The acetylcholinesterase inhibitor, Donepezil, regulates a Th2 bias
- 格式:pdf
- 大小:239.62 KB
- 文档页数:8

中翻英:本研究结果,在5-Aza-CdR 与NaB 联合应用下,RASSF1A 基因表达较单独应用5-Aza-CdR 上调,表明了甲基化酶抑制剂5-Aza-CdR 及去乙酰化酶抑制剂NaB 在转录调控作用上的协同作用。
这与Egger 等[24]的研究结果一致。
研究发现,DNA 甲基化和组蛋白乙酰化对基因表达的调控作用有密切联系。
DNA 甲基化可诱导局部组蛋白去乙酰化,使染色质乙酰化水平降低[4],甲基化CpG 结合蛋白MeCP2 以甲基化依赖的方式结合到染色质上,通过转录抑制结构域与桥蛋白Sin3A 和HDAC 的共同抑制复合体紧密联系[25]Our study shows that the combined administration of 5-Aza-CdR and NaB upregulates the gene expression level of RASSF1A as compared with 5-Aza-CdR alone, demonstrating the synergistic action of methylase inhibitor 5-Aza-CdR and histone deacetylase inhibitor (HDAC) NaB on transcription regulation. Our foundings are also consistent with the study by Egger and colleagues. Numerous studies have found that DNA methylation and histone acetylation correlate closely with gene regulation. DNA methylation can induce local histone deacetylation and therefore reduce the acetylation level of chromatins. Methyl-CpG-binding protein MeCP2 binds to chromatins via a methytation-dependent manner and comes to a close contact with the corepressor complex consisted of HDAC and adaptor protein Sin3A through its transcription repression domain.英翻中A biological indicator for monitoring the effectiveness of a sterilizing, disinfecting, etc., compound or condition is disclosed which comprises a substrate having a surface layer containing functional groups thereon desirably free of silicone linking groups. The functional groups are desirably in the form of a monolayer of a uniformed distribution and of a selected quantity. Various types of microorganisms such as spores and/or etiological agents are covalently bonded to the surface layer functional groups through a crosslinking reagent and thus form a uniform number and distribution of the microorganisms and/or etiological agents. After being subjected to sterilization or other similar treatment, etc., along with various articles such as instruments, the indicator can be cultivated to determine the effectiveness of the sterilization, disinfection, etc. process.本专利公开了一种用于检测杀菌、消毒等化合物有效性的生物指示剂。


缩略语ADABPCI2CNSSPNFTLDHNMDAAPPsAPPBACEPSAITUNELPS02‘’OHPBSPCDBSAFCM缩略语表英文全称Alzheimer’sDiseaseAmyloid13proteinRatPheochromocytomaCentralNerveSystemSenilePlaqueneurofibritarytangleLactatedehydrogenaseN-·methyl·-D-·aspartate0amyloidprecursorproteinSolubleNH2一terminalectodomainofAPPD—siteAPP-cleavingenzymepresenilinApoptoticindexterminal-deoxynucleotidyltransferasemediatednickendlabelingPhosphatidylserinephosphatebuffersalineprogrammedcelldeathbovineserumalbuminflowcytometry中文全称阿尔茨海默病B淀粉样蛋白大鼠嗜铬神经瘤细胞中枢神经系统老年斑神经纤维缠结乳酸脱氢酶N.甲基.D.天冬氨酸淀粉样前体蛋白可溶性多肽B位点剪切酶早老素凋亡指数脱氧核糖核苷酸末端转移酶介导的缺口末端标记法磷脂酰丝氨酸超氧阴离子自由基羟自由基磷酸盐缓冲液程序性细胞死亡牛血清白蛋白流式细胞仪PKCAKPI3·-(4,5--DimethylRhiazol·.2-·yI)-25-diphenyltetrasoliumBromideproteinkinaseCabsorio,}enceKanitzproteaseinhibitorAIApopototicindex2噻唑蓝蛋白激酶C吸光度Kanitz蛋白酶抑制物凋亡指数第四军医大学硕士学位论文AB致PCI2细胞的损伤效应与多奈哌齐的神经保护作用硕士研究生:李珂导师:万琪教授(主任医师)第四军医大学西京医院神经内科,西安710032中文摘要阿尔茨海默病(Alzheimer’sDisease,AD)是发生于老年人群的一种原发性退行性脑病,大于65岁人群的发病率为5%一10%,其确切病因迄今不明。
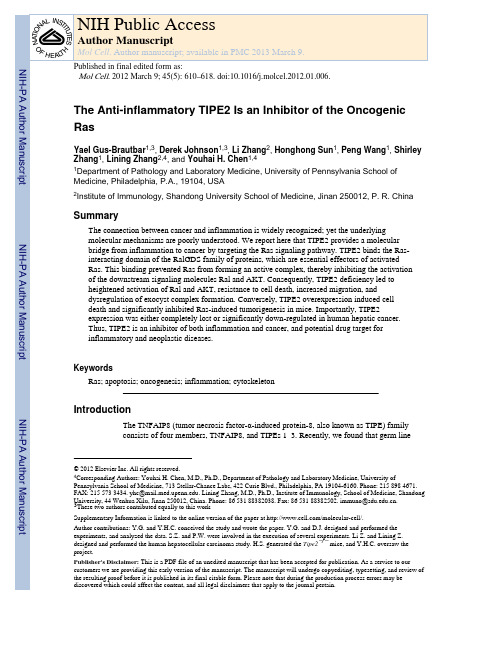
deletion of TIPE2 (a.k.a. TNFAIP8L2) results in fatal inflammation and hypersensitivity to Toll-like receptor and T cell receptor stimulation (Sun et al., 2008). TIPE2 possesses a unique structural fold, shared by all members of the TNFAIP8 family, with no known function or significant homology to other known proteins (Zhang et al., 2009). TIPE2 is down-regulated in patients with chronic inflammatory diseases such as systemic lupus erythematosus and hepatitis, and its expression inversely correlates with disease progression (Li et al., 2009)(unpublished data). Although TIPE2 has emerged as a molecule critical for preventing inflammatory diseases, the mechanisms of its action remained unclear.Ras is a major regulator of cell survival, proliferation, migration, and transformation.Alongside phosphatidylinositol (PI) 3 kinase and Raf1, Ral guanine nucleotide dissociation stimulator (RalGDS) family makes up the third arm of Ras effectors. The RalGDS family members are Guanine nucleotide Exchange Factors (GEFs) for the small GTPases RalA and RalB, switching GDP-bound inactive to GTP-bound active form of Ral (Ferro and Trabalzini, 2010; Spaargaren and Bischoff, 1994). A subset of the RalGEFs, including RalGDS, RGL and RGL2/Rlf, are direct effectors for activated Ras, which binds their C-termini and enhances their GEF activity towards Ral (White et al., 1996; Wolthuis et al.,1996). The RalGEF pathway plays a prominent role in mediating Ras-induced oncogenic transformation in humans. RalGDS deficiency suppresses Ras-mediated tumor formation (Gonzalez-Garcia et al., 2005). In rodent fibroblasts, the RalGEF effector pathway cooperates with the MAPK pathway to promote transformation and metastasis (Ward et al.,2001; White et al., 1996). In humans, the activation of this pathway is essential for transformation in a variety of cell types (Hamad et al., 2002; Rangarajan et al., 2004).The RalGDS transforming ability is mediated by the active RalA and RalB. Active Rals regulate various biological processes including cell proliferation, motility, endo- and exocytosis, and cellular architecture (Feig, 2003). RalA and RalB have distinct and sometimes conflicting roles during oncogenic transformation despite their high sequenceidentity (over 80%). RalA, but not RalB, is critical for RalGDS-mediated transformation,and RalA is more potent than RalB in promoting anchorage-independent growth andtargeted delivery of proteins to basolateral membrane in epithelial cells. RalB is moreeffective than RalA in promoting cell migration and activation of the TBK1 kinase, and insuppressing apoptosis and promoting metastasis (Chien et al., 2006; Chien and White, 2003;Lim et al., 2005; Lim et al., 2006). Constitutively active forms of RalA and RalB cantransform human cell lines (Lim et al., 2005). Both RalA and RalB are activated in humanmalignancies such as bladder, pancreatic and colon cancers, and they collaborate to promoteand maintain oncogenic transformation (Lim et al., 2006; Martin et al., 2011; Smith et al.,2007).Recent studies suggest that the Ral effects on motility, secretion, and cell proliferation arelargely mediated through the regulation of the exocyst complex. This octameric complexregulates targeting and tethering of secretory vesicles to specific plasma membrane domains,such as the leading edge of migrating cells (Rosse et al., 2006; Spiczka and Yeaman, 2008).Two exocyst subunits, Sec5 and Exo84, are bona fide Ral effectors (Moskalenko et al.,2002), each of which belongs to a different sub-complex. One sub-complex contains Exo84and Sec10, and is localized on the plasma membrane, whereas the other contains Sec 5, 6, 8subunits, and is located on secretory vesicles (He and Guo, 2009). Active Ral promotesassembly of these two sub-complexes through dual subunit interaction, leading to vesiculartethering to the plasma membrane (Jin et al., 2005; Moskalenko et al., 2003). As the exocystcomplex is becoming better understood, its involvement in carcinogenesis has been broughtto the forefront. Exocyst subunit interaction with active Ral is required for tumorigenesis ofcolorectal carcinoma, progression of skin cancer, motility, anchorage-dependence, andsurvival of transformed cells (Chien et al., 2006; Martin et al., 2011; Sowalsky et al., 2011).NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author ManuscriptAn important function of the RalGDS family is to promote cell survival. This may bemediated through both Ral GTPases (Chien and White, 2003) and non-canonical activationof AKT (Hao et al., 2008). Canonical AKT activation requires the generation of PIP3 byPI3K at the membrane. The PH domain-containing proteins AKT and PDK1 bind thisphosphoinositide, allowing AKT to be phosphorylated by PDK1 (T308) and mTOR (S473).By contrast, in the non-canonical AKT activation pathway, RalGDS acts as a scaffold forPDK1 and enhances its kinase activity, resulting in increased phosphorylation of AKT.Active AKT phosphorylates a large number of substrates thereby protecting cells from death(Sale and Sale, 2008). RalGDS-mediated AKT activation is responsible for the proliferativeeffect of RalGDS in NIH3T3 cells (Hao et al., 2008). In vivo, RalGDS regulates tumorgrowth by providing survival signals to tumor cells, and consequently, in Ralgds −/− mice,apoptosis of carcinogen-induced papillomas is enhanced (Gonzalez-Garcia et al., 2005).Thus, the regulation of the RalGEF effector pathway is key to Ras-mediated transformation.In this report, we describe an unexpected and previously unknown connection betweenTIPE2 and the RalGDS family, and demonstrate its relevance to cell survival, motility, andRas-induced oncogenesis.Results and Discussion Inflammatory factors significantly down-regulate TIPE2 expression Our previous work indicates that TIPE2 prevents inflammation and maintains immune homeostasis. To test whether TIPE2 itself is regulated by inflammatory signals, we examined TIPE2 mRNA levels in lymphoid and myeloid cells after stimulation with ligands for Toll-like receptors (TLRs) 3, 4, 7, and 9, and mitogenic activators of immune cells (Figure S1, A and D, and unpublished data). We found that TIPE2 expression was significantly down-regulated by all these ligands. Blocking NF-κB activation in cells treated with lipopolysacchride (LPS), a TLR4 ligand, completely rescued the defect in TIPE2expression (Figure S1B), indicating that LPS down-regulates TIPE2 by NF-κB activation.These results indicate that TIPE2 expression can be shut down by inflammatory signals,which may in turn contribute to inflammation-induced pathologies.TIPE2 prevents Ras from binding the Ras-interacting domain of RGLThe mechanisms of TIPE2 function are not clear. To address this issue, we searched forbinding partners of TIPE2 using a two-pronged approach. Firstly, we conducted a yeast two-hybrid screen of a mouse splenic cDNA library using TIPE2 as the bait, and secondly, weperformed a large-scale coimmunoprecipitation of TIPE2-binding proteins followed by massspectrometry. Among several clones isolated in the yeast-two hybrid screen, two were foundto encode the C-terminus region of the RGL. Consistent with this finding, massspectrometry results showed that TIPE2 pulled down with proteins of the RGL-Ral pathway.Together, these results suggested a role for TIPE2 in Ras-mediated signaling.To establish whether endogenous TIPE2 interacts with RGL in mammalian cells, weimmunoprecipitated TIPE2 from the murine macrophage cell line, Raw 264.7. We foundthat endogenous RGL co-precipitated with endogenous TIPE2 (Figure 1A), as did two otherRalGEF family members, RalGDS and RGL2 (Figure S2 and data not shown). To map theregion within RGL that is responsible for TIPE2 interaction, we cloned the murine full-length RGL (amino acids 1–768) or truncated RGL (Figure 1B), in frame with a myc tag andco-transfected the full-length or truncated RGL constructs into cells together with theTIPE2-Flag plasmid. TIPE2 pulled down with full-length RGL and the C-terminus of RGL.However, TIPE2 did not pull down with a truncated RGL that lacked the C-terminal region(Figure 1C). These data indicate that TIPE2 binds to the C-terminus of RGL, which containsthe Ras Interacting Domain (RID) (Murai et al., 1997).NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author ManuscriptActive Ras binds the RID of RalGEFs and activates their GEF activity (Murai et al., 1997;Urano et al., 1996). Our finding that TIPE2 binds the RID of RGL (Figure 1C) suggests thatin the presence of TIPE2, Ras would be unable to bind RGL. We examined the presence ofRas in complex with RGL in 293T cells transiently expressing full-length RGL andincreasing amounts of TIPE2. TIPE2 inhibited endogenous Ras from forming a complexwith RGL, in a dose-dependent manner (Figure 1D). This indicates that TIPE2 can excludeactive Ras from binding to RGL.TIPE2 inhibits RGL-induced activation of Ral and AKT, thereby promoting cell death We then asked whether the outcome of TIPE2 binding to RGL could be inhibition of RGL GEF activity towards its substrate Ral. In 293T cells transiently overexpressing TIPE2, we detected more than 60 percent decrease in Ral GTP level compared to the control (Figure 1E) Similar results were obtained in Raw 264.7 macrophages (data not shown). Active Ras levels were not affected by overexpression of TIPE2 (Figure S2). TIPE2 protein and mRNA levels were downregulated in Raw 264.7 cells treated with LPS (Figures S1 and S5), and Ral activity was elevated as a result of the treatment (Figure S1). Moreover, TIPE2-deficient bone marrow-derived macrophages showed a three-fold increase in active Ral level over wild type control cells (Figure 1G). These results indicate that TIPE2 serves as an inhibitor of RGL activity by blocking active Ras binding to RGL.Knockdown of TIPE2 in T cells confers resistance to Fas ligand-induced apoptosis,indicating that TIPE2 may promote cell death. Tipe2−/− mice suffer from splenomegaly and leukocytosis, caused by increased numbers of myeloid and lymphoid cells (Sun et al., 2008).These abnormalities could be rooted in a decrease of apoptosis in TIPE2-deficient cells. We observed in Raw 264.7 cells that TIPE2 expression was induced upon cell death (Figure S1).We therefore hypothesized that increased expression of TIPE2 might lead to cell death. To test this, we transfected 293T cells with a TIPE2-expressing plasmid. Within 24 hrs, TIPE2expression in these cells induced significant cell death in a dose-dependent manner (Figure2A).Next, we examined the consequence of TIPE2-RGL interaction in cell death. In 293T cells,we found that ectopic TIPE2 expression caused a 2-fold increase in cell death, as assessedby trypan blue staining (Figures 2A and 2B) and cleavage of PARP (data not shown). Atruncated TIPE2 that lacked amino acids 105–132 was not able to bind to RGL (Figure 1F)or induce cell death when expressed in 293T cells (Figure 2C). Importantly, co-transfectionof RGL and TIPE2, rescued the death phenotype (Figure 2B). However, wild type RalA andRalB, or activated mutants of RalA and RalB, had little protective effect against TIPE2-induced death (Figure 2C), suggesting that additional components downstream of RGLcould be involved in the rescue. Previous work showed that RalGDS binds PDK1 andenhances AKT activity. We found that RGL bound to PDK1 through the N-terminal regionof the RGL (Figure S3). Expression of TIPE2 together with RGL ΔN, which did not bindPDK1 could not rescue TIPE2-induced death (Figure 2B). Furthermore, TIPE2 caused areduction in phosphorylated AKT level, which was rescued by RGL (Figure 2E), but not byRGL ΔN, or RalA and RalB, even though RGL ΔN could induce Ral activity (Figure S3).The RGL-PDK1 complex is induced by growth stimuli, and activated Ras plays animportant role in its formation. PDK1 relieves the intra-molecular inhibition of the catalyticdomain of RalGEFs by binding to its N-terminus, and RalGEFs enhance PDK1 kinaseactivity preferentially towards AKT (Hao et al., 2008; Tian et al., 2002). Ras binding toRGL seems to be a necessary but insufficient step in promoting RGL-PDK1 interaction,since a Ras mutant that preferentially binds RalGEFs could not activate AKT under serumstarvation conditions (data not shown)(Tian et al., 2002). Expression of TIPE2 reducedPDK1 binding to RGL by ~44% compared to the control (Figure 2F), implying that theNIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscriptdisruption of Ras binding to RGL may prevent RGL-PDK1 complex formation. PDK1binding to RGL and the subsequent inhibition of AKT could be responsible for TIPE2-induced cell death. To test this hypothesis, we co-expressed TIPE2 with a constitutivelyactive form of AKT (AKT T308D, S473D) and found that activated AKT could rescue fromTIPE2-induced cell death (Figure 2C). Dominant negative AKT (DN-AKT) increased thebasal level of cell death, but when co-expressed with TIPE2 did not induce death beyondthat of TIPE2 alone (Figure 2D, Figure S4A). Furthermore, co-expression of TIPE2 withmyristoylated PDK1 also rescued cells from death (Figure 2C). These results indicate thatTIPE2 promotes cell death primarily through the inhibition of AKT (Figure S4A). Enhancedsusceptibility to serum starvation was observed in Ralgds −/− cells and RGL2 overexpressionrendered resistance to serum withdrawal (Gonzalez-Garcia et al., 2005; Wolthuis et al.,1997). In Tipe2−/− cells, AKT was constitutively phosphorylated, and was unable to befurther induced by lipopolysacchride (LPS) (Figure 2G). We therefore examined the impactof serum deprivation on Tipe2−/− cells (Figure 2H). We found that Tipe2−/− cells wereresistant to serum deprivation-induced death (Figure 2H). Taken together, these resultsindicate that TIPE2 promotes cell death by inhibiting the RGL-PDK1-AKT axis.TIPE2 inhibits cell motility and exocyst complex assemblyA hallmark of Ral function is its regulation of cell motility. Ral activation promotes cellularprotrusions and is essential for directional movement of cells (Rosse et al., 2006; Sugihara etal., 2002). Active Ral mediates chemotaxis in lymphocytes, plays a critical role in tumormetastasis, and contributes to cytokinesis progression (Oxford et al., 2005). Ral mediatesthese effects by regulating both actin dynamics and exocyst complex assembly. Actinpolymerization is essential for maintaining cell shape, internalization processes (endocytosisand phagocytosis), and cell motility. We therefore examined the effects of TIPE2 expressionon actin polymerization. Polymerization of F-actin can be induced by LPS stimulation inmonocytes (Kong and Ge, 2008). Expression of TIPE2 in Raw 264.7 cells resulted in asignificant decrease in the total level of F-actin (Figure 3A). The F-actin polymerization ratein TIPE2-transfected cells was also reduced. Conversely, in Tipe2−/− splenocytes, the rate ofF-actin polymerization was significantly enhanced compared to wild type cells (Figure 3B).These results suggest that TIPE2 can impact both the rate of actin polymerization and thetotal levels of F-actin in immune cells.We next examined the exocyst subunit levels in Tipe2−/− splenocytes and bone marrow-derived macrophages, and detected a significant increase in exocyst subunits Sec 5, 6, 8 and84 (Figure 3C). These results are consistent with previous reports showing decreasedabsolute amounts of Sec5 and Sec6 in RalA- or RalB-depleted rat kidney cells (Rosse et al.,2006). Next, we examined whether TIPE2 impacts the formation of the exocyst complex, bymeasuring its assembly from its two sub-complexes in wild type (WT) and Tipe2−/− cells.While there was no difference in Sec5/Sec6 sub-complex assembly between Tipe2−/− andWT cells, the association between Sec5 and Exo84 increased by about 3-fold in Tipe2−/−cells (Figure 3D). Therefore, the Ral-regulated step of exocyst assembly is defective inTipe2−/− cells. Consistent with this observation, TIPE2 overexpression resulted indestabilization of the exocyst complex. In 293T cells expressing TIPE2, the assembly of thesub-complex Sec5/Sec6 was unchanged, while the assembly of Exo84 and Sec5 wasmarkedly decreased (Figure 3E, 3F).Ral depletion blocks exocyst complex formation at the leading edge of migrating cells, andinhibits cell migration (Rosse et al., 2006; Spiczka and Yeaman, 2008). We testeddirectional cell migration of Tipe2−/− macrophages in a wound-healing assay (Figure 3G).The “wound” was created in confluent Tipe2−/− and wild type cultures (time zero), andmigration of cells into the gap was monitored after 3 and 6 hrs. Wild type macrophagesstarted moving into the wound after 3 hrs, and by 6 hrs the wound was still visible.NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author ManuscriptHowever, Tipe2−/− macrophages moved into the wound faster, and completely closed thegap by 6 hrs. Quantification of the number of cells that moved into the gap showed that therate of Tipe2−/− cell migration was 3-fold higher than that of the wild type (Figure 3H).Moreover, Tipe2−/− macrophages that had moved into the wound were elongated, and hadincreased number of cellular extensions, generally assuming a “migratory” form. In contrast,wild type cells looked round, with smaller number of extensions (Figure S4B). Consistentwith this finding, in vivo migration of TIPE2 knockout leukocytes into skin air-pouchesinjected with the chemokine KC (keratinocyte chemoattractant) was significantly enhancedas compared to wild type controls (unpublished data). The enhanced migratory phenotype ofTipe2−/− cells could be mediated by irregularities of both actin dynamics and exocystcomplex assembly. These abnormalities may partly explain the increased inflammation inTipe2−/− mice.Recently, it has been established that active RalB induces Sec5 dimerization and subsequentactivation of TBK1 kinase (Chien et al., 2006; Ou et al., 2011). This pathway results in AKTactivation, protects cancer cells from apoptosis, and is required for mounting host defenseresponses. RalB and Sec5 are required for TLR3-induced IRF3-dependent interferon-βproduction. We observed reduced interaction between Sec5 and TBK1 in TIPE2-overexpressing cells (Figure 3E), and a reduction in phosphorylated IRF3 (Figure S4C).These results suggest that the Ral/Sec5/TBK1 pathway is inhibited by TIPE2. It was shownpreviously that TIPE2-deficient cells exhibit increased NF-κB activity. Therefore, TIPE2may regulate the NF-κB pathway through the Ral/Sec5/TBK1 axis.TIPE2 inhibits tumorigenesis in vivoActivating mutations of Ras are found in ~30% of all malignant tumors. The best-characterized Ras effector pathways are the Raf-MAPK and PI3K pathways, and theirimportance in Ras-mediated oncogenesis has been extensively studied. However, a growingbody of evidence supports an important role for the RalGDS family in Ras-induced growthand transformation of human cells. To determine the potential roles of TIPE2 intumorigenesis, the Ras-transformed NIH 3T3 fibroblasts (Ras G12V) were used to stablyexpress Flag-tagged TIPE2. Expression of TIPE2 significantly reduced the growth of Ras3T3 cells (Figure 4A). The effect was the most dramatic under low serum conditions, wherecells were more dependent on Ras for survival. Consistent with these results, overexpressionof TIPE2 in Ras 3T3 cells reduced colony formation in soft agar (Figure 4B). Expression ofTIPE2 in NIH 3T3 alone did not result in colony formation. To test the effect of TIPE2 ontumor formation in vivo, Ras 3T3 cell line stably expressing TIPE2 was injected into nudemice. TIPE2 significantly delayed tumor onset in two independent experiments, incomparison to control injections (Figure 4C). NIH 3T3 or NIH 3T3 stably expressing TIPE2did not give rise to tumors. TIPE2 tumors, once formed, could grow to the same weight ascontrol (Figure S5), suggesting that TIPE2 tumors did not have a growth disadvantage ascompared to Ras 3T3 tumors. In addition, while Ral activity was inhibited in the pre-injected cell lines, it was restored in isolated tumor cells (Figure S5). This paradox could beexplained if somehow in mice TIPE2 expression was lost. Indeed, upon examining thetumors 14 and 22 days after tumor cell inoculation, we could not detect any TIPE2 proteinby immunoblotting (Figure 4D). However, by quantitative RT-PCR, we could clearly showthat TIPE2 tumors expressed similar amounts of TIPE2 transcript compared to TIPE2-expressing Ras 3T3 cells before injection (Figure 4E). Therefore, it appears that TIPE2downregulation in the tumor occurred at the protein level. The half-life of TIPE2 protein israther short, around 4 hrs (Figure S5), and the TIPE2 protein is heavily ubiquitinated in cells(Figure 4F). These findings indicate that TIPE2 protein is regulated by ubiquitination andproteasomal degradation. Indeed, the reduced TIPE2 level in tumor cells could be restored tothat of pre-injected cells after treatment with the proteasome inhibitor MG132 (Figure 4G).NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author ManuscriptThis indicates that TIPE2 degradation is enhanced in the tumor cells. Therefore, cells thatformed tumors were those that had TIPE2 protein actively suppressed. These cells werelikely present in the pre-injected pool but were outnumbered by those that did expressTIPE2. However, once injected into the animal, cells that suppressed TIPE2 protein had asignificant survival advantage and were therefore positively selected. Although TIPE2tumors might have originated from less cells, as the delay in tumor onset suggests, theyeventually reached the same size as the control tumors. This unexpected result suggests thatmechanisms responsible for TIPE2 elimination may also result in acquisition of a growthadvantage over Ras3T3 control cells. These results point to a role for TIPE2 as a tumorsuppressor involved in carcinogenesis.TIPE2 is markedly down-regulated in human hepatocellular carcinomaIt was recently published that TIPE2 plays an important role in HBV-induced hepatitis (Xiet al., 2011). Chronic HBV infection is a major cause of HCC and is prevalent among alarge world population. Interestingly, RalGEF plays a more prominent role in transforminghuman cells than murine cells (Hamad et al., 2002). To test the possibility that TIPE2regulates carcinogenesis in humans, we examined the level of TIPE2 expression in the liversof 116 patients suffering from hepatocellular carcinoma. We found that TIPE2 wasexpressed in normal hepatocytes adjacent to carcinoma cells. Remarkably, ~20% ofcarcinoma expressed little or no TIPE2 and the rest expressed significantly lower levels ascompared to adjacent hepatocytes (Figure 4, H–J). TIPE2 re-expression in three culturedhuman HCC cell lines (HepG2, BEL7402, and SMMC-7721) significantly reduced theirgrowth and viability as measured by flow cytometry and MTT [(3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay (unpublished data). Consistent with the murinetumor data, down-regulation of TIPE2 occurred at the protein but not at mRNA level,because RT-PCR revealed no significant difference in TIPE2 mRNA between hepatocellularcarcinoma and its adjacent tissues (unpublished data). Thus, development of humanhepatocellular carcinoma is associated with the down-regulation of TIPE2 protein.In summary, we have discovered a mode of Ras regulation that is carried out by TIPE2, arecently described anti-inflammatory protein containing a unique fold. This mode ofregulation is essential for maintaining an organism’s homeostasis, because its defect leads tosevere inflammation and cancer progression. This finding provides a molecular bridgebetween inflammation and cancer, a connection widely recognized, but poorly understood(Karin and Greten, 2005). Thus, inflammation may cause cancer by inhibiting the expressionof the tumor suppressor TIPE2, in addition to activating the oncogenic NF-κB (Karin andGreten, 2005). Due to its diverse effects on cell survival and motility, the Ras inhibitorTIPE2 represents an attractive drug target for neoplastic and inflammatory diseases.Experimental ProceduresAnimals and human subjectsC57BL/6J (B6) mice that carry a Tipe2 gene null mutation were generated by backcrossingTipe2−/− 129 mice (Sun et al., 2008) to B6 mice for 12 generations. Male nude mice (nu/nu)were purchased from Jackson Laboratories. Mice were housed in the University ofPennsylvania Animal Care Facilities under pathogen-free conditions. All animal proceduresused were pre-approved by the Institutional Animal Care and Use Committee of theUniversity of Pennsylvania.A total of 116 heptocellular carcinoma specimens and 111 normal adjacent hepatic tissuespecimens were obtained from 116 patients aged between 30 and 82 years who underwentoperations at the Qilu Hospital of Shandong University from January 2005 to October 2006.NIH-PA Author Manuscript NIH-PA Author ManuscriptNIH-PA Author ManuscriptThe pathological diagnosis was made according to the current World Health Organization(WHO) criteria for heptocellular carcinoma. None of the patients studied had receivedradiotherapy, chemotherapy, or adjuvant immunotherapy prior to surgery in order toeliminate their effects on gene expression. All human procedures used were pre-approved bythe Institutional Review Board of the Shandong University.ImmunohistochemistryParaffin sections (4μm) were stained with rabbit anti-TIPE2 antibody (IgG) overnight at4°C. Secondary staining was performed with HRP-conjugated anti-rabbit IgG using aMaxVision ™ Kit and a DAB Peroxidase Substrate kit (Maixin Co., Fuzhou, China). Thesections were counterstained with hematoxylin. Unrelated rabbit IgG was used as a controlfor the primary antibody. All slides were independently analyzed by two pathologists in ablinded manner, and scored based on both staining intensity and the percentage of positivecells as follows. Staining intensity: 0, no staining; 1, weak staining; 2, moderate staining; 3,strong staining. The percentage of positive cells: 0, <1%; 1, 1–33%; 2, 34–66%; 3, 67–100%. The two scores for each slide were then combined to produce a final grade of TIPE2expression: 0, total score = 0; 1+, total score = 1 to 2; 2+, total score = 3 to 4; 3+, total score= 5 to 6. When there were discrepancies between the two pathologists, the average score wasused.Cell lines and plasmidsThe 293T, Raw 264.7, NIH 3T3, and Ras V12 NIH 3T3 cells were grown in DMEMsupplemented with 10% FBS, penicillin and streptomycin. To generate stable cell lines, NIH3T3 or Ras NIH3T3 cell lines (gift from Dr. Rotem Karni, Hebrew University of Jerusalem)were infected with pBABE-puro retroviral vector expressing TIPE2-Flag. Culture mediumwas replaced 24 hrs after infection, and after an additional 24 hrs, infected cells wereselected with puromycin (1–1.5 μg/ml) for 3 days. Expression of TIPE2-Flag was verifiedby Western blotting. pRK5 and TIPE2-Flag-pRK5 were described previously (Sun et al.,2008). TIPE2 Δ105–132 was generated from TIPE2 cDNA by PCR and cloned in-framewith a C-terminal Flag tag into vector pRK5. pcDNA3-HA-AKT AAA was a gift from Dr.Morris Birenbaum (University of Pennsylvania). Active AKT (pCDNA3-AKT T308D,S473D), myr-PDK1 (pWZL Myr Flag PDK1), Active RalA (pBABE-RalAV23), ActiveRalB (pBABE-RalBQ72L) were purchased from Addgene GFP-WT-RalA and GFP-WT-RalB plasmids were a gift from Dr. Wei Guo (University of Pennsylvania). Murine RGLcDNA (cDNA clone MGC:18430, IMAGE:4241244, RGL-1 complete CDS) was obtainedfrom ATCC. Full length RGL (amino acids 1–768) was generated from the cDNA clone byPCR, and cloned in-frame with a C-terminal myc tag into vector pRK5 using BamHI-XhoIsites. ΔN RGL (amino acids 300–768), Δ RID RGL (amino acids 86–496), and RID RGL(amino acids 599–768) were generated from the cDNA clone by PCR, and cloned in-framewith a C-terminal myc tag into vector pRK5 using BamHI-XhoI sites. TIPE2-Flag-pBABEwas generated by cloning PCR-amplified TIPE2-Flag fragment into vector pBABE usingBamHI/EcoRI sites.Cell death assaysThe 293T cells, 1×106/dish, were plated in 6-cm dishes and transfected with the followingplasmids: pRK5, TIPE2-Flag-pRK5, TIPE2 Δ3105-132-Flag-pRK5, pcDNA3-HA-AKTAAA, RGL-myc-pRK5, ΔN RGL-myc-pRK5, GFP-RalA, GFP-RalB, RalAV23, RalBQ72,AKT (Thr308D, Ser473D), myr-PDK1 or pEGFP-N3 (Clontech). All transfections werecarried out using FugeneHD reagent (Roche) according to the manufacturer’s instructions.24 hrs later, supernatant was collected, and adherent cells were trypsinized and mixed withthe supernatant. Cells were centrifuged (1200 rpm, 10 minutes), resuspeneded in equalvolume of media, and stained with trypan blue. Dead and live cells were counted on aNIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript。

DOI: 10.1126/science.1094786, 441 (2004);304Science et al.Mitchell S. Abrahamsen,Cryptosporidium parvum Complete Genome Sequence of the Apicomplexan, (this information is current as of October 7, 2009 ):The following resources related to this article are available online at/cgi/content/full/304/5669/441version of this article at:including high-resolution figures, can be found in the online Updated information and services,/cgi/content/full/1094786/DC1 can be found at:Supporting Online Material/cgi/content/full/304/5669/441#otherarticles , 9 of which can be accessed for free: cites 25 articles This article 239 article(s) on the ISI Web of Science. cited by This article has been /cgi/content/full/304/5669/441#otherarticles 53 articles hosted by HighWire Press; see: cited by This article has been/cgi/collection/genetics Genetics: subject collections This article appears in the following/about/permissions.dtl in whole or in part can be found at: this article permission to reproduce of this article or about obtaining reprints Information about obtaining registered trademark of AAAS.is a Science 2004 by the American Association for the Advancement of Science; all rights reserved. The title Copyright American Association for the Advancement of Science, 1200 New York Avenue NW, Washington, DC 20005. (print ISSN 0036-8075; online ISSN 1095-9203) is published weekly, except the last week in December, by the Science o n O c t o b e r 7, 2009w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o m3.R.Jackendoff,Foundations of Language:Brain,Gram-mar,Evolution(Oxford Univ.Press,Oxford,2003).4.Although for Frege(1),reference was established rela-tive to objects in the world,here we follow Jackendoff’s suggestion(3)that this is done relative to objects and the state of affairs as mentally represented.5.S.Zola-Morgan,L.R.Squire,in The Development andNeural Bases of Higher Cognitive Functions(New York Academy of Sciences,New York,1990),pp.434–456.6.N.Chomsky,Reflections on Language(Pantheon,New York,1975).7.J.Katz,Semantic Theory(Harper&Row,New York,1972).8.D.Sperber,D.Wilson,Relevance(Harvard Univ.Press,Cambridge,MA,1986).9.K.I.Forster,in Sentence Processing,W.E.Cooper,C.T.Walker,Eds.(Erlbaum,Hillsdale,NJ,1989),pp.27–85.10.H.H.Clark,Using Language(Cambridge Univ.Press,Cambridge,1996).11.Often word meanings can only be fully determined byinvokingworld knowledg e.For instance,the meaningof “flat”in a“flat road”implies the absence of holes.However,in the expression“aflat tire,”it indicates the presence of a hole.The meaningof“finish”in the phrase “Billfinished the book”implies that Bill completed readingthe book.However,the phrase“the g oatfin-ished the book”can only be interpreted as the goat eatingor destroyingthe book.The examples illustrate that word meaningis often underdetermined and nec-essarily intertwined with general world knowledge.In such cases,it is hard to see how the integration of lexical meaning and general world knowledge could be strictly separated(3,31).12.W.Marslen-Wilson,C.M.Brown,L.K.Tyler,Lang.Cognit.Process.3,1(1988).13.ERPs for30subjects were averaged time-locked to theonset of the critical words,with40items per condition.Sentences were presented word by word on the centerof a computer screen,with a stimulus onset asynchronyof600ms.While subjects were readingthe sentences,their EEG was recorded and amplified with a high-cut-off frequency of70Hz,a time constant of8s,and asamplingfrequency of200Hz.14.Materials and methods are available as supportingmaterial on Science Online.15.M.Kutas,S.A.Hillyard,Science207,203(1980).16.C.Brown,P.Hagoort,J.Cognit.Neurosci.5,34(1993).17.C.M.Brown,P.Hagoort,in Architectures and Mech-anisms for Language Processing,M.W.Crocker,M.Pickering,C.Clifton Jr.,Eds.(Cambridge Univ.Press,Cambridge,1999),pp.213–237.18.F.Varela et al.,Nature Rev.Neurosci.2,229(2001).19.We obtained TFRs of the single-trial EEG data by con-volvingcomplex Morlet wavelets with the EEG data andcomputingthe squared norm for the result of theconvolution.We used wavelets with a7-cycle width,with frequencies ranging from1to70Hz,in1-Hz steps.Power values thus obtained were expressed as a per-centage change relative to the power in a baselineinterval,which was taken from150to0ms before theonset of the critical word.This was done in order tonormalize for individual differences in EEG power anddifferences in baseline power between different fre-quency bands.Two relevant time-frequency compo-nents were identified:(i)a theta component,rangingfrom4to7Hz and from300to800ms after wordonset,and(ii)a gamma component,ranging from35to45Hz and from400to600ms after word onset.20.C.Tallon-Baudry,O.Bertrand,Trends Cognit.Sci.3,151(1999).tner et al.,Nature397,434(1999).22.M.Bastiaansen,P.Hagoort,Cortex39(2003).23.O.Jensen,C.D.Tesche,Eur.J.Neurosci.15,1395(2002).24.Whole brain T2*-weighted echo planar imaging bloodoxygen level–dependent(EPI-BOLD)fMRI data wereacquired with a Siemens Sonata1.5-T magnetic reso-nance scanner with interleaved slice ordering,a volumerepetition time of2.48s,an echo time of40ms,a90°flip angle,31horizontal slices,a64ϫ64slice matrix,and isotropic voxel size of3.5ϫ3.5ϫ3.5mm.For thestructural magnetic resonance image,we used a high-resolution(isotropic voxels of1mm3)T1-weightedmagnetization-prepared rapid gradient-echo pulse se-quence.The fMRI data were preprocessed and analyzedby statistical parametric mappingwith SPM99software(http://www.fi/spm99).25.S.E.Petersen et al.,Nature331,585(1988).26.B.T.Gold,R.L.Buckner,Neuron35,803(2002).27.E.Halgren et al.,J.Psychophysiol.88,1(1994).28.E.Halgren et al.,Neuroimage17,1101(2002).29.M.K.Tanenhaus et al.,Science268,1632(1995).30.J.J.A.van Berkum et al.,J.Cognit.Neurosci.11,657(1999).31.P.A.M.Seuren,Discourse Semantics(Basil Blackwell,Oxford,1985).32.We thank P.Indefrey,P.Fries,P.A.M.Seuren,and M.van Turennout for helpful discussions.Supported bythe Netherlands Organization for Scientific Research,grant no.400-56-384(P.H.).Supporting Online Material/cgi/content/full/1095455/DC1Materials and MethodsFig.S1References and Notes8January2004;accepted9March2004Published online18March2004;10.1126/science.1095455Include this information when citingthis paper.Complete Genome Sequence ofthe Apicomplexan,Cryptosporidium parvumMitchell S.Abrahamsen,1,2*†Thomas J.Templeton,3†Shinichiro Enomoto,1Juan E.Abrahante,1Guan Zhu,4 Cheryl ncto,1Mingqi Deng,1Chang Liu,1‡Giovanni Widmer,5Saul Tzipori,5GregoryA.Buck,6Ping Xu,6 Alan T.Bankier,7Paul H.Dear,7Bernard A.Konfortov,7 Helen F.Spriggs,7Lakshminarayan Iyer,8Vivek Anantharaman,8L.Aravind,8Vivek Kapur2,9The apicomplexan Cryptosporidium parvum is an intestinal parasite that affects healthy humans and animals,and causes an unrelenting infection in immuno-compromised individuals such as AIDS patients.We report the complete ge-nome sequence of C.parvum,type II isolate.Genome analysis identifies ex-tremely streamlined metabolic pathways and a reliance on the host for nu-trients.In contrast to Plasmodium and Toxoplasma,the parasite lacks an api-coplast and its genome,and possesses a degenerate mitochondrion that has lost its genome.Several novel classes of cell-surface and secreted proteins with a potential role in host interactions and pathogenesis were also detected.Elu-cidation of the core metabolism,including enzymes with high similarities to bacterial and plant counterparts,opens new avenues for drug development.Cryptosporidium parvum is a globally impor-tant intracellular pathogen of humans and animals.The duration of infection and patho-genesis of cryptosporidiosis depends on host immune status,ranging from a severe but self-limiting diarrhea in immunocompetent individuals to a life-threatening,prolonged infection in immunocompromised patients.Asubstantial degree of morbidity and mortalityis associated with infections in AIDS pa-tients.Despite intensive efforts over the past20years,there is currently no effective ther-apy for treating or preventing C.parvuminfection in humans.Cryptosporidium belongs to the phylumApicomplexa,whose members share a com-mon apical secretory apparatus mediating lo-comotion and tissue or cellular invasion.Many apicomplexans are of medical or vet-erinary importance,including Plasmodium,Babesia,Toxoplasma,Neosprora,Sarcocys-tis,Cyclospora,and Eimeria.The life cycle ofC.parvum is similar to that of other cyst-forming apicomplexans(e.g.,Eimeria and Tox-oplasma),resulting in the formation of oocysts1Department of Veterinary and Biomedical Science,College of Veterinary Medicine,2Biomedical Genom-ics Center,University of Minnesota,St.Paul,MN55108,USA.3Department of Microbiology and Immu-nology,Weill Medical College and Program in Immu-nology,Weill Graduate School of Medical Sciences ofCornell University,New York,NY10021,USA.4De-partment of Veterinary Pathobiology,College of Vet-erinary Medicine,Texas A&M University,College Sta-tion,TX77843,USA.5Division of Infectious Diseases,Tufts University School of Veterinary Medicine,NorthGrafton,MA01536,USA.6Center for the Study ofBiological Complexity and Department of Microbiol-ogy and Immunology,Virginia Commonwealth Uni-versity,Richmond,VA23198,USA.7MRC Laboratoryof Molecular Biology,Hills Road,Cambridge CB22QH,UK.8National Center for Biotechnology Infor-mation,National Library of Medicine,National Insti-tutes of Health,Bethesda,MD20894,USA.9Depart-ment of Microbiology,University of Minnesota,Min-neapolis,MN55455,USA.*To whom correspondence should be addressed.E-mail:abe@†These authors contributed equally to this work.‡Present address:Bioinformatics Division,Genetic Re-search,GlaxoSmithKline Pharmaceuticals,5MooreDrive,Research Triangle Park,NC27009,USA.R E P O R T S SCIENCE VOL30416APRIL2004441o n O c t o b e r 7 , 2 0 0 9 w w w . s c i e n c e m a g . o r g D o w n l o a d e d f r o mthat are shed in the feces of infected hosts.C.parvum oocysts are highly resistant to environ-mental stresses,including chlorine treatment of community water supplies;hence,the parasite is an important water-and food-borne pathogen (1).The obligate intracellular nature of the par-asite ’s life cycle and the inability to culture the parasite continuously in vitro greatly impair researchers ’ability to obtain purified samples of the different developmental stages.The par-asite cannot be genetically manipulated,and transformation methodologies are currently un-available.To begin to address these limitations,we have obtained the complete C.parvum ge-nome sequence and its predicted protein com-plement.(This whole-genome shotgun project has been deposited at DDBJ/EMBL/GenBank under the project accession AAEE00000000.The version described in this paper is the first version,AAEE01000000.)The random shotgun approach was used to obtain the complete DNA sequence (2)of the Iowa “type II ”isolate of C.parvum .This isolate readily transmits disease among numerous mammals,including humans.The resulting ge-nome sequence has roughly 13ϫgenome cov-erage containing five gaps and 9.1Mb of totalDNA sequence within eight chromosomes.The C.parvum genome is thus quite compact rela-tive to the 23-Mb,14-chromosome genome of Plasmodium falciparum (3);this size difference is predominantly the result of shorter intergenic regions,fewer introns,and a smaller number of genes (Table 1).Comparison of the assembled sequence of chromosome VI to that of the recently published sequence of chromosome VI (4)revealed that our assembly contains an ad-ditional 160kb of sequence and a single gap versus two,with the common sequences dis-playing a 99.993%sequence identity (2).The relative paucity of introns greatly simplified gene predictions and facilitated an-notation (2)of predicted open reading frames (ORFs).These analyses provided an estimate of 3807protein-encoding genes for the C.parvum genome,far fewer than the estimated 5300genes predicted for the Plasmodium genome (3).This difference is primarily due to the absence of an apicoplast and mitochondrial genome,as well as the pres-ence of fewer genes encoding metabolic functions and variant surface proteins,such as the P.falciparum var and rifin molecules (Table 2).An analysis of the encoded pro-tein sequences with the program SEG (5)shows that these protein-encoding genes are not enriched in low-complexity se-quences (34%)to the extent observed in the proteins from Plasmodium (70%).Our sequence analysis indicates that Cryptosporidium ,unlike Plasmodium and Toxoplasma ,lacks both mitochondrion and apicoplast genomes.The overall complete-ness of the genome sequence,together with the fact that similar DNA extraction proce-dures used to isolate total genomic DNA from C.parvum efficiently yielded mito-chondrion and apicoplast genomes from Ei-meria sp.and Toxoplasma (6,7),indicates that the absence of organellar genomes was unlikely to have been the result of method-ological error.These conclusions are con-sistent with the absence of nuclear genes for the DNA replication and translation machinery characteristic of mitochondria and apicoplasts,and with the lack of mito-chondrial or apicoplast targeting signals for tRNA synthetases.A number of putative mitochondrial pro-teins were identified,including components of a mitochondrial protein import apparatus,chaperones,uncoupling proteins,and solute translocators (table S1).However,the ge-nome does not encode any Krebs cycle en-zymes,nor the components constituting the mitochondrial complexes I to IV;this finding indicates that the parasite does not rely on complete oxidation and respiratory chains for synthesizing adenosine triphosphate (ATP).Similar to Plasmodium ,no orthologs for the ␥,␦,or εsubunits or the c subunit of the F 0proton channel were detected (whereas all subunits were found for a V-type ATPase).Cryptosporidium ,like Eimeria (8)and Plas-modium ,possesses a pyridine nucleotide tran-shydrogenase integral membrane protein that may couple reduced nicotinamide adenine dinucleotide (NADH)and reduced nico-tinamide adenine dinucleotide phosphate (NADPH)redox to proton translocation across the inner mitochondrial membrane.Unlike Plasmodium ,the parasite has two copies of the pyridine nucleotide transhydrogenase gene.Also present is a likely mitochondrial membrane –associated,cyanide-resistant alter-native oxidase (AOX )that catalyzes the reduction of molecular oxygen by ubiquinol to produce H 2O,but not superoxide or H 2O 2.Several genes were identified as involved in biogenesis of iron-sulfur [Fe-S]complexes with potential mitochondrial targeting signals (e.g.,nifS,nifU,frataxin,and ferredoxin),supporting the presence of a limited electron flux in the mitochondrial remnant (table S2).Our sequence analysis confirms the absence of a plastid genome (7)and,additionally,the loss of plastid-associated metabolic pathways including the type II fatty acid synthases (FASs)and isoprenoid synthetic enzymes thatTable 1.General features of the C.parvum genome and comparison with other single-celled eukaryotes.Values are derived from respective genome project summaries (3,26–28).ND,not determined.FeatureC.parvum P.falciparum S.pombe S.cerevisiae E.cuniculiSize (Mbp)9.122.912.512.5 2.5(G ϩC)content (%)3019.43638.347No.of genes 38075268492957701997Mean gene length (bp)excluding introns 1795228314261424ND Gene density (bp per gene)23824338252820881256Percent coding75.352.657.570.590Genes with introns (%)553.9435ND Intergenic regions (G ϩC)content %23.913.632.435.145Mean length (bp)5661694952515129RNAsNo.of tRNA genes 454317429944No.of 5S rRNA genes 6330100–2003No.of 5.8S ,18S ,and 28S rRNA units 57200–400100–20022Table parison between predicted C.parvum and P.falciparum proteins.FeatureC.parvum P.falciparum *Common †Total predicted proteins380752681883Mitochondrial targeted/encoded 17(0.45%)246(4.7%)15Apicoplast targeted/encoded 0581(11.0%)0var/rif/stevor ‡0236(4.5%)0Annotated as protease §50(1.3%)31(0.59%)27Annotated as transporter 69(1.8%)34(0.65%)34Assigned EC function ¶167(4.4%)389(7.4%)113Hypothetical proteins925(24.3%)3208(60.9%)126*Values indicated for P.falciparum are as reported (3)with the exception of those for proteins annotated as protease or transporter.†TBLASTN hits (e Ͻ–5)between C.parvum and P.falciparum .‡As reported in (3).§Pre-dicted proteins annotated as “protease or peptidase”for C.parvum (CryptoGenome database,)and P.falciparum (PlasmoDB database,).Predicted proteins annotated as “trans-porter,permease of P-type ATPase”for C.parvum (CryptoGenome)and P.falciparum (PlasmoDB).¶Bidirectional BLAST hit (e Ͻ–15)to orthologs with assigned Enzyme Commission (EC)numbers.Does not include EC assignment numbers for protein kinases or protein phosphatases (due to inconsistent annotation across genomes),or DNA polymerases or RNA polymerases,as a result of issues related to subunit inclusion.(For consistency,46proteins were excluded from the reported P.falciparum values.)R E P O R T S16APRIL 2004VOL 304SCIENCE 442 o n O c t o b e r 7, 2009w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o mare otherwise localized to the plastid in other apicomplexans.C.parvum fatty acid biosynthe-sis appears to be cytoplasmic,conducted by a large(8252amino acids)modular type I FAS (9)and possibly by another large enzyme that is related to the multidomain bacterial polyketide synthase(10).Comprehensive screening of the C.parvum genome sequence also did not detect orthologs of Plasmodium nuclear-encoded genes that contain apicoplast-targeting and transit sequences(11).C.parvum metabolism is greatly stream-lined relative to that of Plasmodium,and in certain ways it is reminiscent of that of another obligate eukaryotic parasite,the microsporidian Encephalitozoon.The degeneration of the mi-tochondrion and associated metabolic capabili-ties suggests that the parasite largely relies on glycolysis for energy production.The parasite is capable of uptake and catabolism of mono-sugars(e.g.,glucose and fructose)as well as synthesis,storage,and catabolism of polysac-charides such as trehalose and amylopectin. Like many anaerobic organisms,it economizes ATP through the use of pyrophosphate-dependent phosphofructokinases.The conver-sion of pyruvate to acetyl–coenzyme A(CoA) is catalyzed by an atypical pyruvate-NADPH oxidoreductase(Cp PNO)that contains an N-terminal pyruvate–ferredoxin oxidoreductase (PFO)domain fused with a C-terminal NADPH–cytochrome P450reductase domain (CPR).Such a PFO-CPR fusion has previously been observed only in the euglenozoan protist Euglena gracilis(12).Acetyl-CoA can be con-verted to malonyl-CoA,an important precursor for fatty acid and polyketide biosynthesis.Gly-colysis leads to several possible organic end products,including lactate,acetate,and ethanol. The production of acetate from acetyl-CoA may be economically beneficial to the parasite via coupling with ATP production.Ethanol is potentially produced via two in-dependent pathways:(i)from the combination of pyruvate decarboxylase and alcohol dehy-drogenase,or(ii)from acetyl-CoA by means of a bifunctional dehydrogenase(adhE)with ac-etaldehyde and alcohol dehydrogenase activi-ties;adhE first converts acetyl-CoA to acetal-dehyde and then reduces the latter to ethanol. AdhE predominantly occurs in bacteria but has recently been identified in several protozoans, including vertebrate gut parasites such as Enta-moeba and Giardia(13,14).Adjacent to the adhE gene resides a second gene encoding only the AdhE C-terminal Fe-dependent alcohol de-hydrogenase domain.This gene product may form a multisubunit complex with AdhE,or it may function as an alternative alcohol dehydro-genase that is specific to certain growth condi-tions.C.parvum has a glycerol3-phosphate dehydrogenase similar to those of plants,fungi, and the kinetoplastid Trypanosoma,but(unlike trypanosomes)the parasite lacks an ortholog of glycerol kinase and thus this pathway does not yield glycerol production.In addition to themodular fatty acid synthase(Cp FAS1)andpolyketide synthase homolog(Cp PKS1), C.parvum possesses several fatty acyl–CoA syn-thases and a fatty acyl elongase that may partici-pate in fatty acid metabolism.Further,enzymesfor the metabolism of complex lipids(e.g.,glyc-erolipid and inositol phosphate)were identified inthe genome.Fatty acids are apparently not anenergy source,because enzymes of the fatty acidoxidative pathway are absent,with the exceptionof a3-hydroxyacyl-CoA dehydrogenase.C.parvum purine metabolism is greatlysimplified,retaining only an adenosine ki-nase and enzymes catalyzing conversionsof adenosine5Ј-monophosphate(AMP)toinosine,xanthosine,and guanosine5Ј-monophosphates(IMP,XMP,and GMP).Among these enzymes,IMP dehydrogenase(IMPDH)is phylogenetically related toε-proteobacterial IMPDH and is strikinglydifferent from its counterparts in both thehost and other apicomplexans(15).In con-trast to other apicomplexans such as Toxo-plasma gondii and P.falciparum,no geneencoding hypoxanthine-xanthineguaninephosphoribosyltransferase(HXGPRT)is de-tected,in contrast to a previous report on theactivity of this enzyme in C.parvum sporo-zoites(16).The absence of HXGPRT sug-gests that the parasite may rely solely on asingle enzyme system including IMPDH toproduce GMP from AMP.In contrast to otherapicomplexans,the parasite appears to relyon adenosine for purine salvage,a modelsupported by the identification of an adeno-sine transporter.Unlike other apicomplexansand many parasitic protists that can synthe-size pyrimidines de novo,C.parvum relies onpyrimidine salvage and retains the ability forinterconversions among uridine and cytidine5Ј-monophosphates(UMP and CMP),theirdeoxy forms(dUMP and dCMP),and dAMP,as well as their corresponding di-and triphos-phonucleotides.The parasite has also largelyshed the ability to synthesize amino acids denovo,although it retains the ability to convertselect amino acids,and instead appears torely on amino acid uptake from the host bymeans of a set of at least11amino acidtransporters(table S2).Most of the Cryptosporidium core pro-cesses involved in DNA replication,repair,transcription,and translation conform to thebasic eukaryotic blueprint(2).The transcrip-tional apparatus resembles Plasmodium interms of basal transcription machinery.How-ever,a striking numerical difference is seenin the complements of two RNA bindingdomains,Sm and RRM,between P.falcipa-rum(17and71domains,respectively)and C.parvum(9and51domains).This reductionresults in part from the loss of conservedproteins belonging to the spliceosomal ma-chinery,including all genes encoding Smdomain proteins belonging to the U6spliceo-somal particle,which suggests that this par-ticle activity is degenerate or entirely lost.This reduction in spliceosomal machinery isconsistent with the reduced number of pre-dicted introns in Cryptosporidium(5%)rela-tive to Plasmodium(Ͼ50%).In addition,keycomponents of the small RNA–mediatedposttranscriptional gene silencing system aremissing,such as the RNA-dependent RNApolymerase,Argonaute,and Dicer orthologs;hence,RNA interference–related technolo-gies are unlikely to be of much value intargeted disruption of genes in C.parvum.Cryptosporidium invasion of columnarbrush border epithelial cells has been de-scribed as“intracellular,but extracytoplas-mic,”as the parasite resides on the surface ofthe intestinal epithelium but lies underneaththe host cell membrane.This niche may al-low the parasite to evade immune surveil-lance but take advantage of solute transportacross the host microvillus membrane or theextensively convoluted parasitophorous vac-uole.Indeed,Cryptosporidium has numerousgenes(table S2)encoding families of putativesugar transporters(up to9genes)and aminoacid transporters(11genes).This is in starkcontrast to Plasmodium,which has fewersugar transporters and only one putative ami-no acid transporter(GenBank identificationnumber23612372).As a first step toward identification ofmulti–drug-resistant pumps,the genome se-quence was analyzed for all occurrences ofgenes encoding multitransmembrane proteins.Notable are a set of four paralogous proteinsthat belong to the sbmA family(table S2)thatare involved in the transport of peptide antibi-otics in bacteria.A putative ortholog of thePlasmodium chloroquine resistance–linkedgene Pf CRT(17)was also identified,althoughthe parasite does not possess a food vacuole likethe one seen in Plasmodium.Unlike Plasmodium,C.parvum does notpossess extensive subtelomeric clusters of anti-genically variant proteins(exemplified by thelarge families of var and rif/stevor genes)thatare involved in immune evasion.In contrast,more than20genes were identified that encodemucin-like proteins(18,19)having hallmarksof extensive Thr or Ser stretches suggestive ofglycosylation and signal peptide sequences sug-gesting secretion(table S2).One notable exam-ple is an11,700–amino acid protein with anuninterrupted stretch of308Thr residues(cgd3_720).Although large families of secretedproteins analogous to the Plasmodium multi-gene families were not found,several smallermultigene clusters were observed that encodepredicted secreted proteins,with no detectablesimilarity to proteins from other organisms(Fig.1,A and B).Within this group,at leastfour distinct families appear to have emergedthrough gene expansions specific to the Cryp-R E P O R T S SCIENCE VOL30416APRIL2004443o n O c t o b e r 7 , 2 0 0 9 w w w . s c i e n c e m a g . o r g D o w n l o a d e d f r o mtosporidium clade.These families —SKSR,MEDLE,WYLE,FGLN,and GGC —were named after well-conserved sequence motifs (table S2).Reverse transcription polymerase chain reaction (RT-PCR)expression analysis (20)of one cluster,a locus of seven adjacent CpLSP genes (Fig.1B),shows coexpression during the course of in vitro development (Fig.1C).An additional eight genes were identified that encode proteins having a periodic cysteine structure similar to the Cryptosporidium oocyst wall protein;these eight genes are similarly expressed during the onset of oocyst formation and likely participate in the formation of the coccidian rigid oocyst wall in both Cryptospo-ridium and Toxoplasma (21).Whereas the extracellular proteins described above are of apparent apicomplexan or lineage-specific in-vention,Cryptosporidium possesses many genesencodingsecretedproteinshavinglineage-specific multidomain architectures composed of animal-and bacterial-like extracellular adhe-sive domains (fig.S1).Lineage-specific expansions were ob-served for several proteases (table S2),in-cluding an aspartyl protease (six genes),a subtilisin-like protease,a cryptopain-like cys-teine protease (five genes),and a Plas-modium falcilysin-like (insulin degrading enzyme –like)protease (19genes).Nine of the Cryptosporidium falcilysin genes lack the Zn-chelating “HXXEH ”active site motif and are likely to be catalytically inactive copies that may have been reused for specific protein-protein interactions on the cell sur-face.In contrast to the Plasmodium falcilysin,the Cryptosporidium genes possess signal peptide sequences and are likely trafficked to a secretory pathway.The expansion of this family suggests either that the proteins have distinct cleavage specificities or that their diversity may be related to evasion of a host immune response.Completion of the C.parvum genome se-quence has highlighted the lack of conven-tional drug targets currently pursued for the control and treatment of other parasitic protists.On the basis of molecular and bio-chemical studies and drug screening of other apicomplexans,several putative Cryptospo-ridium metabolic pathways or enzymes have been erroneously proposed to be potential drug targets (22),including the apicoplast and its associated metabolic pathways,the shikimate pathway,the mannitol cycle,the electron transport chain,and HXGPRT.Nonetheless,complete genome sequence analysis identifies a number of classic and novel molecular candidates for drug explora-tion,including numerous plant-like and bacterial-like enzymes (tables S3and S4).Although the C.parvum genome lacks HXGPRT,a potent drug target in other api-complexans,it has only the single pathway dependent on IMPDH to convert AMP to GMP.The bacterial-type IMPDH may be a promising target because it differs substan-tially from that of eukaryotic enzymes (15).Because of the lack of de novo biosynthetic capacity for purines,pyrimidines,and amino acids,C.parvum relies solely on scavenge from the host via a series of transporters,which may be exploited for chemotherapy.C.parvum possesses a bacterial-type thymidine kinase,and the role of this enzyme in pyrim-idine metabolism and its drug target candida-cy should be pursued.The presence of an alternative oxidase,likely targeted to the remnant mitochondrion,gives promise to the study of salicylhydroxamic acid (SHAM),as-cofuranone,and their analogs as inhibitors of energy metabolism in the parasite (23).Cryptosporidium possesses at least 15“plant-like ”enzymes that are either absent in or highly divergent from those typically found in mammals (table S3).Within the glycolytic pathway,the plant-like PPi-PFK has been shown to be a potential target in other parasites including T.gondii ,and PEPCL and PGI ap-pear to be plant-type enzymes in C.parvum .Another example is a trehalose-6-phosphate synthase/phosphatase catalyzing trehalose bio-synthesis from glucose-6-phosphate and uridine diphosphate –glucose.Trehalose may serve as a sugar storage source or may function as an antidesiccant,antioxidant,or protein stability agent in oocysts,playing a role similar to that of mannitol in Eimeria oocysts (24).Orthologs of putative Eimeria mannitol synthesis enzymes were not found.However,two oxidoreductases (table S2)were identified in C.parvum ,one of which belongs to the same families as the plant mannose dehydrogenases (25)and the other to the plant cinnamyl alcohol dehydrogenases.In principle,these enzymes could synthesize protective polyol compounds,and the former enzyme could use host-derived mannose to syn-thesize mannitol.References and Notes1.D.G.Korich et al .,Appl.Environ.Microbiol.56,1423(1990).2.See supportingdata on Science Online.3.M.J.Gardner et al .,Nature 419,498(2002).4.A.T.Bankier et al .,Genome Res.13,1787(2003).5.J.C.Wootton,Comput.Chem.18,269(1994).Fig.1.(A )Schematic showing the chromosomal locations of clusters of potentially secreted proteins.Numbers of adjacent genes are indicated in paren-theses.Arrows indicate direc-tion of clusters containinguni-directional genes (encoded on the same strand);squares indi-cate clusters containingg enes encoded on both strands.Non-paralogous genes are indicated by solid gray squares or direc-tional triangles;SKSR (green triangles),FGLN (red trian-gles),and MEDLE (blue trian-gles)indicate three C.parvum –specific families of paralogous genes predominantly located at telomeres.Insl (yellow tri-angles)indicates an insulinase/falcilysin-like paralogous gene family.Cp LSP (white square)indicates the location of a clus-ter of adjacent large secreted proteins (table S2)that are cotranscriptionally regulated.Identified anchored telomeric repeat sequences are indicated by circles.(B )Schematic show-inga select locus containinga cluster of coexpressed large secreted proteins (Cp LSP).Genes and intergenic regions (regions between identified genes)are drawn to scale at the nucleotide level.The length of the intergenic re-gions is indicated above or be-low the locus.(C )Relative ex-pression levels of CpLSP (red lines)and,as a control,C.parvum Hedgehog-type HINT domain gene (blue line)duringin vitro development,as determined by semiquantitative RT-PCR usingg ene-specific primers correspondingto the seven adjacent g enes within the CpLSP locus as shown in (B).Expression levels from three independent time-course experiments are represented as the ratio of the expression of each gene to that of C.parvum 18S rRNA present in each of the infected samples (20).R E P O R T S16APRIL 2004VOL 304SCIENCE 444 o n O c t o b e r 7, 2009w w w .s c i e n c e m a g .o r g D o w n l o a d e d f r o m。
![[科普]健忘与阿兹海默症(英文)](https://img.taocdn.com/s1/m/31c3c8d14128915f804d2b160b4e767f5bcf805c.png)
[科普]健忘与阿兹海默症(英⽂)其实是我⼀篇病理学作业,那教授着实与众不同,要求⽤通俗易懂的⽂字来写科学review,结果就写成了⼀篇科普⽂,左右写了,故贴上来,有兴趣看的就当增长见闻吧:)(哪天⼼情好再翻译成中⽂XD)Why People Become so Forgetful Once They Get Alzheimer's Disease?Have you ever heard about a disease called Alzheimer's? I bet most ofyou probably have heard of it from somewhere, even though you mightnot know exactly what it is. For many people who are out of thescience world, "Alzheimer's" means the same thing as "seniledementia", which is actually incorrect if you speak scientifically.More interestingly, "dementia" itself has two different meanings inthe clinical realm and the lay public. "Demented" is generally used bythe lay public synonymously with "mad" or "insane". In clinical termhowever, dementia refers to a specific and pronounced decline ofcognitive function in humans - a decline in mentation. (In Latin,dementia means "apart from mind".) "Senile dementia", often shortenedas simply "dementia", refers to the progressive cognitive declinefound in elderly people beyond what might be expected from normalaging, which is usually due to damage or disease in the brain. Inspecific, dementia can arise from a number of causes such as stroke,vascular problems, some medical conditions, or the abuse of drugs oralcohol. But among them, a pathological condition called Alzheimer’sdisease (AD) is the most common cause of senile dementia. Because ofthis, it becomes that the two terms, AD and dementia, are often usedinterchangeably in public content. However, please keep in mind thatthere are other causes that can lead to dementia besides AD.In 1906, a German psychiatrist Alois Alzheimer first described thedisease that eventually bears his name. He wrote of a 51-year-oldwoman named Mrs. Auguste D who had "a strange disease of the cerebralcortex" that manifested as progressive memory impairment and otherbehavioral and cognitive problems. After Mrs. Auguste D. died in 1906,Dr. Alzheimer carefully examined her brain anatomy and neuropathology.He presented Mrs. Auguste D's case to the German psychiatristcommunity and described the neurofibrillary tangles and amyloidplaques that were found in Mrs. Auguste D’s brain (Alzheimer,1907). “Tangles” and “plaques” have then come to be considered as thetwo main pathological hallmarks of the disease.Simply speaking, the plaques are admixtures of a bunch of junks thatbrain produces. They are found in the extracellular spaces, i.e. theoutside of the brain cells. A typical senile plaque usually containslots of aggregated proteins called amyloid beta (Ab). Scientists haveno idea why our brain produces such protein since it appears to haveno function roles. As this junk protein aggregating into a toxic densecore, the nearby dendrites and axons (the extensions of neurons) areaffected and start dying. In contrast to extracellular plaques, thesecond pathological hallmark of AD is localized intracellulary, i.e.inside of the cells. Many people may have an impression that a cell islike a small squishy water balloon (at least I used to think so),which of course is not true. The cell actually has its own skeletonstructure inside that supports its external shape. It is calledmicrotubule cytoskeleton. In AD, these microtubules and mircofibrilsbecome tangling up with each other for some reason. And eventually thetangles cause the collapse of the cytoskeleton, which leads to thecell death. Remember how your kitten can make a ball of wool into afearful tangle? Same thing can happen in our brain, but we haven’t yetfound that "naughty kitten" which causes the neurofibrillary tangles.Please don't think tangles and plaques are unique in Alzheimer's. Yourbrain is also producing plaques as you reading this line. Tangles andplaques can be found in many normal non-demented elderly, but ratherin a very reduced number and would not cause severe neuronal loss anddramatic cognitive problems. One major task for AD researchers is tofind out why the plaque and tangle forming processes are much moreaccelerated and pronounced in AD.Now you are probably wondering how these pathological alterations atthe cell level can lead to the behavioral and cognitive changes in AD patients. Among the symptoms of dementia caused by AD are losses of learning and memory capacity, decline in reasoning ability, attention problems, language difficulties and problems with perception. Forgetfulness is the most typical presenting symptom showed by AD patients in the beginning stage. These patients can remember how to talk, and may remember events from many years ago, but they have trouble remembering what happened in the past hours. Episodic memory formation is lost as the patients typically lost the recollection oftheir ongoing experiences on a daily basis. Common examples of memory deficits in this stage are the repetition of questions or statementsand the misplacement of items. The patients at this early stage of ADare unable to recall recent conversations or events, whereas the pastlife experience and knowledge learned years ago are still retrievable, clearly showing the impairment in new information acquisition but notin the retention of old memory.These early symptoms of AD remind the scientists of the cognitive changes manifested by another group of patients who lost their hippocampus. Hippocampus (meaning “seahorse” in Greek) is a structure located in the medial temporal lobe of our brain. It got its name fromits curved shape in coronal sections of the brain, which muchresembles a seahorse. Today, the hippocampus is generally believed to play a crucial role in the formation of new declarative memories about experienced events. A fancy scientific term of this process iscalled “memory consolidation”. In brief, the hippocampus serves as a short-term memory store that eventually uploads memory to the neocortex for longer-term storage. I know many people like to draw an analogy between human brain and computer (indeed, in Chinese language, the word “computer” literally means “electronic brain”). If you lookthe brain as a super powerful computer, the hippocampus will be itsRAM (random access memory) which temporarily stores information and later uploads them to the hard disk, i.e. the neocortex, for permanent storage. What if the function of the hippocampus is disrupted? Let’slook at the famous case of a patient named H.M. H.M. is an unfortunate victim of neurosurgical experimentation who had his medial temporal lobes removed as a treatment for epilepsy, the removed parts including most of his hippocami on both sides of his brain. The lesion partially treated the epilepsy but essentially completely destroyed H.M.’scapacity of forming long-term memory. He became unable to form any new long-lasting declarative memories (anterograde amnesia) and has been living a minute-to-minute existence for the past five decades ofyears. However, his prior memories are mostly intact for his lifetimeup to several years before the surgery and he is able to consistentlyrecall them (Scoville & Milner, 2000).Surprisingly, most AD patients in the initial stages have shown thesame memory problems experienced by H.M. You then might be thinking, oh, maybe there’s something wrong with their hippocampus! And that’s exactly what the scientists have found out. Early in the course of AD, plaques and tangles are found particularly abundant in a brain region called “entorhinal cortex” which sits right next to the hippocampus.This region is like a gateway to the hippocampus, and all the information going into or going out from the hippocampus have to pass through it. Without the input from the entorhinal cortex, the hippocampus cannot function properly. Very soon, the hippocampusitself gets occupied by the plaques and tangles, thus loses itsability to establish or consolidate memory for ongoing events. As the disease progresses, the plaques and tangles also start to spread outto other brain structures, meanwhile the initially affected areas are worsening. In brief, the circuitry that is critical for normaldeclarative memory, i.e. the hippocampus and its adjacent regionappear to be the first target affected by AD neuropathology such as plaques and tangles. That is why people start to become so forgetfulonce they get Alzheimer's disease.The sadness is that the “forgetfulness” is only a start for ADpatients. As the plaques and tangles start to spread out to otherlimbic regions and cerebral cortices, patients in the middle stagehave even more profound anterograde memory loss and also start to show retrograde amnesia. That is, recalling remote memories of long pastlife experience and events becomes difficult now. Recognition of knownindividuals progressively declines and verbal communication becomes incoherent. In many cases, AD patients also display mood disorders such as severe depression, since these victims are aware that they are deteriorating mentally and are “losing” loved ones. Eventually in the final stages of AD, during when the whole neocortex as well as other brain structures are profoundly affected by tangles and plaques, the patients become unable to execute the most basic cognitive functions, thus are unable to take care of themselves. They are completely bedridden until death.In these days, AD affects up to half million of people in Canada (Canadian Study of Health and Aging Working Group, 2000), 4 million in US, and 12 million worldwide over the age of 65. It is estimated thatby the year 2025 the number will go up to 20 million worldwide given the fact that at present there is no effective treatment for this disease. These dry statistical numbers probably don’t mean much to you at first glance. Let's look at it in this way then. Right now, aboutone in ten people over the age of 65 in North America have AD. If you live to age 85, you have about a one in two chance of developing this tragically debilitating disease. To me these statistics are sobering, especially given that we don’t have any effective treatment for AD. Since last century, human longevity has been greatly increased worldwide due to our expanding knowledge in biology and great improvement of health care and public hygiene. In the 18th century,the average human lifespan was around 30 years. But in the year 2005, the average life expectancy in Canada was estimated to be 80.1 years. Some scientists predicted that the USA would have 5.3 million people aged over 100 in 2100. Yes, thanks to the great Science. Nowadays we human are able to treat lots of disease and actively change our living environment to accomplish our longevity dream. But, today’s scientific advances are only able to make our bodies live longer, not our brains. It appears that our brain has a shorter “lifespan” than our bodies.Our brain starts to gradually degenerate irreversibly as early as inour 20's. In addition to the “normal aging”, we are facing more aggressive neurodegenerative conditions such as Alzheimer's that we haven't yet found a weapon to fight against. Does it make sense to become a centenarian while losing the ability to recognize your loved ones and losing all the precious memory from your past life? I don't know yours but my answer is a big NO. We need a healthy and functioning brain to make our longevity meaningful. While scientists worldwide are working so hard to seek treatments for Alzheimer’s and other brain diseases, please remember that your brain, just like therest of your body, need to be looked after. It is your own responsibility to take care of your own precious brain.K.FanReference:Alzheimer A. (1907) Uber eine eigenartige Erkrankung der Hirnrinde. Allgemeine Zeitschrift fur Psychiatrie und Psychisch-gerichtliche Medizin. 64:146-48.Canadian Study of Health and Aging Working Group: Canadian Study of Health and Aging Working Group. (2000) The Incidence of Dementia in Canada. Neurology. 55: 66-73.Scoville WB, Milner B. (2000) Loss of recent memory after bilateral hippocampal lesions. 1957. J. Neuropsyychiatry Clin. Neuroscie. 12:103-13.。
Unit 4Drug Therapy in the Older Adult老年人的药物治疗Drug therapy in the older adult population is a complex phenomenon influenced by numerous biopsychosocial factors. The elderly are the largest group of consumers of prescription and over-the-counter (OTC) drugs. The average older adult uses 4.5 prescriptions and 2.1 OTC medications and fills between 12 to 17 prescriptions yearly. The incidence发生率of adverse有害的drug reactions in the elderly is two to three times that found in young adult. This is considered to be a conservative 保守的estimate估计, because drug reactions are less well recognized in older adults and because reactions can often mimic symptoms of specific disease states.药物治疗在老年人口是一个复杂的现象,因其被众多的生物心理社会因素影响。
老年人是处方药和非处方药的最大消费群体。
老年人平均使用4.5张处方和2.1张非处方药,每年填写12到17张处方。
老年人的药物不良反应发生率是年轻人的两到三倍。
这被认为是一个保守的估计,因为药物反应在老年人中较少被认识,因为反应往往可以模拟特定疾病状态的症状。
药学英语Unit 1Inflammatory reaction induced by local ischemic injury is one of the important pathophysiological characteristics after ischemic stroke, so anti-inflammatory therapy may be an effective strategy for acute ischemic stroke. Enlimomab, an anti-ICAM-1 murine monoclonal antibody, can inhibit the recruitment and activity of polymorphonuclear leukocytes, reduce their adhesion and decrease cerebral infarct size in experimental stroke models. However, a much larger efficacy trial including 625 acute ischemic stroke patients has shown that enlimomab was ineffective on ischemic stroke patients even with a worsening outcome. The therapeutic time window of rt-PA is within 3 hours of ischemic onset. Administration of the drug after more than 3 hours of ischemic onset has no significant therapeutic implications and may even end up with an increased hemorrhagic risk. A study using the animal ischemic model indicated that combination of anti-inflammatory therapy and rt-PA could significantly and might as well extend the therapeutic time window of thrombolysis.局部脑缺血损伤引起的炎症反应是缺血性脑卒中发生后的重要病理生理特征,因此,抗炎治疗策略可能是治疗急性缺血性脑卒中的一种有效方法。
Theacetylcholinesteraseinhibitor,Donepezil,regulatesaTh2biasinAlzheimer’sdiseasepatients
MarcellaRealea,*,CarlaIarloria,FrancescoGambia,ClaudioFelicianib,LucciIsabellaa,DomenicoGambia,c
aDepartmentofOncologyandNeuroscience,UnitofImmunology,University‘‘G.d’Annunzio’’,ViadeiVestini31,66123Chieti,Italy
bCatholicUniversity,Rome,Italy
cCentreofExcellenceonAging,Chieti,Italy
Received14September2005;receivedinrevisedform11November2005;accepted14November2005
AbstractTheincreasedpro-inflammatorycytokineproductionwaspreviouslyobservedinAlzheimer’sdisease(AD).Wesoughttoexplorewhetheracetylcholinesteraseinhibitor(AChEI)therapyamelioratesclinicalsymptomsinADthroughdown-regulationofinflammation.Expressionandreleaseofmonocytechemotacticprotein-1(MCP-1),apositiveregulatorofTh2differentiation,andinterleukin(IL)-4,ananti-inflammatorycytokinefromperipheralbloodmononuclearcells(PBMC)inADpatients,wereinvestigated.PBMCwerepurifiedfromADpatientsattimeofenrolment(T0)andafter1monthoftreatmentwithAChEI(T1)andfromhealthycontrols(HC).Supernatantswereanalyzedforcy-tokinelevelsbyELISAmethods.mRNAexpressionweredeterminedbyRTePCR.ExpressionandproductionofMCP-1andIL-4weresig-nificantlyincreasedinADsubjectsundertherapywiththeAChEIDonepezil,comparedtothesameADpatientsattimeofenrolment(P<0.001).OurdatasuggestanotherpossibleexplanationfortheabilityofDonepezil[diethyl(3,5-di-ter-butyl-4-hydroxybenzyl)phosphonate]todelaytheprogressionofAD;infact,DonepezilmaymodulateMCP-1andIL-4production,whichmayreflectageneralshifttowardstypeTh0/Th2cytokineswhichcouldbeprotectiveinADdisease.ThedifferentamountsofMCP-1andIL-4observedmightreflectthedifferentstatesofactivationand/orresponsivenessofPBMC,thatinADpatientscouldbekeptinanactivatedstatebypro-inflammatorycytokines.Ó2005ElsevierLtd.Allrightsreserved.
Keywords:Cytokines;Chemokines;Acetylcholinesteraseinhibitor;Donepezil;Alzheimer’sdisease
1.IntroductionBasicandclinicalresearchhaveprovidedevidenceforaninflammatorymechanisminAlzheimer’sdisease(AD)(Eikelenboometal.,1994;Akiyamaetal.,2000;Eikelenboometal.,2000;Pratico`andTrojanowski,2000;DeLuigietal.,2001;MrakandGriffin,2001)characterizedbymultipledysre-gulationofperipheralbloodmononuclearcells,includingincreasedproductionofpro-inflammatorymolecules(Fillitetal.,1991;Lombardietal.,1999;Streitetal.,2001;Szczepaniketal.,2001).Aroleforneuroinflammatoryprocessesin
Alzheimer’spathogenesishasreceivedfurthersupportfromep-idemiologicstudiesshowingaprotectiveeffectofanti-inflam-matorymedication(Richetal.,1995).Thepresenceofcytokines,acutephaseproteins,complementcomponentsalongwithamyloidbdeposits,suggestacloseassociationbetweenneuriticplaquesandlocalinflammatoryresponses(Lortonetal.,2000;DeLuigietal.,2002;Salaetal.,2003).Recentev-idencesuggeststhatbidirectionalcommunicationoccursbe-tweencellsofthenervousandimmunesystems.Thebasisforthiscommunicationisthereleaseofsolublemoleculesorcyto-kinesbyimmunocompetentcells(Haddadetal.,2002).Inter-leukin(IL)-1,apluripotentproinflammatorycytokine,isincreasedinserumofADpatientsandisoverexpressedinmi-crogliainAlzheimerbrain,andisfrequentlyassociatedwithAbplaques(Shengetal.,1995).Previousstudieshaveshown*Correspondingauthor.Tel./fax:þ398713555287.
E-mailaddress:mreale@unich.it(M.Reale).
0028-3908/$-seefrontmatterÓ2005ElsevierLtd.Allrightsreserved.doi:10.1016/j.neuropharm.2005.11.006
Neuropharmacology50(2006)606e613www.elsevier.com/locate/neuropharmthatAbcanactivatehumanmonocytesandmurinemicroglia,andtriggertheproductionofreactiveoxygenandnitrogenin-termediatesaswellasofproinflammatorycytokines(Klegerisetal.,1994;Medaetal.,1999).Studyconcerninglevelsofcir-culatingcytokines,suchasIL-6andtumornecrosisfactor(TNF)-a,associatedwithADcomparedtoage-matchedcontrolsremainscontroversial(Baueretal.,1991;Griffinetal.,1998;MrakandGriffin,2001;Paganellietal.,2002).TheresponsesofThcellstendtobepolarizedandmutuallyantagonistic,beingcharacterizedbytype1(Th1)ortype2(Th2)cytokineprofiles.Thestudyreportedherewasperformedtodeterminewhetherexpressionandreleaseincirculatingmononuclearcells(PBMC)ofmonocytechemotacticprotein-1(MCP-1),apositiveregulatorofTh2differentiation,andIL-4,ananti-inflammatorycytokine,haveanyrelationshipwithADpatho-genesis.Currentpotentialtreatmentforthediseaseincludestheanticholinesteraseinhibitors.OneofthedrugsapprovedbytheFDAisAricept(Donepezilhydrochloride,Pfizer)(Sugimoto,1999;Maltzetal.,2004).Thelong-actingAChEinhibitorDonepezilisusedtoimprovememoryandotheraspectsofcognitioninADpatients.Interestingly,recentinves-tigationsofcholinergicreceptoractivityinPBMC(Satoetal.,1999)haveprovidedevidencethatthelymphocytespossessanindependent,non-neuronalcholinergicsystemandthatacetyl-cholinesynthesizedandreleasedfromlymphocytesactsasanimmunomodulatorviabothmAChRandnAChR(KawashimaandFuji,2000;FujiandKawashima,2001;DeRosaetal.,2005;Feuerbachetal.,2005).Theexpressionoftheseneuro-transmitterreceptorsonthesurfaceofimmunocompetentcellsisindicativeoftheexistenceofalinkbetweenthenervousandimmunesystems.Activatedimmunecellsmayusethere-leasedAChinself-modulatingautocrineandparacrineloops,soAChdetectedinlymphocytesmayregulatetheactivationofthesecells(Fujietal.,2003;KawashimaandFuji,2003).Basedondatareportedbyotherauthorsthatdescribeanin-hibitoryeffectofAChoncytokinerelease(Borovikovaetal.,2000),weexploredwhetherinvivotreatmentwithAChEImodifiesthereleaseofIL-4andMCP-1fromPBMC.Totestthishypothesiswestudied40ADpatientsbeforeandafter4weeksofAChEItherapy.Ourdatashowincreasedexpres-sionandreleaseofMCP-1andIL-4inPBMCfromAChEI-treatedADpatients.