Organic Syntheses, Coll. Vol. 3, p.846 (1955); Vol. 24, p.94 (1944).
- 格式:pdf
- 大小:109.36 KB
- 文档页数:2

Organic Syntheses, Coll. Vol. 4, p.755 (1963); Vol. 33, p.68 (1953).4-PENTYN-1-OLSubmitted by E. R. H. Jones, Geoffrey Eglinton, and M. C. Whiting 1.Checked by Arthur C. Cope and Ronald M. Pike. 1. ProcedureCaution! This preparation should be conducted in a hood to avoid exposure to ammonia .A solution of sodium amide in liquid ammonia is prepared according to a procedure previously described (Note 1) in a 3-l. three-necked round-bottomed flask equipped with a cold-finger condenser (cooled with Dry Ice) attached through a soda-lime tower to a gas-absorption trap,2 a mercury-sealed stirrer, and an inlet tube. Anhydrous liquid ammonia (1 l.) is introduced from a commercial cylinder through the inlet tube, and 1 g. of hydrated ferric nitrate is added, followed by 80.5 g. (3.5 g. atoms) of clean, freshly cut sodium (Note 1) and (Note 2). The inlet tube is replaced with a 250-ml. dropping funnel, and the mixture is stirred until all the sodium is converted into sodium amide , after which 120.5 g. (1 mole) of tetrahydrofurfuryl chloride 3 (Note 3) is added over a period of 25 to 30 minutes. The mixture is stirred for an additional period of 1 hour, after which 177 g. (3.3 moles) of solid ammonium chloride is added in portions at a rate that permits control of the exothermic reaction. The flask is allowed to stand overnight in the hood while the ammonia evaporates. The residue is extracted thoroughly with ten 250-ml. portions of ether , which are decanted through a Büchner funnel (Note 4). The ether is distilled, and the residue is fractionated at a reflux ratio of about 5 to 1, through a column containing a 20-cm. section packed with glass helices yielding 63–71 g. (75–85%) of 4-pentyn-1-ol , b.p. 70–71° /29 mm., n D1.4443 (Note 5).2. Notes1. Procedures for converting sodium to sodium amide are given on p. 763 and in a previous volume.42. More liquid ammonia should be added through the inlet tube if vaporization reduces the liquid volume to less than 750 ml.3. Freshly distilled tetrahydrofurfuryl alcohol should be used in the preparation of tetrahydrofurfuryl chloride according to the procedure of Organic Syntheses.34. Ether extraction of the solid must be thorough or the yield will be reduced. A large Soxhlet extractor may be used if desired.5. Others have reported b.p. 154–155°, n D 1.4432;5 b.p. 154–155°, n D 1.4450.6 A sample purified through the silver derivative had b.p. 77° /37 mm., n D 1.4464. The α-naphthylurethan of 4-pentyn-1-ol crystallized as needles from 60–80° petroleum ether; m.p. 79–80°. 3. Discussion4-Pentyn-1-ol has been prepared from 4-penten-1-ol 3 by bromination followed by dehydrobromination with alkali;6 by the reaction of 3-bromodihydropyran or 3,4-dihydro-2H-pyran with n -butylsodium , n -butyllithium , or n -butylpotassium ;5,7 by the reaction of dihydropyran or 2-methylenetetrahydrofuran with n -amylsodium or n -butyllithium ;7 by the reduction of ethyl 4-pentynoate with lithium aluminum hydride ;8and by the method used in this preparation.9251922.515References and Notes1.Victoria University of Manchester, Manchester, England.. Syntheses Coll. Vol.2, 4 (1943).. Syntheses Coll. Vol.3, 698 (1955).. Syntheses Coll. Vol.3, 219 (1955).5.Paul and Tchelitcheff, Compt. rend., 230, 1473 (1950); Paul, Angew. Chem., 63, 304 (1951);Paul, Bull. soc. chim. France, 18, 109 (1951).6.Lespieau, Compt. rend., 194, 287 (1932).7.Paul and Tchelitcheff, Bull. soc. chim. France, 19, 808 (1952).8.Colonge and Gelin, Bull. soc. chim. France, 1954, 799.9.Eglinton, Jones, and Whiting, J. Chem. Soc., 1952, 2873.AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)petroleum etherα-naphthylurethan of 4-pentyn-1-olammonia (7664-41-7)ether (60-29-7)ammonium chloride (12125-02-9)sodium (13966-32-0)tetrahydrofurfuryl alcohol (97-99-4)sodium amide (7782-92-5)n-butyllithium (109-72-8)ferric nitratelithium aluminum hydride (16853-85-3)dihydropyran2-methylenetetrahydrofurann-amylsodium4-Penten-1-ol (821-09-0)Tetrahydrofurfuryl chloride(3003-84-7)4-Pentyn-1-ol (5390-04-5)3-bromodihydropyran3,4-dihydro-2H-pyran (110-87-2)ethyl 4-pentynoate (63093-41-4)n-butylsodiumn-butylpotassium Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
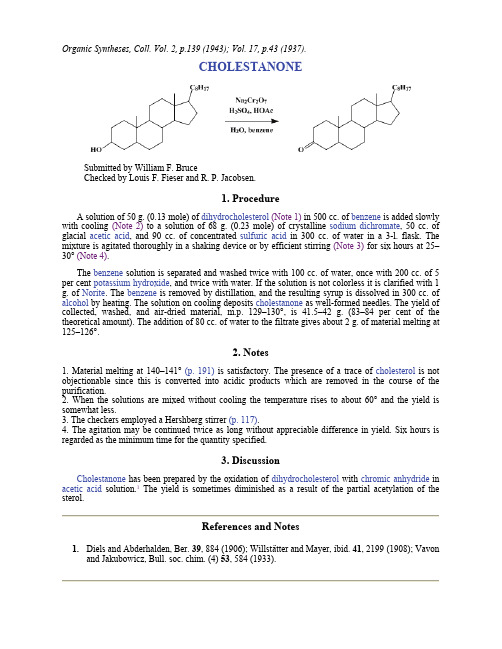
Organic Syntheses, Coll. Vol. 2, p.139 (1943); Vol. 17, p.43 (1937).CHOLESTANONESubmitted by William F. BruceChecked by Louis F. Fieser and R. P. Jacobsen.1. ProcedureA solution of 50 g. (0.13 mole) of dihydrocholesterol(Note 1) in 500 cc. of benzene is added slowly with cooling (Note 2) to a solution of 68 g. (0.23 mole) of crystalline sodium dichromate, 50 cc. of glacial acetic acid, and 90 cc. of concentrated sulfuric acid in 300 cc. of water in a 3-l. flask. The mixture is agitated thoroughly in a shaking device or by efficient stirring (Note 3) for six hours at 25–30° (Note 4).The benzene solution is separated and washed twice with 100 cc. of water, once with 200 cc. of 5 per cent potassium hydroxide, and twice with water. If the solution is not colorless it is clarified with 1 g. of Norite. The benzene is removed by distillation, and the resulting syrup is dissolved in 300 cc. of alcohol by heating. The solution on cooling deposits cholestanone as well-formed needles. The yield of collected, washed, and air-dried material, m.p. 129–130°, is 41.5–42 g. (83–84 per cent of the theoretical amount). The addition of 80 cc. of water to the filtrate gives about 2 g. of material melting at 125–126°.2. Notes1. Material melting at 140–141° (p. 191) is satisfactory. The presence of a trace of cholesterol is not objectionable since this is converted into acidic products which are removed in the course of the purification.2. When the solutions are mixed without cooling the temperature rises to about 60° and the yield is somewhat less.3. The checkers employed a Hershberg stirrer (p. 117).4. The agitation may be continued twice as long without appreciable difference in yield. Six hours is regarded as the minimum time for the quantity specified.3. DiscussionCholestanone has been prepared by the oxidation of dihydrocholesterol with chromic anhydride in acetic acid solution.1 The yield is sometimes diminished as a result of the partial acetylation of the sterol.References and Notes1.Diels and Abderhalden, Ber. 39, 884 (1906); Willstätter and Mayer, ibid. 41, 2199 (1908); Vavonand Jakubowicz, Bull. soc. chim. (4) 53, 584 (1933).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)alcohol (64-17-5)sulfuric acid (7664-93-9)acetic acid (64-19-7)Benzene (71-43-2)Norite (7782-42-5)potassium hydroxide (1310-58-3)sodium dichromate (7789-12-0)chromic anhydrideCholestanone (566-88-1)Dihydrocholesterol (80-97-7)Cholesterol (57-88-5)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。

经典化学合成反应标准操作醛酮的经典合成目录1.前言 (4)2.由醇合成醛酮 (4)2.1铬(VI)试剂 (4)2.1.1 Jones氧化(Cr2O3/H2SO4/acetone) (4)2.1.2 Collins氧化(Cr2O3.2Py) (5)2.1.3 PCC(Pyrindium Chlorochromate)氧化 (8)2.1.4 PDC(Pyrindium Dichromate)氧化 (9)2.2 用活性MnO2氧化 (10)2.2.1 用活性MnO2氧化示例一: (10)2.3用DMSO氧化 (11)2.3.1 DMSO-(COCl)2氧化(Swern Oxidation) (11)2.3.2 DMSO-SO3-Pyridine (12)2.4 用氧铵盐氧化 (13)2.4.1 用氧铵盐氧化示例: (13)2.5 用高价碘试剂氧化 (14)2.5 .1 Dess-Martin氧化反应示例: (14)2.5.2 IBX氧化反应示例: (15)2.6 亚硝酸钠和醋酐氧化 (15)2.6.1 亚硝酸钠和醋酐氧化示例 (15)2.6 TPAP-NMO 氧化 (16)2.6.1 TPAP-NMO 氧化示例 (16)2.7 1,2-二醇的氧化 (16)2.7.1 1,2-二醇的氧化示例一: (17)2.7.1 其他1,2-二醇的氧化相关文献: (18)3.由卤化物合成醛酮 (18)3.1 由伯卤甲基和仲卤甲基的氧化合成醛酮 (18)3.1.1 用DMSO氧化(Kornblum反应) (18)3.1.2用硝基化合物氧化(Hass反应) (20)3.1.3用乌洛托品氧化(Sommelet反应) (21)3.1.4用对亚硝基二甲苯胺氧化吡啶翁盐氧化(Kröhnke反应) (22)3.1.5用胺氧化物氧化 (22)3.2 由二卤甲基或二卤亚甲基合成醛酮 (23)3.2.1 由二卤甲基合成醛反应示例: (23)3.3 由有机金属化合物的酰化合成醛酮 (24)3.3.1 由有机金属化合物的酰化合成醛酮示例 (25)3.4 由Pd催化反应合成醛 (25)4.由活泼甲基或活泼亚甲基烷烃合成醛酮 (25)4.1 用SeO2氧化合成醛酮 (26)4.1.1 用SeO2氧化合成醛酮示例 (26)4.2用空气氧化合成酮 (26)4.2.1用空气氧化合成酮反应示例: (27)4.3 用铬酸氧化合成酮 (27)4.3.1 用铬酸氧化合成酮示例 (27)4.4用高锰酸盐氧化合成酮 (29)4.5 用醌氧化合成酮 (29)5.由羧酸及其衍生物合成醛酮 (30)5.1由羧酸合成醛 (30)5.1.1用金属氢化物还原 (30)5.1.2由脱CO2合成醛 (31)5.1.3由羧酸合成酮 (31)5.2由酰氯及酸酐合成醛酮 (33)5.2.1用Rosenmund法合成 (33)5.2.2用金属氢化物还原 (34)5.3由酯及内酯合成醛 (35)5.3.1 酯通过DIBAL还原为醛示例: (36)5.4由酰胺合成醛酮 (36)5.4.1 由酰胺合成醛酮 (37)5.4.2 McFadyen-Stevens Reaction (38)5.5由酯或酰氯经Weinreb酰胺合成醛酮 (39)5.5.1 由Weinreb酰胺还原合成醛反应示例一 (40)5.5.2由Weinreb酰胺还原合成酮反应示例: (41)5.6由氰合成醛酮 (41)5.6.1DIBAL 还原腈到醛示例(最重要的方法) (42)5.6.2Li(EtO)3AlH 还原腈到醛示例(较重要的方法) (43)5.6.3Ranney Ni 加氢还原氰到合成醛示例 (43)5.6.4有机金属试剂对腈加成合成酮示例 (44)6. 由烯烃、芳环合成醛酮 (46)6.1 由烯烃臭氧氧化合成醛 (46)6.2 烯烃用OsO4/NaIO4氧化合成醛 (47)6.3 烯烃经由有机硼化合物中间体的烯烃甲酰化合成醛 (47)6.5 由烯烃的甲酰化合成醛 (48)6.5.1 Vilsmeyer反应 (48)6.5.2 Duff’s 甲酰化 (51)6.5.3 Reimer-Tiemann 甲酰化 (52)6.5.4 Gattermann甲酰化 (53)6.5.5 多聚甲醛/甲醇镁苯酚甲酰化 (53)6.5.6氯化锡/多聚甲醛苯酚甲酰化 (54)6.5.7重氮化后甲酰化 (54)6.6烯烃经加成-氧化反应合成酮 (56)6.6.1 烯烃经加成-氧化反应合成酮示例 (56)7. 由炔烃合成醛酮 (57)7.1 由加成-氧化反应合成醛酮 (57)7.2 由氧化反应合成酮 (57)7.3 由加成-水解反应合成酮 (58)7.4 由加成-还原反应合成酮 (59)7.5 由加成-烷基化,酰化等反应合成酮 (59)8. 由醚及环氧化合物合成醛酮 (59)8.1 Claisen重排 (59)8.2酸催化下环氧化物重排 (61)8.2.1 酸催化下环氧化物重排合成醛酮示例一 (61)8.3氧化法 (61)8.4 水解法缩醛或酮合成醛酮 (61)9. 由胺合成醛 (62)9.1胺的氧化 (62)9.1.1 胺的氧化合成醛反应示例: (63)9.2 由胺经由西佛碱的方法 (64)9.2.1 由胺经由西佛碱合成醛示例 (64)9.3 自苯胺衍生物合成 (64)10. 由硝基化合物合成醛酮 (64)11. 由Friedel-Crafts反应合成芳基酮 (65)11.1 由Friedel-Crafts反应合成芳基酮示例 (68)12. Dieckmann 缩合脱酸 (69)13. 由合成子合成醛酮 (71)14. 由砜合成醛酮 (71)15. Michael 反应和类似反应(Addition, Condensation) (71)1.前言醛和酮是一类重要的有机化合物,其合成在有机合成中占有非常重要的地位。

Organic Syntheses, Coll. Vol. 4, p.840 (1963); Vol. 36, p.79 (1956).SEBACOIN[Cyclodecanone, 2-hydroxy-]Submitted by Norman L. Allinger1Checked by N. J. Leonard, J. C. Little, and F. H. Owens.1. ProcedureThe apparatus2 consists of a 3-l. three-necked round-bottomed creased flask, with standard ball joints and an indented cone-shaped bottom (Note 1), which is heated by means of an electric mantle and is equipped with a high-speed stirrer of stainless steel driven by a 10,000 r.p.m. motor (Note 2). One side neck is fitted with a bulb-type air-cooled condenser (Note 3), on top of which fits a 1-l. pressure-equalizing Hershberg dropping funnel (Note 4). The top of the dropping funnel is connected in turn to a U-tube containing a 1-cm. head of mercury. The entire apparatus is securely fastened to a sturdy support.In the flask is placed 900 ml. of xylene(Note 5), and a slow stream of purified nitrogen(Note 6) is passed through the system from which the dropping funnel has been temporarily removed. The stirrer is run at slow speed, and the solvent is brought to a gentle reflux. The air stream cooling the condenser is shut off, the mercury valve is disconnected from the condenser, and a few milliliters of solvent is allowed to distil out the top of the condenser (Note 7). The dropping funnel (Note 8), containing a solution of 115 g. (0.50 mole) of dimethyl sebacate(Note 9) and 500 ml. of xylene(Note 5) is then inserted between the top of the condenser and the mercury valve. The air to the condenser is then turned on, and the electric mantle is turned off. The solvent is allowed to cool below its boiling point, and the stirrer is gradually brought to a stop. Throughout these operations the nitrogen flow is adjusted to keep air out of the system. Through a side neck is then added 50.6 g. (2.20 g. atoms) of crust-free sodium metal cut into lumps of convenient size. The side neck is closed, and the stirrer and the heater are turned on. The sodium is dispersed by stirring at about 6000–8000 r.p.m. for 10 minutes, and, with continued heating and stirring at a rate somewhat slower (to give suitable mixing), the dropwise addition of the ester solution is begun at such a rate as to be complete in about 24 hours (Note 10) and (Note 11).Heating and stirring are continued for 1 hour after the addition is completed. The stirrer is then slowed, heating is stopped, the heater is removed, and the reaction flask is allowed to cool for about 15 minutes (Note 12). The reaction flask is then cooled in a water bath, and is finally thoroughly cooled in an ice bath. A solution of 140 ml. of glacial acetic acid in an equal volume of xylene is then added dropwise during about 30 minutes with continued cooling and stirring (Note 13). After addition of 450 ml. of water, stirring is stopped, the nitrogen is turned off, and the flask is disconnected from the apparatus. The two-phase mixture is filtered through a large Büchner funnel with suction to remove a small amount of gum, and the filtrate is then poured into a 3-l. separatory funnel. The aqueous phase is drawn off, extracted with 100 ml. of xylene, and is discarded. The xylene phases are washed in series with 100 ml. of water, and are combined and dried with 10 g. of anhydrous magnesium sulfate. The solution is filtered into a 3-l. round-bottomed flask, and the bulk of the xylene is distilled with the aid ofan aspirator (Note 14) and (Note 15). The residue is transferred to a smaller flask and is distilled through a 2-ft. Vigreux column, the fraction boiling at 134–138°/14 mm. or 124–128°/9 mm. being collected as a yellowish liquid weighing 57–63 g. (67–74%). This material solidifies on standing and is sufficiently pure for most purposes (Note 16) and (Note 18). For further purification it may be crystallized from 150 ml. of pentane by cooling to −10° in an ice-salt bath for several hours. The mixture is filtered, and the crystals are washed with 50 ml. of pentane which has been cooled to −80°. The pure product thus obtained is a white granular crystalline solid, m.p. 38–39°, weighing 53–56 g. (63–66%) (Note 17) and (Note 18).2. Notes1. A flask having this shape gives the most efficient mixing.22. A one-fourth-horsepower motor is adequate. A suitable motor is manufactured by Bodine Electric Company, Chicago, Illinois.3. The use of a water-cooled glass condenser is not recommended since it might accidentally be broken and thereby cause water to flow into the flask. A metal water-cooled condenser has also been used and is satisfactory.4. Adapted from that described in Org. Syntheses, Coll. Vol. 2, 129 (1943).5. The xylene used was purified by heating under reflux with sodium overnight and then distilling, b.p. 137–142°.6. Linde high-purity dry nitrogen was used without further treatment.7. The fumes may be taken off by attaching an aspirator. This procedure assures removal of all moisture from the system.8. The funnel is dried before use with a flame and is then closed with a drying tube and allowed to cool.9. The ester used was Eastman Kodak Company technical grade shaken with sodium carbonate, dried and distilled. The ester boils at 158–160°/11 mm.10. The reaction time can be lengthened considerably without effect. If, however, the time is shortened appreciably, the yield may be markedly lowered.11. Initially the reaction may take on various colors, red, purple, etc., but after a short time a dull gray-brown color appears, which is gradually replaced by a yellow-brown or olive-drab color.12. It is important that the reaction mixture be kept out of contact with the air until it has been acidified.13. When sufficient acetic acid has been added, the dark color of the reaction mixture is replaced by a white color, and the mixture is often quite thick. More acetic acid is not harmful.14. Nitrogen is led through the capillary during the distillations.15. The xylene thus recovered is purified (Note 5) and is used in the next preparation.16. This material slowly decomposes upon standing. It may be stored for at least several months with only slight decomposition if it is kept under nitrogen in the dark and at 0°. The compound appears to be stable when pure.17. Homologs having a ring containing 10 to 18 carbons have been prepared in an analogous manner in yields from 46 to 85%.318. This preparation has also been carried out on a 1.0 mole scale by the checkers, using a 5-l. creased flask. Comparable yields are obtainable.3. DiscussionSebacoin has been prepared by the cyclization of methyl or ethyl sebacate with sodium metal.3,4,5,6,7,8 This preparation is referenced from:z Org. Syn. Coll. Vol. 4, 216z Org. Syn. Coll. Vol. 4, 218z Org. Syn. Coll. Vol. 4, 838z Org. Syn. Coll. Vol. 5, 277References and Notes1.University of California, Los Angeles, California.2.Morton and Redman, Ind. Eng. Chem., 40, 1190 (1948).3.Stoll and Rouvé, Helv. Chim. Acta, 30, 1822 (1947).4.Prelog, Frenkiel, Kobelt, and Barman, Helv. Chim. Acta, 30, 1741 (1947).5.Stoll and Hulstkamp, Helv. Chim. Acta, 30, 1815 (1947).6.Hansley (to E. I. du Pont de Nemours and Company), U. S. pat. 2,228,268 [C. A., 35, 2534(1941)].7.Blomquist, Burge, and Sucsy, J. Am. Chem. Soc., 74, 3636 (1952).8.Prelog, Schenker, and Günthard, Helv. Chim. Acta, 35, 1598 (1952).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)Sebacoinmethyl or ethyl sebacateacetic acid (64-19-7)sodium carbonate (497-19-8)nitrogen (7727-37-9)sodium (13966-32-0)xylene (106-42-3)Pentane (109-66-0)magnesium sulfate (7487-88-9)Cyclodecanone, 2-hydroxy- (96-00-4)dimethyl sebacate (106-79-6)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
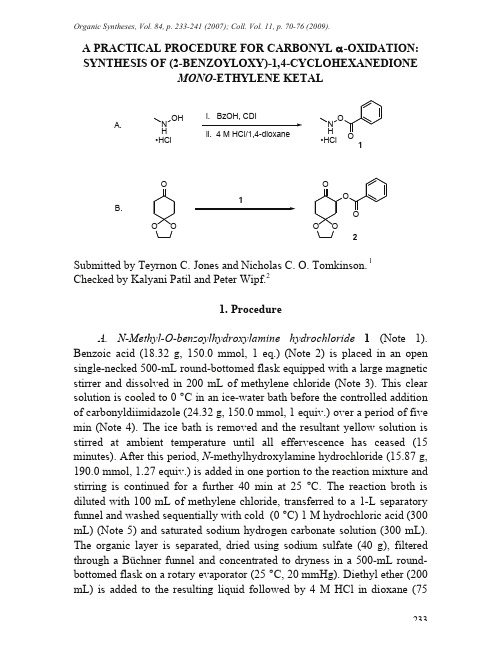
Organic Syntheses, Vol. 84, p. 233-241 (2007); Coll. Vol. 11, p. 70-76 (2009).233A PRACTICAL PROCEDURE FOR CARBONYL -OXIDATION: SYNTHESIS OF (2-BENZOYLOXY)-1,4-CYCLOHEXANEDIONEMONO -ETHYLENE KETALN H OH N H•HCl i. BzOH, CDI ii. 4 M HCl/1,4-dioxaneA.112B.•HClSubmitted by Teyrnon C. Jones and Nicholas C. O. Tomkinson. 1Checked by Kalyani Patil and Peter Wipf.21. ProcedureA. N-Methy -O-benzoy hydroxy amine hydroch oride 1 (Note 1). Benzoic acid (18.32 g, 150.0 mmol, 1 eq.) (Note 2) is placed in an open single-necked 500-mL round-bottomed flask equipped with a large magnetic stirrer and dissolved in 200 mL of methylene chloride (Note 3). This clear solution is cooled to 0 °C in an ice-water bath before the controlled addition of carbonyldiimidazole (24.32 g, 150.0 mmol, 1 equiv.) over a period of five min (Note 4). The ice bath is removed and the resultant yellow solution is stirred at amb ient temperature until all effervescence has ceased (15 minutes). After this period, N -methylhydroxylamine hydrochloride (15.87 g, 190.0 mmol, 1.27 equiv.) is added in one portion to the reaction mixture and stirring is continued for a further 40 min at 25 °C. The reaction b roth is diluted with 100 mL of methylene chloride, transferred to a 1-L separatory funnel and washed sequentially with cold (0 °C) 1 M hydrochloric acid (300 mL) (Note 5) and saturated sodium hydrogen carbonate solution (300 mL). The organic layer is separated, dried using sodium sulfate (40 g), filtered through a Büchner funnel and concentrated to dryness in a 500-mL round-bottomed flask on a rotary evaporator (25 °C, 20 mmHg). Diethyl ether (200 mL) is added to the resulting liquid followed b y 4 M HCl in dioxane (75mL, 0.30 mol, 2 equiv.) (Note 6). After 30 minutes, the solid precipitate is collected in a Büchner funnel (Note 7), washed with 100 mL of ice-cold diethyl ether and dried under high vacuum (0.5 mmHg) for 8 h to give N-methyl-O-benzoylhydroxylamine hydrochloride 1 as a white microcrystalline solid (17.45 g, 62%) (Note 8) that is used in Step B without further purification (Note 9).B.(2-Benzoyloxy)-1,4-cyclohexanedione (mono)ethylene ketal 2. An open 25-mL single-necked round-bottomed flask(Note 10) equipped with a magnetic stirring bar is charged at ambient temperature with 1,4-cyclohexanedione mono-ethylene ketal (4.68 g, 30.0 mmol, 1 equiv.) (Note 11) and dimethyl sulfoxide (7.5 mL). N-Methyl-O-benzoyl-hydroxylamine hydrochloride 1 (5.63 g, 30.0 mmol, 1 equiv., as prepared above) is added to the flask over a period of 15 min (Note 12) and the reaction mixture is stirred for 1 h (Note 13). The solution is subsequently poured into 300 mL of ethyl acetate and transferred to a 1-L separatory funnel where it is washed with 250 mL of water. The layers are separated and retained: the organic layer is washed further with two 250 mL portionsof water, which are discarded; and the original aqueous layer is washed with two 100 mL portions of ethyl acetate which are added to the original organic layer. The combined organic fractions are dried using magnesium sulfate (25 g), filtered through a Büchner funnel and concentrated on a rotary evaporator (40 °C, 20 mmHg) to give a brown solid as a crude product (Note 14).The crude solid is transferred to a 25-mL Erlenmeyer flask and dissolved in 5 mL of hot (80 °C) isopropyl alcohol,then allowed to slowly cool to room temperature. The resultant solid is collected in a Büchner funnel and sequentially washed with ice-cold isopropyl alcohol (50 mL) and ice-cold 35-60 petroleum ether(50 mL) (Note 11) before being dried under high vacuum (0.5 mmHg) for 8 h to yield (2-benzoyloxy)-1,4-cyclohexanedione mono-ethylene ketal 2 (Note 15) as pale yellow needles (6.38 g, 77%) (Note 16).2. Notes1. P rocedure A is a modification of the method of Geffken: Geffken, D. Chem. Ber. 1986, 119, 744.2. Benzoic acid (99+%), carbonyldiimidazole, N-methylhydroxylamine hydrochloride (98%), and 4 M hydrochloric acid in 1,4-dioxane were obtained from Aldrich Chemical Company, Inc. 234M ethylene chloride and diethyl ether were obtained from Fisher Scientific Ltd. Submitters purchased benzoic acid (98%) from Avocado Research Chemicals Ltd. and carbonyldiimidazole (97%) from Lancaster Synthesis Ltd. All chemicals were used as received.3. There was no need to oven-dry the vessels used in the reaction, and all steps were carried out in flasks open to the atmosphere.4. The reaction produced carbon dioxide gas, resulting in vigorous effervescence.5. The hydrochloric acid was prepared by dilution of 12 M hydrochloric acid purchased from EMD Chemicals Inc.6. The submitters generated hydrochloride acid gas from a slow addition of sulfuric acid to ammonium chloride and bubbled the gas directly into the diethyl ether solution.7. Filtration should be carried out in a fume hood as the mother liquor contains dissolved hydrogen chloride.8. The checkers performed the reaction also at 50%, 25%, and 10% scale. The yields ranged from 58-62%.9. Physical properties and spectral data for 1 are as follows: mp 135–137 °C; IR (nujol) 2923, 1770, 1599, 1475, 1260 cm-1; 1H NMR (300 MHz, DMSO-d6) : 2.90 (s, 3 H), 7.57 (t, J = 7.8 Hz, 2 H), 7.72 (t, J = 7.5 Hz, 1 H), 7.95 (d, J = 7.2 Hz, 2 H), 11.38 (brs, 2 H); 13C NMR (75 MHz, DMSO-d6) : 36.7, 126.4, 129.6, 129.8, 135.1, 163.7; M S (EI) m/z (rel intensity) 151 ([M-HCl]+, 11), 136 (25), 123 (24), 122 (87), 107 (8), 106 (70), 105 (56); HRM S (EI) calculated for C8H9NO2 151.0633, found 151.0631; Anal. calcd. for C8H10NO2Cl C, 51.2, H, 5.4, N, 7.5, Cl, 18.9; found C, 51.3, H, 5.5, N, 7.5, Cl, 18.9.10. There was no need to oven-dry the reaction flask, and the reaction was carried out open to the atmosphere.11. 1,4-Cyclohexanedione mono-ethylene ketal (97%) was obtained from Aldrich Chemical Company, Inc. Dimethyl sulfoxide (99.9%) was obtained from Fisher Scientific Ltd. Isopropyl alcohol and 35-60 petroleum ether was obtained from J. T. Baker Chemical Co. Submitters used dimethyl sulfoxide (99%) purchased from Aldrich Chemical Company, Inc., and, isopropyl alcohol and 40-60 petroleum ether purchased from Fisher Scientific Ltd. All chemicals were used as received.12. It was noted that this reaction was exothermic. Measuring of the internal reaction temperature with a thermometer showed that during the addition of 1 the temperature rose to a maximum of 70 °C.23513. The progress of the reaction was monitored by TLC analysis on EMD Silicagel 60 F254 0.25 mm silica plates, using 50% ethyl acetate/35-60 petroleum ether as eluent and visualising with potassium permanganate solution. The ketone starting material had an R f= 0.37 and the oxidized product had an R f= 0.51.14. Occasionally, the crude product was obtained as a brown oil. In these cases the oil may solidify on standing or can be induced to crystallize by the addition of a small amount of 35-60 petroleum ether, which was subsequently removed by rotary evaporation.15. The checkers performed the reaction also at 50%, 25%, and 10% scale. The yields ranged from 78-81%.16. Physical properties and spectral data for 2 are as follows: mp 114-116 °C; IR (dichloromethane) 2964, 1720, 1451, 1274, 1111, 1043 cm-1; 1H NMR (500 MHz, CDCl) : 2.01–2.12 (m, 2 H), 2.27 (t, J = 12.8 Hz, 13H), 2.45–2.51 (m, 2 H), 2.80 (dt, J = 14.2, 6.6 Hz, 1 H), 4.04–4.13 (m, 4 H),5.68 (q, J =6.6 Hz, 1 H),7.44 (t, J = 7.7 Hz, 2 H), 7.57 (t, J = 7.5 Hz, 1 H),8.08 (d, J = 7.8 Hz, 2 H); 13C NMR (75 MHz, CDCl3) : 34.5, 35.8, 40.3, 64.9, 65.0, 73.6, 107.3, 128.4, 129.5, 129.8, 133.2, 165.3, 203.3; MS (EI) m/z (rel intensity) 276 (M+, 16), 219 (25), 171 (22), 155 (23), 126 (16), 122 (20), 115 (12), 105 (100); HRMS (EI) calculated for C15H16O5 276.0997, found 276.1000; Anal. calcd. for C15H16O5 C, 65.2, H, 5.8; found C, 65.1, H, 5.8.3. Discussion-Oxyacylated carbonyl compounds are important functional groups present in many natural products, pharmaceuticals and synthetic intermediates of broad utility. Shi and coworkers have reported the catalytic asymmetric introduction of the oxybenzoyl group - to ketone carbonyl groups by the low temperature epoxidation of preformed enol esters followed by rearrangement under acidic conditions, which furnishes products in good yields and excellent enantioselectivity.3 The preparation of -acetoxy ketones catalyzed by iodobenzene via a hypervalent iodine intermediate has also been described.4 Despite the effectiveness of this method, it has not been extended to aldehyde substrates and is only effective for the introduction of acetoxy groups. Discrimination between primary and non-sterically encumbered secondary carbon centers is also poor using this method.236The procedure described above provides a very practical alternative for the -acyloxylation of carbonyl compounds. The reagents are bench stable and react under mild conditions with both aldehydes and ketones in good yields. Not only do these reagents avoid air-sensitive intermediates and therefore obviate the need for purified solvents and specialized equipment; but the reagents display a remarkable functional group tolerance and useful chemo- and regioselectivity.N-Methyl-O-benzoylhydroxylamine hydrochloride 1 is a general reagent for the oxygenation of both aldehydes and ketones.5 The related N-tert-butyl-O-benzoylhydroxylamine hydrochloride 3 allows the chemoselective -oxybenzoylation of aldehyde substrates in good to excellent yield, since it is completely inert to ketones.6 Both classes of reagents can be used for the introduction of the oxyacyl group of choice by simple modification of the carboxylic acid used in the reagent synthesis. Representative examples for the -functionalization of aldehydes and ketones by N-alkyl-O-acylhydroxylamine hydrochlorides are given in Tables1 and 2.237238-Oxybenzoylation of Aldehydes and Ketones usingN -Methyl-O -Benzoylhydroxylamine Hydrochloride (1)2Et NNSubstrate Product Yield (%)Substrate Product 69a90b75a75a 74a 82c2Entry12345678910Entry Yield (%)a DMSO, rt, 4-24 h, 1 equiv. 1; b DMSO, 50 °C, 24 h, 1 equiv. 1; c DMSO, 50 °C, 48 h, 2 equiv. 174a OBz73b 81b85cN H •HCl 4N H •HCl 5N H •HCl 6H •HCl3-Acyloxylaton of Carbonyl Compounds using N-Alkyl-O-Acyl Hydroxylamine Hydrochlorides (3-6)Substrate Product Yield (%) Entry Reagent1 2 3 4 53330aNone456672a 82a 79a 67b69a THF/H2O (9:1), 50 °C, 24 h;b DMSO, rt, 24 h1.School of Chemistry, Cardiff University, Main Building, Park Place,Cardiff, CF10 3AT, UK; tomkinsonnc@.2.Department of Chemistry, University of Pittsburgh, Pittsburgh, PA15260, USA; pwipf@.3. (a) Zhu, Y.; Shu, L.; Tu, Y.; Shi, Y. J. Org. Chem.2001, 66, 1818; (b)Feng, X.; Shu, L.; Shi, Y. J. Org. Chem.2002, 67, 2831.4. Ochiai, M.; Takeuchi, Y.; Katayama, T.; Sueda, T.; Miyamoto, K. J.Am. Chem. Soc. 2005, 127, 12244.5. Beshara, C. S.; Hall, A.; Jenkins, R. L.; Jones, K. L.; Jones, T. C.;Killeen, N. M.; Taylor, P. H.; Thomas, S. P.; Tomkinson, N. C. O. Org.Lett.2005, 7, 5729.6. Beshara, C. S.; Hall, A.; Jenkins, R. L.; Jones, T. C.; Parry, R. T.;Thomas, S. P.; Tomkinson, N. C. O. Chem. Commun. 2005, 1478239AppendixChemical Abstracts Nomenclature; (Registry Number) Benzoic acid; (65-85-0)Carbonyldiimidazole: 1H-Imidazole, 1,1'-carbonylbis-; (530-62-1)N-Methylhydroxylamine hydrochloride: Methanamine, N-hydroxy-, hydrochloride; (4229-44-1)N-Methyl-O-benzoylhydroxylamine hydrochloride: Methanamine, N-(benzoyloxy)-, hydrochloride; (27130-46-7)1,4-cyclohexanedione mono-ethylene ketal: 1,4-Dioxaspiro[4.5]decan-8-one; (4746-97-8)(2-Benzoyloxy)-1,4-cyclohexanedione (mono)ethylene ketal: 1,4-Dioxaspiro[4.5]decan-8-one, 7-(benzoyloxy)-; (872312-37-3)Nick Tomkinson was born in St Andrews, Scotland in 1969.He studied Chemistry at The University of Sheffield andreceived his BSc in 1992. His Ph.D. studies were under thesupervision of Dr. D. Neville Jones and Professor JimAnderson investigating asymmetric synthesis with unsaturatedsulfur compounds. Postdoctoral studies on Nuclear Receptorswere undertaken with Dr. Tim Willson at GlaxoSmithKline,Research Triangle Park, North Carolina (1996-1998). Nickwas appointed to the staff at Cardiff University in 1999 and in2004 was awarded an EPSRC Advanced Research Fellowship.Teyrnon Jones was born in 1978 and raised in Anglesey, NorthWales. He received his M Chem from The University ofSheffield in 2000, and following completion of hisundergraduate studies chose to remain at Sheffield to researchnew applications of germanium-linked solid-phase organicsynthesis in the laboratory of Dr. Alan Spivey. Subsequent tothe award of his Ph.D. in 2004, an interest in metal-freesynthesis and organocatalysis lead to a successful two-yearpostdoctoral collaboration with Dr. Nick Tomkinson at CardiffUniversity developing new practical methods for carbonyl -oxygenation. He is currently senior scientist in M edicinalChemistry at AstraZeneca, Loughborough UK, working in therespiratory and inflammation therapeutic area.240241Kalyani Patil obtained her B.Sc. (1998) in Chemistry from the University of Mumbai, India. She completed her M.Sc. in 2000 from the Indian Institute of Te chnology, Bombay unde r the guidance of Profe ssor Sujata Bhat. He r M.Sc. the sis involve d the synthe sis of die none s for applications in the synthe sis of bioactive mole cule s. She the n move d to North Dakota State Unive rsity to pursue he r Ph.D. unde r the supe rvision of Professor Mukund Sibi, where her research was focused on the synthe sis of -amino acids via fre e radical che mistry. Afte r comple tion of he r Ph.D. in 2005 she joine d the group of Profe ssor Pe te r Wipf at the Unive rsity of Pittsburgh as a postdoctoral research associate and is currently working on the synthesis of the tetracyclic core of viridin.。
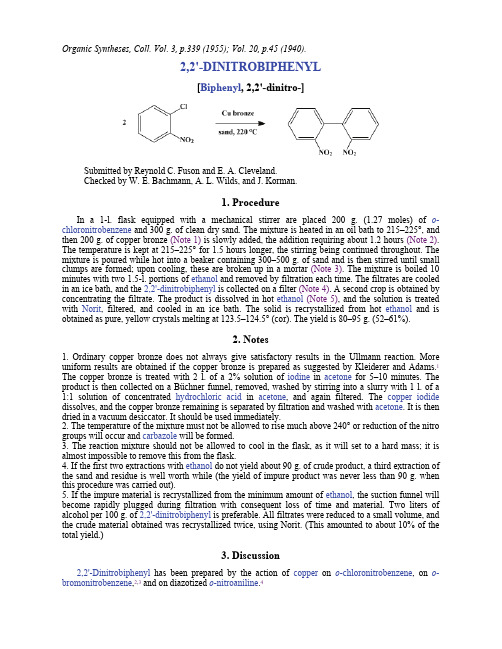
Organic Syntheses, Coll. Vol. 3, p.339 (1955); Vol. 20, p.45 (1940).2,2'-DINITROBIPHENYL[Biphenyl, 2,2'-dinitro-]Submitted by Reynold C. Fuson and E. A. Cleveland.Checked by W. E. Bachmann, A. L. Wilds, and J. Korman.1. ProcedureIn a 1-l. flask equipped with a mechanical stirrer are placed 200 g. (1.27 moles) of o-chloronitrobenzene and 300 g. of clean dry sand. The mixture is heated in an oil bath to 215–225°, and then 200 g. of copper bronze (Note 1) is slowly added, the addition requiring about 1.2 hours (Note 2). The temperature is kept at 215–225° for 1.5 hours longer, the stirring being continued throughout. The mixture is poured while hot into a beaker containing 300–500 g. of sand and is then stirred until small clumps are formed; upon cooling, these are broken up in a mortar (Note 3). The mixture is boiled 10 minutes with two 1.5-l. portions of ethanol and removed by filtration each time. The filtrates are cooled in an ice bath, and the 2,2'-dinitrobiphenyl is collected on a filter (Note 4). A second crop is obtained by concentrating the filtrate. The product is dissolved in hot ethanol(Note 5), and the solution is treated with Norit, filtered, and cooled in an ice bath. The solid is recrystallized from hot ethanol and is obtained as pure, yellow crystals melting at 123.5–124.5° (cor). The yield is 80–95 g. (52–61%).2. Notes1. Ordinary copper bronze does not always give satisfactory results in the Ullmann reaction. More uniform results are obtained if the copper bronze is prepared as suggested by Kleiderer and Adams.1 The copper bronze is treated with 2 l. of a 2% solution of iodine in acetone for 5–10 minutes. The product is then collected on a Büchner funnel, removed, washed by stirring into a slurry with 1 l. of a 1:1 solution of concentrated hydrochloric acid in acetone, and again filtered. The copper iodide dissolves, and the copper bronze remaining is separated by filtration and washed with acetone. It is then dried in a vacuum desiccator. It should be used immediately.2. The temperature of the mixture must not be allowed to rise much above 240° or reduction of the nitro groups will occur and carbazole will be formed.3. The reaction mixture should not be allowed to cool in the flask, as it will set to a hard mass; it is almost impossible to remove this from the flask.4. If the first two extractions with ethanol do not yield about 90 g. of crude product, a third extraction of the sand and residue is well worth while (the yield of impure product was never less than 90 g. when this procedure was carried out).5. If the impure material is recrystallized from the minimum amount of ethanol, the suction funnel will become rapidly plugged during filtration with consequent loss of time and material. Two liters of alcohol per 100 g. of 2,2'-dinitrobiphenyl is preferable. All filtrates were reduced to a small volume, and the crude material obtained was recrystallized twice, using Norit. (This amounted to about 10% of the total yield.)3. Discussion2,2'-Dinitrobiphenyl has been prepared by the action of copper on o-chloronitrobenzene, on o-bromonitrobenzene,2,3 and on diazotized o-nitroaniline.4References and Notes1.Kleiderer and Adams, J. Am. Chem. Soc., 55, 4225 (1933).2.Ullman and Bielecki, Ber., 34, 2174 (1901).3.Mascarelli and Gatti, Gazz. chim. ital., 67, 807 (1937)4.Niementowski, Ber., 34, 3325 (1901).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)copper bronzeethanol (64-17-5)hydrochloric acid (7647-01-0)copper (7440-50-8)iodine (7553-56-2)acetone (67-64-1)Norit (7782-42-5)Biphenyl (92-52-4)o-bromonitrobenzene (577-19-5)o-chloronitrobenzene (88-73-3)carbazole (86-74-8)copper iodide (7681-65-4)o-NITROANILINE (88-74-4)2,2'-DINITROBIPHENYL (2436-96-6)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 6, p.967 (1988); Vol. 51, p.53 (1971).AZIRIDINES FROM β-IODOCARBAMATES: 1,2,3,4-TETRAHYDRONAPHTHALENE(1,2)IMINE[1H -Naphth[1,2-b ]azirine, 1a,2,3,7b-tetrahydro-]Submitted by C. H. Heathcock 1 and A. Hassner 2.Checked by William G. Kenyon and Richard E. Benson. 1. ProcedureA 500-ml., round-bottomed flask equipped with a reflux condenser is charged with a solution of 25 g. of potassium hydroxide in 250 ml. of 95% ethanol , to which is added 16.6 g. (0.0498 mole) of methyl (trans -2-iodo-1-tetralin)carbamate (Note 1). The resulting mixture is heated under reflux on a stream bath for 2 hours, cooled, and added to 500 ml. of water. The clear, yellow solution is shaken three times with 100-ml. portions of diethyl ether . The ether layers are combined, washed three times with 125-ml. portions of water and once with 125 ml. of a saturated sodium chloride , dried over 5 g. of anhydrous potassium carbonate , and filtered. The ether is removed by distillation on a steam bath, giving the crude imine as a yellow-brown oil (Note 2). The oil is transferred to a small flask, the container is rinsed with ether , and the rinse is added to the distillation flask. The product is collected by distillation through a small Vigreux column with warm water circulating through the condenser to prevent crystallization of the product. The fraction boiling at 80–82° (0.15–0.25 mm.) is collected as a solid that forms in the receiver, yielding 4.9–5.1 g. (68–70%) of the imine, m.p. 54–56° (Note 2); the IR spectrum has a band at 3205 cm.−1 (NH) (Note 3).2. Notes1. The methylcarbamate may be prepared by the procedure in Org. Synth., Coll. Vol. 6, 795 (1988).2. The submitters state that product, m.p. 49–51°, can be obtained by direct crystallization of the oil. The oil from a run conducted on a scale twice that described above is cooled to −15° and 30 ml. of pentane is added. Upon scratching the flask, the product crystallizes, is collected by filtration, and washed with a little cold pentane , yielding 9–10 g. (62–69%), m.p. 49–51°.3. The 1H NMR spectrum (CCl 4) shows a broad singlet centered at δ 0.7 (1H) and complex multiplets at 1.1–3.05 (6H) and 6.76–7.30 (4H).3. DiscussionThe procedure reported here, that of Hassner and Heathcock,3 is more convenient than the Wenker synthesis of aziridines 4 and appears to be more general.5 It represents a simple route from olefins to aziridines (via β-iodocarbamates).3,5,6 Aziridines are also useful as intermediates in the synthesis of amino alcohols and heterocyclic systems.5,7,8,9This preparation is referenced from:z Org. Syn. Coll. Vol. 6, 795 References and Notes1.Department of Chemistry, University of California, Berkeley, California 94720.2.Present address: Department of Chemistry, State University of New York, Binghamton, NewYork 13901.3. A. Hassner and C. Heathcock, Tetrahedron, 20, 1037 (1964).4.O. E. Paris and P. E. Fanta, J. Am. Chem. Soc., 74, 3007 (1952).5. A. Hassner and C. Heathcock, J. Org. Chem., 30, 1748 (1965).6.G. Drefahl and K. Ponsold, Chem. Ber., 93, 519 (1960).7.H. W. Heine, Angew. Chem., 74, 772 (1962) [Angew. Chem. Int. Ed. Engl., 1, 528 (1962)].8. A. Hassner, M. E. Lorber, and C. Heathcock, J. Org. Chem., 32, 540 (1967).9.L. A. Paquette and D. E. Kuhla, Tetrahedron Lett., 4517 (1967).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)β-IODOCARBAMATESethanol (64-17-5)potassium carbonate (584-08-7)ether,diethyl ether (60-29-7)sodium chloride (7647-14-5)potassium hydroxide (1310-58-3)Pentane (109-66-0)methylcarbamate1,2,3,4-Tetrahydronaphthalene(1,2)imine,1H-Naphth[1,2-b]azirine, 1a,2,3,7b-tetrahydro- (1196-87-8)Methyl (trans-2-iodo-1-tetralin)carbamate (1210-13-5)Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 10, p.107; Vol. 78, p.82EFFICIENT SYNTHESIS OF HALOMETHYL-2,2'-BIPYRIDINES:4,4'-BIS(CHLOROMETHYL)-2,2'-BIPYRIDINE[ 2,2'-Bipyridine, 4,4'-bis(chloromethyl)- ]Submitted by Adam P. Smith, Jaydeep J. S. Lamba, and Cassandra L. Fraser 1 .Checked by Motoki Yamane and Koichi Narasaka. 1. ProcedureA. 4,4'-Bis[(trimethylsilyl)methyl]-2,2'-bipyridine . A 500-mL, two-necked, round-bottomed flask (Note 1), equipped with a nitrogen inlet, magnetic stirrer, and rubber septum is charged with tetrahydrofuran (THF) (90 mL) (Note 2) and diisopropylamine (9.8 mL, 69.7 mmol) (Note 3). The reaction mixture is cooled to −78°C and a solution of butyllithium (n-BuLi) (1.7 M in hexanes, 36.0 mL, 61.4 mmol) (Note 4) is added. The solution is stirred at −78°C for 10 min, warmed to 0°C and stirred for 10 min, then cooled back to −78°C. A solution of 4,4'-dimethyl-2,2'-bipyridine (5.14 g, 27.9 mmol) (Note 5) in THF (130 mL) (Note 2), prepared in a 250-mL, two-necked, round-bottomed flask under a nitrogen atmosphere, is added via cannula to the cold lithium diisopropylamide (LDA) solution. The resulting maroon-black reaction mixture is stirred at −78°C for 1 hr, then chlorotrimethylsilane (TMSCl) (8.85 mL, 69.7 mmol) (Note 6) is rapidly added via syringe. After the solution becomes pale blue-green (≈10 sec after the TMSCl addition), the reaction is quenched by rapid addition of absolute ethanol (10 mL). (Note: the reaction should be quenched regardless of color change after a maximum of 15 seconds to avoid over silylation). The cold reaction mixture is poured into a separatory funnel (1 L) containing aqueous saturated sodium bicarbonate (NaHCO 3, ≈200 mL) and allowed to warm to ≈25°C. The product is extracted with dichloromethane (CH 2Cl 2, 3 × 300 mL); the combined organic fractions are shaken with brine (≈200 mL) and dried over sodium sulfate (Na 2SO 4). Filtration and concentration on a rotary evaporator affords 8.85 g (97%) of 4,4'-bis[(trimethylsilyl)methyl]-2,2'-bipyridine as a slightly off-white crystalline solid (Note 7).B. 4,4'-Bis(chloromethyl)-2,2'-bipyridine . Into a 500-mL, two-necked, round-bottomed flask (Note 1) equipped with a magnetic stirring bar are placed 5.22 g (15.9 mmol) of 4,4'-bis[(trimethylsilyl)methyl]-2,2'-bipyridine , 15.1 g (63.6 mmol) of hexachloroethane (Cl 3CCCl 3, Note 8) and 9.65 g (63.6 mmol) of cesium fluoride (CsF, Note 9) at 25°C under a nitrogen atmosphere. Acetonitrile (260 mL) (Note 10) is added and the heterogeneous reaction mixture is stirred at 60°C for ≈3.5 hr (or until TLC indicates that all TMS starting material is consumed). After the mixture is cooled to 25°C, it is poured into a separatory funnel containing ethyl acetate (EtOAc) and water (H 2O, ≈100 mL each). The product is extracted with EtOAc (3 × 100 mL); the combined organic fractions are shaken with brine (≈200 mL) and dried over Na 2SO 4. Filtration and concentration on a rotary evaporator, followed by flashchromatography using deactivated silica gel (60% EtOAc: 40% hexanes)(Note 11), gives 3.67 g (91%) of the chloride as a white solid (Note 12).2. Notes1. Before use, all glassware, needles, and syringes were dried overnight in a 120°C oven.2. THF was dried and purified by passage through alumina solvent purification columns 2 or by distillation over sodium /benzophenone .3. Diisopropylamine was purchased from Aldrich Chemical Company, Inc. , and distilled over calcium hydride (CaH 2) prior to use.4. A 1.7 M solution of n-BuLi in hexanes was obtained from Aldrich Chemical Company, Inc. The n-BuLi is titrated prior to its use in each reaction using the following procedure.3 To a 50-mL, round-bottomed flask (Note 1), equipped with nitrogen inlet and a magnetic stirrer is added N-benzylbenzamide (854 mg, 4.0 mmol) (as received from Aldrich Chemical Company, Inc.) and THF (40 mL) (Note 2). The solution is cooled to −42°C (acetonitrile /dry ice) and n-BuLi is added dropwise to the blue endpoint (color persists for >30 sec). The molarity is calculated using a 1:1 stoichiometric ratio of N-benzylbenzamide to n-BuLi. (Just greater than 1 equivalent of alkyllithium is needed to reach the endpoint).5. 4,4'-Dimethyl-2,2'-bipyridine was obtained from GFS Chemicals, Inc. or Tokyo Chemical Industry Co. and used as received.6. Chlorotrimethylsilane (TMSCl) was purchased from Aldrich Chemical Company, Inc. , and used as obtained.7. The following characterization data was obtained: mp 90-92°C; 1H NMR (CDCl 3, 300 MHz) δ: 0.04 (s, 18 H), 2.21 (s, 4 H), 6.94 (d, 2 H, J = 5.01),8.05 (br s, 2 H), 8.46 (d, 2 H, J = 5.00) ; 13C NMR (CDCl 3, 75 MHz) δ: −2.2, 27.1, 120.4, 123.0, 148.3, 150.8, 155.5 . Anal. Calcd for C 18H 28N 2Si 2: C, 65.79; H, 8.59; N, 8.53. Found: C, 65.78; H, 8.43; N, 8.76. It has been noted that desilylation occurs after standing in deuterochloroform (CDCl 3) overnight. The resulting methyl derivatives have also been observed in certain purified TMS bipyridine samples when stored over time. Therefore, it is best to convert these intermediates to the corresponding halides in a timely fashion. 8. Hexachloroethane (Cl 3CCCl 3), obtained from Aldrich Chemical Company, Inc. , was used as received.9. Cesium fluoride was purchased from Acros Organics, Inc. or Soekawa Chemicals Co. and stored in a dry box prior to use. 10. Acetonitrile was distilled over CaH 2 and stored in a 500-mL Kontes flask prior to use. 11. Silica gel used for flash chromatography (particle size 0.035-0.075 mm) was obtained from VWR Scientific Products . Silica chromatography columns were deactivated by flushing with 10% triethylamine in hexanes and then were washed with hexanes prior to use. 12. Spectral properties are as follows: mp 98-100°C; 1H NMR (CDCl 3, 300 MHz) δ: 4.63 (s, 4 H), 7.38 (dd, 2 H, J = 1.9, 5.0), 8.43 (s, 2 H), 8.70 (d, 2 H, J = 4.6) ; 13C NMR (CDCl 3, 75 MHz) δ: 43.9, 120.1, 122.8, 146.7, 149.4, 155.8 . Anal. Calcd for C 12H 10Cl 2N 2: C, 56.94; H, 3.98; N, 11.07. Found: C, 56.82; H, 4.04; N, 11.01. 13. In some cases, particularly if the solvent or reaction conditions are not thoroughly dry, 4,4'-dimethyl-2,2'-bipyridine is formed as a byproduct during the halogenation reaction. This compound may be separated from 4,4'-bis(chloromethyl)-2,2'-bipyridine by flash chromatography on silica gel (not deactivated with Et3N) using EtOAc as the mobile phase. Alternatively, 4,4'-bis(chloromethyl)-2,2'-bipyridine may be purified by recrystallization in hot/cold absolute EtOH, with no evidence of ether formation (e.g., 4,4'-di-Ethoxymethyl-2,2'-bipyridine) by 1H NMR.Waste Disposal InformationAll toxic materials were disposed of in accordance with "Prudent Practices in the Laboratory"; National Academy Press; Washington, DC, 1995.3. DiscussionHalomethylbipyridines, are typically synthesized either by radical halogenation 4 or from hydroxymethylbipyridine precursors.5Radical methods often give rise to mixtures of halogenatedspecies that are difficult to separate with flash chromatography. A solution to this problem, involving the selective reduction of polyhalogenated by-products with diisobutylaluminum hydride (DIBAL-H), has resulted in slight improvements in overall yields.6 While the synthesis of halomethyl compounds from hydroxymethyl precursors is more efficient than radical halogenation, such procedures involve many steps, each of which give intermediates in moderate to high yields.5 Direct trapping of bpy (CH 2Li)n with electrophiles has proved unsuccessful for the generation of halide products.5a The quenching of LDA-generated carbanions with TMSCl prior to halogenation as described here constitutes an efficient, high yield synthesis of halomethyl bpys substituted at various positions around the ring system.7,8Currently, 2,2'-bipyridine derivatives figure prominently in supramolecular assembly,9 in bioinorganic contexts,10 in studies of redox electrocatalysis 4a and in polymeric materials.11 Halomethyl bpys and their various metal complexes have also been used as initiators for controlled polymerizations of several different monomers including styrene and 2-alkyl-2-oxazolines.12TABLE I SYNTHESIS OF (TRIMETHYLSILYL)METHYL-2,2'-BIPYRIDINESProductR 1 R 2 R 3 R 4 Yield (%)4-(Trimethylsilyl)-methyl-2,2'-bipyridineTMSCH 2H H H 935-(Trimethylsilyl)-methyl-2,2'-bipyridineH TMSCH 2H H 996-(Trimethylsilyl)-methyl-2,2'-bipyridineH H TMSCH 2H 974,4'-Bis[(trimethylsilyl)-methyl]-2,2'-bipyridineTMSCH 2H H TMSCH 297TABLE II SYNTHESIS OF HALOMETHYL-2,2'-BIPYRIDINES Product R 1 R 2 R 3 R 4 Yield (%)4-Chloromethyl-2,2'-bipyridineClCH 2H H H94 5-Chloromethyl-2,2'-bipyridineH ClCH 2H H98 6-Chloromethyl-2,2'-bipyridineH H ClCH 2H 95 4,4'-Bis(chloromethyl)-2,2'-bipyridineClCH 2H H ClCH 291 4-Bromomethyl-2,2'-bipyridineBrCH 2H H H925-Bromomethyl-2,2'-bipyridineH BrCH 2H H986-Bromomethyl-2,2'-bipyridine H H BrCH 2H 99References and Notes1.Department of Chemistry, University of Virginia, Charlottesville, VA 22904-4319.2.Pangborn, A. B.; Giardello, M. A.; Grubbs, R. H.; Rosen, R. K.; Timmers, F. J. Organometallics1996, 15, 1518.3.Burchat, A. F.; Chong, J. M.; Nielsen, N. J. Organomet. Chem. 1997, 542, 281.4.(a) Gould, S.; Strouse, G. F.; Meyer, T. J.; Sullivan, B. P. Inorg. Chem. 1991, 30, 2942 andreferences therein; (b) Wang, Z.; Reibenspies, J.; Motekaitis, R. J.; Martell, A. E. J. Chem. Soc., Dalton Trans. 1995, 1511; (c) Rodriguez-Ubis, J.-C.; Alpha, B.; Plancherel, D.; Lehn, J.-M. Helv. Chim. Acta 1984, 67, 2264; (d) Newkome, G. R.; Puckett, W. E.; Kiefer, G. E.; Gupta, V. K.; Xia, Y.; Coreil, M.; Hackney, M. A. J. Org. Chem. 1982, 47, 4116.5.(a) Della Ciana, L.; Hamachi, I.; Meyer, T. J. J. Org. Chem. 1989, 54, 1731; (b) Della Ciana, L.;Dressick, W. J.; Von Zelewsky, A. J. Heterocycl. Chem. 1990, 27, 163; (c) Newkome, G. R.; Kiefer, G. E.; Kohli, D. K.; Xia, Y.-J.; Fronczek, F. R.; Baker, G. R. J. Org. Chem. 1989, 54, 5105; (d) Imperiali, B.; Prins, T. J.; Fisher, S. L. J. Org. Chem. 1993, 58, 1613.6.Uenishi, J.; Tanaka, T.; Nishiwaki, K.; Wakabayashi, S.; Oae, S.; Tsukube, H. J. Org. Chem.1993, 58, 4382.7.Fraser, C. L.; Anastasi, N. R.; Lamba, J. J. S. J. Org. Chem. 1997, 62, 9314.8.Savage, S. A.; Smith, A. P.; Fraser, C. L. J. Org. Chem. 1998, 63, 10048.9.(a) Boulas, P. L.; Gómez-Kaifer, M.; Echegoyen, L. Angew. Chem., Int. Ed. Engl. 1998, 37, 216;(b) Mamula, O.; von Zelewsky, A.; Bernardinelli, G. Angew. Chem., Int. Ed. Engl. 1998, 37, 290. 10.(a) Gray, H. B.; Winkler, J. R. Annu. Rev. Biochem. 1996, 65, 537; (b) Dandliker, P. J.; Holmlin,R. E.; Barton, J. K. Science 1997, 275, 1465.11.For a recent review see: Matyjaszewski, K., Ed. "Controlled Radical Polymerizations"; AmericanChemical Society: Washington, DC, 1998.12.(a) Collins, J. E.; Fraser, C. L. Macromolecules 1998, 31, 6715; (b) McAlvin, J. E.; Fraser, C. L. Macromolecules 1999, 32, 1341; (c) Wu, X.; Fraser, C. L. Macromolecules 2000, 33, 4053.AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)4,4'-Bis(chloromethyl)-2,2'-bipyridines :2,2'-Bipyridine, 4,4'-bis(chloromethyl)- (13); (138219-98-4)4,4'-Bis[(trimethylsilyl)methyl]-2,2'-bipyridine :2,2'-Bipyridine, 4,4'-bis[(trimethylsilyl)methyl]-(14); (199282-52-5) 4,4'-Bis(bromomethyl)-2,2'-bipyridineBrCH 2H H BrCH 297Diisopropylamine (8);2-Propanamine, N-(1-methylethyl)- (9); (108-18-9)Butyllithium:Lithium, butyl- (8,9); (109-72-8)4,4'-Dimethyl-2,2'-bipyridine:2,2'-Bipyridine, 4,4'-dimethyl- (9); (1134-35-6)Chlorotrimethylsilane:Silane, chlorotrimethyl- (8,9); (75-77-4)Hexachloroethane:Ethane, hexachloro- (8,9); (67-72-1)Cesium fluoride (8,9); (13400-13-0)Acetonitrile: TOXIC (8,9); (75-05-8)N-Benzylbenzamide:Benzamide, N-benzyl- (8);Benzamide, N-(phenylmethyl)- (9); (1485-70-7) Copyright © 1921-2002, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 6, p.1019 (1988); Vol. 51, p.142 (1971).TRIMETHYLOXONIUM TETRAFLUOROBORATE[Oxonium, trimethyl- tetrafluoroborate(1-)]Submitted by T. J. Curphey1Checked by A. Eschenmoser, R. Keese, and A. Daniel.1. ProcedureA 500-ml., three-necked flask fitted with a mechanical stirrer, a Dewar condenser (Note 1) connected by a T-tube to a mineral oil bubbler and a source of dry nitrogen, and a gas-inlet tube connected to a source of dry dimethyl ether(Note 2) is charged with 80 ml. of dichloromethane and 38.4 g. (33.3 ml., 0.271 mole) of boron trifluoride diethyl etherate(Note 3). After establishing a nitrogen atmosphere in the flask, the condenser is filled with an acetone–dry ice mixture. With gentle stirring, dimethyl ether is passed into the solution until approximately 75 ml. has collected (Note 4). The gas-inlet tube is replaced with a pressure-equalizing dropping funnel containing 28.4 g. (24.1 ml., 0.307 mole) of epichlorohydrin, which is added dropwise with vigorous stirring over a 15-minute period. The mixture is stirred overnight under an atmosphere of nitrogen(Note 5). The stirrer is replaced by a filter stick, and the supernatant liquid is drawn off from the crystalline trimethyloxonium tetrafluoroborate, while keeping the mixture under nitrogen. The oxonium salt is washed with two 100-ml. portions of anhydrous dichloromethane and two 100-ml. portions of sodium-dried diethyl ether(Note 6), and dried by passing a stream of nitrogen over the salt until the odor of ether is no longer detected, yielding 28–29g. (92.5–96.5%) of a white crystalline solid, m.p. (sealed tube) 179.6–180.0° (dec.), (Note 7) and (Note8).2. Notes1. A Kontes K-45750 condenser was used.2. Dimethyl ether and nitrogen were dried by passage through columns of Drierite. Boron trifluoride etherate (Eastman Practical Grade) was redistilled. Epichlorohydrin (Eastman Organic Chemicals) and dichloromethane (Fisher Scientific Company) were used as received.3. According to 1H NMR analysis the use of boron trifluoride etherate does not cause any detectable introduction of ethyl groups into the product.4. This may conveniently be done by placing, prior to conducting the reaction, a mark on the reaction flask at a level of 190 ml., and collecting dimethyl ether up to the mark. The exact amount of dimethyl ether used is not critical.5. After 2–3 hours of stirring the reaction appears to be over, and the dry ice in the condenser need no longer be renewed. The reaction mixture may be worked up at this point without appreciable reduction in the product yield or purity.6. According to analysis by 1H NMR the use of diethyl ether at this point does not cause any detectable exchange of methyl by ethyl groups in the oxonium salt. A user2 has reported obtaining the best samples of oxonium salt by using boron fluoride dimethyl etherate instead of the diethyl etherate and omitting the diethyl ether washing of the product. Oxonium salt prepared in this way was used to prepare methyl esters (from the corresponding amides) with no detectable (by GC analysis) ethyl esters.7. The melting point of trimethyloxonium tetrafluoroborate apparently depends upon the procedure by which it is prepared and the method of melting-point determination. It has, for example, been reported to melt at 124.5°,3 141–143° [Org. Synth., Coll. Vol. 5, 1096 (1973)], and 175°.4] The 1H NMRspectrum, determined (liquid SO 2, purissimum Fluka AG) in a sealed tube at room temperature shows a single methyl resonance at δ 4.54; a trace of impurity is discernible as a singlet at δ 3.39. 8. When prepared as described, the oxonium salt is stable and nonhygroscopic, and may readily be handled in the air for short periods of time. A sample kept in a desiccator over Drierite for 1 month at −20° showed no change in melting point, and batches stored in this manner for over a year have been successfully used for alkylations.3. DiscussionTrialkyloxonium salts were first discovered by Meerwein,3 who also investigated much of their chemistry. A discussion of the literature prior to 1963 has been published.5 Simple trialkyloxonium cations which have been prepared, other than trimethyl, include triethyl,6 tri-n -propyl,7 and tri-n -butyl,8 with tetrafluoroborate or hexachloroantimonate anions, in most cases. Methods used to prepare trimethyloxonium tetrafluoroborate , which are typical of the class as a whole, include the reaction of boron trifluoride with epichlorohydrin in the presence of dimethyl ether ,3,4,9 the reaction of dimethyloxonium tetrafluoroborate with diazomethane or diazoacetic ester,10 and the alkylation of dimethyl ether by triethyloxonium tetrafluoroborate 11 or dimethoxycarbonium tetrafluoroborate.12 Several of these reactions involve the initial formation of a mixed oxonium ion [R 1R 2OCH 3]+, which then methylates dimethyl ether , providing R 1R 2O and the trimethyloxonium ion. Of the available procedures, the one described here is probably the most convenient, involving as it does a single-step preparation from inexpensive, commercially available, and nonhazardous reagents. Under the proper conditions (Note 8), the resulting product has storage properties comparable to those of the less-accessible trimethyloxonium 2,4,6-trinitrobenzenesulfonate .13The trialkyloxonium salts are powerful alkylating agents. Trimethyl- and triethyloxonium tetrafluoroborates, in particular, have been widely employed for methylation and ethylation of sensitive or weakly nucleophilic functional groups. Alkylations of over 50 such functional groups have been reported in the literature. Examples include amides,4,7,14,15,16 lactams,16,17,18,19 sulfides,3,20 nitro compounds,9 enols and enolates,3,21 ethers,7,11,22 phenols,3 sulfoxides,3,7 amine oxides,3,7,23 carboxylic acids,3 lactones,3,4 ketones,3,16 metal carbonyls,12,24 thiophenes,25 and phosphonitriles.26. Oxonium salts have also been advantageously employed as quarternizing agents for a variety of heterocyclic amines.27,28,29,30,31,32,33,34 In this way the first diquarternary salts of several heterocyclic diazines have been prepared,30,31 as have reagents for peptide synthesis,33,34 for the synthesis of polycyclic ketones,32 and for cyanine dyes.28One of the major advantages of oxonium salts is that alkylations can be effected under reaction conditions that are generally much milder than those necessary with the more conventional alkyl halides or sulfonates. Triethyloxonium tetrafluoroborate , for example, has usually been employed at room temperature in dichloromethane or dichloroethane solution. Occasionally chloroform 17,23 or no solvent at all 4,21 is used. Difficult alkylations can be effected in refluxing dichloroethane .30,31 The less soluble trimethyloxonium tetrafluoroborate has been used as a suspension in dichloromethane or dichloroethane , or as a solution in nitromethane or liquid sulfur dioxide . Reports of alkylations in water 24 and trifluoroacetic acid 22 have also appeared. Direct fusion with trimethyloxonium tetrafluoroborate has succeeded in cases where other conditions have failed.26,31Alkylations by oxonium salts have added several new weapons to the synthetic chemist's armamentarium. For example, the O -alkylated products from amides [R 1C(OR)=NR 2R 3]+ (R=CH 3 or C 2H 5) may be hydrolyzed under mild conditions to amines and esters,15,35 reduced to the amines R 1CH 2NR 2R 3 by sodium borohydride ,14 converted to amide acetals R 1C(OR)2NR 2R 3 by alkoxides,4,16 and (for R 3=H) deprotonated to the imino esters R 1C(OR)=NR 2.17,18,19 Amide acetals and imino esters are themselves in turn useful synthetic intermediates. Indeed, oxonium salts transform the rather intractable amide group into a highly reactive and versatile functionality, a fact elegantly exploited in recent work on the synthesis of corrins.35Other reagents which approach or exceed the oxonium salts in alkylating ability include dialkoxycarbonium ions,36 alkyl trifluoromethanesulfonates,37 alkyl fluorosulfonates,38 dialkylhalonium ions,39 and alkyl halides in the presence of silver salts.25,37,40 In terms of availability, stability, and freedom from hazards,25 oxonium salts often appear to be the reagents of choice. When eithermethylation or ethylation is acceptable, methylation may be preferable. Triethyloxonium tetrafluoroborate must be stored under ether and handled in a dry box,6 whereas the trimethyl salt can be stored solvent-free in the freezing compartment of a refrigerator and dispensed in the open atmosphere. Moreover, while information on the relative alkylating ability of the oxonium salts is not extensive, a few cases have been reported in which trimethyloxonium tetrafluoroborate effected alkylations which the triethyl analog did not.20,31 The trimethyloxonium salt, therefore, appears to be the more potent alkylating agent.This preparation is referenced from:z Org. Syn. Coll. Vol. 5, 1080z Org. Syn. Coll. Vol. 5, 1096z Org. Syn. Coll. Vol. 5, 1099z Org. Syn. Coll. Vol. 6, 576References and Notes1.Department of Chemistry, St. Louis University, St. Louis, Missouri 63156 [Present address:Department of Pathology, Dartmouth Medical School, Hanover, New Hampshire 03755].2.Robert F. Myers (with William S. Johnson), Department of Chemistry, Stanford University,Stanford, California 94305, private communication to the editor-in-chief, May 5, 1970.3.H. Meerwein, G. Hinz, P. Hofmann, E. Kroning, and E. Pfeil, J. Prakt. Chem., [2], 147, 257(1937).4.H. Meerwein, P. Borner, O. Fuchs, H. J. Sasse, H. Schrodt, and J. Spille, Chem. Ber., 89, 2060(1956).5.H. Meerwein, in "Methoden der Organischen Chemie" (Houben-Weyl), Vol. 6/3, Georg ThiemeVerlag, Stuttgart, 1965, p. 325.6.H. Meerwein, Org. Synth., Coll. Vol. 5, 1080 (1973).7.H. Meerwein, E. Battenberg, H. Gold, E. Pfeil, and G. Willfang, J. Prakt. Chem., [2], 154, 83(1939).8.G. Hilgetag and H. Teichmann, Chem. Ber., 96, 1446 (1963).9.N. Kornblum and R. A. Brown, J. Am. Chem. Soc., 86, 2681 (1964).10. F. Klages, H. Meuresch, and W. Steppich, Justus Liebigs Ann. Chem., 592, 81 (1955).11.H. Meerwein, Org. Synth., Coll. Vol. 5, 1096 (1973).12.R. B. Silverman and R. A. Olofson, Chem. Commun., 1313 (1968).13.G. K. Helmkamp and D. J. Pettitt, Org. Synth., Coll. Vol. 5, 1099 (1973).14.R. F. Borch, Tetrahedron Lett., 61 (1968).15.H. Muxfeldt, J. Behling, G. Grethe, and W. Rogalski, J. Am. Chem. Soc., 89, 4991 (1967).16.H. Meerwein, W. Florian, N. Schön, and G. Stopp, Justus Liebigs Ann. Chem., 641, 1 (1961).17.S. Petersen and E. Tietze, Justus Liebigs Ann. Chem., 623, 166 (1959).18. E. Vogel, R. Erb, G. Lenz, and A. A. Bothner-By, Justus Liebigs Ann. Chem., 682, 1 (1965).19.L. A. Paquette, T. Kakihana, J. F. Hansen, and J. C. Philips, J. Am. Chem. Soc., 93, 152 (1971).20.J. E. Baldwin, R. E. Hackler, and D. P. Kelly, J. Am. Chem. Soc., 90, 4768 (1968).21.G. Hesse, H. Broll, and W. Rupp, Justus Liebigs Ann. Chem., 697, 62 (1966).22.P. E. Peterson and F. J. Slama, J. Am. Chem. Soc., 90, 6516 (1968).23. C. Reichardt, Chem. Ber., 99, 1769 (1966).24.R. Aumann and E. O. Fischer, Chem. Ber., 101, 954 (1968).25.R. M. Acheson and D. R. Harrison, J. Chem. Soc. C, 1764 (1970).26.J. N. Rapko and G. Feistel, Inorg. Chem., 9, 1401 (1970).27.H. Balli and F. Kersting, Justus Liebigs Ann. Chem., 647, 1 (1961).28. C. Reichardt, Justus Liebigs Ann. Chem., 715, 74 (1968).29.H. Quast and S. Hünig, Chem. Ber., 99, 2017 (1966); Chem. Ber.101, 435 (1968); H. Quast andE. Schmitt, Chem. Ber., 101, 1137 (1968).30.T. J. Curphey, J. Am. Chem. Soc., 87, 2063 (1965).31.T. J. Curphey and K. S. Prasad, J. Org. Chem., 37, 2259 (1972).32.G. Stork, S. Danishefsky, and M. Ohashi, J. Am. Chem. Soc., 89, 5459 (1967).33.R. B. Woodward, R. A. Olofson, and H. Mayer, Tetrahedron, Suppl. 8, 321 (1966).34.R. A. Olofson and Y. L. Marino, Tetrahedron, 26, 1779 (1970).35. A. Eschenmoser, Q. Rev. Chem. Soc., 24, 366 (1970).36.S. Kabuss, Angew. Chem., 78, 714 (1966) [Angew. Chem. Int. Ed. Engl., 5, 675 (1966)] andreferences therein.37. A. J. Boulton, A. C. G. Gray, and A. R. Katritzky, J. Chem. Soc. B, 911 (1967).38.M. G. Ahmed, R. W. Alder, G. H. James, M. L. Sinnott, and M. C. Whiting, Chem. Commun.,1533 (1968).39.G. A. Olah and J. R. DeMember, J. Am. Chem. Soc., 92, 2562 (1970).40.H. Meerwein, V. Hederich, and K. Wunderlich, Arch. Pharm. Weinheim, Ger., 291, 541 (1958).AppendixChemical Abstracts Nomenclature (Collective Index Number);(Registry Number)Drieriteboron fluoride dimethyl etherateliquid SO2dimethoxycarbonium tetrafluoroborateether,diethyl ether (60-29-7)chloroform (67-66-3)Epichlorohydrin (106-89-8)sulfur dioxide (7446-09-5)nitrogen (7727-37-9)dimethyl ether (115-10-6)Nitromethane (75-52-5)dichloromethane (75-09-2)Diazomethane (334-88-3)boron trifluoride (7637-07-2)boron trifluoride etherate,boron trifluoride diethyl etherate (109-63-7)dichloroethane(75-34-3)trifluoroacetic acid (76-05-1)sodium borohydride (16940-66-2)triethyloxonium tetrafluoroborate (368-39-8)Trimethyloxonium tetrafluoroborate,Oxonium, trimethyl- tetrafluoroborate(1-) (420-37-1)Trimethyloxonium 2,4,6-trinitrobenzenesulfonate (13700-00-0)dimethyloxonium tetrafluoroborateCopyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。
eluant (Note 7) to give 9.0 g (59% overall) of (2R,3S)- and (2S,3S)-1,4-dioxa-2,3-dimethyl-2-(1-methylethenyl)-8-carboethoxy-8-azaspiro[4.5]decane, a 6:1 mixture of diastereomers, as a pale yellow oil (Note 8).B. (2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane. Dry nitromethane (100 mL) (Note 9) is added through a rubber septum by syringe to a vacuum-dried, 500-mL, round-bottomed flask that contains the ketal mixture prepared in Step A (9.00 g, 31.8 mmol) and a magnetic stir bar. The solution is cooled to −23°C, tin(IV) chloride (SnCl4) (11 mL, 94 mmol) is added by syringe and the solution is stirred for 30 min at −23°C (Note 10). At this time the brown solution is warmed to 23°C and stirring is continued for an additional 30 min. Saturated aqueous NH4Cl (200 mL) is added and the mixture is concentrated under reduced pressure using a rotary evaporator to remove nitromethane. The resulting aqueous suspension is extracted with ethyl acetate (200 mL) and the organic extract is washed with brine (200 mL), dried over sodium sulfate (Na2SO4) and concentrated under reduced pressure using a rotary evaporator. The residue is subjected to flash chromatography on silica gel (250 g, 20 cm × 10 cm) using ethyl acetate:hexane (1:1) eluant (Note 7) to give 8.1 g (90%) of (2S,3S)-3-acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane as a pale yellow oil (Note 11) and (Note 12).2. Notes1. Anhydrous tetrahydrofuran was prepared by distillation under argon from sodium benzophenone ketyl.2. 2-Bromopropene, obtained from Aldrich Chemical Company, Inc., was distilled and then passed through a plugof activity IV basic alumina immediately before use.3. The fine white emulsion formed at this stage was collected with the organic phase and was cleared in the subsequent brine washings.4. This crude material was acceptable for use in the second step, although more p-toluenesulfonic acid will be required if large amounts of tributylamine are present. The diol mixture, free from tributylamine, can be obtained by careful chromatography on silica gel using ethyl acetate-hexane (1:1). The purified sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.10 (d, 3 H, J = 6.5, CH3), 1.37 (s, 3 H, CH3), 1.80 (s,3 H, CH3), 2.21 (br s, 2 H, 2 × OH), 3.77 (q, 1 H, J = 5.6, CH), 4.89 (d, 1 H, J = 1.1, CH=C), 5.06 (s, 1 H, CH=C); IR (film) cm−1: 3421, 3397, 3390, 3364, 2981, 2937, 1088; MS (Cl) m/z 113.0936 (113.0966 calcd for C7H14O2, MH –H2O).5. The major isomer is assigned the 3R, 4S stereochemistry on the expectation that the addition would occur preferentially with Cram (Felkin-Ahn) selectivity.3 This assignment was confirmed by 1H NMR DNOE experimentson the isobutyraldehyde acetal.6. 1-Carbethoxy-4-piperidone was obtained from Aldrich Chemical Company, Inc., and used as received.7. A series of 200-mL fractions was collected during flash chromatography. The product was eluted in fractions3–8 as indicated by TLC analysis using 4% ethanolic phosphomolybdic acid stain.8. This sample has the following characteristics: 1H NMR (500 MHz, CDCl3, major isomer) δ: 1.17 (d, 3 H, J =5.1, CH3), 1.26 (t, 3 H, J = 7.1, OCH2CH3), 1.45 (s, 3 H, CH3), 1.77 (s, 3 H, CH3C=), 1.60–1.81 (m, 5 H, 2 × CH2and CH), 3.43–3.75 (m, 4 H, 2 × CH2N), 4.13 (q, 2 H, J = 7.1, OCH2CH3), 4.96 (s, 2 H, CH2=C); IR (film) cm−1: 2977, 1702, 1433, 1238, 1122; MS (Cl) m/z 284.1850 (284.1861 calcd for C15H25NO4, MH). Anal. Calcd forC15H25NO4: C, 63.58; H, 8.89; N, 4.94. Found: C, 63.48; H, 8.90; N, 4.89.9. Nitromethane was dried by distillation of a 10:1 mixture of nitromethane and trifluoroacetic anhydride and collection of the center fraction that distilled at 100°C.10. Tin(IV) chloride (SnCl4) was obtained from Aldrich Chemical Company, Inc., and handled under an atmosphere of argon.11. Gas chromatographic analysis using a 25-m 10% SP 2100 silicone column showed that this sample was 94% pure and contained one major unidentified impurity. Bulb-to-bulb distillation (200°C, 0.6 mm) of a 7.4-g sample of the crude product afforded 7.0 g (85%) of the product as a pale yellow oil, which was shown by GLC analysis to be of 100% purity. This sample has the following spectral characteristics: [α]D−79.1° (MeOH, c 1.0); 1H NMR (500 MHz, CDCl3) δ: 1.17 (d, 3 H, J = 6.6, CH3), 1.25 (m, 6 H, OCH2CH3 and CH3), 1.70–1.90 (m, 4 H, 2 × CH2), 2.19 (s, 3 H,CH3CO), 1.57 (d, 1 H, J = 13.5) 2.36 (d, 1 H, J = 13.5), 3.38–3.70 (m, 4 H, 2 × CH2N), 3.89 (q, 1 H, J = 6.6, CH)4.12 (q, 2 H, J = 7.1, OCH2CH3); 13C NMR (125 MHz, CDCl3) δ: 14.5, 15.6, 22.5, 28.3, 36.0, 37.0, 40.7, 41.1, 47.3, 58.4, 61.0, 79.1, 81.0, 155.5, 210.3; IR (film) cm−1: 2977, 2937, 1705, 1701, 1698, 1472, 1455, 1434, 1365, 1356, 1274, 1237; MS (Cl) m/z 284.1845 (284.1860 calcd for C15H25NO4, MH). Anal. Calcd for C15H25NO4: C, 63.58; H,8.89; N, 4.94. Found: C, 63.38; H, 8.87; N, 4.88.12. The enantiomeric excess of the product is >96%. This was determined by treating a sample of the ketone with sodium borohydride/methanol (NaBH4/MeOH) (23°C) and separating the resulting 3:2 mixture of alcohol diastereomers by flash chromatography (silica gel, 2:3 ethyl acetate-hexane). The major alcohol diastereomer wasconverted to its Mosher ester4 [2.5 eq of (+)-α-methoxytrifluoromethylphenylacetic acid, 3 eq of dicyclohexylcarbodiimide, and 0.2 eq of 4-(dimethylamino)pyridine, CH2Cl2] and the crude esterification reaction mixture was analyzed using 500 MHz 1H NMR. None of the minor diastereomer was observed while doping experiments established that 2% would have been detected [diagnostic signals: δ 1.80 (δ, J = 13.4, major ester diastereomer); δ 1.82 (δ, J = 14.1, minor ester diastereomer)].Waste Disposal InformationAll toxic materials were disposed of in accordance with "Prudent Practices in the Laboratory"; National Academy Press; Washington, DC, 1995.3. DiscussionThis procedure illustrates a fundamentally new method for constructing substituted tetrahydrofurans.5,6,7,8,9,10 This practical method assembles the tetrahydrofuran ring from allylic diol and carbonyl components and in the process forms three ring bonds: C(2)-C(3), C(4)-C(5) and O-C(5). Both aldehydes (eq 1) and ketones (illustrated in the present procedure) can be employed as the carbonyl component. Although it is often convenient to isolate the acetal intermediate, conversion to the 3-acyltetrahydrofuran can also be accomplished in many cases by the direct reaction of the diol and carbonyl components.8 High cis stereoselectivity (at least 20:1) is observed in the preparation of tetrahydrofurans that contain single side chains at carbons 2 and 5 (eq. 1). The kinetically controlled product also has the cis relationship of these side chains and the 3-acyl substituent.A definitive feature of this highly stereoselective new route to substituted tetrahydrofurans is that both syn and anti allylic diol stereoisomers typically afford identical tetrahydrofuran products. Thus, there is no need for stereoselective construction of the allylic diol reaction partner. The construction of substituted tetrahydrofurans in high enantiomeric purity from non-racemic allylic diol precursors has also been established.5,7 The rearrangement illustrated in eq. 2 is the key step in a recent synthesis of (+)-muscarine.The scope and mechanism of the SnCl4-promoted rearrangement of allylic acetals have been investigated in detail and these studies provide considerable guidance for using this new tetrahydrofuran synthesis.5,6,7,8,9 Three major limitations emerge from studies conducted to date: (1) When the tetrahydrofuran construction involves a ketone, and thus forms a quaternary center at C(5), allylic diols with alkene substituents more nucleophilic than terminal vinyl rearrange in highest yield. (2) Allylic acetals that are reluctant to ring open in the presence of acid catalysts to generate oxocarbenium ions often undergo decomposition, rather than conversion to acyltetrahydrofuran products. (3) Allylic acetals that form highly stabilized oxocarbeniums (e.g., cinnamaldehyde-derived acetals) do not undergo conversion to 3-acyltetrahydrofurans.This procedure illustrates the asymmetric synthesis of a spirobicyclic tetrahydrofuran from the reaction of readily available (S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone2 with cyclic ketones. The specific example describedargon (7440-37-1)sodium borohydride (16940-66-2)dicyclohexylcarbodiimide (538-75-0)tributylamine (102-82-9)trifluoroacetic anhydride (407-25-0)p-toluenesulfonic acid (104-15-4)ethyl acetate-hexane (2639-63-6)acetal carbon (463-57-0)Tetrabutylammonium fluoride (429-41-4)2-Bromopropene (557-93-7)4-(dimethylamino)pyridine (1122-58-3)tert-Butyllithium (594-19-4)(+)-α-methoxytrifluoromethylphenylacetic acid (56135-03-6)(2S,3S)-3-Acetyl-8-carboethoxy-2,3-dimethyl-1-oxa-8-azaspiro[4.5]decane (155534-75-1) 3-(S)-[(tert-Butyldiphenylsilyl)oxy]-2-butanone,(S)-3-[[(1,1-dimethylethyl)diphenylsilyl]oxy]-2-butanone (135367-18-9)tert-butyldiphenylsilyl1-Carbethoxy-4-piperidone (29976-53-2)isobutyraldehyde acetal3-methyl-4-pentene-2,3-diolCopyright © 1921-2007, Organic Syntheses, Inc. All Rights Reserved。
Organic Syntheses, Coll. Vol. 3, p.846 (1955); Vol. 24, p.94 (1944).
UNDECYL ISOCYANATE
Submitted by C. F. H. Allen and Alan Bell.
Checked by Nathan L. Drake and John Sterling.
1. Procedure
In a 1-l. three-necked flask, equipped with a stirrer and a thermometer and immersed in an ice bath, is placed 46 g. (0.7 mole) of sodium azide(Note 1) in 150 ml. of water. A mixture of 109 g. (0.5 mole) of lauroyl chloride (b.p. 134–137°/11 mm.) and 150 ml. of acetone is then added from a separatory funnel to the well-stirred solution of the azide at such a rate that the temperature remains at 10–15°. After the mixture has been stirred at this temperature for an hour, the stirrer is stopped and, when the layers have separated, the lower water layer is removed carefully by suction through a glass capillary tube (Note 2). The upper layer is then added slowly to 500 ml. of benzene which has been warmed to 60° (Note 3). A rather rapid evolution of gas results, and the mixture is kept at 60–70° (Note 4) until no more nitrogen is evolved; the conversion of azide to isocyanate requires about an hour. The solution is filtered to remove any insoluble matter, and the benzene is removed by distillation from a modified Claisen flask. Distillation of the residue yields 80–85 g. of ester (81–86%) (Note 5) and (Note 6).
2. Notes
1. A practical grade of sodium azide such as that obtained from the Eastman Kodak Company is satisfactory.
2. It is important that the water be removed as completely as possible before the azide is added to the warm benzene. Failure to remove the water causes formation of the sym-disubstituted urea during decomposition of the azide. If the water is separated carefully, there will be no need to filter the benzene solution before the final distillation.
3. If the azide is added too rapidly, the solution may froth over; it is best to carry out this reaction in a 1-l. beaker.
4. The heat of reaction is usually sufficient to maintain the temperature at 60–70°.
5. On redistillation all the product boils at 103°/3 mm. A second distillation is unnecessary; the original ester is pure enough for all practical purposes.
6. This method is a general one for the preparation of isocyanates.1
3. Discussion
This procedure is one used by Schröter for preparing alkyl isocyanates.1
References and Notes
1.Schröter, Ber., 42, 3356 (1909).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sym-disubstituted urea
Benzene (71-43-2)
nitrogen (7727-37-9)
acetone (67-64-1)
sodium azide (26628-22-8)
UNDECYL ISOCYANATE (2411-58-7)
lauroyl chloride (112-16-3) Copyright © 1921-2005, Organic Syntheses, Inc. All Rights Reserved。