QA TallySheet at Factory-Form
- 格式:xls
- 大小:383.00 KB
- 文档页数:10

WOOLWORTHS LIMITED 澳大利亚沃尔沃斯有限公司Quality Management andEthical Audit Program质量管理和伦理审计程序Vendor Pack供应商材料包内容What we require from you 概述 (3)1.0 Introduction to the Ethical Sourcing Policy & Audit Program关于道德采购政策&审计程序的介绍 (4)2.0 Supplier Responsibilities供应商职责 (12)3.0 Audit Process审计过程 (13)3.1 Supplier Preparation供应商准备 (14)3.2 Audit Scheduling审查时间进度表 (16)3.3 Conducting the Audit进行审计 (17)3.4 Audit Outcome审计结果 (18)4.0 Certification Bodies认证机构 (24)5.0 Confidentiality机密 (26)6.0 Appendix附件 (27)Appendix A – Critical Non-Conformances附录A -关键的不符合项 (28)Appendix B – Pre-Audit Questionnaire附录B -事先审计问卷 (29)Appendix C – Factory Checklist附录C -工厂检查表 (30)Appendix D – Additional Contacts for Approved Certification Bodies附录D其他认证机构33What we require from you 概述Below is a checklist that outlines what we require from you within 2 weeks of receiving this supplier pack:以下是一份清单概括了收到这份供应商材料包后你需要在两个星期内做的Contact detailsShould you have any concerns regarding your specific circumstances, please contact consumergoodsquality@.au or your relevant Woolworths’ business unit buying contact for further assistance.联系细节:如果您有任何关于您具体情况的问题,请联系以下邮箱:consumergoodsquality@.au 或者联系沃尔沃斯的相关业务单位来获得进一步的帮助。
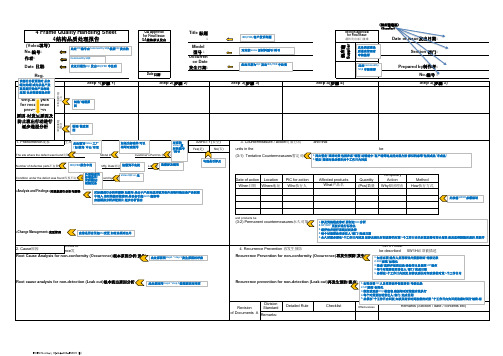
(供应商填写) (Supplier)QA Approval for Final Issue QA 最终承认发出Title 标题 :(Volex 填写)Report by 制作者:Reg.Date 日期:Site 发生场所, number of units 数量, condition(s)条件.Tentative and final countermeasures 暂定和永久对策/ number of units affected 受影响产品数量Yes(是)Action for units in the market, warehouse and factory 对市场及仓库产品的行动 5W1H must be described(5W1H 必须被描述Date of action Location PIC for action Quantity Temporary Action Method When 日期Where 地址Who 执行人(Pcs)数量Why 原因理由How 执行方式All raw materials and products be confimed are OK exdept the contanimated one 所有的原材料和成品都被确认OKOccurrence 发生, detection 检测(leak out)流出5W1H must be described 5W1H 必须被描述Division Standard Detailed Rule Confirm EffectivenessProblem found -> Fill in available information for quick information sharing -> Problem solved -> Submit to QA by request from QA-TL -> Approved, registered and distributed by QA/Confirm effectiveness by QA or QCModel 型号 :供应商S u p p l i e rStep 4(步骤 4)Number of defective parts 不良数量: Mfg. Date 制造日期 : Lot # 批号:Occurren ce Date 发生日期:Step 3(步骤 3)检测Detection(3-1) Tentative Countermeasures 暂定对策Step(步骤)(3-2) Permanent countermeasures 永久对策What 产品名4 Frame Quality Handling Sheet4结构品质处理报告No.编号:Date 日期:Root cause analysis for non-detection (Leak out)根本流出原因分析Root Cause Analysis for non-conformity (Occurrence)根本原因分析(发生)Why-Analysis for recurrence prevention 原因-对发生原因及防止流出行动进行逐步递层分析Step 1(步骤 1)Step 2(步骤 2)Prepared by 制作者:No.编号 :Affected products No(否)发生Occurrence 1. Phenomenon 现象<Change Management>变更管理2. Cause 原因Recurrence prevention for non-detection (Leak out)再发生预防(流出)Recurrence Prevention for non-conformity (Occurrence)再发生预防(发生)Revision of Documents AStep 5(步骤Section Approval for Final Issue 最终发出部门批准Date of issue 发出日期:Section 部门:SAFETY(安全)Checklist The site where the defect was found 发现地: Model # 型号: Customer's Part No.客户产品名:3. Countermeasure / action 对策行动Condition under the defect was found 发现不良条件: Found by 谁发现 :<Analysis and Findings>对现象进行分析与解释Remarks:4. Recurrence Prevention 再发生预防Remarks (Section / date /此处IRR 编号由Commodity SQE 根据IQC 发出的Commodity SQE此处日期为IQC 发出IRR/PRR 中注明的IRR/PRR/客户投诉问题对应的Volex 原材料型号/料号此处日期为IQC 发出IRR/PRR 中注明的日此处Commodity SQE 审核签署此处供应商品质最高管理者审核签署该部分分析原因时,从末端向始端(或为从客户发现问题开始向产品制造过程)由后到前倒退分析制造/制程原因检测/检查原因此处填写Volex 工厂(如横岗/中山/印尼对应的Volex 原材料型号/料号IRR/PRR 报告中的不良数量/比例追溯到不良批次追溯涉及到的不Volex IQC or 生勾选是否涉及安对标题进行分析和解释,如脏污-是由于产品完成后被其他外来物污染还是产品制程中混入,该污染能否被清理,是否会引起RoHS 超标等应做模拟分析并配图片,逐步分析说明改善是否会引起4M 变更-如有必须列出并说此处原因同step1~step 5发生原因相呼应此处原因同step1~step 5检测原因相呼应1.列出暂定/围堵对策,包括在库/制程/运输途中/客户端等地点的处理方法,标识状态等(包括成品/半成品)2.暂定/围堵对策必须在两个工作日内回复1.涉及到流程改善时,要制定DOE 分析2.SIP/SOP 更新后进行标准化3.须评估并进行系统层面改善4.每个对策要注明责任人/部门/完成日期5.永久对策必须在5个工作日内回复,如涉及到长时间改善的对策,5个工作日内先回复改善时间计划表1.如有必要,操作人员再培训并保留培训/考核记录2.SOP 规范/标准化3.检查/监控并保留记录,确保作业员按照SOP 操作4.每个对策要注明责任人/部门/完成日期5.必须在5个工作日内回复,如涉及到长时间改善的对策复改善时间计划表,相应改善措施完成后,更新并提交该报1.如有必要,QC 人员再培训并保留培训/考核记录2.SIP 规范/标准化3.制定或更新IPQC 检查表,确保每项对策被有效执行4.每个对策要注明责任人/部门/完成日期5.必须在5个工作日内回复,如涉及到长时间改善的对策,5个工作日内如果为标准件,可以填写对应型号外观检查的标准条件/何种测试/检测设备受影响产品数量必须被描述)暂定对策永久对策步骤 5)date / contents etc)成品)此表格5W1H 必须填写划表,相应改善措施完成后,更新并提交该对策,5个工作日内先回交该报告,直至关闭作日内先回复改善时间计划表,相应改。

Instructions to Complete Form l:Part Number Accountability 填写表格1 零件编号符合性This form is used to identify the part that is being first—article inspected(FAI part)and associated subassemblies or detail parts.本表格用于说明进行了首件检验(FAM)的零件,和有关部装件或零件。
NOTE注意:1.The ABC Daycode and the Total Sheets Contained in this Report to be at the top of the QADl61 sheet.ABC公司的日期代码和在本报告中的总张数置于QADl61表的顶端.2.Fields 1-4 are repeated on all forms for convenience and traceability.为便于可追溯性,第1-4部分在所有表格上都予以重复。
1) (R) Part Number:Number of the part(FM part).零件编号:零件的编号(用于首件检验的零件)。
2) (R) Part Name:Name of the part as shown on the drawing.零件名称:图样上所示的零件的名称。
3) (CR) Serial number:Serial number of the part.系列编号:零件的系列编号。
4) (R) FAI Report Number:Reference number that identifies the FAI.For ABC this will be Q Number for Subcontractors theirWork Numbers.首件检验报告编号:用于明确首件检验的引用编号。

VENDOR AUDIT FORM 工厂调查表(VAF11.2)Audit date 调查日期:____________________ Audited by 调查人员姓名: _____________________Contact person’s name (English & Chinese) & title 联系人姓名(中英文)和职称:Contact person’s tel联系人电话号码:Contact person’s email 联系人电子信箱:Does the contact person speak, read and write English? 联系人是否会讲, 写, 看英语:Production manager’s name &tel生产主管姓名和电话:Quality manager’s name &tel质检主管姓名和电话:Engineer’s name &tel工程师姓名和电话:How was this vendor found?通过什么渠道获知该厂家:[Attach copies of business cards附上以上人士的名片]Location 地理位置City & Town 城市, 乡镇:Province 省份:Address地址:Zip Code邮编:Nearest major city 离厂家最近的大城市:Distance from nearest major city (km) 距离最近的大城市路程(公里):Recommended nearby hotel name, address &tel (English & Chinese)推荐该厂家附近的酒店名称, 地址(中英文) 和电话:Products & Customers 产品与客户[Attach product list/catalog with price list, if applicable 附上产品目录和价目表]Description of main products manufactured 描写主要产品:% of products produced for export 出口产品的百分率:Main export markets 主要的出口国家:Main customers主要的客户:How does vendor acquire new customers? 加工商如何找到新客户:Planned shows and exhibitions打算参加的展会:Ever visited by foreign customers? 是否接待国外客户:Does vendor have any limits or conditions for cooperation? 厂家是否有合作条件或限制:Physical & Organizational Characteristics & Scope 厂家建筑规模和人员结构[Attach an Organizational Chart附上厂家的结构图]Total # of employees 职工总人数(包括工人):Overall score for condition & cleanliness of facilities 厂房的清洁条件总评分………... _____Manufacturing Practices &Capabilities 厂家的生产方式和能力[Attach a facilities list附上主要设备清单]Main products manufactured & services offered主要产品和服务:Output capacity of main products主要产品设计产量:Current average production output 目前实际平均产量:Current working schedule厂家的工作班次:Primary raw material & components supplier(s) 主要的原料和元器件供应商:Raw materials procurement practices (spot buying or contractual relationships with R.M. suppliers)采购原材料的方式(与固定的供应商有合同还是在现货市场采购):Product warranties & after sale support offered (if applicable)厂家提供的售后维修服务和担保负责事项:In-house tool & die-making capabilities厂家加工模具的能力:Is there a system with records for maintaining & storing molds& dies?厂家有无存放和管理模具与工具的系统记录:In-house testing & measuring devices厂内有无产品测试和测量仪器, 仪表:Are there records of equipment calibration? 质检测试仪器仪表是否定期做校准:ODM/contract manufacturing capabilities能否按客户要求开发生产新产品:Design/R&D capabilities 设计与研发能力:Is there a written preventive maintenance schedule with dated records? 有无书面维修和保养设备的方案的日期纪录: Backup electrical generator or emergency lighting?紧急发电器和照明等:List any outsourced contracted manufacturing services注明该厂外承包的加工服务:Quality Assurance Practices 质量保证措施Does vendor have any Quality Assurance certifications? (list certification type, attainment date, validity date and cert. #) 厂家是否有质检认证书(注明每种证书, 获得日期, 截止日,和证书号码):Is there an independent quality department with managerial authority to initiate preventive action and stop production?厂家有无独立的质检部门; 质检部门是否有权利停止生产和实行预防措施:Number of dedicated quality control personnel 质检专业人员人数:Is there a quality manual or documented quality procedures?有无书面手册或质检程序:Latest revisions and dates stated? 书面质检程序是否更新并记录更新日:Quality manual available to all personnel? 每个员工是否都能随时查看书面质检程序:Is their a formal written corrective action plan? 是否有正式的, 书面的预防措施:Dedicated full-time safety management personnel? 是否有专职负责安全的工作人员:Does the company pay overtime salary in accordance with labor law?厂家是否有按照劳动法支付员工加班工资:Does the company pay social benefits for all employees in accordance with labor law?厂家是否有按照劳动法缴纳所有员工的社会保险费:Does the company have a human resources professional or channel for receiving and dealing with employee complaints& suggestions?厂家是否有人员或渠道接受并处理员工的投诉和建议:Are there written safety rules & regulations?厂家是否有书面安全规章制度:Does vendor provide safety training for workers? 厂家是否有对员工做安全方面的培训:Is there a written emergency response plan?是否有书面的应急预案:Are dangerous machines outfitted with safety apparatus? 危险机器设备上是否有安全防护装置:Are personnel performing dangerous jobs given adequate personal protective equipment?有危险的工种是否有充分的个人保护装置:Are open stairs, platforms and elevated floors guarded by railing on open sides? 楼梯、平台和高台是否有栏杆保护:Are there any records of regular safety inspections? 是否有定期安全检查记录:Is there a system to report injuries and workplace illnesses to management? 员工受伤或生病是否有体系可汇报到相关的管理部门:Are there records of the company’s serious accidents? (e.g. fire, injury, death)厂家是否有严重事故的记录(比如火宅、工伤、死亡等):Do special machine operators have relevant and valid work permits? 特殊工种是否有相关有效的许可证:Are all certificates valid? (e.g. elevators, special machines & equipment) 所有的许可证是否在有效期内(比如电梯、特殊机器设备等):Have all bldgs passed fire safety inspection approval? 是否所有楼都有通过消防验收:Have fire drills been conducted? Are there records? 是否有定期消防演习?是否有记录:Is there a fire extinguisher in each workshop?每个车间是否都有灭火器:Are emergency first aid supplies or facilities available? Any personnel trained in first aid and CPR? 是否有紧急急救设备?是否有员工参加过急救及心肺复苏术培训:Are all the exits (including emergency) and passageways clearly marked and kept clear of any obstructions? 所有出口(包括紧急出口)及通道是否有明显标识并保持通畅:Do the facilities have adequate lighting? 车间是否有充足照明:Do the facilities have adequate ventilation? 车间是否有良好的机械通风:Is the temperature of the workshops excessively hot or cold? 车间的温度会不会过份高或者过份低:Audit Checklist (Items to Collect) 调查时该收集的事项Two brochures or product catalogues 两份样本或产品目录Photos 照片Samples 样品Facilities list 主要设备清单Organizational chart 公司结构图Videos of core processes主要生产过程的录像Photo checklist 照片内容Image of contact person联系人的照片Image of general manager 总经理的照片Image [or photocopy] of business license 营业执照照片或复印件Main facility from the outside 工厂主楼的外观环境Sample room & main products produced样品室或主要生产的产品Anything that stands out, either negatively or positively, with regard to the vendor’s QA or EHS practices任何突出的质量保证体系方面或环境, 健康, 安全及劳工政策方面的问题或特点Overview of inside of facility and each operation 工厂内的环境和各个主要的车间和流水线Raw materials inventory storage area原材料存货区域Main workshops & assembly lines主要的车间和加工流水线Main manufacturing equipment/machines主要生产设备Tools & dies storage, maintenance and fabrication 工具和摸具储存, 修理及加工Testing & inspection area 测试和检验区域Finished goods storage area成品储存区域Product packaging & export packing 产品包装和出口包装Design area产品设计区域Offices/administrative area行政区域和办公室Additional Notes附加注意事项[Attach additional pages as needed如纸张不够请加页]。

3C认证工厂检查调查表中文Factory code:工厂检查调查表Questionnaire for Factory Inspection申请人名称:Name of Applicant:制造商名称:Name of Manufacturer:生产厂名称:Name of Factory:中国质量认证中心China Quality Certification Center1.1 工厂注册名称:Name of the factory:工厂地址:Address of the factory:电子邮件:抵达工厂的最佳交通路线(最近的火车站、机场;如可能,请附一张当地地图)The Optimal route to factory [local Railway station, airport (with the map if possible)]制造商注册名称:Name of the manufacturer:制造商注册地址:Address of the manufacturer:电子邮件:申请人注册名称:Name of the applicant:地址:Address:电子邮件:工厂质量负责人姓名、工作部门及职位、联系电话工厂职员总数(如申证产品生产仅是其中一部分,请注明与申证产品生产、治理有关的职员人数)Total amount of employees in the factory (If the production for the c ertified product is just one part of the whole production, pleaseindicate t he amount of employees for the production and management onthe produ cts applied for certification)申证产品申请编号、名称、型号规格、商标The application No., name, model /specification and trade mark of th e products applied for certification申请产品认证依据的标准Pursuant standards of the products applied for certification工厂是否按认证实施规则的要求建立文件化质量体系?提供①组织机构图②质量手册名目③程序文件名目。
工廠評估報告Manufacturer 工廠:Date of Audit 評估日期:Reason of Audit 評估原因:New Manufacturer 新廠Other 其他Re-Assessment 再次評估Result 結論:Total of Points Possible總分數Products under Consideration 待評估產品:Conclusion 總結:Review Necessary是否需要再次評估:AUDITED BY : _______________________________ DATE : _______________________Following information to be filled by supplier (供應商須填寫以下資料) Head Office Location 總公司地址:Date Company established 公司成立日期:Date this Factory established 工廠成立日期:Other related factories / external supporting 其它工廠:Main Product Lines 主要產品(Please mark product categories with turnover %, 請註明產品類別所佔百分比) :Export Destination 出口地(Please mark destination with turnover %,請註明出口地所佔的百分比):Major Customer 主要客戶(Please mark customer with turnover %,請註明主要客戶所佔的百分比):Major Machineries/Equipment 主要机器設備(Please provide addition list if required, 如有需要, 請提供附頁) :Factory Area 工廠面積:Person contacted/Positions held 負責人姓名及職位:Turnover per Annum每年的營業額:Following information to be filled by supplier (供應商須填寫以下資料)Number of Employees員工人數: HK China(1)Production生產人員:(2)Quality Assurance品保人員:(3)Management & Others管理及其它人員(4)R&D / Engineering研究與開發/工程人員_____________________________________________ QC Department report to 品保部向何人負責:Quality Sampling Plan品質抽樣計劃i) Incoming Inspection進料檢查:Sampling Plan抽樣計劃:A.Q.L. Safety(安全性) : __________________________________________Major (主要性) : __________________________________________Minor (次要性) : __________________________________________ii) Production 生產及流程檢查Sampling Plan抽樣計劃:A.Q.L. Safety(安全性) : ___________________________________________Major(主要性) : ___________________________________________Minor(次要性) : ___________________________________________iii) Pre-Shipment Inspection出貨檢查:Sampling Plan抽樣計劃:A.Q.L. Safety(安全性) : ________________________________________________Major(主要性) : _________________________________________________Minor(次要性) :Audit Details 評估詳情(A)Quality Organization品質体系:___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ Score 得分: __________ / 25(B)Research & Development and Engineering 開發及工程:___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ Score 得分: __________ / 30C) Production & In-Process Controls 生產及流程控制:___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ Score 得分: __________/ 25(D) General 總則___________________________________________________________________________ ___________________________________________________________________________ ___________________________________________________________________________ Score 得分: __________/ 20Any other comments其它評論:________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________Sum of points 得分總計: _________Total actual points 實際總得分: _________Signed: Date : Manufacturer 工廠: _____________________ __________________ Agent 代理商: _____________________ __________________(A) Quality Organization 品質體系Points Is the quality organization sufficiently independent from the manufacturing process?在生產過程中,品質體系是否獨立?(3)Is there a quality manual? Does the working procedure conform to the manual?是否有品質手冊?工序流程是否符合品質手冊? (2)Is there has adequate procedure for incoming, outgoing non-conformance & deviation review?對於不符要求或條件偏差的來料及外送物料, 是否有進行適當的復審程序? (2)Is there any corrective action procedure for internal quality problem and customer complaint?對於內部的質量問題及客戶抱怨, 是否有實施改正行動?Is there evidence that such data is acted upon?是否有證據顯示已經採取相應行動?(2)Is a new vendor approval / evaluation system in place?有沒有新供應商認可/ 評估系統? (2)Is a vendor rating system in place? (Vendor approval list / Vendor performance rating / Vendorevaluation, etc.)供應商的等級系統是否存在?(認可供應商目錄/工作評級/評估, etc) (2)Are incoming materials/components inspected in accordance with the drawings/ specifications,and sampling plan?來料是否根據圖紙/ 規格及抽樣計劃去檢查?(2)Are rejected components segregated and identified?不合格的零件是否分開和區分?(2)Are reworked products subject to a second inspection?翻工的產品是否需作第二次檢查?(2)Is pre-shipment inspection verification of products carried out?是否執行出貨檢查?(2)Is the finding fed back to related department and the improvement scheme will be in place?有關問題是否反饋給有關部門及是否有適當的改善方案? (2)Is the verification carried out in accordance with the drawings / specifications & uniquechecklists with defect classification on pre-shipment inspection?出貨檢查是否根據圖紙/ 規格,和具有次品等級的檢查表?(2)SUB-TOTAL: (本部得分) (25)(B) Research & Development / Engineering開發及工程Points Is the R&D process adequate to ensure reliability?開發程序是否能夠保証產品的可靠性?(5)Are products subjected to reliability testing (accelerated life/destructive testing)?產品是否能通過可靠性測試(加速壽命和破壞性測試)?(5)Is user research carried out?是否執行用戶∕使用者的調查?(5)Does the company have enough facilities (equipment, computer) and manpower for productdevelopment / Engineering?是否擁有足夠的設施(器械,電腦)及人力作為產品開發及工程使用? (5)Does the company have any past experience & capabilities to apply international safetystandards (e.g. UL, CEBEC, BSI, Heavy Metal Content Regulations, etc.)?是否擁有足夠經驗及能力申請國際性安全規格(e.g. UL, CEBEC, BSI, Heavy MetalContent Regulations, etc.)? (5)Are the relevant files (technical and safely standard) available and adequate? Please give detailsbelow.技術資料是否存在以及足夠使用?請填在下面。
HP Pilot系统验收测试表FAT-QA-001 A/0 Page 1 of 9 客户名称:项目编号:DOCUMENT APPROVAL SHEET文件审阅表编制:审核:批准:修订日期版本修订者修订内容客户确认2012.10.25 A/0 初版This Document contains proprietary information of Lisure and shall not be published, reproduced, copied or used for any purpose except as expressly permitted or directed by Lisure本文件包含利穗的专有信息,除非由利穗允许HP Pilot系统验收测试表FAT-QA-001 A/0 Page 2 of 9 客户名称:项目编号:目录文件适用范围 (3)1、设备确认 (4)2.部件检查 (5)3、泵头检查 (6)4.压力测试 (7)5.功能性测试 (8)偏差表 (9)This Document contains proprietary information of Lisure and shall not be published, reproduced, copied or used for any purpose except as expressly permitted or directed by Lisure本文件包含利穗的专有信息,除非由利穗允许HP Pilot系统验收测试表FAT-QA-001 A/0 Page 3 of 9 客户名称:项目编号:文件适用范围本文件用于设备的出厂验收。
列出了设备的检查测试内容和步骤。
This Document contains proprietary information of Lisure and shall not be published, reproduced, copied or used for any purpose except as expressly permitted or directed by Lisure本文件包含利穗的专有信息,除非由利穗允许HP Pilot 系统验收测试表FAT-QA-001A/0 Page 4 of 9客户名称:项目编号:This Document contains proprietary information of Lisure and shall not be published, reproduced, copied or usedfor any purpose except as expressly permitted or directed by Lisure 本文件包含利穗的专有信息,除非由利穗允许1、设备确认测试描述:以下测试用于验证设备与客户订购信息一致。
产品安全认证Product Safety Certification工厂审查调查表The Questionnaire For Factory Inspection申请人名称:Name of Applicant:制造商名称:Name of Manufacturer:制造厂名称:Name of factory:产品安全认证工厂审查调查表The Questionnaire of Factory Inspection forProduct Safety Certification1.1制造厂/商注册名称:Name of the factory / Manufacturer:工厂地址:Address of the factory:电话(含区号): 传真:邮政编码:Tel: Fax: Post Code电子邮件:E-mail:抵达工厂的最佳交通路线(最近的火车站、机场;如可能,请附一张当地地图)The Optimal route to factory [local Railway station, airport (with the map if possible)]1.2制造厂/商的办公地址:Address of the factory/Manufacturer Office :电话(含区号): 传真:邮政编码:Tel : Fax: Post Code:电子邮件:E-mail:2.申请人(持证人)注册名称:Name of the applicant (certificate holder):地址:Address:电话(含区号): 传真:邮政编码:Tel: Fax: Post Code:电子邮件:E-mail:认证责任人:部门及职位:电话:Undertaker of certification: Department/position: Tel:3.制造厂/商质量保证负责人及认证联络工程师(或联络员)姓名、工作部门及职位、联系电话(请附任命书复印件一份及其个人技术工作简历)。
DATE:PSS REP:FACTORY REP:Step 1-Record po batch information:Delivery Date
NST:
NLT:
SO#: COUNTRY:Buyer Approved Sample PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingMONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)Yes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR:
Determine batch size:Sample Size Rejection Critical Number
INSPECTED CASE#:
Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:
DEFECT CODENo. of DefectsCOMMENTSCRMJMN
DISPOSITION:DATE: PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingStep 1-Record po batch information:Delivery DateNST: NLT: MONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)SO#: COUNTRY:Buyer Approved SampleYes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR: Determine batch size:Sample Size Rejection Critical NumberINSPECTED CASE#: Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:DEFECT CODENo. of DefectsCOMMENTSCRMJMNDISPOSITION:PSS REP:FACTORY REP:DATE: PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingStep 1-Record po batch information:Delivery DateNST: NLT: MONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)SO#: COUNTRY:Buyer Approved SampleYes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR: Determine batch size:Sample Size Rejection Critical NumberINSPECTED CASE#: Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:DEFECT CODENo. of DefectsCOMMENTSCRMJMNDISPOSITION:PSS REP:FACTORY REP:DATE: PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingStep 1-Record po batch information:Delivery DateNST: NLT: MONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)SO#: COUNTRY:Buyer Approved SampleYes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR: Determine batch size:Sample Size Rejection Critical NumberINSPECTED CASE#: Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:DEFECT CODENo. of DefectsCOMMENTSCRMJMNDISPOSITION:PSS REP:FACTORY REP:DATE: PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingStep 1-Record po batch information:Delivery DateNST: NLT: MONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)SO#: COUNTRY:Buyer Approved SampleYes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR: Determine batch size:Sample Size Rejection Critical NumberINSPECTED CASE#: Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:DEFECT CODENo. of DefectsCOMMENTSCRMJMNDISPOSITION:PSS REP:FACTORY REP:DATE: PAYLESS SHOESOURCE FOOTWEAR AND ACCESSORY INSPECTION TALLY SHEET Sampling plan forAesthetics and packagingStep 1-Record po batch information:Delivery DateNST: NLT: MONTH: DAY: YEAR: 2017AGENT: PSSINSP#: TALLY SHEET#:PO#:FACTORY:PLACE:(DC-FAC-CFS)SO#: COUNTRY:Buyer Approved SampleYes NoLOT#:DEPT#:COLOR:DESCRIPTION OF PRODUCT: PRODUCT CODE#:PO QTY: PRSABT GIRL S PK BALLETQTY INSPECTED: PRSBATCH QTY: PRSPRICE ON:TICKET:OSR: Determine batch size:Sample Size Rejection Critical NumberINSPECTED CASE#: Batch SizePairs To InspectCritical AQLMajor AQLMinor AQL1-500 PAIRS20124501-1,200 PAIRS321351,201-3,200 PAIRS501463,201-10,000 PAIRS8015810,001-35,000 PAIRS1251610MORE THAN 35,000 PAIRS2001813Step 2 - Record total defects found and comments below:DEFECT CODENo. of DefectsCOMMENTSCRMJMNDISPOSITION:PSS REP:FACTORY REP: