人尿微量白蛋白(m-Alb)检测试剂盒(透射微板法)
- 格式:pdf
- 大小:249.68 KB
- 文档页数:2


医疗器械产品技术要求编号:尿微量白蛋白/肌酐测定试剂盒(透射法)1.产品型号/规格及其划分说明20 人份/盒、40 人份/盒、100 人份/盒2.性能指标2.1试剂外观试剂盒组分应齐全,试剂的包装应整洁;图案与文字应清晰无误;标签应粘贴端正,无污迹;封盖应紧密,无漏液。
测试杯检测区无划痕。
试剂1、试剂3、试剂4 为无色澄清液体、不得有沉淀和絮状物,试剂2 为黄色澄清液体、不得有沉淀和絮状物,试剂 5 为无色至淡黄色澄清液体、不得有沉淀和絮状物。
2.2净含量试剂1 不少于135μl,试剂2 应不少于135μl,试剂3 应不少于120μl,试剂4 应不少于50μl,试剂5 应不少于50μl。
2.3CREA2.3.1试剂空白吸光度试剂的空白吸光度应≤0.1。
2.3.2准确度测试方法一:用本公司CREA 试剂盒和已上市公司CREA 试剂盒同时测定40 个临床样本,用线性回归方法计算两组结果的线性相关系数(r)应不小于0.990,斜率应在[0.9,1.1]内;样本浓度在[30,70]μmol/L 范围内,每个浓度点的线性绝对偏差应不超过±7 μmol/L;在(70,1500] μmol/L 范围内,每个浓度点的线性相对偏差应不超过±10% 。
测试方法二:测试企业准确度参考品,相对偏差应在±10% 内。
2.3.3分析灵敏度测定100μmol/L 的样本时,吸光度变化值(ΔA)应≥0.01。
2.3.4线性范围在[30~1500] μmol/L 线性范围内:a)线性相关系数r 不小于0.990;- 1 -b)测定结果在[30,70] μmol/L 时线性绝对偏差不超过±7μmol/L,在(70,1500]μmol/L范围内的线性相对偏差不超过±10% 。
2.3.5精密度2.3.5.1批内精密度用同一个样本进行检测,所得结果的变异系数CV ≤5%。
2.3.5.2批间差用三个批号的试剂盒测定同一个样本,所得结果的批间相对极差R≤10%。

医疗器械产品技术要求编号:尿微量白蛋白(mALB)测定试剂盒(化学发光-免疫分析法)2性能指标2.1试剂盒2.1.1外观和性状(1)试剂盒各个组分应齐全、完整、液体无渗漏。
(2)中文包装标签应清晰,准确、牢固。
(3)试剂R1 为澄清透明液体,无沉淀、颗粒或絮状物。
(4)试剂R2 为略带乳白色均一性液体,无明显沉淀、颗粒或絮状物。
(5)试剂R3 为澄清透明液体,无沉淀、颗粒或絮状物。
2.1.2装量液体装量应不少于标示值。
2.1.3准确度相对偏差应不超过±10 .0%。
2.1.4空白限应不大于 2.0mg/L。
2.1.5线性(a)在 4.0mg/L~200.0mg/L 范围内,其相关系数r 应不小于0.9900。
(b)[4.0,20.0] mg/L 范围内,线性绝对偏差应不超过±2.0 mg/L;在(20.0,200.0] mg/L 范围内,线性相对偏差应不超过±10 %。
2.1.6批内精密度批内变异系数(CV)应≤10.0%。
2.1.7批间精密度批间变异系数(CV)应≤10.0%。
2.2校准品2.2.1外观和性状澄清透明液体,不得有沉淀、颗粒或絮状物。
2.2.2装量液体装量应不小于标示值。
2.2.3校准品测量准确度(S0 除外)用工作校准品校准测量系统后,以试剂盒配套的校准品作为样本进行检测,其测定结果与标示值的相对偏差应在±10.0%范围内。
2.2.4校准品均一性(S0 除外)瓶内CV 应不大于10.0%;瓶间CV 应不大于10.0%。
2.3质控品2.3.1外观和性状澄清透明液体,不得有沉淀、颗粒或絮状物。
2.3.2装量液体装量应不小于标示值。
2.3.3质控品测定值用试剂盒配套的校准品校准测量系统后,以试剂盒配套的质控品作为样本进行检测,其测定结果应在标示范围内。
2.3.4质控品均一性瓶内CV 应不大于10.0%;瓶间CV 应不大于10.0%。

尿微量白蛋白(MAU)测定试剂盒(胶体金免疫层析法)适用范围:于体外定量测定人尿液中的尿微量白蛋白(MAU)含量。
1.1包装规格20人份/盒1.2 主要组成成分本试剂盒由MAU检测卡、干燥剂和滴管组成。
MAU检测卡由试纸条外壳与试纸条构成。
试纸条由样品垫、胶体金垫(喷有由胶体金标记的MAU单克隆抗体)、层析膜(T线包被有MAU单克隆抗体,C线包被有羊抗鼠IgG抗体)、吸水纸、衬垫构成。
检测卡为20人份/盒,干燥剂为1个/袋,滴管为20个/盒。
2.1 物理性状2.1.1 外观试剂盒各组分齐全、完整;包装袋应密封性好无破损;标签清晰;材料附着牢固,条宽应适应于卡壳且装配紧密。
2.1.2 膜条宽度膜条宽度应不低于4.0mm。
2.1.3 液体移行速度液体移行速度应不低于10mm/min。
2.2 空白检测限应小于5.0mg/L。
2.3 重复性用10mg/L尿微量白蛋白(MAU)参考品和100mg/L尿微量白蛋白(MAU)参考品各重复检测10次,其变异系数(CV)应不大于15%。
2.4 准确度将200.0mg/L尿微量白蛋白(MAU)参考品加入到尿微量白蛋白(MAU)含量5.0mg/L 正常人尿液参考品中,按照体积比1:9混合,对混合后样本进行检测,回收率应在85%~115%范围内。
2.5 线性线性范围为[5.0,200]mg/L,试剂盒的相关系数r应≥0.99。
2.6 批间差用3个批号试剂盒分别对10.0mg/L尿微量白蛋白(MAU)参考品和100.0mg/L 尿微量白蛋白(MAU)参考品各重复检测10次,则3个批号试剂盒之间的批间相对偏差(R)应不大于15%。
2.7 稳定性效期稳定性:2~30℃条件下放置有效期12个月后一个月内,检测物理性状、空白检测限、重复性、准确度、线性应符合2.1~2.6项的要求。
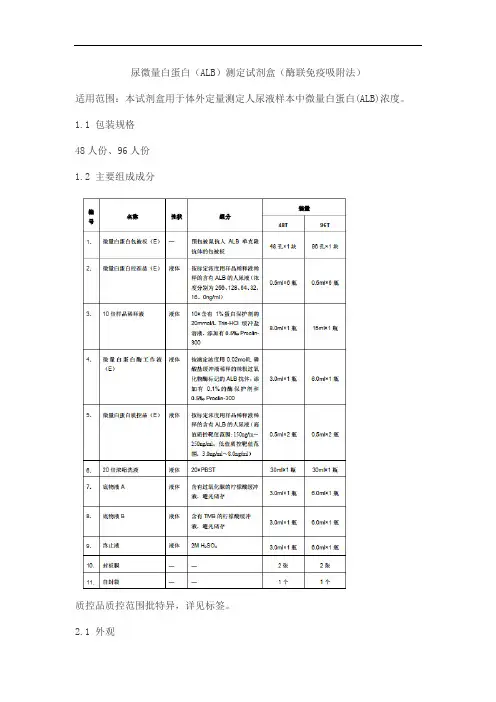
尿微量白蛋白(ALB)测定试剂盒(酶联免疫吸附法)适用范围:本试剂盒用于体外定量测定人尿液样本中微量白蛋白(ALB)浓度。
1.1 包装规格48人份、96人份1.2 主要组成成分质控品质控范围批特异,详见标签。
2.1 外观2.1.1 试剂盒各组分应齐全、完整,液体无渗漏。
2.1.2 标识应清晰,易识别。
2.2 空白检出限空白检出限浓度应不高于2.0ng/ml。
2.3 线性在[0.4,51.2]mg/L范围内线性相关系数r≥0.9900。
2.4 重复性分别用高、低浓度的样品各重复检测10次(样品浓度范围20mg/L±4.0mg/L和12.8mg/L±3.0 mg/L),其变异系数(CV)应不大于10%。
2.5 准确度用中国食品药品检定研究院的人血清白蛋白标准品(270009)对试剂盒进行测试,相对偏差(B)应不超过±20%。
2.6 分析特异性按表1所示交叉反应物规定浓度进行测定,检测结果的浓度值不得超过4.0mg/L。
表1:交叉反应物及浓度列表2.7 溯源性根据GB/T21415-2008《体外诊断医疗器械生物样品中量的测量校准品和控制物质赋值的计量学溯源性》及有关规定提供校准品的来源、赋值过程及测量不确定度等内容。
校准品溯源至企业工作校准品,并与已上市产品比对赋值。
2.8 质控品赋值有效性本试剂盒质控品的测定结果应在质控范围内。
2.9 批间差用3个批号试剂盒检测同一份样品(样品浓度范围20mg/L±4.0mg/L),则三个批号试剂盒之间的批间变异系数CV(%)应不超于15%。
2.10 稳定性将试剂盒各组分置2℃~8℃放置,有效期为12个月,效期后两个月内,检定结果应符合2.1、2.2、2.3、2.4、2.5、2.6的规定。

尿微量白蛋白检测试剂盒(胶体金免疫层析法)产品技术要求
北京库尔
尿微量白蛋白检测试剂盒(胶体金免疫层析法)
适用范围:本品为体外半定量检测人尿液样本中出现的尿微量白蛋白(MAU)的含量。
条型:1人份/袋、1人份/盒、25人份/盒、50人份/盒、100人份/盒;
25人份/筒、4筒/盒;
卡型:1人份/袋、1人份/盒、25人份/盒、50人份/盒。
2.1 物理性状
2.1.1外观
外观应整洁完整,无毛刺,无破损,无污染。
2.1.2膜条宽度
膜条宽度应不小于2.5mm。
2.1.3液体移行速度
液体移行速度应不低于10mm/min。
2.2 准确度
检测结果与相应参考溶液标示值相差同向不超过一个量级,不得出现反向相差。
阳性参考溶液不得出现阴性结果,阴性参考溶液不得出现阳性结果。
2.3 重复性
检测结果的一致性不低于90%。
2.4 检出限
用浓度为20ug/mL MAU样品检测,结果不能为阴性。
2.5 分析特异性
2.5.1与对乙酰氨基酚的交叉反应
检测浓度为20ug/mL的对乙酰氨基酚,结果均应为阴性。
2.5.2与维生素C的交叉反应
检测浓度为20ug/mL的维生素C,结果均应为阴性。
2.5.3与血红蛋白的交叉反应
检测浓度为500ug/mL的血红蛋白,结果均应为阴性。
2.6 批间差
检测结果之间相差不超过一个量级。
2.7 稳定性
2℃~30℃贮存至有效期后2个月内,对产品进行检验,应符合2.1~2.5的要求。

Human microalbunminuria(MAU/ALB) ELISA kit Catalog Number. CSB-E08970hFor the quantitative determination of human microalbunminuria(MAU/ALB) concentrations in serum, plasma, urine.This package insert must be read in its entirety before using this product.If You Have ProblemsTechnical Service Contact informationPhone: 86-27-87582341Fax: 86-27-87196150Email:****************Web: In order to obtain higher efficiency service, please ready to supply the lot numberof the kit to us (found on the outside of the box).1PRINCIPLE OF THE ASSAYThis assay employs the competitive inhibition enzyme immunoassay technique. Antibody specific for MAU has been pre-coated onto a microplate. Standards and samples are pipetted into the wells with biotin-conjugated MAU. A competitive inhibition reaction is launched between MAU (Standards or samples) and biotin-conjugated MAU with the pre-coated MAU antibody. After washing, avidin conjugated Horseradish Peroxidase (HRP) is added to the wells. Following a wash to remove any unbound reagent, a substrate solution is added to the wells and color develops in opposite to the amount of MAU bound in the initial step. The color development is stopped and the intensity of the color is measured.DETECTION RANGE0.078 µg/ml-5 µg/ml.SENSITIVITYThe minimum detectable dose of human MAU is typically less than 0.019 µg/ml. The sensitivity of this assay, or Lower Limit of Detection (LLD) was defined as the lowest human MAU concentration that could be differentiated from zero.SPECIFICITYThis assay has high sensitivity and excellent specificity for detection of human MAU. No significant cross-reactivity or interference between human MAU and analogues was observed.Note: Limited by current skills and knowledge, it is impossible for us to complete the cross-reactivity detection between human MAU and all the analogues, therefore, cross reaction may still exist.2PRECISIONIntra-assay Precision (Precision within an assay): CV%<8%Three samples of known concentration were tested twenty times on one plate to assess.Inter-assay Precision (Precision between assays):CV%<10%Three samples of known concentration were tested in twenty assays to assess.LIMITATIONS OF THE PROCEDUREFOR RESEARCH USE ONLY. NOT FOR USE IN DIAGNOSTIC PROCEDURES.The kit should not be used beyond the expiration date on the kit label.Do not mix or substitute reagents with those from other lots or sources.If samples generate values higher than the highest standard, dilute the samples with Sample Diluent and repeat the assay.Any variation in Sample Diluent, operator, pipetting technique, washing technique, incubation time or temperature, and kit age can cause variation in binding.This assay is designed to eliminate interference by soluble receptors, binding proteins, and other factors present in biological samples. Until all factors have been tested in the Immunoassay, the possibility of interference cannot be excluded.3MATERIALS PROVIDEDReagents QuantityAssay plate (12 x 8 coated Microwells) 1(96 wells) Standard (Freeze dried) 2Biotin-conjugate (100 x concentrate) 1 x 60 µlHRP-avidin (100 x concentrate) 1 x 120 µlBiotin-conjugate Diluent 1 x 10 mlHRP-avidin Diluent 1 x 20 ml Sample Diluent 2 x 20 mlWash Buffer (25 x concentrate) 1 x 20 mlTMB Substrate 1 x 10 mlStop Solution 1 x 10 ml Adhesive Strip (For 96 wells) 4Instruction manual 1STORAGEUnopenedkitStore at 2 - 8°C. Do not use the kit beyond the expiration date.Opened kitCoated assayplateMay be stored for up to 1 month at 2 - 8°C.Try to keep it in a sealed aluminum foil bag,and avoid the damp.Standard May be stored for up to 1 month at 2 - 8° C.If don’t make recent use, better keep it storeat -20°C.HRP-avidinBiotin-conjugateBiotin-conjugateDiluentMay be stored for up to 1 month at 2 - 8°C. HRP-avidinDiluentSample DiluentWash BufferTMB SubstrateStop Solution*Provided this is within the expiration date of the kit.4OTHER SUPPLIES REQUIREDMicroplate reader capable of measuring absorbance at 450 nm, with the correction wavelength set at 540 nm or 570 nm.An incubator which can provide stable incubation conditions up to 37°C±0.5°C.Squirt bottle, manifold dispenser, or automated microplate washer.Absorbent paper for blotting the microtiter plate.100ml and 500ml graduated cylinders.Deionized or distilled water.Pipettes and pipette tips.Test tubes for dilution.PRECAUTIONSThe Stop Solution provided with this kit is an acid solution. Wear eye, hand, face, and clothing protection when using this material.5SAMPLE COLLECTION AND STORAGESerum Use a serum separator tube (SST) and allow samples to clot for30 minutes before centrifugation for 15 minutes at 1000 x g, 2 - 8°C.Remove serum and assay immediately or aliquot and store samples at -20°C or -80°C. Avoid repeated freeze-thaw cycles. Centrifuge the sample again after thawing before the assay.Plasma Collect plasma using EDTA, or heparin as an anticoagulant.Centrifuge for 15 minutes at 1000 x g, 2 - 8°C within 30 minutes of collection. Assay immediately or aliquot and store samples at -20°C or -80°C. Avoid repeated freeze-thaw cycles. Centrifuge the sample again after thawing before the assay.Urine Use a sterile container to collect urine samples. Remove any particulates by centrifugation for 15 minutes at 1000xg, 2 - 8°C and assay immediately or aliquot and store samples at -20°C or -80°C. Avoid repeated freeze-thaw cycles. Centrifuge again before assaying to remove any additional precipitates that may appear after storage.SAMPLE PREPARATIONRecommend to dilute the serum or plasma samples 50000-fold before test.The suggested 50000-fold dilution can be achieved by adding 2µl sample to 398µl of normal saline. Complete the 50000-fold dilution by adding 2µl of this solution to 498µl of Sample Diluent. The recommended dilution factor is for reference only. The optimal dilution factor should be determined by users according to their particular experiments.Recommend to dilute the urine samples with Sample Diluent(1:40) before test. The suggested 40-fold dilution can be achieved by adding 6µl sample to 234µl of Sample Diluent. The recommended dilution factor is for reference only. The optimal dilution factor should be determined by users according to their particular experiments6Note:1. CUSABIO is only responsible for the kit itself, but not for the samplesconsumed during the assay. The user should calculate the possible amount of the samples used in the whole test. Please reserve sufficient samples in advance.2. Samples to be used within 5 days may be stored at 2-8°C, otherwisesamples must be stored at -20°C (≤1month) or -80°C (≤2month) to avoid loss of bioactivity and contamination.3. Grossly hemolyzed samples are not suitable for use in this assay.4. If the samples are not indicated in the manual, a preliminary experiment todetermine the validity of the kit is necessary.5. Please predict the concentration before assaying. If values for these arenot within the range of the standard curve, users must determine the optimal sample dilutions for their particular experiments.6. Tissue or cell extraction samples prepared by chemical lysis buffer maycause unexpected ELISA results due to the impacts of certain chemicals.7. Owing to the possibility of mismatching between antigen from otherresource and antibody used in our kits (e.g., antibody targets conformational epitope rather than linear epitope), some native or recombinant proteins from other manufacturers may not be recognized by our products.8. Influenced by the factors including cell viability, cell number and alsosampling time, samples from cell culture supernatant may not be detected by the kit.9. Fresh samples without long time storage are recommended for the test.Otherwise, protein degradation and denaturalization may occur in those samples and finally lead to wrong results.7REAGENT PREPARATIONNote:Kindly use graduated containers to prepare the reagent. Please don't prepare the reagent directly in the Diluent vials provided in the kit. Bring all reagents to room temperature (18-25°C) before use for 30min.Prepare fresh standard for each assay. Use within 4 hours and discard after use.Making serial dilution in the wells directly is not permitted.Please carefully reconstitute Standards according to the instruction, and avoid foaming and mix gently until the crystals have completely dissolved.To minimize imprecision caused by pipetting, use small volumes and ensure that pipettors are calibrated. It is recommended to suck more than 10µl for once pipetting.Distilled water is recommended to be used to make the preparation for reagents. Contaminated water or container for reagent preparation will influence the detection result.1. Biotin-conjugate (1x) - Centrifuge the vial before opening.Biotin-conjugate requires a 100-fold dilution. A suggested 100-fold dilution is 10 µl of Biotin-conjugate + 990 µl of Biotin-conjugate Diluent.2. HRP-avidin (1x) - Centrifuge the vial before opening.HRP-avidin requires a 100-fold dilution. A suggested 100-fold dilution is 10 µl of HRP-avidin + 990 µl of HRP-avidin Diluent.3. Wash Buffer(1x)- If crystals have formed in the concentrate, warm up toroom temperature and mix gently until the crystals have completely dissolved. Dilute 20 ml of Wash Buffer Concentrate (25 x) into deionized or distilled water to prepare 500 ml of Wash Buffer (1 x).894.StandardCentrifuge the standard vial at 6000-10000rpm for 30s.Reconstitute the Standard with 1.0 ml of Sample Diluent . Do not substitute other diluents. This reconstitution produces a stock solution of 5 µg/ml. Mix the standard to ensure complete reconstitution and allow the standard to sit for a minimum of 15 minutes with gentle agitation prior to making dilutions.Pipette 150 µl of Sample Diluent into each tube (S0-S6). Use the stock solution to produce a 2-fold dilution series (below). Mix each tube thoroughly before the next transfer. The undiluted Standard serves as the high standard (5 µg/ml). Sample Diluent serves as the zero standard (0 µg/ml).Tube S7 S6 S5S4 S3 S2 S1 S0 µg/ml52.51.250.6250.3120.1560.078ASSAY PROCEDUREBring all reagents and samples to room temperature before use. Centrifuge the sample again after thawing before the assay.It is recommended that all samples and standards be assayed in duplicate.1. Prepare all reagents, working standards, and samples as directed in theprevious sections.2. Refer to the Assay Layout Sheet to determine the number of wells to beused and put any remaining wells and the desiccant back into the pouch and seal the ziploc, store unused wells at 4°C.3. Set a Blank well without any solution.4. Add 50µl of standard and sample per well.5. Add 50µl of Biotin-conjugate(1x) to each well(not to Blank well). Coverwith a new adhesive strip. Incubate for 60 minutes at 37°C.(Biotin-conjugate(1x) may appear cloudy. Warm up to room temperature and mix gently until solution appears uniform.)6. Aspirate each well and wash, repeating the process two times for a total ofthree washes. Wash by filling each well with Wash Buffer (200µl) using a squirt bottle, multi-channel pipette, manifold dispenser, or autowasher, and let it stand for 2 minutes, complete removal of liquid at each step is essential to good performance. After the last wash, remove any remaining Wash Buffer by aspirating or decanting. Invert the plate and blot it against clean paper towels.7. Add 100µl of HRP-avidin(1x) to each well(not to Blank well). Cover themicrotiter plate with a new adhesive strip. Incubate for 60 minutes at 37°C.8. Repeat the aspiration/wash process for five times as in step 6.9. Add 90µl of TMB Substrate to each well. Incubate for 20 minutes at 37°C.Protect from light.10. Add 50µl of Stop Solution to each well, gently tap the plate to ensurethorough mixing.1011. Determine the optical density of each well within 5 minutes, using amicroplate reader set to 450 nm. If wavelength correction is available, set to 540 nm or 570 nm. Subtract readings at 540 nm or 570 nm from the readings at 450 nm. This subtraction will correct for optical imperfections in the plate. Readings made directly at 450 nm without correction may be higher and less accurate.*Samples may require dilution. Please refer to Sample Preparation section. Note:1. The final experimental results will be closely related to validity of theproducts, operation skills of the end users and the experimental environments.2. Samples or reagents addition: Please use the freshly prepared Standard.Please carefully add samples to wells and mix gently to avoid foaming. Do not touch the well wall as possible. For each step in the procedure, total dispensing time for addition of reagents or samples to the assay plate should not exceed 10 minutes. This will ensure equal elapsed time for each pipetting step, without interruption. Duplication of all standards and specimens, although not required, is recommended. To avoid cross-contamination, change pipette tips between additions of each standard level, between sample additions, and between reagent additions.Also, use separate reservoirs for each reagent.3. Incubation: To ensure accurate results, proper adhesion of plate sealersduring incubation steps is necessary. Do not allow wells to sit uncovered for extended periods between incubation steps. Once reagents have been added to the well strips, DO NOT let the strips DRY at any time during the assay. Incubation time and temperature must be observed.4. Washing: The wash procedure is critical. Complete removal of liquid ateach step is essential to good performance. After the last wash, remove any remaining Wash Solution by aspirating or decanting and remove any drop of water and fingerprint on the bottom of the plate. Insufficient washing will result in poor precision and falsely elevated absorbance reading. When using an automated plate washer, adding a 30 second soak period following the addition of wash buffer, and/or rotating the plate 180 degrees between wash steps may improve assay precision.115. Controlling of reaction time: Observe the change of color after adding TMBSubstrate (e.g. observation once every 10 minutes), TMB Substrate should change from colorless or light blue to gradations of blue. If the color is too deep, add Stop Solution in advance to avoid excessively strong reaction which will result in inaccurate absorbance reading.6. TMB Substrate is easily contaminated. TMB Substrate should remaincolorless or light blue until added to the plate. Please protect it from light.7. Stop Solution should be added to the plate in the same order as the TMBSubstrate. The color developed in the wells will turn from blue to yellow upon addition of the Stop Solution. Wells that are green in color indicate that the Stop Solution has not mixed thoroughly with the TMB Substrate.1213ASSAY PROCEDURE SUMMARY*Samples may require dilution. Please refer to Sample Preparation section.CALCULATION OF RESULTSUsing the professional soft "Curve Expert" to make a standard curve is recommended, which can be downloaded from our web.Average the duplicate readings for each standard and sample and subtract the average optical density of Blank.Create a standard curve by reducing the data using computer software capable of generating a four parameter logistic (4-PL) curve-fit. As an alternative, construct a standard curve by plotting the mean absorbance for each standard on the x-axis against the concentration on the y-axis and draw a best fit curve through the points on the graph. The data may be linearized by plotting the log of the MAU concentrations versus the log of the O.D. and the best fit line can be determined by regression analysis. This procedure will produce an adequate but less precise fit of the data.If samples have been diluted, the concentration read from the standard curve must be multiplied by the dilution factor.14人尿微量白蛋白(MAU/ALB)酶联免疫试剂盒使用说明书【产品编号】CSB-E08970h【预期应用】ELISA法定量测定人血清、血浆、尿液中MAU含量。
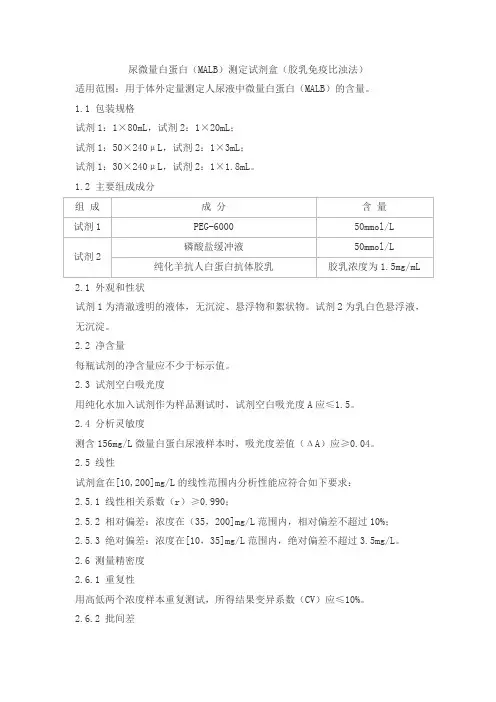
尿微量白蛋白(MALB)测定试剂盒(胶乳免疫比浊法)适用范围:用于体外定量测定人尿液中微量白蛋白(MALB)的含量。
1.1 包装规格试剂1:1×80mL,试剂2:1×20mL;试剂1:50×240μL,试剂2:1×3mL;试剂1:30×240μL,试剂2:1×1.8mL。
1.2 主要组成成分2.1 外观和性状试剂1为清澈透明的液体,无沉淀、悬浮物和絮状物。
试剂2为乳白色悬浮液,无沉淀。
2.2 净含量每瓶试剂的净含量应不少于标示值。
2.3 试剂空白吸光度用纯化水加入试剂作为样品测试时,试剂空白吸光度A应≤1.5。
2.4 分析灵敏度测含156mg/L微量白蛋白尿液样本时,吸光度差值(ΔA)应≥0.04。
2.5 线性试剂盒在[10,200]mg/L的线性范围内分析性能应符合如下要求:2.5.1 线性相关系数(r)≥0.990;2.5.2 相对偏差:浓度在(35,200]mg/L范围内,相对偏差不超过10%;2.5.3 绝对偏差:浓度在[10,35]mg/L范围内,绝对偏差不超过3.5mg/L。
2.6 测量精密度2.6.1 重复性用高低两个浓度样本重复测试,所得结果变异系数(CV)应≤10%。
2.6.2 批间差试剂盒的批间相对极差(R)应≤15.0%。
2.7 准确度在样品中加入一定量的纯品,计算回收率,应在85%~115%范围内。
2.8 稳定性2℃~8℃避光贮存,有效期为12个月,取过有效期后一个月内的试剂盒进行检测,试剂盒应仍能符合2.1、2.3、2.4、2.5、2.6.1、2.7要求。

尿微量白蛋白测定试剂盒(免疫比浊法) 适用范围:用于体外定量测定人尿液中白蛋白的含量。
1.1包装规格1)试剂1:20mL×1 、试剂2:4mL×1;2)试剂1:50mL×1、试剂2:10mL×1;3)试剂1:50mL×3 、试剂2:10mL×3;4)试剂1:50mL×4、试剂2:20mL×2;5)试剂1:65mL×3、试剂2:39mL×1;6)试剂1:70mL×3、试剂2:19mL×3;7)试剂1:100mL×2、试剂2:20mL×2;8)600测试/盒(试剂1:50mL×1、试剂2:9mL×1);9)700测试/盒(试剂1:50mL×3、试剂2:9mL×3);10)840测试/盒(试剂1:50mL×2、试剂2:10mL×2);11)900测试/盒(试剂1:25mL×2、试剂2:5mL×2);12)1050测试/盒(试剂1:72mL×3、试剂2:19mL×3);13)1200测试/盒(试剂1:71mL×2、试剂2:19mL×2);14)1260测试/盒(试剂1:49mL×3、试剂2:10mL×3);15)1300测试/盒(试剂1:50mL×3、试剂2:11mL×3);16)1640测试/盒(试剂1:64mL×3、试剂2:39mL×1);17)1680测试/盒(试剂1:99mL×2、试剂2:20mL×2);18)2400测试/盒(试剂1:71mL×4、试剂2:19mL×4);校准品:1.0mL×1(选配);质控品:1.0mL×1(选配)。
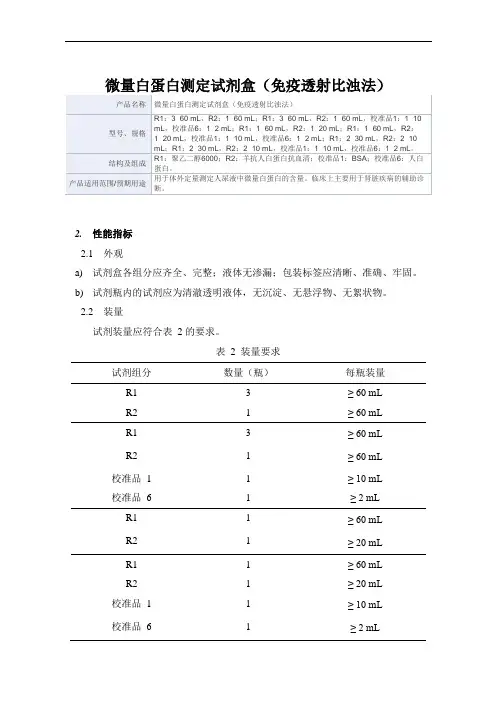
微量白蛋白测定试剂盒(免疫透射比浊法)2.性能指标2.1外观a)试剂盒各组分应齐全、完整;液体无渗漏;包装标签应清晰、准确、牢固。
b)试剂瓶内的试剂应为清澈透明液体,无沉淀、无悬浮物、无絮状物。
2.2装量试剂装量应符合表 2 的要求。
表 2 装量要求试剂组分数量(瓶)每瓶装量R1 3 ≥ 60 mLR2 1 ≥ 60 mLR1 3 ≥ 60 mLR2 1 ≥ 60 mL 校准品 1 1 ≥ 10 mL校准品 6 1 ≥ 2 mLR1 1 ≥ 60 mLR2 1 ≥ 20 mLR1 1 ≥ 60 mLR2 1 ≥ 20 mL 校准品 1 1 ≥ 10 mL校准品 6 1 ≥ 2 mLR1 2 ≥ 30 mL R2 2 ≥ 10 mL R1 2 ≥ 30 mL R2 2 ≥ 10 mL试剂组分数量(瓶)每瓶装量校准品1 1 ≥ 10 mL校准品6 1 ≥ 2 mL2.3空白限不大于0.6 mg/L。
2.4分析灵敏度试剂盒测试30 mg/L 的被测物时,其吸光度差值≥0.040 Abs。
2.5线性区间试剂盒线性在[7,1000] mg/L 区间内,应符合如下要求:a)线性相关系数(r)≥0.990;b) [7,100] mg/L 区间内,线性绝对偏差在±10 mg/L 范围内;(100,1000] mg/L 区间内,线性相对偏差在±10%范围内。
2.6精密度2.6.1重复性试剂盒测试浓度在(25±5)mg/L 和(200±40)mg/L 范围内的样本时,变异系数(CV)≤5.0%。
2.6.2批间差试剂盒测试浓度在(25±5)mg/L 和(200±40)mg/L 范围内的样本时,相对极差(R)≤10.0%。
2.7准确度测试有证参考物质或可溯源至有证参考物质的企业校准品,测定值与理论值的相对偏差应在±10.0%范围内。
2.8校准品外观a)校准品的外观应整洁,标识应清晰、准确、牢固;b)瓶内液体清澈透明,无沉淀、无悬浮物、无絮状物。
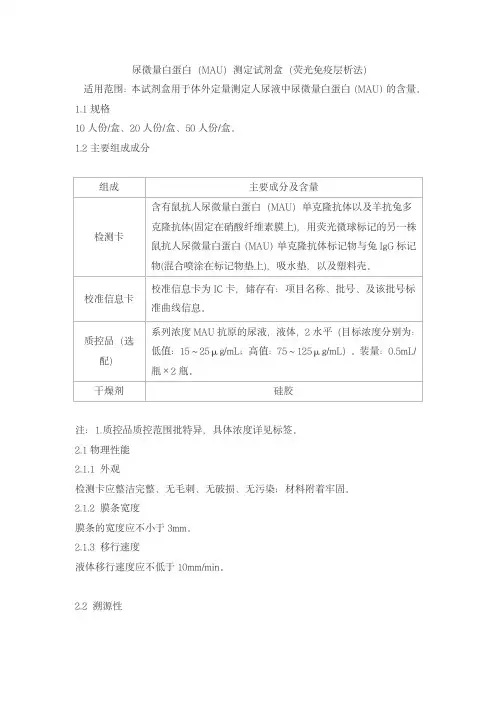
尿微量白蛋白(MAU)测定试剂盒(荧光免疫层析法)
适用范围:本试剂盒用于体外定量测定人尿液中尿微量白蛋白(MAU)的含量。
1.1规格
10人份/盒、20人份/盒、50人份/盒。
1.2主要组成成分
注:1.质控品质控范围批特异,具体浓度详见标签。
2.1物理性能
2.1.1外观
检测卡应整洁完整、无毛刺、无破损、无污染;材料附着牢固。
2.1.2膜条宽度
膜条的宽度应不小于3mm。
2.1.3移行速度
液体移行速度应不低于10mm/min。
2.2溯源性
根据GB/T21415-2008的有关规定,提供所用校准品的来源、赋值过程及测量不确定度等内容,溯源至企业工作校准品,并与已上市产品比对赋值。
2.3空白限
空白限应不高于5μg/mL。
2.4准确度
回收率应在85%~115%之间。
2.5线性
在线性范围[10,200]μg/mL内,相关系数(r)应不低于0.99。
2.6重复性
分别检测高值和低值两个样本,重复性(CV%)应不高于15.0%。
2.7批间差
在三个批次产品之间,样本测定结果的变异系数(CV%)应不高于20.0%。
2.8特异性
2.9质控品赋值有效性
测定高值、低值浓度质控品,其结果均应在质控范围内。
2.10稳定性
10℃~30℃储存(质控品2℃~8℃),有效期12个月,效期后2个月内分别检测2.3~2.6,2.8,2.9项,其结果应符合各项要求。
尿微量白蛋白测定试剂盒(免疫比浊法)适用范围:用于体外定量测定人尿液中微量白蛋白的浓度。
1.1包装规格a) 试剂1:1×40ml,试剂2:1×10ml;b) 试剂1:2×40ml,试剂2:2×10ml;c) 试剂1:4×40ml,试剂2:4×10ml;d) 试剂1:1×32ml,试剂2:1×8ml;e)试剂1:2×200ml,试剂2:2×50ml;f)试剂1:2×16ml,试剂2:2×4ml。
1.2 主要组成成分试剂1主要组成成分:Tris缓冲液(PH 6.0-9.0) 0.1mol/L 聚乙二醇6000 1.1mol/L试剂2主要组成成分:羊抗人MALB抗体适量防腐剂适量表面活性剂适量2.1 外观和性状2.1.1 试剂盒各组分应齐全、完整、液体无渗漏;外包装完好、无破损,标签完好、字迹清晰。
2.1.2 试剂1应为无色透明溶液;试剂2应为无色或乳白色溶液。
2.2 净含量应不低于试剂瓶标示装量。
2.3 试剂空白测定试剂空白吸光度,应<0.5。
2.4 分析灵敏度测试200mg/L的被测物时,吸光度变化(ΔA)应不低于0.05。
2.5 准确性测试可用于评价常规方法的国家标准参考物质,相对偏差<15%。
2.6 重复性变异系数(CV)应不超过5%。
2.7 线性2.7.1在(1,240)mg/L范围内,线性回归的确定系数应不低于0.990;2.7.2[30,240) mg/L范围内,相对偏差≤15%;(1,30)mg/L范围内,绝对偏差≤5mg/L。
2.8 批间差对同一份样品进行重复测定,相对偏差<10%2.9 稳定性该产品在2℃~8℃条件下贮存有效期为18个月,取效期末的产品进行检测,应符合2.1、2.2、2.3、2.4、2.5、2.6、2.7之规定。
尿微量白蛋白测定试剂盒(免疫比浊法)适用范围:本产品适用于体外定量测定人尿液中白蛋白(mALB)的含量。
1.1 产品规格1.2 主要组成成分注:校准品具有批间、赋值特异性,具体值详见靶值单。
2.1外观2.1.1试剂盒标签标识清晰,外包装完整无破损;2.1.2试剂1为无色澄清液体,目测不得有任何沉淀及絮状悬浮物;2.1.3试剂2为无色澄清液体,目测不得有任何沉淀及絮状悬浮物;2.1.4校准品:无色或浅黄色澄清液体,目测不得有任何沉淀及絮状悬浮物。
2.2净含量净含量不低于标示值。
2.3空白吸光度测定待检试剂在主波长340nm、副波长700nm、37℃条件下:A≤0.2。
2.4线性范围(5,280)mg/L范围内,相关系数r≥0.990;(5,30]mg/L范围内,绝对偏差不超过±3mg/L;(30,280)mg/L范围内,相对偏差不超过±10.0%。
2.5分析灵敏度在说明书规定参数设定条件下,测定浓度50mg/L的样本,△A≥0.05。
2.6 精密度2.6.1批内重复性CV≤10.0%。
2.6.2 批间差相对极差R≤10.0%。
2.7 准确度与已上市产品比对:(5,280)mg/L范围内,相关系数r≥0.990;(5,30]mg/L 范围内,绝对偏差不超过±3mg/L;(30,280)mg/L范围内,相对偏差不超过±10.0%。
2.8 校准品2.8.1 均一性:CV≤5.0%;2.8.2 开瓶稳定性:开瓶后3天,相对偏差不超过±10.0%。
2.9 稳定性未开封试剂2℃~8℃储存,可稳定12个月。
取到效期后2个月内产品进行检测, 检测结果应满足2.3、2.4、2.5、2.6.1和2.7的要求。
2.10溯源性依据GB/T 21415—2008《体外诊断医疗器械生物样品中量的测量校准品和控制物质赋值的计量学溯源性》的要求,校准品溯源至工作校准品,工作校准品经与Audit Diagnostics尿微量白蛋白测定试剂盒比对测量赋值。
本试剂盒只能用于科学研究,不得用于医学诊断人(Human)尿微量白蛋白(MAU/ALB)ELISA检测试剂盒使用说明书检测原理试剂盒采用双抗体一步夹心法酶联免疫吸附试验(ELISA)。
往预先包被尿微量白蛋白(MAU/ALB)抗体的包被微孔中,依次加入标本、标准品、HRP标记的检测抗体,经过温育并彻底洗涤。
用底物TMB显色,TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的尿微量白蛋白(MAU/ALB)呈正相关。
用酶标仪在450nm 波长下测定吸光度(OD 值),计算样品浓度。
样品收集、处理及保存方法1. 血清:使用不含热原和内毒素的试管,操作过程中避免任何细胞刺激,收集血液后,3000转离心10分钟将血清和红细胞迅速小心地分离。
2. 血浆:EDTA、柠檬酸盐或肝素抗凝。
3000转离心30分钟取上清。
3. 细胞上清液:3000转离心10分钟去除颗粒和聚合物。
4. 组织匀浆:将组织加入适量生理盐水捣碎。
3000转离心10分钟取上清。
5. 保存:如果样本收集后不及时检测,请按一次用量分装,冻存于-20℃,避免反复冻融,在室温下解冻并确保样品均匀地充分解冻。
自备物品1.酶标仪(450nm)2.高精度加样器及枪头:0.5-10uL、2-20uL、20-200uL、200-1000uL3.37℃恒温箱操作注意事项1. 试剂盒保存在2-8℃,使用前室温平衡20分钟。
从冰箱取出的浓缩洗涤液会有结晶,这属于正常现象,水浴加热使结晶完全溶解后再使用。
2. 实验中不用的板条应立即放回自封袋中,密封(低温干燥)保存。
3. 浓度为0的S0号标准品即可视为阴性对照或者空白;按照说明书操作时样本已经稀释5倍,最终结果乘以5才是样本实际浓度。
4. 严格按照说明书中标明的时间、加液量及顺序进行温育操作。
5. 所有液体组分使用前充分摇匀。
试剂盒组成名称96孔配置48孔配置备注微孔酶标板12孔×8条12孔×4条无标准品0.3mL*6管0.3mL*6管无样本稀释液6mL 3mL 无检测抗体-HRP 10mL 5mL 无20×洗涤缓冲液25mL 15mL 按说明书进行稀释底物A 6mL 3mL 无底物B 6mL 3mL 无终止液6mL 3mL 无封板膜2张2张无说明书1份1份无自封袋1个1个无注:标准品(S0-S5)浓度依次为:0、20、40、80、160、320μg/mL试剂的准备20×洗涤缓冲液的稀释:蒸馏水按1:20稀释,即1份的20×洗涤缓冲液加19份的蒸馏水。
白蛋白(ALB)测定试剂盒(溴甲酚绿法)说明书【产品名称】白蛋白(ALB)测定试剂盒(溴甲酚绿法)【包装规格】a)单一试剂:4×40mLb)单一试剂:5×60mLc)单一试剂:2×100mL【预期用途】用于体外定量测定人体血清中白蛋白的含量。
白蛋白是主要的血清蛋白,主要在肝脏合成,是衡量肝合成功能的一个重要指标。
由于白蛋白独特的分子结构使它成为多种物质如胆红素、脂肪酸、尿酸以及各种药物和抗体的运输载体,此外,白蛋白还具有维持机体渗透压的功能。
白蛋白升高主要见于脱水,降低主要见于营养不良、肝脏疾病、肾功能紊乱和风湿性关节炎等[1]。
【检验原理】在рH为4.2的缓冲液中,白蛋白分子带正电荷,与带负电荷的溴甲酚绿(BCG)生成蓝绿色复合物,在630nm处有吸收峰,复合物的吸收光与白蛋白浓度成正相关。
【主要组成成分】试剂主要组分磷酸氢二钠-柠檬酸缓冲液210mmol/L溴甲酚绿(BCG)0.25mmol/L单十二烷基九乙二醇醚适量注:不同批号试剂盒中各组分未经试验不可互换。
【储存条件及有效期】贮存于2~8℃,有效期为18个月,生产日期、有效期见标签。
【适用仪器】艾威德AS-420/AS-660/AS-1200;日立HITACHI7020型/7060型/7180型/7600型/LABOSPECT008AS型;贝克曼AU400/AU480/AU640/AU680/ AU2700/AU5400/AU5800/AU5811/AU5821;佳能TBA-FX8/TBA-120FR/ TBA-2000FR;罗氏cobas8000c702/cobas8000c701/cobas8000c502;西门子SIEMENS ADVIA1800/ADVIA2400;雅培ABBOTT ARCHITECT c8000/ARCHITECT c16000/ARCHITECT ci8200;西森美康SYSMEX BM6010/C;科华KHB卓越310/卓越330/卓越400/卓越450/ZY-1200/ZY-1280;迪瑞CS-240/CS-T300/CS-300B/CS-380/CS-400A/CS-400B/CS-600A/ CS-600B/CS-800A/CS-800B/CS-1200/CS-1200ISE/CS-1300B/CS-1400;迈瑞MINDRAY BS-220/BS-330/BS-350E/BS-380/BS-390/BS-400/BS-430/BS-600/ BS-800/BS-2000M;颐兰贝ES-200/ES-380/ES-480;赛诺迈德SUNMATIK-9050型;雷杜Chemray420;英诺华D280;特康TC6010L;锦瑞GS400;普康6066。
尿微量白蛋白(mALB)测定试剂盒(免疫比浊法)性能指标1.性能指标1.1外观外观应符合以下要求:a)试剂盒各组分应齐全、完整,液体无渗漏;包装标签文字符号清晰。
b)R1:无色澄清液体。
c)R2:无色至淡黄色透明液体,目测均不得有任何沉淀及絮状悬浮物。
d)校准品、质控品:无色至淡黄色透明液体。
1.2装量液体试剂装量要求不低于标示量。
1.3空白限空白限不高于5 mg/L。
1.4分析灵敏度测定30 mg/L样本时,吸光度差值(△A)应≥0.02。
1.5线性范围1.5.1在[5,400]mg/L范围内,线性相关系数|r|≥0.990;1.5.2在[5,30]mg/L范围内,线性绝对偏差应不超过±3mg/L;在(30,400]mg/L范围内,线性相对偏差应不超过±10%。
1.6重复性变异系数(CV)应≤5%。
1.7批间差批间相对极差R≤10%。
1.8准确度回收率应在90%~110%。
1.9分析特异性当抗坏血酸≤100mg/dL,胆红素≤40mg/dL,肌酐≤100mg/dL,葡萄糖≤800mg/dL,尿素≤400mg/dL时,对试剂检测结果的偏差影响在±10%以内。
1.10量值溯源应明确分析物的量值溯源。
1.11校准品赋值结果及其不确定度的表示方式应使用规范的表示方式,主要表示方式可选择:a)赋值结果±扩展不确定度;b)赋值结果,扩展不确定度。
1.12校准品正确度量值传递的正确度应符合E≤1。
n1.13质控品赋值准确度在用校准品校准后的生化分析仪上测试定值质控品,结果应在制造商指定的赋值范围内。
1.14校准品均匀性应不大于10%。
1.14.1瓶内均匀性:CV瓶内应不大于10%。
1.14.2瓶间均匀性:CV瓶间1.15质控品均匀性应不大于10%。
1.15.1瓶内均匀性:CV瓶内1.15.2瓶间均匀性:CV应不大于10%。
瓶间。
尿微量白蛋白(MAL)测定试剂盒 (免疫比浊法)适用范围:用于体外定量测定人体24小时尿液或随机尿液中微量白蛋白含量。
1.1 试剂盒包装规格试剂1:1×25ml,试剂2:1×5ml;试剂1:2×60ml,试剂2:2×12ml;试剂1:3×40ml,试剂2:3×8ml;试剂1:4×60ml,试剂2:4×12ml;试剂1:2×400ml,试剂2:1×160ml;试剂1:1×10L,试剂2:1×2L;试剂1:2×40ml,试剂2:2×8ml。
校准品(选配):1×1ml,1×1.5ml,1×3ml,1×5ml。
质控品(选配):1×1ml,1×1.5ml,1×3ml,1×5ml。
1.2试剂盒主要组成成分2.1 外观液体双试剂:试剂1无色澄清液体;试剂2浅橙色液体。
校准品:无色至浅黄色澄清液体。
质控品:无色至浅黄色澄清液体。
2.2 净含量液体试剂的净含量不得低于标示体积。
2.3 试剂空白吸光度在37℃、340nm波长、1cm光径条件下,试剂空白吸光度应不大于0.3。
2.4 分析灵敏度测定浓度为25mg/L样本时,吸光度变化值(ΔA)应在(0.005,0.12)范围内。
2.5 线性范围在(2,100)mg/L线性范围内,线性相关系数r不小于0.995。
在(30,100)mg/L区间内线性相对偏差应不大于±10%;(2,30]mg/L区间内线性绝对偏差应不大于±3.0mg/L。
2.6 重复性重复测试两份高低浓度的样本,所得结果的变异系数(CV%)应不大于10%。
2.7 批间差不同批号试剂测试同一份样本,测定结果的批间相对极差应不大于10%。
2.8 准确度相对偏差:相对偏差应不超过±10%。
人尿微量白蛋白(m-Alb)检测试剂盒(透射微板法)
简介:
Viberti 等人发现尿中白蛋白排泄的增加率,可以提示胰岛素依赖性糖尿病肾病发作,但此时尿中总蛋白排泄正常,尿常规蛋白定性实验为阴性,而尿白蛋白排泄增加,称之为微量白蛋白(microablumin ,m-Alb)尿。
Leagene 人尿微量白蛋白(m-Alb)检测试剂盒(透射微板法)其检测原理是尿液中的白蛋白与抗白蛋白特异抗体在液相中反应生成m-Alb 抗原抗体复合物,使反应液呈一定浊度。
本试剂盒专门用于人尿液中的微量白蛋白含量测定。
本试剂盒仅用于科研领域,不宜用于临床诊断或其他用途。
组成:
操作步骤(仅供参考):
1、 准备试剂:临用前,各种试剂均应平衡至室温。
2、 绘制标准曲线:取白蛋白标准(198mg/L),依次稀释后供标准曲线用,其浓度分别为
198mg/L 、99.0mg/L 、49.5mg/L 、19.8mg/L 、9.9mg/L 。
3、 m-Alb 测定操作:酶标仪检测,按下表依次加入试剂:
4、 充分混匀, 盖上塑料膜,37℃孵育。
5、 再次混匀,酶标仪测定波长处的吸光度。
以空白管调零,读取标准管和各待测管的最终
吸光度为A 2。
编号 名称
TC0687100T Storage
试剂(A): m-Alb buffer 10ml RT 试剂(B): 白蛋白标准(198mg/L) 0.1ml -20℃ 试剂(C): m-Alb 抗体工作液 0.15ml -20℃ 试剂(D): m-Alb 生理盐水 10ml
RT 使用说明书
1份
标准管 待测管 m-Alb buffer(μl) 200 200 白蛋白标准(μl) 20 待测样本(μl)
20
混匀,m-Alb 生理盐水调零,读取起始吸光度A 1,然后加入: m-Alb 抗体工作液(μl)
20
20
6、绘制标准曲线:以上述5种浓度的标准液,分别制作5个标准管,同上操作,测定吸
光度。
A标准=A2标准-A1标准
A标准和对应的白蛋白浓度在半对数纸上制图,绘制标准曲线。
计算:A样本=A2样本-A1样本以A样本查标准曲线,即求得尿中白蛋白浓度。
参考区间:
健康成年人尿液白蛋白:
24h尿:<30mg/ 24h。
定时尿:<20μg/min。
随意尿:<30μg/mg肌酐。
注意事项:
1、上述试剂避免反复冻融,以免失效或效率下降。
2、本法属于浊度反应,试剂中有可见混浊出现后,应弃用。
3、如果没有酶标仪,也可以使用分光光度计测定。
使用分光光度计测定蛋白浓度时,每
个试剂盒可以测定的样品数量可能会显著减少。