上市后临床跟踪管理系统程序
- 格式:doc
- 大小:237.98 KB
- 文档页数:19

目录第1章临床路径应用操作 (2)1.1临床路径应用介绍 (2)1。
1.1临床路径介绍 (2)1.1。
2界面整体介绍 (2)1。
2路径功能操作步骤 (5)1。
2。
1路径导入功能 (5)1。
2.2项目生成功能 (8)1.2。
3项目补充功能 (14)1。
2。
4新开医嘱功能 (15)1。
2。
5项目执行功能 (17)1。
2。
6路径评估功能 (18)1.2。
7路径完成功能 (22)1。
2.8打印临床路径表 (24)第2章临床路径跟踪操作 (25)2。
1路径跟踪应用介绍 (25)2。
1。
1路径跟踪介绍 (25)2.1.2跟踪功能界面介绍 (25)2.2跟踪功能操作步骤 (26)2.2.1路径监控功能 (26)2.2。
2查询变异原因功能 (28)2。
2。
3概况分析功能 (31)第1章临床路径应用操作1.1临床路径应用介绍1.1.1临床路径介绍临床路径是针对某种疾病(或手术),以时间为横轴,以入院指导、诊断、检查、用药、治疗、护理、饮食指导、教育、出院计划等理想治疗或护理手段为纵轴,制定标准化治疗护理流程(临床路径表)。
其目的是运用图表的形式来提供有时间的、有序的、有效的照顾,以控制质量和经费,是一种跨学科的、综合的整体医疗护理工作模式。
路径操作流程如图【1—1—1—1】所示:【1-1-1-1】病人导入路径条件是通过病人的诊断、病情、性别、年龄、科室进行判断,如果该病人满足导入条件则允许导入路径,导入路径后根据流程进行一系列的操作包括生成路径项目、执行项目、评估阶段项目、结束路径。
1.1.2界面整体介绍临床路径应用管理模块如图【1-1-2—1】所示,该模块包含了以下几个功能块,分别是操作按钮、病人列表、路径相关信息、路径阶段名称、路径项目列表。
【1—1-2-1】要点说明1.如果当前科室或病区,没有使用的路径表,则不会显示“路径状态"图标和“临床路径”选项卡.“路径状态"图标和“临床路径”选项卡所在位置如图【1—1—2-2】所示。

药品追踪操作规范1. 引言本操作规范旨在确保药品追踪工作的准确性和效率,以满足监管要求并确保患者用药的安全性和追溯能力。
本文档将提供一些基本的操作指导,以帮助执行药品追踪工作的人员如何按照规范进行操作。
2. 药品信息记录在药品追踪工作中,准确记录药品相关信息至关重要。
以下是一些应记录的必要信息:- 药品名称和通用名称- 批次号和生产日期- 供应商和生产商信息- 收货日期- 存储条件和有效期- 出库日期和销售信息- 使用单位和使用者信息- 追溯信息的管理者及其联系方式3. 追踪流程为了保证追溯能力,需要制定良好的追踪流程。
以下是一套常用的追踪流程步骤:1. 药品入库:当收到新的药品时,将其与和其他文档进行核对,并记录药品信息。
2. 药品存储:按照药品的储存要求妥善存放,并定期检查并记录药品的存储条件。
3. 药品销售:在药品出库前,根据销售订单核对药品信息与数量,并记录销售信息。
4. 药品使用:记录药品使用情况,包括使用单位和使用者信息。
5. 追溯查询:在需要追溯药品时,能够根据提供的信息查询到药品的来源和流向。
4. 追溯技术支持为了提高追溯的效率和准确性,可以借助现代技术进行追溯工作。
以下是一些可能的技术支持方式:- 条形码或二维码的应用:将药品信息以条形码或二维码的形式打印在药品包装上,便于追溯查询。
- 数据库管理系统:建立药品信息的数据库,并确保数据的准确性和及时性。
- 追溯软件:使用专门设计的追溯软件,可以更方便地管理和查询药品追溯信息。
5. 培训和审查为了确保操作的规范性,应定期进行药品追踪操作的培训和审查。
培训内容可以包括:- 药品追踪操作规范的介绍和解读- 追溯流程的演示和实操- 追溯技术支持的培训和使用说明同时,定期的审查和反馈可以帮助改进追溯工作中存在的问题和不足之处。
6. 总结药品追踪操作规范是保证药品安全和追溯能力的基础。
通过准确记录药品信息、建立良好的追踪流程和借助现代技术支持,能够有效提高药品追溯工作的效率和准确性。
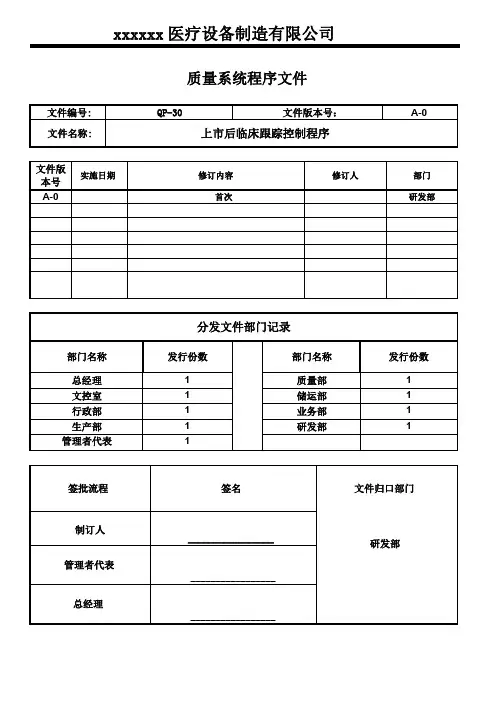
xxxxxx医疗设备制造有限公司质量系统程序文件文件编号:QP-30文件版本号:A-0文件名称:上市后临床跟踪控制程序文件版实施日期修订内容修订人部门本号A-0首次研发部分发文件部门记录部门名称发行份数部门名称发行份数总经理1质量部1文控室1储运部1行政部1业务部1生产部1研发部1管理者代表1签批流程签名文件归口部门制订人_________________研发部管理者代表_________________总经理_________________上市后临床跟踪控制程序发布日期页码第1页共4页1.目的识别和调查与使用投放市场的医疗器械有关的剩余风险,通过系统化的上市后临床随访研究(PMCF)进行调查和评估,确保其投放市场后的器械的长期安全性和性能2.范围本程序规定对上市后临床跟踪研究进行汇编的职责、工作程序、内容和要求。
本程序适用于采用CE标志有关产品上市后临床跟踪研究3.职责3.1研发部负责上市后临床跟踪计划的汇编工作;3.2研发部收集汇总上市后临床数据、进行上市后临床数据评估、维护上市后临床数据库及编制评审上市后临床报告;3.3质量部参与上市后临床跟踪报告评审,组织事故分析评审。
4.工作程序4.1定义:4.1.1临床评价:与医疗器械有关并用来验证器械根据制造商预期使用的临床安全和性能的临床资料的评估和分析。
4.1.2临床数据:临床数据是由医疗器械的使用生成的安全和/或性能信息。
4.1.3临床证据:与医疗器械有关的临床资料和临床评价报告。
4.1.4临床调查:在一个或多个人体受试者上进行的任何系统调查或研究,用于评估医疗器械的安全和/或性能。
4.1.5上市后临床随访(PMCF)的研究:在器械获得CE标识之后的一项研究,用以回答按照已批准的标签使用的器械的临床安全性或性能(即剩余风险)的具体问题。
4.1.6PMCF计划:对于符合93/42/EEC指令,加贴CE标识的医疗器械投放市场后,由制造商设立的文件化的,前瞻性的,组织化的方法和步骤收集其临床资料,目标是在整个医疗器上市后临床跟踪控制程序发布日期页码第2页共4页械的预期寿命期间,被识别风险的可接受性下,确认临床效果和安全性,在事实证据的基础上,探测新出现的风险。

跟踪程序和操作步骤
1. 确定跟踪目标
在开始跟踪程序之前,首先需要明确跟踪的目标是什么。
明确目标可以让您更好地制定相应的策略和操作步骤。
2. 收集相关信息
为了进行有效的跟踪工作,收集相关的信息非常重要。
这些信息可能包括个人身份信息、联系方式、行动计划等。
确保收集到的信息是准确和可靠的。
3. 制定跟踪策略
根据跟踪目标和收集到的信息,制定一个简单而清晰的跟踪策略。
这可以包括确定跟踪的时间范围、跟踪频率以及使用的跟踪工具或技术。
4. 记录跟踪过程
在进行跟踪工作时,及时记录重要的跟踪过程和结果。
这可以帮助您跟踪整个过程,并在需要时进行参考。
5. 定期更新信息
跟踪工作可能需要一段时间才能完成,因此定期更新相关信息
非常重要。
确保及时更新所收集到的信息,并与最初的目标进行比对。
6. 评估和调整
在跟踪过程中,不断评估跟踪策略的有效性,并根据需要进行
调整。
这可以帮助您在整个跟踪过程中保持灵活性,并优化跟踪结果。
7. 保护信息安全
在进行跟踪工作时,确保妥善保护收集到的信息的安全性。
遵
守相关法律法规,并采取适当的安全措施,以防止信息泄露或滥用。
请注意,本文档提供的是一般性的指导,具体的跟踪程序和操
作步骤可能因情况而异。
根据您的具体需求和情况,可能需要进行
进一步的定制和调整。

上市后临床跟踪控制程序(ISO13485-2016)1.0 PURPOSEThe purpose of this work instruction is to define the process to determine and document whether a post-market clinical follow-up study is required forTDI Foot/Ankle Array 8ch medical devices bearing the CE mark. The process will lead to a determination of whether a post-market clinical follow-up study is required and provide guidance for post-market clinical monitoring requirements if a study is not required.2.0 SCOPEThe work instruction applies to all medical device businesses and sites operating under the TDI Foot/Ankle Array 8ch Healthcare Quality Management System.Only medical devices bearing the CE Mark will be required to follow this work instruction.3.0 REFERENCES3.1. External References3.1.1. LawsCouncil Directive 93/42/EEC of 14 June 1993 concerning medical devices including amendments through 05 September 20073.1.2.Guidance DocumentsEuropean Commission Enterprise-Directorate-General MEDDEV 2.12-2 Guidelines on Post Market Clinical Follow-Up dated May 2004 MEDDEV 2.7.1 Rev.3 guidelines on medical device-clinical evaluation-a guide for manufacturers and notified bodies dated April 2009GHTF Post-Market Clinical Follow-Up Studies; SG5(PD)N4R7 (Proposed document 23 July 2008)GHTF Clinical Investigations; SG5(PD)N3R7 (20 January 2008)4.0 ROLES AND RESPONSIBILITIESImportant: When a title of a position is listed in this work instruction, it relates to that position or its equivalent.Below are the roles and responsibilities discussed within this document. Table 错误!文档中没有指定样式的文字。

医院信息追踪和质量服务控制程序1. 简介本文档旨在介绍医院信息追踪和质量服务控制程序。
该程序旨在优化医院内部的信息流动和质量服务,提高医疗服务的效率和质量,确保患者获得高水平的医疗护理。
2. 信息追踪程序医院的信息追踪程序旨在跟踪和记录患者的相关信息,包括诊断结果、治疗方案、药物使用和其他医疗操作等。
以下是该程序的主要步骤:- 患者登记:在患者就诊之前,前台工作人员会要求患者填写基本信息和提供相关证件。
这些信息将被记录在患者档案中。
- 医生咨询:医生将与患者进行详细的咨询,记录病史、症状和其他相关信息。
- 检查和诊断:医生可能会将患者转至其他科室进行特定的检查和诊断。
检查结果将被记录下来,并与患者档案相关联。
- 治疗计划:医生将根据诊断结果制定个性化的治疗计划,并将其记录在患者档案中。
- 随访和进展记录:医生将定期对患者进行随访,并将治疗的进展情况记录下来。
- 档案管理:医院将建立和维护一个完善的患者档案系统,确保患者信息的安全和机密性。
3. 质量服务控制程序医院的质量服务控制程序旨在确保医疗服务的质量和安全,提高患者满意度。
以下是该程序的主要步骤:- 质量标准制定:医院将制定一套质量标准,包括医疗操作规范、感染控制措施、药物使用指南等。
- 质量考核:医院将定期进行内部和外部质量考核,评估医疗服务的质量水平,并对不符合标准的情况进行纠正和改进。
- 不良事件报告和处理:医院将鼓励医务人员报告不良事件,并进行适当的处理和纠正措施,以避免再次发生。
- 患者满意度调查:医院将定期进行患者满意度调查,收集患者对医疗服务的反馈和建议,并进行改进。
- 持续改进:医院将建立一个持续改进的机制,通过定期审核和评估,寻求医疗服务的进一步提升和优化。
4. 总结医院信息追踪和质量服务控制程序是确保医疗服务高效和优质的重要措施。
通过精确追踪患者信息和实施质量服务控制程序,医院能够提供更好的医疗护理,增强患者满意度,同时保障安全和质量。

XXXXXXXXXXX上市后临床跟踪总结报告编制:日期:审核:日期:批准:日期:XXXXX器械有限公司1. 概述XXXXXXXXXXX用于各类痔疮,尤其是重度内痔和部分直肠粘膜脱垂的病人。
其原理是:保留肛垫,将部分内痔及痔上黏膜、黏膜下组织环行切除吻合的同时,进行瞬间吻合。
既阻断了痔的血液供应,又将滑脱组织悬吊固定,将病理状态的肛管直肠恢复到正常的解剖状态。
XXXXX的治疗机理是在脱垂内痔的上方近内痔上缘的地方环形切除直肠下端肠壁的粘膜和粘膜下层组织,并在切除的同时对远近端粘膜进行吻合,使脱垂的内痔及粘膜被向上悬吊和牵拉,不再脱垂。
同时,由于位于粘膜下层来自直肠上供给痔的动脉被切断,术后痔供血减少。
因此,该手术的确切名称应为“痔上粘膜及粘膜下层环切、肛垫悬吊术”。
XXXXXXXXXXX适用于选择性切除直肠齿状线上粘膜和粘膜下组织,恢复直肠下段正常解剖结构,供齿状线上黏膜选择性切除用。
2. 临床跟踪管理目的通过对我公司生产的XXXXXXXXXXX上市后的临床跟踪,建立该产品临床性能的长期安全性和有效性,确定风险的可控性,并且能评估实际临床引用过程中可能出现的风险。
通过研究以解决如下问题:a)在临床应用过程中是否存在风险管理中所提及的风险;b)临床试用过程中是否存在新的风险;c)长期在使用过程中的安全性和有效性。
3. 相关支持性法规及标准《医疗器械注册管理办法》(国家食品药品监督管理总局令第4号)《医疗器械生产监督管理办法》(国家食品药品监督管理总局令第7号)(国家食品药品监督管理总局令第6号)《医疗器械说明书和标签管理规定》关于发布医疗器械生产质量管理规范的公告(2014年第64号)YY/T0316-2016 医疗器械风险管理对医疗器械的要求YY/T 0287-2016 医疗器械质量管理体系用于法规的要求4. 相关部门职责4.1.1质量管理部:负责组织管理报告的会审会签;负责对跟踪管理全过程实施监控;负责核查、汇总验证数据;负责建立档案,及时收存归档。

ISO13485:2016医疗器械上市后临床跟踪程序1、⽬的适当使⽤和执⾏上市后临床跟踪研究,来解决与剩余风险相关的问题,及时修改产品标签,说明书的内容及技术⽂件,确保产品的持续安全有效,满⾜欧盟MEDDEV 2.12/2 rev2(《GUIDELINES ON MEDICAL DEVICES:POST MARKET CLINICALFOLLOW-UP STUDIES A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES》January 2012发布)的要求。
作为产品质量体系的⼀部分,⼀个适当的市场监督程序,对上市产品识别使⽤中风险识别和风险研究的关键,特制定本程序。
2、范围适⽤于本公司带有CE标识的医疗器械产品的销售后的临床数据跟踪。
3、职责3.1 管理者代表:负责组织落实产品符合MDD 93/42/EEC 的要求。
3.2 ⼯程部:负责按本程序及相关法规制定《上市后的临床跟踪计划》及相关⽂件,并根据汇总上市后的临床数据,评价现有⽂件的适合性并修改相关的⽂件。
3.3 品管部:负责按本程序及相关法规对⽂件归档保存,负责产品相关的标签的控制。
3.3 业务部:负责审核《上市后的临床跟踪计划》,并在计划批准后按⽂件要求执⾏。
3.4 总经理:负责对《上市后的临床跟踪计划》的批准。
4、定义4.1 临床调查:在⼀个或更多的⼈类受试者上进⾏的任何系统性的调查或研究,从⽽评估医疗设备的安全性和性能。
4.2 上市后临床跟踪研究:跟随着设备的CE标记⽽执⾏的研究,其意图是回答与设备依照批准的标签使⽤时的临床安全性和性能(如剩余风险)相关的特定问题。
4.3 PMCF计划:制造商建⽴的备⽤证明⽂件的、前瞻性的、有组织的⽅法和程序,其根据与特定设计档案⼀致的CE标记产品的使⽤或属于相同亚类或MDD93/42/EEC指令中定义的⼀般设备组的⼀组医疗设备的使⽤来收集临床数据。
它的⽬标是在医疗设备的预期⽣命期中巩固临床表现和安全性和经鉴定的风险的可接受性,以及在事实证据的基础上探测产⽣的风险。

pmcf法规
PMCF法规是指欧盟的新医疗器械法规(MDR)中对医疗器械的上市后临床跟踪(PMCF)的要求。
PMCF是持续更新的临床评价过程,应在制造商的上市后监督计划中予以设计体现,旨在为器械的临床评价提供最新数据,确保器械获批上市后,其安全性和性能将持续获得监督。
根据MDR 86(1)条规定,PSUR基于PMCF,因此,除I类产品外所有类别的器械都需要PMCF。
器械制造商在设计和运行PMCF研究时,应牢记五个主要目标:在整个预期使用寿命内确认器械的安全性和性能;识别以前未知的副作用并监测已明确的副作用和禁忌症;根据事实证据确定和分析风险;确保MDR附件I(1,9)提到的收益风险比是持续可接受的;识别器械可能的系统误用或标签外使用,以验证器械的预期使用目的是否正确(如果有很多标签外使用,说明之前制定的预期使用可能有误)。
PMCF计划应包括以下信息:上市后临床随访的一般方法和程序;应用PMCF的具体方法和程序,例如评估合适的注册或临床研究;上述方法和程序的适当性的理由;参考临床调查报告和风险管理;上市后临床随访要解决的具体目标;评估与同等或类似设备相关的临床数据;参考任何相关的CS(通用规范),制造商使用的协调标准以及PMCF的相关指南;PMCF 活动的详细且有充分理由的时间表;定期安全更新报告。
需要注意的是,PMCF法规仅是欧盟医疗器械法规的一部分,完整的法规还包括上市后监督计划和定期安全更新报告等要求。
如果你想了解更多关于PMCF法规的信息,可以参考相关的法律法规文件或咨询专业人士。

上市后临床跟踪协议
上市后临床跟踪协议是指在医疗器械或药物上市后,进行后续临床研究以跟踪产品的安全性和有效性的协议。
该协议用于了解产品在实际临床使用中的表现,以确保产品的长期安全性和有效性,并提供进一步的临床数据支持。
上市后临床跟踪协议通常由制造商或申请者与监管机构(如药品监管部门)共同制定,其中包括具体的研究目标、方法、样本规模、研究时间、数据收集与分析等内容。
协议还明确了责任分工和监控机制,以监督和评估后续临床研究的进行。
上市后临床跟踪协议的目的是提供可靠的临床数据,以便评估产品的长期安全性和有效性,并及时发现任何安全性问题或不良事件。
这些数据可以用于更新产品标签,指导医生和患者的使用,以及支持后续的临床决策和监管活动。
上市后临床跟踪协议在药物和医疗器械领域都是常见的做法。
对于一些高风险或新型技术的产品,其上市后临床跟踪协议可能会更加详细和严格,以确保产品的安全性和有效性。
需要注意的是,上市后临床跟踪协议是一项长期的工作,需要耗费大量时间和资源。
然而,通过对产品进行长期的跟踪研究,可以提供对产品性能的更全面和可靠的评估,增强人们对产品的信任和安全性的保障。
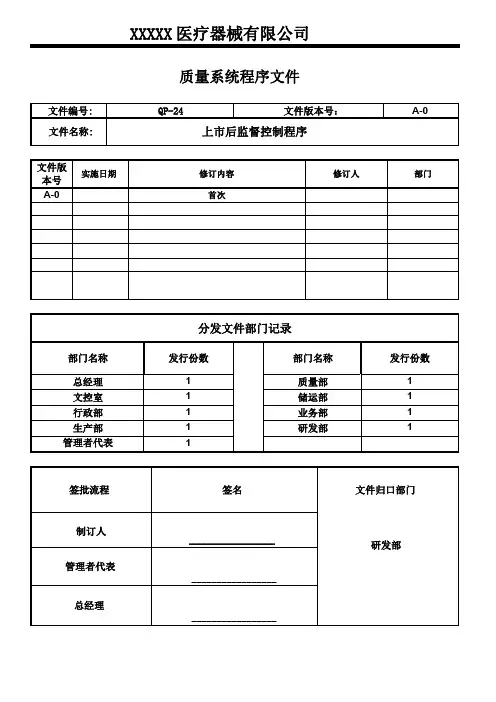
XXXXX医疗器械有限公司质量系统程序文件文件编号:QP-24文件版本号:A-0文件名称:上市后监督控制程序文件版实施日期修订内容修订人部门本号A-0首次分发文件部门记录部门名称发行份数部门名称发行份数总经理1质量部1文控室1储运部1行政部1业务部1生产部1研发部1管理者代表1签批流程签名文件归口部门制订人_________________研发部管理者代表_________________总经理_________________发布日期页码第1页共9页1.目的建立医疗器械不良事件监测、再评价、召回等上市后监管制度,构建了比较全面的、与国际医疗器械监管接轨的产品上市后监管制度,形成了产品上市前、上市后联动,有许可(备案)有退出的医疗器械监管机制,为实现医疗器械全寿命周期监管,奠定坚实的法律基础。
2.范围本程序规定对上市后监督进行汇编的职责、工作程序、内容和要求。
本程序适用于采用CE标志有关产品的临床调查及资料汇编/整理工作。
3.职责3.1业务部和售后组主要负责产品上市后资料的搜集;3.2研发部负责上市后监督的组织及资料的汇编/整理工作;3.3研发部收集汇总产品投诉、不良事件的报告、市场纠正措施及召回等;3.4质量部参与产品投诉、不良事件评审。
3.5涉及部门包括:业务、售后、临床、储运、研发、质量等部门人员;4.工作程序4.1术语和定义:●上市后监督:收集和分析从已经上市的医疗器械中获得经验的过程;●投诉:宣称已从组织的控制中放行的医疗器械存在与标识,质量,耐用性,可靠性,可用性,安全或性能有关的缺陷或宣称影响这些医疗器械性能的服务存在不足的书面,电子或口头的沟通;●抱怨:任何以书面、口头、电讯的形式宣称,已经投放市场的医疗器械在其特性、质量、耐用性、可靠性、安全性及性能等方面存在不足的行为。
●召回:是指产品生产企业按照规定的程序收回已上市销售的存在安全隐患的产品。
●不良事件:是指在正常诊断和治疗过程中,发生本可避免的涉及医疗安全的不良事件/缺陷。
EUROPEAN COMMISSIONDG ENTERPRISEDirectorate GGUIDELINES ON POST MARKET CLINICAL FOLLOW-UP上市后临床跟踪指南The present Guidelines are part of a set of Guidelines relating to questions of application of EC-Directives on medical devices. They are legally not binding. The Guidelines have been carefully drafted through a process of intensive consultation of the various interested parties (competent authorities, Commission services, industries, other interested parties) during which intermediate drafts were circulated and comments were taken up in the document. Therefore, this document reflects positions taken by representatives of interested parties in the medical devices sector.本准则是一个有关的欧共体指令对医疗设备的应用问题指引的一部分。
他们在法律上没有约束力。
该指引已审慎草拟通过各有关方面(主管机关,委员会的服务,工业,其他有关各方)在此期间,中间草案分发和评论的文件采取了密集的磋商进程。
因此,这份文件反映了有关各方的代表在该领域采取的医疗设备的位置。
1.PURPOSEThe purpose of this work instruction is to define the process to determine anddocument whether a post-market clinical follow-up study is required forTDIFoot/Ankle Array 8ch medical devices bearing the CE mark. The process will leadto a determination of whether a post-market clinical follow-up study is required and provide guidance for post-market clinical monitoring requirements if a study is notrequired.2.SCOPEThe work instruction applies to all medical device businesses and sites operatingunder the TDI Foot/Ankle Array 8ch Healthcare Quality Management System.Only medical devices bearing the CE Mark will be required to follow this workinstruction.3.REFERENCES3.1.External Referencesws▪Council Directive 93/42/EEC of 14 June 1993 concerning medical devices including amendments through 05 September 20073.1.2.Guidance Documents▪European Commission Enterprise-Directorate-General MEDDEV 2.12-2 Guidelines on Post Market Clinical Follow-Up dated May 2004▪MEDDEV 2.7.1 Rev.3 guidelines on medical device-clinical evaluation-a guide for manufacturers and notified bodies dated April 2009▪GHTF Post-Market Clinical Follow-Up Studies; SG5(PD)N4R7 (Proposed document 23 July 2008)▪GHTF Clinical Investigations; SG5(PD)N3R7 (20 January 2008)4.ROLES AND RESPONSIBILITIESImportant: When a title of a position is listed in this work instruction, it relates to that position or its equivalent.Below are the roles and responsibilities discussed within this document.Table 4-1: Roles and ResponsibilitiesTable 4-1: Roles and Responsibilities5.WORK INSTRUCTIONPost-market clinical monitoring is an essential element in establishing long termsafety follow-up data and possible emergent risks for medical devices. These risks and data cannot adequately be detected and characterized by relying solely onpre-market clinical investigations.Post market clinical monitoring may include a combination of several strategies: ▪Product complaint review▪Post-market event reporting review of users and patients▪Literature review▪Post-market clinical follow-up studies (PMCFS)This work instruction was created to determine when a PMCFS is necessary tomaintain an adequate post-market surveillance system, as required by the Medical Device Directive 93/42/ECC (MDD) as amended by MDD 2007/47/EC. It will alsoprovide guidance on the post-market clinical monitoring requirements if a PMCFS is not required.Figure 5-1: High-Level Process Overview for Post-Market Clinical Follow-UpPMCFSDetermination5.1.General Requirements5.1.1.Prior to M3 sign-off, the Product Regulatory Affairs Representative in consultationwith the Research Manager or designee and the Design Engineering and/orEngineering Representative shall determine for a given project/program whether a PMCFS is required. They shall also determine the post-market clinical follow-upplan.5.1.2. A PMCFS may not be required for products for which medium/long-term clinicalperformance and safety is already known from previous use of the device or where other appropriate post-market surveillance activities would provide sufficient data to address the risks.5.2.Determining the Type of Post-Market Clinical Follow-UpRequiredPost-market clinical monitoring shall have one of two outcomes, (1) PMCFS required or (2) no PMCFS required.The need for a PMCFS shall be based on a combination of several factors detailed in this section.5.2.1.The Product Regulatory Affairs Representative in consultation with the ResearchManager or designee and Design Engineering and/or Engineering Representativeshall determine whether an equivalent device exists. Equivalence shall bedemonstrated in all the essential characteristics precisely defined below.Equivalence means:▪Clinical▪Used for the same clinical condition or purpose;▪Used at the same site in the body;▪Used in similar population (including age, anatomy, physiology);▪Have similar relevant critical performance according to expected clinical effect for specific intended use▪Technical▪Used under similar conditions of use;▪Have similar specifications and properties;▪Be of similar design;▪Use similar deployment methods▪Have similar principles of operation▪Biological▪Same or similar use of materials in contact with human tissues or body fluids5.2.2.Products for which the medium/long term clinical performance and safety is alreadyknown from previous use of the device, or from fully transferable experience withequivalent devices shall not require a PMCFS.NOTE:If the device quoted as the “equivalent” requires a PMCFS, then the newproduct shall be subject to the same requirement.5.2.3.The need for a PMCFS shall be determined based on the identification of residualrisks that may impact the risk/benefit ratio. A study should always be consideredfor devices where the identification of possible emerging risks and the evaluation of long term safety and performance are essential. The Product Regulatory AffairsRepresentative in consultation with the Research Manager or designee and DesignEngineering and/or Engineering Representative shall identify such emerging risk, the following criteria should be taken into account:▪innovation, e.g., where the design of the device, the materials, the principles of operation, the technology or the medical indications are novel;▪high risk anatomical locations (i.e., heart, central nervous system, etc.);▪severity of disease/treatment challenges;▪sensitivity of target population (i.e., infants, children, pregnant women, etc.);▪identification of an acceptable risk during the pre-CE clinical evaluation, which should be monitored in a longer term and/or through a largerpopulation;▪well known risks identified from the literature or similar marketed devices;▪discrepancy between the pre-market follow-up time scales and the expected life of the product;5.2.4. A properly conducted risk analysis is essential in determining what clinical evidencemay be need ed for a particular device. Any risks identified as an “unacceptable”risk at the conclusion of the development process shall require a PMCFS. A studyshould also be considered for risks identified as “acceptable” or “risk mitigationrequired” if the de vice meets any of the other characteristics identified in 5.2.1 and5.2.2. The risk assessment shall be performed according to the Risk ManagementProcedure. The Product Regulatory Affairs Representative shall review the riskassessment.5.2.5.The Product Regulatory Affairs Representative shall complete the Post MarketClinical Follow-Up Study Determination Form (Appendix A) once the decisionregarding the need for a study has been determined.NOTE:This form may also be used as a guide in making the determination about the need to perform a PMCFS.5.2.6.The Product Regulatory Affairs Representative shall complete the Post-MarketClinical Follow-Up Plan (Appendix B) that details the plan for post-market clinicalfollow-up.5.2.7.The Research Manager or designee and Medical Affairs Representative shall reviewthe Post-Market Clinical Follow-Up Justification Form and The Post-Market ClinicalFollow-Up Plan to confirm the decisions regarding post-market clinical monitoring.5.3.No Post Market Clinical Follow-Up Study Required5.3.1.If it was determined that no PMCFS is required (based on section 5.2), post-marketclinical monitoring is still required for the medical device.5.3.2.Justification regarding the decision not to perform a PMCFS must be clearlydocumented and maintained in the design history/technical file (see 5.2.5).5.3.3.Post-Market Clinical Monitoring Requirements (minimum)5.3.3.1.At a minimum, the following post-market clinical monitoring activities shall becompleted according to TDI Foot/Ankle Array 8ch established procedures/workinstructions. These elements will be inputs into the Post-Market LiteratureEvaluation and Market Analysis Report.▪Review of product complaints according to Complaint Handling Procedure▪Review of post market adverse events according to Post Market Event Reporting Procedure▪Literature review according to TDI Foot/Ankle Array 8ch Evaluation of Clinical Data to Support CE Marking Work Instruction .5.3.3.2.Review of product complaints, post market adverse events and the literature reviewshall be completed at the intervals specified in Table 5-1. The timing outlinedprovides the minimum requirements. The Product Regulatory AffairsRepresentative and/or the Research Manager or designee can determine thatclinical data shall be reviewed more often.Table 5-1: Timing for Review of Clinical Data based on Medical Device Class5.3.3.3.At the interval outlined in Table 5-1, the Research Manager or designee shallcomplete a literature review and analysis of post-market experiences (i.e.complaints and adverse events) and re-evaluate if a PMCFS needs to be conductedbased on this data. The Post Market Literature Evaluation and Market AnalysisConclusion form (Appendix D) shall be completed and maintained as part of thedevice’s design history/technical file. The Product Regulatory AffairsRepresentative and Medical Affairs Representative shall review and approve thisdocument.NOTE:The literature review shall be executed according to the Evaluation of Clinical Data to Support CE Marking Work Instruction, section 5.5. However, the following forms/templates shall be used in place of those specified in this work instruction:a.Instead of using The Literature Evaluation Plan template referenced, use the PostMarket Literature Evaluation and Market Experience Plan form (Appendix C)b.Instead of using The Literature Evaluation Report and Conclusion template, use thePost-Market Literature Evaluation and Market Analysis Report and Conclusion form(Appendix D)5.4.Post Market Clinical Follow-Up Study Required5.4.1.If it was determined that a PMCFS is required, in addition to the requirements listedunder 5.3.3, studies such as extended follow-up of patients enrolled in thepre-market trials, prospective study of a representative subset of patients after thedevice is placed on the market, or an open registry may be performed.5.4.2.The PMCFS shall b e carried out in accordance with TDI Foot/Ankle Array 8ch’sResearch Involving Human Subjects Procedure5.4.3.The Research Manager or designee in consultation with the Regulatory AffairsRepresentative and the Design Engineering and/or Engineering Representative willdetermine the type of PMCFS that will be implemented.5.4.4.The study should take into account the following:▪Results of the clinical investigation including adverse events identified▪Average life expectancy of the device▪The claims made by the manufacturer for the device▪Performances for which equivalence is claimed▪New information becoming available5.4.4.1.At the interval outlined in Table 5-1, the Research Manager or designee shallcomplete a literature review and analysis of post-market experiences (i.e.complaints and adverse events) and review the ongoing results/data of the PMCFS.The Post Market Literature Evaluation and Market Analysis Conclusion form(Appendix D) shall be maintained as part of the device’s design history/technical file.The Product Regulatory Affairs Representative and Medical Affairs Representativeshall review and approve this document.NOTE:The literature review shall be executed according to the Evaluation of Clinical Data to Support CE Marking Work Instruction, section 5.5. However, the following forms/templates shall be used in place of those specified in this work instruction:a.Instead of using The Literature Evaluation Plan template referenced, use the PostMarket Literature Evaluation and Market Experience Plan form (Appendix C)b.Instead of using The Literature Evaluation Report and Conclusion template, use thePost-Market Literature Evaluation and Market Analysis Report and Conclusion form(Appendix D)5.5.Elements of a post-market clinical follow-up study5.5.1.Post-market clinical follow-up studies are performed on a device within its intendeduse/purpose(s) according to the instructions for use.5.5.2. A PMCFS shall include the elements defined in the Writing Clinical InvestigationalPlans and Protocols Work Instruction.5.5.3.The objective(s) of a PMCFS should be stated clearly and should address the residualrisk(s) identified. It should be formulated to address one or more specificquestions relating to the clinical safety or performance of the device.5.5.4.Post-market clinical follow-up studies should be designed to address the objective(s)of the study. The design may vary based on the objective(s) and should bescientifically sound to allow for valid conclusions to be drawn.5.5.5.The study design can take several forms, for example:▪the extended follow-up of patients enrolled in pre-market investigations;▪ a new clinical investigation;▪ a review of data derived from a device registry;▪ a review of relevant retrospective data from patients previously exposed to the device.▪the analysis plan including any interim reporting; and▪procedures for early study termination.5.5.6.The data and conclusions derived from the PMCFS are used to provide clinicalevidence to support the post-market surveillance program. This process may result in the need to reassess whether the device continues to comply with the EssentialPrinciples. Such assessments may result in corrective or preventive actions.6.APPENDIX6.1.Appendix A: Post-Market Clinical Follow-Up Study Determination6.2.Appendix B: Post-Market Clinical Follow-Up PlanExperience Analysis PlanAnalysis Report and Conclusion。
1、目的适当使用和执行上市后临床跟踪研究,来解决与剩余风险相关的问题,及时修改产品标签,说明书的内容及技术文件,确保产品的持续安全有效,满足欧盟MEDDEV 2.12/2 rev2(《GUIDELINES ON MEDICAL DEVICES:POST MARKET CLINICAL FOLLOW-UP STUDIES A GUIDE FOR MANUFACTURERS AND NOTIFIED BODIES》January 2012发布)的要求。
作为产品质量体系的一部分,一个适当的市场监督程序,对上市产品识别使用中风险识别和风险研究的关键,特制定本程序。
2、范围适用于本公司带有CE标识的医疗器械产品的销售后的临床数据跟踪。
3、职责3.1 管理者代表:负责组织落实产品符合MDD 93/42/EEC 的要求。
3.2 工程部:负责按本程序及相关法规制定《上市后的临床跟踪计划》及相关文件,并根据汇总上市后的临床数据,评价现有文件的适合性并修改相关的文件。
3.3 品管部:负责按本程序及相关法规对文件归档保存,负责产品相关的标签的控制。
3.3 业务部:负责审核《上市后的临床跟踪计划》,并在计划批准后按文件要求执行。
3.4 总经理:负责对《上市后的临床跟踪计划》的批准。
4、定义4.1 临床调查:在一个或更多的人类受试者上进行的任何系统性的调查或研究,从而评估医疗设备的安全性和性能。
4.2 上市后临床跟踪研究:跟随着设备的CE标记而执行的研究,其意图是回答与设备依照批准的标签使用时的临床安全性和性能(如剩余风险)相关的特定问题。
4.3 PMCF计划:制造商建立的备用证明文件的、前瞻性的、有组织的方法和程序,其根据与特定设计档案一致的CE标记产品的使用或属于相同亚类或MDD93/42/EEC指令中定义的一般设备组的一组医疗设备的使用来收集临床数据。
它的目标是在医疗设备的预期生命期中巩固临床表现和安全性和经鉴定的风险的可接受性,以及在事实证据的基础上探测产生的风险。
v以上所有信息均为深圳市亿米生命科技有限公司所有,不得外传)11目的规范器械上市后各项信息的来源与输入,通过对上市后的信息进行分析与改善,确保器械上市后监督系统更加完善,从而确保器械的安全性及有效性持续得到提高,特制定本程序。
2范围适用医疗器械上市后的监督管理。
3参考文件3.1《客户反馈处理控制程序》 QP-083.2《数据分析控制程序》QP-143. 3《不合格品控制程序》QP-153.4《纠正与预防措施控制程序》QP-163. 5《风险管理控制程序》QP-173.6《警戒系统控制程序》QP-213.7《忠告性通知、召回及重大变更控制程序》QP-254定义上市后监督:时已投放市场的医疗器械所获取的经验进行收集和分析的系统过程。
5职责5.1综合部:负责上市后信息的统计汇总,并组织各部门进行分析与讨论。
5.2相关部门:负责按照上市后器械信息来源的渠道进行收集与归档,并整理好提交给质量部。
5.3研发部:负责上市后器械信息的风险分析与评价。
6工作内容6.1上市后器械信息来源识别部门依据以下表的要求,至少每年一次对器械上市后各项信息进行收集,并集中交综合部进行整合。
〈以上所有信息均为深圳市亿米生命科技有限公司所有,不得外传》7<以上所有信息均为深圳巾亿米生命科技有限公司所有,不得外传〉〈以上所有信息均为深圳市亿米生命科技有限公司所有,不得外传,〈以上所有信息均为深圳市亿米生命科技有限公司所有,不得外传,深圳市亿米生命科技有限公司内部公开Internal Use Only A6.2上市后信息数据分析综合部依据各部门提供的信息,依据《数据分析控制程序》对上市后关于器械的信息进行分类统计,形成相应的统计表。
7.3上市后信息分析与讨论管代根据统计表的信息组织生产部、研发部、市场部、质量部及综合部召开专门的会议,就器械上市后相关问题进行讨论与分析,并形成相应的会议记录。
6.4风险分析研发部根据会议输出的相关问题点依据《风险管理控制程序》对每一个问题点或潜在的问题点进行风险分析,并评估每一个风险点。
医疗新技术临床应用追踪管理制度为进一步规范医院医疗技术临床应用和完善新技术的准入、评估,保障医疗安全,提高医疗质量和医疗技术水平,根据卫生部《医疗技术临床应用管理办法》(卫医政发[2009第18号])结合我院的实际,特制定本制度。
第一章医疗新技术临床应用追踪管理第一条医教科作为主管部门,对于全院的医疗新技术临床应用进行全程管理和评价,制定医院新技术新项目管理档案。
对新技术实施过程中存在的问题进行分析,并提出指导性建议或意见,及时发现医疗技术风险,并敦促相关科室及时采取相应措施,以避免医疗技术风险或将其降到最低限度。
第二条医疗新技术实施过程中,各级人员必须严格执行技术规范、操作规程及各项规章制度,服从科室管理。
科主任、项目负责人应认真组织、严格把关、定期进行质量监控,检查实施情况,及时发现各种问题并予以有效的解决。
第三条在新技术新项目临床应用过程中,应充分尊重患者的知情权和选择权,并注意保护患者安全,及时履行告知义务。
主管医师应向患者或其委托人详细交待病情,重点交待新技术对于患者的适应性、效益性和可能存在的风险及费用情况,尊重患者及委托人意见,在征得其同意并在《知情同意书》上签字后方可实施。
第四条各科室在开展新技术临床应用过程中做好应用记录和总结分析工作,完善疗效的评价分析,应当(1)认真记录病历资料,随访观察疗效;(2)定期总结病历,每年对新技术实施情况进行评估,填写《新技术、新项目开展情况追踪登记表》,《追踪登记表》中详述开展例数、疗效、经济及社会效益、质量评价等;(3)年终将本年度开展的新技术病例进行分析总结上报,医教科针对汇总情况进行有重点的抽查核实。
第五条经医院评估,符合先进性、安全性等要求的技术项目鼓励继续开展,不符合先进性、安全性等要求的技术项目,医教科根据评估结论决定该技术院内停止使用。
第二章医疗新技术临床应用的暂停、评估与停用、复用第六条医疗新技术应用过程中,出现不良后果或技术问题时,有关人员必须采用措施保证医疗安全并及时向科主任、项目负责人报告。
1.PURPOSEThe purpose of this work instruction is to define the process to determine and document whether a post-market clinical follow-up study is required forTDI Foot/Ankle Array 8ch medical devices bearing the CE mark. The process will lead to a determination of whether a post-market clinical follow-up study is required and provide guidance for post-market clinical monitoring requirements if a study is not required.2.SCOPEThe work instruction applies to all medical device businesses and sites operating under the TDI Foot/Ankle Array 8ch Healthcare QualityManagement System.Only medical devices bearing the CE Mark will be required to follow this work instruction.3.REFERENCES3.1.External Referencesws▪Council Directive 93/42/EEC of 14 June 1993 concerning medical devices including amendments through 05 September 20073.1.2.Guidance Documents▪European Commission Enterprise-Directorate-General MEDDEV 2.12-2 Guidelines on Post Market Clinical Follow-Up dated May 2004▪MEDDEV 2.7.1 Rev.3 guidelines on medical device-clinical evaluation-a guide for manufacturers and notified bodies dated April 2009▪GHTF Post-Market Clinical Follow-Up Studies; SG5(PD)N4R7 (Proposed document 23 July 2008)▪GHTF Clinical Investigations; SG5(PD)N3R7 (20 January 2008)4.ROLES AND RESPONSIBILITIESImportant:When a title of a position is listed in this work instruction, it relates to that position or its equivalent.Below are the roles and responsibilities discussed within this document.Table 4-1: Roles and ResponsibilitiesTable 4-1: Roles and Responsibilities5.WORK INSTRUCTIONPost-market clinical monitoring is an essential element in establishing long term safety follow-up data and possible emergent risks for medical devices. These risks and data cannot adequately be detected andcharacterized by relying solely on pre-market clinical investigations.Post market clinical monitoring may include a combination of several strategies:▪Product complaint review▪Post-market event reporting review of users and patients▪Literature review▪Post-market clinical follow-up studies (PMCFS)This work instruction was created to determine when a PMCFS is necessary to maintain an adequate post-market surveillance system, as required bythe Medical Device Directive 93/42/ECC (MDD) as amended by MDD 2007/47/EC.It will also provide guidance on the post-market clinical monitoringrequirements if a PMCFS is not required.Figure 5-1: High-Level Process Overview for Post-Market Clinical Follow-UpPMCFSDetermination5.1.General Requirements5.1.1.Prior to M3 sign-off, the Product Regulatory Affairs Representative inconsultation with the Research Manager or designee and the DesignEngineering and/or Engineering Representative shall determine for agiven project/program whether a PMCFS is required. They shall alsodetermine the post-market clinical follow-up plan.5.1.2.A PMCFS may not be required for products for which medium/long-termclinical performance and safety is already known from previous use of the device or where other appropriate post-market surveillanceactivities would provide sufficient data to address the risks.5.2.Determining the Type of Post-Market Clinical Follow-UpRequiredPost-market clinical monitoring shall have one of two outcomes, (1) PMCFS required or (2) no PMCFS required.The need for a PMCFS shall be based on a combination of several factors detailed in this section.5.2.1.The Product Regulatory Affairs Representative in consultation with theResearch Manager or designee and Design Engineering and/or Engineering Representative shall determine whether an equivalent device exists.Equivalence shall be demonstrated in all the essential characteristics precisely defined below. Equivalence means:▪Clinical▪Used for the same clinical condition or purpose;▪Used at the same site in the body;▪Used in similar population (including age, anatomy,physiology);▪Have similar relevant critical performance according toexpected clinical effect for specific intended use▪Technical▪Used under similar conditions of use;▪Have similar specifications and properties;▪Be of similar design;▪Use similar deployment methods▪Have similar principles of operation▪Biological▪Same or similar use of materials in contact with human tissues or body fluids5.2.2.Products for which the medium/long term clinical performance and safetyis already known from previous use of the device, or from fullytransferable experience with equivalent devices shall not require aPMCFS.NOTE: If the device quoted as the “equivalent” requires a PMCFS, then the new product shall be subject to the same requirement.5.2.3.The need for a PMCFS shall be determined based on the identification ofresidual risks that may impact the risk/benefit ratio. A study shouldalways be considered for devices where the identification of possible emerging risks and the evaluation of long term safety and performance are essential. The Product Regulatory Affairs Representative inconsultation with the Research Manager or designee and Design Engineering and/or Engineering Representative shall identify such emerging risk, the following criteria should be taken into account:▪innovation, e.g., where the design of the device, the materials, the principles of operation, the technology or the medicalindications are novel;▪high risk anatomical locations (i.e., heart, central nervous system, etc.);▪severity of disease/treatment challenges;▪sensitivity of target population (i.e., infants, children, pregnant women, etc.);▪identification of an acceptable risk during the pre-CE clinical evaluation, which should be monitored in a longer term and/orthrough a larger population;▪well known risks identified from the literature or similar marketed devices;▪discrepancy between the pre-market follow-up time scales and the expected life of the product;5.2.4.A properly conducted risk analysis is essential in determining whatclinical evidence may be needed for a particular device. Any risks identified as an “unacceptable” risk at the conclusion of thedevelopment process shall require a PMCFS. A study should also beconsidered for risks identified as “acceptable” or “risk mitigation required” if the device meets any of the other characteristicsidentified in 5.2.1 and 5.2.2. The risk assessment shall be performed according to the Risk Management Procedure. The Product RegulatoryAffairs Representative shall review the risk assessment.5.2.5.The Product Regulatory Affairs Representative shall complete the PostMarket Clinical Follow-Up Study Determination Form (Appendix A) once the decision regarding the need for a study has been determined. NOTE:This form may also be used as a guide in making the determination about the need to perform a PMCFS.5.2.6.The Product Regulatory Affairs Representative shall complete thePost-Market Clinical Follow-Up Plan (Appendix B) that details the plan for post-market clinical follow-up.5.2.7.The Research Manager or designee and Medical Affairs Representative shallreview the Post-Market Clinical Follow-Up Justification Form and ThePost-Market Clinical Follow-Up Plan to confirm the decisions regarding post-market clinical monitoring.5.3.No Post Market Clinical Follow-Up Study Required5.3.1.If it was determined that no PMCFS is required (based on section 5.2),post-market clinical monitoring is still required for the medical device.5.3.2.Justification regarding the decision not to perform a PMCFS must beclearly documented and maintained in the design history/technical file (see 5.2.5).5.3.3.Post-Market Clinical Monitoring Requirements (minimum)5.3.3.1.At a minimum, the following post-market clinical monitoringactivities shall be completed according to TDI Foot/Ankle Array 8chestablished procedures/work instructions. These elements will beinputs into the Post-Market Literature Evaluation and Market Analysis Report.▪Review of product complaints according to Complaint Handling Procedure▪Review of post market adverse events according to Post Market Event Reporting Procedure▪Literature review according to TDI Foot/Ankle Array 8ch Evaluation of Clinical Data to Support CE Marking Work Instruction .5.3.3.2.Review of product complaints, post market adverse events and theliterature review shall be completed at the intervals specified in Table 5-1. The timing outlined provides the minimum requirements. TheProduct Regulatory Affairs Representative and/or the Research Manager or designee can determine that clinical data shall be reviewed more often.Table 5-1: Timing for Review of Clinical Data based on Medical Device Class5.3.3.3.At the interval outlined in Table 5-1, the Research Manager ordesignee shall complete a literature review and analysis of post-market experiences (i.e. complaints and adverse events) and re-evaluate if aPMCFS needs to be conducted based on this data. The Post MarketLiterature Evaluation and Market Analysis Conclusion form (Appendix D) shall be completed and maintained as part of the device’s designhistory/technical file. The Product Regulatory Affairs Representative and Medical Affairs Representative shall review and approve thisdocument.NOTE:The literature review shall be executed according to the Evaluation of Clinical Data to Support CE Marking Work Instruction, section 5.5. However, the following forms/templates shall be used in place of those specified in this work instruction:a.Instead of using The Literature Evaluation Plan template referenced, usethe Post Market Literature Evaluation and Market Experience Plan form(Appendix C)b.Instead of using The Literature Evaluation Report and Conclusion template,use the Post-Market Literature Evaluation and Market Analysis Report andConclusion form (Appendix D)5.4.Post Market Clinical Follow-Up Study Required5.4.1.If it was determined that a PMCFS is required, in addition to therequirements listed under 5.3.3, studies such as extended follow-up of patients enrolled in the pre-market trials, prospective study of arepresentative subset of patients after the device is placed on the market, or an open registry may be performed.5.4.2.The PMCFS shall be carried out in accordance with TDI Foot/Ankle Array8ch’s Research Involving Human Subjects Procedure5.4.3.The Research Manager or designee in consultation with the RegulatoryAffairs Representative and the Design Engineering and/or EngineeringRepresentative will determine the type of PMCFS that will be implemented.5.4.4.The study should take into account the following:▪Results of the clinical investigation including adverse events identified▪Average life expectancy of the device▪The claims made by the manufacturer for the device▪Performances for which equivalence is claimed▪New information becoming available5.4.4.1.At the interval outlined in Table 5-1, the Research Manager ordesignee shall complete a literature review and analysis of post-market experiences (i.e. complaints and adverse events) and review the ongoing results/data of the PMCFS. The Post Market Literature Evaluation and Market Analysis Conclusion form (Appendix D) shall be maintained as partof the device’s design history/technical file. The Product Regulatory Affairs Representative and Medical Affairs Representative shall review and approve this document.NOTE:The literature review shall be executed according to the Evaluation of Clinical Data to Support CE Marking Work Instruction, section 5.5. However, the following forms/templates shall be used in place of those specified in this work instruction:a.Instead of using The Literature Evaluation Plan template referenced, usethe Post Market Literature Evaluation and Market Experience Plan form(Appendix C)b.Instead of using The Literature Evaluation Report and Conclusion template,use the Post-Market Literature Evaluation and Market Analysis Report andConclusion form (Appendix D)5.5.Elements of a post-market clinical follow-up study5.5.1.Post-market clinical follow-up studies are performed on a device withinits intended use/purpose(s) according to the instructions for use.5.5.2.A PMCFS shall include the elements defined in the Writing ClinicalInvestigational Plans and Protocols Work Instruction.5.5.3.The objective(s) of a PMCFS should be stated clearly and should addressthe residual risk(s) identified. It should be formulated to address one or more specific questions relating to the clinical safety or performance of the device.5.5.4.Post-market clinical follow-up studies should be designed to address theobjective(s) of the study. The design may vary based on the objective(s) and should be scientifically sound to allow for valid conclusions to be drawn.5.5.5.The study design can take several forms, for example:▪the extended follow-up of patients enrolled in pre-marketinvestigations;▪ a new clinical investigation;▪ a review of data derived from a device registry;▪ a review of relevant retrospective data from patients previously exposed to the device.▪the analysis plan including any interim reporting; and▪procedures for early study termination.5.5.6.The data and conclusions derived from the PMCFS are used to provideclinical evidence to support the post-market surveillance program. This process may result in the need to reassess whether the device continuesto comply with the Essential Principles. Such assessments may result in corrective or preventive actions.6.APPENDIX6.1.Appendix A: Post-Market Clinical Follow-Up StudyDetermination6.2.Appendix B: Post-Market Clinical Follow-Up Plan6.3.Appendix C: Post Market Literature Evaluation and MarketExperience Analysis Plan6.4.Appendix D: Post-Market Literature Evaluation and MarketAnalysis Report and Conclusion。