SO2(g) + H2O(l) → H2SO3(aq) 还原性和氧化性
SO2(g) + Br2(aq) → Br-(aq) + SO4-(aq) 2SO2(g) + O2(g) △→ 2SO3(g), slow reaction, catalyzed V2O5 SO3(g) + H2O(l) → H2SO4(aq) 酸雨(Sox, Nox, H2O) H2SO4, 无色腐蚀油状液体;强酸,脱水剂,氧化剂。 SO3(g) + H2SO4(l) → H2S2O7(l) (Oleum, 发烟硫酸) 化肥(2/3),石化产品,染料,清洁剂。
a) Before magnetization, the spins are almost randomly aligned.
b) After magnetization the spins are aligned in the same direction.
9
Trends in Chemical Properties
Cr(II)O basic
Cr2(III)O3 amphoteric
Cr(VI)O3
acidic
10
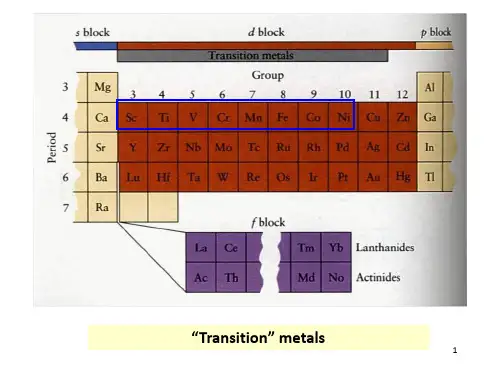
周期 四 五 六
11
These three artifacts represent the progress that has been made in the extraction of d-metals.(a) An ancient bronze (青铜,Cu/Sn) chariot axle cap from China, made from an alloy of metals hat are easy to extract. (b) A nineteenth-century iron steam engine made from a metal that was moderately easy to extract once high temperatures could be achieved. (c) A 20-century airplane engine with titanium components that had to await advanced, hightemperature technology before the element became widely 12 available.