Drug-Device Combination in US Part 1
- 格式:pdf
- 大小:1.55 MB
- 文档页数:77

【审评规范】FDA药-械组合产品桥接指导原则介绍萧惠来国家药品监督管理局药品审评中心,北京100022摘要:美国食品药品管理局(F D A)的“供企业用药-械和生物制品-器械组合产品的桥接指导原则(草案)”详细描述 了药-械组合产品注册申请人,利用另外的开发方案产生的信息,作为拟开发产品的注册资料(即桥接),替代试验研究资 料的方法,支持拟申报产品的批准;推荐渐进式5步骤法,确定桥接策略和信息需求并列举了3个示例予以解读。
详细介绍 该指导原则的内容,期望为中国药-械组合产品研发和监管开辟新思路,建议在合适的条件下可考虑利用“桥接”方法减少 试验研究,加速研宄进程,缩短研宄周期,节省研宄经费,促进药-械组合产品的开发。
关键词:美国食品药品管理局:药-械组合产品:桥接:指导原则中图分类号:R951 文献标志码: A 文章编号:1674-6376 (2021) 03-0461-07DOI :10.7501/j.issn. 1674-6376.2021.03.001Introduction to FDA's bridging for drug-device combination products guidanceXIAO HuilaiCenter for Drug Evaluation, National Medical Products Administration, Beijing 100022, ChinaAbstract: The FDA's Bridging for Drug-Device and Biologic-Device Combination Products Guidance for Industry (Draft) described in detail that the applicant for registration of drug-device combination products can use the information developed by another development program as the registration information of the product to be developed (i. e. bridging) and replace the experimental research data to support the approval of the product to be applied for. That is to say, the stepwise five step approach is recommended to determine the bridging strategy and information requirements are determined, and three examples are given to interpret. This paper introduces the guidance in detail, hoping to open up new ideas for the research and development as well as supervision of drug-device combination products in China. In suitable conditions, it can be considered to use "bridging" method to reduce experimental research, speed up the research process, shorten the research cycle, save research funds, and promote the development of drug-device combination products.Key words: FDA; drug-device combination product; bridging; guidance美国食品药品管理局(FDA)于2019年12月发 布了“供企业用药-械和生物制品-器械组合产品的 桥接指导原则(草案)”[1]。

以医疗器械作用为主的药械组合产品注册审查指导原则英语Guidance Principles for the Registration Review of Medical Device-Drug Combination Products1. IntroductionMedical device-drug combination products are a category of products that combine a medical device and a drug component to achieve the intended use. The registration review of such products requires a comprehensive assessment of both the device and drug components, as well as the interaction between them. This guidance outlines the key principles and considerations for the registration review of medical device-drug combination products.2. ScopeThis guidance applies to medical device-drug combination products where the device component is the primary mode of action. The principles and considerations outlined in this guidance may also be applicable to other types of combination products, such as those where the drug component is the primary mode of action.3. Regulatory FrameworkThe registration of medical device-drug combination products is subject to the regulatory requirements for both medical devices and drugs. Applicants should ensure compliance with the relevant regulations and guidelines for each component, as well as the specific requirements for combination products.4. Product Identification and ClassificationMedical device-drug combination products should be clearly identified and classified based on the primary mode of action and the intended use. Applicants should provide a detailed description of the product, including the device and drug components, their respective functions, and the intended use of the combination product.5. Quality ConsiderationsThe quality of both the device and drug components should be thoroughly evaluated. This includes the design, manufacturing, and control of the individual components, as well as the compatibility and interactions between them. Applicants should provide comprehensive information on thequality attributes, specifications, and control strategies for the combination product.6. Nonclinical EvaluationNonclinical studies should be conducted to assess the safety and performance of the combination product. This may include studies on the device-drug interaction, the local and systemic effects of the combination, and the potential for any adverse interactions or reactions.7. Clinical EvaluationClinical studies are essential to evaluate the safety and efficacy of the combination product. Applicants should design and conduct clinical trials that assess the overall performance of the combination product, including the device and drug components, and the interaction between them.8. Risk ManagementApplicants should implement a comprehensive risk management plan to identify, assess, and mitigate the potential risks associated with the combination product. This includes the risks related to the device and drugcomponents, as well as the risks arising from the interaction between them.9. Labeling and PackagingThe labeling and packaging of the combination product should provide clear and comprehensive information to healthcare professionals and patients. This includes the instructions for use, any special handling or storage requirements, and any warnings or precautions related to the combination product.10. Postmarket SurveillanceApplicants should establish a robust postmarket surveillance system to monitor the performance and safety of the combination product after it is approved for use. This includes the collection and analysis of adverse event reports, as well as the implementation of any necessary corrective or preventive actions.中文版本:医疗器械作用为主的药械组合产品注册审查指导原则1. 引言医疗器械-药物组合产品是一类将医疗器械和药物组合在一起以实现预期用途的产品。

固定剂量复方制剂注册指导原则缩写98绪论 991.适用范围 1002.总体考虑 1003.定义 1104.注册分类 1135.衡量衡量固定剂量复合剂型的优缺点 1136.固定剂量复方制剂上市许可所需要的数据 1167.固定剂量复方制剂产品信息或说明书 1358.上市后研究和上市后的变动变更136参考文献 138 附件1 组合包装的固定剂量复方制剂指导原则 139 附件2 判断科学文献数据是否可以被接受的原则 140附件3 药品研发(或处方前研究) 142 附件4 优效性、等效性和非劣效性临床试验 144缩写AIHW Australian Institute of Health and Welfare (澳大利亚卫生与福利研究所)API Active pharmaceutical ingredient (活性药物成分)BCS Biopharmaceutics Classification Scheme (生物药学分类系统)BCS #1 Biopharmaceutics class number 1(the most favourable) (生物药学分类系统第一类)(受欢迎最有利的一类)CHMP Committee for Medicinal Products for Human Use; see also CPMP (人用医疗产品委员会)(参见CPMP)CPMP Committee for Medicinal Products for Human Use (CHMP), formerly the Committee for Proprietary Medicinal Products (人用医疗产品委员会(CHMP), 前身为专利药品委员会)CPP Certificate of pharmaceutical product (药品证书)EMEA European Medicines Agency, formerly the European Medicines Evaluation Agency (欧洲药品局, 前身为欧洲药品评审局)EU European Union (欧盟)FDA Food and Drug Administration of the USA (美国食品药品管理局)FDC Fixed-dose combination (固定剂量复方治疗)(见词汇表)FDC-FPP Fixed-dose combination finished pharmaceutical product (固定剂量复方药品)(见词汇表)GCP Good clinical practice (药物临床试验管理规范)GLP Good laboratory practice (药物非临床研究质量管理规范)GMP Good manufacturing practice (药品生产质量管理规范)GTDP Good trade and distribution practice (药品贸易和分销管理规范)GSP Good storage practice (药品贮存管理规范)ICH International Conference on Harmonisation (人用药品注册技术要求国际协调会议)IUTLD International Union of Tuberculosis and Lung Disease (国际抗结核病和肺病联盟)MIC Minimum inhibitory concentration (最低抑菌浓度)PP Per-protocol (a form of clinical trial design and analysis) 符合方案集符合方案集(临床试验设计和分析的一种形式)SPC Summary of product characteristics (产品特点总结药品说明书)(见词汇表)TGA Therapeutic Good Administration (澳大利亚药品管理局)WHO World Health Organization (世界卫生组织)绪论随着固定剂量复方治疗(fixed-dose combinations, FDCs)的发展,它们在保护公众健康方面发挥了日益重要的作用。


51文章编号:1671-7104(2020)01-0051-05汤康丽,周俊蕾,李勇,瞿敏明,王钰婕,罗健上海微创医疗器械(集团)有限公司,上海市,201203药械组合产品作为药物和医疗器械的组合体,有效降低了传统医疗器械带来的并发症等风险,在临床应用尤其是植入式治疗领域取得巨大成功。
该研究团队首先阐述了各个国家对药械组合产品的定义和要求,其次对几类常见的药械组合产品的市场应用及其研究进展进行总结分析,对药械组合产品目前存在的技术问题及未来发展趋势进行了分析展望。
药械组合产品;研究和应用 R95;R197.39Adoi: 10.3969/j.issn.1671-7104.2020.01.011TANG Kangli, ZHOU Junlei, LI Yong, QU Minming, WANG Yujie, LUO JianShanghai MicroPort Medical (Group) Co., Ltd., Shanghai, 201203Drug-device combination product, which comprises at least a drug and a medical device, has beenproved to effectively reduce the risk of complications accompanied with conventional medical devices implantation, and has a great clinical success especially in implantable therapeutics. Herein, we firstly elaborated the definitions and requirements of drug-device combination product in different countries, then summarized the market application and research development of typical drug-device combination products. Technical problems and the trend of future development had also been analyzed.drug-device combination products, research and application药械组合产品研究及应用进展【作 者】【摘 要】【关 键 词】【中图分类号】【文献标志码】【 Writers 】【 Abstract 】【Key words 】Progress in Research and Application of Drug-DeviceCombination Product基金项目:上海市科技创新行动计划标准项目(19DZ2202100)通信作者:周俊蕾,E-mail: jlzhou@0 引言药械组合产品通常是指药品和医疗器械共同组成的产品,药械组合产品可以是药物增强型医疗器械,如药物洗脱支架、抗菌导管等,也可以是以器械作为辅助给药手段,如各种透皮贴剂技术等。
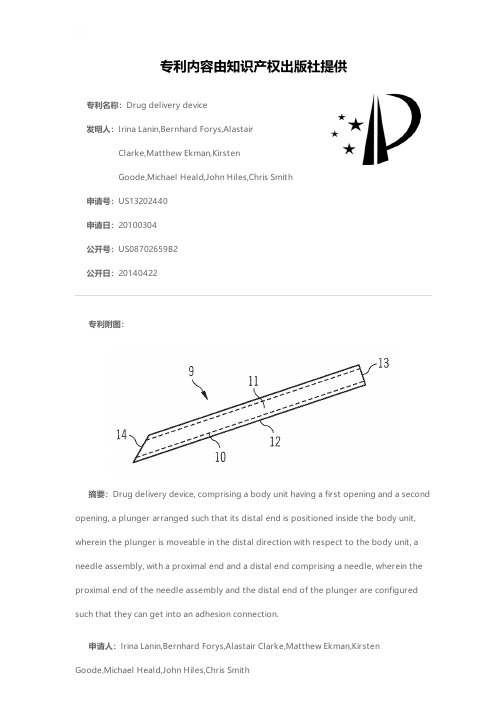
专利名称:Drug delivery device发明人:Irina Lanin,Bernhard Forys,AlastairClarke,Matthew Ekman,KirstenGoode,Michael Heald,John Hiles,Chris Smith申请号:US13202440申请日:20100304公开号:US08702659B2公开日:20140422专利内容由知识产权出版社提供专利附图:摘要:Drug delivery device, comprising a body unit having a first opening and a second opening, a plunger arranged such that its distal end is positioned inside the body unit,wherein the plunger is moveable in the distal direction with respect to the body unit, a needle assembly, with a proximal end and a distal end comprising a needle, wherein the proximal end of the needle assembly and the distal end of the plunger are configured such that they can get into an adhesion connection.申请人:Irina Lanin,Bernhard Forys,Alastair Clarke,Matthew Ekman,KirstenGoode,Michael Heald,John Hiles,Chris Smith地址:Frankfurt am Main DE,Frankfurt am Main DE,Nantwich GB,Macclesfield GB,Frankfurt am Main DE,Crewe GB,South Wirral GB,Holmes Chapel GB 国籍:DE,DE,GB,GB,DE,GB,GB,GB代理机构:McDonnell Boehnen Hulbert & Berghoff LLP更多信息请下载全文后查看。
药学英语- 1 -Table of ContentsLesson 1 Pharmacy in China …………………………………………………Lesson 2 Vitamins ………………………………………………………………Lesson 3 Foods That Fight Cancer …………………………………………Lesson 4 Good Drugs Dangerous DosesA growing threat to public and personal health …………………………Lesson 5 Green Pharmacy Herbal Medicine …………………………………Lesson 6 Traditional Chinese Herbal Medicine …………………………….. Lesson 7 Natural Products ……………………………………………………- 2 -LESSON ONETEXTPharmacy in ChinaThe educational system was perhaps the first shock I encountered in China. The schools of pharmacy in the People’s Republic of China (PRC) are operated by two different governmental agencies. The State Pharmaceutical Administration, an agency of the central government, operates two pharmaceutical universities that have 4000 and 4500 students respectively. These two schools, in Shenyang in Northeast China and in Nanjing in East China, exist to prepare industrial pharmacists for positions within the pharmaceutical industry.In addition, another approximately 50 colleges of pharmacy throughout the various provinces of the PRC are operated by the Ministry of Public Health. There, colleges of pharmacy are almost equally divided between western medicine oriented schools1and those which teach future pharmacists the art and skills of Chinese herbal medicine. Most of the western medicine oriented schools are departments within large medical faculties where the traditional Chinese Medicine schools are sometimes found at medical faculties.SIMILAR TO THE U.S.The next surprise was that the curricula at the colleges of pharmacy in China were not so different from those encountered by pharmacy students in the U. S. In fact, the American student would probably be pleased to learn that the state board examination2prevalent here does not exist in China; rather, graduation and successful completion of studies enable one to become a fully qualified and registered pharmacy practitioner. Furthermore, recent graduates have more options in seeking employment than those who were usually assigned to an employment site based on existing needs of state-run pharmaceutical enterprises. The guided search for employment upon graduation is one of the major changes in China’s higher education, embraced by most college graduates.Virtually all of the graduates of the two pharmaceutical universities operated by the State Pharmaceutical Administration are employed within the- 3 -pharmaceutical industry.Graduates of the other schools of pharmacy generally find employment in hospitals and clinics.You are probably asking: “What about community pharmacy3?”Well, the answer is that people usually receive medical care in clinics at their place of employment, within various neighborhood health centers, or as outpatients at large hospitals. All of these sites employ pharmacists who dispense drugs. Therefore, it is not often that a patient needs to go to a community pharmacy shop to obtain medications.EAST AND WESTMost of the community pharmacies have two departments or two areas, and this is also the case in hospitals and clinics. On one side of the pharmacy we find something which very much resembles any pharmacy that we would see in the U. S. or in Western Europe; but on the other side of the room, or in the next room, is a strange sight - drawers and drawers of herbs and items such as dried portions of small animals, antlers, roots, and pieces of various other dried objects. These are compounded by specialists in traditional Chinese medicine; powders are made, for swallowing by patients.It is easy for us to laugh at this latter type of therapy, but it retains its faithful followers and is considered to be equal in importance to the western drugs and western medicine practiced in China.If one chooses to purchase a drug and does not feel the need to visit the physician at a clinic, one can go to the several community pharmacies which are located on the main shopping streets in downtown areas and ask for drugs. Virtually all drugs except narcotics and other scheduled substances4are legally available without a prescription. The patient may walk into the pharmacy and ask for ampicillin by name and be sold a small bottle containing 12, 16 or 24 capsules.Or he may ask the pharmacist to recommend an antibiotic for a child with an infection, sore throat, etc.While this is rather unusual in our American orientation, it was not a complete surprise to me, as I have seen Rx drugs5 being sold over-the-counter in many parts of the world. I admit, though, that I was curious about the potential for- 4 -abuse. I was assured by numerous individuals that the Chinese population had better things to spend their money on than taking needless drugs, and that there was a minimal if not almost non-existent chemical dependency problem6.SCOPE OF SPASomething ought to be said about the State Pharmaceutical Administration (SPA) which was mentioned earlier. It is a combination of the Food and Drug Administration, the entire pharmaceutical industry, research laboratories, pilot factories7 and educational institutions-all rolled into one.The Beijing-based SPA is a huge unit within the central government. SPA operates thousands of pharmaceutical factories and dictates production schedules8. It arranges contracts for raw materials and intermediate products9and also negotiates for the sale of finished dosage forms to the pharmacies and for export.Other divisions maintain quality control and quality assurance while yet other divisions oversee SPA’s two massive pharmaceutical universities as well as their on campus pilot plants used for training and for the production of drugs for clinical trials.The SPA also has a technology branch which conducts research into improving production techniques and evaluating new technology.During the three-week visit, I gave about a dozen lectures. At many of them, questions about American pharmacy practice were asked. Perhaps the most difficult concept for my Chinese colleagues to grasp was that of Americans deciding individually to move to another part of the country - to quit their jobs, return to school, accept employment elsewhere, or whatever the reason might be. When I mentioned anything of this nature, I was deluged with questions which basically asked what would hap pen if one’s supervisor refused to grant permission to leave one’s job. This says something about the employment situation in the PRC. My audiences were stunned when I indicated that one need only give some notice if there is not a formal contract, as a courtesy, and to select freely another place of employment.SPARTAN, BUT SIMILARDespite the rather spartan conditions found at most pharmacy operations in hospitals and clinics, the work was similar to what we do in that nearly all of the- 5 -products were prefabricated at the factory, including most of the traditional remedies. The pharmacists generally dispensed already manufactured capsules, tablets or powders. Powders were seen more frequently than they are here, but the bulk of the medication was, as it is here, in tablet dosage forms.Most of my Chinese colleagues were professionally well informed, reasonably happy with their jobs, and optimistic about the future.Nearly all of the persons I encountered were enthusiastic and positive about their profession and its future.There is a Chinese Pharmaceutical Association which has its headquarters in Beijing and branches in most other cities and provinces. These groups get together, discuss professional matters, and often publish journals and newsletters.The Association receives a subsidy or support from the national government.Interest was shown in social pharmacy, clinical pharmacy and pharmacy management, as these subjects are not presently taught at the colleges of pharmacy within the PRC. It was my impression, however, that pharmaceutics, biopharmaceutics, medicinal chemistry and pharmacology are similar to what is taught and known in this country.MONTHLY W AGE: $ 70In the 1980s, pharmacist earns about $ 70 per month, an amount that enables one to live reasonably comfortably. Rents are minimal; only a few dollars per month pays for a typical small apartment. Nearly all of the urban population resides in that type of housing.Transportation on buses costs only about a dime a ride, but most people appear to use bicycles as their principal means of conveyance. A sight I found interesting was a parking lot with bicycles arranged in neat rows for as far as the eye could see-similar to the huge automobile parking lots in the U. S. It costs about two pennies to park a bicycle for a day. Most of the other needs are relatively inexpensive, although the cost of food is increasing.The Chinese pharmacists I met appeared well dressed and there is an ever increasing variety of clothing styles, colors and designs. Health care is considered good and the status of the pharmacist is at least equal to that accords to pharmacists in this country.The possibility exists for greater interprofessional relationships. Reason: so - 6 -much of pharmacy is practiced in institutional settings where other health care practitioners function in close coordination with the pharmacist.A large number of the people I met in pharmacy circles had been to the U. S. at one time or another for postgraduate studies, short training classes or professional visits.I was convinced that the warm reception I received was due in part to the fond memories that many of these people had about their kind reception by American families and pharmacists while they visited the U. S.The trip was indeed fascinating and an eye opener.I recommend that my colleagues take such a voyage after they have passed a test in the use of chopsticks, as knives and forks are unavailable at many places.In reflecting about the visit after coming home, I once again came to the conclusion that we are very fortunate with the resources, appliances and status that we have as a profession in the U.S.WORDS AND EXPRESSIONSpharmacy [ ♐❍☜♦♓] n.药学;药店pharmaceutical[ ♐❍☜♦◆♦♓☜●] adj.制药(学)上的药学的;药用的pharmacist [ ♐❍☜♦✋♦♦] n.药剂师,药商;a ~’s药房oriented[ ❒♓♏⏹♦♓♎☜☺] adj. 导向的;以…目的的;重视…的herbal[ ♒☜♌☜●] adj.草的;草药的curriculum[ ☜❒♓◆●☜❍] n.学校的全部课程,(一门)课程prevalent [ ☐❒♏❖☜●☜⏹♦] adj.普遍的;流行的option [ ☐☞☜⏹] n.选择权enterprise [ ♏⏹♦☜☐❒♋♓]n.企业;公司embrace [♓❍♌❒♏♓♦] vi. 拥抱n.拥抱拥抱,接受outpatient [♋◆♦☐♏♓☞☜⏹♦] n. 门诊病人an ~ clinic 门诊所dispense [♎♓♦☐♏⏹♦] vt.分发, 分配;(尤指按处方)配(药)、发(药)medication[ ❍♏♎♓♏♓☞☜⏹] n.药物;药物治疗antler [ ✌⏹♦●☜] n.鹿角, 茸角鹿角therapy [ ♏❒☜☐♓] n.治疗,- 7 -疗法retain [❒♓♦♏♓⏹] vt.保持, 保留narcotic [⏹♦♓] n. 麻醉剂;催眠药prescription[☐❒♓♦❒♓☐☞☜⏹] n.药方,处方ampicillin [ ✌❍☐♓♦♓●♓⏹] n. 氨苄青霉素orientation[ ☎✆❒♓♏⏹♦♏♓☞☜⏹] n.定向位,方向位,熟悉,适应minimal [ ❍♓⏹♓❍☜●] adj.最低限度的,最小的dictate [♎♓♦♏♓♦] v.命令,支配intermediate[ ♓⏹♦☜❍♓♎☜♦] adj.中间的negotiate [⏹♓♈☜◆☞♓♏♓♦] v. 谈判;解决dosage [ ♎☜◆♦♓♎✞] n.剂量,用量assurance [☜☞◆☜❒☜⏹♦] n.保证;自信oversee [ ☜◆❖☜♦♓] v. 监督/视;检查massive [ ❍✌♦♓❖] adj.规模巨大的;大剂量的on-campus [ ✌❍☐☜♦] adj.校园内的clinical [ ●♓⏹♓☜●] adj.临床的deluge [ ♎♏●◆♎✞] v.淹没;泛滥supervisor[ ♦◆☐☜❖♋♓☜] n. 主管人stun [♦♦✈⏹] vt. 使大吃一惊;不知所措courtesy[ ☜♦♓♦♓ ] n.礼貌;好意Spartan [ ♦☐♦☜⏹] adj.简朴的prefabricate[ ☐❒♓♐✌♌❒♓♏♓♦] v.预制;预先构想remedy [ ❒♏❍♓♎♓] n.治疗(法),药物capsule [ ✌☐♦◆●] n.胶囊tablet [ ♦✌♌●♓♦] n.药片powder [ ☐♋◆♎☜] n.药粉,粉剂bulk [♌✈●] n.大批(量);大部分newsletter[ ⏹✞●♏♦☜☎❒✆] n.业务通讯subsidy [ ♦✈♌♦♓♎♓] n. 津贴pharmaceutics[ ♐❍☜♦◆♦♓♦] n.制药学,药剂学biopharmaceutics[ ♌♋♓☜◆♐❍☜♦✞8♦♓♦] [复] n.生物制药学,生物药剂学pharmacology[ ♐❍☜●☜♎✞♓]n.药理学,药物学reside [❒♓♋♓♎] vi.居住dime [♎♋♓❍] n.一角;很少的钱conveyance [ ☜⏹❖♏♓☜⏹♦] n.交通工具accord [☜♎] vt.给予,使一致interprofessional[ ♓⏹♦♏☐❒☜ ♐♏☞☜⏹●] adj.专业之间的;职业之间的institutional[ ♓⏹♦♦♓♦◆☞☜⏹☜●] adj.公共机构的setting [ ♦♏♦♓☠] n. 环境coordination[ ☜◆♎♓⏹♏♓☞☜⏹] n.协作;配合postgraduate[ ☐☜◆♦♦♈❒✌♎◆♓♦☐☜◆♦♦♈❒✌♎✞◆♓♦] adj.研究生的fascinating [ ♐✌♦♓⏹♏♓♦♓☠] adj.迷人的;吸引人的chopsticks [ ♦☞☐♦♦♓♦] n.筷子NOTES1.western medicine oriented schools: schools with western medicine as themain course2.the state board examination: the examination organized by the state board ofpharmacy in each statemunity pharmacy: public drug store on the street4.scheduled substances: Drugs with severe side effects have been classified bythe U.S. Government as the scheduled drugs, hence controlled by law.5.Rx drugs: drugs which must be prescribed by the doctor6....that the Chinese population had better things to spend their money onthan taking needless drugs, and that there was a minimal if not almost non-existent chemical dependency problems: ... that the Chinese people would spend their money on other things rather than on unnecessary drugs, and that the number of the persons addicted to drugs was quite limited, if there did exist such things7.pilot factories: factories in which drugs are produced for clinical trials8.production schedules: contents of drugs to be produced99.intermediate products: half-finished drugsEXERCISESI. Questions:1.Why was the author puzzled about the educational system in China?2.What’s the same characteristic of about 50 colleges of pharmacy throughoutChina?3.What kind of job will students get when they graduate from thepharmaceutical universities? And what about the graduates of other colleges of pharmacy?4.What’s the function of the SPA?5.What questions were often asked at the lectures given by the author?6.Why did the author think that the Chinese pharmacists were well-informed?7.According to the author, the Chinese colleagues were friendly to him. Why?8.The author was much impressed by what he had seen in China. Find the proofin the text.9.What’s the author’s attitude towards traditional Chinese medicine?10.Can you tell, according to the text, something about pharmacy in the USA?II. Translation1.人首次与医生打交道也许是在他出生时。
无源医疗器械技术文件和设计文档指南Whereas the term “Technical File“ is used for Medical Devices of class I, class IIa and class IIb, the term “Design Dossier“ is used for the class III products.标题中的“技术文件”适用于I类,IIa类,IIb类医疗器械,“设计文档”适用于III类医疗器械。
Technical Files are retained in the premises of the manufacturer or the Authorized Representative for potential review of Competent Authorities and Notified Body.Part B of the Technical File may be available at the manufacturer only.技术文件是保留在制造商或授权代表单位的主管部门和认证机构。
部分技术文件B部分只保留在制造商处。
Whereas Design Dossiers have to be submitted to the Notified Body for review prior to CE-Marking of the product (use form Application for CE Conformity Assessment (Product)MED_F_03.03). We will assign a project manager who will entrust one or more further experts with the review of particular modules. All experts are at your disposal directly or indirectly through the project manager. After successful review, the Notified Body issues a design examination certificate according to Annex II.4 of the Council Directive certifying compliance with the relevant provisions of Annex I of the MDD.设计档案材料已被提交到公告机构用于需要CE认证前的产品审查(用CE合格评定(产品)MED_F_03.03规定的格式)。
美国医疗器械促进协会---AAMI(.au/)ProfileAAMI: Leading insurer. Pacesetter in customer benefits and service.AAMI is a leading car, home, compulsory third party (CTP) and small business insurer. We deal directly with our customers and we use innovative business and marketing strategies to provide them with high quality products and excellent customer service. You've already found we're easy to contact.Insurance benefits such as no fault, no penalty; lifetime repair guarantee; lifetime rating one/maximum no claim bonus; valet service; progressive no claim discount on home insurance and the first general insurance customer charter were all introduced by AAMI.Established in 1970, AAMI today has more than 2.5 million policyholders and millions of incoming telephone calls annually.historyIncorporated in Victoria in September 1969, AAMI commenced business on 1 January 1970 as a motor vehicle insurer, offering a major independent alternative to government-owned and motor club-owned insurers. However, it was back in 1934, on 7 January, that the first policy was granted, but not by AAMI as it is known today. The risk was underwritten by a company called Club Motor Insurance Agency (Club Motor).Club Motor was established in 1933 as a highly specialised company to provide motor vehicle insurance for members of the RACV in Victoria, the RACQ in Queensland and the RACT in Tasmania. The Club Motor arrangement continued until 31 December 1969, when the RACV decided to become an insurer in its own right. The RACQ quickly followed suit so that only the RACT remained in the fold. But what was the end of a long liaison, was the beginning for AAMI as it is known today. After the split, and to avoid customer confusion, Club Motor's consortium of shareholders, which read like the who's who of the insurance industry, was no longer able to use the Club Motor name, so they decided on the name Australian Associated Motor Insurers Limited, known as AAMI.In 1978, AAMI branched out into South Australia and then into New South Wales in 1983. The RACT broke away from AAMI in 1985. However AAMI was able to retain 80% of that business. In 1989 came AAMI's decision to diversify. After nearly 20 years of operating solely in car insurance, AAMI launched into the home insurance market. This move was the result of a detailed analysis of the benefits of applying AAMI's strengths, experience and infrastructure to other endeavours without diluting its motoring expertise. The year 1989 also saw the deregulation of the CTP market in New South Wales, with AAMI being granted a licence to operate. In 1993, AAMI entered the Queensland CTP market.AAMI's business has continued to grow and develop so that, today, home insurance and CTP insurance are major components, making increasingly important contributions to the company'sresults.WuXi AppTec 副总裁兼总经理受邀加入美国医疗仪器促进协会(AAMI) 标准委员会在中美两国均有运营实体,全球领先的医药、生物制药以及医疗器械研发外包服务企业药明康德(NYSE: WX),今日荣幸宣布其位于美国的子公司之一WuXi AppTec 亚特兰大运营分部的副总裁兼总经理Trabue D. Bryans 女士受邀加入美国医疗仪器促进协会(AAMI) 标准委员会,任期三年。