reach法规
- 格式:docx
- 大小:19.89 KB
- 文档页数:5

REACH法规要求REACH法规是指欧洲联盟中的一项化学品管理法规,其全称为注册、评估、授权和限制化学品(Registration, Evaluation, Authorisation and Restriction of Chemicals)。
该法规于2024年6月1日在欧盟正式生效,旨在确保化学品的安全使用,保护人类健康和环境。
1. 注册(Registration):根据REACH法规,所有在欧盟市场上生产或进口1吨或以上的化学品都需要进行注册。
注册要求化学品的生产商、进口商或下游使用者提供化学品的详细信息,包括物理化学性质、毒理学特性、使用方法和安全措施等,并提交注册文件给欧洲化学品管理局(ECHA)。
2. 评估(Evaluation):ECHA会对提交的注册文件进行评估,特别关注高风险化学品的安全性和合规性。
如果评估结果显示存在较高的风险,ECHA可以要求制定进一步的测试计划或限制化学品的使用。
3. 授权(Authorisation):对于高度关注的特定化学品,REACH法规要求进行授权管理。
这意味着,使用这些化学品的企业需要向ECHA申请授权,并证明其对于替代物的研究和发展做出了努力。
4. 限制(Restriction):REACH法规对一些特定的化学品或化学物质的使用进行限制。
这些限制可能包括生产、销售、使用和处理的限制,旨在减少其对人类健康和环境的潜在危害。
1.数据收集和共享:化学品的注册要求企业提供详细的物理化学性质、毒理学特性等数据。
这些数据的收集和共享有助于了解化学品的安全风险,促进化学品的合规和创新。
2.替代研究和发展:对于受授权管理的化学品,使用者需要研究和开发替代产品或替代方法。
这推动了化学品行业的创新和绿色发展。
3.安全管理和信息传递:REACH法规要求企业提供化学品的使用方法和安全措施等信息。
这有助于企业和用户更好地管理化学品的安全风险,并确保其正确使用和处理。
4.市场准入和竞争优势:REACH法规规定,没有注册的化学品将无法进入欧盟市场。


欧盟reach法规一、REACH法规背景2001年2月欧盟发布《欧盟未来化学品政策战略》白皮书,介绍了欧委会为实现可持续发展这一首要目标提出的有关欧洲共同体未来化学品政策的战略建议。
1998年欧盟的化学工业占全球第一位,第二位是美国,占28%的产值,贸易顺差为120亿欧元。
2020年欧洲是全球第二大化学品生产方,化工产业是欧盟第四大产业,生产的化学品中有59%直接提供给健康、建筑、汽车、电子和纺织品等其他产业。
一些化学品还对人类健康和环境造成严重损害,导致疾病和早产。
一个众所周知的例子是石棉。
在过去的几十年里,一些疾病的发病率显著增加。
这些疾病的原因尚未查明,担心某些化学物质会引起过敏是合理的。
担忧的原因是缺乏对许多化学物质对人类健康和环境的影响的了解。
这些例子暴露了当前欧盟化学政策的弱点。
1998年4月,在切斯特举行的环境部长非正式理事会会议上,欧洲委员会认识到有必要审查关于化学品的四份现有法律文件,因为人们担心目前的欧盟化学品政策不能提供充分的保护,这引起了越来越大的争议。
为统一协调欧盟对化学品管理的各项法规、政策及措施,维持欧盟化学品工业的竞争性和创造性,减少化学品对环境的污染和人类健康的损害,欧委会制定了REACH法规。
REACH是欧盟的一项对进入其市场的所有化学品进行预防性管理的一项化学品管理法规。
旨在加强对人类健康和环境的保护,使其免受可能由化学品造成的危害,促进安全和可持续化学品的创新,欧盟化学品可持续发展战略,实现无毒环境。
原则“没有数据就没有市场”二、REACH适用范围1.REACH适用于所有化学物质,不仅在工业过程中使用,而且在我们的日常生活中也使用。
例如清洁产品、油漆以及衣服、家具和电器等物品。
因此,该法规对欧盟大多数公司都有影响。
2.适用于企业,无论在供应链中处于什么角色,以及它们生产、进口、出口、供应或使用的产品,这意味着法律很可能在某种程度上适用于你的企业:•涵盖所有制造、进口、分销或使用化学品作为原料或成品的行业(不限于化学工业)•无论你的公司规模有多大,都适用于你•使您对您投放在市场或使用的物质的安全使用负责•要求供应链中的每一个参与者都要就化学品的安全使用进行信息交流•使消费者有权询问贵公司产品中所含的高度关注的物质3.适用于整个欧洲经济区(EEA),包括欧盟成员国、冰岛、列支敦士登和挪威。

欧盟reach最新标准168欧盟REACH最新标准168。
欧盟REACH(Registration, Evaluation, Authorization and Restriction of Chemicals)是欧盟制定的一项旨在保护人类健康和环境的化学品管理法规。
REACH法规的目标是确保化学品的安全使用,并鼓励替代危险化学品。
REACH法规适用于所有在欧盟市场上生产或进口化学品的公司,无论其规模大小。
其中,REACH最新标准168是指针对特定化学品的最新法规要求。
根据欧盟REACH最新标准168,化学品的生产商或进口商需要对其产品进行注册、评估和授权。
其中,注册要求化学品生产商或进口商提交详细的化学品信息,并对其进行风险评估。
评估阶段要求对化学品的危害性和风险进行全面评估,以确定其对人类健康和环境的影响。
授权阶段则是针对特定的高风险化学品,需要获得授权才能在市场上销售和使用。
REACH最新标准168还包括对化学品的限制要求。
针对特定的化学品,欧盟可能会对其使用进行限制,以减少其对人类健康和环境的危害。
这些限制可能涉及化学品的用途、浓度限制、生产和销售限制等方面。
此外,REACH最新标准168还要求化学品生产商或进口商向欧盟化学品管理局提交详细的化学品安全报告。
这些报告需要包括化学品的物理化学性质、毒性、环境行为等方面的信息,以评估其对人类健康和环境的影响。
为了遵守REACH最新标准168,化学品生产商或进口商需要密切关注欧盟对化学品相关法规的更新和变化。
他们需要及时了解最新的法规要求,并对其产品进行相应的注册、评估和授权,以确保其产品在欧盟市场上的合规性。
总之,欧盟REACH最新标准168对化学品生产商或进口商提出了更加严格的要求,以确保化学品的安全使用并保护人类健康和环境。
化学品相关企业需要密切关注最新的法规要求,并采取相应的措施以确保其产品的合规性。
同时,欧盟REACH最新标准168也为化学品的安全管理提供了更加全面和严谨的法规框架,有助于推动化学品行业的可持续发展和健康发展。

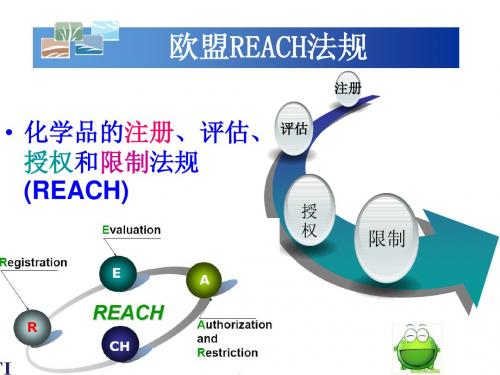
reach法规介绍Reach法规介绍概述:Reach法规(Registration, Evaluation, Authorization and Restriction of Chemicals),中文名为化学品注册、评估、授权和限制法规,是欧洲联盟(EU)针对化学品管理制定的一项法规。
该法规于2007年6月1日生效,旨在保护人类健康和环境,确保化学品的安全使用。
1. 注册(Registration):Reach法规要求在欧洲市场上生产或进口超过1吨/年的化学品进行注册。
注册的目的是收集化学品的相关信息,包括物理化学性质、毒性、环境影响等。
这些信息将用于评估化学品对人类健康和环境的潜在风险。
2. 评估(Evaluation):注册后的化学品需要经过评估,以确定其对健康和环境的风险水平。
评估的范围包括化学品的分类和标识、生态毒性、长期积累性、潜在致畸性和致癌性等。
评估结果将用于制定控制措施和限制授权的决策。
3. 授权(Authorization):某些特别关注的化学品可能需要授权才能继续在市场上销售和使用。
这些化学品被称为特别关注物质(SVHCs,Substances of Very High Concern)。
授权是一项复杂的过程,需要评估化学品的替代品是否可行,并考虑社会经济因素。
只有获得授权的化学品才能继续使用。
4. 限制(Restriction):Reach法规还规定了一些对特定化学品的使用限制。
这些限制是为了保护人类健康和环境免受化学品的危害。
限制的范围包括禁止或限制化学品的使用、生产、进口和销售。
限制的依据是对化学品的评估结果和科学证据。
Reach法规的重要性:Reach法规是全球化学品管理的重要里程碑,对化学品生产和使用行业具有深远影响。
1. 保护人类健康:Reach法规要求对化学品进行全面的评估和控制,以减少人类暴露于有害化学物质的风险。
通过限制和授权机制,尽量减少有害化学品在市场上的使用。
reach法规简介
REACH法规是欧盟对进入其市场的所有化学品进行预防性管理的法规,全
称为《关于化学品注册、评估、许可和限制的法规》。
该法规于2007年6
月1日正式实施,旨在保障人类健康和环境安全,促进化学工业的可持续发展。
根据REACH法规要求,欧盟委员会将建立统一的化学品监控管理体系,对欧盟市场上约3万种化学产品及其下游的纺织、轻工、制药等产品分别纳入注册、评估、许可3个管理监控系统。
未纳入该管理系统的产品不能在欧盟市场上销售。
REACH法规的影响范围非常广,是近年来欧盟实施的各项指
令中影响最大的法规之一。
REACH法规的核心是对化学品的注册、评估和许可。
企业需要对其所生产
的化学品进行注册,提交相关资料和数据,以证明其产品的安全性和合规性。
评估环节则是对企业提交的数据进行审查和评估,以确保产品的安全性和合规性。
如果企业希望在欧盟市场上销售其产品,需要进行许可程序,获得相应的许可证书。
REACH法规的实施将促进化学工业的革新,使企业更加注重产品的安全性
和环保性,同时也将刺激竞争和增长。
与现行复杂的法规体系不同,
REACH将在欧盟范围内创建一个统一的化学品管理体系,使企业能够遵循同一原则生产新的化学品及其产品。
欧盟REACH法规附录17清单是欧盟化学品管理法规REACH (Registration, Evaluation, Authorization and Restriction of Chemicals)的一部分,该清单详细列出了受限制和受监管的化学品物质。
REACH法规是欧盟对化学品的最严格监管法规之一,旨在保护人类健康和环境,同时促进化学品的竞争力和创新。
欧盟REACH法规附录17清单所涉及的化学品物质主要包括受限制物质和优先受关注物质。
受限制物质指的是在欧盟境内生产、销售或进口的化学品中所禁止或受限制使用的物质,这些物质可能对人类健康或环境造成危害,比如致癌物质、致畸物质、激素干扰物质等。
而优先受关注物质则是指那些可能对人类健康或环境产生潜在危害的化学品物质,需要进行更加严格的监管和控制。
在欧盟REACH法规附录17清单中列出的化学品物质受到了严格的管控和限制,包括其在产品中的使用限制、标识要求、禁止使用等。
这些措施旨在减少化学品对人类健康和环境的潜在威胁,促进可持续发展和绿色化学品生产。
从我个人的角度来看,欧盟REACH法规附录17清单的制定和执行对于化学品行业和全球环境都具有重要意义。
这一法规的实施可以促使化学品生产企业更加重视产品安全和环保,避免使用有害物质,从而保障消费者和环境的安全。
欧盟REACH法规的标准和要求也能够影响全球化学品贸易和生产,引导全球化学品行业向更加环保、绿色的方向发展。
这一法规的推行也为其他国家和地区的化学品管理提供了重要借鉴和参考。
欧盟REACH法规附录17清单的制定和实施对于化学品行业、人类健康和环境都具有重要意义。
我们应该更加重视化学品的安全和环保问题,积极遵守和执行类似的法规要求,共同推动全球化学品行业向着更加健康、绿色的方向发展。
在学习欧盟REACH法规附录17清单的过程中,我们应该重点关注受限制和受监管的化学品物质,了解其对人类健康和环境可能产生的影响,并积极采取相应措施保护我们自己和环境。
REACH简介REACH是指:化学品注册、评估、授权和限制的欧洲第1907/2006号(EC)法规,即:R egistration, E valuation, A uthorization and R estriction of Chemicals (REACH) 。
REACH法规是欧盟对进入其市场的所有化学品进行预防性管理的一项强制性管理法规。
它构建了一个庞大繁杂的化学品管理体系,将过去由政府承担的化学品安全责任转移到生产经营者身上,要求生产商、进口商和化学品下游用户对其产品所用到的化学品的安全性负责。
REACH相对于以前欧盟化学品法规最大区别是:一种物质没有被证明它是安全的之前,被认为是危险的。
而以前则相反。
REACH的目的保护人类健康和环境;保持和提高欧盟化学工业的竞争力;增加化学品信息的透明度;减少脊椎动物试验;与欧盟在WTO框架下的国际义务相一致。
从实质意义上讲,REACH法规将促进化学工业的革新,使其生产更安全的产品,刺激竞争和增长。
与现行复杂的法规体系不同,REACH将在欧盟范围内创建一个统一的化学品管理体系,使企业能够遵循同一原则生产新的化学品及其产品。
REACH的适用范围REACH适用于所有在欧盟境内生产或进口的重量超过1吨/年的化学物质、配置品和产品所含有的化学物质(非配置品或产品本身)。
REACH涉及的范围远远超出化工行业的范畴,几乎所有行业都或多或少受到REACH的影响。
REACH法规基本原则:预防性原则(Precautionary Principe)无数据,无市场(“NO DATA,NO Market”)除非物质自身、在配制品中或在物品中的物质根据要求进行了注册,否则不得在欧盟内制造或者投放市场。
一物质,一注册(“One Substance,One Registration”)同一种物质一般只进行一次注册,但行为人出于保密需要,也可以单独注册。
REACH的主要要求:1、注册(R egistration):2、评估(E valuation):3、授权(A uthorization):只有那些高度关注的物质(SVHC)才需要授权。
REACH法规介绍REACH是指欧盟颁布的一项关于化学品的法规,全称为Registration, Evaluation, Authorisation and Restriction of Chemicals(化学品登记、评估、授权和限制)。
这项法规于2024年6月1日正式实施,旨在提高欧洲化学品安全,并确保人类健康和环境的保护。
以下是对REACH法规的详细介绍。
REACH法规的制定是为了填补欧洲化学品管理领域的法律空白,并解决欧洲企业和消费者面临的化学品相关的风险和威胁。
之前的化学品法规,在欧洲范围内存在着各种缺陷和不足,无法提供足够的信息和保障,因此REACH法规的目标是确保全面的化学品管理和安全。
1.全面责任原则:根据REACH法规,化学品的制造商、进口商和使用者都有责任确保其化学品的安全性,并提供相关信息。
2.替代原则:REACH强调替代更危险化学品为较安全和环保的替代品。
3.数据共享原则:根据REACH法规,化学品制造商和进口商要共享化学品相关的信息,以减少动物试验的重复和降低成本。
4.高度保护原则:REACH法规鼓励使用风险较低的替代品,并确保高度易悬浮或易积累的特定物质受到限制。
1.化学品注册:根据REACH法规,制造商和进口商必须对其生产或进口的化学品进行注册。
注册的目的是收集有关化学品的信息,包括有毒性、生态毒性和环境行为等方面的数据。
2.化学品评估:根据REACH法规,化学品登记后将进行评估,以确保其安全性。
这包括评估化学品的风险,并确保根据使用目的采取适当的控制措施,例如限制使用或要求特别授权。
3.化学品授权:对于特定的特别关注物质(SVHCs),根据REACH法规,需要进行授权才能继续使用。
这些物质被认为具有严重的健康和环境风险。
4.物质限制:REACH法规对一些特定物质进行了限制,以减少其在产品和市场上的使用。
这些限制可能针对一些高度有毒物质、易悬浮或易积累的物质,以及对环境和人类健康具有严重潜在危害的物质。
Joint position of the German Government, the Association of the German ChemicalIndustry (VCI) and the Mining, Chemical and Energy Industrial Union (IGBCE)on the consultation document of the European Commission on the Registration,Evaluation and Authorization of Chemicals (REACH)Preliminary remarksIn March 2002, the abovementioned bodies agreed on a joint position on the EU Commission White Paper "Strategy for a future chemicals policy". We reaffirm the statements made in that paper, which also forms the basis of our assessment of the Commission consultation document. The core statement, i.e. that a high level of effective protection of human health and the environment should be provided in such a way that it ensures the chemical industry's innovativeness and competitiveness, remains unchanged. We need regulations which afford effectiveprotection of health and the environment at the lowest possible cost and in quick, simple and reliable administrative procedures.The Commission's consultation document takes up some of our proposals. For example, the envisaged facilitations for intermediates, as well as for research and development, are welcomed. We also support the selection of the groups of substances envisaged for the authorization procedure. However, the shortcomings in the consultation document involve the risk, that without amendments the competitiveness of companies and jobs could be jeopardized (hence impact assessment - cf. below).The key points of criticism can be summarized as follows:- The registration procedure in particular is still too bureaucratic and expensive and fails to fulfil the promise of swift, simple and cost-effective procedures.- The area of application and the requirements for the Chemical Safety Report are toofar-reaching and represent a considerable problem, particularly for SME users in thevalue-added chain.- 2 -- The industrial and commercial confidentiality has not been sufficiently ensured.On the initiative of Chancellor Schr?der, Prime Minister Blair and President Chirac the European Council on 21 March 2003 sent out a clear message that it intends to strengthen the EU's industrial competitiveness. One key element is that the Commission is to subject all major dossiers to a comprehensive impact assessment with regard to industrial competitiveness. This benefit and impact assessment is of central importance to the new chemicals regime. We therefore call upon the European Commission to quickly submit a revised draft. Before this draft is examined in the European Parliament and in the Council, the concrete impact of the envisaged regulations on the European chemicals industry and the economy along the entire value-added chain should be assessed by a third party. In view of the European Parliament elections due to take place in June 2004, the proposed impact assessment would not delay the legislative process.I. Authorization procedure1. Inclusion of further groups of substancesThe proposed regulations in the Commission's consultation document on the inclusionof CMR, PBT and vPvB substances are in accordance with our joint position paperand can be supported. It is proposed that in Point 44 – in connection with an annexlisting the technical criteria – the group of sensitizing and chronically toxic substancesdescribed in more detail in the joint position paper be listed in a separate sub-paragraph.2. Inclusion of further substancesCompared to the chemicals White Paper, further groups of substances (cf. item 1) havebeen included. We must prevent the system from becoming overloaded due to the inclusionof other substances merely suspected of being harmful as this would havenegative consequences for the industry. Against this background, the opening clause(point 44) envisaged by the Commission is too vague.The inclusion of further substances can therefore only be considered according to the following limited criteria:- 3 -- The exceptional character of the regulation must be clear.- In the Regulation itself, the valid sound technical and scientific criteria shouldbe defined which constitute a level of concern comparable to the other groupsof substances.- As with other cases today, a qualified majority in the regulatory committeeshould be required for the inclusion of further substances.3. Immediate decisionAn immediate decision on uses ready for decision should be possible. With regard to a decision on the inclusion of a substance in Annex XIII, it should be examined to whatextent certain uses are directly subject to a restriction. This could reduce the number of authorization procedures to be carried out.4. Authorization time limitsThe so-called sunset date regulation (Point 46 No. 1 c and d) is welcomed. Under it,those uses which were notified up to 18 months before a certain date may be continueduntil a decision on authorization has been made. The Commission should make it clearthat the regulation is to be interpreted in such a way that the normal authorization procedure can be initiated in advance for uses developed during the 18 monthsbetween the stated date and the expiry date.5. National authorizationThe option of national authorization should be examined against the background ofpossible distortions of competition.II. Registration/evaluation1. PolymersThe large number of existing polymers and their wide range of uses in industryaccount for the particular importance of this group of substances to the chemicalindustry and the users in the value-added chain. The review of the obligation to regis-4 -ter proposed in the Commission consultation document involves a lot of costs andefforts. Moreover, it is difficult to establish criteria for identifying a manageable number of the relevant polymers which are both practicable and cost-efficient. Theyshould therefore be initially exempted from the obligation to register until apracticable and cost-efficient selection is possible on the basis of sound technical and valid scientific criteria. As soon as this is feasible at a later date in time, they should be included.2. IntermediatesIn the joint position paper, we expressed our support for exempting intermediates used exclusively in a closed system (including closed transports) from the authorization procedure. This basic line has been confirmed. The Commission's consultationdocument also contains proposals on this which we welcome in principle. It is crucialto ensure that data collection is made easier if the substances are handled in clearly defined and controlled conditions. Against this background, the followingamendments/additions are proposed:- Application of the provisions concerning transported intermediates to intermediatesused on-site in closed systems taking the 28th adapting Directive onthe EU Directive on Dangerous Substances as guidance.- The limitation to only two sites should be waived in the case of transported intermediates.- In view of possible operational accidents, a minimum set of data should berequired for intermediates for which simplifications apply.3. Uniform evaluation standardsEU-wide binding standards for the evaluation of substances should be laid down in the Regulation.4. Obligation to notify research and developmentThe easing of regulations for research and development contained in the consultation document are welcomed. For competitive reasons, however, research projects in com-5 -panies should not be made public as a result of the envisaged obligation to notify such projects. Notifications should therefore be generally treated confidentially.5. Transitional periodsSubstances with EINECS status are treated like phase-in substances if more than 1 t/ahas been produced/imported during the previous ten years. For other substances containedin EINECS of which more than 1 t/a has been produced/imported, an appropriatetransitional period shall apply.6. Intended useThe Commission is requested to develop a system of use and exposure categories tomake the REACH procedure easier to manage, in particular for downstream users.III. Role of the EU Chemicals AgencyThe planned European Chemicals Agency will play a central role in the effective management of the registration, evaluation and authorization of chemicals. There isgreat potential for cost savings here. However, this central role is also important in order to avoid distortions of competition. The Agency should therefore ensure inparticular a uniform application of the rules in all member states. In so far as there are competences for this under the EC Treaty, the Agency should therefore also beallocated decision-making powers. Furthermore, it should also make a key contributionin the administrative procedures towards efficiency and cost savings.It is therefore proposed that the Agency- be able to make binding decisions on any questions arising from the registrationof substances,- confirm receipt of documents, in particular in the case of registrations, if possible, establish whether all documents are complete and inform the registrant ofthis directly (so that, in the interests of companies, an early marketing authorization can be obtained quickly).- 6 -It should be examined whether an appeals committee should be established for disputes on recommendations or decisions made by the Agency.IV. Quality assuranceAn appropriate and effective system of quality assurance should be set up for the documents to be submitted by industry for registration. Depending on the preferencesof those involved, either internal quality assurance measures by the industry with external certification of the system or a prior review of the data to be submitted by independent experts should be considered.V. Safe handling of chemicalsWe support the basic rule on the safe handling of chemicals contained in the consultation document (Point 3) in principle (subject to minor changes). The aim of aresponsible chemicals policy must be to develop safer substances or uses in the case of recognized risks.Taking into consideration the protective purpose of the Chemical Safety Reports, in its drafting the following simplifications for companies are proposed:- Compulsory for substances subject to registration.- Reasonable harmonization of Chemical Safety Reports and safety data sheets,although it is not necessary to provide Chemical Safety Reports to downstreamusers regularly. Rather, only the safety data sheet must be provided on a regularbasis. However, the Chemical Safety Report should be supplied on request.- Summarizing Chemical Safety Report for preparations with reporting obligationsfor those constituent substances of relevance to safetyrecommendations and/or restrictions on use corresponding to the compilationof safety data sheets as laid down in the Directive on preparations.VI. Industrial and commercial confidentialityIn line with the joint position paper, industrial and commercial confidentiality must be ensured. On the other hand, the necessary information must be accessible to the authorities, and the public also has a legitimate right to information. Particularly the - 7 -regulation contained in the consultation document that all information not expressly declared to be confidential should be made available on the Internet, means that,owing to the possible combination of data, information relevant to competition ismade accessible.In order to ensure industrial and commercial confidentiality and, on the other hand, to ensure that the public has the necessary access to information, the following measures are proposed:- All information required to classify substances as hazardous should be madepublic.- Information should be provided on request by the competent authorities. Theyshould refrain from publishing all non-confidential information.- It would suffice if the reasons for handling documents confidentially are made credible.- In order not to reveal business relations in value-added chains, the names and addresses of users (downstream users) should be treated confidentially.VII. SanctionsThe sanctions regulation should be reexamined taking into account the principle of proportionality.。