《物理双语教学课件》Chapter 12 The Kinetic Theory of Gases 理想气体定律
- 格式:doc
- 大小:248.00 KB
- 文档页数:11
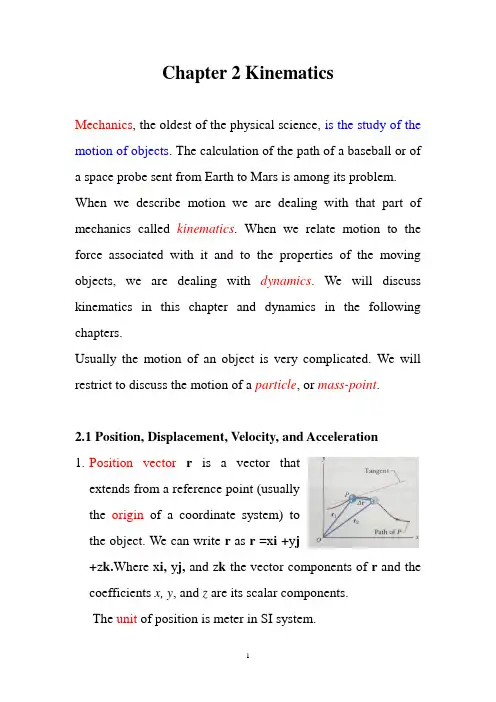
Chapter 2 KinematicsMechanics, the oldest of the physical science, is the study of the motion of objects. The calculation of the path of a baseball or of a space probe sent from Earth to Mars is among its problem. When we describe motion we are dealing with that part of mechanics called kinematics. When we relate motion to the force associated with it and to the properties of the moving objects, we are dealing with dynamics. We will discuss kinematics in this chapter and dynamics in the following chapters.Usually the motion of an object is very complicated. We will restrict to discuss the motion of a particle, or mass-point.2.1 Position, Displacement, Velocity, and Acceleration1.Position vector r is a vector thatextends from a reference point (usuallythe origin of a coordinate system) tothe object. We can write r as r =x i +y j+z k.Where x i, y j, and z k the vector components of r and the coefficients x, y, and z are its scalar components.The unit of position is meter in SI system.2. Displacement vector r ∆: As an object moves, its position vector changes. If the object has position vector r 1at time t 1 and position vector r 2 at later time t t ∆+1, as shown in above figure. The displacement vector is the difference of the two position vectors r 2 and r 1 during the time intervalt ∆, whichcan be written ask z z j y y i x x k z j y i x k z j y i x r r r )()()()()(12121211122212-+-+-=++-++=-=∆ See sample problem 4-1and checkpoint1 of P54 and P55.3. Average velocity vector : If a particle moves through a displacementr ∆ in a time interval t ∆, then its averagevelocity isk t z j t y i t x t k z z j y y i x x t r v ∆∆+∆∆+∆∆=∆-+-+-=∆∆=)()()(121212 Instantaneous velocity vector : is defined as the limit of the average velocity as the time intervalt ∆becomesinfinitesimally short. We can write it ask v j v i v k dt dz j dt dy i dt dx dt r d t r v z y x t ++=++==∆∆=→∆0lim The direction of the instantaneous velocity of a particle is always tangent to the path of the particle, as shown in the figure.The unit of velocity is 1-⋅s m .The magnitude of the velocity of a particle is its speed . Speedis a scalar quantity.dt r d dt r d v v ===Average speed is the total distance covered by the object over the time interval.t cedis total v ∆=tan4. Acceleration vector : When a partic le’s velocity changes from v 1 to v 2 in a time intervalt ∆, its average acceleration a during t ∆ ist v v t v ∆-=∆∆=12Instantaneous acceleration vector is defined as the limit of the average acceleration as the time intervalt ∆ becomes infinitesimally short. It can be express ask a j a i a k dtdv j dt dv i dt dv dt v d t v a z y x z y x t ++=++==∆∆=→∆0lim The unit of acceleration is2-⋅s m .2.2 One Dimensional Motion with Constant Acceleration.1. The rule of one-dimensional motion with constant acceleration can be derived as follow:200000000)(21)()]([)(sin 00t t a t t v s s dt t t a v ds t t a v v adt dv adtdv therefore a dt dv or c a ce t t v v -+-+=⇒-+=-+=⇒====⎰⎰ We also have:)(2])(21)([2)()(202020002020200202s s a v t t a t t v a v t t a t t a v v v -+=-+-+=-+-+= If we suppose t 0=0, Then we haveasv v at t v s s atv v 2212022000=-++=+= 2. Free-falling bodies: If you throw an object either up or down and could somehow eliminate the effects of air on its flight, you would find that the object accelerates downward at a certain rate. We call it free-fall acceleration g. The object is a free-falling body . The value of g change slightly with latitude and with height. At sea level in the mid-latitudes the value is9.8m/s 2, we will use it to solve the problem in this course. If we choose the upward direction along the y axis, then the free fall acceleration is negative. If we suppose t 0=0 and y 0=0, we will have:202200221v v ay gt t v y gtv v -=-=-=2.3 Projectile MotionIf a particle moves in a vertical plane during free fall, it undergoes projectile motion . The object can be called a projectile . Throughout we will assume that the air has no effect on the motion of the projectile.Projectile motion has the feature that the horizontal motion and the vertical motion are independent of each other. So we can deal with them respectively.If we choose the rectangular coordinate as the following figure, then000000sin cos θθv v and v v y x ==1. The horizontal motion is motion without acceleration. So we havet v t v x x x 0000cos θ==-2. The vertical motion is the motion of free fall. So we have)(2)sin ()sin (21)sin (210200200200200y y g v v gt t v v gt t v gt t v y y y y y --=-=-=-=-θθθ 3. The equation of the path : Let x 0=0 and y 0=0, eliminating the t between the equations above, we get20020)cos (2)(θθv gx x tg y -=It is the equation of a parabola , so the path is parabolic.4. The horizontal Range R is horizontal distance the projectile has covered when it returns to its initial height. We have 021)sin (21cos 200200000=-=-=-=-=gt t v gt t v y y tv x x R y θθ Eliminating the time t, we get02000202sin cos sin 2θθθgv g v R ==2.4 Uniform Circular Motion1. A particle is in uniform circular motion if it travels around a circle or circular arc at constant speed. Although the speed does not vary, it means the magnitude of the velocity is a constant quantity, but the direction of the velocity alters with the time. So the particle is accelerating.2. The feature of uniform circular motion: We have)()()(00t v v t r r t t v s s c v ==-=-⇒= 3. The acceleration of uniform circular motion:(1). Direction: (2). Magnitude: r v a 2=4. Another look at the acceleration of uniform circular motion: 0200000)()(n rv r r v v dt t d v dt t d v t dt dv t v dt d dt v d a =-==+=== 5. The figure shows the relation betweenthe velocity and acceleration vectorsat various stages during uniformcircular motion. Both vectors haveconstant magnitude as the motionprogress, but their directions change continuously. The velocity is always tangent to the circle in the direction of motion; the acceleration is always directed radially inward . Because of this, the acceleration associated with uniform circular motion is called a centripetal acceleration .2.5 Relative motion in two or three dimensions1. The figure shows reference frames A and B, now two dimensions, of course, it can be extended to three dimensions if you would like to. Two observers are watching a movingparticle P in the twodifferent frames. Weassume that the two framesare separating at a constantvelocity v BA (both of themare inertial frames ) and wefurther assume that their axes remain parallel to each other for simplicity.2. Position vectors: If two observers on frame A and B each measure the position of particle P at a certain instant. From the vector triangle in the figure, we have the vector equation BA PB PA r r r +=3. Velocity vectors : If we take the time derivative of above equation, we will get the velocity relation of the particle as measure by the two observersBA PB PA v v v +=4. Acceleration vectors : If we take the time derivative of above equation, we will have a connection between the two measured accelerationPB PA a a =。




Corresponding Relation Between SHM and UCMThe simple harmonic motion is the side view of circular motion.Draw x-t Diagram Using Circle of ReferenceExampleUsing the phasorExampleExample: π=+0v >ExampleExampleExampleExample:A wooden block floats in water. We press it until its upper surface2Example ——Vertical SHM:Suppose we hang a spring with force constant k and suspend from it a body with mass m. Oscillation will now be When the body hangs at rest, in equilibriumTake x=0 to be the equilibrium position, andThe body ’s motion is still SHMwith the angular frequency:kmω=ExampleWhen the body is at the position x, the total 0=)0l mg ∆−=0Example cont ’d§6 Damped OscillationsThe dissipative force causes the decrease in amplitude ——damping, the corresponding motion is called damped oscillation.Restoring force:Resistance force:Newton ’s second law:The solution:s F kx=−R bv=−F kx bv ma=−−=∑220d x b dx k x dt m dt m ++=(/2)cos()cos()b m t x Ae t A t ωφωφ−′=+=+22(/2)20, 22b m t k b b A Ae m m m ωω−⎛⎞⎛⎞′==−=−⎜⎟⎜⎟⎝⎠⎝⎠τ=2m /b is called damping time constant, or mean lift time.If the system no longer oscillates,and is called critically dampedDamped Oscillations Cont ’d§7 Forced OscillationsA forced oscillator is damped oscillator driven by an external force that varies periodically.A sinusoidally varying driving force:Newton’s Second Law:The solution:0()sin F t F t ω=202sin dxd xF t b kx m dt dt ω−−=202sin F d x b dx k x tdt m dt m m ω++=cos()x A t ωφ=+()022220/F mA b m ωωω=⎛⎞−+⎜⎟⎝⎠The forced oscillator in its “steady state”isoscillated with the frequency of driven force.The goblet breaks as it vibrates in the resonance In 1940, the Tacoma Narrows Bridge collapsed four months and six days after it was opened for traffic, due to gusty ResonanceUsing Circle of Reference12212cos()A A φφ+−1122122sin sin cos cos A A φφφφ++Chapter 12 Oscillatory Motion are in phase, the resultant amplitude take are out of phase, the resultant amplitude Chapter 12 Oscillatory MotionExample。


»首页 »物理学 »8.01 物理学1999 秋季课程影片教学本课程正在进行字幕听打计划,如需观看成果或协助,请浏览相关网页。
本页翻译进度灯号说明审定:无审定简介:无翻译:李继军译者简介:长江大学物理科学与技术学院。
编辑:朱学恒编辑简介:中央大学电机系毕业奇幻基金会执行长翻译经验超过十年,曾任出版部门总策划。
运行这部分里的 .rm档需要RealOne™ 软件RealOne™ Player software is required to run the .rm files in this section.Walter Lewin 教授悬挂在单摆上证明单摆的周期与单摆的质量无关.这个展示在第十讲的录影里. (图片由Markos Hankin提供,物理系演讲演示组)Professor Walter Lewin demonstrates that the period of a pendulum is independent of the mass hanging from the pendulum. This demonstration can be viewed on the video of Lecture #10. (Image courtesy of Markos Hankin, Physics Department Lecture Demonstration Group).这个录影索引提供了一个关于本课程的细目录.它给出了每一课中相应段落的起始时间.进入某个特别的段落,只需要在RealPlayer视窗中打开该课并调整滑条到指定的起始时间The Video Index gives a breakdown of topics and the start time of the corresponding segment in each lecture. To access a particular segment, play the lecture in a RealPlayer window and adjust the slider bar to the designated start time.课课程单元影片大纲影片1 十的幂-单位- 尺度-测量- 不确定度-空间分析-缩放比例的讨论Powers of Ten - Units - Dimensions -Measurements - Uncertainties - DimensionalAnalysis - Scaling Arguments第1课录影索引Lecture 1VideoIndex(线上- 80K)(线上- 300K)(下载- 300K)2 一维运动学- 速率- 速度- 加速度1D Kinematics - Speed - Velocity - Acceleration第2课录影索引(线上- 80K)(线上- 300K)Forced Oscillations - Normal Modes - Resonance - Natural Frequencies - Musical Instruments 索引Lecture 31VideoIndex(线上- 300K)(下载- 300K)32 热- 热膨胀Heat - Thermal Expansion第32课录影索引Lecture 32VideoIndex(线上- 80K)(线上- 300K)(下载- 300K)33 气体动力论- 理想气体定理- 大气等温线- 相图- 相变Kinetic Gas Theory - Ideal Gas Law - IsothermalAtmosphere - Phase Diagrams - Phase Transitions第33课录影索引Lecture 33VideoIndex(线上- 80K)(线上- 300K)(下载- 300K)34 奇妙的量子世界- 经典力学的崩溃The Wonderful Quantum World - Breakdown ofClassical Mechanics第34课录影索引Lecture 34VideoIndex(线上- 80K)(线上- 300K)(下载- 300K)35 告别特别节目- 高能天体物理Farewell Special - High-energy Astrophysics第35课录影索引Lecture 35VideoIndex(线上- 80K)(线上- 300K)(下载- 300K)RealOne™ 是商标或RealNetworks公司的注册商标RealOne™ is a trademark or a registered trademark of RealNetworks, Inc.。



Chapter 4 Work and EnergyThe concept of energy is one of the most important in the world of science. In everyday usage, the term energy has to do with the cost of fuel for transportation and heating, electricity for lights and appliances, and the foods we consume. However, these ideas do not really define energy. They tell us only that fuels are needed to do a job and that those fuels provide us with something we call energy.Energy is present in the Universe in a variety of forms, including mechanical energy, chemical energy, electromagnetic energy, heat energy, and nuclear energy. Although energy can be transformed from one form to another, the total amount of energy in the Universe remains the same. If an isolated system loses energy in some form, then by the principle of conservation of energy, the system must gain an equal amount of energy in other form. The transformation of energy from one form into another is an essential part of the study of physics, chemistry, biology, geology, and astronomy.In this chapter we are concerned only with mechanical energy. We introduce the concept of kinetic energy, which is defined as the energy associated with motion, and the concept of potential energy, the energy associated with position. We shall see that the ideas of work and energy can be used in place ofNewton’s law to solve certain problems.4.1 Work and Power1. Work W done by a constant force is defined as the product of the component of the force along the direction of displacement and the magnitude of the displacement.ϕcos FS S F W =⋅=Where the force makes an angle of ϕ with displacement .SThe SI unit of work is the joule (J), named for James Prescott Joule, an English scientist of the 1800s. It is derived directly from the units for mass and velocity:1joule=1J=1(kg) (m/s 2) (m)=1 kg m 2/s 22. Work done by a variable force(1). The increment of work dW done on the particle by F during the displacement d r isr d F dW ⋅=, where Force F is function of its position.(2). The work W done by F while the particle moves from an initial position a to a final position b is then⎰⎰⋅==b a b a r d F dW W(3). We use the components ofF and r d to express the forceand displacement, then we havedzF dy F dx F k dz j dy i dx k F j F i F r d F W z y b a x z y b a x b a ++=++⋅++=⋅=⎰⎰⎰)()( 3. Work done by multiple forces : If there are several forces act on a particle, we can replace F in above equation with the net force ∑F , where +++=∑321F F F F , where j F are theindividual forces. Then+++=⋅+++=⋅=⎰⎰321321)(W W W r d F F F r d F W b aba 4. Power(1). The rate at which work is done by a force is said to be the power due to the force . If an amount of work W is done in a time intervalt ∆ by a force, then the average power due to the force is t WP ∆=.(2). The instantaneous power P is the instantaneous rate of doing work, which can be written asv F dtr d F dt dW P ⋅=⋅==. (3). The SI unit of power is the joule per second . This unit is used so often that it has a special name, the watt (W), after James Watt, who greatly improved the rate at which steam engines could do work.4.2 Kinetic Energy and Work-Kinetic Energy TheoremEnergy is a scalar quantity that is associated with a state of oneor more object. The term state here has its common meaning: it is the condition of an object.1. Kinetic energy K is associated with the state of motion of an object. The faster the object moves, the greater is its kinetic energy. For an object of mass m and whose speed v is well below the speed of light , we define kinetic energy as 221mv K = The SI unit of kinetic energy is the same as work —joule.A convenient unit of energy for dealing with atoms or with subatomic particles is the electron-volt (eV).1 electron-volt = 1 eV =1.60 x 10 –19 J.2. Work-kinetic energy theorem : If a force changes the speed of an object, it also changes the kinetic energy of the object. If the kinetic energy is the only type of energy of the object being changed by the force, then the change in kinetic energy is equal to the work W done by the force:W K K K i f =-=∆ Here i K is the initial kinetic energy (=2021mv ) and f K is the kinetic energy (221mv ) after the work is done.3. We can prove work-kinetic energy theorem as follow:002022222221212121)(21k f v v b a z y x z z y y x x b a b a b a K K mv mv v m mvdv W dv v v v d dv v dv v dv v v d v v d v m dt v dtv d m s d f W -=-====++=++=⋅⋅=⋅⋅=⋅=⎰⎰⎰⎰(Numerator and denominator of a fraction)4.3 Work done by weight and by a spring force1. Work done by weight :[]a b a b b a b a m gh m gh h h m g dh m g dW W m gdhm gdl m gdl l d g m dW --=--=-==-=--==⋅=⎰⎰)()cos(cos απαWe can find that the work done by weight on a particle between two points does not depend on the path taken by the particle . Or no matter what path we choose to move the particle, the work done by its weight is the same . In other word if we move a particle around a closed path, the work done by weight on the particle is zero .2. Work done on aparticle-like object by aparticular type of variableforce, namely, springforce —the force exertedby a spring.⎥⎦⎤⎢⎣⎡--=-=-==-=⋅=-=⎰⎰222212121'a b x x x x b a kx kx x k kxdx dW W kxdxs d F dW law s Hook kx F b a b a 3. Conservative force and Non-conservative force : If the workdone by the force is independent of the path the particle moves,the force is a conservative force ; otherwise a non-conservative force . Weight and spring-force are conservative forces; friction is a non-conservative force.4.4 Potential energyPotential energy U is energy that can be associated with the configuration (or arrangement) of a system of objects that exert a force on one another. If the configuration of the system changes, then the potential energy of the system also changes.1. We know that work done by a conservative force has nothing to do with the path the particle taken. So we can introduce a quantity which is the function of the state of the system to indicate this kind of nature for a conservative force. We call it potential .2. Gravitational Potential Energy(1). The work done by weight can be expressed as: []U mgh mgh W a b ∆-=--==, where U ∆ is the change in the gravitational potential energy. Since the work done by weight has definite magnitude from an initial position to a final position, so only a changeU ∆ in gravitational potential energy is physically important .(2). However, to simplify a calculation or a discussion, we cansay that a certain gravitational potential U is associated with any given configuration of the system, with the particle at a given height h. To do so, we rewrite the above equation as:)(i i h h mg U U -=-Then we take i U to be the gravitational potential energy of thesystem when it is in a reference configuration, with the particle at a reference pointi h . Usually, we set 0=i U and 0=i h , then we have mgh U =. So the gravitational potential energy associated with a particle-Earth system depends on the height h of the particle relative to the reference position of h i =0, not the horizontal position.3. Elastic Potential Energy(1). Similar to a particle-Earth system, the work done by the spring force can be rewritten as U kx kx W a b ∆-=⎥⎦⎤⎢⎣⎡--=)21()21(22.(2). To associated a potential energy U with any given configuration of the system, with the block at position x, we set the reference point for the block as x i =0, which is always at the equilibrium position of the block. And we set the corresponding elastic potential energy of the system as U i =0. Thus we have 221)(kx x U =. Attention: (1). Potential energy belongs to the whole system.(2). The magnitude of potential energy depends onthe choice of the reference point.4.5 Work-Energy Theorem and Conservation of Mechanical Energy1. Work-kinetic energy theorem for one particle: We have W K K K i f =-=∆2. Work-Energy Theorem: Suppose we have particles of N in the system we discussed and we use work-kinetic energy theorem for each particle, then we have total amount of N equations like those:jijf j if if K K W K K W K K W -=-=-=222111We can divide the forces exerted on every particle into external forces and internal forces. And the internal forces can also be classified as conservative internal forces and non-conservative forces. Summing the two side of above equations, we will have: i fN j ji jf N j j K K K K W -=-=∑∑==11)(Or to be exactly,K W W W noncon in con in ext ∆=++--We also know that the work done by the conservative internalforce can be written as the minus difference of potential energy. So we get the Work-Energy Theorem :E U K W K W W con in noncon in ext ∆=∆+∆=-∆=+--The work done by external forces and non-conservative internal forces in a given system is exactly equal to the difference of its Mechanical Energy .3. Conservation of Mechanical Energy : When only conservative forces act within a system, the kinetic energy and potential energy can change. However, their sum, the mechanical energy E of the system, does not change . 0=∆+∆=∆U K E4.6 Reading a Potential energy curveConsider a particle that is part of a system in which a conservative force acts. Suppose that the particle is constrained to move along an x axis while the conservative force does work on it.1. Finding the Force Analytically : For one-dimensional motion, the work W done by a conservative force that acts on a particle as the particle moves, and the potential energy have the relation as followdx x F dW x U )()(-=-=∆So can get the force from the potential energytion ensionalmo one dx x dU x F dim )()(--=We can, for example, check this result by putting221)(kx x U =, which is the elastic potentialenergy function for a springforce. Above equation yieldskx x F -=)( as expected.2. The Potential EnergyCurve : The following figureis a plot of a potential energyfunction U(x) for a system inwhich a particle is inone-dimensional motionwhile a conservative forceF(x) does work on it. We caneasily find F(x) by taking theslope of the U(x) curve atvarious points. Fig. (b) is a plot of F(x) found in this way.3. Tuning Point : Since there is only conservative force acting on the particle, the system will remain conservation of its mechanical energy. So we have)()(x U E x K -=. Since kinetic energy)(x K is not less than zero. As the particle moves from 2xto 1x (Fig. a), when the particle reaches 1x , its kineticenergy is zero, meanwhile the force on the particle is positive. It means the particle does not remain atx but instead begins to1move back to the right. Hencex is a tuning point, a place1where K=0 and the particle changes direction of its motion.4.Equilibrium Points:Neutral equilibriumUnstable equilibriumStable equilibrium。
《物理双语教学课件》Chapte...Chapter 11 The First Law of Thermodynamics11.1Thermodynamics1.With this chapter, we begin a new subject-thermodynamics,which deals with an internal energy of systems, thermal energy, and is governed by a new set of laws.2.The central concept of thermodynamics is temperature. Thisword is so familiar that most of us, because of our built-in sense of hot and cold, tend to be overconfident in our understanding of it.3.Our “temperature sense” is in fact not always reliable. On acolder winter day, for example, an iron railing seems much colder to the touch than a wooden fence post, yet both are at the same temperature. This difference in our sense perception comes about because iron removes energy from our fingers more quickly than wood does.4.Temperature is one of the seven SI base quantities. Physicistsmeasure temperature on the Kelvin scale, which is marked in units called kelvins. Although the temperature of a body apparently has no limit, it does have a lower limit; this limiting low temperature is taken as the zero of the Kelvin temperature scale. Room temperature is about 290 kelvins, or290 K as we write it, abovethis absolute zero . The rightfigure shows the wide rangeover which temperatures aredetermined.11.2 Temperature1. The zeroth law ofthermodynamics : If bodies A and B are each in thermal equilibrium with a third body T, then they are in thermal equilibrium with each other, and have the same temperature .2. By international agreement, the triple point of water , in which liquid water, solid ice, and water vapor (gaseous water) coexist, has been assigned a value of 273.16 K as the standard fixed-point temperature for the calibration of thermometers.3. Celsius temperatures are measured in degrees, and the Celsius degree has the same size as the kelvin. However, the zero of the Celsius scale is shifted to a more convenient value than absolute zero. IfC T represents a Celsius temperature, then 015.273-=T T C .In expressing temperature on the Celsius scale, the degree symbol is commonly used. Thus we write C 000.20 for a Celsius reading about 293.15 K for a Kelvinreading.4. The Fahrenheit scale , used in the United States, employsa smaller degree than the Celsius scale and a different zero of temperature. The relation between the Celsius and Fahrenheit scales is,32590+=C F T T where F T is Fahrenheit temperature.11.3 The Absorption of Heat by Solids and Liquids1. Heat capacity C of an object is the proportionality constantbetween an amount of heat and the change in temperature that this heat produces in the object. Thus)(i f T T C Q -=, in which i T and f T are the initial and final temperature of theobject. Heat capacity C has the unit of energy per degree or energy per kelvin .2. Two objects made of the same material will have heatcapacities proportional to their masses. It is therefore convenient to define a “heat capacity per unit mass” or specific heat c that refers not to an object but to a unit mass of the material of which the object is made. The above equation then becomes )(i f T T cm Q -=.3. Molar specific heat : In many instances the most convenientunit for specifying the amount of a substance is the mole (mol),where units y elementarr mol 231002.61?= of anysubstance. When quantities are expressed in moles, the specific heat must also involve moles; it is then called a molarspecific heat.11.4 A Closer Look at Heat and Work1. Let us take as our system a gasconfined to a cylinder with amovable piston, as shown inthe figure. The system startsfrom an initial state i ,described by a pressurei p , a volume i V , and a temperaturei T . You want to change the system to a final state f , describedby a pressure f p , a volume f V , and a temperature f T . We assume that all changes occur slowly, with the result that the system is always in (approximate) thermal equilibrium .2. Suppose that you remove a few lead shot from the piston of the Fig., The differential work done by the gas during the displacement is pdV ds pA s d F dW ==?=))(( , in which dV is the differential change in the volume of the gas owing to the movement of the piston.3. If the gas change its volume fromi V to f V , the total workdone by the gas is ??==fi V V pdV dW W .4. There are actually manyways to take the gasfrom state I to state f, asin the figure. So asystem can be takenfrom a given initial stateto a given final state byan infinite number ofprocesses. Heat may ormay not involved, and ingeneral, the workW and heat Q will havedifferent value for different process . So we say that heat andwork are path-dependent quantities .5. The figure (f) shows a thermodynamics cycle in which the system is taken from some initial state i to some other state f ,and then back to i.11.5 The first law of thermodynamics1. You have just seen that when a system changes from a given initial state to a given final state, both work W done and theheat Q exchanged depend on the nature of the process. Experimentally, however, we find a surprising thing. The quantity )(W Q - is the same for all process. It depends only on the initial and final states and does not depend at all on how the system gets from one to the other.2. The quantity W Q - must represent a change in some intrinsic property of the system. We call this property the internal energy int E and we write W Q E E E i f -=-=?int,int,int . This is the first law of thermodynamics . If the thermodynamics system undergoes only a differential change, we can write the first law as dW dQ dE -=int .3. Adiabatic process : An adiabatic process is the process that occurs so rapidly or occurs in a system that is so well insulated that no transfer of heat occurs between the system and its environment. Putting0=Q in the first law then lead to W E -=?int . This tells us that if the work done by the system, the internal energy of the system decreases by the amount of work. Conversely, if work is done on the system, the internal energy of the system increases by that amount .4. Constant-volume process : If the volume of a system is held constant, that system can do no work. Putting0=W in the first law yieldQ E =?int . Thus if heat is added to a system, theinternal energy of the system increases. Conversely, if heat removed during the process, the internal energy of the system must decrease .5. Cyclical process . There are processes in which, after certain interchanges of heat and work, the system is restores to its initial state. In that case, no intrinsic property of the system can possibly change. Putting0int =?E in the first law yield W Q =. Thus the net work done during the process must exactly equal the net amount of heat transferred .6. Free expansion . See the following figure. These are adiabatic processes in which nowork is done on or by thesystem. Thus 0==W Qand the first law requiresthat 0int =?E . A freeexpansion differs from all other processes we have considered because it cannot be done slowly and in a controlled way. As a result, at any given instant during the sudden expansion, the gas is not in thermal equilibrium and its pressure is not the same everywhere. So although we can plot the initial and final states on a p-V diagram, we cannot plot the expansion itself.。
Chapter 12 The Kinetic Theory of GasesClassical thermodynamics has nothing to say about atoms or molecules. Its laws are concerned only with such macroscopic variables as pressure, volume, and temperature. However, we know that gas is made up of atoms or molecules (groups of atoms bound together). The pressure exerted by a gas must surely be related to steady drumbeat of its molecules on the walls of its container. The ability of a gas to take on the volume of its container must surely be due to the freedom of motion of its molecules. And the temperature and internal energy of a gas must surely be related to the kinetic energy of these molecules. Perhaps we can learn something about gases by approaching the subject from this direction. We call this molecular approach the kinetic theory of gases.12.1 Ideal Gases1、Our goal in this chapter is to explain the macroscopic properties of a gas, such as its pressure and its temperature, in terms of the behavior of the molecules that make it up.2、The experiments show that, at low enough densities, all real gases tend to obey the relation )pV , innRTgas(lawidealwhich p is the absolute pressure, A N N n /= is the number ofmoles of gas present, andR , the gas constant , has the same value for all gases, namely, K mol J R ⋅=/31.8. The temperature T must be expressed in kelvins. Above equation is called the ideal gas law . Provided the gas density is reasonably low, it holds for any type of gas, or a mixture of different types, with n being the total number of moles present.3、 Work done by an ideal gas at constant temperature :(1). Suppose that a sample of n moles of an ideal gas, confined to a piston-cylinder arrangement, is allowed to expand from an initial volume i V to a final volume f V .(2). Suppose further that the temperature T of the gas is held constant throughout the process. Such a process is called an isothermal expansion (and the reverse is called an isothermal compression ).4、Let us calculate the work done by an ideal gas during an isothermal expansion, we have⎰⎰==f i f i V V V V dV V nRT pdV W i fV V nRT ln =. Recall that the symbol ln specifies a naturallogarithm, that is, a logarithm to base e .12.2 Pressure, Temperature, RMS Speed, and Translational Kinetic Energy1、 Let n moles of an ideal gasbeing confined in a cubical box ofvolume V , as in the figure.2、 A typical gas molecule, of mass m and velocity v, is about to collide with the shaded wall. We assume that any collision of a molecule with a wall is elastic , so the change in the particle’s momentum is along the x axis and its magnitude is x x x x mv mv mv p 2)()(-=--=∆. Hence the momentum x p ∆ delivered to the wall by the molecule during the collision is x mv 2+.3、 The average rate at which momentum is delivered to the shaded wall by this single molecule is Lmv v L mv t p x x x x 2/22==∆∆. 4、 F rom Newton’s second law, the rate at which momentum is delivered to the wall is the force acting on that wall. To find the total force, we must add up the contributions of all molecules that strike the wall, allowing for the possibility that they all have different speeds. Dividing the total force x F by the area of the wall then gives the pressurep on that wall. Thus))((///2222132222212xN x x xN x x x v v v Lm L L mv L mv L mv L F p +++=+++== , where N is the number of molecules in the box.5、 Since A nN N = in which A N is Avogadro’s number ,above equation can be re-expressed as223x x A v V nM v L nmN p ==, where A mN M = is the molar mass of the gas , and V is thevolume of the box.6、 For any molecules, 2222z y x v v v v ++=. Because there aremany molecules and because they are all moving in random direction, the average values of the squares of their velocity components are equal, so that3/22v v x =. Thus V v nM p 32=. 7、 The square root of 2v is a kind of average speed, called the root-mean-square speed of the molecules and symbolized by rms v .8、 Using the relationnRT pV = for an ideal gas, it leads to M RT nM pV v v rms 332===.See the RMS speeds ofsome molecules in Table.9、 The averagetranslational kinetic energy of a single molecule of an ideal gas iskT T N R M RT m mv mv K A rms 23)(23)3)(21(212122=====, where the constantK J N R k A /1038.1/23-⨯== is the Boltzmann constant . So we come to the conclusion that at a given temperature T , all ideal gas molecules, no matter what their mass, have the same average translational kinetic energy . When we measure the temperature of a gas, we are also measuring the average translational kinetic energy of its molecules.12.3 Mean Free Path1、 Table gives us the RMS speed of some molecules, A question often arises: If molecules move so fast, why does it take as long as a minute or so before you can smell perfume if someone open a bottle across a room? This is because although the molecules move very fast between collisions, a given molecule will wander only very slowly away from its release point.2、 The right figure shows the path of atypical molecule as it moves through the gas,changing both speed and direction abruptlyas it collide elastically with other molecules.Between collisions, our typical moleculemoves in a straight line at constant speed.Although the figure shows all the other molecules as stationary,they too are moving in much the same way.3、 One useful parameter to describe this random motion is the mean free path λ. As its name implies, λ is the average distance traversed by a molecule between collisions.4、 The expression for the mean free path can be deduced from following steps:(1). First we assume that our molecule is traveling with a constant speed u and that all other molecules are at rest. We assume further that the molecules are spheres of diameter d. A collision will then take place if the centers of the molecules come within a distance d of each other. A help way to look at this situation is to consider our single molecule to have radius of d and all the other molecules to be points.(2). As our single molecule zigzags through the gas, it sweeps out a short cylinder of cross-sectional area 2d π between successive collision. If we watch this molecule for a time interval t ∆, it moves a distance t u ∆. So the volume of the cylinder is t u d ∆2π. The number of collisions that occur is then equal to the number of (point) molecules that lie within this cylinder. It is t u d V N ∆2)/(π.(3). The mean free path is the length of the path divided by the numberof collisions)/()/(22V N u d v V N t u d t v collisions of numbers path of length ππλ=∆∆==.(4). The u in the denominator is the mean speed of our single molecule relative to the other molecules, which are moving. A detailed calculation, taking into account the actual speed distribution of the molecules, gives thatv u 2=. So )/(212V N d πλ=.5、The mean free path of air molecules at sea level is about m μ1.0. At an altitude of km 100, the mean free path is about 16cm. At 300 km, the mean free path is about 20 km.12.4 The Distribution of Molecular Speeds1、 In 1852, Scottish physics James Clerk Maxwell first solved the problem of finding the speed distribution of gas molecules. His result, known as Maxwell’s speed distribution law , isRT v M e v RT M v P 2/22/32)2(4)(-=ππ. Here v is the molecular speed, Tis the gas temperature, M is the molar mass of the gas, and R is the gas constant. The quantity )(v P is a probabilitydistribution function , defined as follow: The product dv v P )( isthe fraction of molecules whose speeds lie in the range v to dv v +.2、 As figure (a) shows, thisfraction is equal to the area of astrip whose height is)(v P and whose width is dv . The totalarea under the distribution curvecorresponds to the fraction ofthe molecules whose speed liebetween zero and infinity. Allmolecules fall into this category,so the value of this total area isunit.3、 There are two otherspeeds. The most probable speed P v is the speed at which )(v P is a maximum. The average speed v is a simple average of the molecular speeds. We will find thatM RT v π8= and M RT v P 2=,so we have relationsrms P v v v <<.12.5 Degree of Freedom and Molar Specific Heats1、 The right figure showskinetic theory models ofhelium (a monatomic gas),oxygen (diatomic), and methane (polyatomic). On the basis of their structure, it seems reasonable to assume that monatomic molecules, which are essentially point-like and have only a very small rotational inertia about any axis, can store energy only in their translational motion. Diatomic and polyatomic molecules, however, should be able to store substantial additional amount of energy by rotating or oscillating.2、To take these possibilities into account quantitatively, we use the theorem of the equipartition of energy, introduced by James Clerk Maxwell: Every kind of molecules has a certain number f of degree of freedom, which are independent ways in which the molecule can store energy. Each such degree of freedom has associated with it, on average, an energy of 2/kT per molecule (or 2/RT per mole).3、For translational motion, there are three degrees of freedom. For rotational motion, a monatomic molecule has no degree of freedom. A diatomic molecule has two rotational degrees of freedom. A molecule with more than two atoms has six degrees of freedom, three rotational and three translational.4、Internal energy is the energy associated with random motion of atoms and molecules. So a sample of n moles of a gas containsnN atoms. The internal energy of the sample is then AnRT f kT f nN E A 2)2)((int ==. Thus, the internal energy int E of anideal gas is a function of the gas temperature only; it does not depend on any other variable .5、 Molar specific heat at constant volume : (1) According to the definition of molar specific heat, we have T nC Q v ∆=. (2) Substituting it into the first law of thermodynamics, we find T nC T nC W Q E V v ∆=-∆=-=∆0int . (3) So we will have the relation R f T n RT f n T n E C v 2)2(int =∆∆=∆∆=. 6、 Molar specific heat at constant pressure : (1) the heat is T nC Q p ∆=. (2) According to the first law of thermodynamics, wehave T nC T nR T nC V p T nC W Q E V p p ∆=∆-∆=∆-∆=-=∆int .(3) So we have relationR C C V p +=.12.6 The Adiabatic Expansion of an Ideal Gas1、 Using the first law of thermodynamics, we have pdV Q dE -=int pdV -=. It means pdV dT nC V -=.2、 From the ideal gas law ()nRT pV = we have nRdT Vdp pdV =+ 3、 Combining these two equations, we have 0)(=+V dV C C p dp V p . Replacing the ratio of the molar specific heats with γ and integrating yieldt cons a pV tan =γ.11 4、 Using the ideal gas law, we also have t cons a TV tan 1=-γ, and t cons a T p tan 1=--γγ.5、 Free expansion: Since a free expansion of a gas is an adiabatic process that involves no work done on or by the gas, and no change in the internal energy of the gas. A free expansion is thus quite different from the type of adiabatic process described above, in which work is done and the internal energy changes. Since the int ernal energy isn’t change for free expansion, we must havef i RT f n RT f n 22=. It means f i T T =, andso f f i i V p V p =.。