(完整版)高中化学各类有机物之间的相互转化关系
- 格式:pdf
- 大小:109.94 KB
- 文档页数:3
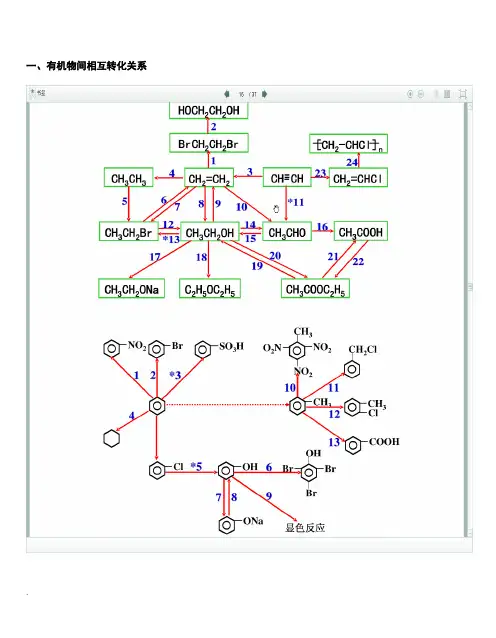
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
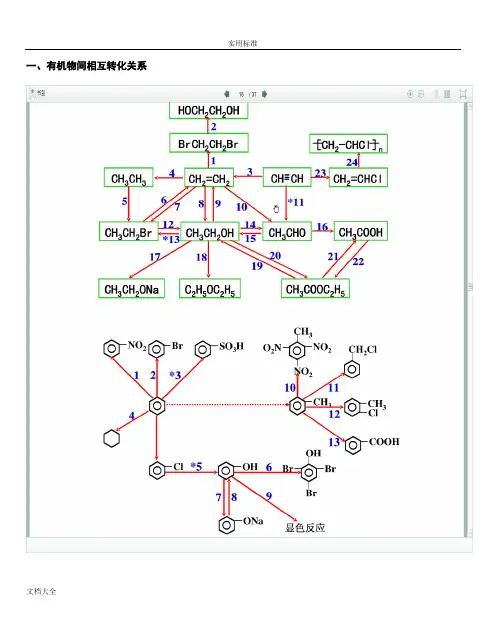
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
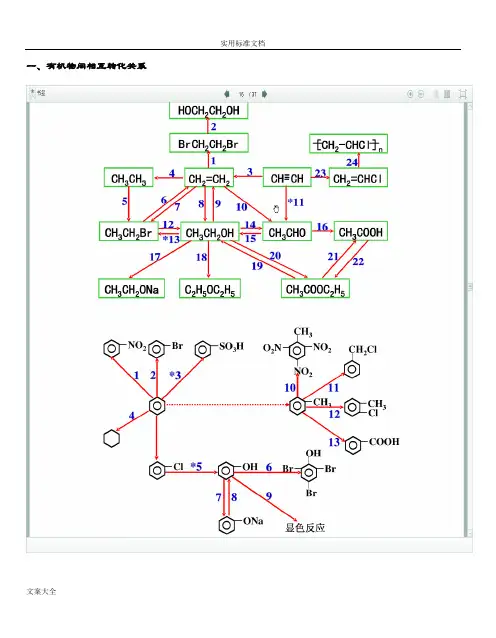
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应) △2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。
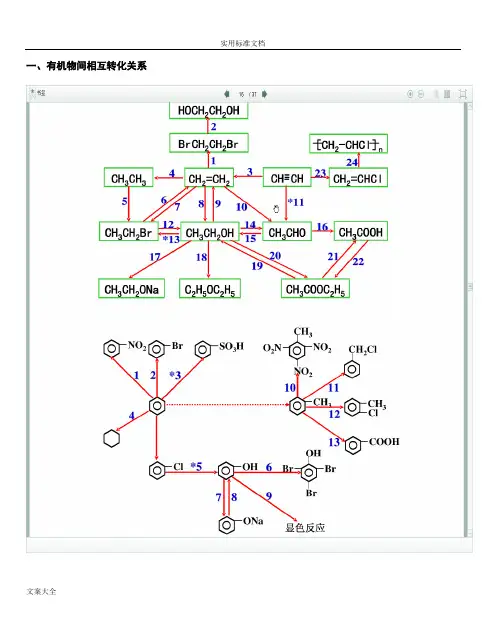
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应) △2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。

一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑸天然橡胶(聚异戊二烯)2、无机物:⑴-2价的S(硫化氢及硫化物)⑵+ 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑶+ 2价的Fe6FeSO4 + 3Br2 = 2Fe2(SO4)3 + 2FeBr36FeCl2 + 3Br2 = 4FeCl3 + 2FeBr32FeI2 + 3Br2 = 2FeBr3 + 2I2⑷Zn、Mg等单质如⑸-1价的I(氢碘酸及碘化物)变色⑹NaOH等强碱、Na2CO3和AgNO3等盐Br2 + H2O = HBr + HBrO2HBr + Na2CO3 = 2NaBr + CO2↑+ H2OHBrO + Na2CO3 = NaBrO + NaHCO3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl4、氯仿、溴苯等)、CS2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷醇类物质(乙醇等)⑸含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑹天然橡胶(聚异戊二烯)⑺苯的同系物2、无机物:⑴氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物)⑵+ 2价的Fe(亚铁盐及氢氧化亚铁)⑶-2价的S(硫化氢及硫化物)⑷+ 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑸双氧水(H2O2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无;通式:C n H2n+2;代表物:CH4B) 结构特点:键角为109°28′,空间正四面体分子。
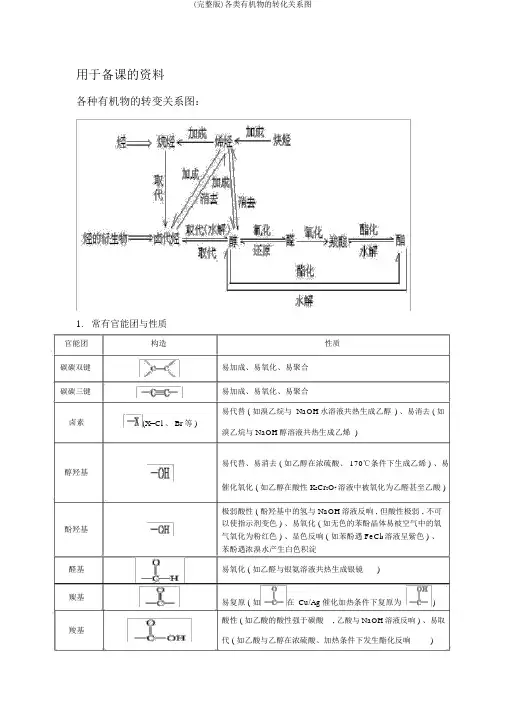
用于备课的资料各种有机物的转变关系图:1.常有官能团与性质官能团碳碳双键碳碳三键卤素醇羟基酚羟基醛基羰基羧基构造性质易加成、易氧化、易聚合易加成、易氧化、易聚合易代替 ( 如溴乙烷与 NaOH水溶液共热生成乙醇) 、易消去 ( 如(X=Cl 、 Br等 )溴乙烷与 NaOH醇溶液共热生成乙烯)易代替、易消去 ( 如乙醇在浓硫酸、 170℃条件下生成乙烯 ) 、易催化氧化 ( 如乙醇在酸性 K2Cr2O7溶液中被氧化为乙醛甚至乙酸 )极弱酸性 ( 酚羟基中的氢与 NaOH溶液反响 , 但酸性极弱 , 不可以使指示剂变色 ) 、易氧化 ( 如无色的苯酚晶体易被空气中的氧气氧化为粉红色 ) 、显色反响 ( 如苯酚遇 FeCl3溶液呈紫色 ) 、苯酚遇浓溴水产生白色积淀易氧化 ( 如乙醛与银氨溶液共热生成银镜)易复原 ( 如在Cu/Ag催化加热条件下复原为)酸性 ( 如乙酸的酸性强于碳酸, 乙酸与 NaOH溶液反响 ) 、易取代 ( 如乙酸与乙醇在浓硫酸、加热条件下发生酯化反响)易水解 ( 如乙酸乙酯在稀硫酸、加热条件下发生酸性水解, 酯基乙酸乙酯在 NaOH溶液、加热条件下发生碱性水解 )烷氧基如环氧乙烷在酸催化下与水一同加热生成乙二醇硝基复原 ( 如酸性条件下 , 硝基苯在铁粉催化下复原为苯胺) 2.有机反响种类与重要的有机反响反响种类重要的有机反响代替反响代替反响反响种类加成反响烷烃的卤代 :CH4+Cl 2CH3Cl+HCl烯烃的卤代 :卤代烃的水解 :CH3CH2Br+NaOH CH3CH2OH+NaBr皂化反响 :+3NaOH3C17H35COONa+酯化反响 : +CH OH +H O2 5 2糖类的水解 :C12H22O11+HOC6H12O6+C6H12O6蔗糖果糖葡萄糖2二肽的水解 :+H2O苯环上的卤代 :+Cl 2+HCl苯环上的硝化 :++H2O苯环上的磺化 :++H2O( 续表 )重要的有机反响烯烃的加成 :+HCl炔烃的加成 :+H2O苯环的加氢 :+3H2醇分子内脱水生成烯烃:C 2H5OH+H2O消去反响卤代烃脱 HX生成烯烃 :CH3CH2Br+NaOH+NaBr+H2O 单烯烃的加聚 :n加聚反响缩聚反响缩聚反响共轭二烯烃的加聚:( 别的 , 需要记着丁苯橡胶、氯丁橡胶的单体 )二元醇与二元酸之间的缩聚:n+nHOCH2CH2OH+2nH2O羟基酸之间的缩聚:+nH2 O氨基酸之间的缩聚:苯酚与 HCHO的缩聚 :n+nHCHO+nH2O氧化催化氧化 :2CH3 CH2OH+O22CH3CHO+2HO反响醛基与银氨溶液的反响:CH3CHO+2[Ag(NH3) 2 ]OH CH3COONH4+2Ag↓+3NH+H2O(注意配平 )醛基与新制氢氧化铜的反响:CH3CHO+2Cu(OH)+NaOH CH3COONa+Cu2↓ +3H2O醛基加氢 :CH3CHO+H2CH3CH2OH复原反响硝基复原为氨基:。
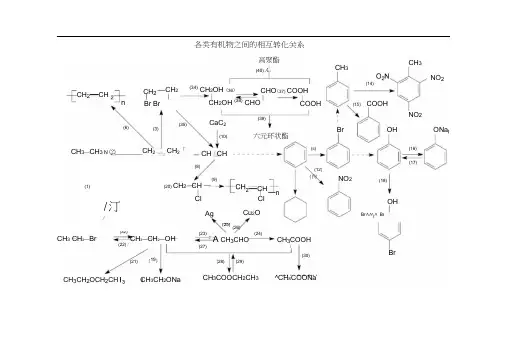
(31)各类有机物之间的相互转化关系CH 3CH 2OCH 2CHCH 3CH 2ONaCH 3COOCH 2CH 3 ^CH s COONa(14)COOHCOOH(39)CaC 2(35)(6)BrOHONa六元环状酯(10)(ii)(16) CH 「CH(17)(8)NO 2(18)Cl OHAgBr(30)(28)(29)高聚酯(40)人CH 2CH 3Cu 2O NO 2NO 2CH 2—CHBr Br(12)(佝 .(9)(20) CH 2—CH —(21)(19)2—CHCH 3 CH 2—Br CH 3—CH 2—OH(22) /CH 3CH 2(34)CH 2OH (36)Br^^\^ BrCHO (37) COOHCH 2OH (38) CHO CH 2二 CH 2「(25)(26)(23) (24)A CH 3CHOCH 3COOH(27)I—nCl(1)/汀/CH 3—CH 3 N ⑵(22) CH 3CH 2OH+HBrCH 3CH 2Br+H 2O光昭 CH 3CH 3+Br 2 光昭.CH 3CH 2Br+HBr CH 2 = CH 2+H 2 催化剂 △ -CH 3CH 3 CH 2 = CH 2+Br 2 ” CH 2BrCH 2Br CH 2 = CH 2+HBr ・CH 3CH 2BrCH 2 =CH 2+H 2O 浓H2S °4 - CH 3CH 2OH 催化剂‘ n CH 2= CH 2 -^CH 2—CH 2十 催化剂 CH 三 CH+H 2 催化-CH 2 = CH 2 CH 三 CH+HCl 催化剂■ CH 2 = CHCl n CH 2=CHCl CH 3(14)(15)+ 3HO —NO 2O 2N浓 H 2SO 4■ △CH 3COOHI 酸性 KMnO 4 II I 或酸性K2C2O7 |2-OH +2Na - 2(16) oH+NaOH OH+Na 2CO 3—ONa +HCl°Na +NaHCO 3 °Na+H 2O—ONa +H 2f催化剂-一CH 2—CH_n Cl ONa +CO 2+H 2OCaC 2+2H 2O » CH 三CH f +Ca(OH )2 + Br 2 催化剂》Br + HBr (18)〉+ HO - NO 2 浓严4.厂:-NO 2 +H 2O催化剂△CH 31 N6Il +3H 2OOH +NaCl"—OH +NaHCO 3—BrJ +3HBr(19) 2CH 3CH 2OH+2Na- 2CH 3CH 2ONa+H 2 f浓H 2SO 4人(20) CH 3CH 2OH 而C »CH 2 = CH 2 f +H 2O浓 H 2SO 4(21) C 2H 5— OH+HO — C 2H 5 荷c" C 2H 5— O — C 2H 5+H 2O (1) (2) (3) (4) (5) (6) (7) (8) (9)(10) (11) (⑵ (13)(30) 2CH 3COOH+2Na ■ 2CH 3COONa+H 2 f2CH 3COOH+Na 2O片 2CH 3COONa+H 2OCH 3COOH+NaOH ■ CH 3COONa+H 2O2CH 3COOH+Na 2CO 3 尸 2CH 3COONa+H 2O+CO 2 fCH 3COOH+NaHCO 3- CH 3COONa+H 2O+CO 2 f(31) CH 3COOC 2H 5+NaOH △» CH s COONa+C z H s OH水(32) CH 3CH 2Br+NaOH —水—CH 3CH 2OH+NaBr 乙醇(33) CH 3CH 2Br+NaOH 醇 CH 2= CH 2 f +NaBr+H 2O(23) 2CH 3CH 2OH+O 2—] 2CH 3CHO+2H 2O CH 3CH 2OH+CUO 仁 CH 3CHO+CU+H 2O (24) 2CH 3CHO+O 2 催化剂》2CH 3COOH(25) CH 3CHO+2Ag(NH 3)2OH 人 CH 3COONH 4+2Ag J(34)(35) (36) +3NH 3+H 2O (37)(26)△CH 3CHO+2CUQH) 2 CH 3COOH+CU 2O J +2H 2O(27)CH 3CHO+H 2 催化剂「CH 3CH 2OH (39)(28)浓 H 2SO 4CH 3COOH+HOC 2H 5 r A J CH 3COOC 2H 5+H 2O(40)(29)稀 H 2SO 4CH 3COOC 2H 5+H 2O ■=△匚 CH 3COOH+C 2H 5OHCH 2Br水CH 2Br + 2N aOH-水CH 2BrCH 2Br+2NaOH CH 2OHCH 2OH +°2CH 2OHCH 2OH +2NaBr催化剂” CHO△CHO +O 2催化剂.COOH +CH2OHCOOH - 一COOH nCOOH 乙醇△ - CH 三 CH f +2NaBr+2H 2OCHO +2H 2。

高三有机化学中有机物间相互转化关系图Prepared on 22 November 2020一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯) 2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 3 6FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2 ⑷ Zn 、Mg 等单质 如⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;变色Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴不饱和烃(烯烃、炔烃、二烯烃等)⑵不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等)⑶石油产品(裂化气、裂解气、裂化汽油等)⑷醇类物质(乙醇等)⑸含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等)⑹天然橡胶(聚异戊二烯)⑺苯的同系物2、无机物:⑴氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物)⑵ + 2价的Fe(亚铁盐及氢氧化亚铁)⑶-2价的S(硫化氢及硫化物)⑷ + 4价的S(二氧化硫、亚硫酸及亚硫酸盐)⑸双氧水(H2O2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A)官能团:无;通式:C n H2n+2;代表物:CH4B) 结构特点:键角为109°28′,空间正四面体分子。
一、有机物间相互转化关系二、能与溴水发生化学反应而使溴水褪色或变色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等)⑷ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑸ 天然橡胶(聚异戊二烯)2、无机物:⑴ -2价的S (硫化氢及硫化物)⑵ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑶ + 2价的Fe6FeSO 4 + 3Br 2 = 2Fe 2(SO 4)3 + 2FeBr 36FeCl 2 + 3Br 2 = 4FeCl 3 + 2FeBr 32FeI 2 + 3Br 2 = 2FeBr 3 + 2I 2⑷ Zn 、Mg 等单质 如 ⑸ -1价的I (氢碘酸及碘化物)变色 ⑹ NaOH 等强碱、Na 2CO 3和AgNO 3等盐 Br 2 + H 2O = HBr + HBrO2HBr + Na 2CO 3 = 2NaBr + CO 2↑+ H 2O HBrO + Na 2CO 3 = NaBrO + NaHCO 3三、能萃取溴而使溴水褪色的物质上层变无色的(ρ>1):卤代烃(CCl 4、氯仿、溴苯等)、CS 2等;下层变无色的(ρ<1):直馏汽油、煤焦油、苯及苯的同系物、低级酯、液态环烷烃、液态饱和烃(如己烷等)等 四、能使酸性高锰酸钾溶液褪色的物质1、有机物:⑴ 不饱和烃(烯烃、炔烃、二烯烃等)⑵ 不饱和烃的衍生物(烯醇、烯醛、烯酸、烯酯、油酸、油酸酯等) ⑶ 石油产品(裂化气、裂解气、裂化汽油等) ⑷ 醇类物质(乙醇等)⑸ 含醛基的化合物(醛、甲酸、甲酸盐、甲酸酯、葡萄糖、麦芽糖等) ⑹ 天然橡胶(聚异戊二烯) ⑺ 苯的同系物变色 Mg + Br 2 === MgBr 2 (其中亦有Mg 与H +、Mg 与HBrO 的反应)△2、无机物:⑴ 氢卤酸及卤化物(氢溴酸、氢碘酸、浓盐酸、溴化物、碘化物) ⑵ + 2价的Fe (亚铁盐及氢氧化亚铁) ⑶ -2价的S (硫化氢及硫化物)⑷ + 4价的S (二氧化硫、亚硫酸及亚硫酸盐) ⑸ 双氧水(H 2O 2)五、常见的各类有机物的官能团,结构特点及主要化学性质(1) 烷烃A) 官能团:无 ;通式:C n H 2n +2;代表物:CH 4B) 结构特点:键角为109°28′,空间正四面体分子。