Acculink颈动脉支架与老保护伞
- 格式:ppt
- 大小:10.72 MB
- 文档页数:64

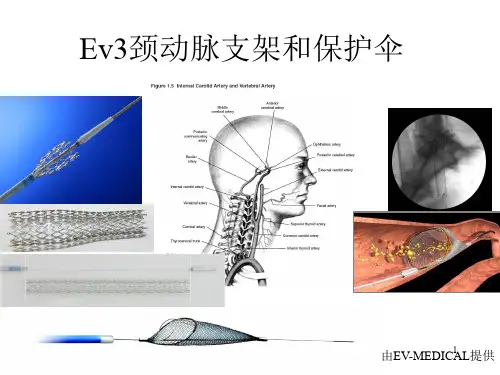




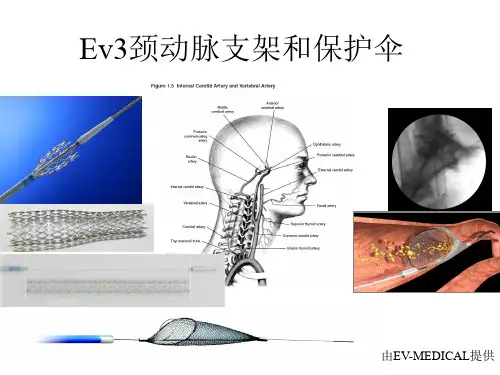
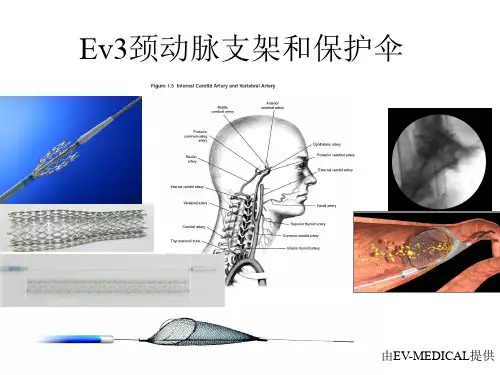



颈动脉支架的现状与展望
赵振心;刘道志;罗七一
【期刊名称】《中国医疗器械信息》
【年(卷),期】2008(014)003
【摘要】颈动脉支架术已成为治疗颈动脉狭窄疾病的主要有效手段,并得到了大规模的临床应用.本文对颈动脉支架的治疗方法、产品设计特点、研究现状及展望等方面进行了简要阐述.
【总页数】5页(P17-20,25)
【作者】赵振心;刘道志;罗七一
【作者单位】微创医疗器械(上海)有限公司,上海,201203;微创医疗器械(上海)有限公司,上海,201203;微创医疗器械(上海)有限公司,上海,201203
【正文语种】中文
【中图分类】R743
【相关文献】
1.颈动脉支架置入术治疗颈动脉狭窄的现状 [J], 张勇;潘旭东;邹英华
2.血管内支架形成术在治疗颈动脉狭窄中的应用探讨与展望 [J], 乐经科;关健伟
3.中国颈动脉支架置入术的文献评价与现状 [J], 宋刚;焦力群;凌锋;谌燕飞;王亚冰;马妍;韩涛;尹国阳
4.颈动脉支架的特点及研究现状 [J], 刘晓纬; 徐学君; 陈科宇; 叶峰; 邓平福
5.颈动脉支架置入术后脑高灌注综合征的临床研究现状及进展 [J], 姚东陂;缪中荣
因版权原因,仅展示原文概要,查看原文内容请购买。
无症状颈动脉重度狭窄患者颈动脉支架置入术后认知功能改变宁雅婵;王春梅;郭建明;郭连瑞;张建;谷涌泉【期刊名称】《中国临床医生杂志》【年(卷),期】2022(50)10【摘要】目的探讨老年无症状颈动脉狭窄患者颈动脉支架置入术(carotid artery stenting,CAS)后认知功能的变化。
方法选择2019年4—10月首都医科大学宣武医院血管外科住院治疗的老年无症状颈内动脉重度狭窄行CAS的患者25例,评估术前1周内和术后3、6、12个月的认知功能的蒙特利尔认知评估(Montreal cognitive assessment,MoCA),并对结果进行比较,同时统计患者12个月内同侧缺血性脑血管事件和再狭窄的发生率。
结果本组25例患者手术过程均顺利,4例患者术后1周复查脑MRI时发现亚临床微栓塞,1例患者术后即出现血流动力学不稳定。
在12个月的随访中,无患者出现死亡、心肌梗死、颅内出血、短暂性脑缺血发作或同侧缺血性脑卒中。
术后3、6、12个月复查血管超声未发现再狭窄发生。
与术前基线检查时相比,术后3、6、12个月时的MoCA总分、注意力和延迟回忆得分均增加(P<0.05)。
画钟表评分在6个月时趋于增加,在12个月时与基线相比显著改善(P<0.05);立方体复制评分在12个月时与术前基线相比有所改善(P<0.05);与术后3个月认知评估相比,术后6个月和12个月MoCA总分进一步有改善(P<0.05)。
12个月时执行功能(复制立方体及画钟表)较3个月时均有改善(P<0.05)。
术后12个月与术后6个月相比,MoCA总分及画钟表改善(P<0.05)。
结论颈动脉支架成形术可能改善老年无症状性颈动脉重度狭窄患者1年内认知功能。
【总页数】3页(P1211-1213)【作者】宁雅婵;王春梅;郭建明;郭连瑞;张建;谷涌泉【作者单位】首都医科大学宣武医院重症医学科;首都医科大学宣武医院血管外科【正文语种】中文【中图分类】R730.5【相关文献】1.颈动脉支架置入术对无症状性颈动脉高度狭窄患者认知功能的影响2.血管内支架置入术对无症状重度颈动脉狭窄患者认知功能的影响3.颈内动脉支架置入对重度颈动脉狭窄患者认知功能影响的临床研究4.颈动脉支架成形术对无症状重度颈动脉狭窄患者认知功能的影响5.支架置入术对重度颈动脉狭窄患者近期脑灌注、认知功能及生活质量的影响因版权原因,仅展示原文概要,查看原文内容请购买。
颈动脉支架成形术治疗症状性颈动脉狭窄患者的疗效观察何国厚;张晓东;罗国君;艾志兵;滕敬华【期刊名称】《临床神经病学杂志》【年(卷),期】2007(20)3【摘要】目的探讨应用颈动脉支架成形术(CAS)治疗症状性颈动脉狭窄患者的可行性及安全性.方法对60例症状性颈动脉狭窄患者(其中颈动脉起始段狭窄52例,颅外段狭窄8例;狭窄程度50%~70%14例,70%~90%28例,≥90%18例)予以CAS治疗,共释放64枚支架,15例应用保护伞.结果狭窄程度由术前(73.4±9.5)%降至术后(12.5±8.2)%,53例斑块消失;5例保护伞中有碎屑.所有患者临床症状在1周内消失,神经功能损害症状改善.术中及术后发生心率减慢53例,血压下降48例,血管痉挛5例,发生脑栓塞、高灌注综合征、低灌注综合征、晕厥各1例;随访3个月~2年,无1例发生短暂性脑缺血发作及脑梗死;20例复查DSA,仅1例出现轻度再狭窄.结论 CAS是治疗症状性颈动脉狭窄安全、有效的方法;严格掌握适应证和熟练操作可降低手术风险.【总页数】3页(P224-226)【作者】何国厚;张晓东;罗国君;艾志兵;滕敬华【作者单位】442000,湖北十堰,郧阳医学院附属太和医院神经内科;442000,湖北十堰,郧阳医学院附属太和医院神经内科;442000,湖北十堰,郧阳医学院附属太和医院神经内科;442000,湖北十堰,郧阳医学院附属太和医院神经内科;442000,湖北十堰,郧阳医学院附属太和医院神经内科【正文语种】中文【中图分类】R743【相关文献】1.颈动脉支架成形术治疗症状性颈动脉狭窄的疗效观察 [J], 赵伟丽;单百会;崔其福2.颈动脉支架成形术治疗症状性颈动脉狭窄的疗效观察 [J], 简崇东;黄荣珍;黄建敏;蒙兰青;李雪斌;黄瑞雅3.症状性颈动脉狭窄患者颈动脉支架成形术前后颅内血流动力学变化 [J], 杨磊;宋存峰4.颈动脉支架成形术治疗症状性颈动脉狭窄的疗效观察 [J], 赵伟丽;单百会;崔其福;5.颈动脉狭窄患者颈动脉支架成形术及诱发症状性脑梗死的危险因素分析 [J], 严澎;魏立平;李文波;李元辉;阮春云因版权原因,仅展示原文概要,查看原文内容请购买。
颈内动脉粥样硬化狭窄血管内支架置入术的疗效观察石进;吕强;张英谦;张卫清;王健;朴龙松;宋东林【期刊名称】《神经损伤与功能重建》【年(卷),期】2006(1)1【摘要】目的:观察颈内动脉血管内支架置入术治疗颈动脉粥样硬化性高度狭窄的安全性和疗效.方法:10例颈内动脉高度狭窄患者均使用保护伞装置后置入自膨式支架,2例患者进行球囊预扩张,1例在支架释放后扩张.结果:患者平均狭窄由(78.3%±9.4%)下降至(12.5%±7.2%).3例患者在术中保护伞装置中回收有黄色片状碎组织物;8例患者有不同程度的心率下降,3例进行了药物处理,其中1例有心率及血压下降持续3 d并出现脑低灌注表现.随访6个月未出现新发脑卒中和支架侧颈内动脉系统短暂性脑缺血发作,超声检查未发现明显颈内动脉支架部位再狭窄,支架形态无改变.结论:颈内动脉自膨式支架置入术治疗颈内动脉粥样硬化狭窄安全有效,但仍需随机对照研究进行长期疗效观察.【总页数】4页(P17-19,24)【作者】石进;吕强;张英谦;张卫清;王健;朴龙松;宋东林【作者单位】北京空军总医院神经内科,北京,100036;北京空军总医院神经内科,北京,100036;北京空军总医院神经内科,北京,100036;北京空军总医院神经内科,北京,100036;北京空军总医院神经内科,北京,100036;北京空军总医院导管室,北京,100036;北京空军总医院导管室,北京,100036【正文语种】中文【中图分类】R741;R743.1【相关文献】1.颈内动脉狭窄血管内支架置入术治疗脑血管病的临床分析 [J], 王梅芳;陈东明2.颈内动脉狭窄血管内支架置入术对缺血性脑血管病患者血管内皮功能的影响 [J], 石玉良;杨玉先;魏统国;曹裕民3.血管内支架置入术治疗颈内动脉狭窄疗效分析 [J], 温宏峰;李继来;杜继臣4.血管内支架置入术治疗颅外段颈动脉粥样硬化狭窄的临床疗效 [J], 张文荣; 牙韩华; 覃保华5.血管内支架置入术治疗颅外段颈动脉粥样硬化狭窄的临床疗效 [J], 张文荣;牙韩华;覃保华因版权原因,仅展示原文概要,查看原文内容请购买。