Guiding Stem Cell Differentiation into Oligodendrocytes
- 格式:pdf
- 大小:2.06 MB
- 文档页数:8


万方数据甄蕾,等.人牙周膜干细胞的初步鉴定及体外成骨诱导ZHENLei.etaLHu眦nPeriodontalLigamentStemCellsDifferentiationintoOsteoblasts/nv/tro・319・RNA提取试剂盒、一步法RT.PCR试剂盒(Tiangen公司。
北京)。
1.2方法1.2.1牙周膜细胞原代培养收集临床12一18岁因正畸需要而拔除的牙周健康、无龋的新鲜前磨牙,PBS洗3次,刮取根中l,3牙周膜组织,采用酶解组织块法培养,每隔3d换液.直至细胞从组织块周围游出。
细胞生长达80%汇合时传代。
1.2.2有限稀释法克隆化培养纯化PDLSCs取对数生长期的第1代细胞。
以1~2个/孔的密度接种于96孔板,常规培养7。
14d,至出现细胞克隆(细胞数≥50为判定标准)后扩大培养。
1.2.3PDLSCs的初步鉴定分别取第2代克隆形成细胞进行爬片。
采用SP法检测波形蛋白和角蛋白的表达。
同时利用兀TC荧光标记二抗检测STRO..1和CDl46的表达。
1.2.4体外诱导分化取第2代对数生长期的克隆形成细胞。
按5xllY个/mL接种于24孔板中,待细胞进入对数生长期后弃去原培养液。
PBS洗3遍,换诱导液(10mmol/LB.甘油磷酸钠、10{mol/L地塞米松、50I.Lg/mL维生素C)培养21d。
对照组细胞常规培养。
1.2.5成骨性能的鉴定1.2.5.1茜素红S染色观察钙结节形成人PDLSCs诱导培养21d后弃去培养液,4%多聚甲醛固定30min后饱和茜素红S溶液染色10min,观察钙结节形成情况。
1.2.5.2定性的细胞ALP活性检测人PDLSCs诱导培养21d后弃去培养液。
按ALP检测试剂盒说明书规范操作。
光学显微镜下观察染色情况。
1-2.5.3免疫细胞化学检测BSP、I型胶原表达人PDLSCs诱导培养21d后常规SP法检测BSP、I型胶原蛋白表达情况。
1.2.5.4RT-PCR检测ALP、BSPmRNA表达收集诱导培养21d后的人PDLSCs.。

核苷酸抗代谢物1. 介绍核苷酸抗代谢物是一类具有重要生物学功能的化合物,它们在细胞内起着调节代谢过程的关键作用。
核苷酸抗代谢物包括多种化合物,如腺苷、鸟苷、尿苷等。
核苷酸抗代谢物在细胞内通过与核酸相关的生化反应发挥作用。
它们可以通过调节核糖核酸(RNA)和脱氧核糖核酸(DNA)的合成和降解来影响基因表达和遗传信息传递。
它们还参与能量代谢、信号传导、细胞增殖和分化等生命活动过程。
2. 功能2.1 调节基因表达核苷酸抗代谢物可以通过影响RNA和DNA的合成来调节基因表达。
腺苷可以通过激活腺苷酸环化酶(adenylyl cyclase)增加环磷腺苷酸(cAMP)水平,进而激活蛋白激酶A(protein kinase A),从而调节转录因子的活性,影响基因的转录。
2.2 能量代谢核苷酸抗代谢物在能量代谢过程中起着重要作用。
腺苷三磷酸(ATP)是细胞内的主要能量储存分子,它可以通过水解释放出能量供细胞使用。
另外,核苷酸抗代谢物还参与糖原合成、糖解和脂肪酸合成等能量代谢途径。
2.3 信号传导核苷酸抗代谢物还参与细胞内外的信号传导过程。
腺苷和鸟苷可以通过结合细胞表面的腺苷酸受体来调节细胞内的信号传导通路。
这些信号传导通路可以影响细胞的增殖、分化、凋亡等生命活动。
3. 相关疾病核苷酸抗代谢物在多种疾病的发生和发展中起着重要作用。
一些遗传性代谢病与核苷酸抗代谢物的异常有关。
丙氨酰-tRNA合成酶缺乏症是一种由核苷酸抗代谢物异常引起的遗传性代谢疾病,患者体内的丙氨酰-tRNA合成酶活性降低,导致蛋白质合成受到影响。
核苷酸抗代谢物还与一些常见疾病的发生和发展相关。
肿瘤细胞通常具有增强的能量需求和异常的代谢特征,核苷酸抗代谢物在肿瘤细胞中扮演着重要角色。
核苷酸抗代谢物可能成为治疗肿瘤的潜在靶点。
4. 药物开发基于对核苷酸抗代谢物功能的理解,科学家们已经开发出一系列与其相关的药物。
这些药物可以通过调节核苷酸抗代谢物水平来治疗相关疾病。
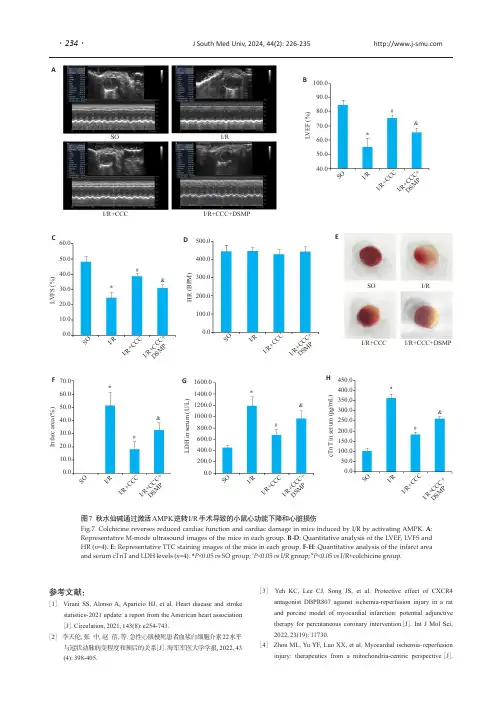
参考文献:[1]Virani SS,Alonso A,Aparicio HJ,et al.Heart disease and strokestatistics-2021update:a report from the American heart association [J ].Circulation,2021,143(8):e254-743.[2]李天伦,张中,赵蓓,等.急性心肌梗死患者血浆白细胞介素22水平与冠状动脉病变程度和预后的关系[J ].海军军医大学学报,2022,43(4):398-405.[3]Yeh KC,Lee CJ,Song JS,et al.Protective effect of CXCR4antagonist DBPR807against ischemia-reperfusion injury in a rat and porcine model of myocardial infarction:potential adjunctive therapy for percutaneous coronary intervention [J ].Int J Mol Sci,2022,23(19):11730.[4]Zhou ML,Yu YF,Luo XX,et al.Myocardial ischemia-reperfusioninjury:therapeutics from a mitochondria-centric perspective [J ].I/R+CCC I/R+CCC+DSMPSOI/R图7秋水仙碱通过激活AMPK 逆转I/R 手术导致的小鼠心功能下降和心脏损伤Fig.7Colchicine reverses reduced cardiac function and cardiac damage in mice induced by I/R by activating AMPK.A :Representative M-mode ultrasound images of the mice in each group.B -D :Quantitative analysis of the LVEF,LVFS and HR (n =4).E :Representative TTC staining images of the mice in each group.F -H :Quantitative analysis of the infarct area and serum cTnT and LDH levels (n =4).*P <0.05vs SO group;#P <0.05vs I/R group;&P <0.05vs I/R+colchicine group.B CEF ADG H I /RS O I /R+C C C I /R +C CC +D SM P L V E F (%)#*&100.090.080.070.060.050.040.0I /RS O I /R +CC CI /R +C C C +D SM P L V F S (%)SO I/RI/R+CCC I/R+CCC+DSMP60.050.040.030.020.010.00.0H R (B P M )500.0400.0300.0200.0100.00.0I /RS O I /R +CC C I /R +C C C +D SM P I /RS O I /R +C C C I /R +C CC +D SM P I /RS O I /R +C C C I /R +C C C +D SM P I /RS O I /R+C C C I /R +C CC +D SM P I n f a r c a r e a (%)70.060.050.040.030.020.010.00.0L D H i n s e r u m (U /L )1600.01400.01200.01000.0800.0600.0400.0200.00.0c T n T i n s e r u m (p g /m L )450.0400.0350.0300.0250.0200.0150.0100.050.00.0#*&#*&#*&#*&J South Med Univ,2024,44(2):226-235··234Cardiology,2021,146(6):781-92.[5]Chen MX,Li XP,Yang H,et al.Hype or hope:Vagus nerve stimulation against acute myocardial ischemia-reperfusion injury [J].Trends Cardiovasc Med,2020,30(8):481-8.[6]Liu X,Xu L,Wu J,et al.Down-regulation of SIK2expression alleviates myocardial ischemia-reperfusion injury in rats by inhibiting autophagy through the mTOR-ULK1signaling pathway [J].J South Med Univ,2022,42(7):1082-8.[7]Lu CH,Guo X,He XH,et al.Cardioprotective effects of sinomenine in myocardial ischemia/reperfusion injury in a rat model[J].Saudi Pharm J,2022,30(6):669-78.[8]Cadenas S.ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection[J].Free Radic Biol Med, 2018,117:76-89.[9]Maximilian Buja L.Mitochondria in ischemic heart disease[J].Adv Exp Med Biol,2017,982:127-40.[10]Peoples JN,Saraf A,Ghazal N,et al.Mitochondrial dysfunction and oxidative stress in heart disease[J].Exp Mol Med,2019,51(12):1-13.[11]Zou RJ,Shi WT,Qiu JX,et al.Empagliflozin attenuates cardiac microvascular ischemia/reperfusion injury through improving mitochondrial homeostasis[J].Cardiovasc Diabetol,2022,21(1): 106.[12]Tong DC,Wilson AM,Layland J.Colchicine in cardiovascular disease:an ancient drug with modern tricks[J].Heart,2016,102(13):995-1002.[13]Elshafei MN,El-Bardissy A,Khalil A,et al.Colchicine use might be associated with lower mortality in COVID-19patients:a meta-analysis[J].Eur J Clin Invest,2021,51(9):e13645.[14]Deftereos SG,Beerkens FJ,Shah B,et al.Colchicine in cardio-vascular disease:In-depth review[J].Circulation,2022,145(1):61-78.[15]Wang LR,Shan YL,Chen L,et al.Colchicine protects rat skeletal muscle from ischemia/reperfusion injury by suppressing oxidative stress and inflammation[J].Iran J Basic Med Sci,2016,19(6):670-5.[16]Tang YJ,Shi CY,Qin YY,et work pharmacology-based investigation and experimental exploration of the antiapoptotic mechanism of colchicine on myocardial ischemia reperfusion injury [J].Front Pharmacol,2021,12:804030.[17]李晨霏,樊迪,杨政,等.AMPK在心肌纤维化相关疾病中的作用及机制研究进展[J].解放军医学杂志,2021,46(12):1239-44.[18]胡淼,童旭辉,黄杰,等.基于铁死亡探讨AMPK抗小鼠脑缺血/再灌注损伤的作用及机制[J].华中科技大学学报:医学版,2021,50(4):418-23.[19]Zhang Y,Wang Y,Xu JN,et al.Melatonin attenuates myocardial ischemia-reperfusion injury via improving mitochondrial fusion/ mitophagy and activating the AMPK-OPA1signaling pathways[J].J Pineal Res,2019,66(2):e12542.[20]曾菲,李强,曾昪,等.氢溴酸加兰他敏介导AMPKα1/Nrf2/HO-1通路对大鼠心肌缺血再灌注损伤的保护作用[J].四川大学学报:医学版,2020,51(3):337-43.[21]Wang Y,Viollet B,Terkeltaub R,et al.AMP-activated protein kinase suppresses urate crystal-induced inflammation and transduces colchicine effects in macrophages[J].Ann Rheum Dis,2016,75(1): 286-94.[22]Lu YY,Chen YC,Kao YH,et al.Colchicine modulates calcium homeostasis and electrical property of HL-1cells[J].J Cell MolMed,2016,20(6):1182-90.[23]Liu HQ,Mo HQ,Yang CB,et al.A novel function of ATF3in suppression of ferroptosis in mouse heart suffered ischemia/reperfusion[J].Free Radic Biol Med,2022,189:122-35.[24]Akodad M,Fauconnier J,Sicard P,et al.Interest of colchicine in the treatment of acute myocardial infarct responsible for heart failure ina mouse model[J].Int J Cardiol,2017,240:347-53.[25]Yu HL,Liu Q,Chen GD,et al.SIRT3-AMPK signaling pathway as a protective target in endothelial dysfunction of early sepsis[J].IntImmunopharmacol,2022,106:108600.[26]Lv DY,Luo MH,Cheng Z,et al.Tubeimoside I ameliorates myocardial ischemia-reperfusion injury through SIRT3-dependentregulation of oxidative stress and apoptosis[J].Oxid Med CellLongev,2021,2021:5577019.[27]Xiang M,Lu YD,Xin LY,et al.Role of oxidative stress in reperfusion following myocardial iIschemia and Its treatments[J].Oxid Med Cell Longev,2021,2021:6614009.[28]Yue HH,Liang WT,Zhan YJ,et al.Colchicine:emerging therapeutic effects on atrial fibrillation by alleviating myocardial fibrosis in a ratmodel[J].Biomedecine Pharmacother,2022,154:113573.[29]Yang MY,Lv H,Liu Q,et al.Colchicine alleviates cholesterol crystal-induced endothelial cell pyroptosis through activating AMPK/SIRT1pathway[J].Oxid Med Cell Longev,2020,2020:9173530.[30]Xin T,Lu CZ.SirT3activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury[J].Aging,2020,12(16):16224-37.[31]Feng LF,Ren JL,Li YF,et al.Resveratrol protects against isoproterenol induced myocardial infarction in rats through VEGF-B/AMPK/eNOS/NO signalling pathway[J].Free Radic Res,2019,53(1):82-93.[32]Tian L,Cao WJ,Yue RJ,et al.Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats withmyocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1alpha signaling pathway[J].J Pharmacol Sci,2019,139(4):352-60.[33]吴志林,朱轶.右美托咪定通过Trx1/AMPK通路减轻心肌缺血再灌注损伤中的氧化应激[J].华中科技大学学报:医学版,2020,49(4):404-7.[34]Wu SN,Zou MH.AMPK,mitochondrial function,and cardio-vascular disease[J].Int J Mol Sci,2020,21(14):4987.[35]秦秀男,秦溱,冉珂,等.七氟醚预处理通过线粒体NAD+-SIRT3通路减轻大鼠心肌缺血再灌注损伤[J].中南大学学报:医学版,2022,47(8):1108-19.[36]韦亚忠,薛晓梅,何斌.活性氧介导心肌缺血再灌注损伤的研究进展[J].上海交通大学学报:医学版,2021,41(6):826-9.[37]Paradies G,Paradies V,Ruggiero FM,et al.Mitochondrial bioenergetics and cardiolipin alterations in myocardial ischemia-reperfusion injury:implications for pharmacological cardiopro-tection[J].Am J Physiol Heart Circ Physiol,2018,315(5):H1341-52.[38]Brenner D,Mak TW.Mitochondrial cell death effectors[J].Curr Opin Cell Biol,2009,21(6):871-7.(编辑:经媛) J South Med Univ,2024,44(2):226-235··235帕金森病(PD )是一种进行性神经退行性疾病,是60岁以上人群中第2常见的神经退行性疾病,其主要原因是黑质致密部(SNc )多巴胺能(DA )神经元的死亡和含α-突触核蛋白的路易体的形成[1]。

Cell differentiation: 是指个体发育过程中细胞之间在形态、结构功能上逐渐产生稳定性差异,形成不同细胞类群的过程。
特点:稳定性特定条件下可产生去分化和转分化时-空性和细胞分裂相关cell determination:细胞分化命运的决定。
是指在个体发育过程中,细胞在发生可识别的分化特征之前,就已确定了未来的发育命运,只能向特定方向分化的状态。
特点:稳定性Transdifferentiation,:高度分化的动物细胞从一种分化状态(序列)转变为另一种分化状态(序列)的现象。
Dedifferentiation:在某些条件下,分化了的细胞并不稳定,其基因活动模式也可发生可逆性的变化,又回到未分化状态。
Housekeeping gene:指导生成维持细胞生存所必须的最基本的蛋白质,并不参与细胞分化方向的确定。
luxury gene:指导细胞特异蛋白的产生。
这些蛋白和分化细胞的特异性状密切相关,但并不是细胞基本生命活动必不可少的。
影响细胞分化的主要因素有哪些?一、细胞质对细胞分化的影响: 母源效应基因产物的极性分布和胚胎细胞的胞质不对称分裂,决定了细胞分化命运二、胚胎细胞间相互作用: 诱导具有层次性、区域特异性和遗传特异性,协调细胞分化方向三、激素:动物发育过程中的变态发育晚期,按预先决定的分化程序调节分化四、环境因素Stem cell: 存在于个体发育过程中,具有长期(或无限)自我更新能力、并可分化产生某种(或多种)特化细胞的原始细胞。
是个体的生长发育、组织器官的结构和功能的动态平衡,以及其损伤后的再生修复的基础。
干细胞的种类: 按分化潜能分类:全能干细胞多能干细胞专能干细胞来源分类:胚胎干细胞成体干细胞肿瘤干细胞totipotent SC:能形成完整的个体哺乳动物——受精卵和8-细胞期之前的细胞,每一个细胞都是全能干细胞。
能形成个体中所有类型的细胞以及起营养作用的胎盘等胚胎外的组织pluripotent SC囊胚中的内细胞团,每一个细胞都能形成个体中所有类型的细胞但不能产生胎盘和其他一些发育时期的支持组织不能形成完整的个体multipotent SC只能分化形成功能上密切相关的一类细胞stem cell niche干细胞通常栖息在体内一个固定的、稳态的、安全的、血供丰富的微环境中,该区域被称为干细胞巢。

小鼠胚胎干细胞体外诱导分化成GABA能神经元目的探讨小鼠胚胎干细胞在体外培养向GABA能神经元定向诱导分化的可能性。
方法将小鼠胚胎干细胞以“无血清”方法培养,用DMEM/F12、N2、B27及NT4作为诱导分化剂定向诱导分化,分化好的细胞利用免疫荧光技术、流式细胞技术和RT-PCR鉴定。
结果在胚胎干细胞诱导分化成神经元后期,免疫荧光显示有GABA能神经元存在;RT-PCR结果证实有GABA能神经元正确分化的重要调控基因Viaat、Gad1和Gad2基因表达;流式细胞仪计数结果显示GABA阳性细胞约占总细胞数的(11.49±6.86)%。
结论小鼠胚胎干细胞经体外培养可以定向诱导分化成GABA能神经元,可作为神经移植的新来源。
[Abstract] ObjectiveTo investigate the possibilities of in vitro culture and differentiation of mouse embryonic stem cell to GABAergic neurons. MethodsMouse embryonic stem cells were cultured and induced into GABAergic neurons in serum-free cultural condition. Immunofluorescence,reverse transcription polymerase chain reaction(RT-PCR) and flow cytometer assay were used to identify the properties of the differentiated cells. ResultsIn the later period of differentiation of embryonic stem cells into neurons,immunofluorescence showed that GABAergic neurons existed,RT-PCR results confirmed the important regulatory genes Viaat,Gad1 and Gad2 gene expression due to the correct differentiation of GABAergic neurons and flow cytometry analysis showed the GABA-positive cells accounted for about(11.49±6.86)% of the total cell number. ConclusionMouse embryonic stem cells can be induced into GABAergic neurons in vitro in serum-free cultural condition,providing a new source of nerve graft.[Key words]Embryonic stem cell; Induce; Differentiate; GABA干细胞移植治疗中枢神经系统疾病的研究方兴未艾,且取得了一定成果,但由于其多取材于胚脑的神经干细胞,为日后临床应用埋下了伦理道德问题之患,并且受到供体来源短缺的限制[1]。

以肿瘤坏死因子α转基因小鼠为类风湿关节炎模型的研究进展王腾腾;徐浩;王拥军;施杞;梁倩倩【摘要】Rheumatoid arthritis (RA) was recognized as a chronic and autoimmune disease.The overexpression of tumor necrosis factor alpha (TNF-α) was of great significance in the pathogenesis of RA and mediated the levels of various inflammatory factors.At present,anti-TNF agents were widely used for the treatment of RA in clinic.The genetically modified mice,overexpressing human TNF-α,could appear pathological manifestations being similar to RA with slow onset,high incidence rate and stablc occurrence.Therefore,TNF transgenic (TNF-Tg) mouse model was significant in the study,generally applied to the curative and mechanism researches of RA,especially some remarkable and fruitful studies over lymphatic vessels.In this study,we summarized the pathophysiological characteristics of the mouse model based on the current research status.%类风湿关节炎(Rheumatoid Arthritis,RA)是一种慢性自身免疫疾病,肿瘤坏死因子α(Tumor Necrosis Factor,TNF-a)过表达在本病的发病过程中起到重要的作用,并介导了多种炎症因子的表达.目前抗TNF药物已广泛应用于RA临床.过表达人TNF-a的转基因小鼠可出现类似RA的病理表现:起病缓慢、发病率高、模型稳定.因此,TNF转基因(TNF-Tg)小鼠模型在RA研究中具有重要的意义,现已广泛应用于RA的诊疗技术和病理机制研究,尤其是淋巴管功能的研究,并取得一系列成果.本文针对该模型小鼠的生理病理特征、研究进展进行综述.【期刊名称】《世界科学技术-中医药现代化》【年(卷),期】2016(018)011【总页数】5页(P1869-1873)【关键词】肿瘤坏死因子α;转基因小鼠;类风湿关节炎;淋巴【作者】王腾腾;徐浩;王拥军;施杞;梁倩倩【作者单位】上海中医药大学附属龙华医院上海200032;上海中医药大学脊柱病研究所上海200032;上海中医药大学附属龙华医院上海200032;上海中医药大学脊柱病研究所上海200032;上海中医药大学附属龙华医院上海200032;上海中医药大学脊柱病研究所上海200032;上海中医药大学康复医学院上海200120;上海中医药大学附属龙华医院上海200032;上海中医药大学脊柱病研究所上海200032;上海中医药大学附属龙华医院上海200032;上海中医药大学脊柱病研究所上海200032【正文语种】中文【中图分类】R274RA是一种以对称性、多发性关节炎为主要临床表现的慢性免疫性疾病,主要的病理改变为:滑膜的增生、炎性因子的浸润、软骨及骨组织的破坏等[1]。

Stemcelltherapy将重新定义医学界Stem Cell Therapy: Redefining the Medical Field Introduction:In recent years, healthcare has witnessed tremendous advancements in regenerative medicine. One groundbreaking innovation that holds immense promise is stem cell therapy. Stem cell therapy employs the use of stem cells to repair damaged tissues and regenerate new cells, offering a potentially revolutionary approach to treating a wide range of medical conditions. This article delves into the potential of stem cell therapy to redefine the medical field, exploring its applications, current challenges, and future prospects.The Power of Stem Cells:Stem cells possess a unique ability to differentiate into various cell types, making them the building blocks of the human body. Through their remarkable regenerative potential, stem cells hold the key to potentially curing previously untreatable diseases. They can be harvested from various sources, including embryos, umbilical cords, and adult tissues, making them accessible for therapeutic use. With the ability to differentiate into specialized cells such as neurons, heart cells, and pancreatic cells, stem cells offer endless possibilities for medical interventions.Application in Disease Treatment:Stem cell therapy has already shown significant promise in multiple medical fields. In neurodegenerative diseases like Parkinson's and Alzheimer's, stem cells can potentially replace damaged neurons and restore brain function. Similarly, in spinal cord injuries, stem cells may provide a means to repair and regenerate damaged nerve tissue, potentially leading to restored mobility for patients. Stem cell therapy also holds the potential torevolutionize the treatment of cardiovascular diseases, diabetes, and even certain types of cancer.Cardiovascular diseases, such as heart failure, can greatly benefit from stem cell therapy. By injecting stem cells into damaged areas of the heart, researchers have observed improved heart function and reduced scar tissue formation. This breakthrough offers hope for millions of individuals suffering from cardiovascular conditions worldwide.Diabetes, a chronic disease affecting millions of people globally, may also find newfound hope through stem cell therapy. By differentiating stem cells into pancreatic beta cells, which produce insulin, scientists are exploring the potential for curing diabetes by replacing dysfunctional or destroyed cells within the pancreas. This approach could transform the lives of patients, dramatically altering the management and control of diabetes.Challenges and Ethical Considerations:Though stem cell therapy holds immense potential, it is not without challenges and ethical considerations. One major challenge lies in the ability to control and direct the differentiation of stem cells into desired cell types. Researchers continue to refine their methodologies and techniques to ensure reliable and predictable outcomes. Additionally, the safety and long-term effects of stem cell therapy require extensive research and clinical trials to establish efficacy and mitigate potential risks.Ethical considerations surrounding the use of embryonic stem cells also pose challenges. The extraction of embryonic stem cells often involves the destruction of embryos, and this raises ethical concerns for some. However, alternative sources of stem cells, such as adult tissues and umbilical cords, offer a more ethically acceptable solution for those who oppose the use of embryonic stem cells.Future Prospects and Conclusion:Stem cell therapy has the potential to redefine the medical field by unlocking endless possibilities for disease treatment and regenerative medicine. As scientists continue to delve deeper into understanding the mechanisms of stem cell differentiation and harness their full potential, the future looks promising. Advances in stem cell research may pave the way for personalized medicine, where therapies are tailored to individual patients based on their unique needs. With ongoing research, clinical trials, and collaborations, stem cell therapy is set to revolutionize healthcare, offering hope for those suffering from currently incurable diseases.In conclusion, stem cell therapy has the potential to redefine the medical field by offering a new paradigm for disease treatment and regeneration. Though challenges and ethical considerations exist, the power of stem cells to repair and regenerate holds immense promise for countless patients worldwide. As research advances and scientific breakthroughs unfold, stem cell therapy may soon become a cornerstone of modern medicine, bringing us closer to a future where previously untreatable conditions become curable.。


细胞分化的重要性英语作文English:Cell differentiation is crucial for the development and functioning of multicellular organisms. It is the process by which stem cells become specialized into different cell types with specific functions. This specialization allows cells to carry out specific tasks within the body, contributing to the overall health and functioning of the organism. Without cell differentiation, an organism would not be able to develop properly and would not be able to maintain homeostasis. In addition, cell differentiation plays a critical role in tissue repair and regeneration. When tissues are damaged, specialized cells are needed to replace the damaged ones and restore normal function. Furthermore, cell differentiation is essential for embryonic development, as it determines the formation of various organs and tissues in the growing organism. Overall, cell differentiation is vital for the proper development, functioning, and maintenance of multicellular organisms.Translated content:细胞分化对多细胞生物的发育和功能至关重要。

纳米材料的应用及毒性研究必要性纳米材料是指三维结构中至少有一维大小在纳米(10-9米)尺度上的材料。
由于纳米材料具有特殊的物理化学特性,使其在很多领域具有广泛的应用,比如:化工、陶瓷、微电子学、计量学、电学、光学以及信息通讯等领域[1]。
近期研究发现纳米技术在生物、医药上也具有巨大的应用潜力,包括疾病诊断、分子成像、生物传感器荧光生物标记,药物和基因传输,蛋白质的检测,DNA结构探讨,组织工程学等[2]。
目前市场上基于纳米技术的产品有很多,包括涂料,化妆品,个人护理品和食品增补剂[3]。
因此人类暴露于纳米颗粒的途径多种多样,吸入,摄取以及皮肤途径。
而且,出于医学的目的,这些颗粒有可能直接被注射进入人体内[4]。
一旦被人体吸收,各种类型的纳米颗粒就会分布到人体的大部分器官,甚至可以通过生物屏障,比如血脑屏障和血睾屏障[5,6]。
2003年,Science和Nature相继发表文章,探讨纳米材料的生物效应、对环境和健康的影响问题[7,8]。
很多研究工作已经证明,纳米材料对生物体会造成负面的影响。
目前为止, 科学家们只对纳米TiO2、SiO2、碳纳米管、富勒烯和纳米铁粉等少数几个纳米物质的生物效应进行了初步的研究[9]。
Vicki Colvin[7]强调:"当这一领域尚处于早期阶段, 并且人类受纳米材料的影响比较有限时, 一定要对纳米材料的生物毒性给予关注. 我们必须现在, 而不是在纳米技术被广泛应用之后, 才来面对这个问题"。
因此对纳米材料毒性的研究,不仅具有必要性而且具有紧迫性,是保证纳米科技顺利发展的前提,可以减少新兴科学对人类及自然界不必要的破坏。
纳米材料毒性研究现状纳米材料具有粒径小、比表面积大的特点,量子效应在纳米尺度上开始支配物质的物理化学性质。
这些特有的性质使得纳米材料的应用领域十分广泛[1]。
然而,纳米材料对生物系统的不利影响引起了越来越多的关注。
已经有很多研究证实,纳米材料并非有益而无害的,它们在细胞、亚细胞以及蛋白质水平上都影响着生物体[10]。
stem cell reviews and reports under review -回复Stem Cell Reviews and Reports: A Comprehensive ReviewIntroduction:Stem cell research has generated significant interest and excitement in the scientific community and the general public alike. The potential of stem cells to treat a wide range of diseases and injuries has sparked numerous investigations and clinical trials. In this article, we will delve into the realm of stem cell reviews and reports, exploring their significance, current state, and potential impact.1. What are Stem Cell Reviews and Reports?Stem Cell Reviews and Reports (SCRR) is a reputable scientific journal that publishes articles related to stem cell research, including reviews, reports, and original research. These publications provide an in-depth analysis and critical evaluation of the latest advancements in stem cell science. The primary aim of SCRR is to bridge the gap between basic stem cell research and clinical applications, fostering a dialogue that promotes the translation of scientific knowledge into therapeutic interventions.2. Significance of Stem Cell Reviews and Reports:Stem Cell Reviews and Reports play a vital role in the dissemination of knowledge within the stem cell field. They serve as a platform for scholars to present their findings, theories, and experimental data. These publications undergo a rigorous peer-review process to ensure the accuracy, validity, and reliability of the information presented. By publishing comprehensive reviews and reports, SCRR helps researchers and clinicians stay up-to-date with the latest discoveries and advances in the field.3. Current State of Stem Cell Reviews and Reports:The field of stem cell research is rapidly expanding, and SCRR reflects this growth. It covers a broad spectrum of topics, ranging from stem cell biology and differentiation to regenerative medicine and tissue engineering. Stem Cell Reviews and Reports also addresses the ethical, legal, and social implications of stem cell research. Furthermore, the journal emphasizes the importance of the translational research, seeking to bridge the gap between laboratory findings and clinical applications.4. Topics Covered in Stem Cell Reviews and Reports:SCRR encompasses a diverse range of topics within the stem cell field. These include:a. Stem Cell Biology: Reviewing the fundamental aspects of stem cell biology, such as stem cell identification, characteristics, and behavior.b. Stem Cell Differentiation: Exploring the mechanisms involved in stem cell differentiation and specialization into specific cell types.c. Stem Cell Therapy: Evaluating the use of stem cells in therapeutic interventions for various diseases and injuries, including neurodegenerative disorders, cardiovascular conditions, and spinal cord injuries.d. Tissue Engineering: Investigating the application of stem cells in tissue engineering approaches to create functional, artificial tissues and organs.e. Stem Cell Ethics: Addressing the ethical considerations surrounding stem cell research, including the use of embryonic stem cells, cloning, and informed consent.5. Potential Impact of Stem Cell Reviews and Reports:Stem Cell Reviews and Reports have the potential to significantly impact the field of stem cell research and regenerative medicine. By providing a comprehensive overview of the current state of thefield, these publications facilitate collaborations, inspire new research directions, and help identify potential gaps in knowledge. Moreover, these reviews and reports serve as a valuable resource for policymakers and funding agencies, aiding in the formulation of evidence-based guidelines and decisions.Conclusion:Stem Cell Reviews and Reports serve as a crucial platform for disseminating knowledge and promoting the translation of stem cell research into clinical applications. With an extensive range of topics covered, these publications help scientists and clinicians stay abreast of the latest advancements in the field. Moving forward, the impact of Stem Cell Reviews and Reports is expected to continue driving progress in stem cell research and regenerative medicine, ultimately benefiting patients worldwide.。
静脉移植骨髓间充质干细胞联合重组人生长激素对充血性心肌病大鼠心肌和血管组织的修复姚巍;王凤芝【摘要】背景:骨髓间充质干细胞静脉移植后,能否归巢至心脏受损部位和分化为心肌样细胞尚无统一定论.目的:探讨重组人生长激素联合骨髓间充质干细胞静脉移植对充血性心肌病大鼠心肌和血管新生的影响.方法:密度梯度离心和贴壁筛选法获得大鼠骨髓间充质干细胞.模型组、细胞移植组、生长激素组、联合组大鼠均在阿霉素诱导下建立心脏衰竭模型.造模后,细胞移植组经静脉注入BrdU标记的骨髓间充质干细胞8×10~(13) L~(-1);生长激素组皮下注射重组人生长激素2 U/(kg·d),连续14 d;联合组行骨髓间充质干细胞移植与重组人生长激素注射.4周后取材,BrdU+MHC及BrdU+Actin免疫组化染色确定骨髓间充质干细胞的归巢情况,评价移植细胞向心肌样细胞和血管内皮细胞的分化,苏木精-伊红染色检测血管密度.结果与结论:与细胞移植组比较,联合组BrdU免疫组化阳性率显著升高(P <0.001);BrdU+MHC双染和BrdU+Actin双染后心肌样细胞、血管内皮细胞均显著增多(P < 0.001).与模型组比较,生长激素组、细胞移植组、联合组的总血管密度、微血管密度、毛细血管密度均显著升高(P < 0.001),后3组间比较无明显差异(P > 0.05).结果证实骨髓间充质干细胞静脉移植后可归巢到心脏,对充血性心肌病大鼠心肌和血管有明显修复作用,能在损伤处区存活、生长,并向心肌样细胞、血管内皮细胞方向分化,增加损伤处血管密度;生长激素可以改善微环境,加强骨髓间充质干细胞向心肌样细胞、血管内皮细胞的转化率.【期刊名称】《中国组织工程研究》【年(卷),期】2010(014)006【总页数】4页(P1064-1067)【关键词】阿霉素;心力衰竭;重组人生长激素;静脉移植;骨髓间充质干细胞【作者】姚巍;王凤芝【作者单位】山西医科大学第二医院干六楼心血管科,山西省太原市,030001;山西医科大学第二医院干六楼心内科,山西省太原市,030001【正文语种】中文【中图分类】R394.20 引言近年来扩张型心肌病的发病率在国内外逐渐呈增高趋势,已成为引起恶性心力衰竭的主要原因之一。
87M.A. Hayat (ed.), Stem Cells and Cancer Stem Cells, Volume 6,DOI 10.1007/978-94-007-2993-3_9, © Springer Science+Business Media B.V . 20129A bstractRetinal and macular degeneration disorders are characterized by a progressive loss of photoreceptors, which causes visual impairment and blindness. In some cases, the visual loss is caused by dysfunction, degen-eration and loss of underlying retinal pigment epithelial (RPE) cells and the subsequent death of photoreceptors. The grim reality is that there is no successful treatment for most of these blindness disorders. Cell therapy aimed at replenishing the degenerating cells is considered a potential ther-apeutic approach that may delay, halt or perhaps even reverse degenera-tion, as well as improve retinal function and prevent blindness in the aforementioned conditions. Human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs) may serve as an unlimited donor source of photoreceptors and RPE cells for transplantation into degenerat-ing retinas and for retinal disease modeling.I ntroductionThe vertebrate eyes form as bilateral evaginations of the forebrain, called optic vesicles (Martínez-Morales et al. 2004 ; Fig. 9.1a ). During develop-ment, the optic vesicles begin to invaginate to form a cup-shaped structure, the optic cup. The inner, thicker neural layer of the optic cup differ-entiates into the neural retina, and the outer, thin-ner pigmented layer forms the retinal pigmentepithelium (RPE). At the early developmental stages, the neuroepithelial cells that compose the optic vesicle are morphologically and molecu-larly identical and are all able to give rise to neu-ral retina and RPE. Exogenous signals coming from the adjacent tissues, including factors from the fi broblast growth factor (FGF) and transform-ing growth factor beta (TGF b ) families, dictate the fate of these cells. The mature vertebrate ret-ina is comprised of six types of neurons and one type of glia (the Müller glia). These seven cell types constitute three nuclear layers: retinal gan-glion cells in the ganglion cell layer (GCL); the horizontal, bipolar and amacrine interneurons, and Müller glial cells in the inner nuclear layer (INL); and rod and cone photoreceptors in the outer nuclear layer (ONL; Harada et al. 2007;M . I delson • B . R eubinoff (*)T he Hadassah Human Embryonic Stem Cell Research Center, The Goldyne Savad Institute of Gene Therapy & The Department of Obstetrics and Gynecology , H adassah University Medical Center ,E in Kerem 12000 ,J erusalem 91120 ,I srael e -mail: b enjaminr@ekmd.huji.ac.il D ifferentiation of HumanPluripotent Stem Cells into Retinal Cells Masha Idelson and Benjamin Reubinoff88M. Idelson and B. ReubinoffFig. 9.1b ). The photoreceptor cells capture lightphotons and transform their energy into electrical signals by a mechanism called phototransduction. The visual pigment which is utilized in this process is located on membranal discs in the outer seg-ments of photoreceptors. The outer segments are continuously renewed: the old discs are shed and new disks form. When the photoreceptors absorb light, they send the signal through the retinal interneurons to the ganglion cells which transmit the electrical impulse to the brain by their axons forming the optic nerve. Rods are responsible for night vision, whereas cones are responsible for color vision and detecting fi ne details. The macula is a small part of the retina which is rich in cones and responsible for detailed central vision.R PE cells that compose the outer layer of the optic cup are pigmented cuboidal cells which lie between the neural retina and the choriocapil-laris, which include the blood vessels supplying the retina. The multiple villi on their apical side are in direct contact with the outer segments ofextraocular mesenchymeabneural retinalensoptic nerveoptic cupsurface ectodermRPEFGFoptic vesiclechoroidBM RPE cone ONLINL GCLlightHC BC MC ACONrod F ig. 9.1 D evelopment and structural arrangement of the retina. ( a ) Schematic representation of retinal development including the transition from optic vesicle to optic cup and retinal patterning. ( b ) Schematic diagram of retinal cells arrangement and connections. A bbreviations :A C amacrinecell, B C bipolar cell, B M Bruch’s membrane, G CL gan-glion cell layer, H C horizontal cell, I NL inner nuclear layer, M C Müller cell, O N optic nerve, O NL outer nuclear layer89 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsthe photoreceptor cells; on their basal side, the RPE is in contact with the underlying basal mem-brane, termed Bruch’s membrane that separates the RPE from the choroid. These cells play cru-cial roles in the maintenance and function of the retina and its photoreceptors. As a layer of pig-mented cells, the RPE absorbs the stray light that was not absorbed by the photoreceptors. The RPE cells form a blood–retinal barrier due to decreased permeability of their junctions. The RPE cells transport ions, water, and metabolic end products from the retina to the bloodstream. They are involved in supplying the neural retina with nutrients from the bloodstream, such as glu-cose, retinol, and fatty acids. Another important function of the RPE is the phagocytosis of shed photoreceptor outer segments. After the outer segments are digested, essential substances such as retinal are recycled. Retinal is also recycled and returned to photoreceptors by the process known as the visual cycle. The precise functioning of the RPE is essential for visual performance. Failure of one of these functions can lead to degeneration of the retinal photoreceptors, vision impairment and blindness.T here are many inherited and age-related eye disorders that cause degeneration of the retina as a consequence of loss of photoreceptor cells. Retinal and macular degeneration disorders can be divided into two main groups. The fi rst group primarily affects the photoreceptors and involves the majority of cases of retinitis pigmentosa. In the second group, the primary damage is to the adjacent RPE cells, and as a consequence of this damage, the photoreceptors degenerate. This group includes age-related macular degeneration, Stargardt’s macular dystrophy, a subtype of Leber’s congenital amaurosis in which RPE65 is mutated, Best’s disease and some cases of retini-tis pigmentosa, as well.W ith regard to retinitis pigmentosa (RP), it is a group of inherited retinal degeneration diseases that are caused, as mentioned above, by a primary progressive loss of rod and cone photoreceptors, followed by a subsequent degeneration of RPE (Hartong et al. 2006). The disease affects approxi-mately 1.5 million patients worldwide and is the most common cause of blindness in people under 70 years of age in the western world. The disease can be characterized by retinal pigment deposits visible on the fundus examination. In most cases, the disease primarily affects rods. At later stages of the disease, the degeneration of cones takes place. As a consequence of disease progression, the patients’ night vision is reduced. Patients initially lose peripheral vision while retaining central vision (a visual status termed “tunnel vision”). In advanced cases, central vision is also lost, commonly at about 60 years of age. The disease affects about 1 in 4,000. The inheritance can be autosomal-recessive, autosomal-dominant or X-linked (in ~50–60%, 30–40%, and 5–15% of cases, respectively). Mutations in more than 140 genes have been iden-tifi ed as causing RP (Hartong et al. 2006).Among these genes are those involved in phototransduc-tion, like rhodopsin, the a- and b- subunits of phos-phodiesterase, the a- and b- subunits of Rod cGMP gated channel and arrestin. The additional muta-tions were found in genes encoding structural pro-teins, like peripherin, rod outer segment protein and fascin. They were also found in transcription factors involved in photoreceptors’ development such as Crx and Nrl, and in other genes, whose products are involved in signaling, cell-cell interac-tion and trafficking of intracellular proteins. Currently, there is no effective cure for RP. Treatment with vitamin A palmitate, omega-3 fatty acids and other nutrients may somewhat slow the rate of the disease progression in many cases. Reduction in exposure to light was also shown to decrease the rate of retinal degeneration.A mong the group of retinal degenerations that are caused by primary loss of RPE cells or their function, age-related macular degeneration (AMD) is the most frequent condition and the leading cause of visual disability in the western world (Cook et al. 2008).Among people over 75 years of age, 25–30% are affected by AMD, with progressive central visual loss that leads to blindness in 6–8%. The retinal degeneration pri-marily involves the macula. The dry form of AMD is initiated by hyperplasia of the RPE and formation of drusen deposits, consisting of meta-bolic end products underneath the RPE or within the Bruch’s membrane. It may gradually progress into the advanced stage of geographic atrophy90M. Idelson and B. Reubinoff with degeneration of RPE and photoreceptorsover large areas of the macula causing central visual loss. Ten percent of dry AMD patients will progress to neovascular (wet) AMD, with blood vessels sprouting through the Bruch’s membrane with subsequent intraocular leakage and/or bleed-ing, accelerating the loss of central vision. While the complicating neovascularization can be treated with anti-VEGF agents, currently there is no effective treatment to halt RPE and photore-ceptor degeneration and the grim reality is that many patients eventually lose their sight (Cook et al. 2008).S targardt’s macular dystrophy (SMD) is the most common form of inherited macular dystro-phy affecting children (Walia and Fishman 2009). The disease is symptomatically similar to AMD. The prevalence of SMD is about 1 in 10,000 chil-dren. The disease involves progressive central visual loss and atrophy of the RPE beneath the macula following accumulation of lipofuscin in RPE cells, which is suggested to consist of non-degradable material, derived from ingested pho-toreceptor outer segments. The inheritance is predominantly autosomal recessive, although an autosomal dominant form has also been described. The mutation in the ABCA4 gene was found to be a most common cause of SMD. The product of the ABCA4 gene is involved in energy transport to and from photoreceptors. The mutated protein cannot perform its transport function and, as a result, photoreceptor cells degenerate and vision is impaired. Currently, there is no effective treat-ment for SMD.C ell therapy to replenish the degenerating cells appears as a promising therapeutic modality that may potentially halt disease progression in the various retinal and macular degeneration dis-orders caused by loss and dysfunction of RPE cells and photoreceptors (da Cruz et al. 2007).I n this chapter we will discuss the potential of human pluripotent cells which includes human embryonic stem cells (hESC) and induced pluripotent stem cells (iPSCs), to gen-erate various types of retinal cells that could be used for transplantation therapy of retinal degen-eration disorders and disease modeling for drug discovery. C ell Therapy of Retinal and Macular DegenerationsT he eye is an attractive organ for cell therapy as it is easily accessible for transplantation and for simple monitoring of graft survival and potential complications by direct fundoscopic visualiza-tion. Anatomically, it is a relatively confi ned organ limiting the potential of unwanted extra-ocular ectopic cell distribution, and a low number of cells are required to replenish the damaged cells. The eye is also one of the immune privi-leged sites of the body.T he concept of replacing dysfunctional or degenerated retina by transplantation has been developing ever since the fi rst retina-to-retina transplant in 1986 (Turner and Blair 1986).In most studies, primary retinal immature (fetal) tissue has been used as donor material. It was demonstrated that such transplants can survive, differentiate, and even establish connections with the host retina to a limited degree (Ghosh et al. 1999). The subretinal transplantation of healthy RPE has some advantages over neural retinal transplantation, as it concerns only one cell type that is not involved in neural networking. Transplantation of RPE has been studied exten-sively in animal models (Lund et al. 2001).The most commonly used animal model of retinal degeneration is the Royal College of Surgeons (RCS) rat model, in which primary dysfunction of the RPE occurs as a result of a mutation in the receptor tyrosine kinase gene M ertk(D’Cruz et al. 2000). This leads to impaired phagocytosis of shed photoreceptor outer segments, with sec-ondary degeneration and progressive loss of pho-toreceptors within the fi rst months of life. It was reported that rat and human RPE cells rescued photoreceptor cells from degeneration when transplanted into the subretinal space of RCS rats (Li and Turner 1988; Coffey et al. 2002).The ability of transplanted RPE cells to restore retinal structure and function has been demonstrated in clinical trials. In humans, autologous transplanta-tions of peripheral RPE as well as macular trans-locations onto more peripheral RPE provide a proof that positioning the macula above relatively91 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellshealthier RPE cells can improve visual functionin AMD patients (Binder et al. 2004; da Cruz et al. 2007). Nevertheless, the surgical procedures for autologous grafting are challenging and are often accompanied by signifi cant complications. In addition, autologous RPE transplants may carry the same genetic background, environmen-tal toxic and aging-related effects that may have led to macular RPE failure and the development of AMD in the patient. It is also problematic to use autologous cells when all the RPE cells are damaged. Cell sources that can be used for such therapy include allogeneic fetal and adult RPE (Weisz et al. 1999; Binder et al. 2004; da Cruz et al. 2007). However, the use of fetal or adult retinal tissues for transplantation is severely lim-ited by ethical considerations and practical prob-lems in obtaining sufficient tissue supply. The search for a cell source to replace autologous RPE such as immortalized cell lines, umbilical cord-derived cells as well as bone marrow-derived stem cells continues.T he derivation of hESCs more than a decade ago has raised immense interest in the potential clinical use of the cells for regeneration (Thomson et al. 1998; Reubinoff et al. 2000).Along the years, signifi cant progress has been made towards the use of hESCs in clinical trials.T he other promising source of cells for transplantation therapy is iPSCs that are simi-lar to hESCs in their stemness characteristics and pluripotency. These cells could be gener-ated from different human somatic cells by transduction of four defi ned transcription fac-tors: Oct3/4, Sox2, Klf4, and c-Myc (Takahashi et al. 2007).G eneration of RPE and neural retina from hESCs and iPSC has numerous advantages, as it can be done from pathogen-free cell lines under good manufacturing practice (GMP) conditions with minimal variation among batches. Such cells can be characterized extensively prior to preclinical studies or for clinical applications, and an unlimited numbers of donor cells can be generated from them. In the following para-graphs, strategies for induction of differentiation of hESCs and iPSCs towards RPE and neural retina fate are reviewed. D ifferentiation into Retinal Pigment EpitheliumI t was reported for the fi rst time in mice and pri-mates that the differentiation of ES cells into RPE could be induced by co-culture with PA6 stromal cells (Kawasaki et al. 2002; Haruta et al. 2004). The resulting cells had polygonal epithelial mor-phology and extensive pigmentation. The cells expressed the markers that are characteristic of RPE. They developed typical ultrastructures and exhibited some functions of RPE. The differenti-ation of hESC into RPE was first reported by Klimanskaya et al. (2004).According to their protocol, hESCs underwent spontaneous differ-entiation by overgrowth on mouse embryonic fibroblasts (MEF), in feeder-free conditions or, alternatively, as embryoid bodies (EBs) in com-bination with withdrawal of bFGF from the medium. The yield of the formation of RPE cells after 4–8 weeks of spontaneous differentiation was relatively low; for example,<1% of EBs con-tained pigmented cells at this stage. However, after 6–9 months in culture, all the EBs contained pigmented cells. The areas of pigmented cells could be further isolated mechanically and prop-agated by passaging as RPE lines. Klimanskaya and colleges characterized the hESC-derived RPE cells by transcriptomics and demonstrated their higher similarity to primary RPE tissue than to human RPE lines D407 and ARPE-19. The low yield of spontaneously differentiating RPE cells was improved by induction of differentia-tion with Wnt and Nodal antagonists, Dkk1 and LeftyA, respectively, the factors that are sug-gested to promote retinal differentiation. This treatment gave rise to pigmented cells within 38% of the hESC colonies after 8 weeks (Osakada et al. 2008). Immunostaining with the ZO-1 anti-body showed that by day 120, hESC-derived pig-mented cells formed tight junctions (about 35% of total cells). We showed that differentiation toward the neural and further toward the RPE fate could be augmented by vitamin B3 (nicotin-amide; Idelson et al. 2009).We further showed that Activin A, in the presence of nicotinamide, effi ciently induces and augments differentiation92M. Idelson and B. Reubinoffinto RPE cells. This is in line with the presumed role of Activin A in RPE development i n vivo .In the embryo, extraocular mesenchyme-secreted members of the TGF b superfamily are thought to direct the differentiation of the optic vesicle into RPE (Fuhrmann et al. 2000).Under our culture conditions, when the cells were grown in suspen-sion as free-fl oating clusters, within 4 weeks of differentiation, 51% of the clusters contained pigmented areas and about 10% of the cells within the clusters were pigmented. When we modifi ed the differentiation conditions to includea stage of monolayer culture growth, the yield of the RPE-like pigmented cells was signifi cantly improved and 33% of the cells were pigmented after 6 weeks of differentiation. The derivation of RPE from hESCs and iPSCs without any external factor supplementation was also demonstrated by other groups (Vugler et al. 2008 ; Meyer et al. 2009 ; Buchholz et al. 2009).T he hESC-derived RPE cells were extensively characterized, including demonstration, both at the mRNA and the protein levels, of the expres-sion of RPE-specifi c markers, such as RPE65, CRALBP, Bestrophin, Tyrosinase, PEDF, PMEL17, LRAT, isoforms of MiTF abundant in RPE, and others. The cells expressed markers of tight junctions that join the adjacent RPE cells: ZO-1, occludin and claudin-1 (Vugler et al. 2008 ) . Electron microscopic analysis revealed that the hESC-derived RPE cells showed features characteristic of RPE. The cells were highly polarized with the nuclei located more basally, and the cytoplasm with the mitochondria and melanin granules of different maturity more api-cally. A formation of basal membrane was observed on the basal surface of the RPE cell. Similar to putative RPE, the hESC-derived RPE basal membrane was shown to be composed of extracellular matrix proteins, collagen IV , lami-nin and fi bronectin (Vugler et al.2008).The appearance of apical microvilli was demonstrated at the apical surface of the RPE. The presence of tight and gap junctions on the apical borders of the RPE cells was also confi rmed by electron microscopy. O ne of the most important functions of RPE cells i n vivo is phagocytosis of shed photoreceptor outer segments, as part of the continuous renewal process of rods and cones. The hESC-derived RPE cells demonstrated the ability to phagocyto-size latex beads or purifi ed photoreceptor outer segments, confi rming that these cells are func-tionali n vitro . It may be concluded from all these studies that human pluripotent stem cells have a potential to give rise to pigmented cells exhibiting the morphology, marker expression and functionof authentic RPE.D ifferentiation into Retinal Progenitors and Photoreceptors O ur group showed, for the fi rst time, the potential of highly enriched cultures of hESC-derived neu-ral precursors (NPs) to differentiate towards the neural retina fate (Banin et al. 2006).We demon-strated that the NPs expressed transcripts of key regulatory genes of anterior brain and retinal development. After spontaneous differentiation i n vitro , the NPs gave rise to progeny expressing markers of retinal progenitors and photoreceptor development, though this was uncommon and cells expressing markers of mature photorecep-tors were not observed. We showed that after transplantation into rat eyes, differentiation into cells expressing specifi c markers of mature photoreceptors occurred only after subretinal transplantation (between the host RPE and pho-toreceptor layer) suggesting that this specifi c microenvironment provided signals, yet unde-fi ned, that were required to support differentia-tion into the photoreceptoral lineage.P rogress towards controlling and inducing the differentiation of hESCs into retinal progenitors and neurons i n vitro was reported in the study of Lamba et al. ( 2006).They treated hESC-derived EBs for 3 days with a combination of factors,including Noggin, an inhibitor of BMP signaling, Dkk1, a secreted antagonist of the Wnt signaling pathway and insulin-like growth factor 1 (IGF-1), which is known to promote retinal progenitor dif-ferentiation. The cultivation of EBs with these factors was followed by differentiation on Matrigel or laminin for an additional 3 weeks in the presence of the combination of the three93 9 Differentiation of Human Pluripotent Stem Cells into Retinal Cellsfactors together with bFGF. Under these culture conditions, the majority of the cells developed the characteristics of retinal progenitors and expressed the specifi c markers Pax6 and Chx10 (82% and 86% of the cells, respectively). The authors showed that after further differentiation, the cells expressed markers of photoreceptor development Crx and Nrl (12% and 5.75%, respectively). About 12% of the cells expressed also HuC/D, the marker of amacrine and ganglion cells. The expression of markers of the other sub-types of retinal neurons was demonstrated, as well. However, only very few cells (<0.01%) expressed markers of mature photoreceptors, blue opsin and rhodopsin. The abundance of cells expressing markers of photoreceptors could be accelerated by co-culture with retinal explants, especially when the explants originated from mice bearing a mutation that causes retinal degeneration.T o better characterize the phenotype of retinal cells obtained with this differentiation protocol, a microarray-based analysis comparing human retina to the hESC-derived retinal cells was per-formed (Lamba and Reh 2011).It was demon-strated that gene expression in hESC-derived retinal cells was highly correlated to that in the human fetal retina. In addition, 1% of the genes that were highly expressed in the hESC-derived cultures could be attributed to RPE and ciliary epithelium differentiation.A n alternative protocol for the derivation of retinal progenitors and photoreceptors was pro-posed by Osakada et al. (2008).Similar to the protocol for the derivation of RPE cells, they used serum-free fl oating cultures in combination with the Dkk1 and LeftyA. After 20 days of cul-ture in suspension, the cells were replated on poly-D-lysine/laminin/fi bronectin-coated slides. Osakada and co-authors demonstrated that on day 35 in culture, about 16% of colonies were positive for retinal progenitor markers Rx and Pax6. Differentiation towards photoreceptor fate was augmented in the presence of N2 by treat-ment with retinoic acid and taurine, which are known inducers of rod fate differentiation. Under these conditions, after an extended culture period of 170 days, about 20% of total cells were positive for Crx, an early photoreceptor marker. On day 200, about 8.5% of the cells expressed the mature rod photoreceptor marker, rhodopsin, as well as cone photoreceptor markers, red/green and blue opsins (8.9% and 9.4%, respectively).A n alternative approach was proposed by the same group based on the use of small molecules. In this method, the chemical inhibitors CKI-7 and SB-431542 that inhibit Wnt and Activin A signaling, respectively, and Y-27632, the Rho-associated kinase inhibitor, which prevents disso-ciation-induced cell death, were used. These molecules were shown to mimic the effects of Dkk1 and LeftyA (Osakada et al. 2009).This strategy, which doesn’t involve the use of recom-binant proteins which are produced in animal or E scherichia coli cells, is more favorable for the gen-eration of cells for future transplantation therapy.I n another study that was published by Meyer et al .(2009), after initial differentiation in sus-pension for 6 days, the aggregates were allowed to attach to laminin–coated culture dishes. After further differentiation as adherent cultures, neu-roepithelial rosettes were formed, which were mechanically isolated and subsequently culti-vated as neurospheres. The authors didn’t use any soluble factors; moreover, they showed that under these conditions, the cells expressed endogenous Dkk1 and Noggin. They also demonstrated that in concordance with the role of bFGF in retinal specifi cation, the inhibition of endogenous FGF-signaling abolished retinal differentiation. Under their differentiation protocol, by day 16, more than 95% of the cells expressed the retinal pro-genitor markers, Pax6 and Rx. The authors dem-onstrated that by day 80 of differentiation, about 19% of all neurospheres contained Crx+ cells and within these Crx+ neurospheres, 63% of all cells express Crx and 46.4% of the cells expressed mature markers, such as recoverin and cone opsin.I n all of the above studies, differentiated cells expressing the retinal markers were obtained; however, the cells were not organized in a three-dimensional retinal structure. In a paper recently published by Eiraku et al. (2011),the authors cul-tured free-fl oating aggregates of mouse ES cells in serum-free medium in the presence of base-ment membrane matrix, Matrigel, that could also94M. Idelson and B. Reubinoffbe substituted with a combination of laminin, entactine and Nodal. Using a mouse reporter ES cell line, in which green fl uorescent protein (GFP) is knocked in at the Rx locus, the authors showed that Rx-GFP+ epithelial vesicles were evaginated from the aggregates after 7 days of differentiation under these conditions. On days 8–10, the Rx-GFP+ vesicles changed their shape and formed optic cup-like structures. The inner layer of these structures expressed markers of the neural retina whereas the outer layer expressed markers of RPE. The authors demonstrated that differen-tiation into RPE required the presence of the adjacent neuroectodermal epithelium as a source of diffusible inducing factors. In contrast, the differentiation into neural retina did not require tissue interactions, possibly because of the intrinsic inhibition of the Wnt-signaling pathway. Eiraku and colleagues showed that the retinal architecture, which was formed within the optic vesicle-like structures, was comparable to the native developing neural retina.R ecently, optic vesicle-like structures were also derived from hESCs and iPSCs using the protocol described above, which is based on iso-lating the neural rosette-containing colonies and culturing them in suspension (Meyer et al. 2011). The cells within the structures expressed the markers of retinal progenitors, and after differen-tiation gave rise to different retinal cell types. It was shown that the ability of optic vesicle-like structures to adopt RPE fate could be modulated by Activin A supplementation. The production of these three-dimensional retinal structures opens new avenues for studying retinal development in normal and pathological conditions.T ransplantation of Pluripotent Stem Cell-Derived Retinal CellsA key step towards future clinical transplanta-tions of hESC-derived RPE and neural retina is to show proof of their therapeutic potential i n vivo. Various animal models of retinal degeneration have been used to evaluate the therapeutic effect of transplanted retinal cells. Human ESC-derived RPE cells were transplanted subretinally to the degenerated eyes of RCS rats. Transplantation of the hESC-derived RPE cells between the RPE and the photoreceptor layer rescued retinal struc-ture and function (Lund et al. 2006; Vugler et al. 2008; Idelson et al. 2009; Lu et al. 2009).The subretinally engrafted hESC-derived RPE cells salvaged photoreceptors in proximity to the grafts as was shown by the measurement of the thick-ness of the ONL, the layer of photoreceptor nuclei, which is an important monitor of photore-ceptor cell survival. The ONL thickness was significantly increased in transplanted eyes in comparison to the degenerated non-treated eyes.I n order to evaluate the functional effect of transplanted cells i n vivo, the electroretinography (ERG) that directly measures the electrical activ-ity of the outer (a-wave) and inner (b-wave) retina in response to light stimulation was used. It was demonstrated that after transplantation of hESC-derived RPE, ERG recordings revealed a signifi -cant preservation of retinal function in the treated eyes as compared to control untreated eyes (Lund et al. 2006; Idelson et al. 2009).The visual func-tion of the animals was also estimated by an optomotor test, which monitors the animal’s refl exive head movements in response to a rotat-ing drum with fi xed stripes. Animals transplanted with hESC-derived RPE showed signifi cantly better visual performance in comparison to con-trol animals (Lund et al. 2006; Lu et al. 2009). The presence of rhodopsin, a major component of photoreceptor outer segments, within the sub-retinaly transplanted pigmented cells suggested that they could perform phagocytosis i n vivo (Vugler et al. 2008; Idelson et al. 2009).B ridging the gap between basic research and initial clinical trials requires immense resources to ensure safety and efficacy. Human ESC-derived RPE cell lines were generated using a current Good Manufacturing Practices (cGMP)-compliant cellular manufacturing process (Lu et al. 2009). Long-term studies analyzing safety and efficacy of transplantation of these GMP-compliant hESC-derived RPE cells revealed that the subretinally transplanted cells survived for a period of up to 220 days and provided prolonged functional improvement for up to 70 days after transplantation. The potential of the hESC-derived。
Cancer stem cells are defined as those with the ability to self-renew while generating a daughter progenitor. They are malignant for the progenitor to undergo further differentiation into more mature progenitors into phenotypically diverse cancer cells. To cure cancer it is imperative to devise therapies that effectively target the cancer stem cells.iPS cells, namely induced pluripotent stem cells, were first produced in 2006 from mouse cells and in 2007 from human cells. They are a type of pluripotent stem cell artificially derived from a non-pluripotent cell, typically an adult somatic cell, by inducing a "forced" expression of specific genes, such as oct4, sox2, nanog, klf4, c-myc and so forth. They are similar to natural pluripotent stem cells, such as ES cells, in many respects, such as the expression of certain stem cell genes, chromatin methylation patterns, doubling time, embryoid body formation and potency of differentiability, but the full extent of their relation to natural pluripotent stem cells is still being assessedcell reprogramming, is the process of directing mature cells to a primitive state of gene expression. Somatic cell nuclear transfer, cell fusion, and introduction of specific transcriptional factors are three main strategies of cell reprogramming.stem cells are biological cells that can differentiate into diverse specialized cell types. There are two types of stem cells: embryonic stem cells that are isolated from the inner cell mass of blastocysts, and adult stem cells that are found in various tissues. The classical definition of a stem cell requires it possess two properties: self-renewal - the ability to go through numerous cycles of cell division while maintaining the undifferentiated state. Potency - the capacity to differentiate into specialized cell types. In the strictest sense, this requires stem cells to be either totipotent or pluripotent - to be able to give rise to any mature cell type, although multipotent or unipotent progenitor cells are sometimes referred to as stem cells.Epigenetic is the study of heritable changes in phenotype or gene expression caused by mechanisms other than changes in the DNA sequence. These changes may remain through cell divisions for the remainder of the cell's life and may also last for multiple generations. However, there is no change in the underlying DNA sequence of the organism; instead, non-genetic factors cause the organism's genes to behave differently.Embryonic stem cells are pluripotent stem cells derived from the inner cell mass of the blastocyst, an early-stage embryo. They have two distinctive properties: the pluripotency of differentiation, and the capacity of self-renewal. They can differentiate into all derivatives of the three primary germ layers, which include more than 220 types of cells in the adult body. They also can maintain their undifferentiated state when they are in the continuous process of division.gene targeting is a genetic technique that uses homologous recombination to change an endogenous gene. The method can be used to delete a gene, remove exons, add a gene, and introduce point mutations. Gene targeting can be permanent or conditional. Gene targetingrequires the creation of a specific vector for each gene of interest. However, it can be used for any gene, regardless of transcriptional activity or gene size.可塑性plasticity refers to the ability of a cell to change its phenotype in response to changes in the environment.Somatic cells nuclear transfer is a laboratory technique for creating a clonal embryo, using an ovum with a donor nucleus. It can be used in embryonic stem cell research, or in regenerative medicine, such as "therapeutic cloning". It can also be used as the first step in the process of reproductive cloning, but it will break human ethics if it is applied in human cloning.Genomic imprinting is a mark about parental origin, namely a genetic phenomenon by which certain genes are expressed in a parent-of-origin-specific manner. It is an inheritance process independent of the classical Mendelian inheritance. Imprinted genes are either expressed only from the allele inherited from the mother , or from the allele inherited from the father. It is also an epigenetic process that involves methylation and histone modifications in order to achieve gene expression without altering the genetic sequence. These epigenetic marks are established in the germline and are maintained throughout all somatic cells of an organism.Transdifferentiation is an irreversible switch of one differentiated cell type to another. It takes place when a non-stem cell transforms into a different type of cell, or when an already differentiated stem cell creates cells outside its already established differentiation path. It is a type of metaplasia, which includes all cell fate switches, including the interconversion of stem cells.Stem cell niche is the microenvironment in which stem cells are found, which interacts with stem cells to regulate stem cell fate. Niche can be in reference to the in vivo or in vitro stem cell microenvironment. During embryonic development, various niche factors act on embryonic stem cells to alter gene expression, and induce their proliferation or differentiation for the development of the fetus. Within the human body, stem cell niches maintain adult stem cells in a quiescent state, but after tissue injury, the surrounding micro-environment actively signals to stem cells to either promote self renewal or differentiation to form new tissues.Aseptic technique refers to a procedure that is performed under sterile conditions. This includes medical and laboratory techniques, such as with microbiological cultures. It includes techniques like flame sterilization.自我更新self-renew means that embryonic stem cells can maintain their undifferentiated state when they are in the continuous process of division. It is the distinctive prosperity of embryonic stem cells or iPS cells.分化differentiation is the process by which a less specialized cell becomes a more specializedcell type. Differentiation occurs numerous times as the organism grows from a simple zygote to a complex system of tissues and cell types. Differentiation dramatically changes a cell's size, shape, membrane potential, metabolic activity, and responsiveness to signals. It is under the exact and rigorous control of the expression of various genes.细胞周期The cell cycle is the series of events that takes place in a cell leading to its division and duplication. It can be divided in two brief periods: interphase, during which the cell grows, accumulating nutrients needed for mitosis and duplicating its DNA; and M phase, during which the cell divides into two distinct cells . The cell cycle is a vital process by which a zygote develops into a mature organism, as well as a process by which various tissues and organs are renewed.治疗性克隆therapeutic cloning means using the technique of cloning to create embryos in the laboratory for medical purposes. The use of cloning techniques to obtain human embryos is in order to extract embryonic stem cells or pluripotent stem cells. These stem cells, in the right conditions, can develop into any types of cells, issues as well as organs, which can be used for medical treatment.。
国际细胞治疗协会定义间充质干细胞的标准文献《 International Society for Cellular Therapy Definition of Mesenchymal Stem Cells Standard Document》IntroductionMesenchymal stem cells (MSCs) are a heterogeneous collection of cells that are capable of self-renewal and differentiation into various cell types. MSCs have the potential to be used in new cellular therapies, as well as to study the underlying mechanisms of diseases and to develop novel therapeutics. The International Society for Cellular Therapy (ISCT) has developed a standard of MSCs to ensure their quality and safety for therapeutic applications. This document outlines the critical criteria for defining, identifying, and characterizing MSCs, as well as strategies for isolating and expanding them.Defining MSCsMSCs are defined as multipotent cells that exhibit a number of characteristics, including (1) self-renewal, (2) plasticity, (3) lineage commitment/differentiation, (4) adhesion, and (5) express certain cell surface markers.Self-Renewal: MSCs have the capacity to divide and replacethemselves, and thus have the potential to generate an indefinite number of cells.Plasticity: MSCs have multilineage differentiation potential, meaning that they can differentiate into a wide variety of cell types.Lineage Commitment/Differentiation: MSCs are capable of differentiating into cells of mesenchymal lineages, such as bone, cartilage, fat, and muscle.Adhesion: MSCs adhere to a variety of surfaces and substrates under in vitro conditions.Cell Surface Markers: MSCs can be identified and characterized by expression of certain cell surface markers, including CD73, CD90, and CD105, as well as the absence of hematopoietic markers such as CD45, CD34, and CD14.Identifying and Characterizing MSCsThe identity and phenotype of MSCs can be determined using a variety of methods, including flow cytometry, immunocytochemistry, and/or molecular techniques such asRT-PCR.Flow cytometry: Flow cytometry is a technique that allows for the identification and characterization of MSCs based on their expression of specific surface proteins. Specificmarkers used to identify MSCs include the cell surface markers previously mentioned, as well as additional markers such as CD29, CD44, and CD166.Immunocytochemistry: MSCs can be identified and characterized based on their expression of specific intracellular proteins. Examples of proteins that are useful for MSC identification and characterization include Oct-4, Nanog, and Sox-2.Molecular techniques: RT-PCR, western blotting, and other molecular techniques can be used to detect and quantify the expression of specific mRNA transcripts.Isolation and ExpansionMSCs can be isolated from a variety of sources, including adipose tissue, bone marrow, and umbilical cord or placenta. Once isolated, MSCs can be expanded in vitro using a variety of techniques, such as culture in serum-free media supplemented with growth factors. Important considerations when expanding MSCs include the type of culture system, the choice of media and supplements, and the conditions used to optimize growth. ConclusionMSCs are a heterogeneous collection of cells with the potential to be used in new cellular therapies. The ISCT hasdeveloped a standard for defining, identifying, and characterizing MSCs, as well as strategies for isolating and expanding them. It is important that MSCs are of high quality and safety when used in therapeutic applications.。
COMMUNICATIONGuiding Stem Cell Differentiation into Oligodendrocytes Using Graphene-Nanofi ber Hybrid ScaffoldsS hreyas S hah ,P erry T. Y in ,T hiers M. U ehara ,S y-Tsong Dean C hueng ,L etao Y ang ,a nd K i-Bum L ee *of the CNS, while eliminating the potential adverse or variable side-effects from growth factors and viral gene vectors, wouldbe highly benefi cial. [ 8]H erein, we report the use of a graphene-based nanomaterial for the design of hybrid nanofi brous scaffolds to guide NSC dif-ferentiation into oligodendrocytes ( F igure 1). Graphene-based nanomaterials, such as graphene oxide (GO), have recently gained considerable interest for tissue engineering applications due to their favorable chemical, electrical and mechanical prop-erties. [ 9] Besides serving as a highly elastic and fl exible struc-tural reinforcement, substrates coated with GO have been dem-onstrated to promote the growth and differentiation of variousstem cell lines, including induced PSCs, MSCs and NSCs.[ 10 ] Based on these considerations, we demonstrate the use of GO as an effective coating material in combination with electro-spun nanofi bers for the selective differentiation of NSCs into oligodendrocytes. By varying the amount of GO coating on the nanofi bers, we observed a GO concentration-dependent change in the expression of key neural markers, wherein coating with a higher concentration of GO was seen to promote differentia-tion into mature oligodendrocytes. Further investigation into the role of GO-coating on the nanofi brous scaffolds showed the overexpression of a number of key integrin-related intracellular signaling molecules that are known to promote oligodendrocyte differentiation in normal development. E lectrospun nanofi ber scaffolds exhibit several key prop-erties that are advantageous for neural tissue engineering, including a high degree of porosity, high surface-to-volume ratio, and a relatively close structural mimic of the native extra-cellular matrix (ECM).[ 11 ] From the wide array of polymeric materials available, we used polycaprolactone (PCL) to generate our nanofi brous scaffolds. PCL is a biodegradable and biocom-patible polyester approved by the FDA for use in the human body as a drug delivery device and suture, and is also widelyused for neural tissue engineering.[ 12 ] In our studies, PCL was electrospun onto a metallic collector and then transferred to glass substrates for cell culture using a medical grade adhe-sive. Nanofi bers with an average diameter of 200–300 nm were generated, which is a fi ber size range that has been reported to be favorable for oligodendrocyte culture, potentially due tothe close morphological resemblance to axons [ 13 ]( F igure 2a ).Thin-layered graphene oxide (GO) was then synthesized and dispersed in deionized water (Figure S1, Supporting Infor-mation). The hydrophobic PCL nanofi bers were exposed to oxygen plasma to render the surface hydrophilic (Figure S2, Supporting Information). The GO was then deposited on the PCL nanofi ber surface, thus allowing for the effi cient and uni-form coating of the PCL nanofi ber surface with GO, as seenS . Shah, S.-T. D. Chueng, L. Yang, Prof. K.-B. LeeD epartment of Chemistry and Chemical Biology, Rutgers The State University of New Jersey P iscataway ,N J 08854 ,U SA E-mail: k blee@P . T. Yin, Prof. K.-B. LeeD epartment of Biomedical EngineeringR utgers, State University of New Jersey P iscataway ,N J 08854 ,U SAT . M. UeharaP hysics Institute of São Carlos University of São PauloCP 369 São Carlos, S ão Paulo 13566 ,B razilDOI: 10.1002/adma.201400523D amage to the central nervous system (CNS) from degenera-tive diseases or traumatic injuries is particularly devastating due the limited regenerative capabilities of the CNS. Among the current approaches, stem-cell-based regenerative medicine has shown great promise in achieving signifi cant functional recovery by taking advantage of the self-renewal and differentia-tion capabilities of stem cells, which include pluripotent stem cells (PSCs), mesenchymal stem cells (MSCs) and neural stemcells (NSCs).[ 1] However, the low survival rate upon transplan-tation has been a longstanding barrier for scientists and clini-cians to overcome.[ 2] To this end, numerous types of natural and synthetic biomaterial scaffolds have been developed, the two main classes being hydrogels and nanofi bers, in an attempt to mimic the cellular microenvironment, support cellulargrowth and improve cellular viability.[ 3] Nevertheless, designing scaffolds with defi ned properties to selectively guide stem cell differentiation towards a specifi c neural cell lineage is still an ongoing challenge.F or CNS regeneration, the selective differentiation of NSCs into either neurons or oligodendrocytes (as opposed to astro-cytes) is highly desirable.[ 4] A number of approaches have been employed to guide differentiation into neurons, including genetic modifi cations, growth factors, cytokines, substratetopography and even nanomaterials.[ 5] However, oligoden-drocyte differentiation has proven to be much more elusive, resulting in only a small percentage of the differentiated cellpopulation.[ 6] The primary approach to guide oligodendro-cyte differentiation has focused on either developing culture media containing a combination of growth factors or the forced expression of key oligodendrocyte-promoting transcription fac-tors via viral gene transfection. [ 7] However, developing a bio-materials-approach to achieve effi cient differentiation of NSCsinto mature oligodendrocytes, which are the myelinating cellsC O M M U N I C A T I O NF igure 1. S chematic diagram depicting the fabrication and application of graphene-nanofi ber hybrid scaffolds. Polymeric nanofi bers (composed of polycaprolactone) generated using electrospinning were subsequently coated with graphene oxide (GO) and seeded with neural stem cells (NSCs). NSCs cultured on the graphene-nanofi ber hybrid scaffolds show enhanced differentiation into oligodendrocyte lineage cells.F igure 2. M orphology of nanofi brous scaffolds and cultured NSCs on the scaffolds. a,b) FE-SEM images of PCL nanofi bers (a) and PCL nanofi bers coated with GO using 1.0 mg/mL GO solution (b). Scale bars: 2 µm. c,d) FE-SEM of differentiated NSCs cultured on PCL nanofi ber scaffolds (c) and graphene-nanofi ber hybrid scaffolds (d) after six days of culture. The cells are pseudo-colored blue for contrast. The differentiated cells on the graphene-nanofi ber hybrid scaffolds (d) show a clear morphological difference in terms of process extension compared with the nanofi ber scaffolds alone (c). Scale bars: 10 µm.COMMUNICATIONwith fi eld emission scanning electron microscopy (FE-SEM) (Figure 2 b and Figure S3, Supporting Information) and helium ion microscopy (Figure S4, Supporting Information).F or the culture of NSCs, the scaffolds were then coated with laminin, a well-established ECM protein which is essen-tial for the adhesion, growth and differentiation of NSCs. [ 14 ]Green fl uorescent protein-labeled adult rat hippocampal NSCs (purchased from Millipore) were then seeded onto the scaf-folds and the morphology was monitored using fl uorescence microscopy. After six days of culture, a signifi cant difference in the cellular morphology was evident on GO-coated nanofi bers compared to the nanofi bers alone (Figure S5, Supporting Infor-mation). FE-SEM shows cell attachment on these surfaces in greater detail, wherein the cells on the GO-coated nanofi bers display extensive branching of cell processes (Figure 2 c ,d and Figure S6, Supporting Information). This type of extensive pro-cess extension is a characteristic attribute reported to distin-guish oligodendrocytes from other neural cells. [ 15 ] This differ-ence in cellular morphology provides evidence for the potential ability of our hybrid scaffolds to enhance NSC differentiationinto oligodendrocytes.T o systematically investigate the effect of GO-coating on NSC differentiation, we generated hybrid scaffolds with varying amounts of GO-coating. Solutions containing three different concentrations of GO (0.1, 0.5 and 1.0 mg/mL) were depos-ited on oxygen plasma-treated PCL nanofi bers. The degree of coating using the various GO concentrations was then observed using FE-SEM ( F igure 3 a ). GO-coating of PCL with 0.1 mg/mL, indicated as PCL-GO (0.1), shows the clear pres-ence of GO compared with the PCL nanofi bers alone, with uniform coating on the surface of individual fi bers. In con-trast, PCL-GO (0.5) and PCL-GO (1.0) exhibit a much greater extent of GO attachment on the nanofi brous surface, showing a high degree of GO coating and connectivity between fi bers. This was confi rmed quantitatively using Raman spectroscopy,where the characteristic peaks of the D band (ca. 1350 cm −1)and G band (ca. 1600 cm −1 ) indicate the presence of GO. Com-parison of the Raman intensity of these peaks further supportsF igure 3. E ffect of concentration-dependent GO-coating on NSC differentiation. a) FE-SEM images of PCL nanofi bers coated with GO solutions ofvarying concentrations: 0.0 mg/mL [PCL], 0.1 mg/mL [PCL-GO (0.1)], 0.5 mg/mL [PCL-GO (0.5)] and 1.0 mg/mL [PCL-GO (1.0)]. Scale bars: 1 µm. b) Raman spectroscopy of glass and PCL nanofi bers coated with varying concentrations of GO. c) Quantitative PCR of NSCs grown on various substrates from RNA isolated after six days of culture. The plot shows fold change in gene expression of markers indicative of neurons (TuJ1), astrocytes (GFAP) and oligodendrocytes (MBP), wherein the PCL-GO substrates show the highest expression of MBP. The gene expression is relative to GAPDH, and normalized to the conventional PLL-coated glass control. Student's unpaired t-test was used for evaluating signifi cance (* = p < 0.05, ** = p < 0.01, n.s. = no signifi cance), compared to the PLL-coated glass control (denoted above the bar) or between different substrates.C O M M U N I C A T I O Nthe trend described above in terms of concentration-dependent GO coating on the PCL nanofi ber surfaces (Figure 3 b ). Moreover, the nanofi brous scaffolds at all three concentrations show signifi cantly higher GO content compared with the con-trol glass surfaces coated with the same respective amounts of GO (Figure 3 b ). The higher surface area-to-volume of the nano-fi bers available for GO attachment, in conjunction with the 3D structure of these scaffolds, may attribute to this difference in coating. T hese various PCL-GO substrates were then used to examine the infl uence of GO-coating on modulating NSC differentiation. For comparison, the following control substrates were used: i) PLL-coated glass (conventional substrate for in vitro neural cultures), ii) PCL nanofi bers alone, and iii) GO-coated glass (at the abovementioned three GO concentrations). All of the sub-strates were coated with laminin to facilitate NSC attachment, and the cells were harvested after six days of culture to compare the gene expression of key neural markers. Quantitative PCR (qPCR) was utilized to compare gene expression of three key markers that are indicative of differentiated NSCs: glial fi bril-lary acidic protein (GFAP; astrocytes), beta-III tubulin (TuJ1; neurons) and myelin basic protein (MBP; mature oligoden-drocytes). First, it is important to note that both the PCL nano-fi bers alone and GO-coated glass (at all three concentrations) individually show enhanced oligodendrocyte gene expression, with about a 2-fold increase in MBP expression (Figure 3 c ). At the same time, TuJ1 shows only about a 1.3-fold increase and GFAP shows about a 0.5-fold decrease in expression, which indicates a stronger preference for differentiation towards oli-godendrocytes rather than neurons and astrocytes (Figure 3 c ). While no reports exist for the effect of graphene-based nano-materials on oligodendrocyte differentiation, previous studies have reported that electrospun nanofi bers can act as permissiveculture platforms for oligodendrocyte culture.[ 16 ] S ince each individual component (nanofi bers and GO) dis-played a favorable trend in NSC differentiation towards oligo-dendrocytes, we hypothesized that the combination of GO and nanofi bers in a single scaffold may have a synergistic effect. In the PCL-GO samples, we observed a remarkable trend in gene expression of these neural markers. The nanofi bers coated at the lowest GO concentration (0.1 mg/mL) showed a 6.5-fold increase in MBP, which is much higher than the expression on PCL nanofi bers alone and GO-coated glass controls (Figure 3 c ). Interestingly, this enhancement in MBP expression was even more pronounced when the concentration of GO was further increased, wherein the cells on PCL-GO (0.5) showed an 8.9-fold increase and PCL-GO (1.0) showed a 9.9-fold increase in MBP expression (Figure 3 c ). Based on the data, there is no statisti-cally signifi cant difference in MBP expression on the PCL-GO (0.5) and PCL-GO (1.0), indicating the saturation of GO on the PCL nanofi ber surface. The overall increase in MBP expression of the cells grown on the PCL-GO substrates points to the role of GO in the observed result, in which the 3D PCL nanotopo-graphy serves to increase the amount of GO coating and the con-sequent surface interface in contact with the NSCs compared with the traditional 2D surfaces. In addition, the simultaneous decrease in GFAP expression and relatively small increase in TuJ1 expression provides further evidence that the hybrid scaffold promotes selective NSC differentiation, with a strong preference towards oligodendrocyte lineage cells (Figure 3 c ). To explore the potential of these hybrid scaffolds as a culture platform for oligodendrocyte differentiation, we elected to use PCL-GO (1.0) for all subsequent experiments (termed PCL-GO hereafter). In regard to biocompatibility, NSCs grown on these scaffolds show excellent survival, as found with cell viability assays (Figure S7, Supporting Information).W e next sought to further characterize the degree of differ-entiation into oligodendrocytes by examining the expression of well-established oligodendrocyte markers at the genetic- and cellular-level. After six days of culture, the cells grown on PCL-GO were immunostained for the early marker Olig2 and the mature marker MBP ( F igure 4a ,b). The immunostained cells show extensive expression of both the nuclear-localized Olig2 and the cytosolic MBP. A similar expression was also observed for the oligodendrocyte-specifi c surface markers O4 (early) and GalC (mature) (Figure S8, Supporting Informa-tion). Expression of these protein markers confi rms the suc-cessful NSC differentiation into oligodendrocytes. The degree of differentiation was further quantifi ed by determining the percentage of cells expressing Olig2 and MBP on the various substrates (Figure 4 c ,d). While the conventional PLL-coated glass substrates showed only about 9% of the cells expressing Olig2, both the PCL only and GO-coated glass substrates showed about 16% Olig2-expressing cells (Figure 4 c ). On the other hand, the PCL-GO substrate displayed about 33% of the cells expressing Olig2, which is signifi cantly higher than all other conditions (Figure 4 c ). A similar trend was also observed for MBP expression, wherein 26% of the cells on PCL-GO were positive for MBP, which corroborates the gene expression results shown earlier (Figure 3 c ). Comparison of the percentage of cells stained for TuJ1 (neurons) and GFAP (astrocytes) fur-ther supports the selective differentiation into oligodendrocytes, with PCL-GO displaying a signifi cant decrease in GFAP-posi-tive cells and a minor increase in the number of TuJ1-positive cells (Figure S9, Supporting Information). Given the diffi culty in achieving the spontaneous differentiation of stem cells into oligodendrocytes, our unique graphene-nanofi ber hybrid scaf-folds exhibit a signifi cant enhancement in oligodendrocyte formation. T o further confi rm that the hybrid scaffolds promote oli-godendrocyte differentiation, we evaluated changes in gene expression for a variety of well-known early and mature oli-godendrocyte-specifi c markers. qPCR was carried out for detecting the gene expression of: i) early markers including 2’,3’-cyclic-nucleotide 3’-phosphodiesterase (CNP), platelet-derived growth factor receptor alpha (PDGFR α), Olig1 and Olig2, and ii) mature markers including proteolipid protein (PLP), MBP, myelin-associated glycoprotein (MAG), myelin oli-godendrocyte glycoprotein (MOG), adenomatous polyposis coli (APC), glutathione S-transferase-pi (GST-π) and galactocerebro-side (GalC). For all genes of interest, NSCs on PCL-GO exhib-ited the strongest level of expression compared with all other control substrates (Figure 4 e ,f and Figure S10, Supporting Information). Interestingly, several of the known genes indica-tive of myelinating oligodendrocytes also showed a substantial increase in gene expression. For instance, MAG and MOG, which are glycoproteins reported to be crucial during the myeli-nation process in the CNS, [ 15 ] were seen to have a 17-fold andCOMMUNICATION19-fold increase in gene expression, respectively (Figure 4f). Taken together, these results confirm that NSCs cultured on PCL-GO substrates exhibit a strong preference towards oligo-dendrocyte differentiation.T hese hybrid scaffolds provide a unique microenvironment that was found to be permissive to oligodendrocyte formation. Nevertheless, how the extracellular cues from these hybrid scaf-folds modulate intracellular signaling pathways to control this selective differentiation remains to be explored. Numerous studies report the importance of stem cell–extracellular matrix interactions in directing oligodendrocyte differentiation. [ 17 ] These interactions have been observed to modulate intracel-lular signaling pathways, primarily through the activation of integrin receptors found on the cellular membrane. Integrin-mediated signaling has been found to be especially important for facilitating fundamental oligodendrocyte processes, suchF igure 4. E nhancement in oligodendrocyte differentiation on PCL-GO. a,b) Fluorescence image of NSCs grown on PCL-GO after six days of culture, stained for the early oligodendrocyte marker Olig2 (a) and the mature oligodendrocyte marker MBP (b). Scale bars: 20 µm. c,d) Quantitative com-parison on various substrates of the percentage of cells expressing Olig2 (c) and MBP (d). The graphs show the mean ± s.e.m, n= 3, comparison by ANOVA – * = p< 0.01. e,f) Quantitative PCR analysis was used to assess the gene expression of early oligodendrocyte markers including CNP, PDGFR, Olig1 and Olig2 (e), and mature oligodendrocyte markers including PLP, MBP, MAG and MOG (f). The gene expression is relative to GAPDH, and normalized to the conventional PLL-coated glass control. A student's unpaired t-test was used for evaluating the signifi cance (* = p< 0.05, ** = p< 0.01), compared to the PLL-coated glass control.C O M M U N I C A T I O NF igure 5. E xpression of integrin-related signaling proteins on nanofi brous scaffolds. a) Schematic diagram depicting the integrin signaling proteins involved in oligodendrocyte differentiation and development. b) Quantitative PCR analysis was used to assess the gene expression of the integrin signaling proteins FAK, Akt, ILK and Fyn. The gene expression is relative to GAPDH, and normalized to the conventional PLL-coated glass control. A student’s unpaired t-test was used for evaluating the signifi cance (* = p < 0.05, ** = p < 0.01), compared to the PLL-coated glass control. c) Confocal images of NSCs grown on various substrates (PLL-coated glass, PCL only, GO-coated glass and PCL-GO) after six days of culture, co-stained for Olig2 (purple) and FAK (orange). Scale bar: 20 µm.COMMUNICATIONas survival, differentiation and myelination. [ 18 ]Culminating evidence from previous reports suggests the role of several key signaling proteins downstream of integrins in regulating oligodendrocyte differentiation and development, including focal adhesion kinase (FAK ), Akt, integrin-linked kinase (ILK )and Fyn kinase (Fyn) [ 18 ]( F igure 5 a ). Therefore, we investigatedwhether oligodendrocyte differentiation-related signal transduc-tion is promoted in NSCs cultured on PCL-GO.A mong the various cell-signaling proteins, we examined the expression of FAK, Akt, ILK and Fyn, which have been found to mediate cytoskeletal remodeling and process extension during oligodendrocyte development. Moreover, disruption of each of these proteins has been reported to cause a variety of developmental defects, including reduced process exten-sion, aberrant myelin formation and attenuated expression ofmyelin proteins. [ 19 ] We found that NSCs cultured on the GO-coated surfaces enhanced the gene expression of all of thesefactors (Figure 5 b ). These signaling molecules exhibited the same trend in expression, wherein the GO-coated glass showed higher expression than PCL, and PCL-GO showed the strongest level of expression with a 2.6-fold increase in FAK and about a 1.7-fold increase in Akt, ILK and Fyn (Figure 5 b ). Additionally, treating the cells grown on PCL-GO scaffolds with cell sign-aling inhibitors showed a signifi cant decrease in gene expres-sion of mature oligodendrocyte markers, which provides fur-ther evidence for the potential role of such cellular signaling in the observed oligodendrocyte differentiation (Figure S11, Sup-porting Information). Collectively, this data supports the role of GO-coating in the upregulation of these downstream molecules in the integrin signaling pathway and may explain, at least in part, the enhanced oligodendrocyte differentiation of NSCs on our hybrid scaffolds.I n order to further elucidate this correlation, we sought to observe cellular co-localization of markers indicative of both integrin signaling and oligodendrocyte differentiation using confocal microscopy. Dual staining was carried out for: i) Olig2, an oligodendrocyte marker, and ii) FAK, one of the main reg-ulators of integrin-ECM signaling[ 19d ] and found in our study to show the highest expression in cells cultured on PCL-GO. The immunostaining for Olig2 (purple) and FAK (orange) was compared for NSCs cultured on PCL-GO with the other con-trol substrates (Figure 5 c ). As observed earlier, cells grown on PCL-GO showed the strongest intensity and highest number of cells expressing Olig2, with minimal expression on the glass control and moderate expression on PCL and GO. A similar trend was also observed in FAK staining, which corresponds to the gene expression levels shown in Figure 5 b . Since the locali-zation of FAK is in the cytoplasm and Olig2 is in the nucleus, the co-localization of the two markers within the same cell can be easily visualized. Interestingly, the cells expressing FAK also expressed Olig2, a phenomenon that was observed on all sub-strates (Figure 5 c ). Moreover, PCL-GO showed the strongest expression of both markers and the highest number of cells co-expressing FAK and Olig2. Together, our data suggests that the GO-coating on the nanofi ber scaffolds may promote oli-godendrocyte differentiation through specifi c microenviron-mental interactions which activate integrin-related intracellular signaling.O verall, we have demonstrated the capability of a unique gra-phene-nanofi ber hybrid scaffold to provide instructive physicalcues that lead to the selective differentiation of neural stemcells into mature oligodendrocytes, without introducing dif-ferentiation inducers in the culture media. The ability to selec-tively guide stem cell differentiation by merely changing the properties of an underlying biomaterial scaffold is a valuable approach for tissue engineering, which can help complement or potentially eliminate the use of exogenous differentiation inducers such as viral gene vectors, growth factors and small molecule drugs. Moreover, our hybrid scaffold is exceptional in that it combines the well-established properties of nanofi bers and graphene-based nanomaterials. For instance, nanofi bers have been shown to provide ideal topography for fabricating nerve guidance conduits, directing neurite outgrowth and pro-moting axonal regeneration. [ 3a ,11 ] On the other hand, graphene-based nanomaterials provide permissive surfaces for proteinand cell adhesion, as well as high conductivity to mediate elec-trical stimulation for supporting neuronal electrophysiology. [ 20 ]In turn, a hybrid scaffold which combines the morphological features of nanofi bers and the unique surface properties of gra-phene-based nanomaterials in a single culture platform, can be highly benefi cial. We envision that such a platform can serve as a powerful tool for developing future therapies for CNS-related diseases and injuries.S upporting InformationSupporting Information is available from the Wiley Online Library or from the author.A cknowled gementsK .-B.L. acknowledges fi nancial support from the NIH Director’s Innovator Award [1DP20D006462–01], National Institute of Biomedical Imaging and Bioengineering of the NIH [1R21NS085569–01], the N.J. Commission on Spinal Cord grant [09–3085-SCR-E-0], and the Rutgers Faculty Research Grant Program. S.S. acknowledges NSF DGE 0801620, Integrative Graduate Education and Research Traineeship (IGERT) on the Integrated Science and Engineering of Stem Cells. T.M.U. acknowledges fi nancial support from FAPESP (Research Foundation from São Paulo State) and Prof. Valtencir Zucolotto (University of São Paulo, Brazil). We acknowledge NIH grant S10RR025424 to Dr. Nilgun Tumer for a confocal microscope. The authors acknowledge NSF grant DMR 1126468 to Prof. Torgny Gustafsson and assistance from Dr. Samir Shubeita for helium ion microscope imaging.[1] a ) J . W. M cDonald ,X . Z. L iu ,Y . Q u ,S . L iu ,S . K. M ickey ,D . T uretsky ,D . I. G ottlieb ,D . W. C hoi ,N at. Med. 1999,5,1410 ;b ) S . P luchino ,A . Q uattrini ,E . B rambilla ,A . G ritti ,G . S alani ,G . D ina ,R . G alli ,U . D el Carro ,S . A madio ,A . B ergami ,R . F urlan ,G . C omi ,A . L. V escovi ,G . M artino ,N ature 2003,422,688 ;c ) L . R. Z hao ,W . M. D uan ,M . R eyes ,C . D. K eene ,C . M. V erfaillie ,W . C. L ow ,E xp. Neurol. 2002,174,11 .[2] T . S hindo ,Y . M atsumoto ,Q . W ang ,N . K awai ,T . T amiya ,S . N agao , J . Med. Invest. 2006,53,42 .[3] a ) H . C ao ,T . L iu ,S . Y . C hew ,A dv. Drug Delivery Rev. 2009,61,1055 ;b ) G . O rive ,E . A nitua ,J . L. P edraz ,D . F. E merich ,N at. Rev. Neurosci. 2009,10,682 .Received: F ebruary 1, 2014Revised: F ebruary 17, 2014 Published online: M arch 26, 2014C O M M U N I C A T I O N[4] a ) H . S. K eirstead ,G . N istor ,G . B ernal ,M . T otoiu ,F . C loutier ,K . S harp ,O . S teward ,J . Neurosci. 2005,25,4694 ;b ) M . A bematsu ,K . T sujimura ,M . Y amano ,M . S aito ,K . K ohno ,J . K ohyama ,M . N amihira ,S . K omiya ,K . N akashima ,J . Clin. Invest. 2010,120,3255 .[5] a ) S . Y . P ark ,J . P ark ,S . H . S im ,M . G. S ung ,K . S. K im ,B . H . H ong ,S . H ong ,A dv. Mater. 2011,23,H 263 ;b ) S . S hah ,A . S olanki ,P . K. S asmal ,K .-B. L ee ,J . Am. Chem. Soc. 2013,135,15682 ;c ) A . S olanki ,S . S hah ,P . T. Y in ,K .-B. L ee ,S ci. Rep. 2013,3,1553 ;d ) J . T akahashi ,T . D. P almer ,F . H . G age ,J . Neurobiol. 1999,38,65 ;e ) A . S olanki ,S . S hah ,K . A. M emoli ,S . Y . P ark ,S . H ong ,K .-B. L ee ,S mall 2010,6,2509 .[6] F . S her ,R . R ößler ,N . B rouwer ,V . B alasubramaniyan ,E . B oddeke ,S . C opray ,S TEM CELLS 2008,26,2875 ;DOI: 10.1634/stemcells.2008-0121 .[7] F . S her ,V . B alasubramaniyan ,E . B oddeke ,S . C opray ,C urr. Opin. Neurol. 2008,21,607 .[8] C . Z hao ,A . T an ,G . P astorin ,H . K. H o ,B iotechnol. Adv. 2013,31,654 .[9] a ) Y . Z hu ,S . M urali ,W . C ai ,X . L i ,J . W. S uk ,J . R. P otts ,R . S. R uoff , A dv. Mater. 2010,22,3906 ;b ) T . H . K im ,K . B. L ee ,J . W. C hoi , B iomaterials 2013,34,8660 .[10] a ) G . Y . C hen ,D . W. P ang ,S . M. H wang ,H . Y . T uan ,Y . C. H u , B iomaterials 2012,33,418 ;b ) W . C. L ee ,C . H . L im ,H . S hi ,L . A. T ang ,Y . W ang ,C . T. L im ,K . P. L oh ,A CS Nano 2011,5,7334 ;c ) A . S olanki ,S .-T. D. Chueng ,P . T. Y in ,R . K appera ,M . C hhowalla ,K .-B. L ee ,A dv. Mater. 2013,25,5477 .[11] J . X ie ,M . R. M acEwan ,A . G. S chwartz ,Y . X ia ,N anoscale 2010,2,35 .[12] A . C ipitria ,A . S kelton ,T . R. D argaville ,P . D. D alton ,D . W. H utmacher ,J . Mater. Chem. 2011,21,9419 .[13] G . T. C hristopherson ,H . S ong ,H . Q. M ao ,B iomaterials 2009,30,556 .[14] Q . S hen ,Y . W ang ,E . K okovay ,G . L in ,S . M. C huang ,S . K. G oderie ,B . R oysam ,S . T emple ,C ell Stem Cell 2008,3,289 .[15] N . B aumann ,D . P ham-Dinh ,P hysiol. Rev. 2001,81,871 .[16]a ) S . L ee ,M . K. L each ,S . A. R edmond ,S . Y . C hong ,S . H . M ellon ,S . J. T uck ,Z . Q. F eng ,J . M. C orey ,J . R. C han ,N at. Methods 2012,9,917 ;b ) D . R. N isbet ,L . M. Y u ,T . Z ahir ,J . S. F orsythe ,M . S. S hoichet ,J . Biomater. Sci., Polym. Ed. 2008,19,623 .[17] H . C olognato ,I . D. T zvetanova ,D ev. Neurobiol. 2011,71,924 .[18] R . W. O'Meara ,J . P. M ichalski ,R . K othary ,J . Signal Transduction 2011,2011,354091 .[19]a ) R . W. O'Meara ,J . P. M ichalski ,C . A nderson ,K . B hanot ,P . R ippstein ,R . K othary ,J . Neurosci. 2013,33,9781 ;b )D .J.O sterhout ,A .W olven ,R .M.W olf ,M .D.R esh ,M .V.C hao ,J . Cell Biol. 1999,145,1209 ;c ) C . S. B arros ,T . N guyen ,K . S. S pencer ,A . N ishiyama ,H . C olognato ,U . M uller ,D evelopment 2009,136,2717 ;d ) A . D. F orrest ,H . E. B eggs ,L . F. R eichardt ,J . L. D upree ,R . J. C olello ,B . F uss ,J . Neurosci. Res. 2009,87,3456 .[20]Y . Z hang ,T . R. N ayak ,H . H ong ,W . C ai ,N anoscale 2012,4,3833 .。