纯化水EP7.0、8.0
- 格式:doc
- 大小:48.50 KB
- 文档页数:3

EUROPEAN PHARMACOPOEIA 8.0Water,purifiedTESTSNitrates :maximum 0.2ppm.Place 5mL in a test-tube immersed in iced water,add 0.4mL of a 100g/L solution of potassium chloride R ,0.1mL ofdiphenylamine solution R and,dropwise with shaking,5mL of nitrogen-free sulfuric acid R .Transfer the tube to a water-bath at 50°C.After 15min,any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of 4.5mL of nitrate-free water R and 0.5mL of nitrate standard solution (2ppm NO 3)R .Aluminium (2.4.17):maximum 10ppb,if intended for use in the manufacture of dialysis solutions.Prescribed solution .To 400mL of the water to be examined add 10mL of acetate buffer solution pH 6.0R and 100mL of distilled water R .Reference solution .Mix 2mL of aluminium standard solution (2ppm Al)R ,10mL of acetate buffer solution pH 6.0R and 98mL of distilled water R .Blank solution .Mix 10mL of acetate buffer solution pH 6.0R and 100mL of distilled water R .Bacterial endotoxins (2.6.14):less than 0.25IU/mL.LABELLINGThe label states,where applicable,that the substance is suitablefor use in the manufacture of dialysis solutions.01/2009:0008WATER,PURIFIEDAqua purificata H 2O M r 18.02DEFINITIONWater for the preparation of medicines other than thosethat are required to be both sterile and apyrogenic,unlessotherwise justified and authorised.Purified water in bulkPRODUCTIONPurified water in bulk is prepared by distillation,by ionexchange,by reverse osmosis or by any other suitable method from water that complies with the regulations on waterintended for human consumption laid down by the competent authority.Purified water in bulk is stored and distributed in conditions designed to prevent growth of micro-organisms and to avoid any other contamination.Microbiological monitoring .During production and subsequent storage,appropriate measures are taken toensure that the microbial count is adequately controlled and monitored.Appropriate alert and action levels are set so as to detect adverse trends.Under normal conditions,an appropriate action level is a microbial count of 100CFU/mL,determined by filtration through a membrane with a nominal pore size not greater than 0.45μm,using R2A agar andincubating at 30-35°C for not less than 5days.The size of the sample is to be chosen in relation to the expected result.R2A agarYeast extract 0.5g Proteose peptone 0.5g Casein hydrolysate 0.5g Glucose0.5gStarch0.5g Dipotassium hydrogen phosphate 0.3g Magnesium sulfate,anhydrous 0.024g Sodium pyruvate 0.3g Agar 15.0g Purified waterto 1000mLAdjust the pH so that after sterilisation it is 7.2±0.2.Sterilise by heating in an autoclave at 121°C for 15min.Growth promotion of R2A agar–Preparation of test strains .Use standardised stable suspensions of test strains or prepare them as stated in Table 0008.-1.Seed lot culture maintenancetechniques (seed-lot systems)are used so that the viable micro-organisms used for inoculation are not more than 5passages removed from the original master seed-lot.Grow each of the bacterial strains separately as described in Table e buffered sodium chloride-peptone solution pH 7.0or phosphate buffer solution pH 7.2to make test e the suspensions within 2h,or within 24h if stored at 2-8°C.As an alternative to preparing and then diluting a fresh suspension of vegetative cells of Bacillus subtilis ,a stable spore suspension is prepared and then an appropriate volume of the spore suspension is used for test inoculation.The stable spore suspension may be maintained at 2-8°C for a validated period of time.–Growth promotion .Test each batch of ready-preparedmedium and each batch of medium,prepared either fromdehydrated medium or from the ingredients described.Inoculate plates of R2A agar separately with a small number (not more than 100CFU)of the micro-organisms indicated in Table 0008.-1.Incubate under the conditions describedin the table.Growth obtained must not differ by a factorgreater than 2from the calculated value for a standardisedinoculum.For a freshly prepared inoculum,growth of themicro-organisms must be comparable to that obtained witha previously tested and approved batch of medium.Table 0008.-1.–Growth promotion of R2A agar Micro-organism Preparation of the test strain Growth promotionPseudomonas aeruginosa such as:ATCC 9027NCIMB 8626CIP 82.118NBRC 13275Casein soyabean digest agar or casein soyabean digest broth 30-35°C 18-24hR2A agar ≤100CFU 30-35°C ≤3daysBacillus subtilis such as:ATCC 6633NCIMB 8054CIP 52.62NBRC 3134Casein soyabean digest agar or casein soyabean digest broth 30-35°C 18-24hR2A agar ≤100CFU 30-35°C ≤3daysTotal organic carbon or oxidisable substances .Carry out the test for total organic carbon (2.2.44)with a limit of 0.5mg/L or alternatively the following test for oxidisable substances:to 100mL add 10mL of dilute sulfuric acid R and 0.1mL of 0.02M potassium permanganate and boil for 5min;the solution remains faintly pink.Conductivity .Determine the conductivity off-line or in-line under the following conditions.EQUIPMENT Conductivity cell :–electrodes of a suitable material such as stainless steel;–cell constant:the cell constant is generally certified by the supplier and is subsequently verified at suitable intervals using a certified reference solution with a conductivity less than 1500μS·cm −1or by comparison with a cell havingGeneral Notices (1)apply to all monographs and other texts3561Water,purified EUROPEAN PHARMACOPOEIA8.0a certified cell constant;the cell constant is confirmed ifthe value found is within2per cent of the certified value, otherwise re-calibration must be performed. Conductometer:accuracy of0.1μS·cm−1or better at the lowest range.System calibration(conductivity cell and conductometer):–against one or more suitable certified reference solutions;–accuracy:within3per cent of the measured conductivity plus0.1μS·cm−1.Conductometer calibration:calibration is carried out for each range of measurement to be used,after disconnectionof the conductivity cell,using certified precision resistors or equivalent devices with an uncertainty not greater than0.1per cent of the certified value.If in-line conductivity cells cannot be dismantled,system calibration may be performed against a calibrated conductivity-measuring instrument with a conductivity cell placed close to the cell to be calibrated in the waterflow. Temperature measurement:tolerance±2°C. PROCEDUREMeasure the conductivity without temperature compensation,recording simultaneously the temperature. Temperature-compensated measurement may be performed after suitable validation.The water to be examined meets the requirements if the measured conductivity at the recorded temperature is not greater than the value in Table0008.-2.Table0008.-2.–Temperature and conductivity requirementsTemperature(°C)Conductivity (μS·cm−1)10 3.620 4.325 5.130 5.440 6.5507.1608.1709.1759.7809.7909.710010.2For temperatures not listed in Table0008.-2,calculate the maximal permitted conductivity by interpolation between the next lower and next higher data points in the table.Heavy metals.If purified water in bulk complies withthe requirement for conductivity prescribed for Water for injections(0169)in bulk,it is not necessary to carry out the test for heavy metals prescribed below.CHARACTERSAppearance:clear and colourless liquid.TESTSNitrates:maximum0.2ppm.Place5mL in a test-tube immersed in iced water,add0.4mL of a100g/L solution of potassium chloride R,0.1mL of diphenylamine solution R and,dropwise with shaking,5mL of nitrogen-free sulfuric acid R.Transfer the tube to a water-bath at50°C.After15min,any blue colour in the solution is not more intense than that in a reference solution prepared at the same time in the same manner using a mixture of4.5mL of nitrate-free water R and0.5mL of nitrate standard solution (2ppm NO3)R.Aluminium(2.4.17):maximum10ppb,if intended for use in the manufacture of dialysis solutions.Prescribed solution.To400mL of the water to be examined add10mL of acetate buffer solution pH6.0R and100mL of distilled water R.Reference solution.Mix2mL of aluminium standard solution(2ppm Al)R,10mL of acetate buffer solution pH6.0R and98mL of distilled water R.Blank solution.Mix10mL of acetate buffer solution pH6.0R and100mL of distilled water R.Heavy metals(2.4.8):maximum0.1ppm.To200mL add0.15mL of0.1M nitric acid and heat in a glass evaporating dish on a water-bath until the volume is reduced to20mL.12mL of the concentrated solution complies with test A.Prepare the reference solution using10mL of lead standard solution(1ppm Pb)R and adding0.075mL of0.1M nitric acid.Prepare the blank solution adding0.075mL of 0.1M nitric acid.Bacterial endotoxins(2.6.14):less than0.25IU/mL,if intended for use in the manufacture of dialysis solutions without a further appropriate procedure for removal of bacterial endotoxins.LABELLINGThe label states,where applicable,that the substance is suitable for use in the manufacture of dialysis solutions.Purified water in containers DEFINITIONPurified water in bulk that has beenfilled and stored in conditions designed to assure the required microbiological quality.It is free from any added substances. CHARACTERSAppearance:clear and colourless liquid.TESTSIt complies with the tests prescribed in the section on Purified water in bulk and with the following additional tests. Acidity or alkalinity.To10mL,freshly boiled and cooled in a borosilicate glassflask,add0.05mL of methyl red solution R. The solution is not coloured red.To10mL add0.1mL of bromothymol blue solution R1.The solution is not coloured blue.Oxidisable substances.To100mL add10mL of dilute sulfuric acid R and0.1mL of0.02M potassium permanganate and boil for5min.The solution remains faintly pink. Chlorides.To10mL add1mL of dilute nitric acid R and 0.2mL of silver nitrate solution R2.The solution shows no change in appearance for at least15min.Sulfates.To10mL add0.1mL of dilute hydrochloric acid R and0.1mL of barium chloride solution R1.The solution shows no change in appearance for at least1h.Ammonium:maximum0.2ppm.To20mL add1mL of alkaline potassium tetraiodomercurate solution R.After5min,examine the solution down the vertical axis of the tube.The solution is not more intensely coloured than a standard prepared at the same time by adding1mL of alkaline potassium tetraiodomercurate solution R to a mixture of4mL of ammonium standard solution(1ppm NH4)R and 16mL of ammonium-free water R.Calcium and magnesium.To100mL add2mL of ammonium chloride buffer solution pH10.0R,50mg of mordant black11 triturate R and0.5mL of0.01M sodium edetate.A pure blue colour is produced.3562See the information section on general monographs(cover pages)EUROPEAN PHARMACOPOEIA 8.0Wheat-germ oil,refinedResidue on evaporation :maximum 0.001per cent.Evaporate 100mL to dryness on a water-bath and dry in an oven at 100-105°C.The residue weighs a maximum of 1mg.Microbial contaminationTAMC:acceptance criterion 102CFU/mL (2.6.12).Use casein soya bean digest agar.LABELLINGThe label states,where applicable,that the substance is suitable for use in the manufacture of dialysis solutions.01/2014:0359WHEAT STARCH (1)Tritici amylum DEFINITIONWheat starch is obtained from the caryopsis of Triticum aestivum L.(T.vulgare Vill.).♦CHARACTERS Appearance :very fine,white or almost white powder thatcreaks when pressed between the fingers.Solubility :practically insoluble in cold water and in ethanol (96per cent).Wheat starch does not contain starch grains of any other origin.It may contain a minute quantity,if any,of tissuefragments of the original plant.♦IDENTIFICATION A.Microscopic examination (2.8.23)using a 50per cent V/V solution of glycerol R .It presents large and small granules,and,very rarely,intermediate sizes (Figure 0359.-1).Thelarge granules,10-60μm in diameter,are discoid or,more rarely,reniform when seen face-on.The central hilum and striations are invisible or barely visible and the granulessometimes show cracks on the edges.Seen in profile,thegranules are elliptical and fusiform and the hilum appears as a slit along the main axis.The small granules,rounded or polyhedral,are 2-10μm in diameter.Between orthogonally orientated polarising plates or prisms,the granules show a distinct black cross intersecting at thehilum.Figure 0359.-1.−Illustration for identification test A of wheatstarchB.Suspend 1g in 50mL of water R ,boil for 1min and cool.A thin,cloudy mucilage is formed.C.To 1mL of the mucilage obtained in identification test B add 0.05mL of iodine solution R1.A dark blue colour is produced,which disappears on heating.TESTSpH (2.2.3):4.5to 7.0.Shake 5.0g with 25.0mL of carbon dioxide-free water R for60s.Allow to stand for 15min.◊Foreign matter .Examined under a microscope using a 50per cent V/V solution of glycerol R ,not more than tracesof matter other than starch granules are present.No starch grains of any other origin are present.◊Total protein :maximum 0.3per cent of total protein(corresponding to 0.048per cent N 2,conversion factor:6.25),determined on 6.0g by sulfuric acid digestion (2.5.9)modifiedas follows:wash any adhering particles from the neck into the flask with 25mL of sulfuric acid R ;continue the heatinguntil a clear solution is obtained;add 45mL of strong sodium hydroxide solution R .Oxidising substances (2.5.30):maximum 20ppm,calculatedas H 2O 2.Sulfur dioxide (2.5.29):maximum 50ppm.Iron (2.4.9):maximum 10ppm.Shake 1.5g with 15mL of dilute hydrochloric acid R .Filter.The filtrate complies with the test.Loss on drying (2.2.32):maximum 15.0per cent,determined on 1.000g by drying in an oven at 130°C for 90min.Sulfated ash (2.4.14):maximum 0.6per cent,determined on 1.0g.Microbial contamination TAMC:acceptance criterion 103CFU/g (2.6.12).TYMC:acceptance criterion 102CFU/g (2.6.12).Absence of Escherichia coli (2.6.13).◊Absence of Salmonella (2.6.13).◊01/2010:1379WHEAT-GERM OIL,REFINED Tritici aestivi oleum raffinatumDEFINITIONFatty oil obtained from the germ of the grain of Triticum aestivum L.by cold expression or by other suitable mechanical means and/or by extraction.It is then refined.A suitable antioxidant may be added.CHARACTERSAppearance :clear,light yellow liquid.Solubility :practically insoluble in water and in ethanol (96per cent),miscible with light petroleum (bp:40-60°C).Relative density :about 0.925.Refractive index :about 1.475.IDENTIFICATIONA.Identification of fatty oils by thin-layer chromatography (2.3.2).Results :the chromatogram obtained is similar to the corresponding chromatogram shown in Figure position of fatty acids (see Tests).TESTSAcid value (2.5.1):maximum 0.9,or maximum 0.3if intended for use in the manufacture of parenteral preparations.(1)This monograph has undergone pharmacopoeial harmonisation.See chapter 5.8.Pharmacopoeial harmonisation .General Notices (1)apply to all monographs and other texts 3563。

Comparison of specifications of purified water (EP, ChP&USP)纯化水质量标准比较(EP, ChP&USP)起草人/日期(Drafted by):审核人/日期(Checked by):批准人/日期(Approved by):XXXXXXXXXXXXXXX有限公司二O一六年十二月纯化水质量标准比较(EP, ChP&USP)1、概述纯化水是我公司重要的制药用水,不同地区均有相应的药典要求,药典是一个国家记载药品标准、规格的法典,一般由国家药品监督管理局主持编纂、颁布实施,国际性药典则由公认的国际组织或有关国家协商编订。
制定药品标准对加强药品质量的监督管理、保证质量、保障用药安全有效、维护人民健康起着十分重要的作用。
制药用水系统的目的之一为“维持制药用水水质在药典要求的可接受范围内”,本文将主要介绍《美国药典》、《欧洲药典》和《中国药典》对制药用水质量的要求。
2、标准比较(ChP2015,EP8,USP38,ICH)3、检测方法及标准Table 1.14、微生物限度检测方法比较(EP&USP ) 4.1 ChP2015纯化水微生物限度R2A 琼脂培养基处方及制备 酵母浸出粉o. 5g 蛋白胨0. 5g酪蛋白水解物0.5g 葡萄糖0. 5g 可溶性淀粉0. 5g 磷酸氢二钾0_3g 无水硫酸镁0. 024g 丙酮酸钠0.3g 琼脂15g纯化水1000ml除葡萄糖、琼脂外,取上述成分,混合,微温溶解,调节pH 值使加热后在25℃的pH 值为7.2±0.2,加人琼脂,加热溶化后,再加人葡萄糖,摇匀,分装,灭菌。
R2A 琼脂培养基适用性检查试验照非无菌产品微生物限度检査:微生物计数法(通则1105)中“计数培养基适用性检查” 的胰酪大豆胨琼脂培养基的适用性检査方法进行,试验菌株为铜绿假单胞菌和枯草芽孢杆菌。
应符合规定。

Comparison of specifications of purified water (EP, ChP&USP)纯化水质量标准比较(EP, ChP&USP)起草人/日期(Drafted by):审核人/日期(Checked by):批准人/日期(Approved by):XXXXXXXXXXXXXXX有限公司二O一六年十二月纯化水质量标准比较(EP, ChP&USP)1、概述纯化水是我公司重要的制药用水,不同地区均有相应的药典要求,药典是一个国家记载药品标准、规格的法典,一般由国家药品监督管理局主持编纂、颁布实施,国际性药典则由公认的国际组织或有关国家协商编订。
制定药品标准对加强药品质量的监督管理、保证质量、保障用药安全有效、维护人民健康起着十分重要的作用。
制药用水系统的目的之一为“维持制药用水水质在药典要求的可接受范围内”,本文将主要介绍《美国药典》、《欧洲药典》和《中国药典》对制药用水质量的要求。
2、标准比较(ChP2015,EP8,USP38,ICH)3、检测方法及标准标准规定检验项目ChP(2015)纯化水纯化水Purified water in bulk (散装,生产出来就通过管道输送使用的)纯化水Purified water in containers 高纯水纯化水纯化水(原料药用于注射液)摇匀,将试管于50℃水浴中放置15分钟,溶液产生的蓝色与标准硝酸盐溶液[取硝酸钾0.163g,加水溶解并稀释至100ml,摇匀,精密量取1ml,加水稀释成100ml,再精密量取10ml,加水稀释成100ml,摇匀,即得(每1ml相当于1μgNO3)]0.3ml,加无硝酸盐的水4.7ml,用同一方法处理后的颜色比较,不得更深(0.000 006%)。
potassium chloride R, 0.1mL of diphenylaminesolution R and, dropwise with shaking, 5mL ofnitrogen-free sulfuric acid R. Transfer the tubeto a water-bath at 50 °C. After 15 min, any bluecolour in the solution is not more intense thanthat in a reference solution prepared at the sametime in the same manner using a mixture of4.5mL of nitrate-free water R and 0.5mL ofnitrate standard solution(2 ppm NO3) R.取5ml纯化水于放置在冰水上的试管中,加入0.4ml的100g/L的氯化钾溶液R、0.1ml的二苯胺溶液R,然后边摇边滴加5ml的无氮的硫酸R。

药典对纯化水的ph范围药典对纯化水的pH范围概述纯化水是制药过程中必不可少的一种重要溶剂。
在制药工艺中,纯化水的质量直接关系到制剂的质量和稳定性。
因此,对于纯化水的质量要求非常高。
其中,pH值是评价纯化水质量的重要指标之一。
本文将介绍药典对纯化水的pH范围。
I. 纯化水的定义II. 纯化水pH值的意义III. 药典对纯化水pH值的要求A. 美国药典(USP)B. 欧洲药典(EP)C. 中国药典(CP)IV. pH值偏高或偏低时可能带来的影响V. pH值异常时应采取措施I. 纯化水的定义纯化水是指经过特殊处理后去除了其中大部分杂质和离子,达到一定纯度要求并符合特定用途需求的水。
其主要用于制药、生物技术、电子等领域。
II. 纯化水pH值的意义在制药工艺中,pH值是一个非常重要的指标。
pH值可以影响制剂的稳定性、药效、生物相容性等多个方面。
因此,对于纯化水而言,pH 值也是一个非常重要的评价指标。
III. 药典对纯化水pH值的要求各国药典对于纯化水的pH值有不同的要求,下面将分别介绍美国药典、欧洲药典和中国药典对于纯化水pH值的要求。
A. 美国药典(USP)美国药典规定,纯化水的pH值应在5.0~7.0之间。
如果使用了气体去除法(如CO2去除法),则其pH值应在5.0~8.0之间。
B. 欧洲药典(EP)欧洲药典规定,纯化水的pH值应在5.0~7.5之间。
C. 中国药典(CP)中国药典规定,注射用水和灭菌用水的pH值应在5.0~7.0之间;其他用途的纯化水则没有明确规定其pH范围。
IV. pH值偏高或偏低时可能带来的影响当纯化水的pH值偏高或偏低时,会影响制剂的稳定性和药效。
例如,当pH值偏高时,会使酸性药物的稳定性降低;而当pH值偏低时,会使碱性药物的稳定性降低。
此外,pH值还会影响生物相容性和溶解度等多个方面。
V. pH值异常时应采取措施当纯化水的pH值超出规定范围时,应及时采取措施进行调整。
一般来说,可以通过加入酸或碱来调整纯化水的pH值。
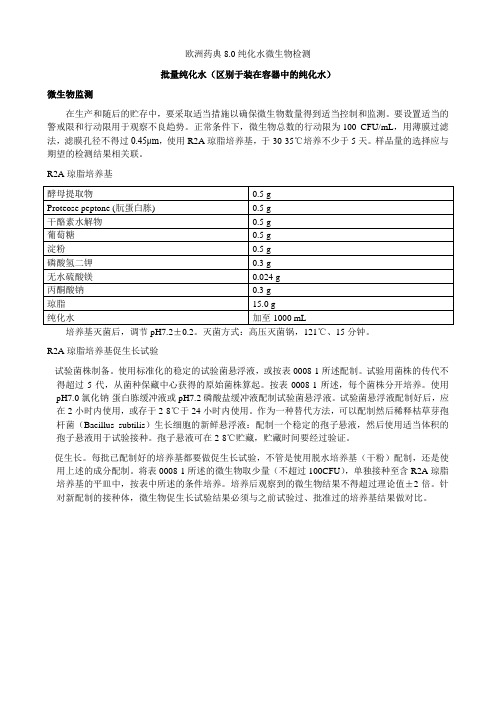
欧洲药典8.0纯化水微生物检测批量纯化水(区别于装在容器中的纯化水)微生物监测在生产和随后的贮存中,要采取适当措施以确保微生物数量得到适当控制和监测。
要设置适当的警戒限和行动限用于观察不良趋势。
正常条件下,微生物总数的行动限为100 CFU/mL,用薄膜过滤法,滤膜孔径不得过0.45μm,使用R2A琼脂培养基,于30-35℃培养不少于5天。
样品量的选择应与期望的检测结果相关联。
R2A琼脂培养基培养基灭菌后,调节pH7.2±0.2。
灭菌方式:高压灭菌锅,121℃、15分钟。
R2A琼脂培养基促生长试验- 试验菌株制备。
使用标准化的稳定的试验菌悬浮液,或按表0008-1所述配制。
试验用菌株的传代不得超过5代,从菌种保藏中心获得的原始菌株算起。
按表0008-1所述,每个菌株分开培养。
使用pH7.0氯化钠-蛋白胨缓冲液或pH7.2磷酸盐缓冲液配制试验菌悬浮液。
试验菌悬浮液配制好后,应在2小时内使用,或存于2-8℃于24小时内使用。
作为一种替代方法,可以配制然后稀释枯草芽孢杆菌(Bacillus subtilis)生长细胞的新鲜悬浮液:配制一个稳定的孢子悬液,然后使用适当体积的孢子悬液用于试验接种。
孢子悬液可在2-8℃贮藏,贮藏时间要经过验证。
- 促生长。
每批已配制好的培养基都要做促生长试验,不管是使用脱水培养基(干粉)配制,还是使用上述的成分配制。
将表0008-1所述的微生物取少量(不超过100CFU),单独接种至含R2A琼脂培养基的平皿中,按表中所述的条件培养。
培养后观察到的微生物结果不得超过理论值±2倍。
针对新配制的接种体,微生物促生长试验结果必须与之前试验过、批准过的培养基结果做对比。
表0008-1R2A琼脂促生长试验微生物试验菌株制备促生长条件。

中国药典2015年版溶液的澄清度与颜色取本品,加水制成每lml中约含阿魏酸钠20m g的溶液,溶液应澄清无色;如显浑浊,与1号池度标准液(通则0902第一法)比较,不得更浓;如显色,与黄色或黄绿色3号标准比色液(通则0901第一法)比较,不得更深。
有关物质避光操作。
取本品,加流动相溶解并稀释制成每l m l中约含0. 7m g的溶液,作为供试品溶液;精密量取lm l,置200m l量瓶中,用流动相稀释至刻度,摇勻,作为对照溶液。
照阿魏酸钠有关物质项下的方法测定。
供试品溶液的色谱图中如有杂质峰,各杂质峰面积的和不得大于对照溶液的主峰面积(0.5%)。
水分取本品,照水分测定法(通则0832第一法1)测定,含水分应为13.0%〜16.0%(供无菌粉末用)或应不超过3.0%(供无菌冻干品用)。
热原取本品,加灭菌注射用水制成每l m l中含阿魏酸钠5m g的溶液,依法检查(通则1142),剂量按家兔体重每l k g 缓慢注射3m l,应符合规定。
无菌照阿魏酸钠项下的方法检査,应符合规定。
其他应符合注射剂项下有关的各项规定(通则0102)。
【含置测定】避光操作。
取装量差异项下的内容物约0.15g,精密称定,加冰醋酸20m l使阿魏酸钠溶解,照阿魏酸钠项下的方法,自“加醋酐3m l”起,依法测定。
每l m l高氣酸滴定液(0.lm o l/L)相当于25.22mg的C10H9N a04•2H20。
【类别】同阿魏酸钠。
【规格】(1)0. lg(2)0. 3g【贮藏】遮光,密封保存。
纯化水ChunhuashuiPurified WaterH2018.02本品为饮用水经蒸馏法、离子交换法、反渗透法或其他适宜的方法制得的制药用水,不含任何添加剂。
【性状】本品为无色的澄清液体;无臭。
【检査】酸碱度取本品10m l,加甲基红指示液2滴,不得显红色;另取10m l,加溴麝香草酚蓝指示液5滴,不得显蓝色。
硝酸盐取本品5m l置试管中,于冰浴中冷却,加10%氣化钾溶液0.4m l与0.1%二苯胺硫酸溶液0.1m l,摇匀,缓缓滴加硫酸5m l,摇勻,将试管于50T:水浴中放置15分钟,溶液产生的蓝色与标准硝酸盐溶液[取硝酸钾0.163g,加水溶解并稀释至100m l,摇匀,精密量取l m l,加水稀释成100m l,再精密量取10m l,加水稀释成100m l,摇匀,即得(每l m l相当于1吨N03)]0.3m l,加无硝酸盐的水4.7m l,用同一方法处理后纯化水的颜色比较,不得更深(0.000006%)。
硫酸庆大霉素定义硫酸庆大霉素是一种绛红色小单胞菌产生的具有抗菌性作用的硫酸混合物。
主要成分包括庆大霉素c1a、c1、c2、c2a、c2b。
按无水物计算,每l mg 的效价不得少于590庆大霉素单位。
性状外观:本品为白色或类白色的粉末;具有引湿性。
溶解性:易溶于水,几乎不溶于乙醇(96%)。
鉴别第一次鉴别:B,C。
第二次鉴别:A,C。
A:薄层色谱法(2.2.27)。
供试品溶液:用纯化水溶解该物质25mg,并用纯化水稀释至溶解到5ml。
溶剂。
对照溶液:用纯化水溶解一小瓶的硫酸庆大霉素对照品并用纯化水稀释至5ml。
板:薄层层析硅胶板R.窗体顶端流动相:浓氨水、甲醇、二氯甲烷的等体积的混合物下层溶液。
体积:10微升。
展开:超过2/3的板。
干燥:在空气中干燥。
检测:用茚三酮溶液显色并在110℃中加热5min。
结果:供试品溶液所得的3个主色谱斑点的位置、颜色、大小均与对照品溶液所得的主色谱斑点相同。
B:观察组成项中供试品溶液所得到的色谱图。
结果:供试品溶液所得的图谱中的5各个主峰和对照品溶液的5个主峰具有相同的保留时间。
C:本品的水溶液显硫酸盐的鉴别反应(2.3.1)测试试液S.溶解0.8克该物质在无二氧化碳水R并用相同溶剂稀释到20ml。
测试结果溶液应澄清,与6号标准比色液(2.2.2,Method II)比较,不得更深。
pH值应为3.5~5.5.比旋度取本品2.5mg,精密称定,加水溶解并定量稀释至25ml,比旋度为+ 107°至+ 121°(无水物质)。
组成液相色谱法(2.2.29):使用归一化过程仅考虑庆大霉素C1a,C1,C2,C2A和C2B的峰。
供试品溶液(a):取25.0mg样品用流动相溶解并稀释至25ml。
供试品溶液(b):取供试品溶液(a)5.0ml,用流动相稀释至25ml。
对照品溶液(a):用流动相溶解5mg的庆大霉素对照品(杂质B),并稀释至25ml。
对照品溶液(b):用流动相溶解20.0mg的硫酸西索米星对照品(杂质B),并稀释至20ml。
纯化水定义是药物的准备用水,而不是那些要求无菌和无热源的,除非另有认证和授权散装纯化水制作散装纯化水是通过蒸馏、离子交换、反渗透或者其他合适的方法依据相关当局制定的水的法律法规和人们消耗水平从水中制的的。
散装纯化水储存和分布于能够抑制微生物生长和避免其他污染的环境中。
微生物检测在生产和之后的储存期间,采取适当的措施确保微生物的数量可监可控。
设置合适的警戒限和行动限来检测不良趋势。
在正常情况下,用不大于0.45μm的滤膜滤过,然后用R2A培养基在30-35℃的条件下培养不少于5天,行动限是100CFU/mL。
样本大小的选择与预期的结果有关。
R2A培养基酵母膏0.5g月示蛋白胨(一种含示的蛋白胨,长用于毒素检测和细菌培养)0.5g干酪素水解物0.5g葡萄糖0.5g淀粉0.5g磷酸氢二钾0.3g无水硫酸镁0.024g丙酮酸钠0.3g琼脂15.0g纯化水到1000mL调整PH,使灭菌之后是7.2±0.2。
在高压锅中121℃灭菌15分钟。
----测试菌株的制备。
使用标准稳定的测试菌株混悬液或者是按照表格0008-1准备。
菌种扩增培养技术(菌种扩增系统)的使用致使用于接种的微生物从源主菌种上的移除不超过5代。
每种微生物菌株的生长分别描述在表格0008-1中。
用PH7.0的钠氯蛋白胨缓冲溶液或者PH7.2的磷酸盐缓冲溶液制作测试悬浮液。
悬浮液的使用不超过2小时或者储存在2-8℃不超过24小时。
作为一个备选项来准备,稀释一个刚配制的枯草芽孢杆菌营养细胞悬浮液,准备一个稳定的孢子悬浮液,然后取适当体积的孢子悬浮液来用于测试接种。
稳定的孢子悬浮液能够在2-8℃条件下保存经过验证的时间。
促生长检测每批准备好的培养基和每批培养基,准备干燥培养基或者配料描述的培养基。
R2A琼脂接种盘分离在表格0008-1中表述的微生物的一小部分。
在表格描述的条件下进行培养。
通过对标准培养基的计算,成长的获得不同于大于系数2的因素。
Comparison of specifications of purified water (EP, ChP&USP)
纯化水质量标准比较
(EP, ChP&USP)
起草人/日期(Drafted by):
审核人/日期(Checked by):
批准人/日期(Approved by):
XXXXXXXXXXXXXXX有限公司
二O一六年十二月
纯化水质量标准比较(EP, ChP&USP)
1、概述
纯化水是我公司重要的制药用水,不同地区均有相应的药典要求,药典是一个国家记载药品标准、规格的法典,一般由国家药品监督管理局主持编纂、颁布实施,国际性药典则由公认的国际组织或有关国家协商编订。
制定药品标准对加强药品质量的监督管理、保证质量、保障用药安全有效、维护人民健康起着十分重要的作用。
制药用水系统的目的之一为“维持制药用水水质在药典要求的可接受范围内”,本文将主要介绍《美国药典》、《欧洲药典》和《中国药典》对制药用水质量的要求。
2、标准比较(ChP2015,EP8,USP38,ICH)
3、检测方法及标准。
纯化水
微生物指导:
在纯化水的制备和随后的贮存中应采用适当的方法来控制和指导微生物数。
应设置适当的警线来检测有害趋势。
在正常情况下,微生物数可接受的水平为100CFU/ml,它是通过膜孔径不大于0.45um的薄膜过滤,用R2A琼脂培养基在30-35℃培养不少于5天的方法测得的。
选择适当的样品体积来测得所希望的结果。
R2A琼脂培养基
酵母浸出胨0.5g
朊间质蛋白胨0.5g
酪蛋白水解物0.5g
葡萄糖0.5g
淀粉0.5g
磷酸氢二钾0.3g
无水硫酸镁0.024g
丙酮酸钠0.3g
琼脂15.0g
纯化水至1000ml
调节pH至灭菌后为7.2±0.2。
然后在121℃下高压灭菌15min。
R2A琼脂培养基的促生长
——测试菌的制备:用标准的稳定的测试菌的悬浮液或按表0008-1所示制备。
运用批种子培养保持技术(批种子系统)来达到用于接种的微生物从原始种子开始不超过5代的培养。
按表0008-1所示独立培养每种细菌。
用pH7.0的氯化钠蛋白胨缓冲液或pH7.2磷酸缓冲液制备菌悬液。
应在2小时内或2-8℃贮存下24小时内使用菌悬液。
同样方法制备、稀释枯草芽孢杆菌营养细胞的新鲜孢子悬液,取适当体积的稳定的孢子悬液用于测试接种。
稳定的孢子悬液可在2-8℃条件下验证时间内贮存。
——促生长:测试每一批准备好的培养基,脱水制备或者由描述的成分制备每一批培养基。
取表0008-1中的微生物少量(不大于100CFU)独立的接种在R2A琼脂培养基中。
按表中的条件培养。
获得的生长结果不得超过由标准接种而来的计算值的两倍范围。
对新制备的接种物,微生物的生长状况应与以前测试过的、经批准的培养基的微生物作比较。
表0008-1——R2A琼脂培养基的促生长
微生物测试菌的制备促生长
铜绿假单胞菌如:ATCC 9027
NCIMB 8626
CIP 82.118
NBrC 13275 酪蛋白大豆琼脂培养基和酪
蛋白大豆肉汤培养基
30-35℃
18-24h
R2A琼脂培养基
≤100CFU
30-35℃
≤3天
枯草芽孢杆菌如:ATCC 6633
NCIMB 8054
CIP 52.62
NBrC 3134 酪蛋白大豆琼脂培养基和酪
蛋白大豆肉汤培养基
30-35℃
18-24h
R2A琼脂培养基
≤100CFU
30-35℃
≤3天
总有机碳或易氧化物:按2.2.44节检测有机碳,其限度为0.5mg/L。
或用易氧化物的检
测代替总有机碳的检测。
方法:取10ml的稀硫酸R和0.02M的高锰酸钾0.1ml至100ml的水中,煮沸5分钟,溶液保持粉红色。
电导率:按以下条件在线或离线检测电导率:
设备
电导池:用合适的材料如不锈钢做电极。
电极常数:供应商一般会提供电极常数,但应用小于1500 µS·cm−1的标准电导率溶液定期的进行校验或与已确认的电极常数的电极作比较。
如果电极常数的校验值在验证值的2%的范围内则校验合格,否则应重新校验。
电导仪:准确度为0.1 µS·cm−1或更好。
系统校验(电导池和电导仪):
用一个或多个标准电导液。
准确性:测定值的3%再加上0.1µS·cm−1。
电导仪的校验:断开电导池,然后用已验证过的不确定度不大于校验值0.1%的精密电阻器或等效的设备校验所需的每个测量值范围。
如果在线电导池不能拆卸,则系统校验应用已校验的有电导池的电导测量仪在水流中关闭电导池进行校验。
温度检测:偏差为± 2 °C。
操作
当没有温度补偿时测量电导应同时记录温度。
而温度补偿测量法应进行验证后再使用。
当用记录温度的方法测量水的电导时,其值不超过下表(0008.-2.)时则符合要求。
表0008.-2.. –温度及电导要求
温度(°C)
电导(µS·cm− 1)
0 2.4 10 3.6 20 4.3 25 5.1 30 5.4 40 6.5 507.1 608.1 709.1 759.7 809.7 909.7 10010.2
对于表0008.-2..中未列出的温度,用在表中相连低点和相连高点之间插入温度来计算电导率限值。
重金属:如果纯化水符合注射用水(0169)中的电导率要求时,可以不按以下要求进行检测。
特性
性状:澄清、无色液体。
测试
硝酸盐:最大值为0.2ppm
取5ml纯化水于放置在冰水上的试管中,加入0.4ml的100g/L的氯化钾溶液R、0.1ml 的二苯胺溶液R,然后边摇边滴加5ml的无氮的硫酸R。
然后将试管放于50°C的水浴上。
15分钟后,测试溶液的蓝色不得深于的标准液的按同种方法试验的颜色。
标准液是用4.5ml的无氮水R和0.5ml的标准硝酸盐溶液R(2ppmNO3)。
铝(2.4.17):当用于透析液的生产时,其最大值为10ppb。
重金属:最大值为0.1ppm。
取200ml纯化水中加入0.15ml的0.1M的硝酸,用玻璃蒸发皿在水域上蒸发至20ml。
取12ml的溶液作为测试液A。
用10ml的标准铅溶液(1ppm Pb)R和0.075ml的0.1M 硝酸作为对照溶液。
在空白溶液中加入0.075ml的0.1M的硝酸做为空白对照。
细菌内毒素(2.6.14):如果用于透析液的生产,而没有更合适的方法消除水中的细菌内毒素时,其小于0.25IU/mL。