the evidence may need to be preserved.For these types of analyses,nondestructive analysis methods are needed,and wet chemical analysis will not do the job.Wet chemical analysis is still used in specialized areas of analysis,but many of the volumetric have been transferred to automated instruments.Classical analysis and instrumental analysis aresimilar in many respects,such as in the need for proper sampling,sample preparation, assessment of accuracy and precision,and proper record keeping.Most analyses today are carried out with specially designed electronic instruments controlled by computers.These instruments make use of the interaction of electromagnetic radiation and matter,or of some physical property of matter,to characterize the sample being analyzed.Often these instruments have automated sample introduction,automated data processing,and even automated sample preparation.To understand how the instrumentation operates and what information it can provide requires knowledge of chemistry,physics, mathematics,and engineering.The analytical chemist must not only know and understand analytical chemistry and instrumentation,but must also be able to serve as a problem solver to colleagues in other scientific areas.This means that the analytical chemist science,food science, geology,and other fields.The field of analytical chemistry is advancing rapidly.To keep up with the advances,the analytical chemist must understand the fundamentals of common analytical techniques,their capabilities,and their shortcomings.The analytical chemist must understand the problem to be solved,select the appropriate technique or techniques to use,design the analytical experiment to provide relevant data to scientists who will use the data.In addition to understanding the scientific problem,the modern analytical chemist often must also consider factors such as time limitations and cost limitations in providing an analysis.Whether one is working for a government regulatory agency,a hospital,a private company,or a university, analytical data must be legally defensible.It must be of know,documented quality,record keeping,especially computer record keeping,assessing accuracy and precision,statistical handing of data,documenting,and ensuring that the data meet the applicable technical standards are especially aspects of the job of modern analytical chemists.Many analytical results are expressed as the concentration of the measured substance in a certain amount of sample.The measured substances is called the monly used concentration units include molarity(moles of substances per liter of solution),weight percent (grams of substance per gram of sample X100%),and units for trace levels of substances.One part per million(ppm)by weight is one microgram of analyze in a gram of sample,that is,1X 10‐6g analyze/g sample.One part per billion(ppb)by weight is one nanogram of element in a gram of sample or1X10‐9g analyze/g sample.For many elements,the technique known as inductively coupled plasma mass spectrometry(ICP‐MS),can detect parts per trillion of the element,that is,picograms of element per gram of sample(1X10‐12g analyze/g sample).To give you a feeling for these quantities,a million seconds is12days(11.57days,to be exact).One part per million in units of seconds would be one second in12days.A part per billion in units of seconds would be1s in32years,and one part per trillion is one seconds in32000years.Today, lawmakers set environmental levels of allowed chemicals in air and water based on measurements of compounds and elements at part per trillion levels because instrumental methods can detect part per trillion levels of analyzes.It is the analytical chemist who is responsible for generating the data that these lawmakers rely on.New words and expressionsacetylsalicylic乙酰水杨酸,阿司匹林alloy合金,使成合金,减低成色amorphous无定形的,非结晶质的,没有明确结晶结构的antacid抗酸剂,中和酸性的,抗酸性的bulk analysis全分析ceramic陶瓷的,陶瓷制品concentration集中,集合,专心,浓缩,浓度crystalline晶体的,晶体状的,水晶的,结晶体,晶态determination测定,滴定discipline纪律,学科,训练electromagnetic radiation电磁辐射forensic法院的,公开辩论的,辩论术forgery伪造物,伪造罪,伪造gravimetric analysis重量分析microgram微观图,显微照片molarity摩尔浓度nanogram毫微克,纳克neutralize使中性,使(溶液)呈中性;中和pharmaceutical药物,制药学上的pharmacology药理学pictogram皮克qualitative定性的,性质上的quantitative定量的,数量的regulatory agency管理机构sample introduction进样surface analysis表面分析throughput生产量,生产能力,吞吐量titration滴定trillionnum万亿volumetric analysis容量分析Part Four Organic ChemistryFriedrich WohlerWhat is Organic ChemistryOrganic chemistry is a subdiscipline within chemistry involving the scientific study of the structure,properties,composition,reactions,and preparation(by synthesis or by other means) of carbon‐based compounds,hydrocarbons,and their derivatives.These compounds may contain any number of other elements,including hydrogen,nitrogen,oxygen,the halogens as well as phosphorus,silicon and sulfur.Organic compounds are structurally diverse.The range of application of organic compounds is enormous.They either form the basis of or are important constituents of many products including plastics,drugs,petrochemicals,food,explosives and paints.They form the basis of all earthly life processes(with very few exceptions).Physical properties of organic compounds typically of interest include both quantitative and qualitative features.Quantitative information include melting point,boiling point,and index of refraction.Qualitative properties include odor,consistency,solubility,and color.Melting and Boiling PropertiesIn contrast to many inorganic materials,organic compounds typically melt and many boil.In earlier times,the melting point(m.p.)and boiling point(b.p.)provided crucial information on the purity and identity of organic compounds.The melting and boiling points correlate with the polarity of the molecules and their molecular weight.Some organic compounds,especially symmetrical ones,sublime,that is they evaporate without melting.A well known example of a sublimable organic compound is para‐dichlorobenzene,the iodiferous constituent of mothballs. Organic compounds are usually not very stable at temperatures above300℃,although some exceptions exist.SolubilityNeutral organic compounds tend to be hydrophobic,that is they are less soluble in water than in organic solvents.Exceptions include organic compounds that contain ionizable groups as well as low molecular weight alcohols,amines,and carboxylic acids where hydrogen bonding anic compounds tend to dissolve in organic solvents.Solvents can be either pure substances like ether or ethyl alcohol,or mixtures,such as the paraffinic solvents such as the various petroleum ethers and white spirits,or the range of pure or mixed aromatic solvents obtained from petroleum or tar fractions by physical separation or by chemical conversion.。
law of mass conservation 质量守恒定律low of definite (or constant) composition 定组成定律low of definite proportions 定比定律low of multiple proportions 倍比定律nucleus ['njuːkl iəs] n 原子核electron [i'lektrɔn] n 电子proton ['prəutɔn] n 质子neutron ['njuːtrɔn] n 中子atomic number 原子序数mass number 质量数subscript ['sʌbskript] n 下标superscript ['suːpəskri pt; 'sjuː pəskript] n 上标isotope ['aisətəup] n 同位素relative atomic mass/atomic weight/average atomic mass 相对原子质量abundance [ə'bʌnd(ə)ns] n 丰度standard atomic weight 标准原子质量unified atomic mass unit/atomic mass unit 规定原子质量单位ion ['aiən] n 离子anion ['ænaiən] n 阴离子cation ['kætaiən] n 阳离子monatomic [,mɔnə'tɔmik] adj 单原子的polyatomic [,pɔliə'tɔmik] adj 多原子的oxyanion 含氧阴离子radical ion 自由基离子light [lait] n 光electromagnetic radiation/radiant energy 电磁辐射diffraction [di'frækʃn] n 衍射interference [intə'fiər(ə)ns] n 干涉frequency ['friːkw(ə)nsi] n 频率wavelength ['wei vleŋθ; 'we i v leŋkθ] n 波长wave number 波数amplitude ['æmpli tjuːd] n 振幅intensity [in'tensiti] n 强度quantum ['kwɔntəm] n 量子Planck's constant 普朗克常数photoelectric effect 光电效应photon ['fəutɔn] n 光子spectrum ['spektrəm] n 光谱continuous spectrum 连续谱,连续光谱discrete spectrum 离散谱,不连续光谱line spectrum 线状谱,光谱线quantum number 量子数ground state 基态excited state 激发态wavelike properties 波动性matter wave 物质波,德布罗意波momentum [mə'mentəm] n 动量Heisenberg's uncertainty principle 海森堡测不准原理Schrödinger's wave equation薛定谔波动方程wavelike and particle-like behavior 波粒二象性quantum mechanic 量子力学wave mechanic 波动力学wave function 波函数probability density 概率密度,几率密度electron density distribution 电子密度分布atomic orbital 原子轨道principal quantum number 主量子数shell [ʃel] n 层azimuthal /angular /orbital quantum number 角量子数subshell ['sʌbʃel] n 亚层magnetic quantum number 磁量子数Cartesian label 笛卡尔坐标spin quantum number 自旋量子数Pauli exclusion principle 泡利不相容原理diamagnetic [,daiəmæg'netik] adj 反磁性的,抗磁性的paramagnetic [pærəmæg'netik] adj 顺磁性的orbital ['ɔːb it(ə)l] n 轨道electron configuration 电子构型Aufbau principle 构造原理orbital diagram 轨道图degenerate orbital 简并轨道Hundred' s Rule 洪特规则valence shell 价层core electrons 核电子periodic table 周期表group/family 族period 周期alkali metals 碱金属alkaline earth metals 碱土金属halogens ['hælədʒənz] n 卤素nitrogens ['naitrədʒ(ə)nz] n 氮族元素chalcogens ['tʃælkɔgənz] n 氧族元素noble gases 稀有气体representative/maingroup elementstransition elements/metals 过渡元素inner transition elements 内过渡元素lanthanide ['lænθənaid] n 镧系元素actinide ['ækti,naid] n 锕系元素nuclear charge 核电荷electrostatic attraction 静电吸引effective nuclear charge 有效核电荷shield [ʃiːld] n /screen [skriːn] n 屏蔽octet [ɔk'tet] n 八隅体atomic radius 原子半径ionization energy 电离能electron affinity 电子亲和性electronegativity [i'lektrəu,neɡə't ivəti] n 电负性isoelectronic [,aisəuilek'trɔnik; ,aisəuelek'trɔnik] adj 等电子的ionization potential 电离电势,电离位first/second ionization energy 第一/第二电离能electropositive [ilektrə(u)'pɔzitiv] adj 带正电的metal ['met(ə)l] n 金属nonmetal ['nɔn,metəl] n 非金属metalloid ['met(ə)lɔid] n 类金属semiconductor [,semikən'dʌktə] n 半导体metallic character 金属性allotrope ['ælətrəup] n 同素异形体peroxide [pə'rɔksaid] n 过氧化物superoxide [,supər'ɔksaid] n 超氧化物chemical bond 化学键valence electron 共价键ionic [ai'ɔnik] adj 离子的covalent [kəu'veil(ə)nt] adj 共价的metallic [mi'tælik] adj 金属的Lewis symbol 路易斯符号electron pairs 电子对octet rule 八隅体规则bonding pairs/shared pair 成键电子对lone pairs/unshared pair 孤电子resonance structure 共轭结构ionic bond 离子键lattice structure 晶格结构lattice energy 晶格能Born-Haber cycle玻恩-哈伯循环covalent bond 共价键bond order 键级single bond 单键double bond 双键triple bond 三键bond energy/enthalpy/strength 键能bond length 键长network covalent solid 网络共价固体bond polarity 键极性nonpolar covalent bond 非极性共价键polar bond 极性键dipole ['daipəul] n 偶极dipole moment 偶极距permanent dipole moment 永久偶极距electric field 电场polarization [,pəulərai'zeiʃən] n极化polarizability ['pəulə,raizə'biləti] n 极化性,极化度metallic bond 金属键valence shell electron-pair repulsion (VSEPR) 价层电子对互斥理论molecular geometry/shape 分子构型steric number (SN) 立体数bond angle 键角linear ['liniə] adj 线性的trigonal ['trig(ə)n(ə)l] adj 三角形的tetrahedral [,tetrə'hiːdrəl] adj 四面体的trigonal bipyramidal 三角双锥的octahedral [,ɔktə'hidrəl] 八面体的bend [bend] 弯的/angular ['æŋgj ulə] adj有棱角的seesaw ['siːsɔː] adj 交互的,前后动的T-shaped T形的square pyramidal 四棱锥的square planar 平面正方形valence-bond (VB) theory 价键理论overlap [əuvə'læp] n 重叠hybridize ['haɪbrɪdaɪz] n 杂交nonequivalent ['nɔni'kwivələnt] adjequivalent [i'kwiv(ə)l(ə)nt] adj 相等的sp hybrid orbital sp杂化轨道sigma (σ) bondσ键pi (π) bondπ键sp3 hybrid orbital sp3杂化轨道sp3d2 hybrid orbital sp3d2杂化轨道sp3d hybrid orbital sp3d杂化轨道sp3d orbital sp3d轨道Molecular orbital (MO) theory 分子轨道理论molecular orbital 分子轨道bonding orbital 成键轨道antibonding orbital 反键轨道sigma (σ) molecular obitalσ分子轨道sigma star (σ*) molecular obital σ*分子轨道pi(π) molecular obitalπ分子轨道pi star(π*) molecular obital π*分子轨道molecular orbital diagram 分子轨道图intramolecular forces 分子内作用力intermolecular force 分子间作用力ion-dipole 离子偶极dipole-dipole force 取向力hydrogen bonding 氢键dispersion force/ London force 色散力/伦敦力Van der Waals’ force范德瓦尔斯力Van der Waals radius 范德瓦尔斯半径chemical reaction 化学反应reactant [ri'ækt(ə)nt] n 反应物product ['prɔdʌkt] n 产物Derect Combination (Synthesis) 化合反应Chemical Decomposition (Analysis) 分解反应Single Displacement (Substitution) 置换反应Double Displacement (Metathesis) 复分解反应Acid-base reaction 酸碱反应Oxidation-reduction (Redox) reaction 氧化还原反应oxidation number(oxidation state) 氧化数oxidizing agent / oxidant/oxidizer 氧化剂reducing agent/reductant/reducer 还原剂combustion [kəm'bʌstʃ(ə)n] n 燃烧,氧化exothermic reaction 吸热反应endothermic reaction 放热反应Isomerization Reaction 异构化反应Disproportionation Reaction 歧化反应Hydrolysis [hai'drɔlisis] n 水解作用chemical equation 化学方程式law of conservation of mass 质量守恒定律law of conservation of charge 电荷守恒定律stoichiometric coefficient 化学计量系数molecular equation 分子方程式ionic equation 离子方程式total ionic equation 总离子方程式spectator ion 不相关离子net ionic equation 净离子方程式yield [jiːld] n 产率theoretical yield 理论产率actual yield 实际产率percent yield产率百分比vapor [veipə] n 蒸汽gas lows 气体定律Boyle’s Low波义耳定律Charles’s Law查理定律Avogadro’s Law阿伏加德罗定律standard temperature and pressure (STP) 标准温度和压强molar volume 摩尔体积ideal gas law 理想气体定律ideal-gas-equation 理想气体方程ideal gas 理想气体proportionality constant 比例常数gas constant 气体常数partial pressure 分压Dalton’s law of partial pressures道尔顿分压定律mole fraction 摩尔分数Kinetic Molecular Theory 分子动理论continuous [kən'tinjuəs] adj 连续的,不间断的random ['rændəm] adj 无规则的,混乱的attractive/repulsive force 引力/斥力elastic [i'læstik] adj 弹性的average kinetic energy 平均动能root-mean-square 均方根most probable speedmean speed 平均速率effusion [i'fjuːʒ(ə)n] n 渗出,泻出Graham’s law格拉哈姆定律diffusion [di'fjuːʒ(ə)n] n 扩散mean free path 平均自由程real gas 真实气体van der Waals equation范德瓦耳斯方程aqueous solution 水溶液solution [sə'luːʃ(ə)n] n溶液solvent ['sɔlv(ə)nt] n溶剂solute ['sɔljuːt; sɔ'ljuːt] n溶质electrolyte [i'lektrəlait] n 电解质non-electrolyte 非电解质strong electrolyte 强电解质weak electrolyte 弱电解质chemical equilibrium 化学平衡concentration [kɔns(ə)n'treiʃ(ə)n] n 浓度percent composition 组成百分比molarity [məu'læriti] n 摩尔浓度molality [mo'læləti] n 质量摩尔浓度grams per liter 克每升mole fraction 摩尔分数parts per million (ppm) 百万分之一concentrated ['kɔnsntreitid] adj 浓缩的dilution [dai'luːʃn] n 稀释Precipitation reaction 沉淀反应Precipitate [pri'sipiteit] n 沉淀物Solubility [,sɔlju'biləti] n 溶解度Insoluble [in'sɔljub(ə)l] adj 不可溶的Titration [tai'treiʃən] n 滴定Analyte ['ænəlait] n 分析物Titrant ['taitrənt] n 滴定剂Primary standard 一级标准Equivalence point 等当点Indicator ['indikeitə] n 指示剂Chemical equilibrium 化学平衡Forward /reverse reaction 正、逆反应Dynamic equilibrium 动力学平衡Incomplete [inkəm'pliːt] / reversible [ri'vɜːs ib(ə)l] 不完全反应,可逆反应Law of chemical equilibrium 化学平衡定律Law of mass action 质量作用定律Equilibrium constant expression 平衡常数表达式Equilibrium constant 平衡常数Homogeneous equilibria 均相平衡Heterogeneous [,het(ə)rə(u)'dʒiːn iəs; -'dʒen-] adj 均匀的Reaction quotient 反应系数Le Châtelier’s principle勒夏特利原理Thermodynamics [,θɜːmə(u)dai'næmiks] n 热力学Thermochemistry [θɜːməu'kemistri] n 热化学Potential energy 势能Kinetic energy 动能System ['sistəm] n 系统Surrounding [sə'raundiŋ] n 环境Boundary ['baund(ə)ri] n 边界,界限Open system 敞开系统Closed system 封闭系统Isolated system 孤立系统State [steit] n 状态State variable 状态变量State function 状态函数Path function 过程方程Heat [hiːt] n 热量Work [wɜːk] n 功Energy ['enədʒi] n 能量Internal energy 内能First law of thermodynamics 热力学第一定律Law of conservation of energy 能量守恒定律State function 状态函数Endothermic [,endəu'θɜːm ik] 放热的Press-volume work 压力体积功Enthalpy ['enθ(ə)lpi; en'θælp i] n 焓Enthalpy of reaction 反应焓Calorimetry [,kælə'rimitri] n 量热法Calorimeter [,kælə'rimitə] n 量热计Heat capacity 热容Molar heat capacity 摩尔热容Specific heat 比热Bomb calorimeter 弹式量热计Hess’s law盖斯定律Enthalpy of formation 生成焓Standard enthalpy 标准焓Standard enthalpy of formation 标准生成焓Molar heat of solution 摩尔溶解热Heat of dilution 稀释热Molar heat of fusion 熔融热Molar heat of vaporization 摩尔汽化热Arrhenius acid/base 阿伦尼乌斯酸、碱Hydronium [hai'droniəm] n 水合氢离子Neutralization reaction 中和反应Salt [sɔːlt; sɔlt] n 盐Amphoteric [,æmfə'terik] adj 两性的Conjugate base/acid 共轭酸/碱Conjugate acid-base pair 共轭酸碱对Autoionization [,ɔːtəu,aiənai'zeiʃən] n 自电离Ion-product constant 电离平衡常数Acidity [ə'siditi] n 酸性Basicity [bei'sisiti] n 碱性Strong acid 强酸Superacid [,sju:pə'æsid] n 过酸Weak acid 弱碱Acid/base-dissociation constant 酸碱电离常数Degree of ionization 电离度Monoprotic /diprotic /triprotic /polyprotic acid 一元/二元/三元/多元酸Oxoacid 含氧酸Carboxylic acid 羧基Leveling effect 拉平效应Leveling solvent 拉平溶剂Differentiating solvent 辨别溶剂Adduct [ə'dʌkt] n 加合物Common-ion effect 同离子效应Buffer solution 缓冲溶液Buffer capacity 缓冲能力Buffer range 缓冲范围Henderson-Hasselbalch equation汉-哈氏公式Acid-base titration 酸碱滴定pH titration curve pH 滴定曲线pH indicator pH 指示剂solubility equilibrium 溶解平衡solubility-product constant(solubility product) 溶解平衡常数insoluble [in'sɔljub(ə)l]不可溶的slightly soluble 微溶的complex ion 配离子formation constant 形成常数amphoteric metal hydroxide 两亲金属氧化物selective precipitation 选择性沉淀qualitative analysis 定性分析metal complex 金属配合物complex ['kɔmpleks] n 络合物coordination compound 配位化合物ligand ['lig(ə)nd] n 配体donor atom供电子原子;配位原子coordination sphere配位层coordinate covalent bond配位共价键complexing agent络合剂;配位剂coordination number配位数monodentate/unidentate 单齿配体bidentate [bai'denteit] n 两齿配体polydentate 多齿配体chelating agent 螯合剂chelate effect 螯合效应isomer ['aisəmə] n 同分异构体isomerism [ai'sɔmərizəm] n 同分异构现象structural isomer 结构异构体linkage isomerism 键合异构ambidentate 两可配位基的coordination isomer 配位异构体stereoisomer [,steriəu'aisəmə; ,stiə-] n 立体异构体geometric isomerism 几何异构optical isomerism 光学异构cis-trans isomer 顺反异构crystal-field theory 晶体场理论crystal field effect 晶体场效应crystal field splitting energy 晶体场分裂能strong-field ligand 强场配体weak-field ligand 弱场配体d-d transition d-d跃迁spectrochemical series光谱化学系列spin-pairing energy 自旋配对能low-spin complex 低自旋配合物high-spin complex 高自旋配合物porphine卟吩porphyrin ['pɔːf irin] n 卟啉chlorophyll ['klɔːrəfil; 'klɔ-] n 叶绿素Hemoglobin [,hiːməu'ɡləubin] n 血红蛋白Nuclear chemistry 核化学Radiochemistry 放射化学Radiation chemistry 辐射化学Nucleon ['njuːkl iɔn] n 核子Nuclide ['njuːkla id] n 核素Nuclear decay 核衰变Radioactive [,reidiəu'æktiv] adj 放射性的Radionuclide [,reidiəu'njuːkla id] n 放射性核素Radioisotope [,reidiəu'aisətəup] n 放射性同位素Strong/nuclear force 核力、强相互作用Parent ['peər(ə)nt] n 母体Daughter ['dɔːtə]Positron ['pɔzitrɔn] n 正电子Antimatter ['ænti,mætə] n 反物质Activity [æk'tiviti] n 活度Becquerel(Bq) ['bɛkərɛl] n 贝克Curie ['kjuəri] n 居里Geiger counters盖革计数器Scintillation counters闪烁计数器Half-life 半衰期Radiocarbon dating放射性碳测定年代Mass defect质量亏损Nuclear binding energy核结合能Binding energy per nucleon 每个核子的结合能Belt of stability 稳定带Magic number 幻数Radioactive series 放射系Nuclear disintegration series核蜕变[分裂]系Nuclear transmutation核嬗变, 核转化Linear accelerator线性加速器、直线加速器Cyclotron回旋加速器Nuclear fission 核裂变Nuclear chain reaction 核链式反应Critical mass临界物质Nuclear power核动力Nuclear weapon核动力Breeder reactor增殖反应堆Nuclear fusion 核聚变Thermo-nuclear reaction 热核反应Ionizing radiation 电离辐射Absorbed dose 吸收剂量Gray(Gy) [grei] n 戈瑞Rad [ræd] n拉德(辐射剂量单位)Equivalent dose 剂量当量Sieverts (Sv) 希沃特Rems雷姆。
a r X i v :n u c l -t h /9704043v 1 22 A p r 1997Proton polarizability contribution to hydrogen Lam
b shift
I.B.Khriplovich
Budker Institute of Nuclear Physics,630090Novosibirsk,Russia
R.A.Sen’kov
Novosibirsk University,630090Novosibirsk,Russia
Abstract The correction to the hydrogen Lamb shift due to the proton electric and magnetic polarizabilities is expressed analytically through their static values,which are known from experiment.The numerical value of the correction is −71±11±7Hz.High experimental precision attained in the hydrogen and deuterium spectroscopy (see,e.g.,[1])stimulates considerable theoretical activity in this field.In particular,the deuteron polarizability contribution to the Lamb shift in deuterium was calculated in Refs.[2–9].An estimate of the proton polarizability contribution to the Lamb shift in hydrogen was made in Ref.[2].A special feature of the corrections obtained in Refs.[2–9]is that they contain logarithm of the ratio of a typical nuclear excitation energy to the electron mass,ln ¯E/m e .In fact,the contribution of the nuclear electric polarizability to the Lamb shift had been obtained with the logarithmic accuracy in Ref.[10]for an arbitrary nucleus.In the present note we consider the problem of the proton polarizability correction to the Lamb shift in hydrogen.The typical excitation energy for the proton ¯E p ∼300MeV is large as compared to other nuclei (to say nothing of the deuteron).So,the logarithm ln ¯E/m
e is not just a mere theoretical parameter,it is truly large,about 6−7,which makes the logarithmic approximation quite meaningful quantitatively.As distinct from Ref.[10],we take into account in our final formula not only the electric polarizability ¯α,but as well the magnetic one ¯β
(though it does not very much influence the result numerically).In our calculation we follow closely the approach of Ref.[7].In particular,we use the gauge A 0=0for virtual photons,so that the only nonvanishing components of the photon propagator are D im =d im /k 2,d im =δim −k i k m /ω2(i,m =1,2,3).The electron-proton forward scattering amplitude,we are interested in,is
T =4πiα d 4k
k 2−2lk M mn .(1)
Here l µ=(m e ,0,0,0)is the electron momentum.The nuclear-spin independent Compton forward scattering amplitude,which is of interest to as,can be written as
M =¯α(ω2,k 2)E ∗E +¯β
(ω2,k 2)B ∗B =M mn e m e n ∗,(2)
1
where¯αand¯βare the nuclear electric and magnetic polarizabilities,respectively.The structureγi(ˆl−ˆk+m e)γj in(1)reduces to−ωδij.Perhaps,the most convenient succession of integrating expression(1)is as follows:the Wick rotation;transforming the integral over the Euclideanωto the interval(0,∞);the substitution k→kω.Then the integration overωis easily performed with the logarithmic accuracy:
∞dω2
.
m2e
The crucial point is that within the logarithmic approximation both polarizabilities¯αand ¯βin the lhs can be taken atω=0,k2=0.Thefinal integration over d3k is trivial.
0The resulting effective operator of the electron-proton interaction(equal to−T)can be written in the coordinate representation as
¯E
V=−αm e[5¯α(0)−¯β(0)]ln
References
[1]K.Pachucki,D.Leibfried,M.Weitz,A.Huber,W.K¨o nig,and T.W.H¨a nsch,J.Phys.
B29,177(1993).
[2]K.Pachucki,D.Leibfried,and T.W.H¨a nsch,Phys.Rev.A48,R1(1993).
[3]K.Pachucki,M.Weitz,and T.W.H¨a nsch,Phys.Rev.A49,2255(1994).
[4]Yang Li and R.Rosenfelder,Phys.Lett.B319,7(1993);ibid,333,564(1994).
[5]W.Leidemann,and R.Rosenfelder,Phys.Rev.C51,427(1995).
[6]J.Martorell,D.W.L.Sprung,and D.C.Zheng,Phys.Rev.C51,1127(1995).
[7]stein,S.S.Petrosyan,and I.B.Khriplovich,Zh.Eksp.Teor.Fiz.109,1146,1996
[Sov.Phys.JETP82,616(1996)].
[8]J.L.Friar,and G.L.Payne,nucl-th/9702019;Phys.Rev.C,in press(1997).
[9]J.L.Friar,and G.L.Payne,nucl-th/9704032.
[10]J.Bernab´e u and T.E.O.Ericson,Z.Phys.A309,213(1983).
[11]B.E.MacGibbon,G.Garino,M.A.Lucas,A.M.Nathan,G.Feldman,and B.Dolbikin,
Phys.Rev.C52,2097(1995).
3。