漂白粉安全技术说明书英文
- 格式:docx
- 大小:76.03 KB
- 文档页数:10


次氯酸钠2013新版安全技术说明书安全技术说明书第一部分化学品及企业标识化学品中文名称: 次氯酸钠溶液,漂白水化学品英文名称: sodium hypochlorite solution 企业名称:地址:邮编:电子邮件地址:联系电话:传真号码:企业应急电话:产品代码: 产品推荐用途: 用于水的净化~以及作消毒剂、纸浆漂白剂等~医药工业中用于制氯胺等。
第二部分危险性概述物理化学危险:本品不燃~受高热分解产生有毒的腐蚀性烟气。
健康危害: 经常用手接触本品的工人~手掌大量出汗~指甲变薄~毛发脱落。
本品有致敏作用。
本品放出的游离氯有可能引起中毒。
环境危害:对环境有危害~对水体、土壤和大气可造成污染。
GHS危险性类别:根据《化学品分类和危险性公示通则》,GB 13690-2009,及化学品分类、警示标签和警示性说明规范系列标准~该产品属于皮肤腐蚀/刺激~类别1,严重眼损伤/眼睛刺激性~类别2A,对水环境的危害~类别1。
标签要素:象形图:警示词: 危险危险信息: 引起严重眼睛刺激; 对水生生物毒性非常大; 引起严重的皮肤灼伤和眼睛损伤。
防范说明: 预防措施:•密闭操作~注意通风~远离高热。
•操作尽可能机械化、自动化。
•操作人员佩戴自吸过滤式防毒面具,全面罩,~穿橡胶耐酸碱服~戴橡胶耐酸碱手套。
•避免与还原剂、酸类接触。
•搬运时要轻装轻卸~防止包装及容器损坏。
•工作场所不得进食、饮水。
•配备相应品种和数量的消防器材及泄漏应急处理设备。
事故响应:•皮肤接触:脱去被污染的衣着~用大量流动清水冲洗。
•眼睛接触:提起眼睑~用大量流动清水或生理盐水冲洗。
就医。
•吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
若呼吸困难~给输氧。
若呼吸停止~进行人工呼吸。
就医。
•食入:饮足量温水~催吐~就医。
安全储存:•储存于阴凉、干燥、通风良好的库房。
•远离火种、热源。
防止阳光直射。
•应与还原剂、酸类等分开存放。
•储区应备有泄漏应急处理设备和合适的收容材料。

化学品安全技术说明书产品名称: 漂白粉按照GB/T 16483、GB/T 17519 编制修订日期: 最初编制日期:版本:第1部分化学品及企业标识化学品中文名:漂白粉化学品英文名:Calcium Hypochlorite企业名称:企业地址:传真:联系电话:企业应急电话:产品推荐及限制用途:For industry use only.。
第2部分危险性概述紧急情况概述:可能加剧燃烧;氧化剂。
吞咽有害。
造成严重皮肤灼伤和眼损伤。
GHS危险性类别:氧化性固体类别 2急性经口毒性类别 4皮肤腐蚀/ 刺激类别1B危害水生环境——急性危险类别 1标签要素:象形图:警示词:危险危险性说明:H272 可能加剧燃烧;氧化剂。
H302 吞咽有害。
H314 造成严重皮肤灼伤和眼损伤。
H400 对水生生物毒性极大。
防范说明:•预防措施:•P210 远离热源/火花/明火/热表面。
禁止吸烟。
•P220 避开/贮存处远离服装/可燃材料。
•P280 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。
•P264 作业后彻底清洗。
•P270 使用本产品时不要进食、饮水或吸烟。
•P260 不要吸入粉尘/烟/气体/烟雾/蒸气/喷雾。
•P273 避免释放到环境中。
•事故响应:•P370+P378 火灾时:使用灭火器灭火。
•P301+P312 如误吞咽:如感觉不适,呼叫解毒中心/ 医生•P330 漱口。
•P301+P330+P331 如误吞咽:漱口。
不要诱导呕吐。
•P303+P361+P353 如皮肤(或头发)沾染:立即脱掉所有沾染的衣服。
用水清洗皮肤/淋浴。
•P363 沾染的衣服清洗后方可重新使用。
•P304+P340 如误吸入:将人转移到空气新鲜处,保持呼吸舒适体位。
•P310 立即呼叫解毒中心/医生•P321 具体治疗 ( 见本标签上的…… )。
•P305+P351+P338 如进入眼睛:用水小心冲洗几分钟。
如戴隐形眼镜并可方便地取出,取出隐形眼镜。
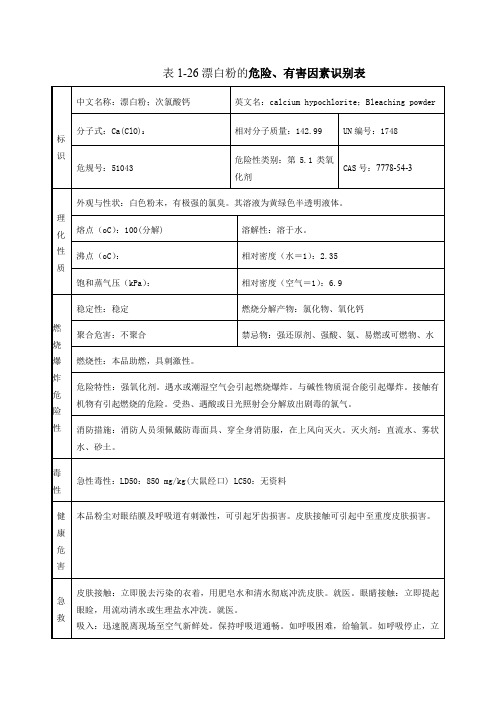
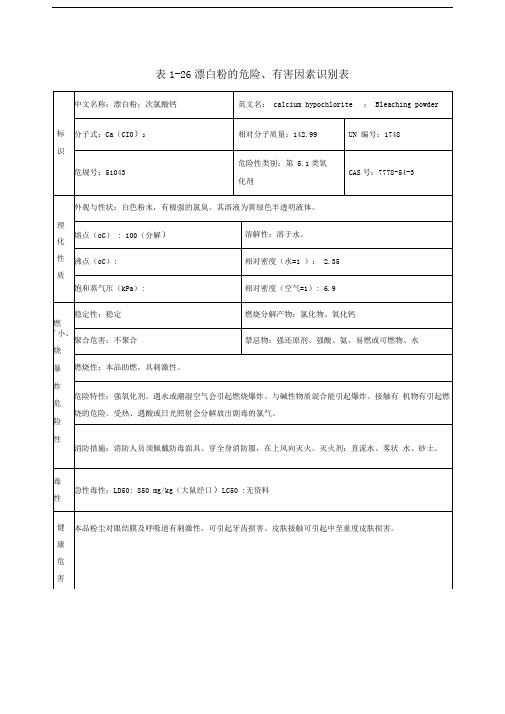
漂白粉危险化学品安全技术说明书第一部分化学品名称化学品中文名称:次氯酸钙化学品英文名称:calcium hypochlorite中文别名:氯化石灰英文别名:chlorine of lime技术说明书编码:051121第二部分成分/组成信息第三部分危险性概述危险性类别:侵入途径:健康危害:本品粉尘对眼结膜及呼吸道有刺激性,可引起牙齿损害。
皮肤接触可引起中至重度皮肤损害。
环境危害:燃爆危险:第四部分急救措施皮肤接触:立即脱去污染的衣着,用肥皂水和清水彻底冲洗皮肤。
就医。
眼睛接触:提起眼睑,用流动清水或生理盐水冲洗。
就医。
吸入:迅速脱离现场至空气新鲜处。
保持呼吸道通畅。
如呼吸困难,给输氧。
如呼吸停止,立即进行人工呼吸。
就医。
食入:饮足量温水,催吐。
就医。
第五部分消防措施危险特性:强氧化剂。
遇水或潮湿空气会引起燃烧爆炸。
与碱性物质混合能引起爆炸。
接触有机物有引起燃烧的危险。
受热、遇酸或日光照射会分解放出剧毒的氯气。
有害燃烧产物:灭火方法:消防人员须佩戴防毒面具、穿全身消防服,在上风向灭火。
灭火剂:直流水、雾状水、砂土。
灭火注意事项及措施:第六部分泄漏应急处理应急处理:隔离泄漏污染区,限制出入。
建议应急处理人员戴防尘面具(全面罩),穿防毒服。
不要直接接触泄漏物。
勿使泄漏物与还原剂、有机物、易燃物或金属粉末接触。
小量泄漏:避免扬尘,用洁净的铲子收集于干燥、洁净、有盖的容器中,转移至安全场所。
大量泄漏:用塑料布、帆布覆盖。
然后收集回收或运至废物处理场所处置。
第七部分操作处置与储存操作注意事项:储存注意事项:储存于阴凉、通风的库房。
远离火种、热源。
库温不超过30℃,相对湿度不超过80%。
包装要求密封,不可与空气接触。
应与还原剂、酸类、易(可)燃物等分开存放,切忌混储。
不宜大量储存或久存。
储区应备有合适的材料收容泄漏物。
第八部分接触控制/个体防护监测方法:工程控制:生产过程密闭,加强通风。
提供安全淋浴和洗眼设备。
次氯酸钠安全技术说明书
第一部分:化学品名称
化学品中文名称:次氯酸钠化学品俗名:漂水、漂白水
化学品英文名称:Sodium hypochlorite solution 英文名称:---------- 供应商名称:--------------------
地址:
电子邮件地址:---------- 邮编:518114
技术说明书编码:---------- 注册号:---------- 生效日期:----------
紧急联络电话:
第二部分:危险性概述
GHS危险性类别:皮肤腐蚀/刺激,类别1
标签象形图:
警示词:危险
侵入途径:吸入、吸食、经皮吸收
次氯酸钠放出的游离氯可引起中毒,亦可引起皮肤病。
已知本品有至敏作用。
用次氯酸钠漂健康危害:
白液洗手的工作,手掌大量出汉,指甲变薄,毛发脱落。
环境危害:对环境有危害,对水体、土壤和大气可造成污染。
燃爆危险:本品不燃。
第三部分:成分/组成信息
有害物成分含量CAS No.
次氯酸钠≥10% 7681-52-9
第四部分:急救措施
皮肤接触:脱去污染的衣着,用大量流动清水冲洗。
化学品安全技术说明书修订日期:2020-6-5 SDS编号:产品名称:次氯酸钙版本:2015第一版第一部分化学品及企业标识化学品中文名:次氯酸钙;漂粉精化学品英文名:calcium hypochlorite;Bleaching powder企业名称:***有限公司企业地址:***邮编:*** 传真: ***联系电话:***电子邮件地址:***企业应急电话:***产品推荐及限制用途:主要用于棉织物、麻织物、纸浆等的漂白。
利用其消毒杀菌作用广泛用于饮水、游泳池水净化、养蚕等方面。
还用于制造化学毒气和放射性的消毒剂。
此外,也用于医药工业等。
第二部分危险性概述紧急情况概述:该品粉尘对眼结膜及呼吸道有刺激性,可引起牙齿损害。
皮肤接触可引起中至重度皮肤损害。
该品粉尘对眼结膜及呼吸道有刺激性,可引起牙齿损害。
皮肤接触可引起中至重度皮肤损害。
GHS危险性类别:氧化性固体-2,皮肤腐蚀/刺激-1B,严重眼睛损伤/眼睛刺激性-1,特异性靶器官系统毒性一次接触-3,对水环境的危害-急性1,对水环境的危害- 长期慢性1,标签要素:象形图:警示词:危险危险信息:可引起燃烧或爆炸;强氧化剂; 引起严重的皮肤灼伤和眼睛损伤;防范说明:预防措施:远离火种、热源,工作场所严禁吸烟。
远离易燃、可燃物。
避免产生粉尘。
避免与还原剂、酸类接触。
事故响应:隔离泄漏污染区,限制出入。
建议应急处理人员戴防尘面具(全面罩),穿防毒服。
不要直接接触泄漏物。
勿使泄漏物与还原剂、有机物、易燃物或金属粉末接触。
安全储存:储存于阴凉、通风的库房。
远离火种、热源。
库温不超过30℃,相对湿度不超过 80%。
废弃处置:本品或其容器以当地法规处置物理和化学危险:该品粉尘对眼结膜及呼吸道有刺激性,可引起牙齿损害。
皮肤接触可引起中至重度皮肤损害。
皮肤接触后立即脱去污染的衣物,用流动清水彻底冲洗皮肤并就医。
健康危害:该品粉尘对眼结膜及呼吸道有刺激性,可引起牙齿损害。
皮肤接触可引起中至重度皮肤损害。
次氯酸钠安全技术说明中文名次氯酸钠英文名Sodiumhypochlorite又名次氯酸钠溶液次氯酸钠(液体)退色,漂白剂安替福民次亚氯酸钠溶液其余名称次氯酸钠溶液次氯酸钠(液体)退色,漂白剂安替福民次亚氯酸钠溶液分子式NaOCl分子量物性数据性状:次氯酸钠为白色粉末,在空气中极不稳固。
受热后快速自行分解,在碱性状态时较稳固。
一般工业品是无色或淡黄色液体,含有效氯为100~140g/L。
密度(g/mL25毒理学数据1.急性毒性[1]LD50:8500mg/kg(大鼠经口)2.刺激性[2] 家兔经眼:10mg,中度刺激。
3.致突变性[3] 微生物致突变:鼠伤寒沙门菌1mg/皿。
DNA损害:大肠杆菌420μmol/L。
细胞遗传学剖析:人淋巴细胞100ppm(24h)。
姐妹染色单体互换:人类胚胎149mg/L。
4.致癌性[4] IARC致癌性议论:G3,对人及动物致癌性凭证不足。
生态学数据1.生态毒性暂无资料2.生物降解性暂无资料3.非生物降解性暂无资料4.其余有害作用[5] 该物质对环境有危害,应特别注意对水体的污染。
分子构造数据计算化学数据性质与稳固性1.防止接触酸,复原剂,易氧化资料,有机资料,二氧化碳,胺,氨。
2.水溶液与碱金属,碱土金属和很多有机无机的活性化学药品是不相容的。
3.易溶于水生成烧碱和次氯酸,次氯酸再分解生成氯化氢和重生氧,因重生氧的氧化能力很强,所以次氯酸钠是强氧化剂。
其稳固度受光、热、重金属阳离子和pH值的影响。
拥有刺激气味。
还没有分别出无水试剂。
碱性溶液为无色液体。
迟缓分解出NaCl,NaClO3和O2。
分解速度与浓度和游离碱相关。
光照或加热能加快分解。
4.高浓度的次氯酸钠溶液在储藏过程中浓度会自动降低。
固体次氯酸钠不论是在含有5个结晶水仍是无水状态下均易发生爆炸。
它也是一种强氧化剂,所以应防止长时间的皮肤接触或吸入。
5.拥有强氧化性和腐化性。
皮肤接触会惹起烧伤。
进入体内会致使黏膜腐化、食管或气管穿孔、咽喉水肿。
安全技术说明书页: 1/13 巴斯夫安全技术说明书按照GB/T 16483编制日期 / 本次修订: 04.04.2022版本: 12.2日期/上次修订: 24.11.2021上次版本: 12.1日期 / 首次编制: 06.05.2006产品: Hydrosulfite F(30667828/SDS_GEN_CN/ZH)印刷日期 29.10.20231. 化学品及企业标识Hydrosulfite F推荐用途和限制用途: 化学品, 纺织工业用助剂, 造纸化学品推荐用途: 还原剂, 漂白剂, 仅用于工业用途, 无机还原剂公司:巴斯夫(中国)有限公司中国上海浦东江心沙路300号邮政编码 200137电话: +86 21 20391000传真号: +86 21 20394800E-mail地址: **********************紧急联络信息:巴斯夫紧急热线中心(中国)+86 21 5861-1199巴斯夫紧急热线中心(国际):电话: +49 180 2273-112Company:BASF (China) Co., Ltd.300 Jiang Xin Sha RoadPu Dong Shanghai 200137, CHINA Telephone: +86 21 20391000Telefax number: +86 21 20394800E-mail address: ********************** Emergency information:Emergency Call Center (China):+86 21 5861-1199International emergency number: Telephone: +49 180 2273-1122. 危险性概述纯物质和混合物的分类:巴斯夫安全技术说明书日期 / 本次修订: 04.04.2022版本: 12.2产品: Hydrosulfite F(30667828/SDS_GEN_CN/ZH)印刷日期 29.10.2023自热物质及其混合物: 分类1急性毒性: 分类5 (口服)皮肤腐蚀/刺激: 分类3严重损伤/刺激眼睛: 分类2A对水环境的急性危害: 分类3标签要素和警示性说明:图形符号:警示词:危险危险性说明:H251自热;可能燃烧。
SAFETY DATA SHEETSAccording to Globally Harmonized System of Classification andLabelling of Chemicals (GHS) - Sixth revised edition1.Identification1.1GHS Product identifierProduct name Calcium Hypochlorite1.2Other means of identificationProduct number-Other names Hypochlorous acid calcium salt1.3Recommended use of the chemical and restrictions on useIdentified uses For industry use only. DisinfectantUses advised against no data available1.4Supplier's detailsCompanyAddressTelephoneFax1.5Emergency phone numberEmergency phone numberService hours Monday to Friday, 9am-5pm (Standard time zone: UTC/GMThours).2.1Classification of the substance or mixtureOxidizing solids, Category 2Acute toxicity - Oral, Category 4Skin corrosion, Category 1BHazardous to the aquatic environment, short-term (Acute) - Category Acute 12.2GHS label elements, including precautionary statementsPictogram(s)Signal word DangerHazard statement(s)H272 May intensify fire; oxidizerH302 Harmful if swallowedH314 Causes severe skin burns and eye damageH400 Very toxic to aquatic lifePrecautionarystatement(s)Prevention P210 Keep away from heat, hot surfaces, sparks, open flames andother ignition sources. No smoking.P220 Keep away from clothing and other combustible materials.P280 Wear protective gloves/protective clothing/eyeprotection/face protection.P264 Wash ... thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P260 Do not breathe dust/fume/gas/mist/vapours/spray.P273 Avoid release to the environment.Response P370+P378 In case of fire: Use ... to extinguish.P301+P312 IF SWALLOWED: Call a POISON CENTER/doctor/…if youfeel unwell.P330 Rinse mouth.P301+P330+P331 IF SWALLOWED: Rinse mouth. Do NOT inducevomiting.P303+P361+P353 IF ON SKIN (or hair): Take off immediately allcontaminated clothing. Rinse skin with water [or shower].P363 Wash contaminated clothing before reuse.P304+P340 IF INHALED: Remove person to fresh air and keepcomfortable for breathing.P310 Immediately call a POISON CENTER/doctor/…P321 Specific treatment (see ... on this label).P305+P351+P338 IF IN EYES: Rinse cautiously with water for severalminutes. Remove contact lenses, if present and easy to do. Continuerinsing.P391 Collect spillage.Storage P405 Store locked up.Disposal P501 Dispose of contents/container to ...2.3Other hazards which do not result in classificationnone3.1Substances4.1Description of necessary first-aid measuresGeneral adviceConsult a physician. Show this safety data sheet to the doctor in attendance.If inhaledFresh air, rest. Half-upright position. Artificial respiration may be needed. Refer for medicalattention.In case of skin contactRemove contaminated clothes. Rinse skin with plenty of water or shower.In case of eye contactFirst rinse with plenty of water for several minutes (remove contact lenses if easily possible),then refer for medical attention.If swallowedRinse mouth. Give one or two glasses of water to drink. Do NOT induce vomiting. Refer formedical attention .4.2Most important symptoms/effects, acute and delayedExcerpt from ERG Guide 140 [Oxidizers]: Inhalation, ingestion or contact (skin, eyes) withvapors or substance may cause severe injury, burns or death. Fire may produce irritating,corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution.(ERG, 2016)4.3Indication of immediate medical attention and specialtreatment needed, if necessaryImmediate first aid: Ensure that adequate decontamination has been carried out. If patient is notbreathing, start artificial respiration, preferably with a demand-valve resuscitator,bag-valve-mask device, or pocket mask, as trained. Perform CPR as necessary. Immediatelyflush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs,lean patient forward or place on left side (head-down position, if possible) to maintain an openairway and prevent aspiration. Keep patient quiet and maintain normal body temperature.Obtain medical attention. /Hypochlorite and Related Compounds/5.1Extinguishing mediaSuitable extinguishing mediaIf material involved in fire: Extinguish using agent suitable for type of surrounding fire.(Material itself does not burn or burns with difficulty.) Use water in flooding quantities as fog.Cool all affected containers with flooding quantities of water. Apply water from as far adistance as possible. /Chlorinated lime, liquid/5.2Specific hazards arising from the chemicalExcerpt from ERG Guide 140 [Oxidizers]: These substances will accelerate burning wheninvolved in a fire. Some may decompose explosively when heated or involved in a fire. Mayexplode from heat or contamination. Some will react explosively with hydrocarbons (fuels).May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode whenheated. Runoff may create fire or explosion hazard. (ERG, 2016)5.3Special protective actions for fire-fightersWear self-contained breathing apparatus for firefighting if necessary.6.1Personal precautions, protective equipment and emergency proceduresUse personal protective equipment. Avoid dust formation. Avoid breathing vapours, mist or gas.Ensure adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust. Forpersonal protection see section 8.6.2Environmental precautionsPersonal protection: chemical protection suit, face shield and filter respirator for organic gasesand vapours adapted to the airborne concentration of the substance. Do NOT let this chemicalenter the environment. Sweep spilled substance into covered air-tight, dry containers. Thenstore and dispose of according to local regulations.6.3Methods and materials for containment and cleaning upPersonal protection: chemical protection suit, face shield and filter respirator for organic gasesand vapours adapted to the airborne concentration of the substance. Do NOT let this chemicalenter the environment. Sweep spilled substance into covered air-tight, dry containers. Thenstore and dispose of according to local regulations.7.1Precautions for safe handlingAvoid contact with skin and eyes. Avoid formation of dust and aerosols. Avoid exposure -obtain special instructions before use.Provide appropriate exhaust ventilation at places wheredust is formed. For precautions see section 2.2.7.2Conditions for safe storage, including any incompatibilitiesWell closed. Store in an area without drain or sewer access. Separated from food and feedstuffs.See Chemical Dangers.Store in a cool, dry, well-ventilated location at a temperature below 120deg F (50°C) to avoid slow decomposition. Separate from oxidizing materils, acids, ammonia,amines, and other chlorinating agents. Immediately remove and properly dispose of any spilledmaterial. /Calcium hypochlorite, dry or calcium hypochlorite, mixtures, dry/8.1Control parametersOccupational Exposure limit valuesno data availableBiological limit valuesno data available8.2Appropriate engineering controlsHandle in accordance with good industrial hygiene and safety practice. Wash hands beforebreaks and at the end of workday.8.3Individual protection measures, such as personal protective equipment (PPE)Eye/face protectionSafety glasses with side-shields conforming to EN166. Use equipment for eye protection testedand approved under appropriate government standards such as NIOSH (US) or EN 166(EU).Skin protectionWear impervious clothing. The type of protective equipment must be selected according to theconcentration and amount of the dangerous substance at the specific workplace. Handle withgloves. Gloves must be inspected prior to use. Use proper glove removal technique(withouttouching glove's outer surface) to avoid skin contact with this product. Dispose of contaminatedgloves after use in accordance with applicable laws and good laboratory practices. Wash anddry hands. The selected protective gloves have to satisfy the specifications of EU Directive89/686/EEC and the standard EN 374 derived from it.Respiratory protectionWear dust mask when handling large quantities.Thermal hazardsno data availablePhysical state white powder with a chlorine-like odourColour PowderOdour Strong chlorine odor100ºCMelting point/ freezingpointBoiling point or initialDecomposes at 100°Cboiling point and boilingrangeFlammability Not combustible but enhances combustion of othersubstances. Many reactions may cause fire or explosion.Gives off irritating or toxic fumes (or gases) in a fire.no data availableLower and upper explosionlimit / flammabilitylimitFlash point no data availableno data availableAuto-ignitiontemperature100°CDecompositiontemperaturepH no data availableKinematic viscosity no data availableSolubility21% in water at 25°Cno data availablePartition coefficientn-octanol/water (logvalue)Vapour pressure no data available2.35g/mLat 25°C(lit.)Density and/or relativedensityRelative vapour density no data availableParticle characteristics n o data available10.1Reactivityno data available10.2Chemical stabilityAll hypochlorite soln are unstable, esp if acidified; slowly decomp on contact with air/hypochlorite soln/10.3Possibility of hazardous reactionsContact with combustible materials will increase fire hazard. May undergo accelerateddecomposition with release of heat above 350 deg F (177°C). /Calcium hypochlorite, dry, orcalcium hypochlorite, mixtures, dry/CALCIUM HYPOCHLORITE is a powerful oxidizingagent, particularly in the presence of water or as it decomposes when heated to release oxygenand chlorine gases. May react vigorously with carbon; reacts potentially explosively with finelydivided carbon. Reacts with acetylene to form explosive chloroacetylenes. Reactions withorganic matter, oil, hydrocarbons; alcohols may lead to explosions. Reactions withnitromethane, methanol, ethanol (and other alcohols) can become violent after a delay. Reactswith possible ignition and/or explosion with organic sulfur compounds and with sulfides.Decomposes evolving oxygen, a change that can be catalyzed by rust on metal containers.Forms highly explosive NCl3 on contact with urea or ammonia. Evolves highly toxic gaseouschlorine gas when heated or on contact with acids [Sax, 9th ed., 1996, p. 1905]. A mixture withdamp sulfur reacted violently, and molten sulfur was ejected [Chem Eng. News, 1965, 46(29),6]. The combination of calcium hypochlorite, sodium hydrogen sulfate, starch, and sodiumcarbonate, when compressed, caused the materials to incandescence, followed by explosion,[Ind. Eng. Chem., 1937, 15, 282].10.4Conditions to avoidno data available10.5Incompatible materialsReacts with water and with acids releasing chlorine. Forms explosive compounds withammonia and amines. Strong oxidizer. Other incompatible materials include organics, nitrogencontaining compounds, dry chemical fire extinguishers containing mono-ammonium phosphate, combustible or flammable materials. /Calcium hypochlorite, dry or calcium hypochlorite,mixtures, dry/10.6Hazardous decomposition productsThe 70% grade may decomp violently if exposed to heat or direct sunlight. Gives off chlorineand chlorine monoxide above 350 deg F (poisonous gases).Acute toxicity•Oral: LD50 Rat (male) oral 790 mg/kg•Inhalation: no data available•Dermal: no data availableSkin corrosion/irritationno data availableSerious eye damage/irritationno data availableRespiratory or skin sensitizationno data availableGerm cell mutagenicityCarcinogenicityEvaluation: There is inadequate evidence for the carcinogenicity of hypochlorite salts inexperimental animals. No data were available from studies in humans on the carcinogenicity of hypochlorite salts. Overall evaluation: Hypochlorite salts are not classifiable as to theircarcinogenicity to humans (Group 3). /Hypochlorite salts/Reproductive toxicityno data availableSTOT-single exposureno data availableSTOT-repeated exposureno data availableAspiration hazardno data available12.1Toxicity•Toxicity to fish: LC50; Species: Lepomis macrochirus (Bluegill) juvenile, weight 3.91 g, length4.6 cm; Conditions: freshwater, renewal, 32°C, pH 7.25-7.55, hardness 41.5-46.3 mg/L CaCO3,alkalinity 42-45 mg/L CaCO3; Concentration: 71 ug/L for 24 hr (95% confidence interval: 68-75 ug/L) /total Cl ion•Toxicity to daphnia and other aquatic invertebrates: EC50; Species: Daphnia magna (Water flea) 1st instar; Conditions: freshwater, static; Concentration: 73 ug/L for 48 hr (95% confidence interval: 67-79 ug/L); Effect: intoxication, immobilization /65% purity•Toxicity to algae: EC50; Species: Pseudokirchneriella subcapitata (Green algae) 3 day algal culture, 1,200,000 cells/cu cm; Conditions: freshwater, 27°C; Concentration: 983 ug/L for 72 hr (95% confidence interval: 805-1210 ug/L); Effect: decreased population growth rate /Chlorinated lime•Toxicity to microorganisms: no data available12.2Persistence and degradabilityno data available12.3Bioaccumulative potentialno data available12.4Mobility in soilno data available12.5Other adverse effects13.1Disposal methodsProductThe material can be disposed of by removal to a licensed chemical destruction plant or bycontrolled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed orseed by storage or disposal. Do not discharge to sewer systems.Contaminated packagingContainers can be triply rinsed (or equivalent) and offered for recycling or reconditioning.Alternatively, the packaging can be punctured to make it unusable for other purposes and thenbe disposed of in a sanitary landfill. Controlled incineration with flue gas scrubbing is possiblefor combustible packaging materials.14.1UN NumberADR/RID: UN1748IMDG: UN1748IATA: UN174814.2UN Proper Shipping NameADR/RID: CALCIUM HYPOCHLORITE, DRY or CALCIUM HYPOCHLORITE MIXTURE, DRY with mo than 39% available chlorine (8.8% available oxygen)IMDG: CALCIUM HYPOCHLORITE, DRY or CALCIUM HYPOCHLORITE MIXTURE, DRY with more th 39% available chlorine (8.8% available oxygen)IATA: CALCIUM HYPOCHLORITE, DRY or CALCIUM HYPOCHLORITE MIXTURE, DRY with more th 39% available chlorine (8.8% available oxygen)14.3Transport hazard class(es)ADR/RID: 5.1IMDG: 5.1IATA: 5.114.4Packing group, if applicableADR/RID: II IMDG: II IATA: II14.5Environmental hazardsADR/RID: yes IMDG: yes IATA: yes14.6Special precautions for userno data available14.7Transport in bulk according to Annex II of MARPOL 73/78 andthe IBC Codeno data available15.1Safety, health and environmental regulations specific for the product in questionInformation on revisionCreation Date Aug 16, 2017Revision Date Aug 16, 2017Abbreviations and acronyms•CAS: Chemical Abstracts Service•ADR: European Agreement concerning the International Carriage of Dangerous Goods by Road •RID: Regulation concerning the International Carriage of Dangerous Goods by Rail•IMDG: International Maritime Dangerous Goods•IATA: International Air Transportation Association•TWA: Time Weighted Average•STEL: Short term exposure limit•LC50: Lethal Concentration 50%•LD50: Lethal Dose 50%•EC50: Effective Concentration 50%Disclaimer: The above information is believed to be correct but does not purportto be all inclusive and shall be used only as a guide. The information in this documentis based on the present state of our knowledge and is applicable to the product withregard to appropriate safety precautions. It does not represent any guarantee ofthe properties of the product. We as supplier shall not be held liable for any damageresulting from handling or from contact with the above product.。