美国FDA药品质量控制微生物实验室检查指南
- 格式:doc
- 大小:40.00 KB
- 文档页数:3

FDA检查员指导手册CP 7356.002:药品生产检查程序目录对现场报告的要求 (35)第一部分背景 (36)第二部分执行 (36)2.1.目的 (36)2.2.策略 (36)2.2.1.对生产企业两年一度的检查(包括重新包装商、合同实验室等) (36)2.2.2.系统性检查 (37)2.2.3.对原料药及制剂生产的系统性检查计划 (38)2.2.3.1.质量系统 (38)2.2.3.2.厂房设施与设备系统 (38)2.2.3.3.物料系统 (38)2.2.3.4.生产系统 (38)2.2.3.5.包装和贴签系统 (38)2.2.3.6.实验室控制系统 (39)2.3.程序管理指导 (39)2.3.1.定义 (39)2.3.1.1.监督性检查 (39)2.3.1.2.达标检查 (40)2.3.1.3.受控状态 (40)2.3.1.4.药品工艺 (40)2.3.1.5.药品生产检查 (41)第三部分检查 (41)3.1.检查活动 (41)3.1.1.总则 (41)3.1.2.检查方法 (42)3.1.2.1.全面性检查的选择 (43)3.1.2.2.简略性检查的选择 (43)3.1.2.3.综合性检查范围 (43)3.1.3.系统性检查范围 (43)3.1.3.1.质量系统 (44)3.1.3.2. 厂房设施与设备系统 (44)3.1.3.3.物料系统 (45)3.1.3.4.生产系统 (46)3.1.3.5.包装和贴签系统 (47)3.1.3.6.实验室控制系统 (48)3.1.4.取样 (49)3.1.5.检查组组成 (49)3.1.6.报告 (49)第四部分分析 (50)第五部分法律性/行政性策略 (50)5.1.质量系统 (51)5.2.厂房设施和设备 (51)5.3.物料系统 (51)5.4.生产系统 (52)5.5.包装和贴签系统 (52)5.6.实验室控制系统 (52)对现场报告的要求作为法律行动的一部分,所有针对因在执行cGMP方面有缺陷而采取的检查,均要向药品评价和研究中心的达标办公室呈交一份现场检查报告(EIR)。



美国FDA高纯水系统检查指南注释:这份文件是检查员和其他FDA人员的参考资料。
这份文件不约束FDA,不授予任何人任何权力、特权、利益或豁免权。
这份指南主要从微生物方面讨论对用于药物制剂和原料药生产的高纯水系统的检查及评价。
它也涉及了对不同类型系统的设计及一些与这些系统相关问题的检查。
如同其它指南一样,它并不是包含一切的,但是提供了对高纯水系统的检查和评价的背景及指导。
微生物实验室的药物质量控制检查指南(1993,5月)提供了另外的指导。
1、系统设计系统设计的一个基本考虑因素是所生产产品的种类。
对于注射剂关注的是热原,这就需要使用注射用水。
注射用水适用于产品配制,以及成分和生产设备的最终清洗。
蒸馏和反渗透膜过滤是USP中列出的生产注射用水的唯一可接受方法。
但是,在原料药和生物技术及一些国外公司中,超滤由于可以使内毒素量减到最少而用于那些针用原料药中。
对于一些眼科药物如眼用冲洗液和一些吸入产品如吸入的无菌水是有热原规定的,我们希望它们的配制使用注射用水。
但是对于大多数吸入剂和眼科用药,它们的配制中使用纯化水。
纯化水也适用于外用药、化妆品和口服制剂。
设计的另一个考虑因素是系统温度。
我们发现热(65-80℃)系统能自身消毒。
虽然公司采用其它系统的花费可能较低,但维护、检测和潜在问题的花费比能源节省的花费要大得多。
系统是否循环或单向也是设计中的一个重要考虑因素。
明显地,持续流动的水受高水平污染的可能性较低。
一个单向水系统基本上是一个死角。
最后,可能最重要的考虑因素是风险评估或要求的质量水平。
同时也应该认识到不同的产品要求不同质量的水。
注射剂要求无内毒素的高纯水。
外用和口服制剂要求较低纯度的水,并无内毒素要求。
即使对于外用和口服制剂也有许多因素决定了不同质量的水。
比如,抗酸剂中的保护剂并不是总是有效的,因此必须制定严格的微生物限度。
质量管理部门应该评估使用他们系统的水生产的每一个产品,并确定在微生物最敏感的产品基础上建立的微生物纠偏限度。

GUIDE(1) TO INSPECTIONSOF FOREIGN PHARMACEUTICAL MANUFACTURERS BACKGROUND背景There has been a significant increase in the number of foreign inspections of pharmaceutical manufacturing plants in the past few years. This trend is attributable mainly to the increase in the number of pre-approval inspections although the increase has been noted in other areas such as routine GMP inspections and compliance follow-up activities. Considering the resource-intensive nature of the foreign inspection program, it has become clear that effective and efficient inspectional coverage is crucial to the successful management of the program and that can be achieved only through maintenance of consistency and uniformity of inspection and enforcement activities.在最近几年医药制造厂外检查数量显著增加。
这一趋势主要是由增加的前置审批检查的次数虽然增加了在其他领域如日常GMP检查和合规性的后续活动记录。
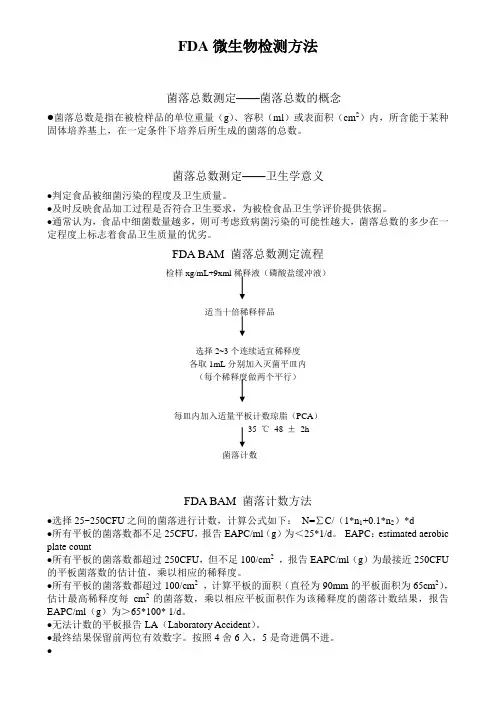
FDA微生物检测方法菌落总数测定——菌落总数的概念●菌落总数是指在被检样品的单位重量(g)、容积(ml)或表面积(cm2)内,所含能于某种固体培养基上,在一定条件下培养后所生成的菌落的总数。
菌落总数测定——卫生学意义●判定食品被细菌污染的程度及卫生质量。
●及时反映食品加工过程是否符合卫生要求,为被检食品卫生学评价提供依据。
●通常认为,食品中细菌数量越多,则可考虑致病菌污染的可能性越大,菌落总数的多少在一定程度上标志着食品卫生质量的优劣。
FDA BAM 菌落总数测定流程检样xg/mL+9xml稀释液(磷酸盐缓冲液)适当十倍稀释样品选择2~3个连续适宜稀释度各取1mL分别加入灭菌平皿内(每个稀释度做两个平行)每皿内加入适量平板计数琼脂(PCA)35 ℃48 ±2h菌落计数FDA BAM 菌落计数方法●选择25~250CFU之间的菌落进行计数,计算公式如下:N=∑C/(1*n1+0.1*n2)*d●所有平板的菌落数都不足25CFU,报告EAPC/ml(g)为<25*1/d。
EAPC:estimated aerobic plate count●所有平板的菌落数都超过250CFU,但不足100/cm2,报告EAPC/ml(g)为最接近250CFU 的平板菌落数的估计值,乘以相应的稀释度。
●所有平板的菌落数都超过100/cm2,计算平板的面积(直径为90mm的平板面积为65cm2),估计最高稀释度每cm2的菌落数,乘以相应平板面积作为该稀释度的菌落计数结果,报告EAPC/ml(g)为>65*100* 1/d。
●无法计数的平板报告LA(Laboratory Accident)。
●最终结果保留前两位有效数字。
按照4舍6入,5是奇进偶不进。
●菌落总数测定几点说明●由于检样中采用30/35℃有氧条件下培养,因而并不是样品中实际的总活菌数,一些特殊营养要求的细菌、厌氧菌、微需氧菌、以及非嗜中温细菌,均难以反映出来。

GUIDE TO INSPECTIONS OF PHARMACEUTICAL QUALITY CONTROL LABORATORIESNote: This document is reference material for investigators and other FDApersonnel. The document does not bind FDA, and does no confer any rights,privileges, benefits, or immunities for or on any person(s).1. INTRODUCTIONThe pharmaceutical quality control laboratory serves one of the mostimportant functions in pharmaceutical production and control. A significantportion of the CGMP regulations (21 CFR 211) pertain to the quality controllaboratory and product testing. Similar concepts apply to bulk drugs.This inspection guide supplements other inspectional information containedin other agency inspectional guidance documents. For example, ComplianceProgram 7346.832 requiring pre-approval NDA/ANDA inspections containsgeneral instructions to conduct product specific NDA/ANDA inspection auditsto measure compliance with the applications and CGMP requirements. Thisincludes pharmaceutical laboratories used for in-process and finishedproduct testing.2. OBJECTIVEThe specific objective will be spelled out prior to the inspection. Thelaboratory inspection may be limited to specific issues, or the inspectionmay encompass a comprehensive evaluation of the laboratory's compliance withCGMP's. As a minimum, each pharmaceutical quality control laboratory shouldreceive a comprehensive GMP evaluation each two years as part of thestatutory inspection obligation.In general these inspections may include-- the specific methodology which will be used to test a new product-- a complete assessment of laboratory's conformance with GMP's-- a specific aspect of laboratory operations3. INSPECTION PREPARATIONFDA Inspection Guides are based on the team inspection approach and ourinspection of a laboratory is consistent with this concept. As part of oureffort to achieve uniformity and consistency in laboratory inspections, weexpect that complex, highly technical and specialized testing equipment,procedures and data manipulations, as well as scientific laboratoryoperations will be evaluated by an experienced laboratory analyst withspecialized knowledge in such matters.District management makes the final decision regarding the assignment ofpersonnel to inspections. Nevertheless, we expect investigators, analystsand others to work as teams and to advise management when additionalexpertise is required to complete a meaningful inspection.Team members participating in a pre-approval inspection must read and befamiliar with Compliance Program 7346.832, Pre-ApprovalInspections/Investigations. Relevant sections of the NDA or ANDA should be reviewed prior to the inspection; but if the application is not availablefrom any other source, this review will have to be conducted using thecompany's copy of the application.Team members should meet, if possible, prior to the inspection to discussthe approach to the inspection, to define the roles of the team members, and to establish goals for completion of the assignment. Responsibilities fordevelopment of all reports should also be established prior to theinspection. This includes the preparation of the FDA 483.The Center for Drug Evaluation and Research (CDER) may have issueddeficiency letters listing problems that the sponsor must correct prior to the approval of NDA/ANDA's and supplements. The inspection team is expected to review such letters on file at the district office, and they are expected to ask the plant for access to such letters. The team should evaluate thereplies to these letters to assure that the data are accurate and authentic. Complete the inspection even though there has been no response to theseletters or when the response is judged inadequate.4. INSPECTION APPROACHA. GeneralIn addition to the general approach utilized in a drug CGMP inspection, the inspection of a laboratory requires the use of observations of thelaboratory in operation and of the raw laboratory data to evaluatecompliance with CGMP's and to specifically carry out the commitments in an application or DMF. When conducting a comprehensive inspection of alaboratory, all aspects of the laboratory operations will be evaluated.Laboratory records and logs represent a vital source of information thatallows a complete overview of the technical ability of the staff and ofoverall quality control procedures. SOPs should be complete and adequate and the operations of the laboratories should conform to the written procedures. Specifications and analytical procedures should be suitable and, asapplicable, in conformance with application commitments and compendialrequirements.Evaluate raw laboratory data, laboratory procedures and methods, laboratory equipment,including maintenance and calibration, and methods validation data to determine the overall quality of the laboratory operation and the ability to comply with CGMP regulations.Examine chromatograms and spectra for evidence of impurities, poortechnique, or lack of instrument calibration.s use systems that provide for the investigation oflaboratory test failures. These are generally recorded in some type of log. Ask to see results of analyses for lots of product that have failed to meet specifications and review the analysis of lots that have been retested,rejected, or reworked. Evaluate the decision to release lots of product when the laboratory results indicate that the lot failed to meet specifications and determine who released them.B. Pre-ApprovalDocuments relating to the formulation of the product, synthesis of the bulk drug substance, product specifications, analysis of the product, and others are examined during the review process in headquarters. However, thesereviews and evaluations depend on accurate and authentic data that trulyrepresents the product.Pre-approval inspections are designed to determine if the data submitted in an application are authentic and accurate and if the procedures listed inthe application were actually used to produce the data contained in theapplication. Additionally, they are designed to confirm that plants(including the quality control laboratory) are in compliance with CGMPregulations.The analytical sections of drug applications usually contain only testresults and the methods used to obtain them. Sponsors are not required tofile all the test data because such action would require voluminoussubmissions and would often result in filing redundant information. Sponsors may deliberately or unintentionally select and report data showing that adrug is safe and effective and deserves to be approved. The inspection team must decide if there is valid and scientific justification for the failure to report data which demonstrates the product failed to meet itspredetermined specifications.Coordination between headquarters and the field is essential for a complete review of the application and the plant. Experienced investigators andanalysts may contact the review chemist (with appropriate supervisoryconcurrence) when questions concerning specifications and standards arise.Inspections should compare the results of analyses submitted with results of analysis of other batches that may have been produced. Evaluate the methods and note any exceptions to the procedures or equipment actually used fromthose listed in the application and confirm that it is the same methodlisted in the application. The analyst is expected to evaluate rawlaboratory data for tests performed on the test batches (biobatches andclinical batches) and to compare this raw data to the data filed in theapplication.5. FAILURE (OUT-OF-SPECIFICATION) LABORATORY RESULTSEvaluate the company's system to investigate laboratory test failures. These investigations represent a key issue in deciding whether a product may bereleased or rejected and form the basis for retesting, and resampling.In a recent court decision the judge used the term "out-of-specification"(OOS) laboratory result rather than the term "product failure" which is morecommon to FDA investigators and analysts. He ruled that an OOS resultidentified as a laboratory error by a failure investigation or an outliertest. The court provided explicit limitations on the use of outlier testsand these are discussed in a later segment of this document., or overcome by retesting. The court ruled on the use of retesting which is covered in alater segment of this document. is not a product failure. OOS results fall into three categories:-- laboratory error-- non-process related or operator error-- process related or manufacturing process errorA. LABORATORY ERRORSLaboratory errors occur when analysts make mistakes in following the method of analysis, use incorrect standards, and/or simply miscalculate the data. Laboratory errors must be determined through a failure investigation toidentify the cause of the OOS. Once the nature of the OOS result has beenidentified it can be classified into one of the three categories above. The inquiry may vary with the object under investigation.B. LABORATORY INVESTIGATIONSThe exact cause of analyst error or mistake can be difficult to determinespecifically and it is unrealistic to expect that analyst error will always be determined and documented. Nevertheless, a laboratory investigationconsists of more than a retest. The inability to identify an error's cause with confidence affects retesting procedures, not the investigation inquiry required for the initial OOS result.The firm's analyst should follow a written procedure, checking off each step as it is completed during the analytical procedure. We expect laboratorytest data to be recorded directly in notebooks; use of scrap paper and loose paper must be avoided. These common sense measures enhance the accuracy and integrity of data.Review and evaluate the laboratory SOP for product failure investigations. Specific procedures must be followed when single and multiple OOS resultsare investigated. For the single OOS result the investigation should include the following steps and these inquiries must be conducted before there is a retest of the sample:o the analyst conducting the test should report the OOS result to thesupervisoro the analyst and the supervisor should conduct an informal laboratoryinvestigation which addresses the following areas:1. discuss the testing procedure2. discuss the calculation3. examine the instruments4. review the notebooks containing the OOS resultAn alternative means to invalidate an initial OOS result, provided thefailure investigation proves inconclusive, is the "outlier" test. However, specific restrictions must be placed on the use of this test.1. Firms cannot frequently reject results on this basis.2. The USP standards govern its use in specific cases only.3. The test cannot be used for chemical testing results. An initial content uniformity test was OOS followed by a passing retest. The initial OOS result was claimed the result of analyst error based on a statistical evaluation of the data. The court ruled that the use of an outlier test is inappropriate in this case..4. It is never appropriate to utilize outlier tests for a statisticallybased test, i.e., content uniformity and dissolution.Determine if the firm uses an outlier test and evaluate the SOP.Determine that a full scale inquiry has been made for multiple OOS results. This inquiry involves quality control and quality assurance personnel inaddition to laboratory workers to identify exact process or non processrelated errors.When the laboratory investigation is inconclusive (reason for the error is not identified) the firm:1. Cannot conduct 2 retests and base release on average of three tests2. Cannot use outlier test in chemical tests3. Cannot use a re-sample to assume a sampling or preparation error4. Can conduct a retest of different tablets from the same sample when aretest is considered appropriate (see criteria elsewhere)C. FORMAL INVESTIGATIONSFormal investigations extending beyond the laboratory must follow an outline with particular attention to corrective action. The company must:1. State the reason for the investigation2. Provide summation of the process sequences that may have caused theproblem3. Outline corrective actions necessary to save the batch and preventsimilar recurrence4. List other batches and products possibly affected, the results ofinvestigation of these batches and products, and any corrective action.Specifically:o examine other batches of product made by the errant employee or machineo examine other products produced by the errant process or operation5. Preserve the comments and signatures of all production and qualitycontrol personnel who conducted the investigation and approved anyreprocessed material after additional testingD. INVESTIGATION DOCUMENTATIONAnalyst's mistakes, such as undetected calculation errors, should bespecified with particularity and supported by evidence. Investigations along with conclusions reached must be preserved with written documentation that enumerates each step of the investigation. The evaluation, conclusion andcorrective action, if any, should be preserved in an investigation orfailure report and placed into a central file.E. INVESTIGATION TIME FRAMESAll failure investigations should be performed within 20 business days ofthe problem's occurrence and recorded and written into a failure orinvestigation report.6. PRODUCT FAILURESAn OOS laboratory result can be overcome (invalidated) when laboratory error has been documented. However, non-process and process related errorsresulting from operators making mistakes, equipment (other than laboratory equipment) malfunctions, or a manufacturing process that is fundamentallydeficient, such as an improper mixing time, represent product failures.Examine the results of investigations using the guidance in section 5 above and evaluate the decision to release, retest, or rework products.7. RETESTINGEvaluate the company's retesting SOP for compliance with scientificallysound and appropriate procedures. A very important ruling in one recentcourt decision sets forth a procedure to govern the retesting program. This district court ruling provides an excellent guide to use in evaluating some aspects of a pharmaceutical laboratory, but should not be considered as law, regulation or binding legal precedent. The court ruled that a firm shouldhave a predetermined testing procedure and it should consider a point atwhich testing ends and the product is evaluated. If results are notsatisfactory, the product is rejected.Additionally, the company should consider all retest results in the context of the overall record of the product. This includes the history of theproduct. The court ordered a recall of one batch of product on the basis of an initial content uniformity failure and no basis to invalidate the testresult and on a history of content uniformity problems with the product.,type of test performed, and in-process test results. Failing assay results cannot be disregarded simply on the basis of acceptable content uniformity results.The number of retests performed before a firm concludes that an unexplained OOS result is invalid or that a product is unacceptable is a matter ofscientific judgment. The goal of retesting is to isolate OOS results butretesting cannot continue ad infinitum.In the case of nonprocess and process-related errors, retesting is suspect. Because the initial tests are genuine, in these circumstances, additionaltesting alone cannot contribute to product quality. The court acknowledged that some retesting may precede a finding of nonprocess or process-basederrors. Once this determination is made, however, additional retesting for purposes of testing a product into compliance is not acceptable.For example, in the case of content uniformity testing designed to detectvariability in the blend or tablets, failing and non-failing results are not inherently inconsistent and passing results on limited retesting do not rule out the possibility that the batch is not uniform. As part of theinvestigation firms should consider the record of previous batches, sincesimilar or related failures on different batches would be a cause ofconcern.Retesting following an OOS result is ruled appropriate only after thefailure investigation is underway and the failure investigation determines in part whether retesting is appropriate. It is appropriate when analysterror is documented or the review of analyst's work is "inconclusive" , but it is not appropriate for known and undisputed non-process or processrelated errors.The court ruled that retesting:o must be done on the same, not a different sampleo may be done on a second aliquot from the same portion of the sample that was the source of the first aliquoto may be done on a portion of the same larger sample previously collectedfor laboratory purposes8. RESAMPLINGFirms cannot rely on resampling. The court ordered the recall of one batch of product after having concluded that a successful resample result alonecannot invalidate an initial OOS result. to release a product that hasfailed testing and retesting unless the failure investigation disclosesevidence that the original sample is not representative or was improperlyprepared.Evaluate each resampling activity for compliance with this guidance.9. AVERAGING RESULTS OF ANALYSISAveraging can be a rational and valid approach when the object underconsideration is total product assay, but as a general rule this practiceshould be avoided. The court ruled that the firm must recall a batch thatwas released for content uniformity on the basis of averaged test results. because averages hide the variability among individual test results. Thisphenomenon is particularly troubling if testing generates both OOS andpassing individual results which when averaged are within specification.Here, relying on the average figure without examining and explaining theindividual OOS results is highly misleading and unacceptable.Content uniformity and dissolution results never should be averaged toobtain a passing value.In the case of microbiological turbidimetric and plate assays an average is preferred by the USP. In this case, it is good practice to include OOSresults in the average unless an outlier test (microbiological assays)suggests the OOS is an anomaly.10. BLEND SAMPLING AND TESTINGThe laboratory serves a vital function in blend testing which is necessary to increase the likelihood of detecting inferior batches. Blend uniformity testing cannot be waived in favor of total reliance on finished producttesting because finished product testing is limited.One court has ruled that sample size influences ultimate blend test results and that the sample size should resemble the dosage size. Any other practice would blur differences in portions of the blend and defeat the object of the test. If a sample larger than the unit must be taken initially, aliquotswhich resemble the dosage size should be carefully removed for the test,retests, and reserve samples. Obviously, the initial larger sample shouldnot be subjected to any additional mixing or manipulation prior to removing test aliquots as this may obscure non-homogeneity.Multiple individual blend uniformity samples taken from different areascannot be composited. However when variation testing is not the object ofassay testing, compositing is permitted.If firms sample product from sites other than the blender, they mustdemonstrate through validation that their sampling technique isrepresentative of all portions and concentrations of the blend. This means that the samples must be representative of those sites that might beproblems; e.g. weak or hot spots in the blend.11. MICROBIOLOGICALThe review of microbiological data on applicable dosage forms is bestperformed by the microbiologist (analyst). Data that should be reviewedinclude preservative effectiveness testing, bioburden data, and productspecific microbiological testing and methods.Review bioburden (before filtration and/or sterilization) from both anendotoxin and sterility perspective. For drug substance labs evaluatemethods validation and raw data for sterility, endotoxin testing,environmental monitoring, and filter and filtration validation. Also,evaluate the methods used to test and establish bioburdens.Refer to the Microbiological Inspection Guide for additional informationconcerning the inspection of microbiological laboratories.12. SAMPLINGSamples will be collected on pre-approval inspections. Follow the sampling guidelines in CP 7346.832, Part III, pages 5 and 6.13. LABORATORY RECORDS AND DOCUMENTATIONReview personal analytical notebooks kept by the analysts in the laboratory and compare them with the worksheets and general lab notebooks and records. Be prepared to examine all records and worksheets for accuracy andauthenticity and to verify that raw data are retained to support theconclusions found in laboratory results.Review laboratory logs for the sequence of analysis versus the sequence of manufacturing dates. Test dates should correspond to the dates when thesample should have been in the laboratory. If there is a computer data base, determine the protocols for making changes to the data. There should be an audit trail for changes to data.We expect raw laboratory data to be maintained in bound, (not loose or scrap sheets of paper), books or on analytical sheets for which there isaccountability, such as prenumbered sheets. For most of those manufacturers which had duplicate sets of records or "raw data", non-numbered loose sheets of paper were employed. Some companies use discs or tapes as raw data andfor the storage of data. Such systems have also been accepted provided they have been defined (with raw data identified) and validated.Carefully examine and evaluate laboratory logs, worksheets and other records containing the raw data such as weighings, dilutions, the condition ofinstruments, and calculations. Note whether raw data are missing, if records have been rewritten, or if correction fluid has been used to conceal errors. Results should not be changed without explanation. Cross reference the data that has been corrected to authenticate it. Products cannot be "tested into compliance" by arbitrarily labeling out-of-specification lab results as"laboratory errors" without an investigation resulting in scientificallyvalid criteria.Test results should not have been transcribed without retention of theoriginal records, nor should test results be recorded selectively. Forexample, investigations have uncovered the use of loose sheets of paper with subsequent selective transcriptions of good data to analyst worksheetsand/or workbooks. Absorbance values and calculations have even been found on desk calendars.Cut charts with injections missing, deletion of files in direct data entry systems, indirect data entry without verification, and changes tocomputerized programs to override program features should be carefullyexamined. These practices raise questions about the overall quality of data.The firm should have a written explanation when injections, particularlyfrom a series are missing from the official work-sheets or from files andare included among the raw data. Multiple injections recorded should be in consecutive files with consecutive injection times recorded. Expect to see written justification for the deletion of all files.Determine the adequacy of the firm's procedures to ensure that all validlaboratory data are considered by the firm in their determination ofacceptability of components, in-process, finished product, and retainedstability samples. Laboratory logs and documents when cross referenced may show that data has been discarded by company officials who decided torelease the product without a satisfactory explanation of the resultsshowing the product fails to meet the specifications. Evaluate thejustification for disregarding test results that show the product failed to meet specifications.14. LABORATORY STANDARD SOLUTIONSAscertain that suitable standards are being used (i.e. in-date, storedproperly). Check for the reuse of stock solutions without assuring theirstability. Stock solutions are frequently stored in the laboratoryrefrigerator. Examine the laboratory refrigerators for these solutions and when found check for appropriate identification. Review records of standard solution preparation to assure complete and accurate documentation. It ishighly unlikely that a firm can "accurately and consistently weigh" to the same microgram. Therefore data showing this level of standardization orpattern is suspect and should be carefully investigated.15. METHODS VALIDATIONInformation regarding the validation of methods should be carefullyevaluated for completeness, accuracy and reliability. In particular, if acompendial method exists, but the firm chooses to use an alternate methodinstead, they must compare the two and demonstrate that the in-house method is equivalent or superior to the official procedure. For compendial methods firms must demonstrate that the method works under the actual conditions of use.Methods can be validated in a number of ways. Methods appearing in the USP are considered validated and they are considered validated if part of anapproved ANDA. Also a company can conduct a validation study on theirmethod. System suitability data alone is insufficient for and does notconstitute method validation.In the review of method validation data, it is expected that data forrepetitive testing be consistent and that the varying concentrations of test solutions provide linear results. Many assay and impurity tests are nowHPLC, and it is expected that the precision of these assays be equal or less than the RSD's for system suitability testing. The analytical performanceparameters listed in the USP XXII, <1225>, under the heading of Validation of Compendial Methods, can be used as a guide for determining the analytical parameters (e.g., accuracy, precision, linearity, ruggedness, etc.) needed to validate the method.16. EQUIPMENTLaboratory equipment usage, maintenance, calibration logs, repair records, and maintenance SOPs also should be examined. The existence of the equipment specified in the analytical methods should be confirmed and its conditionnoted. Verify that the equipment was present and in good working order atthe time the batches were analyzed. Determine whether equipment is beingused properly.In addition, verify that the equipment in any application was in goodworking order when it was listed as used to produce clinical or biobatches. One would have to suspect the data that are generated from a piece ofequipment that is known to be defective. Therefore, continuing to use andrelease product on the basis of such equipment represents a seriousviolation of CGMP's.17. RAW MATERIAL TESTINGSome inspections include the coverage of the manufacturer of the drugsubstance. The safety and efficacy of the finished dosage form is largelydependent on the purity and quality of the bulk active drug substance.Examine the raw data reflecting the analysis of the drug substance including purity tests, charts, etc.Check the impurity profiles of the BPC used in the biobatch and clinicalproduction batches to determine if it is the same as that being used tomanufacture full scale production batches. Determine if the manufacturer has a program to audit the certificate of analysis of the BPC, and, if so, check the results of these tests. Report findings where there is substantialdifference in impurity profiles and other test results.Some older compendial methods may not be capable of detecting impurities as necessary to enable the control of the manufacturing process, and newermethods have been developed to test these products. Such methods must bevalidated to ensure that they are adequate for analytical purposes in thecontrol and validation of the BPC manufacturing process. The drug substance manufacturer must have complete knowledge of the manufacturing process and the potential impurities that may appear in the drug substance. Theseimpurities cannot be evaluated without a suitable method and one that hasbeen validated.Physical tests such as particle size for raw materials, adhesion tests for patches, and extrusion tests for syringes are essential tests to assureconsistent operation of the production and control system and to assurequality and efficacy. Some of these tests are filed in applications andothers may be established by the protocols used to manufacture the product. The validation of methods for such tests are as important as the test forchemical attributes.Physical properties tests often require the use of unique equipment andprotocols. These tests may not be reproducible in other laboratories,therefore, on site evaluation is essential.。

FDA微生物检测方法菌落总数测定——菌落总数的概念●菌落总数是指在被检样品的单位重量(g)、容积(ml)或表面积(cm2)内,所含能于某种固体培养基上,在一定条件下培养后所生成的菌落的总数。
菌落总数测定——卫生学意义●判定食品被细菌污染的程度及卫生质量。
●及时反映食品加工过程是否符合卫生要求,为被检食品卫生学评价提供依据。
●通常认为,食品中细菌数量越多,则可考虑致病菌污染的可能性越大,菌落总数的多少在一定程度上标志着食品卫生质量的优劣。
FDA BAM 菌落总数测定流程检样xg/mL+9xml稀释液(磷酸盐缓冲液)适当十倍稀释样品选择2~3个连续适宜稀释度各取1mL分别加入灭菌平皿内(每个稀释度做两个平行)每皿内加入适量平板计数琼脂(PCA)35 ℃48 ±2h菌落计数FDA BAM 菌落计数方法●选择25~250CFU之间的菌落进行计数,计算公式如下:N=∑C/(1*n1+0.1*n2)*d●所有平板的菌落数都不足25CFU,报告EAPC/ml(g)为<25*1/d。
EAPC:estimated aerobic plate count●所有平板的菌落数都超过250CFU,但不足100/cm2,报告EAPC/ml(g)为最接近250CFU 的平板菌落数的估计值,乘以相应的稀释度。
●所有平板的菌落数都超过100/cm2,计算平板的面积(直径为90mm的平板面积为65cm2),估计最高稀释度每cm2的菌落数,乘以相应平板面积作为该稀释度的菌落计数结果,报告EAPC/ml(g)为>65*100* 1/d。
●无法计数的平板报告LA(Laboratory Accident)。
●最终结果保留前两位有效数字。
按照4舍6入,5是奇进偶不进。
●菌落总数测定几点说明●由于检样中采用30/35℃有氧条件下培养,因而并不是样品中实际的总活菌数,一些特殊营养要求的细菌、厌氧菌、微需氧菌、以及非嗜中温细菌,均难以反映出来。


GUIDE TO INSPECTIONS OF MICROBIOLOGICAL PHARMACEUTICALQUALITY CONTROL LABORATORIES制药行业微生物实验室现场检查指南Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does not confer any rights, privileges, benefits, or immunities for or on anyperson(s).注:该指南是检察官和其他FDA人员的参考材料。
该指南不约束FDA,也不授予任何权利,特权,利益或者任何人的豁免权。
I. INTRODUCTION介绍The Guide to the Inspection of Pharmaceutical Quality Control Laboratories provided very limited guidance on the matter of inspection of microbiological laboratories. While that guide addresses many of the issues associated with the chemical aspect of laboratory analysis of pharmaceuticals, this document will serve as a guide to the inspection of the microbiology analytical process. As with any laboratory inspection, it is recommended that an analyst (microbiologist) who is familiar with the tests being inspected participate in these inspections.“制药行业分析实验室现场检查指南”对如何进行微生物实验室的检查仅提供了非常有限的指导,那份指南文件主要涉及医药行业分析实验室化学分析方面的很多内容,而本指南主要讨论的是微生物分析过程的现场检查指导。

美国微生物检查要求及微生物监控体系在当今的全球化时代,食品安全和医疗卫生领域的重要性日益凸显。
微生物的存在和传播可能对人类健康和产品质量造成严重威胁,因此,建立有效的微生物检查要求和微生物监控体系至关重要。
美国作为全球领先的经济体和科技强国,在这方面拥有一套成熟且严格的体系。
美国的微生物检查要求涵盖了多个领域,包括食品、药品、化妆品、医疗器械以及环境等。
在食品领域,微生物检查主要关注常见的致病菌,如沙门氏菌、大肠杆菌 O157:H7、李斯特菌等。
这些致病菌可能导致严重的食物中毒和疾病爆发。
例如,沙门氏菌污染的鸡肉或鸡蛋可能引发腹泻、呕吐和发热等症状;大肠杆菌 O157:H7 污染的牛肉制品可能导致溶血性尿毒症综合征,对儿童和老年人的危害尤其严重。
为了确保食品的安全性,美国食品药品监督管理局(FDA)制定了一系列严格的微生物检查标准和检测方法。
食品生产企业需要定期对其产品进行微生物检测,并将检测结果报告给监管机构。
此外,监管机构还会进行不定期的抽检,以确保企业遵守相关法规和标准。
如果发现食品中存在超标或有害的微生物,监管机构将采取严厉的措施,包括召回产品、对企业进行罚款甚至关闭企业。
在药品领域,微生物检查同样至关重要。
药品中的微生物污染可能影响药品的疗效,甚至对患者的健康造成严重危害。
美国的药品监管机构要求药品生产企业在生产过程中实施严格的微生物控制措施,包括对原材料、中间产品和成品进行微生物检测。
对于无菌药品,如注射剂和眼用制剂,要求更为严格,必须确保产品在整个生产过程中处于无菌状态。
化妆品和医疗器械领域的微生物检查要求也不容忽视。
化妆品中的微生物污染可能导致皮肤感染和过敏反应,而医疗器械中的微生物污染可能引发交叉感染和医疗事故。
因此,美国相关监管机构对这两个领域的微生物检查也有明确的规定和要求。
美国的微生物监控体系是一个多层次、多部门协同合作的复杂系统。
联邦政府层面,FDA 负责食品、药品、化妆品和医疗器械的监管工作;环境保护署(EPA)则负责环境领域的微生物监控。
GUIDE TO INSPECTIONS OF PHARMACEUTICAL QUALITY CONTROL LABORATORIESNote: This document is reference material for investigators and other FDApersonnel. The document does not bind FDA, and does no confer any rights,privileges, benefits, or immunities for or on any person(s).1. INTRODUCTIONThe pharmaceutical quality control laboratory serves one of the mostimportant functions in pharmaceutical production and control. A significantportion of the CGMP regulations (21 CFR 211) pertain to the quality controllaboratory and product testing. Similar concepts apply to bulk drugs.This inspection guide supplements other inspectional information containedin other agency inspectional guidance documents. For example, ComplianceProgram 7346.832 requiring pre-approval NDA/ANDA inspections containsgeneral instructions to conduct product specific NDA/ANDA inspection auditsto measure compliance with the applications and CGMP requirements. Thisincludes pharmaceutical laboratories used for in-process and finishedproduct testing.2. OBJECTIVEThe specific objective will be spelled out prior to the inspection. Thelaboratory inspection may be limited to specific issues, or the inspectionmay encompass a comprehensive evaluation of the laboratory's compliance withCGMP's. As a minimum, each pharmaceutical quality control laboratory shouldreceive a comprehensive GMP evaluation each two years as part of thestatutory inspection obligation.In general these inspections may include-- the specific methodology which will be used to test a new product-- a complete assessment of laboratory's conformance with GMP's-- a specific aspect of laboratory operations3. INSPECTION PREPARATIONFDA Inspection Guides are based on the team inspection approach and ourinspection of a laboratory is consistent with this concept. As part of oureffort to achieve uniformity and consistency in laboratory inspections, weexpect that complex, highly technical and specialized testing equipment,procedures and data manipulations, as well as scientific laboratory operations will be evaluated by an experienced laboratory analyst with specialized knowledge in such matters.District management makes the final decision regarding the assignment of personnel to inspections. Nevertheless, we expect investigators, analystsand others to work as teams and to advise management when additional expertise is required to complete a meaningful inspection.Team members participating in a pre-approval inspection must read and be familiar with Compliance Program 7346.832, Pre-ApprovalInspections/Investigations. Relevant sections of the NDA or ANDA should be reviewed prior to the inspection; but if the application is not availablefrom any other source, this review will have to be conducted using the company's copy of the application.Team members should meet, if possible, prior to the inspection to discussthe approach to the inspection, to define the roles of the team members, and to establish goals for completion of the assignment. Responsibilities for development of all reports should also be established prior to the inspection. This includes the preparation of the FDA 483.The Center for Drug Evaluation and Research (CDER) may have issued deficiency letters listing problems that the sponsor must correct prior tothe approval of NDA/ANDA's and supplements. The inspection team is expected to review such letters on file at the district office, and they are expectedto ask the plant for access to such letters. The team should evaluate the replies to these letters to assure that the data are accurate and authentic. Complete the inspection even though there has been no response to these letters or when the response is judged inadequate.4. INSPECTION APPROACHA. GeneralIn addition to the general approach utilized in a drug CGMP inspection, the inspection of a laboratory requires the use of observations of thelaboratory in operation and of the raw laboratory data to evaluate compliance with CGMP's and to specifically carry out the commitments in an application or DMF. When conducting a comprehensive inspection of a laboratory, all aspects of the laboratory operations will be evaluated.Laboratory records and logs represent a vital source of information that allows a complete overview of the technical ability of the staff and ofoverall quality control procedures. SOPs should be complete and adequate and the operations of the laboratories should conform to the written procedures. Specifications and analytical procedures should be suitable and, asapplicable, in conformance with application commitments and compendial requirements.Evaluate raw laboratory data, laboratory procedures and methods, laboratory equipment,including maintenance and calibration, and methods validation data to determine the overall quality of the laboratory operation and the abilityto comply with CGMP regulations.Examine chromatograms and spectra for evidence of impurities, poortechnique, or lack of instrument calibration.s use systems that provide for the investigation oflaboratory test failures. These are generally recorded in some type of log.Ask to see results of analyses for lots of product that have failed to meetspecifications and review the analysis of lots that have been retested,rejected, or reworked. Evaluate the decision to release lots of product when the laboratory results indicate that the lot failed to meet specificationsand determine who released them.B. Pre-ApprovalDocuments relating to the formulation of the product, synthesis of the bulk drug substance, product specifications, analysis of the product, and others are examined during the review process in headquarters. However, thesereviews and evaluations depend on accurate and authentic data that trulyrepresents the product.Pre-approval inspections are designed to determine if the data submitted in an application are authentic and accurate and if the procedures listed inthe application were actually used to produce the data contained in theapplication. Additionally, they are designed to confirm that plants(including the quality control laboratory) are in compliance with CGMPregulations.The analytical sections of drug applications usually contain only testresults and the methods used to obtain them. Sponsors are not required to file all the test data because such action would require voluminoussubmissions and would often result in filing redundant information. Sponsors may deliberately or unintentionally select and report data showing that adrug is safe and effective and deserves to be approved. The inspection team must decide if there is valid and scientific justification for the failureto report data which demonstrates the product failed to meet itspredetermined specifications.Coordination between headquarters and the field is essential for a complete review of the application and the plant. Experienced investigators andanalysts may contact the review chemist (with appropriate supervisoryconcurrence) when questions concerning specifications and standards arise.Inspections should compare the results of analyses submitted with results of analysis of other batches that may have been produced. Evaluate the methods and note any exceptions to the procedures or equipment actually used from those listed in the application and confirm that it is the same methodlisted in the application. The analyst is expected to evaluate rawlaboratory data for tests performed on the test batches (biobatches and clinical batches) and to compare this raw data to the data filed in the application.5. FAILURE (OUT-OF-SPECIFICATION) LABORATORY RESULTSEvaluate the company's system to investigate laboratory test failures. These investigations represent a key issue in deciding whether a product may be released or rejected and form the basis for retesting, and resampling.In a recent court decision the judge used the term "out-of-specification" (OOS) laboratory result rather than the term "product failure" which is more common to FDA investigators and analysts. He ruled that an OOS result identified as a laboratory error by a failure investigation or an outliertest. The court provided explicit limitations on the use of outlier testsand these are discussed in a later segment of this document., or overcome by retesting. The court ruled on the use of retesting which is covered in alater segment of this document. is not a product failure. OOS results fallinto three categories:-- laboratory error-- non-process related or operator error-- process related or manufacturing process errorA. LABORATORY ERRORSLaboratory errors occur when analysts make mistakes in following the method of analysis, use incorrect standards, and/or simply miscalculate the data. Laboratory errors must be determined through a failure investigation to identify the cause of the OOS. Once the nature of the OOS result has been identified it can be classified into one of the three categories above. The inquiry may vary with the object under investigation.B. LABORATORY INVESTIGATIONSThe exact cause of analyst error or mistake can be difficult to determine specifically and it is unrealistic to expect that analyst error will alwaysbe determined and documented. Nevertheless, a laboratory investigation consists of more than a retest. The inability to identify an error's causewith confidence affects retesting procedures, not the investigation inquiry required for the initial OOS result.The firm's analyst should follow a written procedure, checking off each stepas it is completed during the analytical procedure. We expect laboratorytest data to be recorded directly in notebooks; use of scrap paper and loose paper must be avoided. These common sense measures enhance the accuracy and integrity of data.Review and evaluate the laboratory SOP for product failure investigations. Specific procedures must be followed when single and multiple OOS resultsare investigated. For the single OOS result the investigation should includethe following steps and these inquiries must be conducted before there is a retest of the sample:o the analyst conducting the test should report the OOS result to the supervisoro the analyst and the supervisor should conduct an informal laboratory investigation which addresses the following areas:1. discuss the testing procedure2. discuss the calculation3. examine the instruments4. review the notebooks containing the OOS resultAn alternative means to invalidate an initial OOS result, provided thefailure investigation proves inconclusive, is the "outlier" test. However,specific restrictions must be placed on the use of this test.1. Firms cannot frequently reject results on this basis.2. The USP standards govern its use in specific cases only.3. The test cannot be used for chemical testing results. An initial content uniformity test was OOS followed by a passing retest. The initial OOS resultwas claimed the result of analyst error based on a statistical evaluation ofthe data. The court ruled that the use of an outlier test is inappropriatein this case..4. It is never appropriate to utilize outlier tests for a statisticallybased test, i.e., content uniformity and dissolution.Determine if the firm uses an outlier test and evaluate the SOP.Determine that a full scale inquiry has been made for multiple OOS results. This inquiry involves quality control and quality assurance personnel in addition to laboratory workers to identify exact process or non process related errors.When the laboratory investigation is inconclusive (reason for the error isnot identified) the firm:1. Cannot conduct 2 retests and base release on average of three tests2. Cannot use outlier test in chemical tests3. Cannot use a re-sample to assume a sampling or preparation error4. Can conduct a retest of different tablets from the same sample when a retest is considered appropriate (see criteria elsewhere)C. FORMAL INVESTIGATIONSFormal investigations extending beyond the laboratory must follow an outline with particular attention to corrective action. The company must:1. State the reason for the investigation2. Provide summation of the process sequences that may have caused the problem3. Outline corrective actions necessary to save the batch and prevent similar recurrence4. List other batches and products possibly affected, the results of investigation of these batches and products, and any corrective action. Specifically:o examine other batches of product made by the errant employee or machineo examine other products produced by the errant process or operation5. Preserve the comments and signatures of all production and quality control personnel who conducted the investigation and approved any reprocessed material after additional testingD. INVESTIGATION DOCUMENTATIONAnalyst's mistakes, such as undetected calculation errors, should be specified with particularity and supported by evidence. Investigations alongwith conclusions reached must be preserved with written documentation that enumerates each step of the investigation. The evaluation, conclusion and corrective action, if any, should be preserved in an investigation orfailure report and placed into a central file.E. INVESTIGATION TIME FRAMESAll failure investigations should be performed within 20 business days ofthe problem's occurrence and recorded and written into a failure or investigation report.6. PRODUCT FAILURESAn OOS laboratory result can be overcome (invalidated) when laboratory error has been documented. However, non-process and process related errors resulting from operators making mistakes, equipment (other than laboratory equipment) malfunctions, or a manufacturing process that is fundamentally deficient, such as an improper mixing time, represent product failures.Examine the results of investigations using the guidance in section 5 above and evaluate the decision to release, retest, or rework products.7. RETESTINGEvaluate the company's retesting SOP for compliance with scientifically sound and appropriate procedures. A very important ruling in one recent court decision sets forth a procedure to govern the retesting program. This district court ruling provides an excellent guide to use in evaluating some aspects of a pharmaceutical laboratory, but should not be considered as law, regulation or binding legal precedent. The court ruled that a firm should have a predetermined testing procedure and it should consider a point at which testing ends and the product is evaluated. If results are not satisfactory, the product is rejected.Additionally, the company should consider all retest results in the contextof the overall record of the product. This includes the history of the product. The court ordered a recall of one batch of product on the basis of an initial content uniformity failure and no basis to invalidate the testresult and on a history of content uniformity problems with the product., type of test performed, and in-process test results. Failing assay results cannot be disregarded simply on the basis of acceptable content uniformity results.The number of retests performed before a firm concludes that an unexplained OOS result is invalid or that a product is unacceptable is a matter of scientific judgment. The goal of retesting is to isolate OOS results but retesting cannot continue ad infinitum.In the case of nonprocess and process-related errors, retesting is suspect. Because the initial tests are genuine, in these circumstances, additional testing alone cannot contribute to product quality. The court acknowledged that some retesting may precede a finding of nonprocess or process-based errors. Once this determination is made, however, additional retesting for purposes of testing a product into compliance is not acceptable.For example, in the case of content uniformity testing designed to detect variability in the blend or tablets, failing and non-failing results are not inherently inconsistent and passing results on limited retesting do not rule out the possibility that the batch is not uniform. As part of the investigation firms should consider the record of previous batches, since similar or related failures on different batches would be a cause of concern.Retesting following an OOS result is ruled appropriate only after thefailure investigation is underway and the failure investigation determinesin part whether retesting is appropriate. It is appropriate when analysterror is documented or the review of analyst's work is "inconclusive" , butit is not appropriate for known and undisputed non-process or process related errors.The court ruled that retesting:o must be done on the same, not a different sampleo may be done on a second aliquot from the same portion of the sample that was the source of the first aliquoto may be done on a portion of the same larger sample previously collected for laboratory purposes8. RESAMPLINGFirms cannot rely on resampling. The court ordered the recall of one batch of product after having concluded that a successful resample result alone cannot invalidate an initial OOS result. to release a product that hasfailed testing and retesting unless the failure investigation discloses evidence that the original sample is not representative or was improperly prepared.Evaluate each resampling activity for compliance with this guidance.9. AVERAGING RESULTS OF ANALYSISAveraging can be a rational and valid approach when the object underconsideration is total product assay, but as a general rule this practice should be avoided. The court ruled that the firm must recall a batch that was released for content uniformity on the basis of averaged test results. because averages hide the variability among individual test results. This phenomenon is particularly troubling if testing generates both OOS and passing individual results which when averaged are within specification. Here, relying on the average figure without examining and explaining the individual OOS results is highly misleading and unacceptable.Content uniformity and dissolution results never should be averaged to obtain a passing value.In the case of microbiological turbidimetric and plate assays an average is preferred by the USP. In this case, it is good practice to include OOS results in the average unless an outlier test (microbiological assays) suggests the OOS is an anomaly.10. BLEND SAMPLING AND TESTINGThe laboratory serves a vital function in blend testing which is necessaryto increase the likelihood of detecting inferior batches. Blend uniformity testing cannot be waived in favor of total reliance on finished product testing because finished product testing is limited.One court has ruled that sample size influences ultimate blend test results and that the sample size should resemble the dosage size. Any other practice would blur differences in portions of the blend and defeat the object of the test. If a sample larger than the unit must be taken initially, aliquotswhich resemble the dosage size should be carefully removed for the test, retests, and reserve samples. Obviously, the initial larger sample shouldnot be subjected to any additional mixing or manipulation prior to removing test aliquots as this may obscure non-homogeneity.Multiple individual blend uniformity samples taken from different areas cannot be composited. However when variation testing is not the object of assay testing, compositing is permitted.If firms sample product from sites other than the blender, they must demonstrate through validation that their sampling technique is representative of all portions and concentrations of the blend. This means that the samples must be representative of those sites that might be problems; e.g. weak or hot spots in the blend.11. MICROBIOLOGICALThe review of microbiological data on applicable dosage forms is best performed by the microbiologist (analyst). Data that should be reviewedinclude preservative effectiveness testing, bioburden data, and product specific microbiological testing and methods.Review bioburden (before filtration and/or sterilization) from both an endotoxin and sterility perspective. For drug substance labs evaluate methods validation and raw data for sterility, endotoxin testing, environmental monitoring, and filter and filtration validation. Also,evaluate the methods used to test and establish bioburdens.Refer to the Microbiological Inspection Guide for additional information concerning the inspection of microbiological laboratories.12. SAMPLINGSamples will be collected on pre-approval inspections. Follow the sampling guidelines in CP 7346.832, Part III, pages 5 and 6.13. LABORATORY RECORDS AND DOCUMENTATIONReview personal analytical notebooks kept by the analysts in the laboratory and compare them with the worksheets and general lab notebooks and records. Be prepared to examine all records and worksheets for accuracy and authenticity and to verify that raw data are retained to support the conclusions found in laboratory results.Review laboratory logs for the sequence of analysis versus the sequence of manufacturing dates. Test dates should correspond to the dates when the sample should have been in the laboratory. If there is a computer data base, determine the protocols for making changes to the data. There should be an audit trail for changes to data.We expect raw laboratory data to be maintained in bound, (not loose or scrap sheets of paper), books or on analytical sheets for which there is accountability, such as prenumbered sheets. For most of those manufacturers which had duplicate sets of records or "raw data", non-numbered loose sheets of paper were employed. Some companies use discs or tapes as raw data and for the storage of data. Such systems have also been accepted provided they have been defined (with raw data identified) and validated.Carefully examine and evaluate laboratory logs, worksheets and other records containing the raw data such as weighings, dilutions, the condition of instruments, and calculations. Note whether raw data are missing, if records have been rewritten, or if correction fluid has been used to conceal errors. Results should not be changed without explanation. Cross reference the data that has been corrected to authenticate it. Products cannot be "tested into compliance" by arbitrarily labeling out-of-specification lab results as "laboratory errors" without an investigation resulting in scientificallyvalid criteria.Test results should not have been transcribed without retention of theoriginal records, nor should test results be recorded selectively. For example, investigations have uncovered the use of loose sheets of paper with subsequent selective transcriptions of good data to analyst worksheetsand/or workbooks. Absorbance values and calculations have even been found on desk calendars.Cut charts with injections missing, deletion of files in direct data entry systems, indirect data entry without verification, and changes to computerized programs to override program features should be carefully examined. These practices raise questions about the overall quality of data.The firm should have a written explanation when injections, particularlyfrom a series are missing from the official work-sheets or from files andare included among the raw data. Multiple injections recorded should be in consecutive files with consecutive injection times recorded. Expect to see written justification for the deletion of all files.Determine the adequacy of the firm's procedures to ensure that all valid laboratory data are considered by the firm in their determination of acceptability of components, in-process, finished product, and retained stability samples. Laboratory logs and documents when cross referenced may show that data has been discarded by company officials who decided to release the product without a satisfactory explanation of the resultsshowing the product fails to meet the specifications. Evaluate the justification for disregarding test results that show the product failed tomeet specifications.14. LABORATORY STANDARD SOLUTIONSAscertain that suitable standards are being used (i.e. in-date, stored properly). Check for the reuse of stock solutions without assuring their stability. Stock solutions are frequently stored in the laboratory refrigerator. Examine the laboratory refrigerators for these solutions andwhen found check for appropriate identification. Review records of standard solution preparation to assure complete and accurate documentation. It is highly unlikely that a firm can "accurately and consistently weigh" to thesame microgram. Therefore data showing this level of standardization or pattern is suspect and should be carefully investigated.15. METHODS VALIDATIONInformation regarding the validation of methods should be carefully evaluated for completeness, accuracy and reliability. In particular, if a compendial method exists, but the firm chooses to use an alternate methodinstead, they must compare the two and demonstrate that the in-house method is equivalent or superior to the official procedure. For compendial methods firms must demonstrate that the method works under the actual conditions of use.Methods can be validated in a number of ways. Methods appearing in the USP are considered validated and they are considered validated if part of an approved ANDA. Also a company can conduct a validation study on their method. System suitability data alone is insufficient for and does not constitute method validation.In the review of method validation data, it is expected that data forrepetitive testing be consistent and that the varying concentrations of test solutions provide linear results. Many assay and impurity tests are now HPLC, and it is expected that the precision of these assays be equal or less than the RSD's for system suitability testing. The analytical performance parameters listed in the USP XXII, <1225>, under the heading of Validationof Compendial Methods, can be used as a guide for determining the analytical parameters (e.g., accuracy, precision, linearity, ruggedness, etc.) neededto validate the method.16. EQUIPMENTLaboratory equipment usage, maintenance, calibration logs, repair records,and maintenance SOPs also should be examined. The existence of the equipment specified in the analytical methods should be confirmed and its condition noted. Verify that the equipment was present and in good working order atthe time the batches were analyzed. Determine whether equipment is being used properly.In addition, verify that the equipment in any application was in goodworking order when it was listed as used to produce clinical or biobatches.One would have to suspect the data that are generated from a piece of equipment that is known to be defective. Therefore, continuing to use and release product on the basis of such equipment represents a seriousviolation of CGMP's.17. RAW MATERIAL TESTINGSome inspections include the coverage of the manufacturer of the drug substance. The safety and efficacy of the finished dosage form is largely dependent on the purity and quality of the bulk active drug substance.Examine the raw data reflecting the analysis of the drug substance including purity tests, charts, etc.Check the impurity profiles of the BPC used in the biobatch and clinical。
GUIDE TO INSPECTIONS OF PHARMACEUTICAL QUALITY CONTROL LABORATORIESNote: This document is reference material for investigators and other FDApersonnel. The document does not bind FDA, and does no confer any rights,privileges, benefits, or immunities for or on any person(s).1. INTRODUCTIONThe pharmaceutical quality control laboratory serves one of the mostimportant functions in pharmaceutical production and control. A significantportion of the CGMP regulations (21 CFR 211) pertain to the quality controllaboratory and product testing. Similar concepts apply to bulk drugs.This inspection guide supplements other inspectional information containedin other agency inspectional guidance documents. For example, ComplianceProgram 7346.832 requiring pre-approval NDA/ANDA inspections containsgeneral instructions to conduct product specific NDA/ANDA inspection auditsto measure compliance with the applications and CGMP requirements. Thisincludes pharmaceutical laboratories used for in-process and finishedproduct testing.2. OBJECTIVEThe specific objective will be spelled out prior to the inspection. Thelaboratory inspection may be limited to specific issues, or the inspectionmay encompass a comprehensive evaluation of the laboratory's compliance withCGMP's. As a minimum, each pharmaceutical quality control laboratory shouldreceive a comprehensive GMP evaluation each two years as part of thestatutory inspection obligation.In general these inspections may include-- the specific methodology which will be used to test a new product-- a complete assessment of laboratory's conformance with GMP's-- a specific aspect of laboratory operations3. INSPECTION PREPARATIONFDA Inspection Guides are based on the team inspection approach and ourinspection of a laboratory is consistent with this concept. As part of oureffort to achieve uniformity and consistency in laboratory inspections, weexpect that complex, highly technical and specialized testing equipment,procedures and data manipulations, as well as scientific laboratoryoperations will be evaluated by an experienced laboratory analyst withspecialized knowledge in such matters.District management makes the final decision regarding the assignment ofpersonnel to inspections. Nevertheless, we expect investigators, analystsand others to work as teams and to advise management when additionalexpertise is required to complete a meaningful inspection.Team members participating in a pre-approval inspection must read and befamiliar with Compliance Program 7346.832, Pre-ApprovalInspections/Investigations. Relevant sections of the NDA or ANDA should bereviewed prior to the inspection; but if the application is not availablefrom any other source, this review will have to be conducted using thecompany's copy of the application.Team members should meet, if possible, prior to the inspection to discussthe approach to the inspection, to define the roles of the team members, andto establish goals for completion of the assignment. Responsibilities fordevelopment of all reports should also be established prior to theinspection. This includes the preparation of the FDA 483.The Center for Drug Evaluation and Research (CDER) may have issueddeficiency letters listing problems that the sponsor must correct prior tothe approval of NDA/ANDA's and supplements. The inspection team is expected to review such letters on file at the district office, and they are expectedto ask the plant for access to such letters. The team should evaluate thereplies to these letters to assure that the data are accurate and authentic.Complete the inspection even though there has been no response to theseletters or when the response is judged inadequate.4. INSPECTION APPROACHA. GeneralIn addition to the general approach utilized in a drug CGMP inspection, theinspection of a laboratory requires the use of observations of thelaboratory in operation and of the raw laboratory data to evaluatecompliance with CGMP's and to specifically carry out the commitments in anapplication or DMF. When conducting a comprehensive inspection of alaboratory, all aspects of the laboratory operations will be evaluated.Laboratory records and logs represent a vital source of information thatallows a complete overview of the technical ability of the staff and ofoverall quality control procedures. SOPs should be complete and adequate and the operations of the laboratories should conform to the written procedures.Specifications and analytical procedures should be suitable and, asapplicable, in conformance with application commitments and compendialrequirements.Evaluate raw laboratory data, laboratory procedures and methods, laboratoryequipment,including maintenance and calibration, and methods validation data to determine the overall quality of the laboratory operation and the abilityto comply with CGMP regulations.Examine chromatograms and spectra for evidence of impurities, poortechnique, or lack of instrument calibration.s use systems that provide for the investigation oflaboratory test failures. These are generally recorded in some type of log.Ask to see results of analyses for lots of product that have failed to meetspecifications and review the analysis of lots that have been retested,rejected, or reworked. Evaluate the decision to release lots of product whenthe laboratory results indicate that the lot failed to meet specificationsand determine who released them.B. Pre-ApprovalDocuments relating to the formulation of the product, synthesis of the bulkdrug substance, product specifications, analysis of the product, and othersare examined during the review process in headquarters. However, thesereviews and evaluations depend on accurate and authentic data that trulyrepresents the product.Pre-approval inspections are designed to determine if the data submitted inan application are authentic and accurate and if the procedures listed inthe application were actually used to produce the data contained in theapplication. Additionally, they are designed to confirm that plants(including the quality control laboratory) are in compliance with CGMPregulations.The analytical sections of drug applications usually contain only testresults and the methods used to obtain them. Sponsors are not required tofile all the test data because such action would require voluminoussubmissions and would often result in filing redundant information. Sponsors may deliberately or unintentionally select and report data showing that adrug is safe and effective and deserves to be approved. The inspection team must decide if there is valid and scientific justification for the failureto report data which demonstrates the product failed to meet itspredetermined specifications.Coordination between headquarters and the field is essential for a completereview of the application and the plant. Experienced investigators andanalysts may contact the review chemist (with appropriate supervisoryconcurrence) when questions concerning specifications and standards arise.Inspections should compare the results of analyses submitted with results of analysis of other batches that may have been produced. Evaluate the methods and note any exceptions to the procedures or equipment actually used fromthose listed in the application and confirm that it is the same methodlisted in the application. The analyst is expected to evaluate rawlaboratory data for tests performed on the test batches (biobatches andclinical batches) and to compare this raw data to the data filed in theapplication.5. FAILURE (OUT-OF-SPECIFICATION) LABORATORY RESULTSEvaluate the company's system to investigate laboratory test failures. These investigations represent a key issue in deciding whether a product may bereleased or rejected and form the basis for retesting, and resampling.In a recent court decision the judge used the term "out-of-specification"(OOS) laboratory result rather than the term "product failure" which is morecommon to FDA investigators and analysts. He ruled that an OOS resultidentified as a laboratory error by a failure investigation or an outliertest. The court provided explicit limitations on the use of outlier testsand these are discussed in a later segment of this document., or overcome byretesting. The court ruled on the use of retesting which is covered in alater segment of this document. is not a product failure. OOS results fallinto three categories:-- laboratory error-- non-process related or operator error-- process related or manufacturing process errorA. LABORATORY ERRORSLaboratory errors occur when analysts make mistakes in following the methodof analysis, use incorrect standards, and/or simply miscalculate the data.Laboratory errors must be determined through a failure investigation toidentify the cause of the OOS. Once the nature of the OOS result has beenidentified it can be classified into one of the three categories above. Theinquiry may vary with the object under investigation.B. LABORATORY INVESTIGATIONSThe exact cause of analyst error or mistake can be difficult to determinespecifically and it is unrealistic to expect that analyst error will alwaysbe determined and documented. Nevertheless, a laboratory investigationconsists of more than a retest. The inability to identify an error's causewith confidence affects retesting procedures, not the investigation inquiryrequired for the initial OOS result.The firm's analyst should follow a written procedure, checking off each stepas it is completed during the analytical procedure. We expect laboratorytest data to be recorded directly in notebooks; use of scrap paper and loosepaper must be avoided. These common sense measures enhance the accuracy and integrity of data.Review and evaluate the laboratory SOP for product failure investigations.Specific procedures must be followed when single and multiple OOS resultsare investigated. For the single OOS result the investigation should includethe following steps and these inquiries must be conducted before there is aretest of the sample:o the analyst conducting the test should report the OOS result to thesupervisoro the analyst and the supervisor should conduct an informal laboratoryinvestigation which addresses the following areas:1. discuss the testing procedure2. discuss the calculation3. examine the instruments4. review the notebooks containing the OOS resultAn alternative means to invalidate an initial OOS result, provided thefailure investigation proves inconclusive, is the "outlier" test. However,specific restrictions must be placed on the use of this test.1. Firms cannot frequently reject results on this basis.2. The USP standards govern its use in specific cases only.3. The test cannot be used for chemical testing results. An initial contentuniformity test was OOS followed by a passing retest. The initial OOS result was claimed the result of analyst error based on a statistical evaluation ofthe data. The court ruled that the use of an outlier test is inappropriatein this case..4. It is never appropriate to utilize outlier tests for a statisticallybased test, i.e., content uniformity and dissolution.Determine if the firm uses an outlier test and evaluate the SOP.Determine that a full scale inquiry has been made for multiple OOS results. This inquiry involves quality control and quality assurance personnel inaddition to laboratory workers to identify exact process or non processrelated errors.When the laboratory investigation is inconclusive (reason for the error isnot identified) the firm:1. Cannot conduct 2 retests and base release on average of three tests2. Cannot use outlier test in chemical tests3. Cannot use a re-sample to assume a sampling or preparation error4. Can conduct a retest of different tablets from the same sample when aretest is considered appropriate (see criteria elsewhere)C. FORMAL INVESTIGATIONSFormal investigations extending beyond the laboratory must follow an outline with particular attention to corrective action. The company must:1. State the reason for the investigation2. Provide summation of the process sequences that may have caused the problem3. Outline corrective actions necessary to save the batch and preventsimilar recurrence4. List other batches and products possibly affected, the results ofinvestigation of these batches and products, and any corrective action.Specifically:o examine other batches of product made by the errant employee or machine o examine other products produced by the errant process or operation5. Preserve the comments and signatures of all production and qualitycontrol personnel who conducted the investigation and approved anyreprocessed material after additional testingD. INVESTIGATION DOCUMENTATIONAnalyst's mistakes, such as undetected calculation errors, should bespecified with particularity and supported by evidence. Investigations alongwith conclusions reached must be preserved with written documentation that enumerates each step of the investigation. The evaluation, conclusion andcorrective action, if any, should be preserved in an investigation orfailure report and placed into a central file.E. INVESTIGATION TIME FRAMESAll failure investigations should be performed within 20 business days ofthe problem's occurrence and recorded and written into a failure orinvestigation report.6. PRODUCT FAILURESAn OOS laboratory result can be overcome (invalidated) when laboratory error has been documented. However, non-process and process related errorsresulting from operators making mistakes, equipment (other than laboratoryequipment) malfunctions, or a manufacturing process that is fundamentallydeficient, such as an improper mixing time, represent product failures.Examine the results of investigations using the guidance in section 5 aboveand evaluate the decision to release, retest, or rework products.7. RETESTINGEvaluate the company's retesting SOP for compliance with scientificallysound and appropriate procedures. A very important ruling in one recentcourt decision sets forth a procedure to govern the retesting program. Thisdistrict court ruling provides an excellent guide to use in evaluating someaspects of a pharmaceutical laboratory, but should not be considered as law, regulation or binding legal precedent. The court ruled that a firm shouldhave a predetermined testing procedure and it should consider a point atwhich testing ends and the product is evaluated. If results are notsatisfactory, the product is rejected.Additionally, the company should consider all retest results in the contextof the overall record of the product. This includes the history of theproduct. The court ordered a recall of one batch of product on the basis ofan initial content uniformity failure and no basis to invalidate the testresult and on a history of content uniformity problems with the product.,type of test performed, and in-process test results. Failing assay resultscannot be disregarded simply on the basis of acceptable content uniformity results.The number of retests performed before a firm concludes that an unexplained OOS result is invalid or that a product is unacceptable is a matter ofscientific judgment. The goal of retesting is to isolate OOS results butretesting cannot continue ad infinitum.In the case of nonprocess and process-related errors, retesting is suspect.Because the initial tests are genuine, in these circumstances, additionaltesting alone cannot contribute to product quality. The court acknowledgedthat some retesting may precede a finding of nonprocess or process-based errors. Once this determination is made, however, additional retesting forpurposes of testing a product into compliance is not acceptable.For example, in the case of content uniformity testing designed to detectvariability in the blend or tablets, failing and non-failing results are notinherently inconsistent and passing results on limited retesting do not ruleout the possibility that the batch is not uniform. As part of theinvestigation firms should consider the record of previous batches, sincesimilar or related failures on different batches would be a cause ofconcern.Retesting following an OOS result is ruled appropriate only after thefailure investigation is underway and the failure investigation determinesin part whether retesting is appropriate. It is appropriate when analysterror is documented or the review of analyst's work is "inconclusive" , butit is not appropriate for known and undisputed non-process or processrelated errors.The court ruled that retesting:o must be done on the same, not a different sampleo may be done on a second aliquot from the same portion of the sample that was the source of the first aliquoto may be done on a portion of the same larger sample previously collectedfor laboratory purposes8. RESAMPLINGFirms cannot rely on resampling. The court ordered the recall of one batchof product after having concluded that a successful resample result alonecannot invalidate an initial OOS result. to release a product that hasfailed testing and retesting unless the failure investigation disclosesevidence that the original sample is not representative or was improperlyprepared.Evaluate each resampling activity for compliance with this guidance.9. AVERAGING RESULTS OF ANALYSISAveraging can be a rational and valid approach when the object underconsideration is total product assay, but as a general rule this practiceshould be avoided. The court ruled that the firm must recall a batch thatwas released for content uniformity on the basis of averaged test results.because averages hide the variability among individual test results. Thisphenomenon is particularly troubling if testing generates both OOS andpassing individual results which when averaged are within specification.Here, relying on the average figure without examining and explaining theindividual OOS results is highly misleading and unacceptable.Content uniformity and dissolution results never should be averaged toobtain a passing value.In the case of microbiological turbidimetric and plate assays an average ispreferred by the USP. In this case, it is good practice to include OOSresults in the average unless an outlier test (microbiological assays)suggests the OOS is an anomaly.10. BLEND SAMPLING AND TESTINGThe laboratory serves a vital function in blend testing which is necessaryto increase the likelihood of detecting inferior batches. Blend uniformitytesting cannot be waived in favor of total reliance on finished producttesting because finished product testing is limited.One court has ruled that sample size influences ultimate blend test resultsand that the sample size should resemble the dosage size. Any other practice would blur differences in portions of the blend and defeat the object of thetest. If a sample larger than the unit must be taken initially, aliquotswhich resemble the dosage size should be carefully removed for the test,retests, and reserve samples. Obviously, the initial larger sample shouldnot be subjected to any additional mixing or manipulation prior to removingtest aliquots as this may obscure non-homogeneity.Multiple individual blend uniformity samples taken from different areascannot be composited. However when variation testing is not the object ofassay testing, compositing is permitted.If firms sample product from sites other than the blender, they mustdemonstrate through validation that their sampling technique isrepresentative of all portions and concentrations of the blend. This meansthat the samples must be representative of those sites that might beproblems; e.g. weak or hot spots in the blend.11. MICROBIOLOGICALThe review of microbiological data on applicable dosage forms is bestperformed by the microbiologist (analyst). Data that should be reviewedinclude preservative effectiveness testing, bioburden data, and productspecific microbiological testing and methods.Review bioburden (before filtration and/or sterilization) from both anendotoxin and sterility perspective. For drug substance labs evaluatemethods validation and raw data for sterility, endotoxin testing,environmental monitoring, and filter and filtration validation. Also,evaluate the methods used to test and establish bioburdens.Refer to the Microbiological Inspection Guide for additional informationconcerning the inspection of microbiological laboratories.12. SAMPLINGSamples will be collected on pre-approval inspections. Follow the samplingguidelines in CP 7346.832, Part III, pages 5 and 6.13. LABORATORY RECORDS AND DOCUMENTATIONReview personal analytical notebooks kept by the analysts in the laboratoryand compare them with the worksheets and general lab notebooks and records. Be prepared to examine all records and worksheets for accuracy andauthenticity and to verify that raw data are retained to support theconclusions found in laboratory results.Review laboratory logs for the sequence of analysis versus the sequence ofmanufacturing dates. Test dates should correspond to the dates when thesample should have been in the laboratory. If there is a computer data base,determine the protocols for making changes to the data. There should be anaudit trail for changes to data.We expect raw laboratory data to be maintained in bound, (not loose or scrapsheets of paper), books or on analytical sheets for which there isaccountability, such as prenumbered sheets. For most of those manufacturerswhich had duplicate sets of records or "raw data", non-numbered loose sheetsof paper were employed. Some companies use discs or tapes as raw data andfor the storage of data. Such systems have also been accepted provided theyhave been defined (with raw data identified) and validated.Carefully examine and evaluate laboratory logs, worksheets and other recordscontaining the raw data such as weighings, dilutions, the condition ofinstruments, and calculations. Note whether raw data are missing, if recordshave been rewritten, or if correction fluid has been used to conceal errors.Results should not be changed without explanation. Cross reference the datathat has been corrected to authenticate it. Products cannot be "tested intocompliance" by arbitrarily labeling out-of-specification lab results as"laboratory errors" without an investigation resulting in scientificallyvalid criteria.Test results should not have been transcribed without retention of theoriginal records, nor should test results be recorded selectively. Forexample, investigations have uncovered the use of loose sheets of paper withsubsequent selective transcriptions of good data to analyst worksheetsand/or workbooks. Absorbance values and calculations have even been found on desk calendars.Cut charts with injections missing, deletion of files in direct data entrysystems, indirect data entry without verification, and changes tocomputerized programs to override program features should be carefullyexamined. These practices raise questions about the overall quality of data.The firm should have a written explanation when injections, particularlyfrom a series are missing from the official work-sheets or from files andare included among the raw data. Multiple injections recorded should be inconsecutive files with consecutive injection times recorded. Expect to seewritten justification for the deletion of all files.Determine the adequacy of the firm's procedures to ensure that all validlaboratory data are considered by the firm in their determination ofacceptability of components, in-process, finished product, and retainedstability samples. Laboratory logs and documents when cross referenced may show that data has been discarded by company officials who decided torelease the product without a satisfactory explanation of the resultsshowing the product fails to meet the specifications. Evaluate thejustification for disregarding test results that show the product failed tomeet specifications.14. LABORATORY STANDARD SOLUTIONSAscertain that suitable standards are being used (i.e. in-date, storedproperly). Check for the reuse of stock solutions without assuring theirstability. Stock solutions are frequently stored in the laboratoryrefrigerator. Examine the laboratory refrigerators for these solutions andwhen found check for appropriate identification. Review records of standardsolution preparation to assure complete and accurate documentation. It ishighly unlikely that a firm can "accurately and consistently weigh" to thesame microgram. Therefore data showing this level of standardization orpattern is suspect and should be carefully investigated.15. METHODS VALIDATIONInformation regarding the validation of methods should be carefullyevaluated for completeness, accuracy and reliability. In particular, if acompendial method exists, but the firm chooses to use an alternate methodinstead, they must compare the two and demonstrate that the in-house method is equivalent or superior to the official procedure. For compendial methodsfirms must demonstrate that the method works under the actual conditions of use.Methods can be validated in a number of ways. Methods appearing in the USP are considered validated and they are considered validated if part of anapproved ANDA. Also a company can conduct a validation study on theirmethod. System suitability data alone is insufficient for and does notconstitute method validation.In the review of method validation data, it is expected that data forrepetitive testing be consistent and that the varying concentrations of testsolutions provide linear results. Many assay and impurity tests are nowHPLC, and it is expected that the precision of these assays be equal or lessthan the RSD's for system suitability testing. The analytical performanceparameters listed in the USP XXII, <1225>, under the heading of Validationof Compendial Methods, can be used as a guide for determining the analytical parameters (e.g., accuracy, precision, linearity, ruggedness, etc.) neededto validate the method.16. EQUIPMENTLaboratory equipment usage, maintenance, calibration logs, repair records,and maintenance SOPs also should be examined. The existence of the equipment specified in the analytical methods should be confirmed and its conditionnoted. Verify that the equipment was present and in good working order atthe time the batches were analyzed. Determine whether equipment is beingused properly.In addition, verify that the equipment in any application was in goodworking order when it was listed as used to produce clinical or biobatches.One would have to suspect the data that are generated from a piece ofequipment that is known to be defective. Therefore, continuing to use andrelease product on the basis of such equipment represents a seriousviolation of CGMP's.17. RAW MATERIAL TESTINGSome inspections include the coverage of the manufacturer of the drugsubstance. The safety and efficacy of the finished dosage form is largelydependent on the purity and quality of the bulk active drug substance.Examine the raw data reflecting the analysis of the drug substance includingpurity tests, charts, etc.Check the impurity profiles of the BPC used in the biobatch and clinicalproduction batches to determine if it is the same as that being used tomanufacture full scale production batches. Determine if the manufacturer hasa program to audit the certificate of analysis of the BPC, and, if so, checkthe results of these tests. Report findings where there is substantialdifference in impurity profiles and other test results.Some older compendial methods may not be capable of detecting impurities asnecessary to enable the control of the manufacturing process, and newermethods have been developed to test these products. Such methods must bevalidated to ensure that they are adequate for analytical purposes in thecontrol and validation of the BPC manufacturing process. The drug substancemanufacturer must have complete knowledge of the manufacturing process and the potential impurities that may appear in the drug substance. Theseimpurities cannot be evaluated without a suitable method and one that hasbeen validated.Physical tests such as particle size for raw materials, adhesion tests forpatches, and extrusion tests for syringes are essential tests to assureconsistent operation of the production and control system and to assurequality and efficacy. Some of these tests are filed in applications andothers may be established by the protocols used to manufacture the product.The validation of methods for such tests are as important as the test forchemical attributes.Physical properties tests often require the use of unique equipment andprotocols. These tests may not be reproducible in other laboratories,therefore, on site evaluation is essential.。
FDA 制剂厂检查指南对制剂企业的检查-CGMP备注:这个文件是对检查者和其他的FDA 官员的参照。
这份文件并不限制FDA,并不猎取任何利益,义务,权利,或豁免某人1简介这个文件介绍到一个大约的药厂检查指导他们是否符合药物的CGMP。
这个指导应当被用来作IOM 的介绍,其他的药物检查指导,和复核工程。
这个检查的指导相对于IOM 的第十章,一些指导是:A 原料药视察指导B 高纯水系统视察的指导C QC 试验室视察的指导D 微生物QC 试验室的指导E 冻干注射剂视察的指导F清洁验证视察的指导G 制药过程中的计算机系统的视察的指导H 一般工艺过程验证的指导2CGMP处方和非处方全部的药物的生产过程要对应于CGMP 否则就被认为在FDC 实施中掺假。
501(a)(2)(B)依据处方药物的记录肯定要比照于704(a)(1)(B)章。
假设药物是OTC 药物被NDA 或ANDA 掩盖,FDA 可以回忆,复制,验证在505(k)(2)章下的FDC。
然而,假设产品是在FDA 没注册过的OTC 药物。
并没有法定的严格要求在视察过程中给视察者看的的记录要按704 章FDC 的要求。
而且,全部的处方药和OTC 药的生产必需符合CGMP 的要求,包括那些记录。
视察者应当回忆这些记录作为打算是否符合CGMP 要求的一局部。
在很少的场合,可以拒绝回忆OTC 的记录由于并没有法定要求这样做。
当工厂在没有法定义务去供给回忆这些记录时,没有减轻需要符合在501(a)(2)(B)CGMP的要求,包括对主体文件的要求。
假设一个工厂拒绝回忆OTC 的记录,视察者应当被其他的视察方法打算特别符合CGMP 的要求。
视察者观看和查找对于处方药和非处方药并不依据FDA-483 确定的检查表中做的行为。
组织和人员工厂肯定要有QC 把握文件描述CFR 的责任和权威。
QC 文件肯定要在说明它的相对于生产文件的独立性,在书面说明它的责任权属。
说明操作者的官员的名字,头衔和内在的责任,其他的主要的职员在IOM 中列出。
GUIDE TO INSPECTIONS OF MICROBIOLOGICAL PHARMACEUTICAL QUALITY CONTROLLABORATORIESNote: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does not confer any rights, privileges, benefits, or immunities for or on any person(s).I. INTRODUCTIONThe Guide to the Inspection of Pharmaceutical Quality Control Laboratories provided very limited guidance on the matter of inspection of microbiological laboratories. While that guide addresses many of the issues associated with the chemical aspect of laboratory analysis of pharmaceuticals, this document will serve as a guide to the inspection of the microbiology analytical process. As with any laboratory inspection, it is remended that an analyst (microbiologist) who is familiar with the tests being inspected participate in these inspections.II. MICROBIOLOGICAL TESTING OF NON-STERILE PRODUCTSFor a variety of reasons, we have seen a number of problems associated with the microbiological contamination of topical drug products, nasal solutions and inhalation products. The USP Microbiological Attributes Chapter <1111> provides little specific guidance other than "The significance of microorganisms in non-sterile pharmaceutical products should be evaluated in terms of the use of the product, the nature of the product, and the potential hazard to the user." The USP remends that certain categories be routinely tested for total counts and specified indicator microbial contaminants. For example natural plant, animal and some mineral products for Salmonella, oral liquids for E. Coli, topicals for P. aeruginosa and S. Aureus, and articles intended for rectal, urethral, or vaginal administration for yeasts and molds. A number of specific monographs also include definitive microbial limits.As a general guide for acceptable levels and types of microbiological contamination in products, Dr. Dunnigan of the Bureau of Medicine of the FDA mented on the health hazard. In 1970, he said that topical preparations contaminated with gram negative organisms are a probable moderate to serious health hazard. Through the literature and through our investigations, it has been shown that a variety of infections have been traced to the gram negative contamination of topical products. The classical examplebeing the Pseudomonas cepacia contamination of Povidone Iodine products reported by a hospital in Massachusetts several years ago.Therefore, each pany is expected to develop microbial specifications for theirnon-sterile products. Likewise, the USP Microbial Limits Chapter <61> provides methodology for selected indicator organisms, but not all objectionable organisms. For example, it is widely recognized that Pseudomonascepacia is objectionable if found in a topical product or nasal solution in high numbers; yet, there are no test methods provided in the USP that will enable the identification of the presence of this microorganism.A relevant example of this problem is the recall of Metaproterenol Sulfate Inhalation Solution. The USP ##II monograph requires no microbial testing for this product. The agency classified this as a Class I recall because the product was contaminated with Pseudomonas gladioli/cepacia. The health hazard evaluation mented that the risk of pulmonary infection is especially serious and potentially life-threatening to patients with chronic obstructive airway disease, cystic fibrosis, and immuno-promised patients. Additionally, these organisms would not have been identified by testing procedures delineated in the general Microbial Limits section of the pendia.The USP currently provides for retests in the Microbial Limits section <61> however there is a current proposal to remove the retest provision. As with any other test, the results of initial test should be reviewed and investigated. Microbiological contamination is not evenly dispersed throughout a lot or sample of product and finding a contaminant in one sample and not in another does not discount the findings of the initial sample results. Retest results should be reviewed and evaluated, and particular emphasis should be placed on the logic and rationale for conducting the retest.In order to isolate specific microbial contaminants, FDA laboratories, as well as many in the industry, employ some type of enrichment media containing inactivators, such as Tween or lecithin. This is essential to inactivate preservatives usually present in these types of product and provides a better medium for damaged or slow growing cells. Other growth parameters include a lower temperature and longer incubation time (at least 5 days) that provide a better survival condition for damaged or slow-growing cells. For example, FDA laboratories use the test procedures for cosmetics in the Bacteriological Analytical Manual (BAM), 6th Edition, to identify contamination in non-sterile drug products. This testing includes an enrichment of a sample in modified letheen broth. After incubation, further identification is carried out on Blood Agar Plates and MacConkey Agar Plates. Isolated colonies are then identified. This procedure allows FDA microbiologists to optimize the recovery of all potential pathogens and to quantitate and speciate all recovered organisms. Another important aspect of proceduresused by FDA analysts is to determine growth promotion characteristics for all of the media used.The selection of the appropriate neutralizing agents are largely dependent upon the preservative and formulation of the product under evaluation. If there is growth in the enrichment broth, transfer to more selective agar media or suitable enrichment agar may be necessary for subsequent identification.Microbiological testing may include an identification of colonies found during the Total Aerobic Plate Count test. Again, the identification should not merely be limited to the USP indicator organisms.The importance of identifying all isolates from either or both Total Plate Count testing and enrichment testing will depend upon the product and its intended use. Obviously, if an oral solid dosage form such as a tablet is tested, it may be acceptable to identify isolates when testing shows high levels. However, for other products such as topicals, inhalants or nasal solutions where there is a major concern for microbiological contamination, isolates from plate counts, as well as enrichment testing, should be identified.III. FACILITIES, EQUIPMENT, AND MEDIABegin the inspection with a review of analyses being conducted and inspect the plates and tubes of media being incubated (caution should be exercised not to inadvertently contaminate plates or tubes of media on test). Be particularly alert for retests that have not been documented and “special projects〞 in which investigations of contamination problems have been identified. This can be evaluated by reviewing the ongoing analyses (product or environmental) for positive test results. Request to review the previous day’s plates and media, if available and pare your observations to the recorded entries in the logs. Inspect the autoclaves used for the sterilization of media. Autoclaves may lack the ability to displace steam with sterile filtered air. For sealed bottles of media, this would not present a problem. However, for non-sealed bottles or flasks of media, non-sterile air has led to the contamination of media. In addition, autoclaving less than the required time will also allow media associated contaminants to grow and cause a false positive result. These problems may be more prevalent in laboratories with a heavy workload.Check the temperature of the autoclave since overheating can denature and even char necessary nutrients. This allows for a less than optimal recovery of already stressed microorganisms. The obvious problem with potential false positives is the inability to differentiate between inadvertent medium contamination and true contamination directly associated with the sample tested.IV. STERILITY TESTINGOn 10/11/91, the Agency published a proposed rule regarding the manufacture of drug products by aseptic processing and terminal sterilization. A list of contaminated or potentially contaminated drug products made by aseptic processing and later recalled was also made available. Many of the investigations/inspections of the recalled products started with a list of initial sterility test failures. FDA review of the manufacturer's production, controls, investigations and their inadequacies, coupled with the evidence of product failure (initial sterility test failure) ultimately led to the action.The USP points out that the facilities used to conduct sterility tests should be similar to those used for manufacturing product. The USP states, "The facility for sterility testing should be such as to offer no greater a microbial challenge to the articles being tested than that of an aseptic processing production facility". Proper design would, therefore, include a gowning area and pass-through airlock. Environmental monitoring and gowning should be equivalent to that used for manufacturing product.Since a number of product and media manipulations are involved in conducting a sterility test, it is remended that the inspection include actual observation of the sterility test even though some panies have tried to discourage inspection on the grounds that it may make the firm's analyst nervous. The inspection team is expected to be sensitive to thisconcern and make the observations in a manner that will create the least amount of disruption in the normal operating environment. Nevertheless, such concerns are not sufficient cause for you to suspend this portion of the inspection.One of the most important aspects of the inspection of a sterility analytical program is to review records of initial positive sterility test results. Request lists of test failures to facilitate review of production and control records and investigation reports. Particularly, for the high risk aseptically filled product, initial positive sterility test results and investigations should be reviewed. It is difficult for the manufacturer to justify the release of a product filled aseptically that fails an initial sterility test without identifying specific problems associated with the controls used for the sterility test.Examine the use of negative controls. They are particularly important to a high quality sterility test. Good practice for such testing includes the use of known terminally sterilized or irradiated samples as a system control. Alternatively, vials or ampules filled during media fills have also been used.Be especially concerned about the case where a manufacturer of aseptically filled products has never found an initial positive sterility test. While such situations may occur, they are rare. In one case, a manufacturer's records showed that they had never found a positive result;their records had been falsified. Also, the absence of initial positives may indicate that the test has not been validated to demonstrate that there is no carryover of inhibition from the product or preservative.Inspect robotic systems or isolation technology, such as La Calhene units used for sterility testing. These units allow product withdrawal in the absence of people. If an initial test failure is noted in a sample tested in such a system, it could be very difficult to justify release based on a retest, particularly if test controls are negative.Evaluate the time period used for sterility test sample incubation. This issue has been recently clarified. The USP states that samples are to be incubated for at least 7 days, and a proposal has been made to change the USP to require a period of 14 days incubation. You are expected to evaluate the specific analytical procedure and the product for the proper incubation period. Seven days may be insufficient, particularly when slow growing organisms have been identified. Media fill, environmental, sterility test results and other data should be reviewed to assure the absence of slow growing organisms. Also, you should pare the methods being used for incubation to determine if they conform to those listed in approved or pending applications.V. METHODOLOGY ANDVALIDATION OF TESTPROCEDURESDetermine the source of test procedures. Manufacturers derive test procedures from several sources, including the USP, BAM and other microbiological references. It would be virtually impossible to pletely validate test procedures for every organism that may be objectionable. However, it is a good practice to assure that inhibitory substances in samples are neutralized.During inspections, including pre-approval inspections, evaluate the methodology for microbiological testing. For example, we expect test methods to identify the presence of organisms such as Pseudomonascepacia or other Pseudomonas species that may be objectional or present a hazard to the user. Where pre-approval inspections are being conducted, pare the method being used against the one submitted in the application. Also verify that the laboratory has the equipment necessary to perform the tests and that the equipment was available and in good operating condition on the dates of critical testing.The USP states that an alternate method may be substituted for pendial tests, provided it has been properly validated as giving equivalent or better results.You may find that dehydrated media are being used for the preparation of media. Good practice includes the periodic challenge of prepared media with low levels of organisms. This includes USP indicator organisms as well as normal flora. The capability of the media to promote the growthof organisms may be affected by the media preparation process, sterilization (overheating) and storage. These represent important considerations in any inspection and in the good management of a microbiology laboratory.VI. DATA STORAGEEvaluate the test results that have been entered in either logbooks or on loose analytical sheets. While some manufacturers may be reluctant to provide tabulations, summaries, or printouts of microbiological test results, this data should be reviewed for the identification of potential microbial problems in processing. When summaries of this data are not available the inspection team is expected to review enough data to construct their own summary of the laboratory test results and quality control program.Some laboratories utilize preprinted forms only for recording test data. Some laboratories have also pointed out that the only way microbiological test data could be reviewed during inspections would be to review individual batch records. However, in most cases, preprinted forms are in multiple copies with a second or third copy in a central file. Some panies use log-books for recording data. These logbooks should also be reviewed.Additionally, many manufacturers are equipped with an automated microbial system for the identification of microorganisms. Logs of such testing, along with the identification of the source of the sample, are also of value in the identification of potential microbial problems in processing.The utilization of automated systems for the identification of microorganisms is relatively mon in the parenteral manufacturer where isolates from the environment, water systems, validation and people are routinely identified.Microbiologists in our Baltimore District are expert on the use of automated microbic analytical systems. They were the first FDA laboratory to use such equipment and have considerable experience in validating methods for these pieces of equipment. Contact the Baltimore District laboratory for information or questions about these systems. Plants with heavy utilization of these pieces of equipment should be inspected by individuals from the Baltimore District laboratory.VII. MANAGEMENT REVIEWMicrobiological test results represent one of the more difficult areas for the evaluation and interpretation of data. These evaluations require extensive training and experience in microbiology. Understanding the methodology, and more importantly, understanding the limitations ofthe test present the more difficult issues. For example, a manufacturer found high counts of Enterobactercloacae in their oral dosage form product derived from a natural substance. Since they did not isolate E.coli, they released the product. FDA analysis found E.cloacae in most samples from the batch and even E.coli in one sample. In this case management failed to recognize that microbiological contamination might not be uniform, that other organisms may mask the presence of certain organisms when identification procedures are performed, and that microbiological testing is far from absolute. The inspection must consider the relationship between the organisms found in the samples and the potential for the existence of other objectionable conditions. For example, it is logical to assume that if the process would allow E.cloacae to be present, it could also allow the presence of the objectionable indicator organism. The microbiologist should evaluate this potential by considering such factors as methodology, and the growth conditions of the sample as well as other fundamental factors associated with microbiological analysis.Evaluate management's program to audit the quality of the laboratory work performed by outside contractors.VIII. CONTRACT TESTING LABORATORIESMany manufacturers contract with private or independent testing laboratories to analyze their products. Since, these laboratories willconduct only the tests that the manufacturer requests, determine the specific instructions given to the contractor. Evaluate these instructions to assure that necessary testing will be pleted. For example, in a recent inspection of a topical manufacturer, total plate count and testing for the USP indicator organisms were requested. The control laboratory performed this testing only and did not look for other organisms that would be objectionable based on the product's intended use.Analytical results, particularly for those articles in which additional or retesting is conducted, should be reviewed. Test reports should be provided to the manufacturer for tests conducted. It is not unusual to see contract laboratories fail to provide plete results, with both failing as well as passing results.Bacteriostasis/fungiostasis testing must be performed either by the contract lab or the manufacturer. These test results must be negative otherwise any sterility test results obtained by the contractor on the product may not be valid.DOC版.。
FDA检查员指导手册原料药生产检查(药品质量保证)FDA检查员指导手册7356.002F原料药生产检查(药品质量保证)第一部分背景总则法案的501(a)(2)(B)条款要求所有药品的生产都必须遵守现行GMP的要求,而原料药也不例外。
对于原料药和制剂这两者的要求,法案并没有区别对待,而任何原料药或制剂方面的GMP缺陷都构成了对法案的偏离。
对于原料药或药物成分来说,FDA并没有为此而专门发布cGMP法规文件(就像我们现在有的制剂cGMP法规一样)。
因此,本文提到的“cGMP”指的是法案要求,而并非美国联邦法规(CFR)第21部分210和211条款中关于制剂的要求。
其实,FDA早就意识到cGMP对制剂的要求(美国联邦法规第21部分210、211条款)在理念上对于原料药生产来说同样适用且有效。
这些理念包括使用合适的设备;聘用经过培训且通过资质确认的人员;建立充分合理的书面程序和控制,确保生产工艺和控制的有效性,从而保证产品质量;建立一套中间体和最终药品检测方法的体系,确保药品在规定的使用期限内保持质量的稳定性。
2001年,FDA在人用药物注册技术要求国际协调会议(ICH)上与其他政府监管部门共同努力,采用了针对API行业cGMP的国际性指南,也就是ICH Q7A,活性药物成分的药品质量管理的指南。
ICH Q7A正体现了FDA对于原料药现行GMP体系的要求。
因此,遵循该指南要求的API及其相关生产和检验设施是符合法定cGMP要求的。
然而,1只要是能符合法案501(a)(2)(B)的要求,并能确保API符合其纯度、均一性和质量特性的方法都可以采用。
在本程序中所使用的术语“活性药物成分”(原料药)的含义与ICH Q7A中的定义一致。
在ICH Q7A中活性药物成分被定义为“旨在用于药品生产的任何物质或混合物,当用于药品生产时,这些物质即成为药品中的活性成分。
这种物质被用来提供药学活性或在诊断、治疗、止痛、缓解、处理或疾病预防中起着直接作用或用于影响机体结构和功能。
美国FDA药品质量控制微生物实验室检查指南1993年I.导言《药品质量控制实验室检查指南》主要涉及许多有关药品实验室分析的化学方面的问题,对微生物实验室的检查仅提供了有限的指导,而本指南则是微生物分析检查过程的指导。
本指南建议,如同对任何实验室检查—样,在检查微生物实验室时,应有—名熟悉检验的分析学家(微生物学家)参与。
II.非无菌药品的微生物检验由于种种原因,局部药品、滴鼻剂和吸入剂存在许多微生物污染方面的问题.美国药典‘‘微生物属性”章(1111)指出“应该从药品用法、药品性质及时患者的潜在危害等方面评价微生物在非无菌药品中的重要性”,除此之外,没有提供具体的指导。
美国药典还建议应对某些种类的非无菌药品做常规的总菌数检验及某些特定的污染指示微生物的检验:例如:植物、动物和某些矿物质中的沙门氏菌属;口服液体中的大肠杆菌;局部用药品小的金黄色倘萄球菌和绿脓杆菌污染;以及:自肠、尿道、阴道用药中的酵母菌和霉菌:大量的专题文章还沦及厂微生物的限度。
作为非无菌药品受微生物污染的可接受程度和类型的—般性指导,美国食品和药品管理局药品分局的邓尼根博士曾强调其对健康的危害问题。
1970年他提出被革兰氏阴性细菌污染的局部制剂可能引起中度至重度的健康危害:文献和调查表明,许多感染都源于这种局部药品的革兰氏阴性细菌污染。
几年前马萨诸塞州的—家医院就报道过—宗络合碘(Povidonelodine)被洋葱假恤孢菌(Pseudomonas cepacia)污染的典型病例。
因此,每家公司都希望为自己的非无菌药品制订出一种关于微生物限度的标准,美国药典中“微生物限度”(USP61)提供了检验几种指示微生物的方法,但并末涉及所有有害微生物。
例如医药界普遍认为,洋葱假中孢菌在局部药品或滴鼻剂斗,大量存在是有害的,但美国药典没有提供证明这种微生物存在的检验方法。
间羟异丙肾上腺素硫酸盐吸入剂溶液的收回就是这方面的—个例子。
美国药典第XⅫ版各论部分没有要求对这种药品进行微生物检验。
药品管理局将其列为一级收回,因为此药受到了洋葱假单孢菌污染。
健康危害评估表明,这种微生物对肺部感染的风险很大,特别是对某些患有慢性气管阻塞、囊性纤维变性和免疫缺陷等病的患者具有潜在的生命危险。
此外,药典的微生物限度部分所描述的检验程度不能鉴别这些微生物。
现行美国药典在“微生物限度”部分(61)做了复检的规定,但是许多建议要求取消这种复检条款。
类似于其他检验,初次检验结果应予以审查与研究。
微生物污染并不是均匀地分布在一批药品或样品中的。
如果在一个样品中发现污染而在另—次取样样品中没有发现,则不应忽视初次发现的结果。
复检的结果应予以审查与评估,并且应特别注意进行复检的逻辑性和合理性。
为了分离出特定的微生物污染物,FDA实验室和制药工业的许多实验室,使用某些自钝化剂如吐温或卵磷脂的营养培养基,这对于钝化药品中常有的防腐剂的作用是非常必要的,还能为受损的或生长缓慢的微生物提供更好的基质;其他生长参数包括降低培养温度以及延长培养时间(至少5天),因为它们可以为这类微生物提供良好的生存条件。
例如:在《细菌学分析手册》(BAM)第Ⅵ版中,FDA实验室采用化妆品的检验方法鉴别非无菌药品中的污染物。
这项检验包括将样品置于改良Letheen肉汤中培养。
培养结束后,再用血琼脂平皿和麦球凯琼脂平皿进一步鉴定,然后再鉴别分离出的菌落。
FDA微生物学家用这种方法使所有潜在的病原体的复活达到最佳,并且测定复活的微生物数量及其类别。
FDA分析学家使用这种方法的另—个重要方面就是要确定所有用过的培养暴促进微生物生长的性能。
选择适当的中和剂很大程度上取决于防腐剂的性质和被评价药品所智的配方:如果在营养肉汤出现微生物生长,那么下一步的鉴别就可将样品转到更有选择性的琼脂培养基或适当的增菌琼脂上。
微生物检验可包括对在需氧菌总数检验小发现的菌落的鉴定。
另外上述鉴定不应仅限于美国药典中的指示微生物。
鉴别从微生物总数检验或(和)富集培养检验中分离的各种菌落的重要性,将取决于药品的种类及共其途。
很明显,如果检验—个口服固体制剂如片剂,当检验显示微生物污染量很大时,就可以考虑对分离出的菌落进行鉴别,然而对其他被微生物污染后易引起危害的药品,如局部用药品、吸人剂、滴鼻剂,应鉴别从前落计数及富集检验中分离的菌落。
III.设备、器材和培养基首先审查正在进行的分析,并检查培养中的培养皿.和试管(操作过程中不要疏忽大意而污染检验中的培养皿和试管)。
特别注意那些没有文字记录的复检和经凋查已经鉴别出有污染问题的“特殊项目”。
这种评价可以通过审查正在进行的分析是否有阳性结果来实现。
如有可能要审查前一天的培养皿和培养基,还要把观察结果与实验记录本中的记录进行比较。
检查用于培养基灭菌的高压蒸汽灭菌器。
高压蒸汽灭菌器可能缺少以无菌过滤的空气来代替蒸汽的能力。
对于密封的培养瓶,不会出现这种问题;然而对于不密封的培养瓶,非无菌的空气会导致培养基的污染。
此外,如高压灭菌的时间少于要求的时间,也会使培养基中的污染物生长,并得出错误的阳性结果。
在任务繁重的实验室里,这些问题可能更容易出现。
要检查高压蒸汽灭菌器的温度,温度过高会使必需的营养素变性甚至炭化,这可能使受到抑制的微生物达不到最佳复活。
潜在的假阳性结果带来的明显问题,是无法区别因疏忽大意而产生的培养基污染和直接来源于被检验样品的真正的污染。
Ⅳ。
无菌检验1991年10月11 日,药品管理局颁布了关于经无菌加工艺和最终灭菌生产药品的规定,并列出—份经无菌加工工艺生产的受污染或潜在污染而致收回的药品清单。
许多对收回药品的调查或检查都起因于初次无菌检验不合格。
FDA对生产厂的生产、质控、调查及其缺陷进行中核的结果以及最初无菌检验不合格的证据最终导致厂对这些药品的收回。
美国药典规定,用于进行无菌检验的设施与用于制药的设施应该是一致的,并规定,用于无菌检验的设施不应比无菌加工生产设施造成更多的微生物污染机率。
因此,合理的设计应该包括更衣区域和通过气闸。
环境的监测和着装应该与药品生产中相一致。
由于无菌检验中包括大量药品和培养基的操作,因此检查应包括对无菌检验的实地观察;即使有些公司曾借口实地检查可能会使分析人员紧张而试图阻止,检查组应注意此问题,使实地观察能以尽可能不干扰正常操作环境的方式进行。
然而这种考虑不足以成为放弃这部分检查的充分理由。
检查无菌检验程序最重要的方面之一就是审查元菌检验初次阳性结果的记录。
要求列出不合格的检验结果以便于审查生产、质控记录和调查报告,特别是对于风险性很大的无菌分装的药品,应审查无菌检验初次阳性结果和调查结果。
对于生产厂来说,如果无菌分装的产品在初次无菌检验时出现阳性结果,但无菌检验中的对照未发现异常,则很难决定是否发放该种产品。
应检查是否设立阴性对照品,设立阴性对照对高质量的无菌检验儿为重要;无菌检验规范的做法包括使用已知的最终灭菌或经辐射灭菌的样品作为系统对照,或者使用培养基模拟分装试验中分装的安瓿或西林瓶。
要特别注意生产无菌分装药品的厂家从未发现初次阳性无菌检验结果的情况,尽管这种情况可能发生,但毕竟少见。
曾经有一个案例,某生产厂的记录表明没有阳性结果,实际上他们的记录被改动了。
不存在初次阳性结果,还说明这项检查未通过验证来显示有没有来自于药品或防腐剂的残留抑制物的存在。
检查自动化无菌检验系统或无菌隔离技术,如用于无菌检验的La Calhene装置,这些装置保证了不需要人直接移取样品。
如果被检样品在此系统中检验发现初次检验不合格,很难根据—次复检决定药品是否发放,特别是检验对照是阴性的时候。
评价无菌检验样品的培养时间。
该问题已被阐明,美国药典提出样品至少需要培养7天,而—项提议要求美国药典将其改为14天;希望诸位能评价—项具体的分析过程和产品以确定其最适培养时间。
7天可能不够充分,特别是发现有生长过程缓慢的微生物存在时,培养基灌装、环境、无菌检验结果和其他数据应加以审查以确保没有发现生长缓慢的微生物。
此外,还应比较各种培养方法,以确定它们是否符合已被批准或将被批准的申请书中所列的方法。
V.方法和检验程序的验证确定检验程序的来源。
生产厂的检验程序有几个方面的来源,如美国药典、《细菌学分析手册》及其他微生物参考资料。
事实上对各种有害微生物进行的检验程序不可能得到全面验证,但是应确保样品中的抑制物被中和。
在检查过程中,包括批准前的检查中,要评价微生物检验的方法。
例如我们希望检验方法可以鉴别一些微生物的存在,如洋葱假单孢菌或其他有害的假单孢菌属。
在进行批准前检查时,要将正在使用的方法和申请中呈递的方法进行比较,同时证实实验室拥有必要的检验设备,这些设备在进行关键检验时确实可用并处于良好的运行状态。
美国药典规定,如果已经过正确的验证,另一种方法可以得出同样甚至更好的结果,它可以用来代替法定检验方法。
你也许会发现人们已使用脱水的培养基来制备培养基。
良好的操作包括定期用极少量微生物去测试制备好的培养基,包括使用美国药典规定的指示微生物和正常菌群。
培养基促进生长的能力可能受到培养基制备程序、灭菌过程(过热)和储存的影响,它们是对任何实验室进行检查的重要指标,也是实现微生物实验室优良管理的重要考虑事项。
Ⅵ.数据储存评价检验结果是记录在实验记录本(logbooks)上还是记录在活页检验单上,由于有些生产厂可能不愿提供表明微生物检验结果的表格、总结或打印稿,应当审查数据来鉴别生产过程中潜在的微生物。
当得不到此数据的总结时,检验组应审查充分的数据来建立他们自己对于实验室结果和质量控制程序的总结。
一些实验室将事先印好的表格用于记录检验数据。
有些实验室还指出在检查中审查微生物检验数据的唯一途径是审查单批记录。
然而在大多数情况下,事先印出的表格都是一式几份,而第二,三份总是存在中心档案中。
一些公司以实验记录本记录资料,这些实验记录本也应该被查阅。
另外,很多生产厂装备厂自动化微生物系统,如用于鉴别微生物的Vitek装置。
这种检验的记录和对样品来源的鉴别对于识别生产过程中潜在的微土物问题具有同样价值。
鉴别微生物的自动化系统比较普遍地用于制造非肠道药品的生产厂,用于对从环境、水系统、验证过程和人员的分离物的常规鉴别。
我们巴尔的摩地区的微生物学家都是自动化微生物分析系统使用方面的专家。
他们最先在FDA实验室里使用这种设备并且对于验证这些设备的方法有丰富的经验。
关于该系统的情况或有关问题,可以向巴尔的摩地区实验室查询。
另外,大规模使用这类设备的应当接受来自巴尔的摩地区实验室专家的检查。
Ⅶ.管理审查微生物检验结果是数据评价及解释中一个较难的领域。
评价微生物检验结果需要受过微生物学的专业训练并具备经验。
要厂解检验的方法,而且更重要及更难的是要厂解检验的局限性。