Chapter 5-22The friction factor chart 天大化工原理上册英文版课件
- 格式:ppt
- 大小:875.50 KB
- 文档页数:46



General T eaching Outline forPrinciples of Chemical EngineeringCourse Number:Suitable for:Majors of chemical engineering and technology, biochemical engineering, food engineering, environment engineering, applied chemistry, industry equipment and control engineering, pulp and paper, polymer and inorganic material engineering.Course character: Basic course for technologyAcademic Credits: 7Academic Hours: 114Written by Hao Shixiong Writing Date: 2006.03.06 Proofread by Proofreading Date: 2006.03.06Section ⅠBasic requirements1. The Course objectiveThe ‘principles of chemical engineering’is a requirement course for general chemical engineering speciality. It is suitable for undergraduate students in the senior years who have the usual training in mathematics, physics, chemistry, and mechanics. It includes the principles of a fluid flow, heat transfer, principles of mass transfer and separation processes, the construction and operating principle of typical equipment, the experimental and researching methods of unit operation, and the calculation and selection of typical equipment. The course aims are to train and educate students to know or understand basic unit operations of chemical engineering. The course emphasizes the combination between the theory and practices, and ability of analysis and solution to practical process.2. Previous coursesAdvanced mathematics, physics, physical chemistry, mechanics, mechani cal drawi ng3. The basic requirements and contents for each chapterChapter 1 Definitions and principlesBasic law; Material balance; Law of motion; Energy balance; Equilibrium; Units and dimensions; Physical quantities; Primary and secondary quantities; Dimensions and dimensional formulas; Conversion of units; Dimensionless equations and consistent units; Dimensi onal equati ons.Chapter 2 Fluid statics and its applicationsNature of fluids; Hydrostatic equilibrium; Applications of fluid statics; Manometers continuous gravity decanter.Chapter 3 Fluid flow phenomenaThe velocity field; Laminar flow; Shear rate, and shear stress; Newtonian and non-Newtonian fluids; Viscosity; Kinematic viscosity.Turbulence; Laminar and turbulent flow; Reynolds number and transition from laminar to turbulence flow; Nature of turbulence; Deviating velocities in turbulence flow; Eddy viscosity; Flow in boundary layers; Laminar and turbulent flow in boundary layers; Boundary-layer formation in straight tubes; Boundary-layer separation and wake formation.Chapter 4 Basic equations of fluid flowOne-dimensional flow; Mass balance; Macroscopic momentum balance; Layer flow with free surface; Momentum balance in potential flow; Discussion of Bernoulli equation; Bernoulli equation: correction for effects of solid boundaries; Kinetic-energy correction factor; Correction of Bernoulli equation for fluid friction; Pump work in Bernoulli equation.Chapter 5 Incompressible flow in pipes and channelsShear stress and skin friction in pipes; Relation between skin friction and wall shear; Relations between skin-friction factor; Laminar flow of Newtonian fluids; V elocity distribution in a pipe;A verage velocity for laminar flow in a pipe; Hagen-Poiseuille equation; Relations between maximum velocity and average velocity; Laminar flow in an annulus; Friction factor in flow through channel of noncircular cross section; Turbulent flow in pipes and channels; Effect of roughness; Hydraulically smooth; The friction factor and friction coefficient chart; Friction from changes in velocity or direction; Friction loss from sudden expansion of cross section; Friction loss from sudden contraction of cross section; Effect of fittings and valves; Form-friction losses in the Bernoulli equation.Chapter 6 Flow past immersed bodiesDrag, Drag coefficients; Drag coefficients of typical shapes; Mechanics of particle motion, Equation for one-dimensional motion of particle through fluid; Terminal velocity, drag coefficient, movement of spherical particles; The terminal velocities at the different Reynolds number; Criterion for settling regime.Chapter 7 Separation equipmentsGravity settling processes; Centrifugal settling processes; Separation of solids from gases; cyclones, filtration; Clarifying filters; Gas cleaning; Liquid clarification, discontinuous pressure filters; Filter press; Shell-and-leaf filters; Continuous pressure filters; Principles of cake filtration; Pressure drop through filter cake; Filter medium resistance; Constant-pressure filtration; Continuous filtration; Washing filter cakes.Chapter 8 T ransportation and metering of fluidsPipe and tubing; Selection of pipe sizes; Fluid-moving machinery; Developed head; Power requirement; Suction lift and cavitation; Suction lift; Positive-displacement pumps; V olumetric efficiency; Rotary pumps; Centrifugal pumps; Centrifugal pump theory; Head-flow relations for an ideal pump; The relation between head and volumetric flow; Effects of speed and impeller sizechange; Characteristic curves; Head-capacity relation; Efficiency; Centrifugal-pump characteristics; System head curve; Operating point; Operating point change; Operation in parallel and in series of centrifugal pump; Multistage centrifugal pumps; Pump priming; Fans; Blowers.Measurement of flowing fluids; Full-bore meters; V enturi meter; The basic equation for venturi meter; V enturi coefficient; Flow rate; Pressure recovery; Orifice meter; Pressure recovery; Area meters: rot meters; Theory and calibration of rotameters; Inserti on meters; Pi cot tube.Chapter 10 Heat T ransferNature of heat flow; Heat transfer by conduction; Basic law of conduction; Unsteady-state conduction; Steady-state conduction; Thermal conductivity; Steady-state conduction; Compound resistance in series; Heat flow through a cylinder.Chapter 11 Principles of heat flow in fluidsTypical heat-exchange equipment; Countercurrent and parallel-current flows; Single-pass shell-and-tube condenser; Energy balances, heat flux and heat transfer coefficient; Heat flux, A verage temperature of fluid stream; Overall heat-transfer coefficient; Mean temperature difference; Individual heat-transfer coefficients; Special cases of the overal l coeffi ci ent.Chapter 12 Heat transfer to fluids without phase changeRegimes of heat transfer in fluids; Heat transfer by forced convection in turbulent flow; Empirical equation; Effect of tube length; Estimation of wall temperature t w; Cross sections other than circular; Heat transfer in transition region between laminar and turbulent flow; Heating and cooling of fluids in forced convection outside tubes, fluids flowing normal to a single tube; Natural convection; Natural convection to air from vertical shapes and hori zontal pl ates.Chapter 13 Heat transfer to fluids with phase changeHeat transfer from condensing vapors; Dropwise and film-type condensation; Coefficients for film-type condensation; V ertical tubes, Horizontal tubes; Effect of noncondensables; Heat transfer to boiling liquids; Pool boiling of saturated liquid.Chapter 14 Radiation heat transferFundamental facts concerning radiation; Emission of radiation; Wavelength of radiation; Emissive power; Blackbody radiation; Emissivities of solids; Practical source of blackbody radiation; Laws of blackbody radiation; Absorption of radiation by opaque solids; Radiation between surfaces.Chapter 17 Principles of Diffusion and Mass T ransfer Between PhasesTheory of diffusion; Comparison of diffusion and heat transfer; Diffusion quantities; V eloc ities in diffusion; Molal flow rate, velocity, and flux; Relations between diffusivities; Interpretation of diffusion equations; Equimolal diffusion; One-component mass transfer (one-way di ffusi on).Prediction of Diffusivities; Diffusion in gases; Diffusion in liquids; Turbul ent di ffusi on.Mass transfer theories; Mass transfer coefficient; Film theory; Two-fi l m theory.Chapter18. Gas AbsorptionDefinition of absorption; Principles of absorption; Material balances; Limiting gas-liquid ratio; Rate of absorption; Calculation of tower height; Number of transfer units; Alternate forms of transfer coefficients; Effect of pressure; Temperature variations in packed towers; Stripping factor method for calculating the number of transfer units; Absorption efficiency A.Empirical correlations for mass transfer coefficients in absorption.Chapter 19 Introduction to Mass T ransfer and Separation ProcessesDefinition of separation processes; Importance and variety of separations; Economic significance of separation processes; Categorizations of separation processes; General separation process; Technological maturity of processes; Terminology and symbols.Supplementary:Phase equilibria: Phase rule; Equilibrium and equilibrium stage; Thermodynamic relationships: Equilibrium ratio ( or equilibrium constant or K value); Relative volatility----key separation factor in distillation; Ideal system and Dalton’s law, Raoult’s law; Phase equilibrium diagrams for ideal systems(t-x-y diagram; x-y diagram); Henry’s law; Azeotropes; Effect of total pressure on vapor/liquid equilibrium.Chapter 20 Equilibrium-Stage OperationsCascades. Ideal stage/equilibrium stage/theoretical stage; Equipment for stage contacts; Principles of stage processes; Terminology for stage-contact plants; Material balances; Enthalpy balances; Graphical methods for two-component system; Operating line diagram; Ideal contact stages; Determining the number of ideal stages; Absorption factor method for calculating the number of ideal stages.Supplementary:Introduction to distillation: Process description; Equilibrium/flash distillation; Principles and flow diagram of distillation.Chapter 21 DistillationContinuous distillation with Reflux. Material balances in plate columns: Overall material balances for two-component systems; Net flow rates; Operating linesNumber of ideal plates; McCabe-Thiele Method. Constant molal overflow; Reflux ratio; Condenser and top plate; Bottom plate and reboiler; Feed plate; Feed line; Construction of operating lines; Optimum feed plate location; Heating and cooling requirements; Minimum number of plates/total reflux; Minimum reflux/infinite number of plates; Invariant zone; Optimum reflux; Nearly pure products; Some special cases of distillation (Multiple feeds and side-stream drawoffs; Direct steam heating); Use of Murphree efficiency/determining the number of actual plates.Batch distillation. Simple distillation; Batch distillation with reflux. Calculation and analysisfor the operation of a distillation column.Chapter 24 Drying of SolidsIntroduction to methods for removing liquid from solid materials; Purposes and applications of drying; Classification of drying processes; Drying conditions for convecti ve dryers.Properties of moist air and humidity chart. Moist air properties: Humidity; Relative humidity; Humid volume; Humid heat; Total enthalpy of moist air; Dry-bulb temperature and wet-bulb temperature; Adiabatic saturation temperature; Dew point. Humidity chart of Air-Water system. Applications of H-I diagram.Material and energy balances; Expressions of water (moisture) content of solids; Material balances; Heat balances; Thermal efficiency of drying process; Air states when passing through the drying system.Phase equilibria and drying rates. Phase equilibria: Equilibrium water(moisture) and free water(moisture); Equilibrium-moisture curves; Bound and unbound water; Drying curves and drying rate curves under constant drying conditions; Drying mechanism of wet solids and the influencing factors: Constant-rate period (Period of controls of surface water vaporization); Drying in the falling-rate period (period of controls of water diffusing from interior to solid surface); Critical water(moisture) content and its influencing factors. Methods for increasing rate of drying.Calculation of drying time under constant drying conditions.4. T extbook and reference booksT extbook:Unit operation of chemical engineering(Sixth edition) Author: Warren L. McCabe, Julian C. Smith and Peter HarriottReference books:[1]. 姚玉英主编. 化工原理(上、下册)(新版)[M] . 天津: 天津大学出版社, 1998[2]. 赵汝溥, 管国锋. 化工原理[M] . 北京: 化学工业出版社, 1995.[3]. 大连理工大学化工原理教研室编. 化工原理(上、下册)[M]. 大连:大连理工大学出版社, 1992[4]. 陈敏恒,丛德滋,方图南,齐鸣斋编. 化工原理(上、下册)[M].(第二版).北京: 化学工业出版社, 1999[5]. 朱家骅,叶世超等编. 化工原理(上、下册)[M]. 北京:科学技术出版社, 2002[6]. 姚玉英. 化工原理例题与习题[M](第三版). 北京: 化学工业出版社, 2003[7]. 柴成敬,王军,陈常贵,郭翠梨编.化工原理课程学习指导[M]. 天津: 天津大学出版社, 2003[8]. 匡国柱. 化工原理学习指导[M]. 大连: 大连理工大学出版社, 20025. Periods for Every Unitl. Fluid flow 20 hours2. Fluid transportation 10 hours3. Separation of heterogeneous mixture 10 hours4. Heat transfer 20 hours5 Gas Absorption 24 hours6 Distillation 18 hours7 Drying of Solids 12 hours6. Evaluation Methods of the CourseThe assess method: quiz, homework and course report et al. which are determined by the teacher, and the unified final examination。
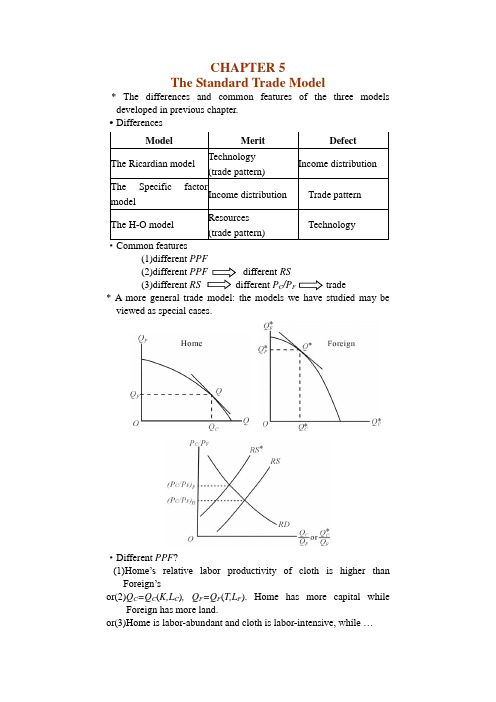
CHAPTER 5The Standard Trade Model* The differences and common features of the three models developed in previous chapter.·Differences·Common features(1)different PPF(2)different PPF different RS(3)different RS different P C/P F trade* A more general trade model: the models we have studied may be viewed as special cases.·Different PPF?(1)Home’s relative labor productivity of cloth is higher thanForeign’sor(2)Q C=Q C(K,L C), Q F=Q F(T,L F). Home has more capital while Foreign has more land.or(3)Home is labor-abundant and cloth is labor-intensive, while …Model Merit DefectThe Ricardian modelTechnology(trade pattern)Income distribution The Specific factormodelIncome distribution Trade patternThe H-O modelResources(trade pattern)Technology·Different Pc/P F?At any given Pc/P F, (Q C/Q F)>(Q C*/Q F*), RS lies to the right of RS*, that is (P C/P F)H<(P C/P F)F。


the five factors of supersuasion文章-回复【The Five Factors of Supersuasion】In today's fast-paced world, effective persuasion is a vital skill to possess. Whether in business negotiations, personal relationships, or social interactions, the ability to influence others can greatly impact the outcome. However, what separates ordinary persuasion from exceptional persuasion, often referred to as "supersuasion," lies within the mastery of five key factors. In this article, we will explore each of these factors and provide a step-by-step analysis of how to apply them to achieve success in any persuasive endeavor.Factor 1: Emotional ConnectionThe first factor of supersuasion is establishing a powerful emotional connection. Emotions have a profound influence on decision-making processes, often guiding individuals towards favorable outcomes. To create an emotional connection, it is essential to understand the desires, fears, and values of the person you are trying to persuade. By tapping into their emotions and demonstrating a genuine empathy towards their needs, you can forge a powerful bond, increase trust, and ultimately sway theirdecisions in your favor.Step 1: Understand your audience: Conduct thorough research to identify the emotional triggers most prevalent within your target audience. Consider their background, experiences, and aspirations to gain valuable insight into their desires and fears.Step 2: Craft your message: Tailor your persuasive message to resonate with the emotions you have identified. Utilize storytelling techniques, personal anecdotes, or relatable scenarios to evoke an emotional response from your audience.Step 3: Demonstrate empathy: During your interaction, actively listen to your audience and show genuine understanding and compassion towards their concerns. Validate their emotions and demonstrate your commitment to addressing their needs.Factor 2: CredibilityCredibility plays a vital role in successful persuasion. People are more likely to be influenced by those they trust and perceive to beknowledgeable and reliable. Building credibility requires a combination of expertise, transparency, and consistency.Step 4: Establish expertise: Display your expertise in the relevant field by offering evidence, sharing experiences, or presenting data-driven arguments. Demonstrating deep knowledge instills confidence in your audience and enhances your overall credibility.Step 5: Be transparent: Honesty and transparency are key aspects of building trust. Clearly communicate your intentions, provide accurate information, and be open about the potential risks or limitations involved. This level of transparency enhances your credibility and reduces skepticism.Step 6: Consistency in messaging: Consistency reinforces your credibility. Ensure that your message remains consistent across various platforms and interactions. Inconsistencies raise doubts about your trustworthiness and may hinder your persuasive efforts.Factor 3: Social ProofSocial proof refers to the phenomenon where individuals adopt the opinions or behaviors of others to validate their own judgments. Utilizing social proof can enhance the persuasive impact by leveraging the power of conformity.Step 7: Provide testimonials or endorsements: Gather testimonials, endorsements, or case studies from credible sources or influential individuals within the field. These external validations serve as evidence of your claims and increase the perceived value of your message.Step 8: Utilize statistics and peer pressure: Presenting statistics or data that indicate a consensus among a majority of people can establish a sense of social proof. Moreover, highlighting the growing popularity or trendiness of a particular idea or course of action can exert additional persuasive pressure.Factor 4: ReciprocityReciprocity is a powerful psychological principle that states peopleare more likely to respond positively if they have received a favor or gesture of goodwill. Harnessing this principle can significantly increase your persuasive influence.Step 9: Give first: Initiate the persuasive interaction by providing value or assistance to your audience, without expecting anything in return. This creates a feeling of indebtedness and increases the likelihood of reciprocation.Step 10: Personalize favors: Tailor your acts of reciprocation to the specific needs or preferences of your audience. This demonstrates thoughtfulness and enhances the perceived value of the goodwill gesture.Factor 5: Frame of ReferenceThe final factor of supersuasion is the ability to frame your message in a way that aligns with the existing belief systems or ideologies of your audience. People are more open to accepting ideas that are congruent with their pre-existing views, making framing an essential component of persuasion.Step 11: Understand existing beliefs: Conduct research or engage in conversations to understand the belief system or ideology of your audience. Identify the shared values or perspectives that your message can align with.Step 12: Re-frame your message: Utilize language and examples that appeal to the existing beliefs of your audience. Demonstrate how your message supports or enhances their current worldview, making it easier for them to accept and adopt your ideas.In conclusion, supersuasion is not simply about convincing others; it requires a deep understanding of human psychology and the application of five key factors. By establishing emotional connections, building credibility, leveraging social proof, harnessing reciprocity, and framing your message effectively, you can become a master persuader. Remember, while persuasion can be a powerful tool, it is important to use it responsibly and ethically, always keeping the best interests of others in mind.。