
Cosmetic ingredients found safe as used (1513 total, through June, 2013)
Ingredient#"As used" concentration for safe as used conclusion
Acacia Senegal Gum and Acacia Senegal Gum Extract 2 up to 9%
Acetic Acid 1 up to 0.3%
Acetylated Lanolin 1 up to 7%
Acetylated Lanolin Alcohol 1 up to 16%
Acetyl Tributyl Citrate and Acetyl Triethyl Citrate 2 up to 7%
Acetyl Trihexyl Citrate 1 not in use at the time*
Acetyl Trioctyl Citrate 1 not in use at the time*
Acrylates/Dimethiconol Acrylate Copolymer (Dimethiconol and its Esters and
1 up to 0.5%
Reaction Products)
Actinidia Chinensis (Kiwi) Seed Oil 1 up to 0.1%
Adansonia Digitata Oil 1 up to 0.01%
Adansonia Digitata Seed Oil 1 not in use at the time*
Adipic Acid (Dicarboxylic Acids and their Salts and Esters) 1 0.000001% in leave on; 18% in rinse off
adipic acid/1,4 butanediol/terephthalate copolymer 1 not in use at the time*
Alanine 1 3 x 10-7 to 0.1%
4 up to 99%
Alcohol Denat. denatured with t-Butyl Alcohol, Denatonium Benzoate, Diethyl
Phthalate, or Methyl Alcohol
Aleurites Moluccanus Bakoly Seed Oil 1 not in use at the time*
Aleurities Moluccana Seed Oil 1 0.00001 to 5%
Allantoin 1 up to 2%
Allantoin Ascorbate 1 up to 0.05%
Allantoin Biotin and Allantoin Galacturonic Acid 2 not in use at the time*
3 concentration not reported*
Allantoin Glycyrrhetinic Acid, Allantoin Panthenol, and Allantoin Polygalacturonic
Acid
Almond Meal (aka- Prunus Amygdalus Dulcis (Sweet Almond) Seed Meal) 0 up to 76%
Alumnina Magnesium Metasilicate 1 up to 0.01%
Aluminum Calcium Silicate 1 up to 6%
Aluminum Citrate 1 concentration not reported*
Aluminum Dimyristate 1 up to 3%
Aluminum Distearate 1 up to 5%
Aluminum Iron Silicates 1 not in use at the time*
13 not in use at the time*
Aluminum Isostearates/Myristates, Calcium Myristate, Decyl Myristate, Ethylhexyl
Myristate, Ethyl Myristate, Glyceryl Dimyristate, Glyceryl Isostearate/Myristate,
Isostearyl Myristate, Isotridecyl Myristate, Methyl Myristate, Oleyl Myristate,
Tetradecyloctadecyl Myristate, Tridecyl Myristate
Aluminum Myristate 1 up to 1%
Alumnium Myristates/Palmitates 1 up to 6%
Aluminum Silicate 1 up to 37% in dentifrices; up to 3% in other uses
Aluminum Stearate 1 up to 8%
Aluminum Tristearate 1 up to 10%
Amaranthus Hypochondriacus Seed Oil 1 not in use at the time*
Amino Bispropyl Dimethicone 1 not in use at the time*
5-Amino-4-Chloro-o-Cresol 1 up to 2% in oxidative and non-oxidative hair dyes
5-Amino-6-Chloro-o-Cresol 1 up to 2% in oxidative and non-oxidative hair dyes
4-Amino-m-Cresol 1 up to 0.7% in oxidative and non-oxidative hair dyes
6-Amino-m-Cresol 1 up to 2.4% in oxidative and non-oxidative hair dyes
4-Amino-2-Hydroxytoluene 1 up to 5%
Aminomethyl Propanediol 1 up to 2%
Aminomethyl Propanol 1 up to 7%
2-Amino-3-Nitrophenol 1 up to 2%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
2-Amino-4-Nitrophenol 1 concentration not reported*
2-Amino-4-Nitrophenol Sulfate 1 not in use at the time*
2-Amino-5-Nitrophenol 1 not in use at the time*
4-Amino-3-Nitrophenol 1 up to 9%
m-Aminophenol 1 up to 5%
o-Aminophenol 1 up to 1%
p-Aminophenol 1 up to 1%
Aminopropyl Dimethicone 1 not in use at the time*
Ammonium Bisulfite 1 up to 32%
11 not in use at the time*
Ammonium Cocomonoglyceride Sulfate, Coconut Oil Decyl Esters, Decyl Cocoate,
Hydrogenated Coco-Glycerides, Isodecyl Cocoate, Lauryl Cocoate, Octyldodecyl
Cocoate, Potassium Hydrogenated Cocoate, Sodium Cocomonoglyceride Sulfate,
Sodium Hydrogenated Cocoate, and Tridecyl Cocoate
9 not in use at the time*
Ammonium Coco-Sulfate, Ammonium Myristyl Sulfate, Magnesium Coco-Sulfate,
Sodium Coco/Hydrogenated Tallow Sulfate, Sodium Ethylhexyl Sulfate, Sodium
Oleyl Sulfate, Sodium Tallow Sulfate, Sodium Tridecyl Sulfate, Zinc Coco-Sulfate
Ammonium Cumenesulfonate 1 not in use at the time*
Ammonium Glycyrrhizate 1 up to 5%
Ammonium Laureth Sulfate 1 up to 36%
Ammonium Stearate 1 up to 10%
Ammonium Sulfite 1 not in use at the time*
Ammonium Tallate 1 not in use at the time*
Ammonium Xylenesulfonate 1 up to 3%
Amodimethicone 1 up to 3%
Amodimethicone Hydroxystearate 1 not in use at the time*
Amyl Acetate 1 up to 10%
Amyl Benzoate 1 not in use at the time*
Anacardium Occidentale (Cashew) Seed Oil 1 0.002 to 1%
10 not in use at the time*
apricot kernel amino acids
corn gluten amino acids
elastin amino acids
garcinia mangostana amino acids
jojoba amino acids
lycium barbarum amino acids
sesame amino acids
spirulina amino acids
sweet almond amino acids
yeast amino acids
Arachidyl Glycol 1 not in use at the time*
Arachidyl Propionate 1 up to 10%
Arachis Hypogaea (Peanut) Oil 1 0.0001 to 30%
Arctium Lappa Seed Oil 1 not in use at the time*
Argania Spinosa Kernel Oil 1 0.001 to 10%
Arginine 1 0.00002 to 2% in leave on; 0.00004 to 18% in rinse off Arginine HCl 1 0.004 to 0.1%
Ascorbic Acid 1 up to 10%
Ascorbyl Dipalmitate 1 not in use at the time*
Ascorbyl Palmitate 1 up to 0.2%
Ascorbyl Stearate 1 not in use at the time*
Asparagine 1 concentration not reported*
Aspartic Acid 1 0.000005 to 0.6% in leave on; 0.0001 to 1% in rinse off Astragalus Gummifer Gum (aka Tragacanth (Astragalus Gummifer) Gum) 1 up to 3%
Astrocaryum Murumuru Seed Butter 1 0.001 to 7%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Attapulgite 1 up to 8%
Avena Sativa (Oat) Kernel Oil 1 0.01-3%
Avocado Oil (aka Persea Gratissima (Avocado) Oil) 1 0.0001 to 98%
Azelaic Acid (Dicarboxylic Acids and their Salts and Esters) 1 up to 0.3% in leave on; 10% in rinse off
Babassu Acid 1 not in use at the time*
Basic Blue 99 1 up to 2%
Bassia Butyracea Seed Butter 1 not in use at the time*
Bassia Latifolia Seed Butter 1 0.001 to 2%
Batyl Alcohol 1 0.03 to 3%
Beeswax 1 up to 56%
Behenoxy Dimethicone 1 up to 3%
Behenyl Alcohol 1 up to 50%
Behenyl Benzoate 1 not in use at the time*
Bentonite 1 12-80% in mud packs; up to 8% for other uses Benzaldehyde 1 up to 0.5%
Benzoic Acid 1 0.000002 to 5%
Benzophenone-1 1 up to 2%
Benzophenone-2 1 up to 6%
Benzophenone-3 1 up to 7%
Benzophenone-4 1 up to 2.5%
Benzophenone-5, -6 2 up to 0.3%
Benzophenone-8 1 up to 0.2%
Benzophenone-9 1 up to 0.4%
Benzophenone-11 1 up to 0.2%
Benzyl Alcohol 1 0.000006 to 10%
Benzyl Benzoate 1 0.000005 to 4%
Benzylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%) Bertholletia Excelsa Seed Oil 1 0.0003 to 0.5%
BHA (aka Butylated Hydroxyanisole) 1 up to 0.2%
BHT (Butylated Hydroxytoluene) 1 up to 0.5%
Biotin 1 up to 1%
Bisabolol 1 up to 1%
N,N-Bis(2-Hydroxyethyl)-p- Phenylenediamine Sulfate 1 up to 5%
Borago Officinalis Seed Oil 1 0.001 to 1%
Boron nitride 1 Up to 25% in leave on
Brassica Campestris (Rapeseed) Oil Unsaponifiables 1 not in use at the time*
Brassica Campestris (Rapeseed) Seed Oil 1 0.007 to 17%
Brassica Napus Seed Oil 1 not in use at the time*
Brassica Oleracea Acephala Seed Oil 1 not in use at the time*
Brassica Oleracea Italica (Broccoli) Seed Oil 1 0.001 to 3%
n-Butane (aka Butane) 1 up to 92%
1,2-Butanediol 1 not in use at the time*
Butoxyethyl Acetate 1 not in use at the time*
Butyl Acetate 1 up to 72%
t-Butyl Acetate 1 up to 10%
n-Butyl Alcohol 1 up to 15% in nail products and 0.002% in other products
t-Butyl Alcohol 1 up to 0.5%
Butyl Benzoate 1 not in use at the time*
Butyl Benzyl Phthalate 1 not in use at the time*
Butylene Glycol 1 0.00007 to 89%
Butylene Glycol Cocoate 1 up to 2%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Butylene Glycol Diisononanoate 1 17%
Butyl Ester of PVM/MA Copolymer 1 up to 14%
Butyl Myristate 1 concentration not reported*
Butyloctyl Benzoate 1 not in use at the time*
Butylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%)
Butyl Stearate 1 up to 9%
Butyrospermum Parkii (Shea) Butter 1 0.0005 to 60%
Butyrospermum Parkii (Shea) Butter Unsaponifiables 1 0.06 to 3%
Butyrospermum Parkii (Shea) Oil 1 0.01 to 15%
C12-15 Alkyl Benzoate 1 0.0008-59%
C14-18 Glycol 1 not in use at the time*
C15-18 Glycol 1 concentration not reported*
C16-17 Alkyl Benzoate 1 concentration not reported*
C18-30 Glycol 1 not in use at the time*
C20-30 Glycol 1 not in use at the time*
C30-45 Alkyl Dimethicone 1 2%
C24-28 Alkyl Methicone 1 not in use at the time*
C30-45 Alkyl Methicone 1 not in use at the time*
Caesalpinia spinosa gum 1 0.002 to 0.5% in leave-on; 0.03% in rinse-off
Caesalpinia spinosa hydroxypropyltrimonium chloride 1 not in use at the time*
Calcium Acetate 1 concentration not reported*
Calcium Ascorbate 1 not in use at the time*
Calcium Aspartate 1 not in use at the time*
Calcium Benzoate 1 0.002 to 0.004%
Calcium Carboxymethyl Cellulose 1 not in use at the time*
4 not in use at the time*
Calcium Citrate
Copper Citrate
Disodium Cupric Citrate
Manganese Citrate
Calcium Disodium EDTA 1 not in use at the time*
Calcium Glycinate 1 3%
Calcium Silicate 1 up to 10%
Calcium Sodium PVM/MA Copolymer 1 concentration not reported*
Calcium Stearate 1 up to 23%
Calcium Xylenesulfonate 1 not in use at the time*
Calendula Officinalis Extract 1 not in use at the time*
Calendula Officinalis Flower 1 not in use at the time*
Calendula Officinalis Flower Extract 1 up to 6%
Calendula Officinalis Flower Oil 1 up to 0.1%
Calendula Officinalis Seed Oil 1 not in use at the time*
Camelina Sativa Seed Oil 1 0.002 to 1%
Camellia Japonica Seed Oil 1 0.01 to 0.2%
Camellia Kissi Seed Oil 1 0.1 to 10%
Camellia Oleifera Seed Oil 1 0.003 to 3%
Camellia Sinensis Seed Oil 1 up to 0.1%
Canarium Indicum Seed Oil 1 not in use at the time*
Candelilla (Euphorbia Cerifera) Wax 0 up to 27%
Canola Oil 1 0.0002 to 73%
Canola Oil Unsaponifiables 1 up to 0.001%
Caprylic/Capric Triglyceride 1 up to 84%
Caprylic/Capric/Coco Glycerides 1 up to 4%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Caprylyl Glyceryl Ether 1 not in use at the time*
Caprylyl Glycol 1 0.00003 – 5%
Carbomer-910, -962 2 not in use at the time*
Carbomer-934, -934P, -940, -941 4 up to 2%
Carboxymethyl Hydroxyethylcellulose 1 up to 3%
Carboxymethyl hydroxypropyl guar 1 not in use at the time*
Carica Papaya Seed Oil 1 up to 0.1%
Carthamus Tinctorius (Safflower) Seed Oil 1 0.00005 to 84%
Carya Illinoensis (Pecan) Seed Oil 1 not in use at the time*
Caryocar Brasiliense Fruit Oil 1 0.0005-0.2
Cassia gum 1 not in use at the time*
Cassia hydroxypropyltrimonium chloride 1 0.06 to 0.4% in rinse-off
Cellobiose Octanonanoate 1 not in use at the time*
Cellulose 1 up to 88%
Cellulose Acetate 1 up to 2%
Cellulose Acetate Butyrate 1 up to 15%
Cellulose Acetate Propionate 1 not in use at the time*
Cellulose Gum 1 up to 20%
Cellulose Succinate 1 not in use at the time*
Ceratonia siliqua gum 1 0.0003 to 0.05% in leave-on; 0.0003 to 0.07% in rinse-off Ceresin 1 up to 20%
Cetearyl Alcohol 1 up to 25%
Cetearyl Methicone 1 up to 1%
Cetyl Acetate 1 up to 17%
Cetyl Alcohol 1 up to 50%
Cetyl Dimethicone 1 0.01 to 17%
Cetyl Glyceryl Ether 1 0.002 to 5%
Cetyl Glycol 1 not in use at the time*
Cetyl Hydroxyethylcellulose 1 up to 2%
Chenopodium Quinoa Seed Oil 1 up to 0.3%
Chimyl Alcohol 1 0.002 to 5%
4-Chloro-2-Aminophenol 1 safe as used in oxidative hair dyes, but not in use at the time*;
insufficient data for use in non-oxidative hair dyes
2-Chloro-p-Phenylenediamine 1 up to 0.1%
2-Chloro-p-Phenylenediamine Sulfate 1 up to 1.0%
4-Chlororesorcinol 1 ≤1% in hair dyes
Chloroxylenol 1 up to 0.5%
Cholesterol 1 up to 3%
Cholesteryl Nonanoate 1 up to 30%
Choleth-24 1 up to 5% topical
Citric Acid 1 0.0000005 to 4% in leave-on; 0.000002 to 10% in rinse-off Citrullus Lanatus (Watermelon) Seed Oil 1 up to 2%
Citrus Aurantifolia (Lime) Seed Oil 1 not in use at the time*
Citrus Aurantifolia (Lime) Seed Oil Unsaponifiables 1 not in use at the time*
Citrus Aurantium Dulcis (Orange) Seed Oil 1 not in use at the time*
Citrus Aurantium Dulcis (Orange) Seed Oil Unsaponifiables 1 not in use at the time*
Citrus Grandis (Grapefruit) Seed Oil 1 not in use at the time*
Citrus Grandis (Grapefruit) Seed Oil Unsaponifiables 1 not in use at the time*
Citrus Limon (Lemon) Seed Oil 1 1 to 5%
Citrus Paradisi (Grapefruit) Seed Oil 1 0.01 to 20%
Cocoamphoacetate (aka Sodium Cocoamphoacetate) 1 up to 10%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Cocoamphodiacetate (aka Disodium Cocoamphodiacetate) 1 up to 50%
Cocoamphodipropionate (aka Disodium Cocoamphodipropionate) 1 up to 25%
Cocoamphopropionate (aka Sodium Cocoamphopropionate) 1 not in use at the time*
Cocoglycerides 1 up to 14%
Coconut Acid 1 0.03 to 14%
Coconut Alcohol 1 up to 0.9%
Cocos Nucifera (Coconut) Oil 1 0.0001 to 80%
Cocos Nucifera (Coconut) Seed Butter 1 not in use at the time*
Coix Lacryma-Jobi (Job’s Tears) Seed Oil 1 not in use at the time*
collagen amino acids 1 Up to 6% in leave ons
collodion 1 Up to 14% in nail products
Copernicia Cerifera (Carnauba) Wax 1 up to 20%
Corn Acid 1 not in use at the time*
Corn Glycerides 1 not in use at the time*
Corylus Americana (Hazel) Seed Oil 1 concentration not reported*
Corylus Avellana (Hazel) Seed Oil 1 0.005 to 98%
Cottonseed Acid 1 not in use at the time*
Cotton (Gossypium Herbaceum) Seed Oil 0 0.004 to 32%
Crambe Abyssinica Seed Oil 1 concentration not reported*
Cucumis Sativus (Cucumber) Extract 1 0.0002 to 0.003%
Cucumis Sativus (Cucumber) Fruit and Cucumis Sativus (Cucumber) Juice 2 concentration not reported*
Cucumis Sativus (Cucumber) Fruit Extract 1 0.0000001 to 1%
Cucumis Sativus (Cucumber) Fruit Water 1 0.05 to 3%
Cucumis Sativus (Cucumber) Seed Extract 1 0.01 to 0.08%
Cucumis Sativus (Cucumber) Seed Oil 1 concentration not reported*
Cucurbita Pepo (Pumpkin) Seed Oil 1 0.003 to 0.1%
Cyamopsis tetragonoloba (guar) gum 1 0.02 to 1% in leave-on; 0.09 to 5% in rinse-off
Cyclomethicone 1 up to 89%
Cycloheptasiloxane 1 concentration not reported*
Cyclohexasiloxane 1 up to 48%
Cyclopentasiloxane 1 up to 93%
Cyclotetrasiloxane 1 up to 28%
Cynara Cardunculus Seed Oil 1 not in use at the time*
Cysteine 1 0.0001 to 0.05% in leave on; 0.0001 to 5% in rinse off
Cysteine HCl 1 0.0001% in leave on; 0.0001 to 6% in rinse off
Cystine 1 0.001% in leave on; 0.001 to 3% in rinse off
Decylene Glycol 1 concentration not reported*
Decyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dehydroacetic Acid 1 up to 0.7%
Denatonium Benzoate 1 1/16 avoidupois ounce per 100 gal alcohol
2,4-Diaminophenoxyethanol Dihydrochloride and 2,4-Diaminophenoxyethanol
2 up to 2%
Sulfate
Diammonium citrate 1 concentration not reported*
Diammonium EDTA 1 not in use at the time*
Dibehenyl Fumarate 1 not in use at the time*
Dibutyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 concentration currently not reported;* previously reported up to 8% Dibutyloctyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dibutyl Phthalate 1 up to 15%
Dibutyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Di-C12-15 Alkyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Di-C12-15 Alkyl Fumarate 1 up to 5%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Dicapryl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 concentration not reported*
Dicaprylyl/Capryl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dicapryl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 concentration not reported*
Dicetearyl Dimer Dilinoleate 1 up to 7%
Dicetearyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dicetyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diethyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diethylene Glycol Diethylhexanoate/Diisononanoate 1 up to 19%
Diethylene Glycol Diisononanoate 1 not in use at the time*
Diethylhexyl Adipate (aka Dioctyl Adipate) (Dicarboxylic Acids and their Salts and
1 up to 14% in leave on; 0.6% in rinse off
Esters)
Diethylhexyl Dimer Dilinoleate (aka Dioctyl Dimer Dilinoleate) 1 up to 12%
Diethylhexyl Fumarate 1 not in use at the time*
Diethylhexyl Sodium Sulfosuccinate (aka Dioctyl Sodium Sulfosuccinate) 1 ≤5%
Diethylhexyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 5% in leave on; 1% in rinse off
Diethylhexyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 6% in leave on; 5% in rinse off
Diethyl Malonate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 0.02% in leave on; 0.01% in rinse off
Diethyl Phthalate 1 up to 2%
Diethyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 1.5% in leave on
Diethyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diheptylundecyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 6% in leave on
Dihexyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 concentration in leave on not reported;* up to 3% in rinse off Dihexyldecyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dihexyldecyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dihydrocholesteryl Nonanoate 1 not in use at the time*
Dihydrogenated tallow benzylmonium hectorite 1 not in use at the time*
Diisobutyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 0.001 to 3%
Diisobutyl Glutarate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diisobutyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diisocetyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diisocetyl Dodecanedioate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 7% in leave on
Diisodecyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 concentration in leave on not reported*
Diisononyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Disooctyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diisooctyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 3% in leave on
Diisopropyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 up to 7% in leave on; 8% in rinse off
Diisopropyl Dimer Dilinoleate 1 up to 53%
Diisopropyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 10% in leave on; 2% in rinse off
Diisostearyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 10% in leave on; 3% in rinse off
Diisostearyl Dimer Dilinoleate 1 up to 12%
Diisostearyl Fumarate 1 up to 20%
Diisostearyl Glutarate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Diisostearyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dilauryl citrate 1 concentration in rinse-off not reported*
Dimethicone 1 up to 80% in hair preparations; up to 24% in makeup Dimethicone Copolyol** 0 up to 1%
2 up to 0.5%
Dimethicone PEG-7 Phosphate and Dimethicone PEG-8 Benzoate (aka Dimethicone
Copolyol)
Dimethicone PEG-10 Phosphate,
3 concentration not reported*
Dimethicone PEG-6 Acetate,
and Dimethicone PEG-8 Adipate (aka Dimethicone Copolyol)
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion Dimethicone PEG/PPG-7/4 Phosphate,
3 not in use at the time*
Dimethicone PEG/PPG-12/4 Phosphate,
and Dimethicone PEG/PPG-20/23 Benzoate (aka Dimethicone Copolyol)
Dimethiconol (Dimethiconol and its Esters and Reaction Products) 1 0.004 to 36%
Dimethiconol Arginine (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Beeswax (Dimethiconol and its Esters and Reaction Products) 1 0.8 to 0.9%
Dimethiconol Behenate (Dimethiconol and its Esters and Reaction Products) 1 Up to 0.5%
Dimethiconol Borageate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Candelillate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Carnaubate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Cysteine (Dimethiconol and its Esters and Reaction Products) 1 Up to 0.07%
Dimethiconol Dhupa Butterate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Hydroxystearate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Illipe Butterate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Isostearate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Kokum Butterate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Lactate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Meadowfoamate (Dimethiconol and its Esters and Reaction Products) 1 0.5 to 1%
Dimethiconol Methionine (Dimethiconol and its Esters and Reaction Products) 1 concentration not reported*
Dimethiconol/Methylsilanol/Silicate Crosspolymer (Dimethiconol and its Esters and
1 not in use at the time*
Reaction Products)
Dimethiconol Mohwa Butterate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Dimethiconol Panthenol (Dimethiconol and its Esters and Reaction Products) 1 Up to 0.07%
Dimethiconol Sal Butterate (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
1 not in use at the time*
Dimethiconol/Silica Crosspolymer (Dimethiconol and its Esters and Reaction
Products)
1 Up to 0.3%
Dimethiconol/Silsesquioxane Copolymer (Dimethiconol and its Esters and Reaction
Products)
Dimethiconol Stearate (Dimethiconol and its Esters and Reaction Products) 1 Up to 1%
Dimethiconol/Stearyl Methicone/Phenyl Trimethicone Copolymer (Dimethiconol
1 not in use at the time*
and its Esters and Reaction Products)
Dimethoxysilyl Ethylenediaminopropyl Dimethicone 1 not in use at the time in non-coloring hair care products* Dimethyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 0.2% in rinse off
Dimethyl Glutarate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 15% in rinse off
8 not in use at the time*
Dimethyl Lauramine, Dimethyl Myristamine, Dimethyl Palmitamine, Dimethyl
Behenamine, Dimethyl Cocamine, Dimethyl Hydrogenated Tallowamine, Dimethyl
Tallowamine, Dimethyl Soyamine
Dimethyl Phthalate 1 up to 2%
Dimethyl Stearamine 1 up to 4% in non-coloring hair care products
Dimethyl Succinate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dioctyl Adipate(aka Diethylhexyl Adipate) 0 up to 38%
Dioctyl Dimer Dilinoleate (aka Diethylhexyl Dimer Dilinoleate) 0 up to 12%
Dioctyldodecyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 concentration in leave on not reported*
Dioctyldocecyl Dimer Dilinoleate 1 up to 10%
Dioctyldodecyl Dodecanedioate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 6% in leave on
Dioctyldodecyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 8% in leave on
Dioctyl Sodium Sulfosuccinate (aka Diethylgexyl Sodium Sulfosuccinate) 0 ≤5%
Dioleyl Tocopheryl Methylsilanol 1 ≤6%
Dioscorea Villosa (Wild Yam) Root Extract (aka Wild Yam (DV) Extract 1 up to 15% of max. 2% plant solids
Dipentaerythrityl Pentaisononanoate 1 up to 13%
Dipotassium Aspartate 1 not in use at the time*
Dipotassium Azelate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Dipotassium EDTA 1 less than 0.1%
Dipotassium Glycyrrhizate 1 up to 1%
Dipropyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Dipropylene Glycol 1 up to 50%
Disodium Azelate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Disodium Cocoamphodiacetate 1 0.0006 to 12%
Disodium Cocoamphodipropionate 1 0.008 to 15%
Disodium EDTA 1 less than 1%
Disodium Fumarate 1 not in use at the time*
Disodium Glycyrrhizate 1 not in use at the time*
Disodium Lauriminodipropionate 1 not in use at the time*
Disodium Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Disodium Succinate (Dicarboxylic Acids and their Salts and Esters) 1 up to 0.4% in leave on; 0.0005% in rinse off
Disodium Succinoyl Glycyrrhizate 1 concentration not reported*
Disperse Black 9 1 0.3 - 0.5% in hair coloring products
Disperse Violet 1 1 up to 0.7%
Disteardimonium hectorite 1 0.04 to 28% in leave-on; 0.7 to 4% in rinse-off
Ditridecyl Adipate (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Ditridecyl Dimer Dilinoleate 1 not in use at the time*
DMDM Hydantoin 1 up to 1%
Dodecanedioic Acid (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Drometrizole 1 0.07%
EDTA 1 up to 2%
Elaeis Guineensis (Palm) Butter 1 not in use at the time*
Elaeis Guineensis (Palm) Kernel Oil 1 0.05 to 23%
Elaeis Guineensis (Palm) Oil 1 0.002 to 48%
Elaeis Oleifera Kernel Oil 1 concentration not reported*
Elaeis (Palm) Fruit Oil 1 not in use at the time*
Emulsifying Wax N.F. 1 up to 21%
Erythorbic Acid 1 up to 1%
Ethoxydiglycol 1 up to 80%
Ethyl Acetate 1 up to 85%
Ethyl Benzoate 1 0.0008-0.01%
Ethylcellulose 1 up to 4%
Ethyl citrates 1 0.5 to 1% in rinse-off
Ethyl Ester of PVM/MA Copolymer 1 up to 30%
Ethyl Hexanediol 1 ≤5%
Ethylhexyl Benzoate 1 3 to 4%
Ethylhexylglycerin 1 0.000001 to 8% (up to 2% in leave-on)
Ethyl myristate 1 not in use at the time*
Ethylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%)
4 not in use at the time*
Ethyl Pelargonate, Isobutyl Pelargonate, Methyl Pelargonate,
Neopentyl Glycol Dicaprylate/Dipelargonate/Dicaprate
Ethyl Ricinoleate 1 not in use at the time*
Euphorbia Cerifera (Candelilla) Wax 1 up to 27%
Euterpe Oleracea Fruit Oil 1 0.00001 to 0.5%
Ferric citrate 1 Up to 0.5% in rinse-off; concentration in rinse-off not reported* Ferrous Fumarate 1 up to 0.0003%
Fossil and Synthetic Waxes (e.g.- paraffin) 0 varies
Fragaria Ananassa (Strawberry) Seed Oil 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Fragaria Chiloensis (Strawberry Seed Oil 1 not in use at the time*
Fragaria Vesca (Strawberry) Seed Oil 1 not in use at the time*
Fragaria Virginiana (Strawberry) Seed Oil 1 not in use at the time*
Fuller’s Earth 1 up to 50%
Fumaric Acid 1 up to 5%
Garcinia Indica Seed Butter 1 0.1 to 2%
Gevuina Avellana Seed Oil 1 0.1 to 2%
Gevuina Avellana Oil 1 0.002 to 0.2%
Glutamic Acid 1 0.000004 to 0.4% in leave on; 0.00003 to 0.1% in rinse off Glutamine 1 0.002% in leave on; 0.005% in rinse off
Glutaric Acid (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Glycereth-7 Diisononanoate 1 not in use at the time*
Glyceryl Adipate 1 not in use at the time*
Glyceryl Alginate and Glyceryl Erucate 2 up to 0.5%
Glyceryl Allyl Ether 1 not in use at the time*
Glyceryl Arachidate 1 not in use at the time*
Glyceryl Behenate 1 up to 5%
Glyceryl Caprate 1 not in use at the time*
Glyceryl Caprylate 1 not reported*
Glyceryl Caprylate/Caprate 1 up to 2%
Glyceryl Capryl Ether 1 not in use at the time*
Glyceryl Citrate/Lactate/ Linoleate/ Oleate 1 not in use at the time*
Glyceryl Cocoate 1 up to 5%
Glyceryl Collagenate 1 not in use at the time*
Glyceryl Glycyrrhetinate 1 not in use at the time*
Glyceryl Hydrogenated Rosinate 1 not in use at the time*
Glyceryl Hydrogenated Soyate 1 not in use at the time*
Glyceryl Hydroxystearate 1 up to 2%
Glyceryl Isopalmitate 1 not in use at the time*
Glyceryl Isostearate 1 up to 6%
Glyceryl Isostearates 1 not in use at the time*
Glyceryl Isostearate/Myristate 1 not in use at the time*
Glyceryl Isotridecanoate/Stearate/ Adipate 1 not in use at the time*
Glyceryl Lanolate 1 not in use at the time*
Glyceryl Laurate 1 up to 4%
Glyceryl Laurate/Oleate, Glyceryl Laurate SE, and Glyceryl Oleate SE 3 not in use at the time*
Glyceryl Lauryl Ether 1 up to 7% in rinse-off
Glyceryl Linoleate and Glyceryl Linolenate 2 up to 1%
Glyceryl Montanate 1 not in use at the time*
Glyceryl Myristate 1 concentration not reported*
Glyceryl Oleate 1 up to 5%
Glyceryl Oleate/Elaidate 1 up to 2%
Glyceryl Palmitate 1 concentration not reported*
Glyceryl Palmitate/Stearate and Glyceryl Palmitoeleate 2 not in use at the time*
Glyceryl Pentadecanoate 1 not in use at the time*
Glyceryl Polyacrylate 1 up to 2%
Glyceryl Ricinoleate 1 up to 12%
Glyceryl Ricinoleate SE 1 not in use at the time*
Glyceryl Rosinate 1 up to 7%
Glyceryl Sesquioleate 1 not in use at the time*
Glyceryl/Sorbitol Oleate/Hydroxystearate 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Glyceryl Stearate 1 up to 50%
Glyceryl Stearate/Acetate 1 up to 7%
Glyceryl Stearate Diacetate 1 not in use at the time*
Glyceryl Stearate/Maleate 1 not in use at the time*
Glyceryl Stearate SE 1 up to 25%
Glyceryl Tallowate 1 not in use at the time*
Glyceryl Thiopropionate 1 not in use at the time*
Glyceryl Triacetyl Hydroxystearate 1 9%
Glyceryl Triacetyl Ricinoleate 1 8%
Glyceryl Undecylenate 1 concentration not reported*
Glycine 1 0.0005 to 4%
Glycine Soja (Soybean) Oil 1 0.0002 to 95%
Glycine Soja (Soybean) Oil Unsaponifiables 1 0.0001 to 0.2%
Glycol Distearate 1 up to 9%
Glycol Ricinoleate 1 not in use at the time*
Glycol Stearate 1 up to 6%
Glycol Stearate SE 1 up to 12%
Glycyrrhetinic Acid 1 up to 2%
Glycyrrhetinyl Stearate 1 not in use at the time*
3 0.0001 to 0.4%
Glycyrrhiza Glabra (Licorice) Rhizome/Root, Glycyrrhiza Glabra (Licorice) Root
and Glycyrrhiza Glabra (Licorice) Root Extract
Glycyrrhiza Glabra (Licorice) Leaf Extract 1 concentration not reported*
5 not in use at the time*
Glycyrrhiza Glabra (Licorice) Root Juice, Glycyrrhiza Glabra (Licorice) Root
Powder, Glycyrrhiza Glabra (Licorice) Root Water, Glycyrrhiza Inflata Root Extract,
Glycyrrhiza Uralensis (Licorice) Root Extract
Glycyrrhiza Glabra (Licorice) Root Extract [polyol soluble] 1 0.0001 to 0.06%
Glycyrrhiza Inflata Root Extract [polyol soluble] 1 0.0001 to 15%
Glycyrrhizic Acid 1 up to 0.1%
Gossypium Herbaceum (Cotton) Seed Oil 1 0.004 to 32%
Guar hydroxypropyltrimonium chloride 1 0.005 to 2% in leave-on; 0.002 to 2% in rinse-off
Guizotia Abyssinica Seed Oil 1 not in use at the time*
hair keratin amino acids 1 Up to 3% in leave ons
HC Blue No. 2 1 2% in a hair dye (diluted in use to 1%)
HC Red No. 7 1 up to 1%
HC Yellow No. 4 1 ≤3%
HC Yellow No. 5 1 up to 1.6%
Hectorite 1 100% in a skin cleaning preparation; up to 15% in other uses HEDTA 1 concentration not reported*
Helianthus Annuus (Sunflower) Seed Oil 1 0.000007 to 96%
Helianthus Annuus (Sunflower) Seed Oil Unsaponifiables 1 0.005 to 2%
Hexacosyl Glycol 1 not in use at the time*
1,2-Hexanediol 1 0.00005 – 10%
Hexyldecyl Benzoate 1 not in use at the time*
Hexylene Glycol 1 up to 6%
Hexyl Methicone 1 not in use at the time*
Hippophae Rhamnoides Fruit Oil 1 6 to 35%
Hippophae Rhamnoides Oil 1 0.2 to 0.7%
Hippophae Rhamnoides Seed Oil 1 not in use at the time*
Histidine 1 0.00009 to 0.05% in leave on; 0.00004 to 0.0008% in rinse off Histidine HCl 1 0.00003 to 0.07% in leave on
Hyaluronic Acid 1 up to 1%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Hydrated Silica 1 up to 34%
Hydrogenated Adansonia Digitata Seed Oil 1 not in use at the time*
Hydrogenated Apricot Kernel Oil 1 concentration not reported*
Hydrogenated Apricot Kernel Oil Unsaponifiables 1 not in use at the time*
Hydrogenated Argania Spinosa Kernel Oil 1 not in use at the time*
Hydrogenated Avocado Oil 1 up to 0.5%
Hydrogenated Black Currant Seed Oil 1 not in use at the time*
Hydrogenated Camelina Sativa Seed Oil 1 not in use at the time*
Hydrogenated Camellia Oleifera Seed Oil 1 concentration not reported*
Hydrogenated Canola Oil 1 concentration not reported*
Hydrogenated Castor Oil 1 up to 39%
Hydrogenated Cocoglycerides 1 up to 31%
Hydrogenated Coconut Acid 1 6 to 10%
Hydrogenated Coconut Oil 1 0.001 to 50%
Hydrogenated Cottonseed Oil 1 0.001 to 24%
Hydrogenated Cranberry Seed Oil 1 not in use at the time*
Hydrogenated Evening Primrose Oil 1 concentration not reported*
Hydrogenated Grapefruit Seed Oil 1 not in use at the time*
Hydrogenated Grapefruit Seed Oil Unsaponifiables 1 not in use at the time*
Hydrogenated Grapeseed Oil 1 0.3 to 0.5%
Hydrogenated Hazelnut Oil 1 not in use at the time*
Hydrogenated Jojoba Oil 1 up to 31%
Hydrogenated Kukui Nut Oil 1 not in use at the time*
Hydrogenated Lanolin 1 up to 10%
Hydrogenated Lime Seed Oil 1 not in use at the time*
Hydrogenated Lime Seed Oil Unsaponifiables 1 not in use at the time*
Hydrogenated Macadamia Seed Oil 1 not in use at the time*
Hydrogenated Meadowfoam Seed Oil 1 not in use at the time*
Hydrogenated Olive Oil 1 0.0005 to 12%
Hydrogenated Olive Oil Unsaponifiables 1 0.05 to 5%
Hydrogenated Orange Seed Oil 1 not in use at the time*
Hydrogenated Orange Seed Oil Unsaponifiables 1 not in use at the time*
Hydrogenated Palm Acid 1 not in use at the time*
Hydrogenated Palm Kernel Oil 1 0.4 to 13%
Hydrogenated Palm Oil 1 0.2 to 30%
Hydrogenated Passiflora Edulis Seed Oil 1 not in use at the time*
Hydrogenated Peach Kernel Oil 1 not in use at the time*
Hydrogenated Peanut Oil 1 2 to 5%
Hydrogenated Pistachio Seed Oil 1 not in use at the time*
Hydrogenated Polyisobutene 1 up to 96%
Hydrogenated Pumpkin Seed Oil 1 not in use at the time*
Hydrogenated Punica Granatum Seed Oil 1 not in use at the time*
Hydrogenated Rapeseed Oil 1 0.3 to 4%
Hydrogenated Raspberry Seed Oil 1 not in use at the time*
Hydrogenated Rice Bran Oil 1 not in use at the time*
Hydrogenated Rice Bran Wax 1 concentration not reported*
Hydrogenated Rosa Canina Fruit Oil 1 not in use at the time*
Hydrogenated Safflower Seed Oil 1 not in use at the time*
Hydrogenated Sesame Seed Oil 1 not in use at the time*
Hydrogenated Shea Butter 1 up to 1%
Hydrogenated Soybean Oil 1 0.001 to 42%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Hydrogenated Sunflower Seed Oil 1 6 to 35%
Hydrogenated Sweet Almond Oil 1 up to 0.5%
Hydrogenated Sweet Almond Oil Unsaponifiables 1 not in use at the time*
Hydrogenated Tallow Glyceride 1 up to 25%
Hydrogenated Tallow Glycerides 1 up to 25%
Hydrogenated Vegetable Oil 1 0.0004 to 60%
Hydrogenated Wheat Germ Oil 1 not in use at the time*
Hydrogenated Wheat Germ Oil Unsaponifiables 1 not in use at the time*
Hydrolyzed caesalpinia spinosa gum 1 0.002 to 0.4% in leave-on; 0.008% in rinse-off
Hydrolyzed Cellulose Gum 1 not in use at the time*
Hydrolyzed ceratonia siliqua gum extract 1 not in use at the time*
Hydrolyzed Collagen 1 up to 6%
1 not in use at the time*
Hydrolyzed Collagen PG-Propyl Dimethiconol (Dimethiconol and its Esters and
Reaction Products)
Hydrolyzed Corn Protein and Hydrolyzed Corn Starch 2 not in use at the time*
Hydrolyzed guar 1 0.03 to 3% in leave-on; 0.2 to 2% in rinse-off
Hydrolyzed Jojoba Esters 1 up to 2%
Hydrolyzed Rice Bran Extract 1 up to 0.0004%
Hydrolyzed Rice Bran Protein 1 not in use at the time*
Hydrolyzed Rice Extract 1 up to 0.3%
Hydrolyzed Rice Protein 1 up to 2%
Hydroquinone (Amended) 1 up to 0.5% in nail care products
C18-22 Hydroxyalkyl hydroxypropyl guar 1 not in use at the time*
Hydroxybenzomorpholine 1 0.03%
Hydroxybutyl Methylcellulose 1 not in use at the time*
Hydroxyethylcellulose 1 up to 6%
Hydroxyethyl Ethylcellulose 1 up to 3%
6-Hydroxyindole 1 up to 0.5%
Hydroxylated Lanolin 1 up to 28%
4-Hydroxypropylamino-3-Nitrophenol 1 up to 2.6%
Hydroxypropylcellulose 1 up to 4%
Hydroxypropyldimethicone 1 not in use at the time*
Hydroxypropyl guar 1 0.05 to 2% in leave-on; 0.1 to 93% in rinse-off Hydroxypropyl guar hydroxypropyltrimonium chloride 1 0.04 to 0.9% in leave-on; 0.2 to 0.8% in rinse-off Hydroxypropyl Methylcellulose 1 up to 4%
Hydroxypropyl Methylcellulose Acetate/Succinate 1 not in use at the time*
Hydroxystearic Acid 1 up to 10%
hypericum perforatum extract 1 Up to 0.01% in leave on
hypericum perforatum flower extract 1 Up to 0.005% in leave on
hypericum perforatum flower/leaf/stem extract 1 Up to 0.007% in leave on
hypericum perforatum oil 1 Up to 0.00005% in leave on
hypericum perforatum flower/leaf extract
3 not in use at the time*
hypericum perforatum flower/twig extract
hypericum perforatum leaf extract
Imidazolidinyl Urea 1 up to 1%
Irvingia Gabonensis Kernel Butter 1 0.003 to 0.4%
Isoamyl Acetate 1 up to 10%
Isobutane 1 up to 85%
Isobutyl Acetate 1 6 to 45%
Isobutyl Benzoate 1 0.01%
Isobutylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%)
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Isodecyl citrate 1 concentration in leave-on not reported*
Isodecyl Glyceryl Ether 1 not in use at the time*
Isoleucine 1 0.0003 to 0.002%
Isomerized Jojoba Oil 1 not in use at the time*
Isopentane 1 up to 37%
Isopolyglyceryl-3 Dimethiconol (Dimethiconol and its Esters and Reaction Products) 1 not in use at the time*
Isopropyl Acetate 1 0.5 to 2%
Isopropyl Alcohol 1 up to 100% (in manicuring preparations)
Isopropyl Benzoate 1 not in use at the time*
7 not in use at the time*
Isopropyl citrate
Distearyl citrate
Trihexyldecyl citrate
Triisopropyl citrate
Trilauryl citrate
Trioleyl citrate
Tristearyl citrate
Isopropyl Ester of PVM/MA Copolymer 1 not in use at the time*
Isopropyl Lanolate 1 up to 26%
Isopropylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%) Isostearic Acid 1 up to 26%
Isostearyl Acetate 1 0.002 to 5%
Isostearyl Alcohol 1 up to 50%
Isostearyl Benzoate 1 1%
Isostearyl Glyceryl Ether 1 0.001 to 0.02%
Isostearyl Sebacate (Dicarboxylic Acids and their Salts and Esters) 1 Up to 0.7%
Japan (Rhus Succedanea) Wax (aka Rhus Succedanea Fruit Wax) 1 up to 34%
Jojoba Alcohol 1 up to 1%
Jojoba Esters 1 up to 44%
Juglans Regia (Walnut) Seed Oil 1 0.00003 to 0.2%
Kaolin 1 up to 100%
keratin amino acids 1 Up to 0.5% in leave on and rinse off
Laneth-9 Acetate, and Laneth-10 Acetate 2 not in use at the time*
Lanolin 1 up to 37%
Lanolin Acid 1 up to 10%
Lanolin Alcohol 1 up to 4%
Lanolin Oil 1 up to 65%
Lanolin Wax 1 up to 25%
Lapyrium Chloride 1 0.03% in a body wash
Lauric Acid 1 up to 25%
Lauriminodipropionic Acid 1 not in use at the time*
Lauryl Glycol 1 not in use at the time*
Lauryl/Myristyl Benzoate 1 not in use at the time*
Leucine 1 0.0009 to 0.001% in leave on
Limnanthes Alba (Meadowfoam) Seed Oil 1 0.002 to 74%
Linseed Acid 1 concentration not reported*
Linum Usitatissimum (Linseed) Seed Oil 1 0.001 to 10%
Lithium Magnesium Silicate and Lithium Magnesium Sodium Silicate 2 not in use at the time*
Lithium Stearate 1 up to 3%
Locust bean hydroxypropyltrimonium chloride 1 0.4% in rinse-off
Luffa Cylindrica Seed Oil 1 up to 0.01%
lupine amino acids 1 Up to 0.1% in leave on and rinse off
Lupinus Albus Oil Unsaponifiables 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Lupinus Albus Seed Oil 1 concentration not reported*
Lycium Barbarum Seed Oil 1 concentration not reported*
Lysine 1 0.00002 to 0.7% in leave on; 1 x 10-7 to 0.04% in rinse off Lysine HCl 1 0.00003 to 0.6% in leave on; 0.0008 to 0.1% in rinse off Macadamia Integrifolia Seed Oil 1 0.00006 to 5%
Macadamia Ternifolia Seed Oil 1 0.0003 to 30%
Magnesium Acetate 1 0.02 to 0.03%
Magnesium Aluminum Silicate 1 up to 5%
Magnesium Ascorbate 1 not in use at the time*
Magnesium Ascorbyl Phosphate 1 up to 3%
Magnesium Aspartate 1 0.0003 to 0.1% in leave on; 0.00005 to 0.06% in rinse off Magnesium Benzoate 1 not in use at the time*
Magnesium citrate 1 0.01 to 2% in leave-on; up to 0.5% in rinse off
Magnesium Cocoate 1 concentration not reported*
Magnesium Coco-Sulfate 1 not in use at the time*
Magnesium Glycinate 1 not in use at the time*
Magnesium Laureth Sulfate 1 up to 7%
Magnesium Myreth Sulfate 1 not in use at the time*
Magnesium Myristate 1 up to 10%
Magnesium Oleth Sulfate 1 up to 0.2%
Magnesium Silicate and Magnesium Trisilicate 2 not in use at the time*
Magnesium Stearate 1 up to 8%
Maleic Acid 1 up to 0.004% as a pH adjuster
Malonic Acid (Dicarboxylic Acids and their Salts and Esters) 1 not in use at the time*
Maltitol 1 up to 15%
Maltitol Laurate 1 not in use at the time*
Mangifera Indica (Mango) Seed Butter 1 0.0005 to 3%
Mangifera Indica (Mango) Seed Oil 1 0.003 to 6%
Methicone 1 up to 5%
Methionine 1 0.0001 to 0.005% in leave on; 0.0001 to 0.07% in rinse off Methoxyisopropanol 1 up to 35% in nail care products; fragrance use not addressed Methoxyisopropyl Acetate 1 not in use at the time*
Methyl Acetate 1 10 to 60%
3-Methylamino-4-Nitrophenoxyethanol 1 0.15%
p-Methylaminophenol and p-Methylaminophenol Sulfate 2 up to 0.7%
Methyl Benzoate 1 0.0005-0.3%
Methylcellulose 1 up to 20%
Methyl Cocoate 1 up to 0.06%
Methyl Ethylcellulose 1 not in use at the time*
Methyl Glycyrrhizate 1 not in use at the time*
2-Methyl-5-Hydroxyethylaminophenol 1 up to 5%
Methyl Hydroxyethylcellulose 1 not in use at the time*
Methyl Isobutyl Ketone (aka- MIBK) 0 up to 21% in nail care products; up to 4% as a denaturant Methyl Methacrylate Crosspolymer 1 up to 14%
Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer 1 up to 3%
Methyl Myristate 1 not in use at the time*
Methylparaben 1 up to 0.4% if used alone (paraben mixes up to 0.8%)
Methyl Pelargonate 1 not in use at the time*
2-Methyl Resorcinol 1 up to 2%
Methyl Ricinoleate 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
MIBK (aka Methyl Isobutyl Ketone) 1 up to 21% in nail care products; up to 4% as a denaturant Microcrystalline Cellulose 1 up to 57%
Microcrystalline Wax 1 up to 50%
milk amino acids 1 Concentration not reported*
Mink Oil 1 up to 3%
Monosodium citrate 1 0.004 to 5% in leave-on; 0.8 to 5% in rinse-off
Montan Wax 1 up to 11%
Montmorillonite 1 not in use at the time*
Morinda Citrifolia Seed Oil 1 not in use at the time*
Moringa Oleifera Seed Oil 1 up to 0.001%
Moringa Pterygosperma Seed Oil 1 0.003 to 3%
Myristamide DEA 1 0.8%
Myristic Acid 1 up to 50%
Myristyl Acetate 1 not in use at the time*
Myristyl Alcohol 1 up to 5%
Myristyl Glycol 1 not in use at the time*
1-Naphthol 1 up to 3% in hair coloring products
Neopentyl Glycol Diisononanoate 1 1%
Neopentyl Pelargonate 1 not in use at the time*
Niacin and Niacinamide 2 Niacin ≤0.1%; Niacinamide ≤3%
Nitrocellulose 1 Up
3-Nitro-p-Hydroxyethylaminophenol 1 up to 10% (5% after dilution)
2-Nitro-p-Phenylenediamine 1 up to 1%
4-Nitro-o-Phenylenediamine 1 up to 0.2%
Nonoxynol-9 1 up to 50%
Nonoxynol-10, -50 2 up to 25%
Nonoxynol-12 1 up to 5%
Nonoxynol-14 1 up to 1%
Nonoxynol-15, -30 2 ≤0.1%
Nonoxynol-40 1 not in use at the time*
Nonyl Acetate 1 0.0004%
nylon-6 1 Up to 20% in leave ons
nylon-11 1 Up to 4% in leave ons
nylon-12 1 Up to 35% in leave ons
nylon 6/12 1 Up to 1% in leave ons
nylon-66 1 Up to 8% in leave ons
nylon-10/10, nylon-611, nylon-12/6/66 3 not in use at the time*
oat amino acids 1 up to 0.0002% in leave on
Octacosanyl Glycol 1 not in use at the time*
Octoxynol-9 1 up to 5%
Octoxynol-10 1 up to 25% in hair bleaches
Octoxynol-11 1 up to 1%
Octoxynol-13 and -30 2 up to 2%
Octoxynol-40 1 up to 0.02%
Octoxynol-12, -16, -20, -25, -33, and -70 6 not in use at the time*
Octoxynol-9 and -20 Carboxylic Acid 2 not in use at the time*
Octyldodecanol 1 up to 85%
Octyldodecyl Benzoate 1 3 to 4%
Octyldodecyl Stearoyl Stearate 1 up to 15%
Oenothera Biennis (Evening Primrose) Oil 1 0.00002 to 58%
Olea Europaea (Olive) Fruit Oil 1 0.0005-100%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Olea Europaea (Olive) Husk Oil 1 not in use at the time*
Olea Europaea (Olive) Oil Unsaponifiables 1 0.0001-3%
Oleic Acid 1 up to 50%
Oleyl Alcohol 1 up to 62%
Oleyl Glyceryl Ether 1 not in use at the time*
Olive Acid 1 not in use at the time*
Orbignya Cohune Seed Oil 1 concentration not reported*
Orbignya Oleifera Seed Oil 1 0.0009 to 27%
Orbignya Speciosa Kernel Oil 1 0.5 to 0.9%
Oryza Sativa (Rice) Bran Oil 1 0.0003 to 78%
Oryza Sativa (Rice) Germ Oil 1 0.003 to 3%
5 not in use at the time*
Oryza Sativa (Rice) Bran Acid, Oryza Sativa (Rice) Bran Wax, Oryza Sativa (Rice)
Bran Extract,
Oryza Sativa (Rice) Bran, and Oryza Sativa (Rice) Extract
Oryza Sativa (Rice) Germ Powder and Oryza Sativa (Rice) Starch 2 up to 97%
Oryza Sativa (Rice) Seed Oil* 1 not in use at the time*
Ozokerite 1 up to 22%
Palm Acid 1 1 to 17%
Palm (Elaeis Guineensis) Oil (aka Elaeis Guineensis (Palm) Oil) 0 up to 2%
Palmitic Acid 1 up to 25%
Palm Kernel Acid 1 0.2 to 12%
Palm Kernel (Elaeis Guineensis) Oil (aka Elaeis Guineensis (Palm) Kernel Oil) 0 up to 25%
Panthenol 1 up to 25%
Pantothenic Acid 1 not in use at the time*
Paraffin (Fossil and Synthetic Waxes) 1 0.03 to 99%
Passiflora Edulis Seed Oil 1 0.0007 to 3%
Peanut Acid and Peanut Glycerides 1 not in use at the time*
Peanut (Arachis Hypogaea) Oil (aka Arachis Hypogaea (Peanut) Oil) 1 up to 25%
PEG≥40 because PEGs of various chain lengths are likely to be added to the
International Cosmetic Ingredient Dictionary and Handbook in the
future, the CIR Expert Panel concluded that any PEG≥4 would be
safe for use in cosmetics
PEG-4 1 0.01 to 67%
PEG-6 1 0.01 to 51%
PEG-7, -16, -18, -33, -45, -55, -60, -80, -100, -135, -200, -500, -800, -9M, -25M, -
18 not in use at the time*
65M, 115M, and 160M
PEG-8 1 0.0002 to 85%
PEG-9 1 0.02 to 0.9%
PEG-10 1 0.1 to 0.2%
PEG-12 1 0.04 to 56%
PEG-14 1 0.1 to 2%
PEG-20 1 0.02 to 27%
PEG-32 1 0.03 to 15%
PEG-40 1 0.001 to 6%
PEG-75 1 0.2 to 36%
PEG-90 1 0.05 to 21%
PEG-150 1 0.0009 to 6%
PEG-180 1 0.05 to 5%
PEG-220 1 0.3 to 0.4%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
PEG-240 1 4 to 10%
PEG-350, PEG-450 2 1%
PEG-400 1 1 to 3%
PEG-2M 1 0.5%
PEG-5M 1 0.01%
PEG-7M, PEG-14M 2 0.05 to 0.5%
PEG-20M 1 0.4 to 3%
PEG-23M 1 0.05%
PEG-45M 1 0.03 to 0.3%
PEG-90M 1 0.0002 to 0.3%
PEG-180M 1 0.05 to 0.08%
PEG-2 Diisononanoate 1 2%
PEG-3 Dimethicone, PEG-9 Dimethicone, PEG-14 Dimethicone, and PEG-17
4 not in use at the time*
Dimethicone
PEG-7 Dimethicone 1 2%
PEG-8 Dimethicone 1 up to 1%
PEG-10 Dimethicone 1 21%
PEG-12 Dimethicone 1 up to 4%
PEG-2, -3, -4, -6, -8, -12, -20, -32, -50, -150 Distearate 10 up to 5%
PEG-9, -75, -120, -175 Distearate 4 not in use at the time*
PEG-5, -10, -70 Hydrogenated Lanolin 3 not in use at the time*
PEG-20, -24, -30 Hydrogenated Lanolin 3 up to 5%
PEG-5 Isononanoate 1 1%
PEG-5, -24, -25, -100 Lanolin 4 up to 5%
PEG-10, -35, -55, -150 Lanolin 4 not in use at the time*
PEG-20, -60, -85 Lanolin 3 up to 10%
PEG-27, -40, -50 Lanolin 3 up to 5%
PEG-30 Lanolin 1 up to 1%
PEG-75 Lanolin 1 up to 25%
PEG-75 Lanolin Oil 1 up to 5%
PEG-75 Lanolin Wax 1 not in use at the time*
PEG/PPG-4/12 Dimethicone and PEG/PPG-14/4 Dimethicone 2 up to 1%
PEG/PPG-17/18 Dimethicone 1 up to 0.2%
PEG/PPG-18/18 Dimethicone 1 up to 10%
PEG/PPG-20/6 Dimethicone 1 up to 0.3%
PEG/PPG-20/15 Dimethicone 1 up to 0.08%
PEG/PPG-22/23 Dimethicone 1 0.005%
13 concentration not reported*
PEG/PPG-3/10 Dimethicone, PEG/PPG-6/11 Dimethicone, PEG/PPG-8/14
Dimethicone,
PEG/PPG-15/15 Dimethicone, PEG/PPG-16/2 Dimethicone, PEG/PPG-19/19
Dimethicone,
PEG/PPG-20/20 Dimethicone, PEG/PPG-20/23 Dimethicone, PEG/PPG-20/29
Dimethicone,
PEG/PPG-22/24 Dimethicone, PEG/PPG-23/6 Dimethicone, PEG/PPG-25/25
Dimethicone,
and PEG/PPG-27/27 Dimethicone
PEG-10 Propylene Glycol, PEG-75 and -120 Propylene Glycol Stearate 3 not in use at the time*
PEG-8 Propylene Glycol Cocoate and PEG-25 Propylene Glycol Stearate 2 1 to 5%
PEG-55 Propylene Glycol Oleate 1 up to 10%
PEG-6, and -8 Sorbitan Beeswax 2 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
PEG-20 Sorbitan Beeswax 1 up to 11%
18 not in use at the time*
PEG-20 Sorbitan Cocoate, PEG-40 Sorbitan Diisostearate, PEG-2, and
-5 Sorbitan Isostearate, PEG-40 Sorbitan Laurate, PEG-3 and -6 Sorbitan Oleate,
PEG-80 Sorbitan Palmitate, PEG-40 Sorbitan Perisostearate, PEG-3, -6, -40, and -60
Sorbitan Stearate,
PEG-20 and -30 Sorbitan Tetraoleate PEG-60 Sorbitan Tetrastearate, PEG-20
Sorbitan Triisostearate, and PEG-18 Sorbitan Trioleate
PEG-20 Sorbitan Isostearate 1 1 to 10%
PEG-40 Sorbitan Lanolate 1 0.1 to 1%
PEG-75 Sorbitan Lanolate 1 up to 10%
PEG-10 Sorbitan Laurate 1 ≤10%
PEG-44 and -75 Sorbitan Laurate 2 1 to 5%
PEG-80 Sorbitan Laurate 1 concentration not reported*
PEG-40 Sorbitan Peroleate 1 up to 25%
PEG-40 and -60 Sorbitan Tetraoleate and PEG-160 Sorbitan Triisostearate 3 0.5 to 10%
PEG-40 and -50 Sorbitol Hexaoleate (aka Sorbeth-40 and -50 Hexaoleate) 2 concentration not reported*
PEG-30 Sorbitol Tetraoleate Laurate (aka Sorbeth Tetraoleate Laurate) 1 concentration not reported*
PEG-60 Sorbitol Tetrastearate (aka Sorbeth Tetrastearate) 1 concentration not reported*
PEG-5, -10, and -25 Soy Sterol 3 up to 2%
PEG-16 Soy Sterol 1 up to 0.5%
PEG-30 and -40 Soy Sterol 2 not in use at the time*
PEG-2 Stearate 1 up to 2%
PEG-8 Stearate 1 up to 3%
PEG-12 Stearate 1 up to 1%
PEG-20 Stearate 1 up to 4%
PEG-40 Stearate 1 up to 7%
PEG-50 Stearate 1 up to 9%
PEG-5, -10, -30, -55, -75, -90 Stearate 6 concentration not reported*
PEG-6, -32, -100, -150 Stearate 4 up to 6%
Pelargonic Acid (aka nonanoic acid) 1 not in use at the time*
Pentaerythrityl Cocoate 1 concentration not reported*
Pentaerythrityl Tetrabehenate 1 2 to 3%
Pentaerythrityl Tetrabehenate/Benzoate/Ethylhexanoate 1 0.5 to 16%
Pentaerythrityl Tetrabenzoate 1 not in use at the time*
Pentaerythrityl Tetra C5-9 Acid Esters 1 not in use at the time*
Pentaerythrityl Tetra C5-10 Acid Esters 1 not in use at the time*
Pentaerythrityl Tetracaprylate/Tetracaprate 1 0.07 to 5%
Pentaerythrityl Tetracocoate 1 not in use at the time*
Pentaerythrityl Tetraethylhexanoate 1 0.06 to 50%
Pentaerythrityl Tetraethylhexanoate/Benzoate 1 40%
Pentaerythrityl Tetraisononanoate 1 not in use at the time*
Pentaerythrityl Tetraisostearate 1 0.1 to 55%
Pentaerythrityl Tetramyristate 1 not in use at the time*
Pentaerythrityl Tetraoleate 1 not in use at the time*
Pentaerythrityl Tetrapelargonate 1 2%
Pentaerythrityl Tetrastearate 1 7%
Pentasodium Pentetate 1 up to 3%
Pentetic Acid 1 up to 0.03%
Pentylene Glycol 1 0.001 to 5%
Perilla Ocymoides Seed Oil 1 concentration not reported*
Persea Gratissima (Avocado) Butter 1 concentration not reported*
Persea Gratissima (Avocado) Oil (aka Avocado Oil) 0 0.0001 to 98%
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
Ingredient#"As used" concentration for safe as used conclusion
Persea Gratissima (Avocado) Oil Unsaponifiables 1 0.2 to 6%
Petroleum Distillates (aka Petroleum Distillate) 1 up to 82%
Phenoxyethanol 1 ≤0.0002 to 1%
Phenylalanine 1 0.00009 to 0.03% in leave on; 0.0004 to 0.0008% in rinse off p-Phenylenediamine*** 1 up to 0.04%
p-Phenylenediamine HCl and p-Phenylenediamine Sulfate 2 not in use at the time*
Phenyl Methyl Pyrazolone 1 up to 1%
Phenyl Trimethicone 1 up to 36%
Phytantriol 1 up to 3%
Phytosteryl Nonanoate 1 not in use at the time*
Picramic Acid 1 up to 0.6%
Pistacia Vera Seed Oil 1 0.003 to 1%
Plukenetia Volubilis Seed Oil 1 0.05 to 0.6%
Poloxamer 105 1 up to 3%
Poloxamer 181 and 182 2 up to 6%
Poloxamer 184 and 234 2 up to 10%
Poloxamer 185 1 up to 9%
Poloxamer 188 and 212 2 up to 2%
Poloxamer 217, 237, 238, 335, 338, and 401 6 concentration not reported*
Poloxamer 333 1 1%
Poloxamer 334 1 0.3%
Poloxamer 407 1 up to 20%
Poloxamer 101, 108, 122, 123, 124, 183, 215, 231, 235, 282, 284, 288, 331, 402,
17 not in use at the time*
403, Poloxamer 105 Benzoate and Poloxamer 182 Dibenzoate
Polyamino Sugar Condensate 1 up to 1%
Polybutene 1 up to 92%
polybutylene terephthalate 1 up to 12% in leave ons
Polyethylene 1 0.09 to 24%
polyethylene isoterephthalate 1 up to 0.5% in leave ons
polyethylene terephthalate 1 Concentration not reported*
Polyglyceryl-20 Octaisononanoate 1 3%
Polyisobutene 1 up to 76%
Polymethyl Methacrylate (PMMA) 1 up to 30%
polypentaerythrityl terephthalate 1 not in use at the time*
polypropylene terephthalate 1 Concentration not reported*
Polyquaternium-7 1 up to 0.4%
Polyquaternium-10 1 up to 5%
Polyquaternium-11 1 up to 10%
Polysorbate-20, -85 2 > 50%
Polysorbate-21 1 up to 1%
Polysorbate-40 1 up to 10%
Polysorbate-60, -80 2 up to 25%
Polysorbate-61, -65, -81 3 up to 5%
Polyvinyl Acetate 1 <25% of 55-60% emulsion
Polyvinyl Alcohol 1 up to 10%
Potassium Acetate 1 3%
Potassium Ascorbyl Tocopheryl Phosphate 1 ≤0.02%
Potassium Babassuate 1 not in use at the time*
Potassium Benzoate 1 0.0002 to 0.0003%
Potassium Butyl Ester of PVM/MA Copolymer 1 not in use at the time*
Potassium Cellulose Succinate 1 not in use at the time*
Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
国家安全审查之目的在于,维护本国的,经济安全、国防安全进而保护国家主权,维护国家利益。其审查的内容包括四个方面,即对国家安全的影响,对国家经济运行的影响,对社会基本生活秩序的影响,以及对涉及国家安全关键技术研发能力的影响。本文以凯雷收购徐工案和FAG并购西北轴承为例,研究外资并购中国家安全问题。 案例:凯雷收购徐工案 徐工案是外资并购我国国有企业典型的案例,本案并购方为美国美国凯雷投资集团,它是全球最大的私募股权投资基金(PE)之一。而徐工集团是我国生产大型装备制造业的公司,也是我国工程装备制造业的龙头企业。对该企业的并购是否危及我国装备制造业的产业安全,进而危及我国经济安全国家安全是整个案件讨论的焦点。 2005年10月25日,凯雷集团准备以3.75亿美元的价格收购徐工工程机械有限公司85%的股权,并签署战略投资协议。这一行为引发了国家有关部委和舆论的普遍关注。 2006年7月商务部在审查徐工集团的并购方案后,提出了20个问题,并要求徐工补充相应的材料。这些问题主要表现为,凯雷收购徐工的动机、收购后的安排、凯雷集团是否涉及美国军事产业、效益完成后中方控制力、是否涉及业绩对赌条款等。 随后调查得出,凯雷作为私募(PE)并不具备运营重型机械的能力,其目的在于转卖徐工集团。转卖的后手是卡特彼勒公司。经过细致的调查,卡特彼勒公司被认定在中国重型工程机械行业进行着战略性并购侵蚀活动。进而国家有关部委叫停凯雷收购徐工案。 徐工案发生在国有企业改制时期,一方面,通过外资并购可以获得大量的资金和技术,促进国有企业改制升级。另一方面外资可能利用我国并购法律不完善,资产评估股权作价不完善,以较低的并购价格收购我国不同行业的龙头企业,对我国国家安全造成不利的影响。 随着我国经济的发展和对外开放的进一步扩大,外资并购我国国内企业的数量也在不断增长,可是外资并购犹如一把双刃剑,它既有利于国内企业引进资金、技术、先进管理经验,也有可能带来限制竞争、滥用市场支配地位等。而且有的外资在并购国内企业时采用“空手套白狼”的模式,甚至以“退货”的方式抛弃所并购企业,为了保障国内企业的利益我国法律规定了在外资并购境内企业时应对其进行反垄断审查,若涉及国家安全的还应接受由商务部、发改委等部委共同负责的国家安全审查。 一、外资并购的反垄断审查与国家安全审查的联系
国外化妆品标签要求 https://www.doczj.com/doc/0317238324.html, 2004-3-3 一、美国对化妆品标签的要求 美国联邦食品和药品管理局(FDA)依据1938年颁布的“食品、药品和化妆品法”以及“商品包装和标签法”,颁布了对化妆品标签的严格而明确的规定。所有市场上销售的化妆品必须符合规定,这是评价化妆品是否合格的主要依据,也是对化妆品进行管理的主要内容。凡标签不符合要求的化妆品禁止在市场上销售。现将对化妆品标签的要求摘录如下: (一)对标签内容的要求 1.在标签的主显示面 主显示面系指在通常销售的情况下最容易看到的标签标识的正面。在该标签的主显示面必须注明下列各项: (1)产品名称。 (2)鉴别项目:包括描述名称或说明产品性质或用途。 (3)内容物准确的净重:固体、半固体或粘稠状化妆品重量以磅和盎司表示;液体以美国加仑、夸脱、品脱和液体盎司为单位表示。净重也可另外再以公制系统表示。 (4)警告:如果产品的安全性未经充分检测,就需注明:本产品的安全性未经确定。 2.在标签的信息说明面 通常在包装的侧面和背面,需注明以下项目: (1)生产厂或将产品投放州间贸易的经销商名称和地址。地址必须包括街道、城市、州和邮政编码,如果经销商不是生产厂或批发商,则必须在标签中按规定的语句说明。 (2)产品成分:在美国,要求在供个人使用的零售化妆品标签上注明成分。但是对于在专业机构用于专业人员的发用产品或化妆品以用在工作场所供个人使用的清洁、护肤产品,如果不将其销售给消费者作为家庭使用时可以不需注明成分。 注明成分必须使用英语。成分的排列顺序是根据其用量和主要用途决定的。属于药品的化妆品,需把活性药品成分排在化妆品成分之前。色素和含量小于或等于百分之一的成分可不考虑用量次序排列。通常排列次序为:活性药品成分、用量小于等到于1%的成分、色素、其他成分。注明成分时对所使用的成分名称需采用法定名称。经美国联邦食品和药品管理局(FDA)同意不需注明的成分可以注明为“其他成分”。(3)警告:美国联邦食品和药品管理局(FDA)要求在某些产品标签上标注法规规定的警告和注意事项,如气溶胶产品、女用除臭喷雾剂、儿童用泡沫浴产品等。 (4)其他警告:为防止对人体健康造成危害,确保使用安全,有的产品还应该有其他需要注明的警告。(5)安全使用指南:有些产品当被消费者误用时可能带来危害,美国联邦食品和药品管理局(FDA)要求对于这些产品除需标注“警告”外,尚需说明安全使用方法,以指导消费者正确和安全地使用。 (6)含煤焦油染料的染发剂需按法规规定说明注意事项和斑贴试验方法。
美国国家安全法 (1947年7月26日) 本法旨在促进国家安全。规定了国防部长、国家军事体制、陆军部、海军部和空军部的条款,调整国家军事体制和有关国家安全的政府各部、局的活动。 经美利坚合众国参议院和众议院通过,成为法律。 简短的标题 〔美国法典第50卷第401章〕本法可称为《1947年国家安全法》 目录 第2条政策宣言 第一章国家安全的协调 第101条国家安全的协调 第102条中央情报局 第103条国家安全资料委员会 第二章国防部 第201条国防部 第202条国防部长 第203条国防部长军事助理 第204条文职人员 第205条陆军部 第206条海军部 第207条空军部 第208条美国空军 第209条调动有效日期 第210条战争委员会 第211条参谋长联席会议 第212条联合参谋 第213条军火局
第214条研究与发展委员会 第三章杂项 第301条部长薪金 第302条次长与助理次长 第303条顾问委员会与成员 第304条被调文职人员的身份 第305条保留条款 第306条转帐 第307条经费使用的授权 第308条定义 第309条可分性 第310条有效日期 第311条总统职务的接续 第411条废除与保留条款 第五章情报活动的责任 第501条国会监督 第六章保护某些国家安全的情报 第601条美国间谍、情报员及其它来源的秘密情报的保护 第602条辩护与例外 第603条报告 第604条治外法权 第605条向国会提供情报 第606条定义 政策宣言 第2条〔50美国法典401〕国会制订本法的目的是为美国未来的安全提供一个全面的纲领性文件;确定与国家安全有关的政策和手段的统一体制以及各部、局、政府的职能;设立包括陆军部、海军部(包括海军航空兵和美国海军陆战队)、空军部三个军事部的国防部,并隶属于国防部长的领导、管辖与控制;确立每一个军事部在各部长的领导下成为独立的组织,并在国防部
金家律师修订 本协议或合同的条款设置建立在特定项目的基础上,仅供参考。实践中,需要根据双方实际的合作方式、项目内容、权 利义务等,修改或重新拟定条款。本文为Word格式,可直接使用、编辑或修改 化妆品原料采购合同 供方:__________________________________________ 需方:__________________________________________ 供需双方依据《中华人民共和国合同法》、《中华人民共和国产品质量法》、及其他有关法 律、法规的规定,在平等、自愿、协商一致的基础上,就原材料的采购事宜,订立本合同。 第一条产品明细。 第二条产品质量要求 1.产品质量标准,按国家标准、行业标准或双方约定标准中最高标准执行。(约定标准) 2.保质期限: 供方所提供的产品,保质期限不少于____个月(自产品出厂之日起计算)。 第三条产品包装 1.包装要求:在外包装上应注明产品名称、规格、配料、产品批准号、生产厂名、地址、 生产日期、保质期、净含量、贮藏要求等。
2.包装物由_______方提供,费用由__ ______方承担。 3.包装物处理按下列第_________项执行。 3.1 包装物不回收。 3.2 包装物由供方回收。 第四条交提货方式 交提货方式,按下列第_________项履行。 1.供方送货:产品运输由供方负责办理,供方送至_________,运费由供方承担。 2.供方代办运输:供方根据双方约定的协议代办运输,运费由需方承担(代办运输协议另订附件)。 3.需方自提:需方前往供方指定地点_________,自行提货、自行运输。 第五条验收方法 1.供方提供的每一批产品都须附随根据双方确认的质量标准进行检验的检验合格报告,但若有下列情况之一,必须附随计量认证合格的检验机构出具的检验合格报告。 1.1 供方首次向需方所销售的产品(不包括同品牌不同规格的产品); 1. 2 供方停产一个生产周期又恢复生产的产品(一个生产周期为__个月); 1.3 供方所供产品,在生产制造时工艺及原辅材料有较大改变的; 1.4 其他。 2.产品到达目的地后,需方应指定专人按双方约定的质量标准(应包括主要卫生指标、内外包装、保质期、标识感官等)及时进行抽样检验,完成验收程序。如有质量问题,需方应在收货后一个月内书面通知供方,同协商处理办法。如对产品质量认定发生异议,由需方所在地供需双方共同认可的经计量认证的机构检验报告为准。 3.需方根据合同规定或要货通知单及供方送货单做好收货工作,并及时通知质量验收员验收产品。 第六条付款方式 需方选择下述第_______种方式付款,并按该方式所定时间如期足额将货款支付给供方。 1.一次性付款方式:日支付全部货款,计人民币_______元。 2.分期付款方式: 2.1 签署本合同时,支付货款全部的_________%,计人民币_________元, 2.2 日前支付货款全部的______%,计人民币_________元。
外资并购国家安全审查三篇 随着我国经济的发展和对外开放的进1步扩大,外资并购我国国内企业的数量也在不断增长,可是外资并购犹如1把双刃剑,它既有益于国内企业引进资金、技术、先进管理经验,也有可能带来限制竞争、滥用市场安排地位等。本站今天为大家精心准备了,希望对大家有所帮助!外资并购国家安全审查1国家安全审查之目的在于,保护本国的,经济安全、国防安全进而保护国家主权,保护国家利益。其审查的内容包括4个方面,即对国家安全的影响,对国家经济运行的影响,对社会基本生活秩序的影响,和对触及国家安全关键技术研发能力的影响。本文以凯雷收购徐工案和FAG并购西北轴承为例,研究外资并购中国家安全问题。 案例:凯雷收购徐工案 徐工案是外资并购我国国有企业典型的案例,本案并购方为美国美国凯雷投资团体,它是全球最大的私募股权投资基金(PE)之1。而徐工团体是我国生产大型设备制造业的公司,也是我国工程设备制造业的龙头企业。对该企业的并购是不是危及我国设备制造业的产业安全,进而危及我国经济安全国家安全是全部案件讨论的焦点。 2005年10月25日,凯雷团体准备以3.75亿美元的价格收购徐工工程机械有限公司85%的股权,并签署战略投资协议。这1行动引发了国家有关部委和舆论的普遍关注。 2006年7月商务部在审查徐工团体的并购方案后,提出了20个问题,并要求徐工补充相应的材料。这些问题主要表现为,凯雷收购徐工的动机、收购后的安排、凯雷团体是不是触及美国军事产业、效益完成后中方控制力、是不是触及事迹对赌条款等。
随后调查得出,凯雷作为私募(PE)其实不具有运营重型机械的能力,其目的在于转卖徐工团体。转卖的后手是卡特彼勒公司。经过细致的调查,卡特彼勒公司被认定在中国重型工程机械行业进行着战略性并购腐蚀活动。进而国家有关部委叫停凯雷收购徐工案。 徐工案产生在国有企业改制时期,1方面,通过外资并购可以取得大量的资金和技术,增进国有企业改制升级。另外一方面外资可能利用我国并购法律不完善,资产评估股权作价不完善,以较低的并购价格收购我国不同行业的龙头企业,对我国国家安全造成不利的影响。 外资并购国家安全审查2随着我国经济的发展和对外开放的进1步扩大,外资并购我国国内企业的数量也在不断增长,可是外资并购犹如1把双刃剑,它既有益于国内企业引进资金、技术、先进管理经验,也有可能带来限制竞争、滥用市场安排地位等。而且有的外资在并购国内企业时采取“空手套白狼”的模式,乃至以“退货”的方式抛弃所并购企业,为了保障国内企业的利益我国法律规定了在外资并购境内企业时应对其进行反垄断审查,若触及国家安全的还应接受由商务部、发改委等部委共同负责的国家安全审查。 1、外资并购的反垄断审查与国家安全审查的联系 (1)两种审查均对同1项商业活动,即都是对外资并购境内企业进行审查。 虽然反垄断审查旨在避免经营者集中、垄断市场,而国家安全审查旨在保护国家经济安全,二者审查的具体内容不相同,但二者审查实质上都是对外资并购境内企业进行审查。 (2)两种审查均触及政府部门。 我国《反垄断法》第21条规定:“经营者集中到达国务院
化妆品监管法规 一、我国化妆品监管法律体系现状 目前,我国关于化妆品监管方面的法律主要有:1993年施行2000年修订的《产品质量法》、1989年施行的《标准化法》、1994年施行的《消费者权益保护法》等。行政法规主要有:1990年施行的《化妆品卫生监督条例》、2005年施行的《工业产品生产许可证管理条例》等。地方性法规、规章主要有:卫生部的《化妆品卫生监督条例实施细则》、国家质量监督检验检疫总局的《化妆品标识管理规定》、国家出入境检验检疫局的《进出口化妆品监督检验管理办法》、国家工商行政管理局的《化妆品广告管理办法》、《四川省化妆品卫生监督管理办法》等。 二、我国化妆品监管法律体系存在的问题 首先,缺乏专门立法。化妆品关系到人体健康,具有风险性,是特殊产品,因此,对其监管立法也应要求有较高的专业性。但截至目前,我国还没有出台一部专门规范化妆品监督管理的法律,而是与普通产品一样受《产品质量法》的调整。专业性较强的法规《化妆品卫生监督条例》至今实施已有20余年了,虽然是行政法规,但却是以卫生部令的形式颁布,与《立法法》关于行政法规的要求不相适应。《化妆品卫生监督条例》的内容总共只有三十五条,其中关于化妆品概念的规定过于笼统,化妆品风险监测与评估、安全事故处置、广告监管、不合格化妆品召回等许多重要制度都没有涉及,或散落于其他规章或规范性文件中,对化妆品违法行为的责任追究力度太轻,起不到应有的震慑作用。 其次,监管职能交叉。我国目前施行的是化妆品卫生监督与化妆品质量监督并行的监管体制。化妆品质量监管由质量技术监督、出入境检验检疫和工商行政管理部门负责,化妆品卫生监管先后由卫生部门和食品药品监管部门负责。一个生产化妆品的企业要同时获得化妆品卫生监督部门的《化妆品生产卫生许可证》和质量技术监督部门的《生产许可证》,生产特殊用途的化妆品还需要取得化妆品卫生监督部门的批准文号。化妆品的标准既有卫生标准,还有质量标准。这种监管体制既造成了监管资源、检验资源的重复利用和浪费,也给化妆品生产企业带来了诸多不便。 三、建立健全我国化妆品监管法规体系的建议 首先,将“化妆品卫生”与“化妆品质量”统一为“化妆品安全”,为化妆品监管立法指明方
布什新国家安全战略的主要内容 一.确立了美国国家安全最主要的任务:打击恐怖主义和防止大规模毁灭性武器的扩散。 布什在《报告》中明确指出:“我们所面临的严重威胁是激进主义和技术的结合”。“9·11”事件表明,即使没有装备大规模毁灭性武器的恐怖分子,利用飞机作为武器对美国发动袭击,就对美国造成了巨大的创伤,如果恐怖分子掌握了大规模毁灭性武器,将对美国的国家安全造成极其严重的威胁。“当生、化和核武器随着弹道导弹技术一起扩散时,即使弱国和小的团体也能够获得对大国进行灾难性打击的能力”。布什政府认为,“据信伊拉克拥有化学武器,并计划获得核武器和生物制剂;朝鲜已经成为世界上主要的弹道导弹生产商,并在积极的发展自己的大规模毁灭性武器;其他的…无赖国家?也在寻求核、生、化武器”。 为了击败恐怖分子对美国威胁,美国必须使用所拥有的各种手段——军事力量、国土防御能力、司法制度、情报以及积极的措施。“我们反恐的重点次序是:破坏和摧毁全球的恐怖组织,并且打击它们的领导人,破坏它们的指挥、控制和通信,切断对它们的物资供应,断绝它们的财源。这样就能做到瘫痪恐怖分子的计划和他们实施恐怖行动的能力。” 另外,布什强调,对待恐怖分子和“无赖国家”,美国“要
在威胁真正形成之前将其摧毁,并将这种思想作为一种共识和自卫的手段”。要求美国不能再像过去那样仅采取“反应”的姿态,要在威胁形成之前对它们进行先发制人的打击,不能让敌人先发制人。 二.通过与世界大国保持良好的关系以保持和平 “9·11”事件发生后,美国在阿富汗发动了打击基地组织和塔利班的军事行动,美国在阿富汗反恐战争的胜利与盟国及其他国家如俄罗斯、中国的支持和帮助是分不开的。布什政府认识到,美国要完成国家安全的主要任务——打击恐怖主义和大规模毁灭性武器的扩散,需要得到世界主要大国的支持,为了维护美国的国家安全,必须与世界大国保持良好的关系。 《报告》指出,美国和俄罗斯的战略利益在许多领域都已经交叠在一起,美俄之间已经建立了一种新的战略关系,美俄将通过新的关系框架摆脱冷战的敌意,抛弃相互确保摧毁的旧观念,建立一种持久的战略伙伴关系。对于中国,布什政府认为,“美中关系是我们战略中一个重要的部分。我们欢迎一个强大、和平而繁荣的中国的出现。”中美之间尽管存在很多分歧,但是在目前的反恐战争和推动朝鲜半岛稳定等方面进行了非常好的合作,而且中美之间也存在许多共同的利益和挑战。而同样是地区大国的印度,美国认为,美印之间有着良好关系,两国有着共同的利益并在维护地区稳
日本化妆品管理规定 2009年11月21日 日本化妆品管理规定 日本对化妆品进行管理的法规主要是日本厚生劳动省颁布的《药事法》(The pharmaceutical affairs law),但是涉及化学物质管理、容器生产和销售等法律也必须遵守。2001年之前,日本对化妆品和医药部外品都实行审批制,2001年日本《药事法》进行了修订,自2001年4月1日起取消了对化妆品的审批。 厚生省负责全国的化妆品管理工作。厚生省内的医药安全局具体负责化妆品的管理,该局设有审查管理课、安全对策课、监督指导课等7个处室。地方政府的生局负责化妆品的监督执法工作,其工作任务是:①对生产经营场所监督检查:②对无证企业的管制;③进口化妆品的监管;④广告宣传的监督。全国有3500名监督员,监督员90%是药剂师,分布在地方卫生局。 1 化妆品分类 化妆品在日本被分为两类,一类叫“化妆品”(cosmetics),类似于我国所称的普通化妆品,包括香皂、洗发香波、护发素、雪花膏、化妆水、彩妆化妆品、牙膏等;另一类被称为“医药部外品”(quasi—drugs),类似于我国所称的特殊用途化妆品,包括药皂、去屑 洗发香波、药用牙膏、染发剂、烫发剂、生发剂等。 2 监督管理简介 2.1 化妆品 2.1.1 日本对化妆品不实行审批制,企业按照政府的有关规定自行规范自己的生产行为,企业对产品的质量和安全性负全部责任。但企业在生产任何新产品之前,必须向当地卫生部门备案(仅备案产品名称),进口商进口新化妆品则要求进口商向当地卫生部门备案。企业对产品安全性负全部责任。 2.1.2 对于化妆品生产所使用的原料,厚生劳动省将其分为两类来管理,第一类原料是“化妆品使用的防腐剂、紫外线吸收剂和焦油色素”,另一类是“除防腐剂、紫外线吸收剂和焦油色素之外的其他化妆品原料”。对于第一类原料,厚生劳动省发布“许可原料名单”,企业生产化妆品要使用此类原料时只能使用名单之内的原料,使用名单之外的原料必须经过审批。对于第二类原料,厚生劳动省发布“化妆品禁止使用成分和限制使用成分名单”,企业生产化妆品不得使用禁用物质,选用限用物质必须符合限用标准(包括浓度、用途、规格等),
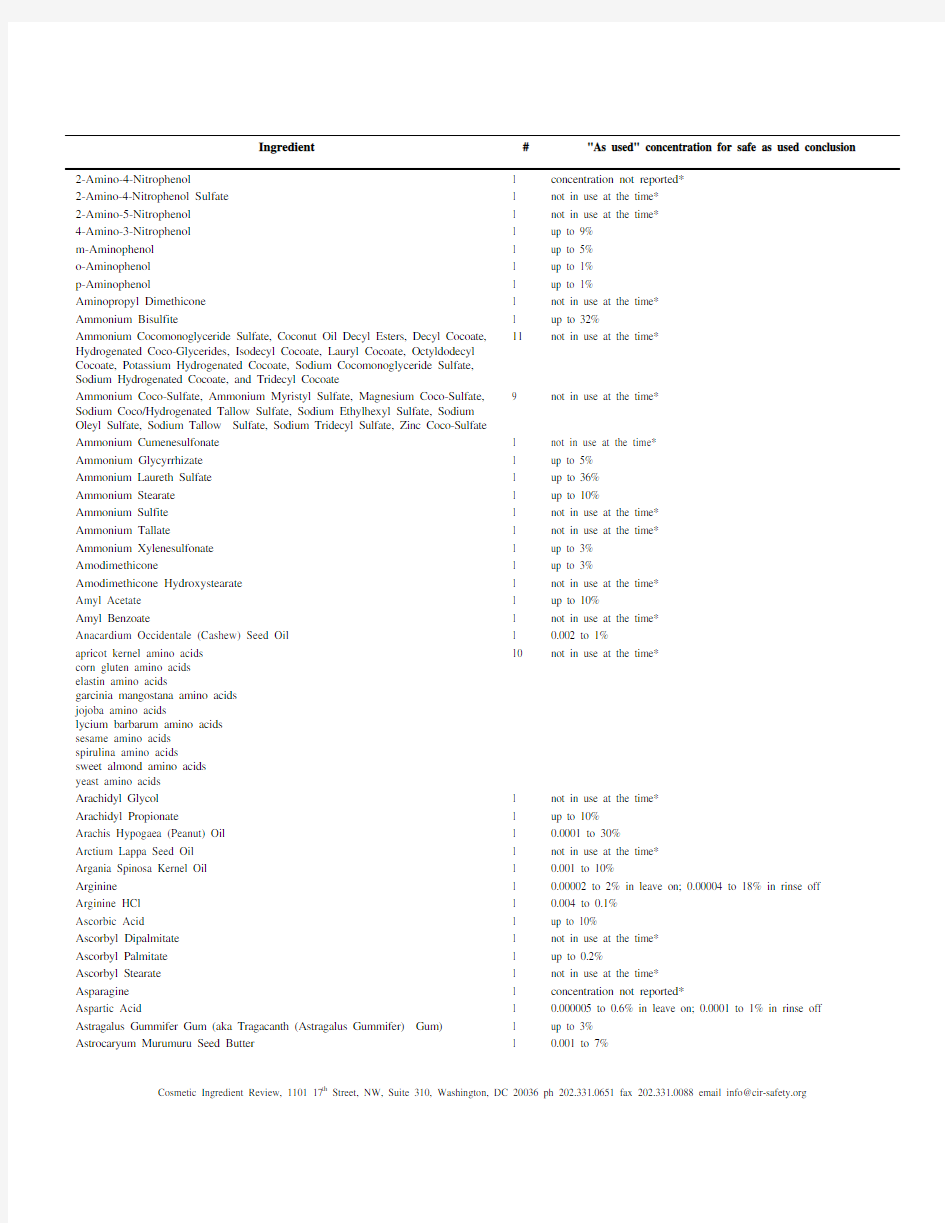
Cosmetic ingredients found safe as used (1513 total, through June, 2013) Ingredient#"As used" concentration for safe as used conclusion Acacia Senegal Gum and Acacia Senegal Gum Extract 2 up to 9% Acetic Acid 1 up to 0.3% Acetylated Lanolin 1 up to 7% Acetylated Lanolin Alcohol 1 up to 16% Acetyl Tributyl Citrate and Acetyl Triethyl Citrate 2 up to 7% Acetyl Trihexyl Citrate 1 not in use at the time* Acetyl Trioctyl Citrate 1 not in use at the time* Acrylates/Dimethiconol Acrylate Copolymer (Dimethiconol and its Esters and 1 up to 0.5% Reaction Products) Actinidia Chinensis (Kiwi) Seed Oil 1 up to 0.1% Adansonia Digitata Oil 1 up to 0.01% Adansonia Digitata Seed Oil 1 not in use at the time* Adipic Acid (Dicarboxylic Acids and their Salts and Esters) 1 0.000001% in leave on; 18% in rinse off adipic acid/1,4 butanediol/terephthalate copolymer 1 not in use at the time* Alanine 1 3 x 10-7 to 0.1% 4 up to 99% Alcohol Denat. denatured with t-Butyl Alcohol, Denatonium Benzoate, Diethyl Phthalate, or Methyl Alcohol Aleurites Moluccanus Bakoly Seed Oil 1 not in use at the time* Aleurities Moluccana Seed Oil 1 0.00001 to 5% Allantoin 1 up to 2% Allantoin Ascorbate 1 up to 0.05% Allantoin Biotin and Allantoin Galacturonic Acid 2 not in use at the time* 3 concentration not reported* Allantoin Glycyrrhetinic Acid, Allantoin Panthenol, and Allantoin Polygalacturonic Acid Almond Meal (aka- Prunus Amygdalus Dulcis (Sweet Almond) Seed Meal) 0 up to 76% Alumnina Magnesium Metasilicate 1 up to 0.01% Aluminum Calcium Silicate 1 up to 6% Aluminum Citrate 1 concentration not reported* Aluminum Dimyristate 1 up to 3% Aluminum Distearate 1 up to 5% Aluminum Iron Silicates 1 not in use at the time* 13 not in use at the time* Aluminum Isostearates/Myristates, Calcium Myristate, Decyl Myristate, Ethylhexyl Myristate, Ethyl Myristate, Glyceryl Dimyristate, Glyceryl Isostearate/Myristate, Isostearyl Myristate, Isotridecyl Myristate, Methyl Myristate, Oleyl Myristate, Tetradecyloctadecyl Myristate, Tridecyl Myristate Aluminum Myristate 1 up to 1% Alumnium Myristates/Palmitates 1 up to 6% Aluminum Silicate 1 up to 37% in dentifrices; up to 3% in other uses Aluminum Stearate 1 up to 8% Aluminum Tristearate 1 up to 10% Amaranthus Hypochondriacus Seed Oil 1 not in use at the time* Amino Bispropyl Dimethicone 1 not in use at the time* 5-Amino-4-Chloro-o-Cresol 1 up to 2% in oxidative and non-oxidative hair dyes 5-Amino-6-Chloro-o-Cresol 1 up to 2% in oxidative and non-oxidative hair dyes 4-Amino-m-Cresol 1 up to 0.7% in oxidative and non-oxidative hair dyes 6-Amino-m-Cresol 1 up to 2.4% in oxidative and non-oxidative hair dyes 4-Amino-2-Hydroxytoluene 1 up to 5% Aminomethyl Propanediol 1 up to 2% Aminomethyl Propanol 1 up to 7% 2-Amino-3-Nitrophenol 1 up to 2% Cosmetic Ingredient Review, 1101 17th Street, NW, Suite 310, Washington, DC 20036 ph 202.331.0651 fax 202.331.0088 email info@https://www.doczj.com/doc/0317238324.html,
美国外国投资国家安全审查制度研究 进入21世纪以来,我国在吸收外资、经济高速发展的同时对外投资也进入了高速增长阶段。2015年我国对外直接投资达到1180.2亿美元,较上年增长14.7%,仅居美、日之后成为全球第三大对外投资国。未来几年,我国对外投资有望继续增速。我国对外投资在全球范围内的崛起既是我国经济长期持续、快速增长的结果,也是我国经济进一步发展的必然要求。 通过对外投资在全球范围内有效地利用和整合资源已成为中国经济持续发展、再上台阶的必然要求。美国目前是世界第一大经济体,是世界上最大的投资市场。能源成本相对较低,有众多受过良好教育的劳动力,有世界一流的技术和管理经验。投资美国市场,寻求国内产业结构调整所需要的核心技术,学习美国先进的管理经验,获得美国的能源、知识产权、品牌等战略资源是中国企业对外投资的必然趋势。 与此同时,在后金融危机时代美国的产业结构正向高端服务业升级,当地资 本开始从一些制造业领域流出,需要外来资本的注入,这对于中国企业进入是一 个好时机。中国企业可以通过并购等方式以较低的经济成本快速实现海外并购利益最大化。根据美国咨询机构荣鼎公司的最新统计数据,2015年中国对美直接投资达157亿美元,与2014年相比增长了30%。中国对美国的直接投资已从2000 年每年的几亿美元增加到2015年的157亿美元,增速非常惊人。 近年来,美国以国家安全为由不断地阻挠和干扰中国企业在美国的投资活动。中国企业在赴美投资的过程中遭受美国国家安全审查的案件数量越来越多,中国企业被审查的案件数量已从2005年的1件占美国当年国家安全审查案件总量的1.56%,猛增到2014年的24件占美国当年国家安全审查案件总量的16.32%。美 国的外国投资国家安全审查已经成为我国企业,特别是国有企业、高科技企业和能源企业,投资美国时面临的最大障碍和阻力。中国企业一方面需要投资美国, 另一方面又不得不面对美国的外国投资国家安全审查。 只有深入地研究美国的外国投资国家安全审查制度,才能更好的了解和应对中国企业赴美投资过程中的国家安全审查。本研究从六个部分展开。第一章从宏观方面介绍了与国家安全审查相关的理论,包括国家安全的概念、国家安全涉及的领域、国际投资的概念与分类、国际投资对投资国和东道国经济的影响、外国
The 2015 National Security Strategy 美国国家安全战略(2015版) Today, the United States is stronger and better positioned to seize the opportunities of a still new century and safeguard our interests against the risks of an insecure world. The President’s new National Security Strategy provides a vision and strategy for advancing the nation’s interests, universal values, and a rules-based international order through strong and sustainable American leadership. The strategy sets out the principles andpriorities that describe how America will lead the world toward greater peace and a new prosperity. 今天,美国更加强大,所处的形势更加有利,可以更好的把握仍然处于新世纪时代的各种机会,捍卫我们的国家利益,抵御这个不安全世界中的各种风险。总统的《国家安全战略》为利用美国强有力、可持续的领导地位促进国家利益、普世价值、基于原则的国际秩序提供了一个愿景和战略。该战略为美国领导世界走向更加和平、更加繁荣设定了原则和优先事项。并针对安全、繁荣、价值观和国际秩序这四个主要方面进行了阐述。 ?We will lead with purpose, guided by our enduring national interests and values and committed to advancing a balanced portfolio of priorities worthy of a great power. ?我们经通过清晰的目的实现领导,将以我们持久国家利益和价值为指导,致 力于维持优先事项的平衡组合,投入我们的伟大力量; ?We will lead with strength, harnessing a resurgent economy, increased energy security, an unrivaled military, and the talent and diversity of the American people. ?我们将通过强大的力量实现领导,利用好正在复兴的经济、已经改善的能源 安全和无以伦比的军事和人才实力; ?We will lead by example, upholding our values at home and our obligations abroad. ?我们要发挥典范作用实现领导,在国内发扬我们的价值、在国外履行我们的 责任; ?We will lead with capable partners, mobilizing collective action and building partner capacity to address global challenges. ?我们要通过有力的伙伴来实现领导,动员集体行动、打造伙伴能力,应对全 球挑战; ?We will lead with all instruments of U.S. power, leveraging our strategic advantages in diplomacy, development, defense, intelligence, science and technology, and more. ?我们要通过各种美国的力量工具来实现领导,发挥我们在外交、发展、国防、 情报、科技和其他方面的优势; ?We will lead with a long-term perspective, influencing the trajectory of major shifts in the security landscape today in order to secure our national interests in the future.
化妆品配料间操作规范公司标准化编码 [QQX96QT-XQQB89Q8-NQQJ6Q8-MQM9N]
配料操作规程 1. 目的 对配料工作进行标准化管理。 2.范围 此规程适合于本公司所有产品的配料工作。 3.权责 生产技术部:及时通知配料人员相应的配方,按配方要求准确称量。 生产人员:按照该工作规范操作。 4.工作规程 4.1 卫生清洁、准备工作。 4.1.1 物料清场,不得有上批次生产产品及所用原料。 4.1.2去污溶液清洁操作台、地面、配料设备、配料用具并用清水冲洗干净。 4.1.3用75%的酒精消毒配料设备、配料用具、干燥备用。 4.1.4检查配料设备是否正常,校正好计量器具。 4.1.5空间空气消毒、开紫外灯照射0.5-1个小时,关紫外灯20-30分钟后可进行配料操作。 4.2领料 4.2.1生产技术部发布生产指令后,配料主操作人员按着需配制产品的配方要求及配料量计算出所需各种原料量,并填制原料领料单。 4.2.2领料单经技术员校对所需原料名称,并进行审核确认后,凭领料单到原料仓库提领出所需原料。 4.2.3提取原料时,配料操作人员与仓库人员共同复核各种原料的重量,并记录在领料单上。 4.2.4原料外包装拆除后,由物流通道进入配料室。 4.3配料 4.3.1配料操作人员按要求更衣、帽、鞋,清洁消毒手,戴口罩进入配料室。 4.3.2严格按配料产品工艺流程要求,进行配料操作,并及时记录每一工艺操作。产品配制过程的称量由主操作人员执行,副操作人员复核。 4.3.3配料原始记录填写要求及时准确,并如实记录。配料过程的设备操作要严格按设备操作说明书进行。配料员根据配方工艺要求投料生产,按工艺要求填写相应的生产工艺表,完成一道工序立即作好记录。 4.3.4配料过程中发现异常情况要及时通知技术员。在技术员指导下正确处理异常情况,并及时记录。 4.3.5达到工艺操作要求后,操作完成后,用经过清洗的半成品贮存桶(用75%的酒精擦洗),消毒后装半成品。填写送检单,通知品质部取样检测。 4.3.6在拉入贮料间前要灌装组长、配料组长共同监秤称重制品重量,并填写《半成品入库单》。 4.4配料结束 4.4.1配料完毕,通知质检部门检验,检验合格后方可出料并贮存于储料罐中,并作好记录。 4.4.2操作完毕后需清洗机器设备。 4.4.3配料量交至生产负责人,并转交至灌装装工序。 4.4.4剩余原料办理退库手续,操作人员与仓管人员共同复核每个原料的重量,并记录在原始配料单上。 4.4.5物料及包装清场,操作现场卫生整洁。 4.4.6下班前关掉电源,打扫车间内卫生。
《美国外国投资委员会国家安全审查指南》 (财政部于2008年12月8日颁布, 联邦纪事第73卷, 第236号) 译者:张力行﹑黎军 一﹑指南的立法要求 《2007年外国投资与国家安全法》(以下简称“FINSA”)对《1950年国防生产法》第721节(以下简称“721节”)(美国联邦法规汇编,第50卷,第2170页)做了修订。根据第721节(b)(2) (E)的要求,美国财政部作为美国外国投资委员会(以下简称“CFIUS”)的主席,须就CFIUS审查过的和引起国家安全考虑的交易类型颁布如下《国家安全审查指南》。1 本指南将从如下三个部分,对CFIUS的外资审查程序的目的和性质做一个概述。但是, 本指南不应为任何人设立或赋予任何权利,也应不对美国政府构成约束。 如有任何问题, 可以同美国商务部的官员联络, 联络方式如下: Nova Daly,副助理部长 电话:(202)622-2752 电邮:Nova.Daly@https://www.doczj.com/doc/0317238324.html, Welby Leama,高级顾问 电话:(202)622-0099 电邮: Welby.Leaman@https://www.doczj.com/doc/0317238324.html,. 地址:美国财政部,宾夕法尼亚大道1500号, NW., 华盛顿特区,邮编:20220 二﹑CFIUS审查程序的目的和性质 1. CFIUS审查程序的目的 欢迎外国投资是美国的一项长期承诺。2007年5月,布什总统所作的有关开放型经济的声明重申了这一承诺,指出“我们的繁荣和安全是建立在开放经济的基础之上的”。而CFIUS则是在这一开放投资政策的前提下履行其职责的。在FINSA序言中,国会也指出,该法的目的是“为了确保国家安全,同时促进外国投资,创造和维护就业,以及对可能影响国家安全的外资审查程序进行改革”。
一、中美关系与国家安全 1、美国全球战略变化 背景: 第一阶段:二战结束——1968年之前。美国全球战略确立和初步发展的时期。以苏联为主要对手,以欧洲为战略重点,以亚、非、拉美广大地区为扩张目标,以控制西欧和日本为重要基础,最终达到称霸世界的目的。 第二阶段:1968年尼克松上台——70年代末。美国全球战略大调整的时期. 美苏之间的力量对比不利于美国,盟友自主性增强,陷入越战泥潭。美国全球战略重大调整,采取守势的缓和战略。恢复中美关系。外交政策灵活、务实与内向,取得了相当的成功,对后来美国对外关系的发展产生了深远的影响。 第三阶段:1981年里根上台——20世纪80年代末,是“对抗共产主义”新遏制战略时期。为“扩军抗苏”,“重整国威”,改变同苏联争霸所处的不利局面,把遏制苏联作中心环节,加强了对苏联的进攻态势,同时也借助中国抗衡苏联。老布什提出“超越遏制”战略,并把“和平演变”作为实现美国对外战略的最主要的手段。 第四阶段:克林顿上台执政时期。美国在不改变其争夺全球霸权的总目标的基础上,逐步提出了以“经济”、“实力”、“民主”为美国外交政策核心支柱的“参与和扩张”战略取代过去长期推行的遏制战略,强化了经济安全的色彩,企图在政治、外交、军事、经济贸易和科技文化各个领域建起“美国第一”的霸权。 第五个阶段:小布什上台执政时期。2001年他上台后,就表现出强烈的冷战思维和新保守主义色彩。“9·11”事件发生后,美国的外交政策表现出更强的进攻性、冒险性、独断专行和单边主义倾向。不断在世界范围内推进美式民主和价值观,进行意识形态扩张,维护美国全球领导作用。追求美国的绝对安全、绝对军事优势、对世界的绝对主导。结果是国内经济所出现的巨大“黑洞”、糟糕的国际形象及急剧下降的国际影响力。 第六个阶段:奥巴马上台执政时期美国全球战略新变化——战略重点东移 2008年奥巴马上台以后,采取了务实的外交政策,在“巧实力”的新外交理念下,从维护和巩固一朝独霸的地位出发,以“3D”(国防、外交和发展)为支柱,明确了现阶段美国外交需要优先解决的“一个中心,两场战争,三个重点”。即以解决国内经济问题,创造就业,振兴经济为中心。力争尽快从伊拉克战争和阿富汗战争中脱身。以解决巴以冲突、朝鲜核问题和伊朗核问题为外交重点。 2009年11月,奥巴马首次称美国“世世代代是一个太平洋国家”,自己是美国第一位“太平洋总统”,称“这个地区的未来与我们利害攸关”。2011年11月,进一步表示“美国是太平洋地区的重要一员,我们绝不会离开”。 2010年国务卿希拉里·克林顿宣布,南海是美国的关键利益所在。2011年,她提出“太平洋世纪”的概念。认为“未来的政治将决定于亚洲,今后10年美国外交方略的最重要使命之一将是把大幅增加的投入——在外交、经济、战略和其他方面——锁定于亚太地区。”为此,太平洋地区“必须开创一种有章可依的秩序——一种开放、自由、透明而公平的秩序。”并表示,只有美国才有能力充当这样一种秩序的开创者和维护者。 美国全球战略新变化的原因 一、亚太地区对全球而言重要性日趋凸显亚太地区既是全球人口最多,经济力量最大、最重要、最具活力的地区,也已经成为世界政治发展新的战略重心,亚洲地区有可能出现一个有巨大潜力的军事竞争者,东亚沿海是特别具有挑战性的地区,这对美国未来具有生死攸关的意义。 二、面对中国综合国力的迅速提升和日益扩大的国际影响力,美国要确保自己在亚太
美国化妆品法规概述 1、美国化妆品法规历史 1938年美国正式颁布《食品药品和化妆品法案》(FD&C法案),第一次将化妆品纳入食品和药品管理局(FDA)的管制范围之内。法案规定化妆品不能掺假伪劣和错误标注,也就是说,化妆品按照预期用途必须是安全的,而且必须进行正确的标注。另外,法案对于在药品和化妆品里使用色素也进行了管理,建立了一个可用于化妆品和药品的色素添加剂清单,并且针对每种色素添加剂的化学成分规格做出了规定。自1938年以来,《FD&C法案》已经多次修正。特别是1960年《色素添加剂修正案》,要求业界提供每种着色剂安全的科学数据,FDA由此可以确定着色剂在产品里的纯度限制。1966年的《正确包装和标识法案》(FPLA),该法案增加了对成品标注的要求,并且使公众更容易获取产品信息来对产品进行比较。FPLA要求并不仅限于针对化妆品和药品,而是针对所有进入流通的消费品。 2、化妆品法规的实施 美国FDA负责《FD&C法案》的实施,通过保证食品、药品和化妆品的质量、安全性和有效性来保护美国消费者的利益。此外,FDA还通过发布规章、指南、政策声明、函件和讲话来为《FD&C法案》的具体实施提供框架。其中规章用于正式贯彻实施《FD&C法案》,它具有法律约束力,在《联邦规章法典》(CFR)中发布,CFR每年更新一次。指南是用于解释规章的技术或政策性文件,并且代表FDA在某个问题上的最新思想和科学技术的最新发展,指南没有法律约束力,但是它们对于了解FDA的标准动态提供了有用的参考。FDA 下属多个科学家组成的中心,负责食品、药品和化妆品规章的实施,FDA食品安全与应用营养中心(CFSAN),负责化妆品的安全性和标的管理,而FDA药品评价与研究中心(CDER)则负责处方药、非处方药和非专利药的管理。FDA对化妆品和非处方药的管理偏重于标签管理,另一个政府机构,联邦贸易委员会(FTC)主要负责化妆品和非处方药的广告管理。FTC主要负责对不正当和欺诈行为的管制。所有广告行为必须真实可靠、不带有误导性,并且必须有可以证明其宣传合理性的依据。 3、国内化妆品生产销售管理 (1)自愿注册计划(The Voluntary Cosmetic Registration Program)。包括自愿化妆品 生产设施注册和自愿报告计划。自愿进行化妆品生产设施注册是指任何化妆品公司 在自愿的情况下,对涉及生产或包装销售的化妆品的生产设施应予以注册。设施应 在化妆品开始投产后天内注册,一旦FDA完成全部文件的审核,将会给该生产设 施指定一个永久性注册号。自愿报告计划是化妆品生产厂家自愿注册化妆品成分声 明(配方),即在生产销售前化妆品生产厂家既可自愿注册,又可不经FDA批准 直接生产上市。该计划鼓励化妆品制造商在化妆品首次进入市场60天内将新产品 说明报告提交给FDA。停止出售或生产的产品也应向FDA报告。这项计划的目的 是为了让FDA了解有关在美国市场上出售的化妆品的最新情况。自愿注册厂家将