D116一致收敛46183
- 格式:ppt
- 大小:430.02 KB
- 文档页数:29




离子交换色谱-积分脉冲安培法测定大蒜中的蒜氨酸李存芝;黄雪松【摘要】为测定新鲜大蒜中蒜氨酸,采用离子交换色谱-积分脉冲安培(IEC-IPA)分析法,以离子交换分离柱分离,积分脉冲安培电化学检测,柱温:30℃;流动相:离子水、250 mmol/L NaOH及1 mol/L醋酸钠进行梯度洗脱:流速:0.25 mL/min.测得结果:蒜氨酸保留时间为5.100~5.200 min,浓度在0.45~3.0μg/mL内线性关系良好,相关系数0.997 9,检测限0.05 μg/mL,加样回收率为96.7%,标准差1.7%;测得的广东蒜及山东蒜的蒜氨酸含量分别是1.262 9%、1.511 4%.【期刊名称】《食品与发酵工业》【年(卷),期】2010(036)012【总页数】4页(P169-172)【关键词】大蒜(Allium sativum L.);蒜氨酸;离子色谱法;积分脉冲安培检测【作者】李存芝;黄雪松【作者单位】暨南大学食品科学与工程系,广东,广州,510632;暨南大学食品科学与工程系,广东,广州,510632【正文语种】中文大蒜(Allium sativum L.)是百合科葱属植物蒜的鳞茎,含有丰富的营养物质[1]。
据报道新鲜大蒜中含有大量大蒜素的前体物质——蒜氨酸(S-烯丙基-L-半胱氨酸亚砜)[2]。
研究亦表明,蒜氨酸是大蒜中的主要活性成分,具有抗氧化、清除自由基、降脂、抗肿瘤、抗血栓等多项功能[3]。
所以测定蒜氨酸的含量已成为评定大蒜及其制品品质的重要指标。
由于大蒜成分的多样性和不稳定性,直接测定蒜氨酸含量存在一定困难,且各种分析结果有很大差异,至今尚未有统一的标准方法测定大蒜中蒜氨酸含量。
传统的定硫法精确度极低;薄层扫描法[4]、高效液相色谱法[5~8]、液质联用法[9]、高效毛细管电泳法[10]、4-MP[11]测定法、高效离子对色谱法[12]、柱前或柱后衍生化后测定[13]等方法前处理复杂,检测周期长,或需特殊的仪器,在应用中受到限制。

NSF/ANSI 42 – 2005饮用水处理设备–审美效果NSF国际标准/美国国家标准iv 目录前言........... ........... ........... ........... ........... ........... ........... ................................. ................................. ........... ........... .. (vii)1 大纲........... ........... ............................................ ........... ........... ............................... ........... ........... ........... ........... .. (1)1.1 宗旨 ........... ........... ........... ........... ........... ........... ........... ....................................... ........... ................................ .. (1)1.2 范围 .. ........... ........... ........... ........... ........... ........... ........... ........... ........... ........................................................ .. (1)1.3 替代材料、设计和建筑 (1)1.4 降低化学和机械性能要求 (1)1.5 最低要求 (2)2 参考标准........ ........ ........ ........ ........ ........ .......................... ........ ........ ........ ........ ........ .. (2)3 定义 (3)4 材料 (6)4.1 接触饮用水的材料 (6)4.2 材料评估 (7)表1 –萃取测试参数 (8)表2 –依据分子式萃取测试参数 (10)表3 –美国联邦环境保护法令中列出的材料 (12)表21 - 不需要配方审查 (12)表4 –非特定的萃取测试参数 (13)5 结构性能 (14)5.1 结构的整体性 (14)5.2 接受 (14)5.3 工作压力 (14)5.4 结构整体性测试方法 (14)表5 –结构整体性测试要求 (18)6 最小性能要求 (19)6.1 原理 (19)6.2 下水管道连接 (19)6.3 产出水出水口 (19)6.4 伤害........ ........ ........ .. (20)6.5 工作温度 (20)6.8 额定压力下降 (20)6.9 最小工作流量 (20)表6 –最小工作流量 (21)6.10 活化剂和添加剂 (21)6.11 介质 (22)7 可选测试要求的强制性测试 (22)7.1 一般要求 (22)7.2 抑菌性能 (24)7.3 化学还原测试 (25)表8 –减少氯胺的要求 (28)表9 –减少氯 (32)表10 –减少硫化氢和苯酚的要求 (34)表11 –减少铁、锰的要求 (36)表12 –调节pH值的要求 (38)表13 –减少锌的要求 (40)7.4 机械还原测试 (42)v 表14 –极微颗粒减少(85%) 种类 (42)表15 –极微颗粒减少所需测试灰尘规格(85%) (43)7.5 水垢控制测试 (44)表16–用于控制水垢的添加剂 (44)8 说明和信息 (45)8.1 安装、操作和维护说明 (45)8.2 参数标牌 (46)8.3 可更换部件 (48)8.4 运行数据表 (48)表17 –运行数据表减少要求 (50)表18 –运行数据表减少要求 (50)附件A 水处理系统及其部件申请认证计划的关键要素.A1A.1 产品标记 ........................................................................................................................................................................A1A.2 列入获得认证的公司名..................................................................................................................................................A1A.3 年度审计 ........................................................................................................................................................................A1A.4 测试 ................................................................................................................................................................................A2A.5 原料配方的毒理学鉴定 ................................................................................................................................................A2A.6 校正 ................................................................................................................................................................................A2A.7 执行A2A.10 投诉 ..............................................................................................................................................................................A3A.11 广告 ...............................................................................................................................................................................A3A.12 记录…………………………………………………………………………………………………………………….A3A.13 公示................................................................................................................................................................................A3A.14 保密…………………………………………………………………………………………………………………..A3B HPLC色谱分析一氯代胺程序.............................................................................................................................................B1B.1 分析方法概述....................................................................................................................................................................B1B.2 分析仪器和材料 ..............................................................................................................................................................B1B.3 试剂和耗材........................................................................................................................................................................B1B.4 安全………………………………………………………………………………………………………………………B4B.5 程序………………………………………………………………………………………………………………………B4B.6 数据分析............................................................................................................................................................................B5B.7 品质控制 ..........................................................................................................................................................................B5B.8 参考文献…………………………………………………………………………………………………………………B7表B1 –通过品质控制的标准................................................................................................................................................B7vi前言2本标准的目的是:对于旨在降低影响公共或私有供水系统的感官效果的有害物质(非健康影响)的饮用水处理设备,本标准制订关于该类设备有关的材料、设计、建筑和运行的最低要求。
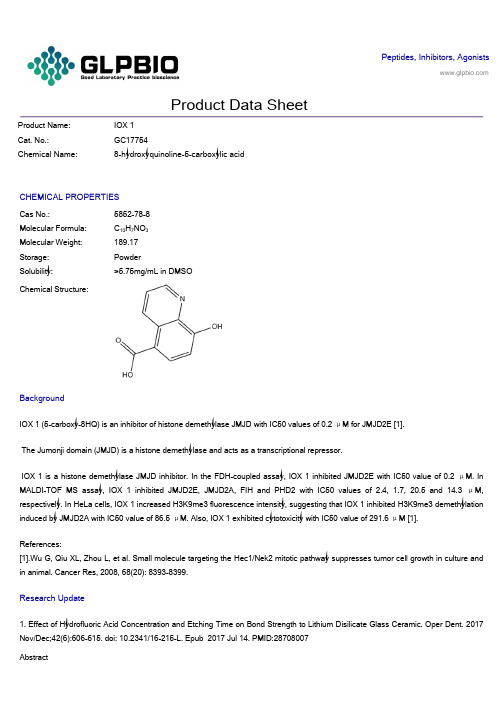
Peptides, Inhibitors, AgonistsProduct Data SheetProduct Name: IOX 1Cat. No.:GC17754Chemical Name: 8-hydroxyquinoline-5-carboxylic acidCHEMICAL PROPERTIESCas No.: 5852-78-8Molecular Formula: C10H7NO3Molecular Weight: 189.17Storage: PowderSolubility: >5.75mg/mL in DMSOChemical Structure:BackgroundIOX 1 (5-carboxy-8HQ) is an inhibitor of histone demethylase JMJD with IC50 values of 0.2 μM for JMJD2E [1].The Jumonji domain (JMJD) is a histone demethylase and acts as a transcriptional repressor.IOX 1 is a histone demethylase JMJD inhibitor. In the FDH-coupled assay, IOX 1 inhibited JMJD2E with IC50 value of 0.2 μM. In MALDI-TOF MS assay, IOX 1 inhibited JMJD2E, JMJD2A, FIH and PHD2 with IC50 values of 2.4, 1.7, 20.5 and 14.3 μM, respectively. In HeLa cells, IOX 1 increased H3K9me3 fluorescence intensity, suggesting that IOX 1 inhibited H3K9me3 demethylation induced by JMJD2A with IC50 value of 86.5 μM. Also, IOX 1 exhibited cytotoxicity with IC50 value of 291.6 μM [1].References:[1].Wu G, Qiu XL, Zhou L, et al. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res, 2008, 68(20): 8393-8399.Research Update1. Effect of Hydrofluoric Acid Concentration and Etching Time on Bond Strength to Lithium Disilicate Glass Ceramic. Oper Dent. 2017 Nov/Dec;42(6):606-615. doi: 10.2341/16-215-L. Epub 2017 Jul 14. PMID:28708007AbstractThe aim of this study was to evaluate the influence of different concentrations of hydrofluoric acid (HF) associated with varied etching times on the microshear bond strength (μSBS) of a resin cement to a lithium disilicate glass ceramic. Two hundred seventy-five ceramic blocks (IPS e.max Press [EMX], Ivoclar Vivadent), measuring 8 mm × 3 mm thickness, were randomly distribut ed into five groups according to the HF concentrations (n=50): 1%, 2.5%, 5%, 7.5%, and 10%.2. Does acid etching morphologically and chemically affect lithium disilicate glass ceramic surfaces? J Appl Biomater Funct Mater. 2017 Jan 26;15(1):e93-e100. doi: 10.5301/jabfm.5000303. PMID:27647389AbstractBACKGROUND: This study evaluated the surface morphology, chemical composition and adhesiveness of lithium disilicate glass ceramic after acid etching with hydrofluoric acid or phosphoric acid.METHODS: Lithium disilicate glass ceramic specimens polished by 600-grit silicon carbide paper were subjected to one or a combination of these surface treatments: airborne particle abrasion with 50-μm alumina (AA), etching with 5% hydrofluoric acid (HF) or 36% phosphoric ac id (Phos), and application of silane coupling agent (Si).3. Fatigue failure load of feldspathic ceramic crowns after hydrofluoric acid etching at different concentrations. J Prosthet Dent. 2018 Feb;119(2):278-285. doi: 10.1016/j.prosdent.2017.03.021. Epub 2017 May 26. PMID:28552291AbstractSTATEMENT OF PROBLEM: Hydrofluoric acid etching modifies the cementation surface of ceramic restorations, which is the same surface where failure is initiated. Information regarding the influence of hydrofluoric acid etching on the cyclic loads to failure of ceramic crowns is lacking.PURPOSE: The purpose of this in vitro study was to evaluate the influence of different hydrofluoric acid concentrations on the fatigue failure loads of feldspathic ceramic crowns.。
2NMR 试剂A111246氘代乙酸Acetic acid-d4 99.5 atom % D 1186-52-310x0.5ml 5g, 10g, 50g A111247氘代乙酸Acetic acid-d4 99.93 atom % D1186-52-35ml10x0.75ml A100962氘代丙酮 Acetone-d6 (D,99.8%) + TMS (0.03%)666-52-410ml A100963氘代丙酮 Acetone-d6 (D,99.96%)(+0.03% V/V TMS)666-52-410x0.6ml 25g A100965氘代丙酮 Acetone-d6 99.9 atom % D 666-52-410x0.6ml A100968氘代乙腈Acetonitrile-d399.8 atom % D2206-26-010×0.5ml 10×0.6ml 10×0.75ml 10×1g10g, 25g, 50g A100969氘代乙腈Acetonitrile-d399.96 atom % D 2206-26-010×0.5ml 10×0.6ml 10×0.75ml 5×0.75ml A100970氘代乙腈Acetonitrile-d3(D,99.8%) (0.03% v/v TMS)2206-26-010×0.6ml 10g A121289氘代乙酰基氯-d3 Acetyl chloride-D399 atom % D 19259-90-65g 25g A121270氘代氨水-d3Acetyl chloride-d399 atom % D 13550-49-75L 25L A121278氘代乙酸铵-d7Ammonium acetate-D7 98 atom % D 194787-05-81g 5g A121292 氯化铵-d4Ammonium-d4 chloride 98 atom % D12015-14-45g 25g A117711氯化铵-15NAmmonium chloride-15N 10 atom % D , ≥98.5%(cp)39466-62-11g, 5g 25g,100g A117712氯化铵-15NAmmonium chloride-15N 99 atom % D , ≥98.5%(cp)39466-62-11g 5g 25gB100912氘代苯-d6 Benzene-d699.6 atom % D 1076-43-310*0.5ml 10*0.6ml 10*0.75ml 10*1g, 5g10g,25g, 100g B100913氘代苯-d6 Benzene-d6 D,99.6% (0.03% v/v TMS)1076-43-310×0.6ml 10g B100918氘代苯-d6 Benzene-d6 99.96 atom % D1076-43-310×0.5ml 10×0.6ml 10×0.75ml B121294 氘代溴化苯-d5 Bromobenzene-D599.5 atom % D4165-57-55g 25g C109593氘代氯仿-d Chloroform-d (D,99.8%) +1% V/V TMS865-49-610×1g 50g 100g C109594氘代氯仿-d Chloroform-d (D,99.8%) +0.03% V/V TMS 865-49-610 X 0.6ML 10×1g 50g,100g C109595氘代氯仿-d Chloroform-d 99.8 atom % D 865-49-610 X 0.6ML 10×1g 50g, 100g C121272氘代环己醇-d12Cyclohexanol-D1299.5 atom % D 66522-78-91g D118378十氢化萘-d18Decahydronaphthalene-d1899 atom % D 28788-42-31g 5g D121297溴化氘Deuterium bromide 99 atom % D 13536-59-95L D113903氧化氘Deuterium oxide99.96 atom % D7789-20-010×1ml 10g, 50g3NMR 试剂D113904氧化氘Deuterium oxide 99.9 atom % D7789-20-010×0.55ml 25g, 100g D113905氧化氘Deuterium oxide (D, 99.8%) STERILITY TESTED 7789-20-0250ml D113906氧化氘Deuterium oxide (D, 99.9%) LOW PARAMAGNETIC 7789-20-0100g D1151091,2-二氯乙烷(氘4) 12-Dichloroethane-d499 atom % D 17060-07-05×1g 5g D1022571,2-二氯代苯(氘4) 12-Dichlorobenzene-d499 atom % D 2199-69-11g 5g D121273 1,4- 二溴苯-d41,4- Dibromobenzene-D4 98 atom % D 4165-56-41g 5g D102265二氯甲烷-d2Dichloromethane-D299.9 atom % D1665-00-510×0.5ml 10×0.75ml 1g, 5g E118380乙醚-d10 Ether-d1099 atom % D 2679-89-21g 5g D121274二甲基乙酰胺-d9Dimethylacetamide-D999 atom % D116057-81-91g 5g N102258氘代N,N-二甲基甲酰胺-d7 N,N-Dimethylformamide-d799.5 atom % D 4472-41-710×1g 5g D121275 甲基硫酸酯-d6Dimethylsulfate-D699 atom % D 15199-43-6 1g 5gD106263二甲基亚砜-d6Dimethylsulfoxide-D699.9 atom % D2206-27-110*0.6ml 10*0.75ml 5*3ml 10*1g ,5g 10g ,25g ,50g D106267二甲基亚砜-d6Dimethylsulfoxide-D6 D.99.9% septum 2206-27-110g 25g D106265二甲基亚砜-d6Dimethylsulfoxide-D6 D.99.9% +0.05%TMS 2206-27-110×1g5g,10g, 25g D106264二甲基亚砜-d6Dimethylsulfoxide-D6 D.99.9% +0.03%TMS 2206-27-15g10g ,25g D106266二甲基亚砜-d6Dimethylsulfoxide-D6D.99.9% +1%TMS2206-27-110×0.6ml 10×0.75ml 10×1g5g ,10g, 25g E102259氘代乙醇-d6 Ethanol-d699 atom % D 1516-08-11g 5g E102260氘代乙醇-d6 Ethanol-d6D,99% anhydrous 1516-08-11g 5g D121276氘代甲酸-d2 Formic acid-D2D98%(95 wt.% in D2O)920-42-31g 5g H118382氘代正庚烷-d16 Heptane-d1699 atom % D 33838-52-71g 5g H121303氘代六氟-2-丙醇-d2Hexafluoro-2-propanol-D299 atom % D 38701-74-51g 5g H121283氘代正己烷-d14 n-Hexane-D1499 atom % D 21666-38-61g 5g L121279 氘代锂铝-d4Lithiumaluminumdeuterid 98 atom % D, 95%14128-54-2 1g 5g M121280 氘代丙二酸-d4 Malonic acid-D4 99 atom % D 813-56-910g M118385氘代甲基环己烷-d14 Methylcyclohexane-d1499.5 atom % D 10120-28-21g 5gM102262氘代甲醇-d4Methanol-12C,d499.8 atom % D811-98-310×0.5ml 10×0.75ml 10×1g,5g,25g M102263氘代甲醇-d4Methanol-12C,d4D,99.8% septum811-98-325g 50gM102264氘代甲醇-d4Methanol-12C,d4D,99.8% (0.05% v/v TMS)811-98-32×0.6ml10×0.6ml, 10g4NMR 试剂N114091氘萘-d8Naphthalene-D8分析标准品1146-65-2100mg N121305氘代硝酸-dNitric acid-D. 50 wt % in D20D,99%(65 wt.% in D2O)13587-52-55g 25g n118386氘代硝基苯-d5 Nitrobenzene-d599 atom % D 4165-60-05g10g, 25g N102268氘代硝基甲烷Nitromethane-d399 atom % D 13031-32-810g 25g O118387氘代正辛烷-d18 Octane-d1899 atom % D 17252-77-61g 5g P118388氘代正戊烷-d12 Pentane-d1298 atom % D 2031-90-51g 5g S102269氘代硫酸Sulfuric acid-d2 solution 99 atom % D 13813-19-950g P121281氘代苯酚-d6 Phenol-D699 atom % D 13127-88-31g 5g P118389氘代磷酸-d3Phosphoric acid-D3 85 wt % in D20 99 atom % D 14335-33-250g 100g p118383 氘代异丙醇-d8 2-Propanol-D898 atom % D 22739-76-05g 25g P113721氘代吡啶-d5 Pyridine-D599.5 atom % D7291-22-71g, 5g 25g P113720氘代吡啶-d5 Pyridine-D5(D,99.5%) +0.05% V/V TMS7291-22-710×0.6ml 10×1g 10g ,25g S121307氘代醋酸钠-d3Sodium acetate-D398 atom % D39230-37-05g 25g S121310氘氧化钠Sodium deuterium oxide 30 wt % in D20D,99.5%(40 wt.% in D2O)14014-06-310g 50g S121282氘代苯乙烯-d8 Styrene-D898 atom % D 19361-62-7 1g 5gT1022701,1,2,2-四氯乙烷-d21,1,2,2-Tetrachloroethane-d299.6 atom % D 33685-54-010×0.5ml 1g, 5g, 25g T106828四甲基硅Tetramethylsilane NMR 级75-76-325g 100g T102271氘代四氢呋喃-d8 Tetrahydrofuran-d899.95 atom % D1693-74-910×0.5ml,5g 10×0.75ml 10×1g T102273氘代四氢呋喃-d8 Tetrahydrofuran-d899.95 atom % D 1693-74-910×0.5ml 10×0.75ml 5ml T102274氘代甲苯-d8 Toluene-d899.60 atom % D 2037-26-55g10g, 25g T102276氘代甲苯-d8 Toluene-d8D,99.5%(0.03% TMS)2037-26-510g T102275氘代甲苯-d8 Toluene-d899.94 atom % D2037-26-510×0.5ml 10×0.75ml T109782氘代三氟乙酸Trifluoroacetic acid-d 99.5 atom % D+0.03%TMS 599-00-85g10g, 25g T109783氘代三氟乙酸Trifluoroacetic acid-d99.5 atom % D599-00-810×0.5ml 10×0.75ml 10×1g 10g, 25g T109782氘代三氟乙酸Trifluoroacetic acid-d D,99.5% (0.03% TMS)599-00-85g10g, 25g O102277氘代邻二甲苯-d10 o-Xylene-d1098 atom % D56004-61-65gP102278氘代对二甲苯-d10 p-Xylene-d1098 atom % D 41051-88-15g。
学 报Journal of China Pharmaceutical University 2023,54(6):749 - 756749多黏菌素E甲磺酸钠的色谱指纹图谱与质量一致性研究李宣1,2,3#,黄敏文1,2#,周杰4,袁耀佐1,2*,杭太俊3**(1江苏省食品药品监督检验研究院,南京 210019;2国家药品监督管理局化学药物杂质谱研究重点实验室,南京 210019;3中国药科大学药物分析教研室,南京 210009;4正大天晴药业集团股份有限公司,连云港 222062)摘 要 建立了多黏菌素E甲磺酸钠的UPLC指纹图谱用于质量一致性研究,色谱柱为Acquity UPLC® Peptide CSH C18(2.1 mm × 150 mm,1.7 µm),以磷酸盐缓冲液-乙腈(19∶1)为流动相A,磷酸盐缓冲液-乙腈(1∶1)为流动相B,梯度洗脱,流速为0.3 mL/min,柱温为30 ℃,检测波长为210 nm;采用“中药色谱指纹图谱相似度评价系统(2012版)”进行相似度评价,结合多种指标性组分含量测定结果,对原研原料药和国产仿制原料药进行质量一致性评价。
研究结果显示,原研原料药和仿制原料药指标性组分含量均满足欧洲药典标准的要求,二者的UPLC指纹图谱具有高度的相似性,表明两者质量基本一致。
建立指纹图谱进行相似度评价,并与指标性组分含量测定结果相结合作为综合评价方法,用于复杂组分药物质量一致性的研究,具有快速、准确、全面的特点,有助于药品质量评价,为复杂组分抗生素质量一致性评价提供了思路。
关键词多黏菌素E甲磺酸钠;指纹图谱;相似度评价;多种成分含量测定;质量评价方法中图分类号R917 文献标志码 A 文章编号1000 -5048(2023)06 -0749 -08doi:10.11665/j.issn.1000 -5048.2023071903引用本文李宣,黄敏文,周杰,等.多黏菌素E甲磺酸钠的色谱指纹图谱与质量一致性研究[J].中国药科大学学报,2023,54(6):749–756.Cite this article as:LI Xuan,HUANG Minwen,ZHOU Jie,et al. Chromatographic fingerprint and quality consistency of colistimethate sodium [J].J China Pharm Univ,2023,54(6):749–756.Chromatographic fingerprint and quality consistency of colistimethate sodium LI Xuan1,2,3#, HUANG Minwen1,2#, ZHOU Jie4, YUAN Yaozuo1,2*, HANG Taijun3**1Jiangsu Institute for Food and Drug Control, Nanjing 210019; 2NMPA Key Laboratory for Impurity Profile of Chemical Drugs, Nanjing 210019; 3Department of Pharmaceutical Analysis,China Pharmaceutical University,Nanjing 210009; 4Chia Tai Tianqing Pharmaceutical Group Co Ltd, Lianyungang 222062, ChinaAbstract The UPLC fingerprint of colistimethate sodium was established for the study of quality consistency. The chromatographic column was Acquity UPLC® Peptide CSH C18 (2.1 mm × 150 mm, 1.7 µm).The mobile phase A was phosphate buffer-acetonitrile (19∶1), and the mobile phase B was phosphate buffer-acetonitrile (1∶1). The mobile phase was in gradient elution at a flow rate of 0.3 mL/min.The column temperature was set at 30 °C and the detection wavelength was 210 nm.The similarity of the fingerprints was analyzed with the Similarity Eval⁃uation System for Chromatographic Fingerprint of Tradition Chinese Medicine (Version 2012) in combination with content determination of multiple index components to evaluate the quality consistency of imported and domestic bulk drugs.The result showed that both the original and generic bulk drugs met the specified limit requirements in the European Pharmacopoeia standards, and that their UPLC fingerprints were highly similar, indicating that the quality of the two substances was consistent.Establishing a fingerprint for similarity evalua⁃tion and combining it with the results of indicator component content determination as a comprehensive evalua⁃tion method for the study of drug quality consistency of complex components has the characteristics of fast, accu⁃收稿日期2023-07-19 通信作者*Tel:************E-mail:yuanyaozuo@**Tel:138****2961E-mail:hangtj@基金项目江苏省药品监督管理局药品监管科学科研计划资助项目(No.202122)#李宣与黄敏文为共同第一作者学 报Journal of China Pharmaceutical University 2023,54(6):749 - 756第54 卷rate, and comprehensive, which is helpful for drug quality evaluation and provides ideas for the evaluation of anti⁃biotic quality consistency of complex components.Key words colistimethate sodium; fingerprint; similarity evaluation; multi-component content determination;quality evaluation methodThis study was supported by Jiangsu Provincial Drug Administration’s Drug Regulatory Scientific Research Program (No.202122)#LI Xuan and HUANG Minwen contributed equally to this work随着全球耐药问题的日益严峻,多黏菌素类药物成为临床上治疗严重革兰氏阴性耐药菌感染的最后一道防线[1],同时也被WHO列为最高优先至关重要的抗微生物药物。