2015鲁教版九年级+期末测试
- 格式:doc
- 大小:72.50 KB
- 文档页数:10

济南30中2015届九年级化学上学期期末考试试卷(带解析鲁教版)济南30中2015届九年级化学上学期期末考试试卷(带解析鲁教版)一、选择题(每题只有一个答案正确,请选出填入下表.共40分)1.下列过程中,只发生物理变化的是()A.火箭发射B.节日焰火C.风力发电D.葡萄酿酒考点:化学变化和物理变化的判别..专题:物质的变化与性质.分析:有新物质生成的变化叫化学变化,没有新物质生成的变化叫物理变化.化学变化的特征是:有新物质生成.判断物理变化和化学变化的依据是:是否有新物质生成.解答:解:A、火箭发射中,燃料燃烧属于化学变化,故选项错误;B、节日焰火,火药燃烧属于化学变化,故选项错误;C、风力发电后,还是风,属于物理变化,故选项正确;D、葡萄酿酒,酒是新物质,故选项错误;故选C点评:本考点考查了物理变化和化学变化的区别,基础性比较强,只要抓住关键点:是否有新物质生成,问题就很容易解决.本考点主要出现在选择题和填空题中.2.(2分)《环境空气质量标准》新增PM2.5指标,PM2.5是指大气中直径小于或等于2.5mm的颗粒物.为实现空气质量达标,合理的做法是()A.煤炭直接燃烧B.秸杆焚烧还田C.提倡多开汽车D.开发清洁能源考点:防治空气污染的措施..专题:空气与水.分析:A、煤炭直接燃烧会加剧空气污染;B、秸秆焚烧还田会加剧空气污染;C、提倡多开汽车会加剧空气的污染;D、开发清洁能源,可以保护环境.解答:解:A、煤炭直接燃烧会产生大量的空气污染物,加剧空气污染,不利于实现空气质量达标,故A错误;B、秸秆焚烧还田会加剧空气污染,不利于实现空气质量达标,故B错误;C、提倡多开汽车会加剧空气的污染,不利于实现空气质量达标,故C错误;D、开发清洁能源,可以保护环境,利于实现空气质量达标,故D正确.故选:D.点评:环境问题是社会关注的焦点问题,与之相关的问题就成为中考的热点之一.3.(2分)(2012河北)下图所示的实验操作不正确的是()A.检查气密性B.加入固体粉末C.滴加液体D.过滤粗盐水考点:检查装置的气密性;固体药品的取用;液体药品的取用;过滤的原理、方法及其应用..专题:常见仪器及化学实验基本操作.分析:A、根据检查装置气密性的方法进行分析判断.B、根据固体粉末状药品的取用方法进行分析判断.C、根据胶头滴管的使用方法进行分析判断.D、过滤液体时,注意“一贴、二低、三靠”的原则.解答:解:A、检查装置气密性的方法:把导管的一端浸没在水里,双手紧贴容器外壁,若导管口有气泡冒出,装置不漏气;反之则相反.图中所示操作正确.B、取用粉末状药品,试管横放用药匙或纸槽把药品送到试管底部,图中所示操作正确.C、使用胶头滴管滴加少量液体的操作,注意胶头滴管的位置是否伸入到容器内或接触容器内壁.应垂直悬空在容器口上方滴加液体,防止污染胶头滴管,图中所示操作正确.D、过滤液体时,要注意“一贴、二低、三靠”的原则,图中缺少玻璃棒引流,漏斗下端没有紧靠在烧杯内壁上,图中所示操作错误.故选D.点评:本题难度不大,熟悉各种仪器的用途及使用注意事项、掌握常见化学实验基本操作的注意事项是解答此类试题的关键.4.(2分)(2012济南)化学与我们的生产、生活息息相关,以下说法中,不合理的是()A.工业常用铁桶来储运烧碱B.冬天室内用煤炉取暖要严防煤气中毒C.自来水是纯净物,不含任何杂质D.可用食醋除去热水瓶内壁上的水垢考点:常见中毒途径及预防方法;酸的化学性质;纯净物和混合物的判别..专题:课本知识同类信息.分析:A、金属铁和烧碱不反应;B、煤炭不完全燃烧的产物是一氧化碳,有毒;C、自来水是一种混合物;D、食醋的主要成分是醋酸,可以和碳酸钙以及氢氧化镁反应.解答:解:A、金属铁和烧碱不反应,可以用铁桶来储运烧碱,故A正确;B、煤炭不完全燃烧的产物是有毒的一氧化碳气体,冬天室内用煤炉取暖要严防煤气中毒,故B正确;C、纯净水是纯净物,但在生活中通常所说的纯净物都不是绝对纯净物,纯净物一般指含杂质很少的较纯物质,自来水是一种混合物,故C错误;D、食醋的主要成分是醋酸,可以和水垢的主要成分碳酸钙以及氢氧化镁反应,从而达到除垢的目的,故D正确.故选C.点评:本题是一道化学和生活相结合的题目,可以根据所学知识进行回答,要求学生熟记教材知识,灵活运用.5.(2分)下列物质中,属于纯净物的是()A.液氧B.稀盐酸C.河水D.矿泉水考点:纯净物和混合物的判别..专题:物质的分类.分析:本题考查利用纯净物的概念来判断物质是否为纯净物,宏观上看只有一种物质,微观上只有一种分子.解答:解:A、液氧是由氧气一种物质组成,属于纯净物,故A正确;B、稀盐酸中含有氯化氢和水,属于混合物,故B错;C、河水中含有水和多种可溶性杂质,属于混合物,故C 错;D、矿泉水中含有水和多种矿物质,属于混合物,故D错.故选A.点评:在熟悉概念的基础上能从宏观和微观两个方面来判断纯净物和混合物,还要从社会实践中了解生活中常见物质的组成6.(2分)(2008青岛)减少污染,净化空气,“还我一片蓝天”,已成为世界各国人民的共同心声.下列气体会造成空气污染的是()A.一氧化碳B.二氧化碳C.氧气D.氮气考点:空气的污染及其危害..专题:结合课本知识的信息.分析:本题主要考查空气的污染和防治,并要了解空气的组成,解答时要回顾旧知识,对每一个选项进行比对后再做选择.空气的主要污染物有颗粒物质、一氧化碳、二氧化硫、氮的氧化物、碳氢化合物等,防治空气污染的措施有控制污染源,减少化石燃料的使用,使用清洁能源等.解答:解:A、一氧化碳,该气体是有毒气体,是空气的首要污染物之一,故A正确;B、二氧化碳,是空气成分之一,无毒,对植物光合作用起着重要作用,故B不正确;C、氧气,空气主要成分之一,对人和动物的呼吸有重要作用,故C不正确;D、氮气,空气主要成分之一,无毒,化学性质不活泼,故D不正确;故选A.点评:本题较为简单,只要能记住空气的组成和空气的污染物即可正确解答.7.(2分)对过氧化氢(H2O2)组成的叙述中,正确的是()A.由1个氢分子和1个氧分子组成B.由氢、氧两种元素组成C.由1个水分子和1个氧原子结合而成D.由两个氢元素和两个氧元素组成考点:化学式的书写及意义..专题:化学用语和质量守恒定律.分析:根据化学式的意义进行分析:①宏观意义:a.表示一种物质;b.表示该物质的元素组成;②微观意义:a.表示该物质的一个分子;b.表示该物质的分子构成;进行分析判断.解答:解:A.根据过氧化氢的化学式(H2O2)可知一个过氧化氢分子由由两个氢原子和两个氧原子构成;故说法错误;B.根据过氧化氢的化学式(H2O2)可知过氧化氢是由氢元素和氧元素组成的;故说法正确;C.由过氧化氢的化学式可知,过氧化氢是由过氧化氢分子构成的,不含水分子,故选项说法错误.D.元素是个宏观概念,只讲种类不讲个数,故错误.故选B.点评:本题难度不大,掌握化学式的宏观与微观意义并能灵活运用是正确解答本题的关键.8.(2分)下列关于分子、原子、离子的说法,不正确的是()A.分子是保持物质性质的一种微粒B.原子失去电子就变成阳离子C.原子是构成物质的一种微粒D.在化学反应中原子不可以再分考点:分子、原子、离子、元素与物质之间的关系;原子和离子的相互转化..专题:物质的微观构成与物质的宏观组成.分析:A、根据分子是保持物质化学性质的一种微粒进行解答;B、根据原子和离子的相互转化进行解答;C、根据构成物质的微粒进行解答;D、根据在化学变化中,原子不能再分进行解答.解答:解:A、分子是保持物质化学性质的一种微粒,故A错误;B、原子失去电子就变成阳离子,故B正确;C、原子是构成物质的一种微粒,故C正确;D、在化学变化中,原子不能再分而分子能够再分,故D正确.故选:A.点评:了解分子、原子、离子、元素与物质之间的关系;了解物质的组成和物质的构成;了解在化学变化中,原子与离子的相互转化.9.(2分)下列物质的化学名称与俗名,对应错误的是()A.汞﹣﹣水银B.氧化钙﹣﹣熟石灰、消石灰C.碳酸钠﹣﹣纯碱D.氢氧化钠﹣﹣烧碱、火碱、苛性钠考点:化学式的书写及意义..专题:化学用语和质量守恒定律.分析:根据常见化学物质的名称、俗称进行分析判断即可.解答:解:A.汞俗称水银,故说法正确;B.氧化钙俗称生石灰,故对应错误;C.碳酸钠俗称纯碱、苏打,故说法正确;D.氢氧化钠俗称烧碱、火碱、苛性钠,故D对应正确;故选:B.点评:本题考查了物质的名称与俗称,完成此题,可以依据已有的知识进行.要求同学们加强对基础知识的储备,以便灵活应用.10.(2分)(2007北京)在X+2O2CO2+2H2O的反应中,根据质量守恒定律可判断出X的化学式为()A.CH4B.C2H5OHC.CD.CO考点:有关化学式的计算和推断;质量守恒定律及其应用..专题:元素质量守恒.分析:此题根据质量守恒定律,反应前后原子种类和个数都不变,分别统计出反应前后原子种类及个数,比较分析就可以求出所求物质的化学式.解答:解:根据质量守恒定律,反应前后原子种类和个数都不变,由方程式知生成物中含有的原子种类及原子个数为C,1;H,4;O,4;已知反应物中含有的原子种类及原子个数为O,4;比较分析可知X中含有C和H元素,其原子个数分别为1和4,故X的化学式为CH4.故选A点评:此题主要考查学生对质量守恒定律的实际运用,只有掌握了这一知识的内涵,才能自由驾御,正确解答.11.(2分)(2003济南)曾经有人把工业用盐如亚硝酸钠(NaNO2)误作食盐,用于烹调,引发多次中毒事件.在亚硝酸钠中氮元素的化合价()A.+2价B.﹣3价C.+3价D.+5价考点:化学式的书写及意义;常见元素与常见原子团的化合价;有关元素化合价的计算;有关化学式的计算和推断..专题:化学式的计算;化学用语和质量守恒定律.分析:在化合物中,元素的化合价代数和为零.解答:解:在亚硝酸钠中,钠元素的化合价是+1,氧元素的化合价是﹣2,可以求出氮元素的化合价是+3.故选C.点评:解答本题要理解在单质中,元素化合价为零,在化合物中,各种元素的化合价不为零,但是代数和为零.12.(2分)(2011黔南州)生活中处处有化学,下列说法中不合理的是()A.用食醋可以除去水壶中的水垢B.用肥皂水涂抹蚊子叮咬处,可以减轻痛痒C.用洗洁精清洗餐具,可以除去油污D.油锅着火时,可以用水浇灭考点:酸的化学性质;乳化现象与乳化作用;中和反应及其应用;灭火的原理和方法..专题:化学与生活.分析:A、根据食醋与碳酸钙反应分析B、根据肥皂水与蚁酸发生中和反应分析C、根据洗洁精的乳化功能分析D、根据灭火方法分析解答:解:A、食醋与水垢的主要成分碳酸钙能反应,故A正确B、肥皂水显碱性,蚊子叮咬后,在人的皮扶内分泌出蚁酸,能与肥皂水发生中和反应,故B正确C、洗洁精具有乳化功能,能乳化油污,故C正确D、油锅着火时不能用水浇灭,因为油比水的密度小,当水倒入锅中后,油恢浮在水上面,使得油仍然可以与空气的氧接触,从而起不到灭火的作用.如果遇到这种情况,应该用锅盖把锅盖上或倒入菜,故D错误;故答案为D点评:本题考查与生活紧密相关的化学知识,让学生体会化学来源于生活服务于生活.13.(2分)(2012济南)某粒子结构示意图如图所示,下列对该粒子的判断中,错误的是()A.原子核内有12个质子B.该粒子是阴离子C.在化学变化中易失电子D.该粒子属于金属元素考点:原子结构示意图与离子结构示意图..专题:化学用语和质量守恒定律.分析:因为粒子种类与粒子结构间的关系是:阳离子的核内质子数>核外电子数;阴离子的核内质子数<核外电子数;原子的核内质子数=核外电子数.所以可知该粒子为镁原子.金属元素的原子的最外层电子一般少于4个,在化学变化中容易失去电子,达到8电子(或2电子)稳定结构;解答:解:A、从图示可以看出,该元素核内质子数为12,故正确;B、该粒子核内质子数等于核外电子数,都为12,为镁原子,说法错误;C、该元素的原子最外层上有2个电子,易失去2个电子达到稳定结构,故正确.D、该元素的核内存在12个质子,属于镁元素,镁元素属于金属元素,故正确;故选B.点评:本题主要考查原子结构示意图的意义、掌握核内质子数和核外电子数的关系,学会区别阳离子、阴离子、原子的微粒的电子层排布特点是解题的关键.14.(2分)下列除杂所选用的试剂和操作方法错误的一组是(括号内为杂质)()选项待提纯物质选用试剂和操作方法ACaO[Ca(OH)2]加入适量的水BCO(CO2)将混合气体通过足量的NaOH溶液CFe(Zn)加入足量的FeSO4溶液,过滤DNaCl(Na2CO3)加入适量的HCl溶液A.AB.BC.CD.D考点:物质除杂或净化的探究;常见气体的检验与除杂方法;金属的化学性质;碱的化学性质;盐的化学性质..专题:物质的分离、除杂、提纯与共存问题.分析:根据原物质和杂质的性质选择适当的除杂剂和分离方法,所谓除杂(提纯),是指除去杂质,同时被提纯物质不得改变.除杂质题至少要满足两个条件:①加入的试剂只能与杂质反应,不能与原物质反应;②反应后不能引入新的杂质.解答:解:A、CaO能与水反应生成氢氧化钙,反而会把原物质除去,不符合除杂原则,故选项所采取的方法错误.B、CO2能与氢氧化钠溶液反应生成碳酸钠和水,CO不与氢氧化钠溶液反应,能除去杂质且没有引入新的杂质,符合除杂原则,故选项所采取的方法正确.C、Zn能与足量的FeSO4溶反应生成硫酸锌和铁,再进行过滤,能除去杂质且没有引入新的杂质,符合除杂原则,故选项所采取的方法正确.D、适量的HCl溶液能与Na2CO3反应生成氯化钠、水和二氧化碳,能除去杂质且没有引入新的杂质,符合除杂原则,故选项所采取的方法正确.故选:A.点评:物质的分离与除杂是中考的重点,也是难点,解决除杂问题时,抓住除杂质的必需条件(加入的试剂只与杂质反应,反应后不能引入新的杂质)是正确解题的关键.15.(2分)下列叙述中,正确的是()A.二氧化碳分子是由一个碳原子和一个氧分子构成的B.原子在化学变化中也是可分的C.核外电子排布相同的粒子一定属于同种元素D.不同元素的原子的核电荷数一定不同考点:分子和原子的区别和联系;原子的定义与构成;元素的概念..专题:物质的微观构成与物质的宏观组成.分析:根据已有的物质微观粒子的关系即物质的组成进行分析解答即可.解答:解:A、二氧化碳分子是由碳原子和氧原子构成的,错误;B、原子是化学变化中的最小的微粒,在化学变化中不能再分,错误;C、核外电子排布相同的微粒不一定是同一种元素,例如钠离子和氖原子,错误;D、不同的元素的原子核内质子数不同,故核电荷数一定不同,正确;故选D.点评:本题考查的是物质的微观构成粒子的知识,完成此题,可以依据已有的知识进行.16.(2分)(2007南充)废旧计算机的某些部件含有Zn,Fe、Cu、Ag、Pt(铂)、Au(金)等金属,经物理方法初步处理后,与足量稀盐酸充分反应,然后过滤,剩余的固体中不应有的金属是()A.Cu、AgB.Fe、ZnC.Pt、CuD.Ag、Au考点:金属活动性顺序及其应用..专题:结合课本知识的信息.分析:在金属活动性顺序中,氢前的金属可以与酸发生置换反应.解答:解:在金属活动性顺序中,Zn、Fe、Cu、Ag、Pt、Au的活动性顺序为Zn>Fe>H>Cu>Ag>Pt>Au,与足量稀盐酸充分反应,则锌和铁参加反应,剩余的固体中不可能含有锌和铁,分析选项,故选B.点评:本题考查了金属活动性顺序的应用,完成此题,可以依据金属活动性顺序及其意义进行.17.(2分)学习化学时会遇到许多“相等”,下列有关“相等”的说法中错误的()A.原子中核内质子数和核外电子数一定相等B.溶液稀释前后溶质的质量一定相等C.中和反应中参加反应的酸和碱质量一定相等D.物质发生化学变化前后,质量总和一定相等考点:原子的定义与构成;用水稀释改变浓度的方法;中和反应及其应用;质量守恒定律及其应用..专题:物质的微观构成与物质的宏观组成;化学用语和质量守恒定律;溶液、浊液与溶解度;常见的酸酸的通性.分析:A、根据原子中各微粒间的数量关系分析解答;B、根据溶液稀释的方法分析解答;C、根据中和反应的知识分析解答;D、根据质量守恒定律分析解答即可.解答:解:A、在原子中核内质子数和核外电子数一定相等,正确;B、溶液稀释前后溶质的质量一定相等,正确;C、中和反应中参加反应的酸和碱质量不一定相等,例如氢氧化钠和盐酸反应,只要二者的质量比满足80:73,二者即可恰好完全反应,错误;D、物质发生化学变化前后,质量总和不变,故质量一定相等,正确;故选C.点评:本题考查是化学中常见的相等的关系,完成此题,可以依据已有的知识进行.18.(2分)(2012烟台)实验小组探究盐酸和氢氧化钠反应过程中溶液pH的变化规律,得到如图所示曲线.下列有关该实验事实的说法正确的是()A.该实验是将氢氧化钠溶液滴入盐酸中B.B点表示盐酸和氢氧化钠恰好完全反应C.A点时的溶液能使酚酞试液变红D.C点时,溶液中的溶质只有氯化钠考点:中和反应及其应用;酸碱指示剂及其性质;溶液的酸碱性与pH值的关系..专题:常见的酸酸的通性.分析:氢氧化钠溶液呈碱性,其pH大于7,盐酸呈酸性,其pH小于7,氢氧化钠溶液和盐酸可以发生中和反应,恰好完全反应时其pH等于7,氢氧化钠过量时显碱性,盐酸过量时显酸性.解答:解:A、根据图象可以看出,开始时溶液的pH大于7,溶液呈碱性,故是向氢氧化钠溶液中加入稀盐酸,故A错误;B、B点对应的pH等于7,表明氢氧化钠溶液和稀盐酸恰好完全反应,故B正确;C、从图象可以看出,A点溶液呈碱性,酚酞试液在碱性溶液中显红色,故C说法正确;D、C点的pH继续减小,说明酸还没有完全反应,故溶液中含有氯化氢溶质,故D错误,故选BC.点评:本题以图象的形式考查了酸碱中和反应过程中溶液pH的变化,完成此题,要抓住曲线变化的趋势和关键点的意义.19.(2分)(2011济南)甲、乙、两种不含结晶水的固体物质的溶解度曲线如图所示.根据图示判断,下列说法中,不正确的是()A.甲、乙两种物质的溶解度都随温度的升高而增大B.在t1时,甲、乙两物质饱和溶液溶质的质量分数相等C.将t1时甲的饱和溶液升温(溶剂不蒸发),溶液浓度增大D.当甲含有少量乙时,可用冷却热饱和溶液的方法提纯甲考点:固体溶解度曲线及其作用..专题:溶液、浊液与溶解度.分析:A、根据溶解度曲线图回答本题;B、根据饱和溶液溶质质量分数计算方法考虑;C、根据升温后各量的变化情况考虑;D、根据所提纯物质溶解度变化情况考虑.解答:解:A、通过图示可知甲、乙两种物质的溶解度都随温度的升高而增大,故A说法正确;B、饱和溶液溶质质量分数与溶解度有关,而在t1时,甲、乙两物质溶解度相等,所以饱和溶液溶质的质量分数相等,故B说法正确;C、将t1时甲的饱和溶液升温后由饱和溶液变为不饱和溶液,溶质、溶剂都不变,所以溶液浓度不变,故C说法错误;D、由于甲溶解度随着温度变化比较大,所以结晶析出的方法是冷却热饱和溶液的方法,故D说法正确.故选C.点评:通过回答本题知道了溶解度曲线表示的意义,知道了溶解度如何比较大小,饱和溶液溶质质量分数的计算方法,固体物质从溶液中结晶析出的方法.20.(2分)(2013江西模拟)碳酸氢钠用于焙制糕点,在270℃时分解:2NaHCO3Na2CO3+H2O+CO2↑.现取NaHCO316.8g,在敞口容器中加热到质量不再改变为止,减少的质量为()A.4.4gB.6.2gC.8.8gD.10.6g考点:根据化学反应方程式的计算..专题:有关化学方程式的计算.分析:根据碳酸氢钠在270℃时分解的化学方程式可知2NaHCO3Na2CO3+H2O+CO2↑,在敞口容器中加热到质量不再改变为止时,剩余固体为碳酸钠,由所取碳酸氢钠的质量计算反应后生成碳酸钠的质量,根据质量守恒定律,反应前后固体的质量差即减少的质量.解答:解:设反应生成碳酸钠的质量为x2NaHCO3Na2CO3+H2O+CO2↑16810616.8gx=x=10.6g则减少的质量=16.8g﹣10.6g=6.2g故选:B.点评:解答本题时,由于在敞口容器中加热,反应生成的二氧化碳和水都会导致质量减少,因此,可利用质量守恒定律计算反应前后固体的质量差,求得减少的质量.二、填空题(化学方程式每空2分,其余每空1分,共30分)21.(4分)按要求写出下列名称或符号.(1)2个氧分子2O2;(2)2N两个氮原子;(3)2个钙离子2Ca2+;(4)氧化镁中镁元素呈正二价O.考点:化学符号及其周围数字的意义..专题:化学用语和质量守恒定律.分析:(1)根据分子的表示方法:正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字,进行解答;(2)根据标在元素符号前面的数字表示原子的个数;进行解答;(3)根据离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.若表示多个该离子,就在其元素符号前加上相应的数字;进行解答;(4)根据元素化合价的表示方法:确定出化合物中所要标出的元素的化合价,然后在其化学式该元素的上方用正负号和数字表示,正负号在前,数字在后,进行解答.解答:解:(1)根据分子的表示方法:正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字,因此2个氧分子表示为:2O2;故答案为:2O2;(2)根据标在元素符号前面的数字表示原子的个数;因此2N表示为:两个氮原子;故答案为:两个氮原子;(3)根据离子的表示方法:在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.若表示多个该离子,就在其元素符号前加上相应的数字;因此2个钙离子表示为:2Ca2+;故答案为:2Ca2+;(4)根据元素化合价的表示方法:确定出化合物中所要标出的元素的化合价,然后在其化学式该元素的上方用正负号和数字表示,正负号在前,数字在后,因此氧化镁中镁元素呈正二价表示为:O;故答案为:O.点评:本题主要考查学生对化学用语的书写和理解能力,题目设计既包含对化学符号意义的了解,又考查了学生对化学符号的书写,考查全面,注重基础.22.(5分)选择C、H、O、Na四种元素中的适当元素,组成符合下列要求的物质,将其化学式填在空格中:(1)一种金属单质Na(2)天然气的主要成分CH4(3)一种碱NaOH(4)纯碱Na2CO3。
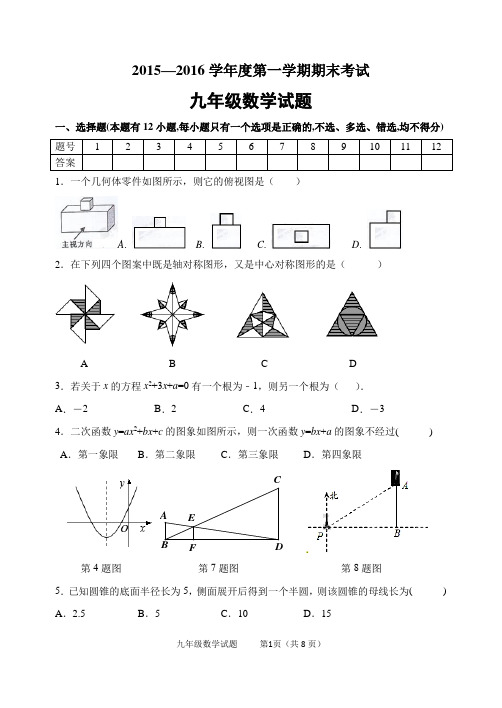
2015—2016学年度第一学期期末考试九年级数学试题一、选择题(本题有12小题,每小题只有一个选项是正确的,不选、多选、错选,均不得分)1.一个几何体零件如图所示,则它的俯视图是()A. B. C. D.2.在下列四个图案中既是轴对称图形,又是中心对称图形的是()A BCD3.若关于x的方程x2+3x+a=0有一个根为﹣1,则另一个根为().A.-2 B.2 C.4D.-34.二次函数y=ax2+bx+c的图象如图所示,则一次函数y=bx+a的图象不经过( ) A.第一象限B.第二象限C.第三象限D.第四象限第7题图第4题图第7题图第8题图5.已知圆锥的底面半径长为5,侧面展开后得到一个半圆,则该圆锥的母线长为( ) A.2.5 B.5 C.10 D.156.一个不透明的盒子中装有6个大小相同的乒乓球,其中4个是黄球,2个是白球.从该盒子中任意摸出一个球,摸到黄球的概率是( )7.如图,已知AB 、CD 、EF 都与BD 垂直,垂足分别是B 、D 、F ,且AB =1,CD =3,那么EF 的长是( )A .13B .23 C .34 D .458.如图,一艘海轮位于灯塔P 的北偏东方向55°,距离灯塔为2 海里的点A 处.如果海轮沿正南方向航行到灯塔的正东位置,海轮航行的距离AB 长是( ) (A )2 海里 (B )2sin 55°海里 (C )2cos 55°海里 (D )2tan 55°海里 9.如图,圆O 是△ABC 的外接圆,∠A =68°,则∠OBC 的大小是( ) A .22° B .26° C .32° D .68°BB第9题图 第11题图 第12题图10.若二次函数y =x 2+bx 的图像的对称轴是经过点(2,0)且平行于y 轴的直线,则关于x的方程x 2+bx =5的解为( )A .120,4x x ==B .121,5x x ==C .121,5x x ==- D .121,5x x =-=11.如图,D 、E 分别是△ABC 的边AB 、BC 上的点,DE ∥AC ,若S △BDE :S △CDE =1:3,则S △DOE:S △AOC 的值为()A .B .C .D .12.如图,A 、B 是双曲线y =上的两点,过A 点作AC ⊥x 轴,交OB 于D 点,垂足为C .若△ADO 的面积为1,D 为OB 的中点,则k 的值为( ) A . B . C . 3 D . 4二、填空题(共5小题,每小题4分,满分20分)13.如图,在⊙O中,AB=CD,∠DCB=28°,则∠ABC= 度.第13题图第15题图第16题图第17题图14.使用计算器进行计算时:在计算器显示DEG状态下,依次按键,结果显示为15.如图,圆内接四边形ABCD中两组对边的延长线分别相交于点E,F,且∠A=55°,∠E=30°,则∠F= .16.如图,在平面直角坐标系xoy中,四边形ODEF和四边形ABCD都是正方形,点F 在x轴的正半轴上,点C在边DE上,反比例函数y=(k≠0,x>0)的图象过点B,E.若AB=2,则k的值为.17.二次函数23=xy的图象如图,点O为坐标原点,点A在y轴的正半轴上,点B、C在二次函数23=xy的图象上,四边形OBAC为菱形,且∠OBA=120°,则菱形OBAC 的面积为.三、解答题(共7小题,共52分)18.计算:1-0)30(sin41)140(tan-45cos2°+++°°;19.为弘扬“齐鲁文化”,某单位开展了“齐鲁文化之都”演讲比赛,在安排1位女选手和3位男选手的出场顺序时,采用随机抽签方式.(1)请直接写出第一位出场是女选手的概率;(2)请你用画树状图或列表的方法表示第一、二位出场选手的所有等可能结果,并求出他们都是男选手的概率.20.如图,在△ABC中,AB=AC,以AC为直径的⊙O交AB于点D,交BC于点E.(1)求证:BE=CE;(2)若BD=2,BE=3,求AC的长.yxAO 21.已知:如图,在平面直角坐标系xOy 中,正比例函数y =34x 的图像经过点A ,点A 的纵坐标为4,反比例函数y =xm的图像也经过点A ,第一象限内的点B 在这个反比例 函数的图像上,过点B 作BC ∥x 轴,交y 轴于点C ,且AC =A B .求:(1)这个反比例函数的解析式; (2)直线AB 的表达式.22.我市准备在相距2千米的M,N两工厂间修一条笔直的公路,但在M地北偏东45°方向、N地北偏西60°方向的P处,有一个半径为0.6千米的住宅小区(如图),问修筑公路时,这个小区是否有居民需要搬迁?(参考数据:≈1.41,≈1.73)23.如图,一条公路的转弯处是一段圆弧().(1)用直尺和圆规作出所在圆的圆心O ;(要求保留作图痕迹,不写作法)(2)若的中点C 到弦AB 的距离为20m ,80 AB m ,求所在圆的半径.)题23第(ABC24.如图隧道的截面由抛物线和长方形构成,长方形的长是12m ,宽是4m .按照图中所示的直角坐标系,抛物线可以用c bx x y ++-=261表示,且抛物线上的点C 到OB 的水平距离为3m ,到地面OA 的距离为217m 。

实验中学2015-2016上学期九年级英语期末综合测试题一. 单项选择。
1. Loud music may make people _______ fastA. to eatB. eatC. ateD. eating2. I am sorry that I have kept you ________ me so long.A. waitingB. waiting forC. to waitD. to wait for3.The old man lived ______ in the village but he didn’t feel ______.A.alone;alone B.lonely;lonely C.alone;lonely D.lonely;alone 4.—Do you like red or pink?—______.I like black.A.Both B.All C.Either D.Neither5.I failed the exam.What _______ news! My parents said that they were ______ at my grades.A.disappointing;disappointing B.disappointing;disappointedC.disappointed;disappointed D.disappointed;disappointing6.My mother often hears me _______ in my room.A.sing B.sang C.to sing D.singing7.—I would rather _______ you the secret right now.—Why not?A.don’t tell B.not to tell C.not tell D.didn’t tell8.I found it boring _______ the lecture.I nearly fell asleep.A.1istening to B.to listen to C.listen to D.listened to 9.The _______ he learns,the _______ knowledgeable he will be.A.more;more B.more;less C.most;most D.most;least10. Mr. Brown always makes his class _________ and keeps his students _________ in class.A. alive; interestingB. lively; interestingC. alive; interestedD.lively; interested11. It’s impolite to laugh at, stare at or play _____on disabled people.A. funB. jokesC. tricksD. pa rts12. We ______ four thousand new words by the end of last year.A. learnedB. had learnedC. have learnedD. will learn13. When I got there, I realized that I ______ my glasses at home.A. forgotB. had forgotC. leftD. had left14. The little girl felt _______ at being the center of attention.A. embarrassingB. embarrassedC. exhaustedD. exhausting15. Do you know your new teacher is one of my ______. He is my uncle.A. relativesB. friendsC. classmatesD. members16.We must do something useful to _______ pollution.A. cut offB. cut upC. cut downD. cut in17.—There are only a few old city walls left.—Yeah.Most of them _______ in the 1960s.A.pulled down B.have pulled downC.had pulled down D.were pulled down18.—What are the _______ of bike riding? —It can help cut down air pollution.A. advantagesB. reasonsC. resultsD. ideas 19.—Are you going to _____ any of the events?—Yes. Maybe long jump and high jump.A. take part inB. joinC. attendD. join in 20.Many overseas Chinese look forward to _______ more about their roots in the summer camp.A.learn B.be learning C.learning D.be learned21.Grandma _______ us stories when we were very young.A.used to tell B.was used to tell C.is used to tell D.was used to telling22.I can’t afford _______ him an expensive birthday present.A. to buyB. buyingC. for buyingD. buy23. Whatever we do, first, we need to have a right _________ towards everything.A. abilityB. attitudeC. memoryD. concentrate24. —Sam, our daughter has grown up. Should we do everything for her?—Oh, dear. Let her _________ her own life.A. behaveB. shareC. organizeD. do25. Scientists can’t _________ on why we need to sleepA. dependB. explainC. guideD. agree26. The amount of sleep we need _________ different from person to person.A. have beenB. isC. areD. has been27. —You look so sleepy. I think you shouldn’t often stay up.—Yes, I find the less I sleep, _________ I do in the work during the day.A. the moreB. the betterC. the fewerD. the worse28. —Let’s go to the KFC, OK?—We’d better eat junk food _________ possible. It’s not healthy. A.as much as B.as little as C.as soon as D.as quikly as29. —Hi, Lucy. Would you like to _________ with me in the shopping center thisweekend?—Certainly, I’d like to.A. help outB. get throughC. hang outD. give out30. I almost forget the worst thing that _______ me last year.A. happenedB. happened toC. took placeD. take place31. The workers were made _____ day and night in the old days.A. workB. workedC. to workD.works32.—Miss Li, I have trouble with my English. What can I do?—Study harder from now on. It’s never _________ late _________ catch up with your classmates.A. too; toB. so; thatC. as; asD. such; that33. —Do you still remember _____ me somewhere in Kunming?—Yes, of course. Two years ago.A. to seeB. seeC. seeingD. saw34. It’s hard for me ______ the teacher when she talked to the class.A. understandB. understoodC. to understandD. understanding35. He used to _____ problems ____ English, but he can speak English very well now.A. have, speakB. has, speaksC. have, speakingD. having, speaking二、阅读理解ADifferent weather makes people feel different.It influences health,intelligence and feelings.In August,it is very hot and wet in the southern part of the United States.People there have heart trouble and other kinds of health problems during this month.In the Northeast and the Middle West,it is very hot at some times and very cold at other times.People in these states have more heart trouble after the weather changes in February or March.The weather can also influence intelligence.For example,in a 1983 report by scientists,IQ of a group of students was very high when a very strong wind came,but after the strong wind,their IQ was 10% lower.The wind can help people have more intelligence.Very hot weather,on the other hand,can make it lower.Students in many schools of the United States often get worse on exams in the hot months of the year(July and August).Weather also has a strong influence on people’s feelings.Winter may be a bad time for thin people.They usually feel cold during these months.They might feel unhappy during cold weather.But fat people may have a hard time in hot summer.At about 18℃ people become stronger.Low air pressure may make people forgetful.People leave more bags on buses and in shops on low-pressure days.People feel best at a temperature of about 18℃. Are you feeling sad,tired,forgetful,or unhappy today? It may be the weather’s problem.1._______ can cause health problems.A.Hot and wet weather B.A strong windC.Warm weather D.Low air pressure2.A report shows that people may have more intelligence when _______ comes. A.rain B.a strong windC.very hot weather D.low air pressure3.According to the writer,fat people may feel bad in ______ weather.A.cold B.cool C.warm D.hot4.The writer wants to tell us that _______.A.hot and cold weather influences all people in the same wayB.weather influences people’s behaviorC.IQ changes when weather changesD.people feel good on low-pressure days5.The best title for this passage is“_______”.A.Hot Weather Causes Health ProblemsB.Different Weather Makes People Feel BadC.Weather Influences FeelingsD.Weather Influences Health,Intelligence and FeelingsBSocial customs and ways of behaving change all the time. Things that were considered impolite many years ago are now acceptable. Just a few years ago, it was considered to be impolite behavior for a man to smoke on the street. No man who thought of himself as being a gentleman would make a fool of himself by smoking when a lady was in the room.Customs also differ from country to country. Does a man walk on the left or the right of a woman in your country? Or doesn’t matter? What about table manners?The Americans and British people not only speak the same language but also share a large number of social customs. For example, in both America and England, people shake hands when they meet each other for the first time. Also most Englishmen will open a door for a woman or offer their seats to women, and so will most Americans. Promptness(准时) is important both in England and in America. That is, the dinner guest either arrive close to the time that has been made or calls up to explain his delay(耽误).The important thing to remember about social customs is not to do anything that might make other people feel uncomfortable—especially if they are your guests. There is an old story about a man who gave a very formal dinner party. When the food was served, one of the guests started to eat his peas with a knife. The other guests were amused or greatly surprised, but the host calmly picked up his knife and began eating in the same way. It would have been bad manners to make his guest feel foolish or uncomfortable.6. The underlined phrase make a fool of himself in the first paragraph means .A. make himself strongB. make himself richC. make himself comfortableD. cause himself to seem like a fool7. According to the passage, the American and the British .A. don’t speak the same languageB. don’t have social customs in commonC. do share a lot of social customsD. do have the exactly same social customs8. If a dinner invitation is for six o’clock, the guest is supposed to arrive at _____ six.A. nearly or a minute later thanB. a quarter pastC. a quarter toD. much later than9. The last example in the passage shows . .A. the correct way to use a knife at tableB. that it is good manners not to make your guest feel foolish or uncomfortableC. that social customs and ways of behaving change too fastD. that different counties have different customs10 .The best title of the passage is .A. Different CustomsB. Social CustomsC. Customs are Changing FastD. Different Countries, Different CustomsCBefore the elevator was invented in the late 1800s,buildings were much smaller and lower,as people did not want to walk up and down stairs(楼梯) all day.With the invention of the elevator came high-rise buildings and skyscrapers.Some people find elevators make them a little uncomfortable because there are many people all standing close in a small room.It is best just to relax and enjoy the ride.When the elevator doors open,stand aside and let everyone out before you try to get in.Even if you are in a hurry,it’s impolite to push someone so that you can get into an elevator.Don’t stare at(盯着看) people or stand too close.Try to keep your eyes looking ahead or you could make others feel nervous or uncomfortable.If you have to move past people to get out of the elevator,say“Excuse me,please”or“I’m sorry”rather than just push them out of the way.Others will think you’r e welcome if you show some politeness.If you are standing close to the buttons(按钮),ask others what floors they are going to and press the buttons for them.This is considered to be polite.In case of emergency(紧急情况),follow the instructions written inside the elevator and try to keep others calm.11. The elevator was invented _______.A.in 1800 B.in 1899 C.in the 18th century D.in the 19th century 12. Before the elevator was invented,buildings were _______.A.big enough B.tall enough C.low and small D.huge but low13. The underlined word“skyscrapers”means“_______”in Chinese.A.天窗 B.摩天大楼 C.天桥 D.冲天火箭14. According to the passage,if you stare at someone in an elevator,he or she may feel _______.A.uncomfortable B.angry C.strange D.calm15. If you have an emergency in an elevator,you should _______.A.cry for help loudlyB.knock on the door loudlyC.follow the elevator instructionsD.call the police for helpDNick is a 14-year-old school boy. His life is full of exams and studies on weekdays. He has little free time. He thinks playing computer games is the best way to make him relax. When he has free time, he sits in front of the computer. Just like that way, he neither eats nor drinks for several hours.Last weekend, he played games on the computer again. He was too excited and didn't want to move. He didn't have anything for six hours. When he had to go to the bathroom, he found he could not move. He was taken to the hospital. The doctor told him he should do some more different kinds of activities. In other words, he needs more exercise and outdoor activities to make him have a healthy body.After coming back from the hospital, Nick follows the doctor's advice. He often plays soccer with his friends. Sometimes he still plays computer games on weekends, but he never does it for long. Now, he lives a happy and healthy life.16.How old is Nick?A.13. B.14. C.15. D.16.17.Nick likes _________ in his free time.A.riding a bike B.playing the guitarC.playing computer games D.having a school trip18.Who took Nick to the hospital?A.His dad. B.His mom.C.His neighbor. D.We are not sure.19.Why was Nick taken to the hospital?A.Because he was too excited.B.Because he had a cold in the morning.C.Because he was too nervous of his studies.D.Because he couldn't move after playing computer games so long.20.Nick should ________ to follow the doctor's advice.A.go to a movie B.listen to music C.take more exercise D.play computer games三、词汇运用I.根据句意和汉语提示写出所缺的单词。

2015~2016学年度第二学期期末试题练习一相对原子质量:1,12,14,16,27,31,65,39,40,35.5,55一.选择题1.在下列各项中,化学反应前后肯定没有发生改变的是()①原子的数目②分子的数目③元素的种类④物质的总质量⑤物质的种类⑥原子的种类A.①③④⑤B.①③④⑥C.①④⑥D.①③⑤2.镁带在耐高温的密闭容器中(内含空气)加热,下列图中能正确表示容器内所有物质总质量(m)与时间(t)的变化关系是()A.B.C.D.3.化学方程式3 + 2O23O4可读作()A.铁加氧气等于四氧化三铁B.三个铁加两个氧气等于一个四氧化三铁C.铁和氧气点燃后生成四氧化三铁D.铁加氧气点燃等于四氧化三铁4.某物质在纯氧中燃烧生成了氮气和水蒸气,该物质一定含有()A.氮元素和氧元素B.氢元素和氧元素C.氮元素和碳元素D.氮元素和氢元素5.化学方程式a C2H2+ b O2 c H2O + d 2配平后a、b、c、d 之和为( )A.15B.14C.13 D.106.在化学反应X + 2Y = Z中,3g X和足量Y充分反应后生成8g Z,则参加反应的Y的质量为()A.2.5 g B.3 g C.5 g D.6 g7.下列各式中,正确表示铝与稀硫酸反应的化学方程式是()A.+ H24 4 + H2↑B.2 3H24 2(4)3 + 3H2↑C.+ 2H24 (4)2 + 2H2↑D.2 + H24 24 + H2↑8.在2 = 2C反应中,已知A的式量为24,C的式量为40,则B 的相对分子质量为()A.16 g B.32 g C.16 D.329.下列说法能用质量守恒定律解释的是()A.水受热变为的水蒸气B.62g磷和80g的氧反应生成142g五氧化二磷C.20g食盐溶于80g水得到100g的食盐水D.拧开盛酒精的瓶盖一段时间后质量变小10.9g水在通电条件下可生成氢气的质量是()A.1 g B.2 g C.3 g D.4 g11.由化学方程式+ 5O242+4H2O,可知x、y、z的值分别是( )mt mtmtmtA.1、2、3 B.2、4、1 C.4、8、2 D.4、4、112.常用燃烧法测定有机物的组成。

九年级模拟试题(六)相对原子质量: H-1 O-16 C-12 Ca-40 Cl-35.5一、选择题(本题包括12小题,每小题2分,共40分。
每小题只有一个....选项符合题意。
)1.化学改变世界的途径是使物质发生化学变化。
下列属于化学变化的是A .石油分馏出汽油、柴油B .利用膜法从海水中获得淡水C .铁矿石冶炼成铁D .工业上从空气中分离出氧气2.下列物质均属于纯净物的是:A .“蒙牛”纯牛奶 矿泉水B .“白佛山”岩石 纯净空气C .“克利策”啤酒 干冰与冰的混合物D .“飘雪”蒸馏水 冰水混合物 3.生活中一些物质的pH 如下表所示,其中显碱性的是柠檬 糖水 牙膏 牛奶 pH 2~3 7 8~9 6~7 A .柠檬 B .糖水 C .牙膏 D .牛奶4.PM2.5是指大气中直径不超过2.5μm 的颗粒物,主要来源是化石燃料的燃烧和扬尘。
它是造成雾霾天气的元凶之一,吸入人体后能直接进入支气管,因而对人体健康影响更大。
下列措施能减少PM2.5污染的是A .鼓励开私家车出行 B .鼓励燃煤火力发电。
C .鼓动使用太阳能热水器D .鼓励焚烧秸秆节约能源5、垃圾分类有利于资源的回收、利用及环境保护。
下列垃圾分类错误..的是 6.下列物质露置于空气中,没有发生化学变化而质量增大的是( )A .浓硫酸B .浓盐酸C .生石灰D .氢氧化钠7、下图所示实验操作中,正确的是( ) 选项 A B C D 垃圾废旧塑料瓶 废旧易拉罐 废旧电池 枯枝落叶垃圾分类 可回收物 其他垃圾 有害垃圾 可堆肥垃圾A.取用液体 B.点燃酒精灯 C.称量氯化钠固体 D.加热固体8.溶液在生产,生活中应用广泛。
下列有关溶液的说法正确的是A.均一、稳定的液体都是溶液 B.溶液中不能同时存两种溶质C.只有固体和液体可以作为溶质 D.外界条件不改变,溶质溶剂不会分离9.物质的性质决定其用途。
下列叙述错误的是A.熟石灰能和酸发生反应,可用熟石灰改良酸性土壤B.氮气化学性质不活泼,可将氮气充入食品包装袋内延长食品保质期C.“洗洁精”有乳化功能,可用“洗洁精”洗涤餐具上的油污D.甲醛能使蛋白质失去生理活性,可用甲醛溶液浸泡水产品防腐10.下列有关原子的说法正确的是A.原子能构成分子,但不能直接构成物质B.原子在化学变化中不能再分,只能重新组合C.碳- 12原子和碳一13原子的中子数相同D.原子中一定含有质子、中子、电子三种粒子11.化学变化的结果是有“新物质”生成.如果把反应物看成是“旧物质”,下面对“新物质”和“旧物质”的理解,正确的是()A.新物质是指世界上没有的物质B.新物质不会再变成其它物质C.新物质和旧物质的性质及用途不可能完全相同D.新物质的元素组成与旧物质的元素组成肯定不同12.下列化学用语中,数字“2”的说法正确的是A.表示离子个数的是⑤⑥ B.表示离子所带电荷数的是④⑤C.表示分子中原子个数的是③⑦ D.表示分子个数的是①②13.下列有关化合价的叙述正确的是A.化合价与原子最外层电子数无关B.氨气(NH3)中氮元素的化合价为+3 C.氧气中氧元素的化合价为-2 D.有些元素在不同条件下可表现出不同化合价14.下列实验方案或措施不合理的是A.用稀盐酸清除铁制品表面的铁锈 B.用氯化钡溶液鉴别稀盐酸和稀硫酸C.用CO2鉴别NaOH和Ca(OH)2溶液 D.用无色酚酞试液检验NaOH溶液是否变质15.下列对宏观现象的微观解释正确的是A .水和过氧化氢的化学性质不同,因为组成元素和分子构成不同B .稀有气体可做保护气,因为原子最外层电子层达到稳定结构C .NaOH 溶于水温度升高,因为Na +、OH -扩散吸收的热量大于水合放出的热量D .水通电生成氢气和氧气,因为水分子中含有氢分子和氧分子16.在一个密闭容器内有X 、Y 、Z 、Q 四种物质,在一定条件下充分反应,测得反应前后各物质的质量见下表。

2015-2016学年山东省济宁市曲师大附中九年级(上)期末化学试卷一、选择题(共10小题,每小题1分,1-4每小题各1分,5-10每小题各2分,满分16分)1.化学改变世界的途径是使物质发生化学变化.下列属于化学变化的是()A.从空气中分离得到氧气和氮气B.从石油中分离得到汽油和柴油C.C从煤碳中焦化得到焦炭和煤焦油D.从海水中利用膜法获得淡水2.2015年联合国发布报告:到2030年,全球将有40%的国家和地区面临干旱问题.节约用水是每个公民应尽的责任和义务.下列关于水的认识不正确的是()A.水是一种常见的溶剂B.将活性炭放入硬水中可使其软化C.工业废水要经过处理后再排放D.肥皂水可鉴别硬水和软水3.生活中的下列现象,用分子的有关知识解释不正确的是()A.湿衣服在太阳底下干得快,说明分子运动速率与温度有关B.金秋时节,人们在桂花树旁会闻到怡人的香味,说明分子在不断运动C.液化石油气须加压后贮存在钢瓶中,说明分子之间有间隙D.水沸腾时能掀起壶盖,说明分子的大小随温度升高而增大4.下列说法错误的是()A.原子的质量几乎都集中在原子核上B.原子通过得失电子形成离子,但离子不能形成原子C.原子、分子、离子、电子都是构成物质的微粒D.由分子构成的物质发生化学变化时,分子本身没有变5.下列对化学式NO2的各种表述中,不正确的是()A.表示二氧化氮是由一个氮元素与两个氧元素组成的B.表示一个二氧化氮分子C.表示二氧化氮这种物质D.表示一个二氧化氮分子由一个氮原子和两个氧原子构成6.如图是四种微粒结构示意图,下列有关各微粒的说法中,错误的是()A.①的化学性质比较稳定 B.③④属于同种元素C.④是一种阴离子D.②容易得到电子7.化学是一门以实验为基础的自然科学,学会正确的实验观察方法十分重要,下列实验现象描述中正确的是()A.磷在空气中燃烧产生大量的白色烟雾B.铁在空气中燃烧火星四射,产生黑色固体C.镁在空气中燃烧产生耀眼白光,生成白色固体D.碳在氧气中燃烧发出白光,产生黑色气体8.在密闭容器中有甲、乙、丙、丁四种物质,在一定条件下充分反应,测得反应前后各物质的质量分数如图所示,下列说法正确的是()A.丙可能是单质B.在该反应中丁一定没有参加化学反应C.该反应是化合反应D.甲和乙的质量之和一定等于生成丙的质量9.A、B、C三种物质的溶解度曲线如图所示.下列分析正确的是()A.t1℃时,A、C两种物质的饱和溶液中溶质的质量相等B.t2℃时,把50gA放入50g水中能得到A的饱和溶液,其中溶质和溶液的质量比为1:3C.将t2℃时A、B、C三种物质的饱和溶液降温至t1℃,所得溶液的溶质质量分数的大小关系是B>C=A•D.将C的饱和溶液变为不饱和溶液,可采用升温的方法向收集满二氧化碳的集气瓶中加入约体积的水,振荡二、填空题(共5小题,每小题4分,满分17分)11.化学用语是学习化学的重要工具,按要求用化学用语填空;(1)2个氮分子;(2)2个硫酸根离子,(3)医疗上用来治疗胃酸过多的一种盐,(4)常用于改良酸性土壤的一种碱.12.(1)某校学生在研究物质燃烧的条件时,进行了以下实验:点燃一支蜡独,竖直放在桌面上,用烧杯罩住(如图l所示),请依据此实验回答问题:①蜡烛燃烧时,观察到烧杯内壁产生水雾,说明石蜡中含有元素②蜡烛熄灭的瞬间,观察到烛芯上方产生一缕白烟,白烟的主要成分是;(2)在老师的指导下,同学们利用氧气传感器测定了蜡烛燃烧过程中氧气含量的变化情况,设计并完成了如图2所示的实验,得出了如图3所示的氧气含量的变化曲图,根据图3所示的曲线图,同学们得出了以下结论.下列说法正确的是填序号)A.只有当氧气耗尽时.蜡烛才会熄灭B.蜡烛熄灭时,集气瓶内仍有氧气C.氧气浓度小于一定值时,蜡烛无法燃烧.13.现有 A、B、C、D、E五种溶液,它们分別是氢氧化钠的溶液、硫酸铜溶液、碳酸钠溶液、.氯化钠溶液和稀硫酸中的一种,鉴别它们可按如图所示的步骤进行.请回答下列问题:(1)B中的溶质是(填化学式);(2)不能与A溶液发生反应的物质类别有;①单质②氧化物③酸④碱⑤盐(3)C溶液为;(4)用X鉴别D、E时,X可以选用不同的物质.①若X为稀盐酸,写出反应的化学方程式;②若X为澄清石灰水,写出反应的化学方程式.14.某氯化钾样品含有杂质氯化钙和氯化镁,设计提纯的流程图如图:请认真分析流程中各步骤的信息作答:(1)溶剂X是;Y溶液中溶质的化学式为:.(2)蒸发操作的目的是除去(填物质的化学式).(3)写出加入过量KOH溶液反应的化学方程式:.15.今年我国多地都不同程度地出现雾霾天气,它严重影响了人们的生活和生命安全,这是2016年元旦前后曲阜空气质量指数变化趋势图.从图中可以看出,近一个月我们这里空气质量能达到优良的天气很少,治理污染,刻不容缓,下列治理雾霾措施切实可行的是.①鼓励开私家车出行②外出时佩戴反雾霾口罩③减少燃煤发电,增大太阳能发电④禁止使用化石燃料⑤控制烟花爆竹的燃放⑥推广乙醇汽油⑦将农田中产生的秸秆就地焚烧⑧将工业废气处理后再排放.三、实验探究题16.如图1装置适当组合可用于O2、H2、CO2等气体的制备和收集.(1)仪器①的名称是,若A装置内药品为MnO2和过氧化氢溶液,反应的方程式为.若A装置内药品为Zn和稀盐酸,反应的方程式为.(2)B装置正放桌面,用于收集A生成的O2,则a接(选填“b”或“c”);若B装满水,用于收集A生成的H2,则a接(选填“b”或“c”).(3)如果要制取和收集干燥的CO2,需要在A装置之后连接两个B装置,则在前一个B装置内加入的试剂是(选填“浓硫酸”或“氢氧化钠”).(4)某白色固体A可能含有碳酸钠、氯化钡(水溶液呈中性),氢氧化钠中的一种或几种,进行如图2实验.完成下列填空:①操作Ⅰ是,根绝上述实验分析,A中一定含有的物质是,C中一定含有的溶质是.四、计算与应用17.2015年12月10日,在瑞典斯德哥尔摩,诺贝尔奖颁奖典礼举行,如图为我国女药学家屠呦呦领奖,谱贝尔生理学或医学奖评産委员会主席齐拉特対新华社记者说“中国女科学家屠呦呦从中药中分离出青蒿素应用子疟疾治疗,这表明中国传统的中草药也能给科学家们带来新的启发.”她表示,经过现代技术的提纯和与现代医学相结合,中草药在疾病治疗方面所取得的成就“很了不起”,青蒿素的分子式为C15H22O5,请计算:(1)青蒿素中碳、氧元素的质量比;(2)青蒿素中元素的质量分数最大.18.某化学兴趣小组的同学在老师的指导下,对一瓶出现变质硬化的氢氧化钙固体变质程度进行探究,测定3g样品中含有杂质的质量,设计实验装置(气密性良好)如图1所示.(1)取3克部分变质的氢氧化钙样品放入烧瓶中.滴入稀盐酸,当观察到(填实验现象),停止滴加稀盐酸;(2)实验结束后,量简内进入水的体积如图2所示,其演数为165mL,已知在该实验条件下,生成气体的密度为2g•L﹣1,则生成C02气体的质量为;(精确到 0.01)(3)请计算3克变质的氢氧化钙祥品中杂质的质量.(精确到0.01)2015-2016学年山东省济宁市曲师大附中九年级(上)期末化学试卷参考答案与试题解析一、选择题(共10小题,每小题1分,1-4每小题各1分,5-10每小题各2分,满分16分)1.化学改变世界的途径是使物质发生化学变化.下列属于化学变化的是()A.从空气中分离得到氧气和氮气B.从石油中分离得到汽油和柴油C.C从煤碳中焦化得到焦炭和煤焦油D.从海水中利用膜法获得淡水【考点】化学变化和物理变化的判别.【专题】物质的变化与性质.【分析】化学变化是指有新物质生成的变化,物理变化是指没有新物质生成的变化,化学变化和物理变化的本质区别是否有新物质生成;据此分析判断.【解答】解:A、从空气中分离得到氧气和氮气过程中没有新物质生成,属于物理变化.B、从石油中分离得到汽油和柴油过程中只是状态发生改变,没有新物质生成,属于物理变化.C、从煤碳中焦化得到焦炭和煤焦油过程中有新物质生成,属于化学变化.D、从海水中利用膜法获得淡水过程中没有新物质生成,属于物理变化.故选C.【点评】本题难度不大,解答时要分析变化过程中是否有新物质生成,若没有新物质生成属于物理变化,若有新物质生成属于化学变化.2.2015年联合国发布报告:到2030年,全球将有40%的国家和地区面临干旱问题.节约用水是每个公民应尽的责任和义务.下列关于水的认识不正确的是()A.水是一种常见的溶剂B.将活性炭放入硬水中可使其软化C.工业废水要经过处理后再排放D.肥皂水可鉴别硬水和软水【考点】硬水与软水;水资源的污染与防治;常见的溶剂.【专题】性质决定用途;空气与水;化学与生活.【分析】A、根据水的作用分析;B、根据活性炭的作用解答;C、根据工业废水要经过处理后再排放可以防止水污染解答;D、根据软硬水的鉴别方法分析.【解答】解:A、水能溶解许多物质,是最常见的溶剂,故A正确;B、加热煮沸可以将硬水转化为软水,活性炭具有吸附性,能够吸附色素和异味,不能将硬水软化,故B错误;C、工业废水要经过处理后再排放可以防止水污染,故C正确;D、加入肥皂水振荡,泡沫丰富的为软水,泡沫少的为硬水,故肥皂水可鉴别硬水和软水,故D正确.故选B【点评】本题考查硬水软水的鉴别、活性炭的作用及环境的保护,属基础知识考查.3.生活中的下列现象,用分子的有关知识解释不正确的是()A.湿衣服在太阳底下干得快,说明分子运动速率与温度有关B.金秋时节,人们在桂花树旁会闻到怡人的香味,说明分子在不断运动C.液化石油气须加压后贮存在钢瓶中,说明分子之间有间隙D.水沸腾时能掀起壶盖,说明分子的大小随温度升高而增大【考点】利用分子与原子的性质分析和解决问题.【专题】物质的微观构成与物质的宏观组成.【分析】根据已有的知识进行分析,闻到气味是因为分子在不断的运动,分子的运动与温度有关;分子间有一定的间隔,分子间的间隔与温度有关,据此解答.【解答】解:A、湿衣服在太阳底下干得快,说明分子运动速率与温度有关,温度越高,运动的越快,故A正确;B、闻到气味是因为分子在不断的运动,故B正确;C、液化石油气须加压后贮存在钢瓶中,说明分子之间有间隙,故C正确;D、水沸腾时能掀起壶盖,说明分子的间隔随温度升高而增大,不是分子的大小,故D错误;故选D.【点评】本题考查了分子的基本性质,完成此题,可以依据已有的知识进行.4.下列说法错误的是()A.原子的质量几乎都集中在原子核上B.原子通过得失电子形成离子,但离子不能形成原子C.原子、分子、离子、电子都是构成物质的微粒D.由分子构成的物质发生化学变化时,分子本身没有变【考点】原子的定义与构成;分子、原子、离子、元素与物质之间的关系;原子和离子的相互转化;分子的定义与分子的特性.【专题】物质的微观构成与物质的宏观组成.【分析】根据已有的物质的微观构成粒子之间的关系进行分析解答即可.【解答】解:A、原子的质量几乎都集中在原子核上,正确;B、原子通过得失电子形成离子,离子能得失电子形成原子,错误;C、电子不是构成物质的微粒,错误;D、由分子构成的物质发生化学变化时,分子本身没有变,正确;故选BC.【点评】本题考查的是物质的微观构成粒子的知识,完成此题,可以依据已有的知识进行.5.下列对化学式NO2的各种表述中,不正确的是()A.表示二氧化氮是由一个氮元素与两个氧元素组成的B.表示一个二氧化氮分子C.表示二氧化氮这种物质D.表示一个二氧化氮分子由一个氮原子和两个氧原子构成【考点】化学式的书写及意义.【专题】物质的微观构成与物质的宏观组成;化学用语和质量守恒定律.【分析】根据由分子构成的物质的化学式的含义:微观表示物质的一个分子;表示一个分子的构成;宏观:表示一种物质;表示该物质的元素组成;进行解答.【解答】解:A.可以表示二氧化氮是由氮元素和氧元素组成的,而元素是个宏观概念,只讲种类、不讲个数,故错误;B.可以表示一个二氧化氮分子,故正确;C.可以表示二氧化氮这种物质,故正确;D.可以表示一个二氧化氮分子是由一个氮原子和两个氧原子构成的,故正确.故选A.【点评】本题考查学生对物质化学式含义的理解与在解题应用的能力6.如图是四种微粒结构示意图,下列有关各微粒的说法中,错误的是()A.①的化学性质比较稳定B.③④属于同种元素C.④是一种阴离子D.②容易得到电子【考点】原子结构示意图与离子结构示意图.【专题】化学用语和质量守恒定律.【分析】A、若粒子的最外层电子数为8(氦为2个),属于相对稳定结构.B、元素是质子数(即核电荷数)相同的一类原子的总称,同种元素的粒子是质子数相同,据此进行分析判断.C、当质子数=核外电子数,为原子;当质子数>核外电子数,为阳离子;当质子数<核外电子数,为阴离子.D、若原子的最外层电子数≥4,在化学反应中易得电子,若最外层电子数<4,在化学反应中易失去电子.【解答】解:A、①的最外层电子数为8,化学性质比较稳定,故选项说法正确.B、元素是质子数(即核电荷数)相同的一类原子的总称,决定元素种类的是质子数(即核电荷数),③④的质子数相同,属于同种元素,故选项说法正确.C、④质子数=11,核外电子数=10,质子数>核外电子数,为阳离子,故选项说法错误.D、②的最外层电子数是7,大于4,在化学反应中易得到1个电子而形成阴离子,故选项说法正确.故选:C.【点评】本题难度不大,考查学生对粒子结构示意图及其意义的理解,明确粒子中核内质子数和核外电子数之间的关系是解题的关键.7.化学是一门以实验为基础的自然科学,学会正确的实验观察方法十分重要,下列实验现象描述中正确的是()A.磷在空气中燃烧产生大量的白色烟雾B.铁在空气中燃烧火星四射,产生黑色固体C.镁在空气中燃烧产生耀眼白光,生成白色固体D.碳在氧气中燃烧发出白光,产生黑色气体【考点】氧气与碳、磷、硫、铁等物质的反应现象.【专题】实验现象的观察和记录.【分析】A、根据磷在空气中燃烧的现象进行分析判断.B、根据铁在空气中燃烧的现象进行分析判断.C、根据镁在空气中燃烧的现象进行分析判断.D、根据碳在氧气中燃烧的现象进行分析判断.【解答】解:A、磷在空气中燃烧,产生大量的白烟,而不是白色烟雾,故选项说法错误.B、铁丝在空气中只能烧至发红,不会产生火星,故选项说法错误.C、镁在空气中燃烧产生耀眼白光,生成白色固体,故选项说法正确.D、碳在氧气中燃烧,发出白光,生成能使澄清石灰水变浑浊的气体,故选项说法错误.故选:C.【点评】本题难度不大,掌握常见物质燃烧的现象即可正确解答,在描述物质燃烧的现象时,需要注意光和火焰、烟和雾的区别.8.在密闭容器中有甲、乙、丙、丁四种物质,在一定条件下充分反应,测得反应前后各物质的质量分数如图所示,下列说法正确的是()A.丙可能是单质B.在该反应中丁一定没有参加化学反应C.该反应是化合反应D.甲和乙的质量之和一定等于生成丙的质量【考点】质量守恒定律及其应用;反应类型的判定.【专题】化学用语和质量守恒定律.【分析】本题可分析甲~丁四种物质反应前后各物质的质量分数,根据质量守恒定律,确定是反应物还是生成物,据此结合题意进行分析判断即可.【解答】解:由四种物质反应前后各物质的质量分数可知,反应前后甲的质量分数减少了70%﹣42%=28%,故甲是反应物;同理可以确定乙的质量分数减少了14%﹣8%=6%,故乙是反应物;丙的质量分数增加了40%﹣6%=34%,丙是生成物;丁的质量分数不变,可能作该反应的催化剂,也可能没有参加反应.A、该反应中甲、乙是反应物,丙为生成物,符合“多变一”的特征,属于化合反应,则其一定是化合物,故选项说法错误.B、丁的质量分数不变,可能没有参加反应,也可能作该反应的催化剂,故选项说法错误.C、该反应的反应物为甲和乙,生成物是丙,符合“多变一”的特征,属于化合反应,故选项说法正确.D、该反应的反应物为甲和乙,生成物是丙,根据质量守恒定律,参加反应的甲、乙的质量之和(而不是甲、乙的质量之和)一定等于生成丙的质量,故选项说法错误.故选:C.【点评】本题难度不大,考查的是质量守恒定律的应用,解题的关键是分析图中数据,灵活运用质量守恒定律9.A、B、C三种物质的溶解度曲线如图所示.下列分析正确的是()A.t1℃时,A、C两种物质的饱和溶液中溶质的质量相等B.t2℃时,把50gA放入50g水中能得到A的饱和溶液,其中溶质和溶液的质量比为1:3C.将t2℃时A、B、C三种物质的饱和溶液降温至t1℃,所得溶液的溶质质量分数的大小关系是B>C=A•D.将C的饱和溶液变为不饱和溶液,可采用升温的方法【考点】固体溶解度曲线及其作用;饱和溶液和不饱和溶液相互转变的方法;溶质的质量分数、溶解性和溶解度的关系.【专题】溶液、浊液与溶解度.【分析】根据固体的溶解度曲线可以:①查出某物质在一定温度下的溶解度,从而确定物质的溶解性,②比较不同物质在同一温度下的溶解度大小,从而判断饱和溶液中溶质的质量分数的大小,③判断物质的溶解度随温度变化的变化情况,从而判断通过降温结晶还是蒸发结晶的方法达到提纯物质的目的.【解答】解:A、t1℃时,A、C两种物质的饱和溶液的质量不能确定,所以溶质质量也不能确定,故A错误;B、t2℃时,A物质的溶解度是50g,所以把50gA放入50g水中能得到A的饱和溶液,其中溶质和溶液的质量比为:25g:75g=1:3,故B正确;C、将t2℃时A、B、C三种物质的饱和溶液降温至t1℃,t1℃时,B物质的溶解度大于A物质的溶解度,C物质的溶解度随温度的升高而减小,所以C物质降温后,没有晶体析出,所得溶液的溶质质量分数的大小关系是B>A>C,故C错误;D、C物质的溶解度随温度的升高而减小,所以将C的饱和溶液变为不饱和溶液,可采用降温的方法,故D错误.故选:B.【点评】本题难度不是很大,主要考查了固体的溶解度曲线所表示的意义,及根据固体的溶解度曲线来解决相关的问题,从而培养分析问题、解决问题的能力.向收集满二氧化碳的集气瓶中加入约体积的水,振荡【考点】化学实验方案设计与评价;吸入空气与呼出气体的比较;二氧化碳的化学性质;生石灰的性质与用途;中和反应及其应用.【专题】实验设计题;简单实验方案的设计与评价.【分析】二氧化碳能使澄清石灰水变浑浊;二氧化碳能够溶于水;稀硫酸和氢氧化钠反应生成氯化钠和水,稀硫酸和硝酸钡反应生成白色沉淀硫酸钡和硝酸,硫酸钠和硝酸钡反应生成白色沉淀硫酸钡和硝酸钠;氧化钙和水反应生成氢氧化钙,氢氧化钙溶液显碱性,pH大于7.【解答】解:A、常温下,同时分别向同体积的盛有空气样品和呼出气体样品的集气瓶中滴加相同滴数的饱和澄清石灰水,振荡,呼出气体样品的集气瓶中,澄清石灰水变浑浊,而盛有空气样品的集气瓶中无明显现象,因此可以用澄清石灰水探究人体吸入的空气与呼出的气体中二氧化碳含量的不同;B、向收集满二氧化碳的集气瓶(玻璃瓶,不会发生形变)中加入约体积的水,振荡,无明显现象,因此不能用向收集满二氧化碳的集气瓶中加入水的方法验证二氧化碳与水反应生成碳酸;C、稀硫酸和氢氧化钠反应生成氯化钠和水,稀硫酸和硝酸钡反应生成白色沉淀硫酸钡和硝酸,硫酸钠和硝酸钡反应生成白色沉淀硫酸钡和硝酸钠,因此不能用向稀硫酸与氢氧化钠溶液反应后所得的溶液中满加硝酸钡溶液的方法探究稀硫酸与氢氧化钠钠溶液恰好完全反应;D、氧化钙和水反应生成氢氧化钙,氢氧化钙溶液显碱性,pH大于7,因此不能用先加足量水溶解,振荡,用洁净干操玻璃棒蘸取溶液.滴到 pH试纸上的方法探究生石灰是否变质.故选:A.【点评】合理设计实验,科学地进行实验、分析实验,是得出正确实验结论的前提,因此要学会设计实验、进行实验、分析实验,为学好化学知识奠定基础.二、填空题(共5小题,每小题4分,满分17分)11.化学用语是学习化学的重要工具,按要求用化学用语填空;(1)2个氮分子2N2;(2)2个硫酸根离子2SO42﹣,(3)医疗上用来治疗胃酸过多的一种盐NaHCO3,(4)常用于改良酸性土壤的一种碱Ca(OH)2.【考点】化学符号及其周围数字的意义.【专题】化学用语和质量守恒定律.【分析】(1)分子的表示方法,正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字.(2)离子的表示方法,在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.若表示多个该离子,就在其离子符号前加上相应的数字.(3)医疗上用来治疗胃酸过多的一种盐是碳酸氢钠,写出其化学式即可.(4)氢氧化钙是常用于改良酸性土壤的一种碱,写出其化学式即可.【解答】解:(1)由分子的表示方法,正确书写物质的化学式,表示多个该分子,就在其化学式前加上相应的数字,则2个氮分子可表示为2N2.(2)由离子的表示方法,在表示该离子的元素符号右上角,标出该离子所带的正负电荷数,数字在前,正负符号在后,带1个电荷时,1要省略.若表示多个该离子,就在其离子符号前加上相应的数字,故2个硫酸根离子可表示为:2SO42﹣.(3)医疗上用来治疗胃酸过多的一种盐是碳酸氢钠,其化学式为:NaHCO3.(4)氢氧化钙是常用于改良酸性土壤的一种碱,其化学式为:Ca(OH)2.故答案为:(1)2N2;(2)2SO42﹣;(3)NaHCO3;(4)Ca(OH)2.【点评】本题难度不大,掌握常见化学用语(分子符号、化学式、离子符号等)的书写方法是正确解答此类题的关键.12.(1)某校学生在研究物质燃烧的条件时,进行了以下实验:点燃一支蜡独,竖直放在桌面上,用烧杯罩住(如图l所示),请依据此实验回答问题:①蜡烛燃烧时,观察到烧杯内壁产生水雾,说明石蜡中含有氢元素②蜡烛熄灭的瞬间,观察到烛芯上方产生一缕白烟,白烟的主要成分是石蜡颗粒;(2)在老师的指导下,同学们利用氧气传感器测定了蜡烛燃烧过程中氧气含量的变化情况,设计并完成了如图2所示的实验,得出了如图3所示的氧气含量的变化曲图,根据图3所示的曲线图,同学们得出了以下结论.下列说法正确的是BC 填序号)A.只有当氧气耗尽时.蜡烛才会熄灭B.蜡烛熄灭时,集气瓶内仍有氧气C.氧气浓度小于一定值时,蜡烛无法燃烧.【考点】燃烧的条件与灭火原理探究;蜡烛燃烧实验;质量守恒定律及其应用.【专题】科学探究.【分析】(1)根据质量守恒定律反应前后元素的种类不变解答;(2)根据白烟的主要成分是石蜡颗粒进行解答;(3)根据已有的知识结合图示进行分析,反应前氧气的体积分数是19.56%,当氧气的体积分数达到15.96%时,蜡烛熄灭,说明氧气的浓度低于一定值时,蜡烛无法燃烧,任何化学反应都遵循质量守恒定律.【解答】解:(1)蜡烛燃烧时,观察到烧杯内壁产生水雾,根据质量守恒定律反应前后元素的种类不变,说明石蜡中含有氢元素;(2)(白烟的主要成分是石蜡颗粒,所以用火柴点燃白烟,观察到的现象是白烟能燃烧;(3)A、由图示可知,蜡烛熄灭时,集气瓶内仍有氧气,故错误;B、蜡烛熄灭时,集气瓶内仍有氧气,故对;C、当氧气的体积分数达到15.96%时,蜡烛熄灭,说明氧气的浓度低于一定值时,蜡烛无法燃烧,故正确;答案:(1)氢;(2)石蜡颗粒;(3)BC.【点评】此题是一道实验过程的考查题,解题的关键是对每项实验过程的详细分析,结合题目的要求作出判断,属基础性实验考查题.。
2014-2015学年第一学期九年级质量检测化学试题(时间:60分钟,满分:100分)一、选择题(本题包括12题,每小题3,共36分.每小题只有一个选项符合题意)1、下列物质的溶液长期放置在空气中,溶液质量因发生化学变化而减少的是 ( )A.烧碱B.石灰水C.浓盐酸D.氯化钾2、稀盐酸和稀硫酸具有相似的化学性质,其本质原因是()A 都能与指示剂作用B.都能解离出酸根离子C.都能解离出氢离子D.都含有氢元素3、以下说法正确的是()A.溶液一定是均一、无色、稳定的B.均一、稳定的液体一定是溶液C.溶液的溶质一定是固体D.溶液一定是混合物4、光亮的铁钉在下列几种情况下,最不容易生锈的是()5、生活中许多物质都与化学密切相关,如“雪碧”等碳酸饮料的pH小于7.若将“雪碧”饮料晃动后再打开瓶盖,其pH将()A.变小B.变大C.不变D.无法确定6、2008年9月27日,太空第一次留下了“中国人的脚印”.我国研制的航天员舱外服为航天员成功进行太空行走提供了可靠的保证.航天员舱外服内含有与氢氧化钠性质相似的氢氧化锂(LiOH),它不可能具有化学性质是()A.与二氧化碳反应B.与盐酸反应C.与氧化铁反应 C.与氯化铜溶液反应7、下列关于实验操作中先后顺序的叙述错误的是 ( )A.实验室制取气体时,先装药品,再检查装置气密性B.用托盘天平称量药品时,先调节天平平衡,再称量C.实验室用CO与 Fe2O3反应制取铁时,先通CO,再点燃酒精灯D.稀释浓硫酸时,先在烧杯内倒入水,再缓慢注入浓硫酸,并不断搅拌8、以下是人体几种体液的pH,其中呈酸性的是()A.胰液7.5~8.0 B.胃液0.9~1.5C.血浆7.35~7.45 D.胆汁7.1~7.39、有下列四种实验设计及操作,实验过程中其现象不足以说明C02与Na0H溶液发生了反应的是( )A. B. C. D10逻辑推理是化学学习中常用的思维方法,下列推理正确的是 ( )A.中和反应有盐和水生成,因此有盐和水生成的反应一定是中和反应B.氧化物中含有氧元素,而含氧元素的化合物不一定是氧化物C.碱中都含有氢元素,所以含有氢元素的化合物一定是碱D.置换反应一定有单质生成,所以有单质生成的反应一定是置换反应11、是氢氧化钠溶液与稀盐酸恰好完全反应的微观示意图,由此得出的结论不正确的是()A.反应结束时溶液的pH=7B.反应前后元素的种类没有变化C.氢氧化钠溶液中存在的粒子是Na+和OH-D.该反应的实质是H+和OH-结合生成H2O分子12、将质量相等的A、B、C三种金属,同时分别放入三份溶质质量分数相同且足量的稀盐酸中,反应生成H2的质量与反应时间的关系如图所示。
14--15年九年级化学第一学期期末检测题<三>可能用到的相对原子质量:H:1 O:16 C:12 Mn:55一、填空题(每小题只有一个正确答案,每小题3分,共36分)可能用到的相对原子质量:H 1 O l6 C l2 Zn 65 S 32 Cu 64 Mg 241.氧元素和硫元素的本质区别是()A.中子数不同B.质子数不同C.电子数不同D.相对原子质量不同2.下列变化中,属于化学变化的是()A.滴水成冰B.酒精燃烧C.水乳交触D.苹果榨计3.下列物质中属于纯净物的是()A.白酒B.加碘盐C.干冰颗粒D.食醋4.下图是用来表示物质间发生化学变化的模型示意图,圈中“●“○”分别表示两种不同元素的原子。
能用该示意图表示的反应是()A.2H2O2 MnO2 2H2O+O2↑B. 2C0十02点燃 2C02C.H2+Cl2 点燃 2HClD. C+02 点燃 C025.用分子的观点解释下列现象,不合理的是()A.食物变质——分子本身发生了变化 B.汽油挥发——分子大小发生了变化C.热胀冷缩——分子间隔发生了变化 D.花香四溢——分子作扩散运动6.雄伟壮观的国家大剧院主体建筑表面安装了近两万块钛(Ti)金属板。
下列关于钛及其化合物的说法正确的是()A.Ti可以表示钛元素,也可以表示一个钛原子,还可以表示钛单质B.TiO2中含有氧分子 C.CaTiO3属于氧化物D.Ti原子核内有22个质子,则Ti3+核外有25个电子7.用氯化钠配制100g溶质质量分数为0.9%的生理盐水,现有下列操作:①配制溶液;②称取氯化钠固体;③过滤;④量取蒸馏水;⑤计算。
正确的操作顺序是()A.①②③④ B.⑤②④①C.⑤①②④D.③②④①8.下列分别为二氧化碳的制取、干燥、收集和性质检验的装置图,其中错误的是()9.下图形象地表示某反应前后反应物与生成物分子及其数目的变化,其中分别表示A、B、C三种不同的分子。
该反应的化学方程式中A、B、C前的化学计量数之比为()A.4:1:3 B.3:1:2 C.4:1:2 D.3:1:310.丙氨酸是一种氨基酸,其相对分子质量是89,其中氮元素的质量分数是15.8%,则每个丙氨酸分子中氮原子个数是() A.1 B.2 C.3 D.411.用计算机模拟确认,60个原子可结合成一个N60分子。
2015年期末模拟化学试题(四)可能用到的相对原子质量:Na 23 O 16 S 32一、单项选择(每小题2分,共16分)题号 1 2 3 4 5 6 7 8答案1.(2014菏泽)家庭生活中处处都有物质变化,下列发生在家庭生活中的变化属于物理变化的是()A.鲜牛奶变质B.酵母粉发酵C.钢丝球刷碗D.洁厕精除垢2.(2014定西)下列图中所示的实验操作正确的是()A.取用大理石B.加热液体C.测定溶液的pHD.稀释浓硫酸3.(2014兰州)某市5月26日的空气质量日报如下:项目空气污染指数空气质量级别空气质量可吸入颗粒物65 Ⅱ良二氧化硫 6二氧化氮20.下列各项对表中三个空气质量指标不会产生影响的是()A.露天焚烧垃圾B.用煤做燃料C.用氢气做燃料D.用洒水车洒水4.(2014抚州)下列物质属于纯净物的是()A.冰水B.海水C.盐水D.雨水5.(2014安徽)高铁酸钠(Na2FeO4)是一种新型高效的水处理剂。
下列有关高铁酸钠的说法正确的是()A.属于氧化物B.钠、铁、氧三种元素质量比是2:1:4C.铁元素化合价为+6 D.由两种金属和一种非金属组成6.(2014赤峰)对于下列几种化学符号,有关说法正确的是()①N ②Na+③④P2O5⑤KClO3.A.表示物质组成的化学式有①④⑤B.表示阳离子的有②③C.④中数字“5”表示五氧化二磷中有5个氧原子D.⑤中各元素的质量比为1:1:37.(2014齐齐哈尔)下列实际应用中,利用中和反应原理的是()①用氢氧化钠溶液洗涤石油产品中的残余硫酸②用碳酸氢钠治疗胃酸过多③用熟石灰改良酸性土壤④用稀氨水涂抹在蚊子叮咬处(分泌出蚁酸)止痒.A.①②B.②③④C.②③D.①③④8.(2014德阳)金属单质M与非金属单质硫发生如下反应为2M + S △M2S。
甲、乙二组学生在实验室分别进行该实验,加入的M和硫的质量各不相同。
充分反应后,实验数据记录如下表,则M的相对原子质量是()A.64 B.56C.39 D.23二、填空题(每空一分,共22分)9.(2014梅州,5分)化学与生活息息相关。
莱芜市莱城区南冶中学2015-2016学年上学期九年级英语期末综合检测试卷(1-10单元)一、单项选择1. –My son seldom has breakfast.--It is unhealthy habit. You must ask him to change it.A. /; anB. the; anC. /; aD. the; a2. If you meet some new words, you’d better in a dictionary.A. look out themB. look them outC. look them upD. look up them3. Her son coke, but now he milk.A. used to drink; is used to drinkingB. used to drinking; drinksC. is used to drinking; used to drinkD. is used to drink; is drinking4. –Let me help you carry the box, Granny.--Thank you, Li Lei. It’s very nice you me.A. for; to helpB. of; to helpC. of; helpingD. for; helping5. my way school, I happened to meet one of my old friends.A. In; atB. On; inC. In; toD. On; to6. The shop sells flowers is at the end of the street.A. whoB. whereC. whichD. what7. –Is Tom in the next room?--Well, it’s hard to say. But I heard him loudly when I passed by just now.A. speakB. to speakC. spokenD. speaking8. Do you believe that paper is made wood?--Yes, I do. And can see that books are made paper.A. from; fromB. from; ofC. of; fromD. of; of9. We need some players for the game. you your brother can join us.A. Not butB. Neither; norC. Either; orD. Not only; but also10. I regret out too late.A. stayingB. staysC. to stayingD. stay11. The woman you saw at the park be Jane. She is at work now.A. mustB. mightC. canD. can’t12. At present, lots of people would rather in the country because there ispollution in the city.A. live; moreB. to live; lessC. live; lessD. to live; more13. Miss Wang is patient me and always encourages me hard.A. to; studyB. with; to studyC. with; studyD. in; studying14.–Do you know the movie Harry Potter?--Yes. I it twice. It’s funny.A. sawB. seeC. have seenD. will see15. –Is the classroom yours?--No. is on the second floor.A. weB. ourC. ours.D. us16. The children were hungry and the salad up quickly.A. was eatingB. ateC. was eatenD. is eating17. The traffic accident happened on Saturday. , no one was hurt.A. LuckB. LuckilyC. LuckyD. Unlucky18. -- of volunteers will be needed for Olympic Games.--Let’s go and them.A. Thousands; joinB. Thousand; join inC. Three thousand; take part inD. Thousands; take part in19. –We get on well with our friends in our daily life.--You are right. One finger cannot lift a small store.A. supposed toB. are supposed toC. suppose toD. are suppose to20. I have some tickets for the basketball match. I wonder .A. where you buy the ticketsB. why you like to go thereC. if you’d like to come alongD. when you watch the match21. Remember to hand in your homework time.A. aboutB. forC. onD. by22. –We must keep our classroom every day.-Yes, Miss Gao.A. cleanB. cleanedC. to cleanD. cleaning23. Sam saw a wallet on the ground and he it up quickly.A. putB. gaveC. pickedD. kept24. You have done very well. You have just learned Russian for only two months .A. at allB. after allC. in allD. above all25. –You are under great pressure, aren’t you?-Yes. I have trouble every night.A. sleepsB. to sleepC. sleepingD. slept26. This made me angry. She didn’t say , either.A. a little of; somethingB. a little of; anythingC. a little; somethingD. a little; anything27. Flooding is one of in the world.A. biggest problemsB. biggest problemC. the biggest problemsD. the biggest problem28. – is the height of the tree?– It’s more than .A. What; 20 meters longB. How; 20 meters tallC. How much; tall 20 metersD. What; 20 meters tall29. We never visit a friend’s house without first.A. callB. callingC. callsD. called30. We should try our best to prevent him from the same mistake again.A. makeB. to makeC. makingD. made31. Students are not supposed at school.A. to smokeB. smokingC. smokeD. smokes32. –Let’s go to the KFC, OK? -We’d better eat junk food possible. It’s not healthy.A. as little asB. as much asC. as often asD. as well as33. We are all looking forward to senior high school.A. to goB. to goingC. for goD. for going34. Vera hospital because she broke her arm.A. takes toB. took toC. was takenD. was taken to35. The Great Wall lots of tourists every day.A. causesB. attractsC. bringD. carry36. Oh, Mom. My cake is three times than Lucy’s. Why do you give her that large one?A. as small asB. the smallestC. biggerD. smaller37. I can’t understand it. The question which Wang Xiaoya asked just now is really easy to answer. , many people gave the wrong answer.A. HoweverB. ButC. ThereforeD. And38. – ?– I cut my hand and it’s bleeding.A. What’s the matter with youB. What’s wrong with youC. What’s your troubleD. All of the above39. –We often talk with each other on QQ.-Really? Will you please show me it?A. what to useB. how to useC. what I can useD. how can I use40. – It seems that the old man has .– Yes, he is to build a big house for himself.A. enough money; rich enoughB. enough money; enough richC. money enough; enough richD. money enough; rich enough二、阅读理解AGood afternoon, and welcome to England. We hope that your visit here will be a pleasant one. Today, I would like to draw your attention to a few of our laws. The first one is about drinking. Now, you may not buy alcohol(酒) in this country if you are under 18 years of age, nor may your friends buy it for you.Secondly, noise. Enjoy yourselves by all means, but please don’t make unnecessary noise, particularly at night. We ask you to respect other people who may wish to be quiet.Thirdly, crossing the road. Be careful! The traffic moves on the left side of the road in this country. Use pedestrian crossings(人行横道) and do not take any chances when crossing the road.My next point is about litter. It is an offence(违法行为) to drop litter in the street. When you have something to throw away, please put it in your pocket and take it home, or put it in a litter bin.Finally, as regards something, it is against the law to buy cigarettes or tobacco(烟草) if you are under 16 years of age.I’d like to finish by saying that if you require any sort of help, you should contact your local police station, who will be pleased to help you.1. The main purpose of this speech would be to .A. prepare people for international travelB. tell the laws of different kindsC. tell people of the punishment for breaking lawsD. give advice to travelers to the country2. How many laws are there discussed in the speech?A. Three.B. FourC. FiveD. Six.3. The underlined word “contact” in the seventh paragraph means .A. 保持B.参加C.报告D. 联系4. From the speech we learn that .A. in this country, if you are under 18 years of age, you may not buy alcohol, but your friend can buy it for youB. you may not buy cigarettes of tobacco unless you are above 16 years of ageC. because the traffic moves on the left side of the road, you must use pedestrian crossings when crossing the road.D. you can’t make noise except at night5. Who do you think is probably to make the speech?A. A policeman.B. A worker at a hotelC. A lawyer.D. An hostess(空姐)BFun dayTo celebrate the Year of the TigerFree SoftDrinksOrganized by Finding Fun Youth Centre and Nowhere HighSchoolDate: 17 February 2015Time: 11am-9pmPlace: Nowhere High School PlaygroundFee: ﹩20(buy three get one free)Programs: drama, lion dance, magic show and balletperformanceHighlights: 1) enter the lucky draw(抽奖) to win a digitalcamera2) learn to make festival foodJoin us on the Fun day! All are welcome!Join us on the Fun day! All are welcome!6. What you have just read is a(an) .A. noteB. reportC. noticeD. poster7. What is going to take place on 17 February 2015?A. A big event to welcome a Chinese new year.B. A social gathering to raise money for wildlife.C. A party for close friends to meet and have fun.D. A meeting of Nowhere High School students.8. How much do you have to pay in total if five of you go together?A. $40.B. $60.C. $80.D. $1009. Which performance can’t you watch?A. Lion danceB. Dolphin showC. Magic showD. Ballet performance10. If you want to join in the event, you can’t get there at .A. 1pm.B. 11am.C. 9pm.D. 8pm.CAs China’s influence grows, many students in the US are working hard to learn Chinese. Parents and education experts(专家) in the US think Chinese speakers may have greater chances.The number of schools that teach Chinese has grown. Ten years ago, only about 300 schools in the US had Chinese lessons. Today, about 1,600 schools teach Chinese. Lomond Elementary School in Ohio is one of them. Each week, all students in grades one through five have a one? hour Chinese class. They learn the language through songs, games and lots of talking.“We’re trying to make students interested in the language,”teacher James told Times For kids. “We think it’s really important that all children receive these lessons,” he says. “We’d like to do even more.”“Schools are looking to the future,”says Nancy, a US education expert. “China is becoming a really powerful country. Young people should not only know the language but understand the culture.”Students may not be thinking about the future, but they think learning Chinese language and culture is fun.“We wish we could go to China one day.” said Karl and his classmate, Walt, from Miami.More than 40 million foreigners around the world are studying Chinese. About 50,000 people in the US are learning the language. Chinese has become the second most spoken foreign language in the US after Spanish.11. At present, many American students are learning Chinese because .A. they wish to work in ChinaB. they would like to travel all over ChinaC. it is the most useful language in the worldD. China is becoming a powerful country12. Which of the following is TRUE about Lomond Elementary School?A. All students have a Chinese class everydayB. Chinese culture is not taught in schoolC. Teachers think interest is important in learning Chinese.D. Only the students in the fifth grade can learn Chinese.13. is an American education expert.A. JamesB. NancyC. KarlD. Walt14. We can learn from the passage thatA. students are looking to the futureB. there are more Spanish people than Chinese in the USC. Chinese is becoming popular in the USD. Chinese is the most popular foreign language in the US15. What is the best title of the passage?A. US Kids Learn ChineseB. Foreigners Study ChineseC. Learning Chinese Is FunD. Chinese Speakers Have GreaterChancesDDo you know why different animals or pests (昆虫) have their special colors? Colors in them seem to be used mainly to protect themselves.Some birds like eating locusts (蝗虫), but birds cannot easily catch them. Why? It is because locusts change their colors together with the change of the colors of crops(庄稼). When crops are green, locusts look green. But as the harvest time comes, locusts change to the same brown color as crops have. Some other pests with different colors from plants are easily found and eaten by others. So they have to hide themselves for lives and appear only at night.If you study the animal life, you’ll find the main use of coloring is to protect themselves. Bears, lions and other animals move quietly through forests. They cannot be easily seen by hunters (猎人). This is because they have the colors much like the trees.Have you ever noticed an even stranger act? A kind of fish in the sea can send out a kind of very black liquid (液体)when it faces danger. While the liquid spreads over (散开), its enemies (敌人)cannot find it. And it immediately swims away. So it has lived up to now though it is not strong at all.16.From the passage we learn that locusts________.A. are small animalsB. are easily found by birdsC. are dangerous to their enemiesD. change their colors to protect themselves17.How can pests with different colors from plants keep out of danger?A. They run away quickly.B. They have the colors much like their enemies.C. They hide themselves by day and appear at night.D. They have to move quietly.18.Bears and lions can keep safe because _________.A. they have the colors much like the treesB. they move quietlyC. they like brown and gray colorsD. they live in forests19.Why can the kind of fish live up to now?A. Because it is very and strong.B. Because the liquid it sends out can help it escape from its enemies.C. Because the liquid it sends out can kill its enemies.D. Because it swims faster than any other fish.20.Which is the best title for this passage?A. The Change of Colors for Animals and PestsB. Colors of Different Animals and PestsC. The Main Use of Colors for Animals andPestsD. Some Animals and Pests三、单词拼写根据句义完成句中已给出首字母的单词。
期末综合测试卷I.单项选择(共15题,计15分)1. I won the speech competition and my classmates were proud _______ me.A. inB. toC. ofD. for2. The old man wanted to live somewhere quiet. He always asked us to keep _______.A. quickB. silentC. activeD. healthy3. The students are interested in English and they all hope _____ an English club.A. joinB. joiningC. to joinD. joined4. I can't understand what the book is about because it ____ in English.A. writesB. will writeC. is writtenD. will be written5. I can’t go to the movie with you. I have to stay at home and ______ my sick sister.A. look atB. look afterC. look forD. look up6.My aunt is a writer. She _____ ten books since 2003.A. writesB. wroteC. will writeD. has written7. They _______a lot of people since they became volunteers in 2010.A. helpB. helpedC. will helpD. have helped8. Chinese ______ at many high schools in that country today.A. is taughtB. is teachingC. will teachD. was taught9. —Mary, is this your MP5?—Let me see. Oh, no. ______ is in my schoolbag.A. MyselfB. MeC. MyD. Mine10. My father ______ Shanghai on business. He ______ back in two weeks.A. has gone to; comesB. has gone to; will beC. has been to; comesD. has been to; will be11.—Have you seen the funny movie Let the Bullets Fly?—Yes, it made me ______ many times.A. laughB. cryC. sleepD. sing12. —What were you doing when the earthquake happened in Japan?—I ______ in the street when I heard the news.A. walkB. walkedC. am walkingD. was walking13. — ______ have you been away from your hometown, Alan?—Since 5 years ago.A. How manyB. How longC. How muchD. How often14. —Hello, Mike. Long time no see. Where were you?—Oh, not only my parents but also I ______ Hainan for a month.A. have gone toB. have been toC. has been inD. have been in15. —Could you tell me ______ to the bank, please?—Yes. It’s down Central Street on your right.A. how I gotB. how I can getC. how can I getD. how did I getII.完形填空(共10题,计15分)The best thing to start your day is a good breakfast. There are different 1 of breakfasts around the world.“Eat breakfast like a king, lunch like a prince and dinner like a pauper(穷人).” 2 the old saying tells us, breakfast is the most important meal of the day. It 3 us with energy after a long night without food.If people don’t have breakfast in the morning, they will feel tired and get angry easily. Breakfast helps children stay more focused in school. A study showed that children who eat breakfast regularly score 4 in most subjects.Breakfast is also important for weight loss. If you have a balanced breakfast, you will eat 5 throughout the day.A healthy and 6 breakfast should include some protein(蛋白质) like eggs, milk or a little meat, which makes you energetic all day.Carbohydrates(碳水化合物)like rice or bread can keep you active. Vegetables and fruit 7 the vitamins that humans need.8 ,in China, breakfast is different from region to region. Porridge with pickles(咸菜),baozi, noodles, soybean milk and youtiao are common throughout the 9 country. Although Western culture has influenced China a lot, most people still 10 traditional Chinese breakfast. They think that porridge and noodles are not only easily digested(消化), but also can provide enough energy in the morning.1.A. ways B. types C. rules D. methods2.A. So B. Though C. As D. When3.A. provides B. gives C. offers D. has4.A. lower B. less C. higher D. more5.A. less B. fewer C. more D. much6.A. experienced B. balanced C. excited D. different7.A. has B. have C. is D. are8.A. Therefore B. Thus C. Because D. However9.A. all B. one C. whole D. total10.A. dislike B. know C. want D. preferIII.阅读理解(共15题,计30分)AThe days are getting shorter and shorter, and temperatures are dropping. Winter is coming and it is easy for people to catch flu. Many people have started taking vitamin C pills as a precautionary(预防的)way. But a research has shown that vitamin C pills do not provide as much protection as they think. They are even not as good as other ways, like often washing your hands. Some German scientists have given people information and a test on the subject helping to stop incorrect ways.Whether it is caused by cold or flu(流行性感冒), a runny nose and sore throat are signs of catching a cold. Many people overestimate(高估) the advantages of vitamin C and other pills. For years it was believed that taking vitamin C pills not only provided protection against colds but also against cancers, helping people to live longer. But the result of the research showed disagreement on these beliefs(看法). The result has now come out on the website . “Some pills may not help people live longer. Some pills may even lead to earlier death,” says Professor Peter Sawicki. Scientists also say that the best way to get vitamins is through food instead of having vitamin pills. That is to say, we disagree with the way of taking vitamin C pills. There are many simple but useful ways to lower the risk of catching a cold. These include often washing your hands with normal soap(肥皂) and water and not touching your face with your hands. People who have already had a cold can stop it from spreading by throwing away tissues(纸巾) at once after using them and not shaking hands with other people.1.Most people believe that taking vitamin C pills can _______.A.make them catch a cold easilyB.provide protection against coldsC.make them clevererD.prevent all illnesses2.A runny nose and sore throat mean someone ________.A.will catch a cold in a few daysB.would never catch a coldC.has caught a coldD.would never spread colds to others3.The best way to get vitamins is _______.A.eating food which contains VitaminsB.taking vitamin pillsC.drinking water which contains vitaminsD.eating as much pork as possible4._______ is NOT an effective way to lower the risk of catching a cold.A.Not touching your face with your handsB.Often washing your hands with normal soap and waterC.Not shaking hands with those people who have already had a coldD.Not throwing away tissues at once after using them5.About taking vitamin C pills, _______ is shown by the research.A.fighting against sore throatB.fighting against colds and cancersC.helping people live longerD.leading to earlier death by some pillsBSince the beginning of time, man has invented many interesting things. Some of these inventions, like the number and the radio, have certainly changed history. Since 1946, one of the most important inventions has been the computer.The first computer was built at Harvard University in 1944. It was as large as a room and quite difficult and slow to operate. But since the invention of the silicon chip(硅片), computers have become smaller, easier to use, and faster to operate. Some computers are as small as television sets. Some computers can be made smaller than a book. And computers are getting smaller all the time.There are several reasons why the computer is useful. First, it can store very, very large amount of information. Second, the computer can operate very quickly. Third, modern computers can be built into other kinds of machines, like radios, cars and planes. They can do a lot of work for us.Soon, almost everyone, either at home or at work, will use some kind of computer. The lives of all of us will be changed by this invention.1. Which has been one of the most important inventions since 1946?A. The number.B. The radio.C. The computer.D. The silicon chip.2. The first computer was built ______.A. in the middle of the 19th centuryB. in the middle of the 20th centuryC. at the beginning of timeD. at the beginning of this century3. A modern computer can be made ______.A. as large as a roomB. smaller than a bookC. smaller than silicon chipsD. like a radio4. The computer has been made much better since the invention of ______.A. the silicon chipB. the radioC. the color TV setD. the dictionary5. The computer is useful because ______.A. it can store lots of informationB. it can store much moneyC. it can operate very quickly and can be built into other machinesD. both A and CCHaving Fun “Growing” and “Stealing”Recently, an Internet game has become a new fashion among young office workers and students.People can “farm” on a piece of “land” and “grow”, “sell” or even “steal” “vegetables”, “flowers” and “fruit” on the Internet.They can earn some e-money and buy more “seeds”, “pets” and even “houses”.Joyce interviewed some young people.Here are their opinions.Harold:I don’t quite understand why they are so mad about the childish game. Maybe they are just not confident enough to face the real world.Allan:I enjoy putting some “bugs” in my friends’ gardens and we’ve become closer because of the game.Having fun together is the most exciting thing about it.Laura:You know, people in the city are longing for(渴望) the life in the countryside.It reduces my work pressure(压力);besides, it gives me the exciting experience of being a “thief”.Ivy:Well, it’s ju st a waste of time.Teenagers playing the game spend so many hours on it that they cannot focus on (专注于)their study.1.According to the passage, people can’t ______ things in this game.A.grow B. borrow C. steal D.sell2.Among the people Joyce interviewed, ______ likes the game while ______ dislikes the game.A. Laura; Allan B.Allan; Harold C.Harold; Ivy D. Ivy; Allan3. From Laura’s words, we can guess that she’s most probably ______.A. a student B.an office worker C. a farmer D. a thief4.Which is NOT the reason why people like the game?A. They are longing for country life.B.They can have fun with friends.C. The game can relax people and give them a new experience.D.They are confident enough to face the real world.5. Where can you find this passage?A. In a car magazine. B.In an advertisement.C.In a newspaper. D. In a science book.IV.完成句子(共5题,计5分)1.我逐渐习惯了这些事,不再觉得它们这样奇怪。