聚甲基丙烯酸甲酯之结构制备
- 格式:ppt
- 大小:355.50 KB
- 文档页数:20

年产1000吨聚甲基丙烯酸甲酯生产工艺设计本文旨在介绍聚甲基丙烯酸甲酯的生产工艺设计的背景和目的。
聚甲基丙烯酸甲酯是一种重要的聚合物,在化工行业中具有广泛的应用。
它具有优异的耐候性、耐化学性和机械性能,被广泛用于涂料、粘合剂、纺织品、塑料、橡胶等领域。
为了满足市场需求,设计一个年产1000吨的聚甲基丙烯酸甲酯生产工艺是必要的。
本文将详细介绍该生产工艺的设计方案,以确保高效、稳定和可持续的生产。
聚甲基丙烯酸甲酯的生产过程通常包括单体合成、聚合反应、分离和精制等步骤。
每个步骤都需要精确的控制和优化,以确保产品的质量和产量。
目前,聚甲基丙烯酸甲酯的生产工艺已经相对成熟,但仍存在一些挑战和改进的空间。
例如,如何提高单体的合成效率、提高聚合反应的选择性和降低分离过程中的能耗等。
本文的目的是设计一个可行的年产1000吨聚甲基丙烯酸甲酯的生产工艺。
通过对各个步骤的优化和改进,以及引入新的技术和设备,以提高生产效率、降低能耗和减少环境影响。
在设计过程中,我们将充分考虑工艺的简单性、可行性和经济性。
同时,也要确保产品的质量符合相关标准和要求。
通过本文的生产工艺设计,预计能够实现年产1000吨聚甲基丙烯酸甲酯的高效、稳定和可持续生产,满足市场的需求和产品的质量要求。
本工艺所需的主要原材料为聚甲基丙烯酸甲酯(也称甲基丙烯酸甲酯聚合物),其制备过程需要以下原材料以及对原材料的一些要求和准备工作:甲基丙烯酸甲酯单体:甲基丙烯酸甲酯单体是聚甲基丙烯酸甲酯的主要组成成分,因此其纯度和质量直接影响最终产品的质量。
在准备过程中,应选择纯度高、无杂质的甲基丙烯酸甲酯单体。
甲基丙烯酸甲酯单体:甲基丙烯酸甲酯单体是聚甲基丙烯酸甲酯的主要组成成分,因此其纯度和质量直接影响最终产品的质量。
在准备过程中,应选择纯度高、无杂质的甲基丙烯酸甲酯单体。
溶剂:在聚甲基丙烯酸甲酯的制备过程中,常常需要使用溶剂来控制反应速率和溶解性。
选择合适的溶剂是确保反应进行顺利的重要因素,例如可以选择无水甲醇作为溶剂。

聚甲基丙烯酸甲酯合成方程式
聚甲基丙烯酸甲酯(PMMA)是一种常见的合成树脂,其合成方程式如下:
CH2=C(CH3)COOCH3。
这是甲基丙烯酸甲酯的结构式。
合成PMMA的过程通常涉及甲基丙烯酸甲酯的聚合反应。
聚合反应通常由过氧化物类引发剂引发,生成高分子量的聚合物。
这个过程可以用化学方程式表示为:
n(CH2=C(CH3)COOCH3) → -[CH2-C(CH3)COOCH3]n-。
在这个方程式中,n代表重复单元的数量,-[CH2-
C(CH3)COOCH3]n-代表聚合物链。
这个方程式显示了甲基丙烯酸甲酯单体的聚合过程,形成了聚合物PMMA。
需要注意的是,这里只给出了聚合的基本方程式,实际的合成过程中可能会涉及到不同的反应条件和催化剂。
此外,还有其他合成PMMA的方法,比如通过甲基丙烯酸的酯化反应等。
总的来说,聚
甲基丙烯酸甲酯的合成是一个复杂的过程,需要在实验室中进行精确控制。

聚甲基丙烯酸甲酯结表面张力一、概述聚甲基丙烯酸甲酯(PMMA)是一种常见的有机高分子材料,具有良好的透明度、耐腐蚀性和机械强度等优良性能,在工业生产和科学研究中得到广泛应用。
而表面张力作为表征物质表面性质的重要参数,在PMMA的制备及应用过程中也扮演着重要角色。
本文将从PMMA结构、表面张力的定义及测量方法、PMMA结构对表面张力影响等方面进行探讨。
二、PMMA结构PMMA是由甲基丙烯酸甲酯单体经过自由基聚合反应制得的,其化学式为(C5O2H8)n,其中n为聚合度。
PMMA分子主链由甲基丙烯酸甲酯单体中的丙烯酸部分组成,侧链则是由甲基部分组成。
这种结构使得PMMA具有较高的玻璃转移温度和较低的熔点,同时也使其易于加工和成型。
三、表面张力定义及测量方法1. 定义表面张力是指液体分子间的相互作用力引起的液体表面处产生的张力,其大小决定了液体表面形态和液滴形成等现象。
表面张力与液体种类、温度、压强等因素都有关系。
2. 测量方法常用的测量表面张力的方法有静态法、动态法和悬滴法。
其中静态法是最常用的方法,其原理是在一定条件下测量液体与空气之间形成平衡时所需要施加的最小外力,即为表面张力。
而动态法则是通过测量液体在表面活性剂或固体表面上运动时所受到的阻力来计算表面张力。
四、PMMA结构对表面张力影响PMMA分子结构中含有酯基团,这种化学结构使得PMMA分子在空气中形成一个相对稳定的界面层。
同时,PMMA分子链上还带有甲基基团,这些基团可以与水分子发生一定程度的相互作用。
这些因素共同影响了PMMA材料的表面张力。
实验研究发现,在一定条件下(如温度、湿度等),PMMA材料的表面张力随着甲基丙烯酸甲酯单体聚合度的增加而增大。
这是由于聚合度的增加会使PMMA分子链更加紧密,表面张力也随之增大。
此外,PMMA材料的表面张力还受到环境湿度、温度等因素的影响。
五、结论PMMA作为一种常见的有机高分子材料,在制备和应用过程中都需要考虑其表面张力对物理化学性质和应用效果的影响。

聚甲基丙烯酸甲酯的聚合研究姓名唐小峰班级高化0801学院化学与材料科学学院聚甲基丙烯酸甲酯的聚合研究目录摘要 (2)Contents (3)History (3)Synthesis (4)Processing (4)Handling, cutting, and joining (4)Properties (5)Poly(methyl acrylate) (7)1.1前言 (7)1.2 聚甲基丙烯酸甲酯的制备 (7)1.2.1原料使用条件 (8)1.2.2聚甲基丙烯酸甲酯的制备 (8)1.3 PMMA的物理性能 (9)1.4 PMMA化学性能 (9)1.5 压克力性能 (10)1.6 工艺特性 (10)1.7 聚甲基丙烯酸甲酯的用途 (10)摘要聚甲基丙烯酸甲酯(PMMA)是由甲基丙烯酸甲酯自由聚合而成,有浇铸成型、注塑成型、挤出成型、热成型等制备方法。
研究了聚甲基丙烯酸甲酯的物理化学性能以及在不同条件下制的的聚甲基丙烯酸甲酯的工艺特性。
最后,介绍了聚甲基丙烯酸甲酯生产和市场现状,包括(生产厂家、产品牌号、市场消费)等。
简述了聚甲基丙烯酸甲酯生产技术及市场发展并提出建议。
关键词 PMMA 浇铸注塑挤出热工艺特点市场发展Poly(methyl methacrylate) (PMMA) is a transparent thermoplastic, often used as a light or shatter-resistant alternative to glass. It is sometimes called acrylic glass. Chemically, it is the synthetic polymer of methyl methacrylate. The material was developed in 1928 in various laboratories, and was first brought to market in 1933 by Rohm and Haas Company, under the trademark Plexiglas.[4] It has since been sold under many different names including Lucite and Perspex.The often-seen spelling poly(methyl 2-methylpropanoate)with -an-is an error for poly(methyl 2-methylpropenoate), based on propenoic acid.PMMA is an economical alternative to polycarbonate(PC) when extreme strength is not necessary. Additionally, PMMA does not contain the potentially harmful bisphenol-A subunits found in polycarbonate. It is often preferred because of its moderate properties, easy handling and processing, and low cost, but behaves in a brittle manner when loaded, especially under an impact force, and is more prone to scratching compared to conventional inorganic glass.Contents•1Historyo 1.1Names•2Synthesis•3Processing•4Handling, cutting, and joining•5Acrylate resin casting•6Propertieso 6.1Modification of properties•7Poly(methyl acrylate)•8Useso8.1Transparent glass substituteo8.2Daylight redirectiono8.3Medical technologies and implantso8.4Artistic and aesthetic useso8.5Other uses•9See also•10Referenceso10.1Bibliographyo10.2External linksHistoryThe first acrylic acid was created in 1843. Methacrylic acid, derived from acrylic acid, was formulated in 1865. The reaction between methacrylic acid and methanol results in the ester methyl methacrylate. The German chemists Fittig and Paul discovered in 1877 the polymerization process that turns methyl methacrylate into polymethyl methacrylate. In 1933 the German chemist Otto Röhm patented and registered the brand name PLEXIGLAS. In 1936 the first commercially viable production of acrylic safety glass began. During World War II acrylic glass was used for submarine periscopes, windshields, canopies, and gun turrets for airplanes.[5]NamesPMMA has been sold under a variety of brand names and generic names. It is often generically called acrylic glass,[6]although it is chemically unrelated to glass. It is sometimes called simply acrylic, although acrylic can also refer to other polymers or copolymers containing polyacrylonitrile. Other notable trade names include:•Lucite[7]•Plexiglas[8]•Optix (Plaskolite)[9]•Perspex[10]•Altuglas[11][unreliable source?] (Arkema)SynthesisPMMA is routinely produced by emulsion polymerization, solution polymerization and bulk polymerization. Generally radical initiation is used (including living polymerization methods), but anionicpolymerization of PMMA can also be performed. To produce 1 kg (2.2 lb) of PMMA, about 2 kg (4.4 lb) ofpetroleum is needed. PMMA produced by radical polymerization (all commercial PMMA) is atactic and completely amorphous.ProcessingThe glass transition temperature (T g) of atactic PMMA is 105 °C. The T g values of commercia l grades of PMMA range from 85 to 165 °C (185to 329 °F); the range is so wide because of the vast number of commercial compositions which are copolymers with co-monomers other than methyl methacrylate. PMMA is thus an organic glass at room temperature —i.e., it is below its T g. The forming temperature starts at the glass transition temperature and goes up from there.[12] All common molding processes may be used, including injection molding, compression molding and extrusion. The highest quality PMMA sheets are produced by cell casting, but in this case, the polymerization and molding steps occur concurrently. The strength of the material is higher than molding grades owing to its extremely high molecular mass. Rubber toughening has been used to increase the strength of PMMA owing to its brittle behavior in response to applied loads.Handling, cutting, and joiningPMMA can be joined using cyanoacrylate cement, more commonly known as superglue, with heat (welding), or by using solvents such as di- or trichloromethane to dissolve the plastic at the joint which then fuses and sets, forming an almost invisible weld. Scratches may easily be removed by polishing or by heating the surface of the material.Laser cutting may be used to form intricate designs from PMMA sheets. PMMA vaporizes to gaseous compounds (including its monomers) upon laser cutting, so a very clean cut is made, and cutting is performed very easily. However, the pulsed lasercutting introduces a high internal stresses along the cut edge, which when exposed to solvents produces undesirable "stress-crazing" at the cut edge and several millimetres deep. Even ammonium-based glass-cleaner and almost everything short ofsoap-and-water produces similar undesirable crazing, sometimes over the entire surface of the cut parts, at great distances from the stressed edge. Annealing the PMMA sheet/parts is therefore an obligatory post-processing step when intending to chemically bond lasercut parts together. This involves heating the parts in an air circulating oven from room temperature up to 90°C (at a rate of no more than 18 degrees per hour) down to room temperature (at a rate of no more than 12 degrees per hour). Temperature should be maintained as follows: one hour for 3mm thickness, two hours for up to 6mm thickness, four hours for up to 12mm thickness, and six hours for up to 20mm thickness. A rapid annealing cycle is reliablefor thin sheets and involves placing them in a pre-heated oven to 80°C for one hour, then removing parts from oven and allowing to cool to room temperature. This added time component should be factored into the whole fabrication process, and the alternative Zero-rake sawcutting technique may provide better cost-effectiveness, unless complex non-straight line edges are required. In this respect PMMA has an advantage over competing polymers such aspolystyrene and polycarbonate, which require higher laser powers and givemore messy and charred laser cuts.In the majority of applications, it will not shatter. Rather, it breaksinto large dull pieces. Since PMMA is softer and more easily scratchedthan glass, scratch-resistant coatings are often added to PMMA sheets toprotect it (as well as possible other functions).Methyl methacrylate "synthetic resin" for casting (simply the bulkliquid chemical) may be used in conjunction with a polymerization catalystsuch as MEKP, to produce hardened transparent PMMA in any shape, from amold. Objects like insects or coins, or even dangerous chemicals inbreakable quartz ampules, may be embedded in such "cast" blocks, fordisplay and safe handling.PropertiesSkeletal structure of methyl methacrylate, the monomer that makes up PMMAPMMA is a strong and lightweight material. It has a density of1.17–1.20 g/cm3,[1][13] which is less than half that of glass.[1] It alsohas good impact strength, higher than both glass and polystyrene; however, PMMA's impact strength is still significantly lower than polycarbonateand some en gineered polymers. PMMA ignites at 460 °C (860°F) and burns,forming carbon dioxide, water, carbon monoxide and low molecular weight compounds, including formaldehyde.[14]PMMA transmits up to 92% of visible light (3 mm thickness), and gives a reflection of about 4% from each of its surfaces on account of its refractive index(1.4914 at 587.6 nm).[3]It filters ultraviolet(UV) light at wavelengths below about 300 nm(similar to ordinary window glass). Some manufacturers[15] add coatings or additives to PMMA to improve absorption in the 300–400 nm range. PMMA passes infrared light of up to 2800 nm and blocks IR of longer wavelengths up to 25000 nm. Colored PMMA varieities allow specific IR wavelengths to pass while blocking visible light (for remote control or heat sensor applications, for example).PMMA swells and dissolves in many organic solvents; it also has poor resistance to many other chemicals on account of its easily hydrolyzed ester groups. Nevertheless, its environmental stability is superior to most other plastics such as polystyrene and polyethylene, and PMMA is therefore often the material of choice for outdoor applications.[16]PMMA has maximum water absorption ratio of 0.3–0.4% by weight.[13] Tensile strength decreases with increased water absorption.[17] Its coefficient of thermal expansion is relatively high as (5–10)×10−5/K.[18]Modification of propertiesPure poly(methyl methacrylate) homopolymer is rarely sold as an end product, since it is not optimized for most applications. Rather, modified formulations with varying amounts of other comonomers, additives, and fillers are created for uses where specific properties are required. For example,A small amount of acrylate comonomers are routinely used in PMMA grades destined for heat processing, since this stabilizes the polymer to depolymerization ("unzipping") during processing.Comonomers such as butyl acrylate are often added to improve impact strength.omonomers such as methacrylic acid can be added to increase the glass transition temperature of the polymer for higher temperature use such as in lighting applications.Plasticizers may be added to improve processing properties, lower the glass transition temperature, or improve impact properties.Dyes may be added to give color for decorative applications, or to protect against (or filter) UV light.Fillers may be added to improve cost-effectiveness.Poly(methyl acrylate)The polymer of methyl acrylate, PMA or poly(methyl acrylate), is similar to poly(methyl methacrylate), except for the lack of methyl groups on the backbone carbon chain.[19] PMA is a soft white rubbery material that is softer than PMMA because its long polymer chains are thinner and smoother and can more easily slide past each other.1.1前言聚甲基丙烯酸甲酯英文名称:PolymethylMethacrylate简称(PMMA),PMMA树脂是无毒环保的材料,可用于生产餐具,卫生洁具等,具有良好的化学稳定性、和耐候性。

复合相变材料/聚甲基丙烯酸甲酯微胶囊的制备及表征胡广东1,邹黎明2,徐速1,郭立富1(1.东华大学材料科学与工程学院,上海201620;2.纤维材料改性国家重点实验室,上海201620)摘要:以二元复合相变材料为芯材,聚甲基丙烯酸甲酯(PMMA)为壳材,采用原位聚合法制备出具有蓄热、调温功能的相变材料微胶囊。
通过SEM、DSC、TGA、FTIR等手段对这种微胶囊进行了结构性能表征。
关键词:相变材料;聚甲基丙烯酸甲酯;微胶囊;原位聚合法;结构性能中图分类号:TQ342文献标识码:A文章编号:1001-7054(2011)01-0020-04所谓相变材料(Phase Change Materials,PCM),是指在一定狭窄明确的温度范围(即通常所说的相变范围)内可以改变物理形态,如从固态转变为液态或从液态转变为固态的材料[1]。
微胶囊相变材料(Microencapsulated phase change material,MCPCM)是应用微胶囊技术在固-液/固-固相变材料颗粒表面包覆一层性能稳定的高分子膜而构成的具有芯壳结构的复合材料,这种材料有效解决了相变材料存在的易泄露、相分离及腐蚀性等问题,提高了相变材料的使用效率,并拓宽了相变材料的应用领域[2,3]。
目前,利用相变材料微胶囊具有吸收存储和重新释放热量的特性,相变材料微胶囊在电子设备[4]、建筑[5]、纺织服装[6]、军事[7]等领域均具有广泛的应用前景。
目前,大多数相变材料微胶囊都是以单一的相变材料为芯材,蜜胺树脂、脲醛树脂等为壳材,采用原位聚合法制备得到。
这些相变材料微胶囊存在以下缺点:(1)单一的相变材料相变温度范围过窄,限制了相变材料的应用领域;(2)以蜜胺树脂、脲醛树脂为囊材制备的微胶囊在使用过程中会释放出甲醛等有害气体。
为了克服以上缺点,新型相变材料和聚合物的选择就显得尤为重要。
Ahmet Sari等[8]以单一的正十七烷为芯材、聚甲基丙烯酸甲酯(PMMA)为壳材制备出一种平均粒径0.26μm、相变焓81.5J/g的相变微胶囊。


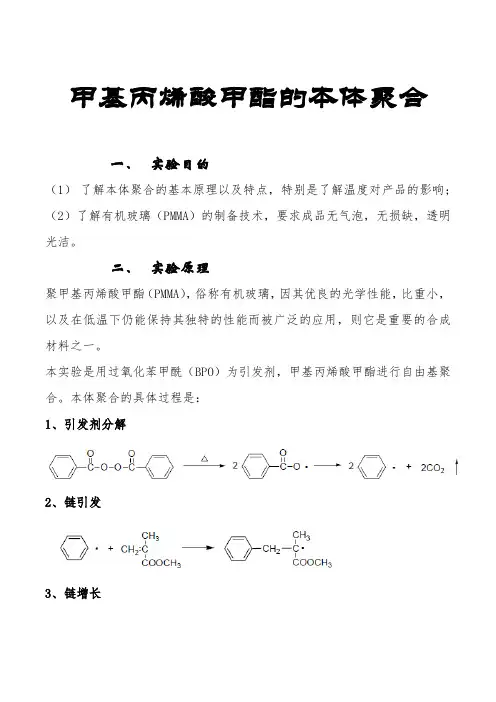
甲基丙烯酸甲酯的本体聚合一、实验目的(1)了解本体聚合的基本原理以及特点,特别是了解温度对产品的影响;(2)了解有机玻璃(PMMA)的制备技术,要求成品无气泡,无损缺,透明光洁。
二、实验原理聚甲基丙烯酸甲酯(PMMA),俗称有机玻璃,因其优良的光学性能,比重小,以及在低温下仍能保持其独特的性能而被广泛的应用,则它是重要的合成材料之一。
本实验是用过氧化苯甲酰(BPO)为引发剂,甲基丙烯酸甲酯进行自由基聚合。
本体聚合的具体过程是:1、引发剂分解2、链引发3、链增长4、链终止A.偶合终止B.歧化终止其中,甲基丙烯酸甲酯在60℃以上时聚合,以歧化终止为主。
本体聚合反应是一个连锁反应,反应速度很快,伴随着聚合物的生成出现自动加速现象,并且甲基丙烯酸甲酯不是聚合物的良溶剂,长脸自由基有一定程度的卷曲,自动加速效应更加明显。
因为引发是通过小分析的单分子的分解发生的,而生长只需要单体移动到生长链的末端,所以这两个过程的聚合速率再聚合初期并不特别依赖相应反应物在在介质中扩散的能力。
另一方面,双分子终止需要在粘度增加到一定程度后,终止速率将被扩散速率所控制,而引发和生长速率则不受影响。
这种在速率上的不连续性突然破坏了连锁反应的稳定状态,终止生长的链段数少于开始生长的链段数,导致反应速率与放热速率随反应进行而增加。
这种效应称之为“自动加速效应”。
由于粘度增加,散热困难,会发生“爆聚”。
因此,本体聚合要求严格控制不同反应阶段的温度,随时排除反应热是很有必要的。
在本体聚合反应开始前,通常有一段诱导期,聚合速度为零,体系无粘度变化。
然后反应逐步进行。
当转化率超过20%之后,聚合速度显著加快,称为自加速效应,此时若控制不当,体系易发生暴聚而使产品性能变坏。
而转化率达80%之后,聚合速率显著减小,最后几乎停止聚合反应,需升高温度才能使之完全聚合。
三、 实验药品及仪器药品:过氧化苯甲酰(BPO )(0.05g )---甲基丙烯酸甲酯(MMA )(15mL )---仪器:恒温水浴锅、三口烧瓶、直型冷凝管、磨口锥形瓶、牛角管、温度计、天平、小试管等。

聚甲基丙烯酸甲酯分子式聚甲基丙烯酸甲酯(Poly(methyl methacrylate),简称PMMA)是一种广泛应用的高分子材料,其分子式为C5H8O2。
它具有优良的透明性、耐候性、机械强度和加工性能,被广泛应用于建筑、汽车、电子、医疗器械等领域。
一、PMMA的制备方法1. 自由基聚合法:将甲基丙烯酸甲酯(MMA)与自由基引发剂在溶剂中进行聚合反应。
2. 溶液聚合法:将MMA溶解在适当的溶剂中,加入引发剂进行聚合反应。
3. 悬浮聚合法:将MMA悬浮于水中,加入引发剂进行聚合反应。
4. 均相聚合法:将MMA与溶剂和引发剂混合后,在高温下进行聚合反应。
二、PMMA的物理化学性质1. 密度:1.18 g/cm³;2. 熔点:160-165℃;3. 抗张强度:60-90 MPa;4. 弹性模量:2400-3000 MPa;5. 抗冲击强度:2-4 kJ/m²;6. 折射率:1.49;7. 透明度:92%以上。
三、PMMA的应用领域1. 建筑领域:作为建筑材料,PMMA可用于制作天窗、墙板、隔断等。
2. 汽车领域:PMMA可用于汽车灯罩、后视镜等部件。
3. 电子领域:PMMA可用于制作光纤、显示屏等。
4. 医疗器械领域:PMMA可用于制作人工眼球、牙齿修复材料等。
四、PMMA的加工方法1. 注塑成型法:将加热融化的PMMA注入模具中,冷却后得到所需形状的制品。
2. 挤出成型法:将加热融化的PMMA通过挤压机挤出成所需形状的制品。
3. 热成型法:将PMMA板材加热软化后,通过吸塑或热弯成所需形状的制品。
五、PMMA的注意事项1. PMMA易受紫外线影响而变黄变脆,应注意避免长时间暴露在阳光下;2. PMMA不耐高温,不宜接触火源;3. PMMA易受到有机溶剂的腐蚀,应避免接触酸碱等化学物质。
总之,PMMA作为一种广泛应用的高分子材料,在各个领域都有着重要的应用价值。
在使用过程中,需要注意其物理化学特性和加工方法,并避免对其产生损害。

聚甲基丙烯酸甲酯聚甲基丙烯酸甲酯(Poly(methyl methacrylate)简称PMMA)是一种广泛应用的聚合物材料。
它具有优异的透明度、高强度和良好的耐候性等特点,被广泛应用于建筑、汽车、光学器件等领域。
本文将从聚甲基丙烯酸甲酯的制备方法、物性表征以及应用领域等方面进行介绍。
一、聚甲基丙烯酸甲酯的制备方法1. 逐步聚合法:首先将甲基丙烯酸甲酯单体与引发剂加入反应釜中,经过一系列的反应步骤,得到聚合物。
这种方法常用于小规模的实验室制备。
2. 均相聚合法:在适当的溶剂中,将甲基丙烯酸甲酯单体与引发剂进行均相溶液聚合。
该方法适用于大规模生产,能获得更高的聚合度和更均匀的分子量分布。
3. 残留体系聚合法:通过将甲基丙烯酸甲酯单体与引发剂固定在聚合剂载体上,通过体系热解或紫外辐射等方式来释放聚合物。
这种方法能够实现可控的聚合反应,得到具有特定结构的聚甲基丙烯酸甲酯。
二、聚甲基丙烯酸甲酯的物性表征1. 透明度:聚甲基丙烯酸甲酯具有优异的透明度,其透明度与玻璃相当,而密度只有玻璃的一半。
这使其广泛应用于自动车窗、光学仪器和观察窗等领域。
2. 强度:聚甲基丙烯酸甲酯具有较高的强度,比普通玻璃更耐冲击,减少了由于碎裂而造成的伤害。
3. 耐候性:聚甲基丙烯酸甲酯具有良好的耐候性,不易受紫外线照射、湿度、高温等环境因素的影响。
因此,它常用于室外标牌、车身配件等需要耐候性的领域。
4. 气密性:聚甲基丙烯酸甲酯具有很好的气密性,能够有效阻挡氧气和水蒸气的渗透,保护容器内部物品的质量。
三、聚甲基丙烯酸甲酯的应用领域1. 建筑领域:聚甲基丙烯酸甲酯常用于建筑领域中的采光板、隔热材料和玻璃替代品等产品。
其透明度和强度使其成为理想的建筑材料之一。
2. 汽车领域:在汽车制造过程中,聚甲基丙烯酸甲酯被广泛应用于车窗、后视镜、仪表盘等部位。
其高强度和耐候性保证了汽车零部件的长期使用。
3. 光学器件:由于其透明度和光学性能,聚甲基丙烯酸甲酯在光学器件领域有着广泛的应用。

PMMA复合材料制备与合成摘要:PMMA复合材料制备是采用本体聚合原理来制备聚甲基丙烯酸甲酯(PMMA)。
通过改变预聚合温度以及引发剂的用量来确定实验的最佳反应方案是30g甲基丙烯酸甲酯(MMA)在85℃水浴下,偶氮二异丁腈(AIBN)0.015g作用下预聚合30min。
采用预聚浆体模腔浇注法来制备试样,并通过对所制试样进行冲击性能、透光率、硬度等物性测试来对其表征。
从物理化学光学方面全面了解PMMA复合材料的基本特征和性能,总结PMMA复合材料在现代化生活中重要角色以及中国国情下的PMMA材料发展。
关键词:聚甲基丙烯酸甲酯膨润土本体聚合引发剂一、PMMA复合材料简单理解聚丙烯酸酯类透明塑料一般系指聚甲基丙烯酸甲酯(即PMMA),其单体甲基丙烯酸甲酯(MMA)是一种活性高而且易于均聚和共聚的单体,它主要用于制造有机玻璃,也是广泛用于制造模塑料、工程塑料、涂料及粘合剂等的原料。
甲基丙烯酸甲酯(简称MMA)的均聚物或共聚物的片状物俗称为有机玻璃,它是目前塑料中透明性最好的品种。
俗名特殊处理有机玻璃。
亚克力的研究开发,距今已有一百多年的历史。
1872年丙烯酸的聚合性始被发现;1880年甲基丙烯酸的聚合性为人知晓;1901 年丙烯聚丙酸脂的合成法研究完成;1927年运用前述合成法尝试工业化制造;1937年甲基酸脂工业制造开发成功,由此进入规模性制造。
二战期间因亚克力具有优异的强韧性及透光性,首先,被应用于飞机的挡风玻璃,坦克司机驾驶室的视野镜。
1948年世界第一只压克力浴缸的诞生,樗着压克力的应用进入了新的里程碑。
20世纪80年代,中国压克力(亚克力)有机玻璃年销售量不足2万吨,消费市场不以建筑业为主。
进入90年代以来,PMMA 在建筑业中的市场应用有了较大的发展,消费量增长很快,2000年已达到8.5万吨,其中浇注板2.9万吨、挤出板2.5万吨、模塑料3.1万吨(不包括挤出板用模塑料)。
预计今后几年中,中国压克力有机玻璃市场发展速度年均将保持在6%以上。
聚甲基丙烯酸甲酯的静态浇铸工艺1. 聚甲基丙烯酸甲酯简介聚甲基丙烯酸甲酯(Poly(methyl methacrylate),简称PMMA)是一种常见的有机高分子材料,具有良好的透明性、耐候性和机械性能。
它广泛应用于建筑、汽车、光学等领域。
PMMA的制备方法有多种,其中静态浇铸工艺是一种常用且经济有效的方法。
2. 静态浇铸工艺原理静态浇铸工艺是通过将液态或溶液状态的原料注入到模具中,经过一定时间的固化,得到所需形状和尺寸的制品。
在聚合物材料中,由于其高分子量和粘度较大,在静态浇铸过程中需要控制好温度、压力和固化时间等参数,以确保制品质量。
3. 静态浇铸工艺步骤3.1 模具准备选择合适的模具对于静态浇铸工艺至关重要。
模具应具备良好的耐高温性能、平整度和尺寸精度。
常用的模具材料包括金属(如铝合金)和硅胶等。
3.2 原料准备将聚甲基丙烯酸甲酯原料按照一定比例与溶剂混合,以得到适合浇铸的液态或溶液状态。
溶剂的选择应考虑其对聚合物的溶解性和挥发性。
3.3 模具涂层处理为了防止原料与模具黏附,需要在模具表面进行涂层处理。
常用的涂层材料有硅油、聚四氟乙烯等,可以提高模具表面的光滑度并减少黏附力。
3.4 原料注入将经过预处理的原料缓慢注入到模具中,控制注入速度和压力,以避免产生气泡或残留物。
同时需注意填充整个模腔,并确保原料均匀分布。
3.5 固化过程在注入完成后,需要将模具放置在恒温环境中进行固化。
固化时间取决于聚合物材料的性质和厚度,一般需要数小时至数天不等。
固化过程中应控制好温度,以避免产生内部应力和变形。
3.6 模具分离待固化完成后,将模具从固化室中取出,并进行模具分离操作。
根据模具材料的不同,可以采用剥离剂、冷却等方式辅助分离。
3.7 后处理经过模具分离后,得到的制品可能存在一些瑕疵或不平整。
此时可以进行切割、研磨、抛光等后处理工艺,以得到最终的产品形态和表面质量。
4. 工艺参数及控制在静态浇铸工艺中,需要控制以下参数以确保制品质量:•温度:影响聚合物的流动性和固化速度,应根据材料特性选择合适的温度范围。
甲基丙烯酸甲酯的结构甲基丙烯酸甲酯由甲基丙烯酸和甲醇反应得到。
它的结构由一个甲基丙烯酸根基团和一个甲醇根基团组成。
甲基丙烯酸根基团是一个无环烯酮结构,包括一个丙酮结构和一个甲基基团。
甲醇根基团由一个甲基基团和一个羟基团组成。
甲基丙烯酸甲酯分子中,甲基丙烯酸和甲醇通过一个双键连接在一起,形成酯的酯键。
甲基丙烯酸甲酯的结构如下所示:CH3OCH2=C--C--O--CH3CH3H甲基丙烯酸甲酯是一种无色液体,具有特殊的香味。
它具有较低的沸点和熔点,可挥发。
甲基丙烯酸甲酯在常温下具有较低的粘度和表面张力,易于流动和涂覆。
它具有良好的化学稳定性,可以在很多化学物质中稳定存在。
此外,甲基丙烯酸甲酯的分子中含有甲基基团和丙酮结构,使其具有较好的亲油性,能够与有机溶剂相容,如醇类、醚类、酮类等。
甲基丙烯酸甲酯具有广泛的应用领域。
由于其低粘度和良好的物理性质,甲基丙烯酸甲酯被广泛用于涂料和油漆工业。
它可以用作溶剂、稀释剂和增塑剂,用于改善涂料和油漆的流动性和延展性。
此外,甲基丙烯酸甲酯还可以用于制备UV固化涂料,作为重要的基础材料之一、UV固化涂料能够在紫外线照射下迅速形成固态薄膜,具有干燥快、无溶剂和低能耗的优点,广泛应用于印刷、电子、家具等领域。
除了涂料和油漆工业,甲基丙烯酸甲酯在其他领域也有重要的应用。
在塑料工业中,甲基丙烯酸甲酯是一种重要的合成树脂原料,可用于制备聚甲基丙烯酸甲酯(PMMA)。
PMMA是一种透明、硬度高、耐候性强的塑料,具有良好的光学性能和物理性能,广泛应用于汽车、光学仪器、建筑材料等领域。
此外,甲基丙烯酸甲酯还可以用于制备高性能的工程塑料,如聚甲醛、聚醚醚酮等。
总之,甲基丙烯酸甲酯是一种重要的有机化合物,具有较好的化学稳定性和物理性质,广泛应用于涂料、油漆、塑料等工业领域。
随着科学技术的不断发展,对甲基丙烯酸甲酯的研究和应用将会进一步拓展。
聚甲基丙烯酸甲酯概述聚甲基丙烯酸甲酯(Polymethyl Methacrylate,PMMA),俗称“有机玻璃”或“亚克力(acrylic)”。
它是由丙烯酸甲酯自由基聚合得到的聚合物,是迄今为止合成透明材料中质地最优异、价格较低的品种。
1927年,德国罗姆-哈斯公司的化学家在两块玻璃板之间将丙烯酸酯加热,丙烯酸酯发生聚合反应,生成黏性的橡胶状夹层,可用作防破碎的安全玻璃。
当他们用同样的方法使甲基丙烯酸甲酯聚合时,得到了透明度极好、其他性能也良好的有机玻璃板。
1931年,罗姆-哈斯公司建厂生产聚甲基丙烯酸甲酯,首先在飞机工业得到应用,取代了赛璐珞塑料,用作飞机座舱罩和挡风玻璃。
第二次世界大战期间因PMMA具有优异的强韧性及透光性,被应用于飞机的挡风玻璃、坦克司机驾驶室的视野镜。
1948年世界第一个PMMA浴缸的诞生,标志着PMMA的应用进入新的里程碑。
1.聚甲基丙烯酸甲酯的结构与性能(1)结构聚甲基丙烯酸甲酯的分子结构式如下:PMMA大分子链上的甲基和甲酯基破坏了分子链的空间规整性,使其呈无定形态,难以结晶。
大分子链上不对称取代基妨碍了大分子的内旋转,因而分子链具有一定的刚性,其T比PE高得多。
PMMA大分子结构中没有叔氢原子,具有较好g的耐候性,即耐老化性能。
如果采用配位聚合也可以得到全同立构或间同立构的聚甲基丙烯酸甲酯。
(2)性能PMMA无毒无味,密度在1.15~1.19 g/m3,是玻璃(2.40~2.80 g/m3)的一半,同样大小的材料,其重量只有普通玻璃的一半,金属铝的43%。
①光学性能。
PMMA是高度透明的无定形热塑性塑料,具有十分优异的光学性能,透光率可达90%~92%,比玻璃的透光度高,折射率为1.49,雾度不大于2%,可透过大部分紫外线和红外线。
石英能完全透过紫外线,但价格高昂,普通玻璃只能透过0.6%的紫外线,但PMMA却能透过73%。
在照射紫外光的状况下,与聚碳酸酯相比,PMMA具有更佳的稳定性。