高中化学各类有机物之间的相互转化关系
- 格式:doc
- 大小:425.50 KB
- 文档页数:3
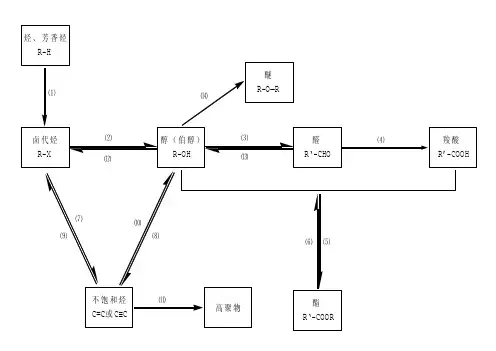
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反响而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃〔烯烃、炔烃、二烯烃等〕⑵ 不饱和烃的衍生物〔烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等〕 ⑶ 石油产品〔裂化气、裂解气、裂化汽油等〕⑷ 含醛基的化合物〔醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等〕 ⑸ 天然橡胶〔聚异戊二烯〕 2、无机物:⑴ -2价的S 〔硫化氢及硫化物〕⑵ + 4价的S 〔二氧化硫、亚硫酸及亚硫酸盐〕 ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3+ 2FeBr 3 6FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 3 2FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2 ⑷ Zn 、Mg 等单质 如⑸ -1价的I 〔氢碘酸及碘化物〕变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O变色Mg + Br 2 === MgBr 2 〔其中亦有Mg 与H +、Mg 与HBrO 的反响〕△HBrO + Na2CO3 = NaBrO + NaHCO3三、能萃取溴而使溴水褪色的物质上层变无色的〔ρ>1〕:卤代烃〔CCl4、氯仿、溴苯等〕、CS2等;下层变无色的〔ρ<1〕:直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃〔如己烷等〕等四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴不饱和烃〔烯烃、炔烃、二烯烃等〕⑵不饱和烃的衍生物〔烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等〕⑶石油产品〔裂化气、裂解气、裂化汽油等〕⑷醇类物质〔乙醇等〕⑸含醛基的化合物〔醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等〕⑹天然橡胶〔聚异戊二烯〕⑺苯的同系物2、无机物:⑴氢卤酸及卤化物〔氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物〕⑵ + 2价的Fe〔亚铁盐及氢氧化亚铁〕⑶-2价的S〔硫化氢及硫化物〕⑷ + 4价的S〔二氧化硫、亚硫酸及亚硫酸盐〕⑸ 双氧水〔H 2O 2〕五、常见的各类有机物的官能团,构造特点及主要化学性质(1)烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 构造特点:键角为109°28′,空间正四面体分子。
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的64 + 32 = 22(4)3 + 2362 + 32 = 43 + 2322 + 32 = 23 + 2I 2⑷ 、等单质 如⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ 等强碱、23和3等盐 2 + H 2O = +2 + 23 = 2 + 2↑+ H 2O + 23 = + 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(4、氯仿、溴苯等)、2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 + 2 2 (其中亦有与、与的反应) △2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的(亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:22;代表物:4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑸天然橡胶(聚异戊二烯)2、无机物:⑴-2价的S(硫化氢及硫化物)⑵ + 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑶ + 2价的Fe6FeSO4 + 3Br2 = 2Fe2(SO4)3 + 2FeBr36FeCl2 + 3Br2 = 4FeCl3 + 2FeBr32FeI2 + 3Br2 = 2FeBr3 + 2I2⑷ Zn、Mg等单质如⑸-1价的I(氢碘酸及碘化物)变色⑹ NaOH等强碱、Na2CO3和AgNO3等盐Br2 + H2O = HBr + HBrO2HBr + Na2CO3 = 2NaBr + CO2↑+ H2OHBrO + Na2CO3 = NaBrO + NaHCO3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl4、氯仿、溴苯等)、CS2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷醇类物质(乙醇等)⑸含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑹天然橡胶(聚异戊二烯)⑺苯的同系物2、无机物:⑴氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物)⑵ + 2价的Fe(亚铁盐及氢氧化亚铁)⑶-2价的S(硫化氢及硫化物)⑷ + 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑸双氧水(H2O2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
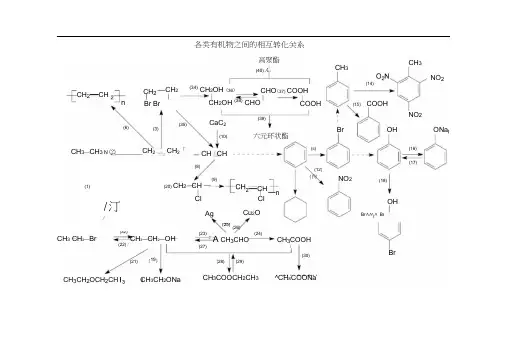
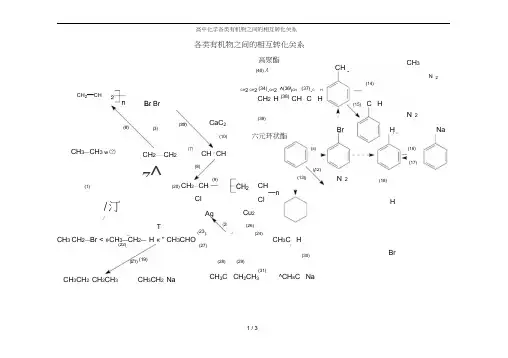
(31)各类有机物之间的相互转化关系CH 3CH 2OCH 2CHCH 3CH 2ONaCH 3COOCH 2CH 3 ^CH s COONa(14)COOHCOOH(39)CaC 2(35)(6)BrOHONa六元环状酯(10)(ii)(16) CH 「CH(17)(8)NO 2(18)Cl OHAgBr(30)(28)(29)高聚酯(40)人CH 2CH 3Cu 2O NO 2NO 2CH 2—CHBr Br(12)(佝 .(9)(20) CH 2—CH —(21)(19)2—CHCH 3 CH 2—Br CH 3—CH 2—OH(22) /CH 3CH 2(34)CH 2OH (36)Br^^\^ BrCHO (37) COOHCH 2OH (38) CHO CH 2二 CH 2「(25)(26)(23) (24)A CH 3CHOCH 3COOH(27)I—nCl(1)/汀/CH 3—CH 3 N ⑵(22) CH 3CH 2OH+HBrCH 3CH 2Br+H 2O光昭 CH 3CH 3+Br 2 光昭.CH 3CH 2Br+HBr CH 2 = CH 2+H 2 催化剂 △ -CH 3CH 3 CH 2 = CH 2+Br 2 ” CH 2BrCH 2Br CH 2 = CH 2+HBr ・CH 3CH 2BrCH 2 =CH 2+H 2O 浓H2S °4 - CH 3CH 2OH 催化剂‘ n CH 2= CH 2 -^CH 2—CH 2十 催化剂 CH 三 CH+H 2 催化-CH 2 = CH 2 CH 三 CH+HCl 催化剂■ CH 2 = CHCl n CH 2=CHCl CH 3(14)(15)+ 3HO —NO 2O 2N浓 H 2SO 4■ △CH 3COOHI 酸性 KMnO 4 II I 或酸性K2C2O7 |2-OH +2Na - 2(16) oH+NaOH OH+Na 2CO 3—ONa +HCl°Na +NaHCO 3 °Na+H 2O—ONa +H 2f催化剂-一CH 2—CH_n Cl ONa +CO 2+H 2OCaC 2+2H 2O » CH 三CH f +Ca(OH )2 + Br 2 催化剂》Br + HBr (18)〉+ HO - NO 2 浓严4.厂:-NO 2 +H 2O催化剂△CH 31 N6Il +3H 2OOH +NaCl"—OH +NaHCO 3—BrJ +3HBr(19) 2CH 3CH 2OH+2Na- 2CH 3CH 2ONa+H 2 f浓H 2SO 4人(20) CH 3CH 2OH 而C »CH 2 = CH 2 f +H 2O浓 H 2SO 4(21) C 2H 5— OH+HO — C 2H 5 荷c" C 2H 5— O — C 2H 5+H 2O (1) (2) (3) (4) (5) (6) (7) (8) (9)(10) (11) (⑵ (13)(30) 2CH 3COOH+2Na ■ 2CH 3COONa+H 2 f2CH 3COOH+Na 2O片 2CH 3COONa+H 2OCH 3COOH+NaOH ■ CH 3COONa+H 2O2CH 3COOH+Na 2CO 3 尸 2CH 3COONa+H 2O+CO 2 fCH 3COOH+NaHCO 3- CH 3COONa+H 2O+CO 2 f(31) CH 3COOC 2H 5+NaOH △» CH s COONa+C z H s OH水(32) CH 3CH 2Br+NaOH —水—CH 3CH 2OH+NaBr 乙醇(33) CH 3CH 2Br+NaOH 醇 CH 2= CH 2 f +NaBr+H 2O(23) 2CH 3CH 2OH+O 2—] 2CH 3CHO+2H 2O CH 3CH 2OH+CUO 仁 CH 3CHO+CU+H 2O (24) 2CH 3CHO+O 2 催化剂》2CH 3COOH(25) CH 3CHO+2Ag(NH 3)2OH 人 CH 3COONH 4+2Ag J(34)(35) (36) +3NH 3+H 2O (37)(26)△CH 3CHO+2CUQH) 2 CH 3COOH+CU 2O J +2H 2O(27)CH 3CHO+H 2 催化剂「CH 3CH 2OH (39)(28)浓 H 2SO 4CH 3COOH+HOC 2H 5 r A J CH 3COOC 2H 5+H 2O(40)(29)稀 H 2SO 4CH 3COOC 2H 5+H 2O ■=△匚 CH 3COOH+C 2H 5OHCH 2Br水CH 2Br + 2N aOH-水CH 2BrCH 2Br+2NaOH CH 2OHCH 2OH +°2CH 2OHCH 2OH +2NaBr催化剂” CHO△CHO +O 2催化剂.COOH +CH2OHCOOH - 一COOH nCOOH 乙醇△ - CH 三 CH f +2NaBr+2H 2OCHO +2H 2。
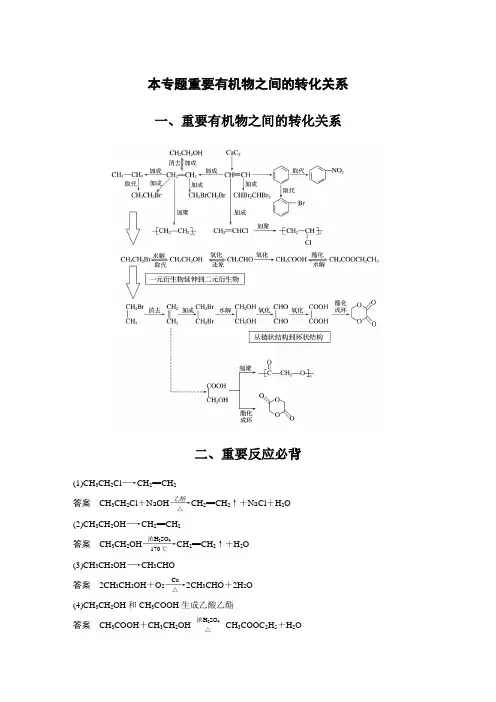
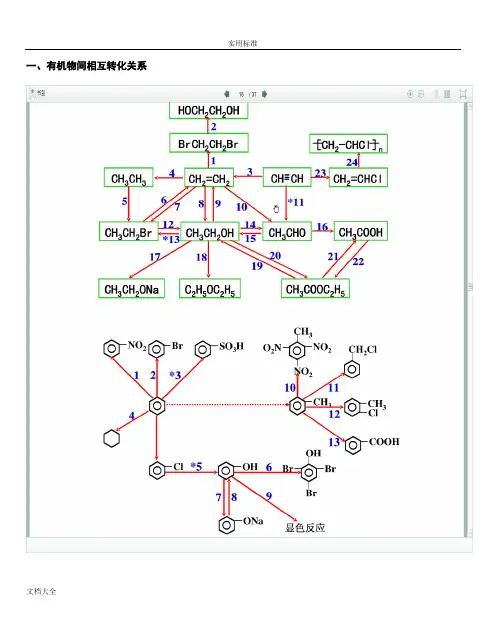
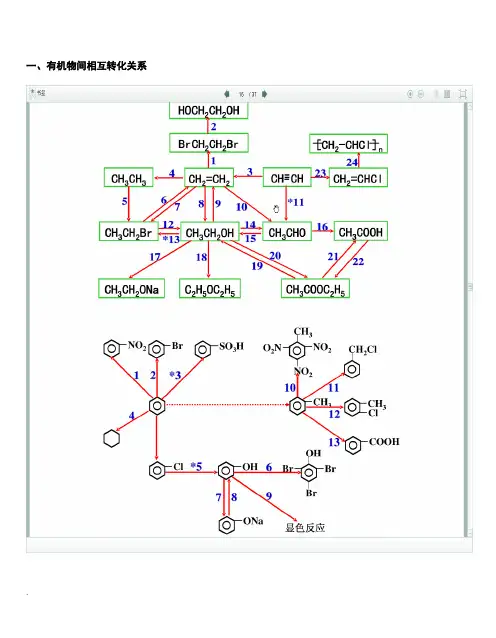
本专题重要有机物之间的转化关系一、重要有机物之间的转化关系二、重要反应必背(1)CH 3CH 2Cl ―→CH 2==CH 2答案 CH 3CH 2Cl +NaOH ――→乙醇△CH 2==CH 2↑+NaCl +H 2O (2)CH 3CH 2OH ―→CH 2==CH 2答案 CH 3CH 2OH ――――→浓H 2SO 4170 ℃CH 2==CH 2↑+H 2O (3)CH 3CH 2OH ―→CH 3CHO答案 2CH 3CH 2OH +O 2――→Cu △2CH 3CHO +2H 2O (4)CH 3CH 2OH 和CH 3COOH 生成乙酸乙酯答案 CH 3COOH +CH 3CH 2OH 浓H 2SO 4△CH 3COOC 2H 5+H 2O(5)OHC —CHO ―→HOOC —COOH答案 OHC —CHO +O 2――→催化剂△HOOC —COOH (6)乙二醇和乙二酸生成聚酯答案 n HOCH 2—CH 2OH +n HOOC —COOH 一定条件+2n H 2O(7)乙醛和银氨溶液的反应答案 CH 3CHO +2Ag(NH 3)2OH ――→△CH 3COONH 4+2Ag ↓+3NH 3+H 2O(8)乙醛和新制Cu(OH)2悬浊液的反应答案 CH 3CHO +2Cu(OH)2+NaOH ――→△CH 3COONa +Cu 2O ↓+3H 2O(9)―→ 答案 +2NaOH ――→醇△+2NaCl +2H 2O (10)―→ 答案 +Br 2――→FeBr 3+HBr(11) 和饱和溴水的反应答案 +3Br 2―→↓+3HBr(12) 和溴蒸气(光照)的反应答案 +Br 2――→光 +HBr(13) 和HCHO 的反应答案 n +n HCHO ――→H + +n H 2O(14)酯在碱性条件的水解(以乙酸乙酯在NaOH 溶液中为例)答案 CH 3COOC 2H 5+NaOH ――→△CH 3COONa +C 2H 5OH(15)和NaOH 的反应 答案 +2NaOH ――→△CH3COONa ++H2O三、有机物的检验辨析1.卤代烃中卤素的检验取样,滴入NaOH 溶液,加热至分层现象消失,冷却后加入稀硝酸酸化,再滴入AgNO 3溶液,观察沉淀的颜色,确定是何种卤素。