有机化学合成02第二章官能团化和官能团转换汇总
- 格式:ppt
- 大小:3.06 MB
- 文档页数:96

有机化学官能团相互转化5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O +OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O + JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi,fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2Robinson Annulation German invention, as acylating agent LDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every week HORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C PhMe CPhJOC, 1978, 43, 364.ArAr'H Br Ar C C Ar'NaOEtvia:Ar Ar'Br i. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement; no regiosiomerC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // Zn CCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl 148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAcJOC, 1978, 43, 364.HOAc/doc/e915102442.html,, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CNPPh 3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction) Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KH RO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)1. H 2S2. Pb(OAc)431. H 2S2. Pb(OAc)4OON SN N N OSN NSON ON NNN NON NO OSO ON NOO OO OTiCl 3N 2H 4。

有机化合物的合成与官能团转化在化学的广袤世界里,有机化合物的合成与官能团转化是极其重要的研究领域。
这不仅是实验室里的科学探索,更是与我们日常生活息息相关的技术应用。
有机化合物的合成,简单来说,就是通过一系列的化学反应,将相对简单的起始原料转化为具有特定结构和功能的复杂有机分子。
这个过程就像是搭积木,我们选择合适的“积木块”(起始原料),然后按照一定的规则和方法把它们拼接在一起,形成我们想要的“建筑物”(目标化合物)。
而官能团转化则是在已经存在的有机化合物分子中,对特定的官能团进行化学变化,从而改变分子的性质和功能。
官能团就像是有机分子的“功能部件”,它们决定了分子的化学性质和反应活性。
先来说说有机化合物的合成方法。
最常见的方法之一是逐步合成法,也称为线性合成。
这种方法就像是一步一个脚印,从起始原料开始,通过一系列的化学反应,逐个引入所需的官能团,逐步构建目标分子的结构。
比如,要合成一种含有苯环和羟基的化合物,我们可能先从苯开始,通过一系列的取代反应引入羟基等官能团。
另一种重要的合成方法是会聚合成法。
它有点像先分别制作好几个“模块”,然后将这些模块拼接在一起,形成最终的产物。
这种方法可以大大缩短合成路线,提高合成效率。
在合成过程中,选择合适的反应条件至关重要。
温度、压力、溶剂、催化剂等因素都会影响反应的进行和产物的生成。
比如,有些反应需要在高温高压下进行,而有些则在常温常压下就能顺利进行;有的反应在极性溶剂中效果好,有的则在非极性溶剂中表现更佳。
接下来谈谈官能团转化。
醇可以通过氧化反应转化为醛或酮,醛进一步氧化可以得到羧酸。
例如,乙醇在适当的氧化剂作用下可以被氧化为乙醛,乙醛继续氧化则能变成乙酸。
而羧酸和醇可以在一定条件下发生酯化反应,生成酯类化合物。
烯烃是一类重要的官能团,它们可以通过加成反应引入其他原子或基团。
比如,乙烯和氯化氢加成可以得到氯乙烷。
胺类官能团也有丰富的转化方式。
例如,通过酰化反应可以在胺基上引入酰基。
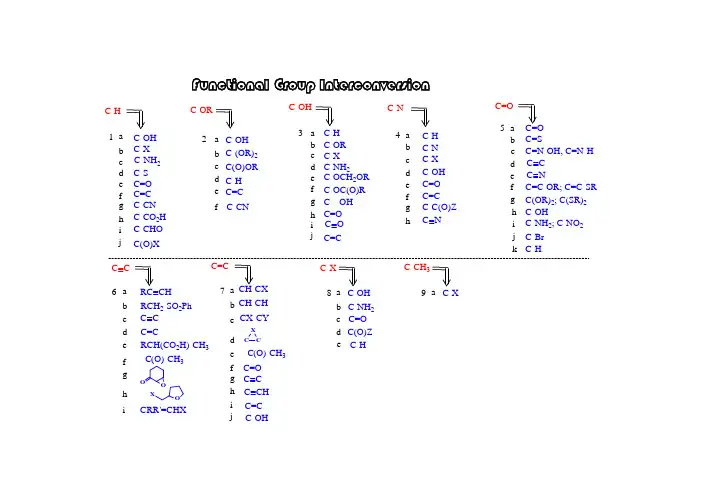


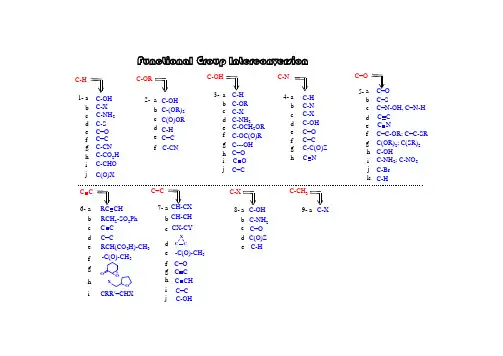
有机反应中的官能团互换有机反应中的官能团互换是一种常见的有机合成反应,通过官能团的互换,可以合成出各种功能性化合物,为有机化学领域的研究和应用提供了广阔的可能性。
官能团互换反应的研究不仅有助于人们对有机化合物结构和性质的理解,还可以为新药物、高分子材料等领域的研究提供有力支持。
一、醇的官能团互换醇是一类非常重要的官能团,它在有机化合物合成中起着至关重要的作用。
醇的官能团互换反应是有机合成中一种常见的反应类型,通过醇的官能团互换反应,可以合成出不同种类的醇类化合物。
例如,苯酚和溴代烷在氢氧化钠存在下发生互换反应,得到对溴苯醇。
二、醛和酮的官能团互换醛和酮是有机化合物中另一类常见的官能团,它们在生物体内具有重要的生物活性。
醛和酮的官能团互换反应可以通过亚硫酸氢钠等还原剂的作用来实现,产物通常是醇。
例如,苯甲醛和苯酮在硫代氢氨基的还原反应中分别得到苯甲醇和亚苯甲醇。
三、羧酸和酯的官能团互换羧酸和酯是有机化合物中另外两类常见的官能团,它们在药物合成、香料合成等方面有着广泛的应用。
羧酸和酯的官能团互换反应可以通过醇的加成反应或氢化反应来实现。
例如,乙酸和乙酰氯反应得乙酰乙酸乙酯。
四、烃和卤代烃的官能团互换烃和卤代烃是有机化合物中最基本的官能团,它们通过官能团互换反应可以得到不同种类的化合物。
例如,氯代烷和溴乙烷可以在碘乙烷的存在下通过卤代烷的置换反应得到溴代烷。
通过对有机反应中的官能团互换反应的研究,我们可以更深入地了解有机化合物的结构和性质,为有机合成领域的发展提供新的思路和方法。
官能团互换反应的应用不仅可以为很多领域的研究提供帮助,还可以为人类社会的发展和进步做出积极的贡献。


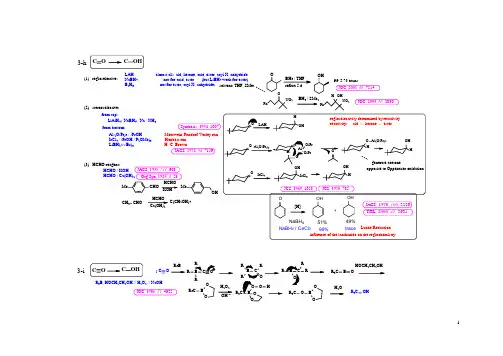
JA C S, 1972, 94, 7159.L A H ------------ alm ost all: ald, ketone, acie, ester, acyl X, anhydrideN aB H4 --------------- not for acid, ester (but L iB H4 w ork for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:L iA lH4; N aB H4; N a / N H3A l (O i Pr)3 / i PrO H ----------- M eerw ein-Pondorf-V erley rxnIrC l4 / i P rO H / P(O M e)3 ------ H enbest rxnL iB H(sec Bu)3 ------------------ H. C. B row nfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). H C H O reagent:M e C H O M eO HH C H OJA C S, 1935, 511, 903.C H3C H O C(C H2O H)42O rg.Syn, 1925, 4, 53.H C H O / K O HH C H O / C a(O H)2S ynthesis, 1994, 1007.PhN O2OPhN O2H O HB H / SM eJO C, 2001, 66, 7514.JO C, 2003, 68, 2030.OB H3 / T H F99.5 % transsolvent: T H F, S M e23-iR3B, H O C H2C H2O H // H2O2 // N aO HJO C, 1986, 51, 4925.C O RRR3BRRRRR3C B OH O C H2C H2O HR3C BOOH2O2O HR3HO BOOR3CH2OR3C O Hp ra c tic e3-k OO HHO HO HOO HO HHO HO HJO C , 1967, 32, 3452.H 2O 2: dangerous,skin w hiten, m etal decom poseH g (O A c)2: toxic, hard to rem ove (3). B 2H 6, H 2O 2 / O H -, H 2O(2). H g(O A c)2, H 2O // N aB H 4(1). H 3O +3-j3-j.13-j.2hydration:(1). K M nO 4 / N aO H (2). O sO 4(3). H 2O 2/H C O 2H (4). N a / E tO HH Hcis tran cis +trancisM e 2NNNC H 3HC lH 3N C H 3H H N C H 3CH H+NC H 3C l N H H C H OCO O HA C H 21. L A H R 3C N H 2RCN R 2R C N H R R 3CO HR 2C O H R C O H R C N H 2tertiarysecondary prim ary C om pare nom enclature class:not a very useful reactionC -NC -H C -N C -X C -O H C =OC =C 4-abc d ef g 4-aSO 2N H 2Ph IO A c O A cS O ON H S OON I P h Fe (T PP )C lS O ON H 2(insertion)T P PN NNNP h2. N aN 3N C O1. SO 2C l 2O 2CCO O h iC N C (O )X C -C (O )XN H 2H 2R C N O 2R C N H 2i ii4-b C F 3C O 3H // F e / H O A c1. m an y red u cin g ag en ts4-b.14-b.21.2.3.4.F e 3(C O )12 / C H 3O HJO C , 1972, 37, 930.N aB H 4 / P d -C N a 2S S n / H C l V o g el's 12.57V o g el's 12.58V o g el's 12.595.H 2 / P t (S )-CJA C S , 1965, 87, 2767.su lfid ed p latiu mn o t affect: aro m atic rin g s, k eto n es, h alid es, n itriles, am id e, eastersJA CS, 1933, 55, 4579.2H CH O N M e 2CO 2EtN H 2CO 2EtRC N CC NRCCRC N H 2iC N R N N+-C N R R'ii 1. H CH O / H CO 2H 1. RBCl 2 / base1. H C(O Et)3 // N aBH 4;2. R 2CO // N aBH 3CN N H 2NCH 3CH 3H CH O H CO O HN 3N HBCl 2N H 2C O O HN CO O HHCH 3H C(O Et)N aBH 4b.3 2. H CH O // H 2 / Pd-CN 3N O 2M eO 2CN aBH 4CoCl 26H 2O (cat)rtN H 2N O 2M eO 2CSynthesis, 1979,537.m ild conditionhigh yieldnot affect:: N O 2, C=C, CN , CO O R, CO O H2. N aBH 4 / CoCl 2-6H 2Ono t g o od , u su ally co n tain p o lyalk ylatio n p ro d u cts2. D elep in e3. N aN 3 / R E D4-d4-c 5. U n p o lu n g4. N aN 3 / R E D3. D elep in e2. G ab riel:1. N H 3N OK N 2H 42Oi. L A H , N aB H 4ii. H 2 / catiii Z n / H C l; A l (H g )i. M g // N H 2C lii. M g // P h S C H 2-N 3co m m ercial av ailab le, tetram er o f M e 3N24. C B r 4, P P h 3, N aN 3, D M F // P P h 3 / T H FJO C , 2000, 65, 7110.u ro tro p in e (乌洛托品)m eth en am in e (六甲烯胺)h ex am eth ylen etetram in e (环六亚甲基四胺)内服后遇酸分解出 H C H O ,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2N O SO 3HB 2H 6 / H 2N OC H 3C N / H 3O +B 2H 6 / N H 2C l C =CC -C -N H C O C H 3C =C C -C -N H 24-f4-e5. P 4S 10 // R aN i4. E t 3O + B F 4- // N aB H 43. B 2H 62. N aB H 3(O C O R )1. L iA lH 46. L aw esson's reagent // R 4-h4-g4-g.a4-g.b RC N H 2R C N H 2R 'formformA lH 3 / T H FB rCNB r N H 2JO C , 2000, 65, 8152.A lH T H FT H , 1989, 30, 5137.JO C , 1987, 52, 3901.R 'L i // N aB H 4R 'M gX // N aB H 4R 'M gX // L i/N H 3(l)R '2C uL i // N aB H 4T H , 1989, 30, 5139.JO C , 1993, 58, 4313.RCNR CN H 2R 'R 'M // H4-iN H 2ON HO C H 3O PhI(O A c)23JO C , 1993, 58, 2478.RCON H 2RCONIPhO A c RNCOR N HCOO C H 3C H 3O HPhI, O A ccPhI(O A c)4-i.2CN H 2R C H 2PhI(O A c)2 // K O H / C H 3O HC(O R)2C(S R)2hC-N H2C-N O2C NC C5-agfdcba5-C=C-O RC=C-S RC-O HC=N-O HC=N-HC=SC=OC=Ov. via: epox ysilan eR COC RR COC H2R242H3O+C O2H3OOO2-4OO3H3O+Z nT sN H N H2M eL i T M S C l M C P B A L A H24C H2ORRC H2ORRaq C H3C N/C H2CORRi. via:α-C O2Hii. via: α-haloketon eiii. via: ald ol p ro cessiv. via: thioen ol etherROC H2Rd raw back: req uire sim ple stru cture, use m an y p ow erful ag ents: M eL i, L A H, M C P B Aeij C-B rk C-Hii. M C P B Ai. h yd ro lysis 5-b5-c C =N -O HC =N -Hi. R aN i ii. T iC l 3iii. K M n O 4 / A l 2O 3H 3O+5-dH g 2+ / H 2OJO C , 1972, 37, 2138.JO C , 1970, 35, 858.H g S O 4 / H 2O / H 2O5-c.15-c.2T H L , 2001, 42, 4775.1. D IB A L / H 3O +5-eS tenphen reductionm ostly forJ.O rg.S yn , 1925, 3, 1874.2. H C l./ S nC l 2 / E t 2O 5-e.1R -CH 2-CN5-e.25-e.3-C H 2-C OHR -C H -C OH R 'R -C H -C OR "R 'R 'X / n -B uL i C H 3I R ''M gB r H 3O+3.O HO HH 3O +O HO H5-fH 3O +H g 2+ / C H 3C N (aq)C =C -O RC =C -SRO C H 3OH 3O +SC H 3OH g 2+3H 3O H g2+3H 3O+OO O SSS S SR SR O O O R O R 5-gH g 2+/ H 3O+H 3O + / solv (aq)H 3O + / solv (aq)H g 2+ / H 3O +O R O RO H O HH 3O +/ solv (aq)acid catalysta very com m on protecting group, deprotect back to ketoneHCO E t O E tO E tR M gX / H 3O +HCO E t O E tO E tR M gXRCHO。


了解有机化合物的官能团转化反应有机化合物的官能团转化反应是有机化学中的重要内容之一。
它指的是通过一系列化学反应,将有机分子中的一种官能团转变为另一种官能团的过程。
这种转化反应对于有机合成和药物研发等领域具有重要的意义。
本文将介绍一些常见的有机化合物官能团转化反应,并探讨其在实际应用中的意义。
一、醇的官能团转化反应醇是一种常见的有机化合物官能团,通过一系列反应,可以将醇转化为其他官能团,如醛、酮、酯等。
其中,醇的氧化反应是一个重要的官能团转化方法。
一种常用的氧化剂是酸性高锰酸钾溶液,它可以将主链上的一级醇氧化为醛,将主链上的二级醇氧化为酮。
此外,还可以通过醇的酯化反应将醇转化为酯,该反应通常使用酸催化剂,如硫酸。
二、醛和酮的官能团转化反应醛和酮是有机化合物中常见的官能团,它们可以通过多种反应转化为其他官能团。
例如,可以通过还原反应将醛和酮还原为相应的醇。
还原反应通常使用金属氢化物作为还原剂,如氢气和催化剂。
此外,醛和酮还可以通过羟醇化反应将其转化为醇和醚。
羟醇化反应通常使用亲核试剂,如醇和醚等。
三、酸和酸衍生物的官能团转化反应酸和酸衍生物是有机化合物中常见的官能团,它们可以通过多种反应转化为其他官能团。
例如,可以通过酸的酯化反应将酸转化为酯。
酯化反应通常使用醇和酸催化剂。
此外,酸还可以通过酸的羰基化反应或酸的卤代反应转化为醛或酰卤。
四、胺的官能团转化反应胺是有机化合物中重要的官能团,它可以通过一系列反应转化为其他官能团。
例如,可以通过胺的酰胺化反应将胺转化为酰胺。
酰胺化反应通常使用酸催化剂。
此外,胺还可以通过烷基化反应将其转化为胺的烷基衍生物。
五、烯烃的官能团转化反应烯烃是有机化合物中具有双键结构的官能团,它可以通过多种反应转化为其他官能团。
例如,可以通过烯烃的加成反应将烯烃转化为环烷烃。
加成反应通常使用亲电试剂,如酸、醛等。
此外,烯烃还可以通过烯烃的氧化反应或烯烃的卤代反应转化为相应的官能团。
总结起来,有机化合物的官能团转化反应是有机合成中的重要内容,通过这些反应,可以将有机分子中的一种官能团转变为另一种官能团。
有机合成中的新型官能团官能团转化方法有机合成中的新型官能团转化方法有机合成是化学领域中的一个重要研究方向,它涉及到合成有机化合物的方法和技术。
在有机合成中,官能团的转化是一个关键步骤,它可以将一个官能团转化成另一个特定的官能团。
随着科学技术的不断发展,越来越多的新型官能团转化方法被开发出来,本文将介绍其中一些新颖的方法。
1. 环合反应法环合反应是一种重要的有机合成方法,它能够在分子中形成环状结构。
在有机合成中,环合反应可以通过使用适当的试剂和条件,将已有的官能团转化为环状结构。
例如,氧化环合反应可以将醇转化为环醚,羧酸转化为内酯。
这些环合反应不仅可以构建多样化的化合物结构,还可以有效地提高合成反应的产率和选择性。
2. 氧化还原法氧化还原反应在有机合成中是一种常用的官能团转化方法。
通过氧化还原反应,可以在分子中引入或去除电荷,从而改变分子的性质和反应活性。
例如,通过还原醛或酮可以得到对应的醇,而通过氧化醇可以得到对应的醛或酮。
氧化还原反应在制备药物、天然产物合成等方面有着广泛的应用。
3. 反应活化法反应活化是一种通过引入特定官能团,提高官能团转化反应活性的方法。
例如,可以通过在分子中引入活化基团来加速亲核试剂的加成反应。
活化基团可以通过共轭系统、杂环结构等方式引入。
反应活化法在有机合成中具有重要的应用价值,能够提高反应速率和选择性。
4. 催化剂法催化剂的引入可以加速特定官能团转化反应的进行,同时在反应中起到节约原料、提高选择性等作用。
催化剂可以选择各种金属催化剂、酶等物质,根据反应类型的不同选择不同的催化剂。
例如,通过金属催化剂,在羰基化合物中引入卤素原子,从而实现烯醇和烯醇醚的高效官能团转化。
5. 等离子体法等离子体是一种高能粒子形式,在有机合成中可以用于官能团转化反应。
通过等离子体法,可以在分子中产生超过10000K的高温,实现官能团转化反应。
等离子体法广泛应用于化学合成、光化学反应等领域,可以有效地实现官能团转化反应。
5-fH 3O +Hg 2+ / CH 3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O +OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2Robinson Annulation German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:Me CPhJOC, 1978, 43, 364.ArAr'H Br Ar C C Ar'NaOEtvia:Ar Ar'Br i. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAcJOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CNPPh 3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)1. H 2S2. Pb(OAc)431. H 2S2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNNCH3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)SN SF O OOO Chem. Rev., 1996, 96, 1737.2FCH 3SO O OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 22. HOAc / i PrOH$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SFO OF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N 33ONXR 1R 3OOR 2HMg(ClO 4)2R 1R 3O OR 2XNBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 Valready industrilized NF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.9-a9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem. Rev., 1996, 4, 47.CH 2Cl 2bridgehead methylationB 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.RCO NH 2RCO NIPh OAcRN C OR NH COOCH 3CH 3OHPhI, OAcRPhI(OAc)4-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2h C-NH 2C-NO 2C N C C 5-ag f d c b a 5-C=C-OR C=C-SR C-OH C=N-OH C=N-H C=S C=O C=Ov. via: epoxysilaneRCO CRRCO CH 2R42SOCl H 3O +23OO2-CrO 4OONaBH 3H 3O +3ZnTsNHNH 2MeLiTMSCl MCPBA LAH324CH 2CORRCH 2CORRMsClKOtBuHgCl 3SSCH 2CORRPhCHOi. via: α-CO 2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherRCO CH 2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAe i j C-Br k C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-CN5-e.25-e.3-CH 2-C OHR -CH -C OH R'R -CH -C O R"R'R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 46. Lawesson's reagent // RaNi4-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.RCO NH 2RCO NIPh OAcRN C OR NH COOCH 3CH 3OHPhI, OAcRPhI(OAc)4-i.2C NH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2h C-NH 2C-NO 2C N C C 5-ag f d c b a 5-C=C-OR C=C-SR C-OH C=N-OH C=N-H C=S C=O C=Ov. via: epoxysilaneRCO CRRCO CH 2R42SOCl H 3O +23OO2-CrO 4OONaBH 3H 3O +3ZnTsNHNH 2MeLiTMSCl MCPBA LAH324CH 2CORRCH 2CORRMsClKOtBuHgCl 3SSCH 2CORRPhCHOi. via: α-CO 2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherRCO CH 2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAe i j C-Br k C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-CN5-e.25-e.3-CH 2-C OHR -CH -C OH R'R -CH -C O R"R'R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH3CN (aq)C=C-OR C=C-SROCH 3OH 3O+SCH 3OHg 2+3OH 3O Hg 2+3H 3O+OO O SSS SSRSR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRMgXRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3OHPDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBr DMSO / AgBF 4- Me 2SBull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2CC R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)O(MVK)CH 3CH CH 2Robinson Annulation German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)use: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:MeCPhJOC, 1978, 43, 364.ArAr'HBr Ar C C Ar'NaOEtvia:Ar Ar'Bri. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?heatRCH=CH 2:PBu RCH 2CH 2-O-PBu 3RCH 2CH 2-OHPh-Se-PBu 3Ph-Se-CNmechanism:MCPBA OAc CO 2MeOAcMeO MeO 2CNO 2SeNOAcCO 2MeOAc MeOMeO 2Cminorapplied for reactions: without rearrangement;no regiosiomerCC (CO 2H)2 / benzeneOH PhPhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CS OR ORC C BrOAcZn / HOAcOAcO AcOAcOBrOAcZn OOAc OAcOAcJOC, 1978, 43, 364.HOAc, 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionNa R C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R 2R 1R 2(EtO)3Puse: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CNPPh 3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:Zn-CuP(OEt)1. H 2S2. Pb(OAc)431. H 2S2. Pb(OAc)4OON SN N N OSN NSON ON NNSN NON NO OSO ON NOO OO OTiCl 3N 2H 4g7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNNCH3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH OH CH I PI 38-a.12. F 3S-NEt 21.(DAST)SN SF O OOO Chem. Rev., 1996, 96, 1737.2FCH 3SO O OH ONCHCl 2CH 3 1. CF 3CHFCF 2NEt 22. HOAc / i PrOH$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OH1. AgNO 3/KOH 2R Br Ber. 1942, 75, 296.8-d.2ClOClRhCl(PPh 3)38-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39, 437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SFO OF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N 33OO N XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 Valready industrilized NF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.9-a9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem. Rev., 1996, 4, 47.CH 2Cl 2bridgehead methylation。