阿托伐他汀钙片CTD格式模板资料(制剂)
- 格式:doc
- 大小:336.50 KB
- 文档页数:29
![阿托伐他汀钙片CTD格式模板资料[制剂]](https://uimg.taocdn.com/56627beef12d2af90342e678.webp)
CTD格式8号申报资料主要研究信息(药学部分:制剂)3.2.P.1 剂型选择依据及产品组成(1)本品为普通片剂,规格为:10mg每片XXXXX片的处方组成见表3.2.P.1。
附表3.2.P.1XXXXX片药物组成表(2)本品在制备过程中没有使用专用的溶剂。
(3)XXXXX片的内包装材料为铝塑板,有避光和防潮的作用,与XXXXX 的内包装材质相同。
内包材的生产厂家具有内包材注册证,执行国家标准。
3.2.P.2 产品开发XXXXX是HMG-CoA还原酶的一选择性、竞争性抑制剂,可以显著降低胆固醇水平,并降低心肌梗死或脑卒中的发病危险。
临床试验已经证实XXXXX 降低胆固醇的临床疗效明显优于其它汀类药物,对原发性高胆固醇血症、包括家族性高胆固醇血症或混合型高脂血症患者以及纯合子家族性高胆固醇血症者有明显疗效。
XXXXX(商品名)作为目前世界上顶级降血脂药物,由美国华纳-兰伯特公司研制开发。
1997年上市,之后并入辉瑞公司。
自1998年以来取得了优异的业绩,成为当今世界增长最快的药品,连续三年名列全球畅销处方药第一位,2003年全球销售92.3亿美元,2004年高达108.6亿美元。
华纳-兰伯特公司的XXXXX在1999年9月获准中国申请药品行政保护,在国内由XX辉瑞生产销售。
目前,国内原红惠制药(现更名嘉林药业)已获得XXXXX及片剂产品的生产批文,天方药业研制开发XXXXX胶囊,于2005年9月29日获得新药证书和药品注册批件。
我公司立项仿制XXXXX片,规格与辉瑞制药(XX)XX的XXXXX相同,为:10mg/片。
3.2.P.2.1 处方组成3.2.P.2.1.1 原料药性状:白色至类白色结晶性粉末。
溶解性:XXXXX不溶于PH≤4的水溶液,能微溶于蒸馏水、PH为7.4的磷酸盐缓冲液、乙腈,轻度微溶于乙醇,易溶于甲醇。
贮藏:遮光,密封保存。
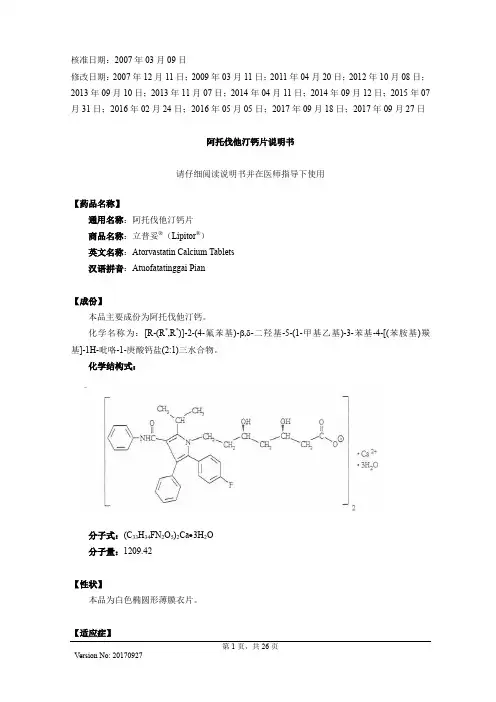
有效期:48个月分子式:XXXXX分子量:XXXX化学名:XXXXXXXXXXXX英文名:Atorvastatin Calcium化学结构式:XXXXXXXXXXXX生产厂家:XXXXX批准文号:XXXXXX质量标准:药品注册标准YBH00262010原料检验:依据药品注册标准XXXX检测XXXX原料,有关物质图谱于参比制剂的有关物质图谱进行对比。

目录3.2.S.1 基本信息 (3)3.2.S.1.1药品名称 (3)3.2.S.1.2结构 (3)3.2.S.1.3理化性质 (3)3.2.S.2 生产信息 (3)3.2.S.2.1生产商 (3)3.2.S.2.2生产工艺和过程控制 (4)3.2.S.2.3物料控制 (8)3.2.S.2.4关键步骤和中间体的控制 (8)3.2.S.2.5工艺验证和评价 (9)3.2.S.2.6生产工艺的开发 (9)3.2.S.3. 特性鉴定 (10)3.2.S.3.1结构和理化性质 (10)3.2.S.3.2杂质 (10)3.2.S.4 原料药的质量控制 (11)3.2.S.4.1质量标准 (11)3.2.S.4.2分析方法 (12)3.2.S.4.3分析方法的验证 (12)3.2.S.4.4批检验报告 (13)3.2.S.4.5质量标准制定依据 (13)3.2.S.5 对照品 (13)3.2.S.6 包装材料和容器 (13)3.2.S.7 稳定性 (13)3.2.S.7.1稳定性总结 (13)3.2.S.7.2上市后稳定性承诺和稳定性方案 (14)3.2.S.7.3稳定性数据汇总 (14)3.2.S.1 基本信息3.2.S.1.1药品名称中文名称:阿托伐他汀钙英文名称:Atorvastatin Calcium化学名称:calcium (3R,5R)-7-[2-(4-fluorophenyl)-3-phenyl-4-(phenylcarbamoyl) -5-propan- 2-ylpyrrol-1-yl]-3,5-dihydroxyheptanoateCAS号:134523-03-83.2.S.1.2 结构化学结构式:分子式:C66H68Ca2F2N4O10分子量:1195.4197264手性中心:具有2个手性碳3.2.S.1.3 理化性质性状:白色或类白色粉末,无色,无味,稍有吸湿性。
溶解性:易溶于酒精甲醇,微溶于氯仿,几乎溶于水,乙酸乙酯,乙醚和环己烷。

阿托伐他汀钙片说明书【药品名称】通用名称:阿托伐他汀钙片商品名称:阿托伐他汀钙片(立普妥)英文名称:Atorvastatin Calcium Tablets拼音全码:ATuoFaTaTingGaiPian(LiPuTuo)【主要成份】本品的主要成分是阿托伐他汀钙,化学名称为:EP,-(R’,R’)]-2-(4-氟苯基)一B·6一二羟基一5一(1-甲基乙基)一3-苯基-4-[(苯胺)基关]-1-氢一吡咯-1-庚酸钙三水台物。
【性状】本品为白色椭圆形薄膜衣片。
【适应症/功能主治】高胆固醇血症原发性高胆固醇血症患者。
包括家族性高胆固醇血症(杂合子型)或混合型高脂血症(相当于Fredrickson分类法的IIa和IIb型)患者,如果饮食治疗和其他非药物治疗疗效不满意,应用本品可治疗其总胆固醇(TC)升高、低密度脂蛋白胆固醇(LDL-C)升高、载脂蛋白B(Apo B)升高和甘油三酯(TG)升高。
在纯合子家族性高胆固醇血症患者,阿托伐他汀可与其他降脂疗法(如LDL血浆透析法)合用或单独使用(当无其他治疗手段时),以降低TC和LDL-C。
冠心病冠心病或冠心病等危症(如:糖尿病、症状性动脉粥样硬化疾病等)合并高胆固醇血症或混合型血脂异常的患者,本品适用于:降低非致死性心肌梗死的风险,降低致死性和非致死性卒中的风险、降低血管重建术的风险,降低因充血性心力衰竭而住院的风险,降低心绞痛的风险。
【规格型号】 20mg*7片【用法用量】病人在开始本品治疗前,应进行标准的低胆固醇饮食控制,在整个治疗期间也应维持合理膳食。
应根据低密度脂蛋白胆固醇基线水平、治疗目标和患者的治疗效果进行剂量的个体化调整。
常用的起始剂量为10mg,每日一次。
剂量调整时间间隔应为4周或更长。
本品最大剂量为每天一次80mg。
可在一天内的任何时间服用,并不受进餐影响。
对于心血管事件的低危患者治疗目标是LDL-C<4.14mmol/L(或<160mg/dL)和总胆固醇<6.62mmol/L(或<240mg/dL)。


in mg, of atenolol (C 14H 22N 2O 3) and chlorthalidone ASSAY(C 14H 11ClN 2O 4S) in each Tablet taken by the formula:•P ROCEDUREBuffer:3.9g/L of ammonium acetate in water. Adjust 2C (V /10)(r U /r S )with glacial acetic acid to a pH of 5.0 ± 0.1.Solution A:Acetonitrile, stabilizer-free tetrahydrofuran,in which C is the concentration, in mg per mL, of the ap-and Buffer (21:12:67)propriate USP Reference Standard in the Standard prepara-Solution B:Acetonitrile, stabilizer-free tetrahydrofuran,tion; V is the volume, in mL, of the volumetric flask used to and Buffer (61:12:27)prepare the stock solution for the Assay preparation; and r U Diluent:N,N-dimethylformamideand r S are the responses for the corresponding analyte ob-Mobile phase:See the gradient table below.tained from the Assay preparation and the Standard prepara-[N OTE —If necessary, adjust the Mobile phase by increas-tion, respectively.ing or decreasing the percentage of acetonitrile or the NOTE —If a trailing peak or shoulder is observed on the pH of the ammonium acetate solution to achieve a chlorthalidone peak with a relative retention time of not retention time of 26–34 min for the atorvastatin peak.]more than 1.1 in the chromatograms of both the Standard preparation and the Assay preparation, sum the areas for the Time Solution ASolution Bchlorthalidone peak with the trailing peak or shoulder to (min)(%)(%)report the peak responses for chlorthalidone.01000401000702080850100Atorvastatin Calcium10001001051000115100System suitability solution:0.05mg/mL of USP Atorva-statin Calcium RS and 0.06mg/mL of USP Atorvastatin Related Compound B RS in DiluentStandard solution:0.4mg/mL of USP Atorvastatin Cal-cium RS in Diluent . [N OTE —Use sonication if necessary.]Sample solution:0.4mg/mL of Atorvastatin Calcium in Diluent . [N OTE —Use sonication if necessary.]Chromatographic system(See Chromatography 〈621〉, System Suitability .)[N OTE —If significant fronting of the peaks for atorvasta-tin related compound B and atorvastatin is observed,C 66H 68CaF 2N 4O 10·3H 2O 1209.42use the following Diluent to prepare the Sample solu-C 66H 68CaF 2N 4O 101155.34tion , Standard solution , and System suitability solution : 1H -Pyrrole-1-heptanoic acid, 2-(4-fluorophenyl)-β,δ-acetonitrile, stabilizer-free tetrahydrofuran, and water dihydroxy-5-(1-methylethyl)-3-phenyl-4-[(phenylami-(1:1:2).]no)carbonyl]-, calcium salt (2:1), trihydrate [R -(R *,R *)]-;Mode:LCCalcium (βR ,δR )-2-(p -fluorophenyl)-β,δ-dihydroxy-5-isopro-Detector:UV 244 nmpyl-3-phenyl-4-(phenylcarbamoyl)pyrrole-1-heptanoate Column:4.6-mm × 25-cm; 5-µm packing L7(1:2), trihydrate [344423-98-9].Column temperature:35° Anhydrous [134523-03-8].Flow rate:1.5mL/min Injection size:20µL DEFINITIONSystem suitabilityAtorvastatin Calcium contains NLT 98.0% and NMTSamples:System suitability solution and Standard 102.0% of C 66H 68 CaF 2N 4O 10, calculated on the anhydrous solutionbasis.Suitability requirementsResolution:NLT 1.5 between the peaks for atorvasta-IDENTIFICATIONtin related compound B and atorvastatin, System suit-•A . I NFRARED A BSORPTION 〈197K 〉 ability solution•B . C ALCIUMTailing factor:NMT 1.6, Standard solutionDiluent:Methanol, water, and hydrochloric acid Relative standard deviation:NMT 0.6%, Standard (75:25:2)solution Blank:DiluentAnalysisSample solution:0.05mg/mL of Atorvastatin Calcium Samples:Standard solution and Sample solutionin Diluent Calculate the percentage of C 66H 68CaF 2N 4O 10 in the por-Analysistion of Atorvastatin Calcium taken:Samples:Sample solution and Blank Spectrometric conditionsResult = (r U /r S ) × (C S /C U ) × 100(See Spectrophotometry and Light-Scattering 〈851〉.)Mode:Atomic absorption spectrophotometry r U = peak response from the Sample solution Analytical wavelength:Calcium emission line at r S = peak response from the Standard solution422.7 nmC S= concentration of USP Atorvastatin Calcium RSFlame:Air–acetylenein the Standard solution (mg/mL)Acceptance criteria:The Sample solution exhibits a sig-C U = concentration of Atorvastatin Calcium in thenificant absorption at the calcium emission line at 422.7Sample solution (mg/mL)nm.Acceptance criteria:98.0%–102.0% on the anhydrous basisIMPURITIESAcceptance criteriaInorganic Impurities Individual impurities:See Impurity Table 1.•H EAVY M ETALSTotal impurities:NMT 1.0%. [N OTE —This total does not Diluent:Methanol and water (9:1)include atorvastatin related compound E, as determined Sample solution:Dissolve 250mg of the sample in in the test for Enantiomeric Purity .]30mL of Diluent .Standard lead solution:Prepared as directed under Impurity Table 1Heavy Metals 〈231〉.Relative Acceptance Reference solution:Dilute 0.5mL of the Standard lead Retention Criteria,solution with Diluent to Time NMT (%)Blank solution:20mL of DiluentAtorvastatin related compound A a 0.80.3Monitor solution: Dissolve 250mg of Atorvastatin Cal-cium in 0.5mL of the Standard lead solution , and dilute Atorvastatin related compound B b 0.90.3with Diluent to 30mL.Atorvastatin1.0n/a AnalysisAtorvastatin related compound C c 1.20.3Samples:Sample solution, Reference solution, Blank so-Atorvastatin related compound D d, e 2.10.1lution, and Monitor solutionAny other individual impurity—0.1To each solution, add 2mL of pH 3.5 Acetate Buffer ,a Desfluoro impurity.prepared as directed under Heavy Metals 〈231〉. Mix,b3S,5R isomer.add to 1.2mL of thioacetamide–glycerin base TS, and c Difluoro impurity.mix immediately. Pass the solutions through a mem-d Epoxide impurity.brane filter of 0.45-µm pore size. Compare the spots e Atorvastatin related compound D may undergo a transformation equi-on the filters obtained with the different solutions:librium with its cyclic hemiketal form. The cyclic hemiketal of atorvastatin the brown color of the spot from the Sample solution related compound D elutes about 1–2 min before atorvastatin related is not more intense than that of the spot from the compound D. Use the sum of the areas of the two peaks as a peakReference solution . The test is invalid if the Reference response for atorvastatin related compound D in the Standard solution and the Sample solution .solution does not show a slight brown color com-pared to the Blank solution , or if the color of the Mon-SPECIFIC TESTSitor solution is not at least as intense as the color of •E NANTIOMERIC P URITYthe Reference solution .Mobile phase:Hexane, dehydrated alcohol, and trifluor-Acceptance criteria:NMT 20ppm oacetic acid (940:60:1)Organic Impurities System suitability stock solution:5mg/mL of USP•P ROCEDUREAtorvastatin Calcium RS and 37.5µg/mL of USP Atorva-Buffer, Solution A, Solution B, Diluent, Mobile phase,statin Related Compound E RS in methanol. [N OTE —System suitability solution, and Chromatographic Atorvastatin related compound E is the 3S,5S enanti-system:Proceed as directed for the Assay .omer of atorvastatin.]Standard solution:1.5µg/mL each of USP Atorvastatin System suitability solution:Transfer 2.0mL of the Sys-Related Compound A RS, USP Atorvastatin Related tem suitability stock solution to a 10-mL volumetric flask,Compound B RS, USP Atorvastatin Related Compound add 2.0mL of dehydrated alcohol, and dilute with hex-C RS, and USP Atorvastatin Related Compound D RS in ane to volume.DiluentSample solution:Transfer 10mg of Atorvastatin Cal-Sample solution:1mg/mL of Atorvastatin Calcium in cium to a 10-mL volumetric flask, dissolve in 2.0mL of Diluent . [N OTE —Use sonication if necessary.]methanol, add 2.0mL of dehydrated alcohol, and dilute Analysiswith hexane to volume.Samples:Standard solution and Sample solutionChromatographic systemChromatograph the Standard solution, and identify the (See Chromatography 〈621〉, System Suitability .)components on the basis of their relative retention Mode:LCtimes, given in Impurity Table 1.Detector:UV 244 nmCalculate the percentage of each of the atorvastatin re-Column:4.6-mm × 25-cm; packing L51lated compounds A, B, C, and D in the portion of Flow rate:1.0mL/min Atorvastatin Calcium taken:Injection size:20µL System suitabilityResult = (r U /r S ) × (C S /C U ) × 100Samples:System suitability solution[N OTE —The elution order of the peaks is atorvastatin r U= peak response of the relevant atorvastatinrelated compound E followed by atorvastatin.]related compound from the Sample solutionResolution:NLT 2.0 between the peaks for atorvastatin r S = peak response of the relevant atorvastatinrelated compound E and atorvastatin related compound from the Standard AnalysissolutionSamples:Sample solutionC S = concentration of the relevant atorvastatinCalculate the percentage of atorvastatin related com-related compound in the Standard solution pound E in the portion of Atorvastatin Calcium taken:(mg/mL)C U = concentration of Atorvastatin Calcium in theResult = (r U /r T ) × 100Sample solution (mg/mL)Calculate the percentage of any other individualr U= peak response for atorvastatin relatedimpurity in the portion of Atorvastatin Calcium taken:compound Er T= sum of the responses of the peaks for Result = (r U /r T ) × 100atorvastatin related compound E and atorvastatinr U= peak response of any other individualAcceptance criteria:NMT 0.3% of atorvastatin related impurity from the Sample solutioncompound Er T= sum of the responses of all the peaks from the Sample solution[N OTE —Disregard any peak observed in the blank; the reporting level for impurities is 0.05%.]•W ATER D ETERMINATION , Method Ia 〈921〉: 3.5%–5.5%Water, Method I 〈921〉: not more than 1.0%.Residue on ignition 〈281〉: not more than 0.1%.ADDITIONAL REQUIREMENTSHeavy metals—•P ACKAGING AND S TORAGE : Preserve in well-closed contain-Test preparation—Thoroughly mix 1.0g of Atovaquone ers, and store at room temperature.with 0.5g of magnesium oxide in a silica crucible. Ignite to •USP R EFERENCE S TANDARDS 〈11〉dull redness until a homogeneous white or grayish-white USP Atorvastatin Calcium RSmass is obtained. If the mixture remains colored after USP Atorvastatin Related Compound A RS30minutes, allow to cool, mix using a fine glass rod, and Desfluoro impurity, or (3R ,5R )-7-[3-(phenylcarbamoyl)-repeat the ignition. If necessary, repeat the operation. Heat 2-isopropyl-4,5-diphenyl-1H -pyrrol-1-yl]-3,5-dihydrox-the residue at 800° for about 1hour. Cool, take up the yheptanoic acid, calcium salt.residue in two 5-mL portions of 6N hydrochloric acid, add C 66H 70CaN 4O 101119.380.1mL of phenolphthalein TS, and then add 13.5 N ammo-USP Atorvastatin Related Compound B RSnium hydroxide until a pink color is obtained. Cool, add 3S ,5R Isomer, or (3S ,5R )-7-[3-(phenylcarbamoyl)-glacial acetic acid until the solution is decolorized, and add 5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H -pyrrol-1-yl]-0.5mL in excess. Filter, if necessary, and wash the filter with 3,5-dihydroxyheptanoic acid, calcium salt.water. Dilute with water to 20mL.C 66H 68CaF 2N 4O 101155.34USP Atorvastatin Related Compound C RSStandard preparation—Add 1.0mL of Standard Lead Solu-Difluoro impurity, or (3R ,5R )-7-[3-(phenylcarbamoyl)-tion (see Special Reagents under Heavy Metals 〈231〉) to 0.5g 4,5-bis(4-fluorophenyl)-2-isopropyl-1H -pyrrol-1-yl]-3,5-of magnesium oxide, and dry between 100° and 105°. Pro-dihydroxyheptanoic acid, calcium salt.ceed as directed for Test preparation, starting with “Ignite to C 66H 66F 4N 4O 101191.34dull redness”.USP Atorvastatin Related Compound D RSBlank preparation—Proceed as directed for Test prepara-Epoxide impurity, or 3-(4-fluorobenzoyl)-2-isobutyryl-tion, omitting the Atovaquone.3-phenyl-oxirane-2-carboxylic acid phenylamide.Procedure—Transfer 12.0mL of the Test preparation to a C 26H 22FNO 4431.4650-mL color-comparison tube, 10.0mL of the StandardUSP Atorvastatin Related Compound E RSpreparation to another, and 10.0mL of the Blank preparation 3S ,5S Enantiomer, or (3S ,5S )-7-[3-(phenylcarbamoyl)-to a third. Then add 2.0mL of the Test preparation to the 5-(4-fluorophenyl)-2-isopropyl-4-phenyl-1H -pyrrol-1-yl]-Standard preparation as well as to the Blank preparation . Add 3,5-dihydroxyheptanoic acid, calcium salt.2mL of pH 3.5 Acetate Buffer (see Heavy Metals 〈231〉) to C 66H 68CaF 2N 4O 101155.34each of the three tubes, mix, add 1.2mL ofthioacetamide–glycerin base TS, and mix. Allow to stand for 2minutes, and view downward over a white surface: the solution from the Standard preparation is slightly brown when compared with the solution from the Blank prepara-tion, and the color of the solution from the Test preparation Atovaquoneis not darker than that of the solution from the Standard preparation (10µg per g).Limit of residual organic solvents—Standard solution—Transfer 1.0mL of methanol and 1.0mL of glacial acetic acid to a 100-mL volumetric flask,dilute with dimethylformamide to volume, and mix. Transfer 5.0mL of this solution to a second 100-mL volumetric flask,dilute with dimethylformamide to volume, and mix.C 22H 19ClO 3366.84Test solution—Transfer about 100mg of Atovaquone, ac-1,4-Naphthalenedione, 2-[4-(4-chlorophenyl)cyclohexyl]-curately weighed, to a 2-mL volumetric flask, dissolve in and 3-hydroxy-, trans -.dilute with dimethylformamide to volume, and mix.2-[trans -4-(p -Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-Chromatographic system (see Chromatography 〈621〉)—The naphthoquinone [95233-18-4].gas chromatograph is equipped with a flame-ionization de-tector and a 4-mm × 2.8-m column that contains 10% liq-» Atovaquone contains not less than 97.5percent uid phase G16 on support S2. The carrier gas is nitrogen,and not more than 101.5percent of C 22H 19ClO 3,flowing at a rate of about 42.5mL per minute. The column calculated on the anhydrous and organic solvent-temperature is maintained at about 180° and the detector free basis.block temperature is maintained at about 250°. Chromato-graph the Standard solution, and record the peak responses Packaging and storage—Preserve in tight, light-resistant as directed for Procedure: the relative retention times are containers.about 0.4 for methanol and 1.0 for acetic acid; the resolu-USP Reference standards 〈11〉—tion, R, between methanol and acetic acid is not less than USP Atovaquone RS14; the column efficiency calculated from the acetic acid USP Atovaquone Related Compound A RSpeak is not less than 700; and the tailing factor for acetic cis -2[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-acid is not less than 0.8.naphthoquinone.Procedure—Separately inject equal volumes (about 1µL)Identification—of the Standard solution and the Test solution into the chro-matograph, record the chromatograms, and measure the A: Infrared Absorption 〈197M 〉.peak areas for methanol and acetic acid. Calculate the per-B: The retention time of the major peak in the chromato-centage, by weight, of methanol and acetic acid in the por-gram of the Assay preparation corresponds to that in the tion of Atovaquone taken by the formula:chromatogram of the Standard preparation, as obtained in the Assay.0.1(G/W )(r U /r S )in which G is either 0.79, the specific gravity of methanol,or 1.05, the specific gravity of glacial acetic acid, as appro-priate; W is the weight, in mg, of Atovaquone taken to pre-pare the Test solution; and r U and r S are the peak area re-sponses of methanol or acetic acid, as appropriate, obtained Atovaquone Oral Suspensionfrom the Test solution and the Standard solution, respectively:not more than 0.2% of methanol or of acetic acid is found.» Atovaquone Oral Suspension contains not less Related compounds—Using the chromatograms of theAssay preparation and the Resolution solution obtained in the than 90.0percent and not more than 110.0per-Assay, calculate the percentage of atovaquone related com-cent of the labeled amount of atovaquone pounds in the portion of Atovaquone taken by the formula:(C22H19ClO3).100(r i/r s)Packaging and storage—Preserve in tight, light-resistantcontainers.in which r i is the individual peak response of a related com-USP Reference standards 〈11〉—pound, if any, in the chromatogram of the Assay prepara-USP Atovaquone RStion; and r s is the sum of the responses of all the peaks inUSP Atovaquone Related Compound A RSthe chromatogram of the Assay preparation, including thecis-2[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-atovaquone peak. Not more than 1.0% of any related com-naphthoquinone.pound with a retention time corresponding to that of atova-Identification—quone related compound A, as determined from the chro-matogram of the Resolution solution, is found; not more A: Ultraviolet Absorption 〈197U〉—than 0.5% of any related compound with a retention time Medium: a mixture of methanol and water (1:1).of 0.63 or 1.8 relative to that of atovaquone is found; and Solution—Transfer 5.0mL of the Assay preparation and not more than 0.3% of any related compound with a reten- 5.0mL of the Standard preparation, prepared in the Assay, tion time of 0.89 relative to that of atovaquone is found.to separate 50-mL volumetric flasks, dilute with Medium to Not more than 0.2% of any other individual related com-volume, and mix.pound is found; and the sum of all other such related com-B: The retention time of the major peak in the chromato-pounds is not more than 1.0%. The sum of all related com-gram of the Assay preparation corresponds to that in the pounds is not more than 1.5%.chromatogram of the Standard preparation, as obtained in Assay—the Assay.Mobile phase—Prepare a mixture of acetonitrile, water,Uniformity of dosage units 〈905〉—methanol, and phosphoric acid (525:300:175:5). Make ad-FOR ORAL SUSPENSION PACKAGED IN SINGLE-UNIT CONTAINERS: justments if necessary (see System Suitability under Chroma-meets the requirements.tography 〈621〉).Deliverable volume 〈698〉—Diluent—Prepare a mixture of acetonitrile and water(80:20).FOR ORAL SUSPENSION PACKAGED IN MULTIPLE-UNIT CONTAINERS:meets the requirements.Standard preparation—Dissolve an accurately weighedquantity of USP Atovaquone RS in Diluent, and dilute quan-pH 〈791〉: between 3.5 and 7.0.titatively, and stepwise if necessary, with Diluent to obtain a Sedimentation—solution having a known concentration of about 0.25mgFOR ORAL SUSPENSION PACKAGED IN MULTIPLE-UNIT CONTAINERS—per mL.Transfer 50mL of well-mixed Oral Suspension to a glass-Resolution solution—Prepare a solution in Diluent contain-stoppered graduated cylinder, and allow to stand foring about 0.25mg of USP Atovaquone RS and 0.02mg of16hours. Measure the volume, if any, of clear liquid ob-USP Atovaquone Related Compound A RS per mL. Store in served in the cylinder: not more than 1mL of clear liquid is a low-actinic glass container.found.Assay preparation—Transfer about 25mg of Atovaquone,Related compounds—Using the chromatograms of the accurately weighed, to a low-actinic, 100-mL volumetric Resolution solution, the Standard preparation, and the Assay flask, dissolve in and dilute with Diluent to volume, and mix.preparation obtained in the Assay, calculate the percentage Chromatographic system (see Chromatography 〈621〉)—The of atovaquone-related compounds, based on the labeled liquid chromatograph is equipped with a 220-nm detector strength of atovaquone, by the formula:and a 4.6-mm × 25-cm column that contains packing L1.The flow rate is about 3mL per minute. Chromatograph theResolution solution, and record the peak areas as directed forProcedure: the relative retention times are about 0.85 foratovaquone related compound A and 1.0 for atovaquone;and the resolution, R, between atovaquone related com-in which C is the concentration, in mg per mL, of USP pound A and atovaquone is not less than 4. Chromatograph Atovaquone RS in the Standard preparation; D is the density the Standard preparation, and record the peak responses as of Oral Suspension, in g per mL (1.04g per mL at 20° to directed for Procedure: the column efficiency is not less than25°); S is the weight, in g, of Oral Suspension taken to 9000 theoretical plates; the tailing factor is not more than prepare the Assay preparation; L is the labeled amount, in 1.5; and the relative standard deviation for replicate injec-mg per mL, of atovaquone in the Oral Suspension; F i is the tions is not more than 2%.response factor of an individual atovaquone related com-pound relative to the response of atovaquone, specifically, Procedure—Separately inject equal volumes (about 20µL)1.08 for any peak observed at a relative retention time ofof the Standard preparation and the Assay preparation intoabout 0.65, 0.85 for any peak observed at a retention time the chromatograph, record the chromatograms, and meas-corresponding to that of atovaquone related compound A, ure the areas for the major peaks. Calculate the quantity, inas determined from the chromatogram of the Resolution so-mg, of C22H19ClO3 in the portion of Atovaquone taken bylution, and 1.0 for any other related compound peak; r i is the formula:the individual peak response of an atovaquone related com-pound, if any, in the chromatogram of the Assay prepara-100C(r U/r S)tion; and r S is the peak response of atovaquone in the chro-matogram of the Standard preparation. Disregard any peak in which C is the concentration, in mg per mL, of USPhaving a relative retention time of about 0.3, which is due Atovaquone RS in the Standard preparation; and r U and r Sto photodegradation during preparation of the Assay prepa-are the atovaquone peak areas obtained from the Assayration. Not more than 0.5% of an atovaquone related com-preparation and the Standard preparation, respectively.。

制剂CTD格式申报资料4、5.2类3.2.P 制剂3.2.P.1 剂型及产品组成(1)说明具体的剂型,并以表格的方式列出单位剂量产品的处方组成,列明各成分在处方中的作用,执行的标准。
如有过量加入的情况需给予说明。
对于处方中用到但最终需去除的溶剂也应列出。
表1(注:表格依次编号,下同):处方(2)如附带专用溶剂,参照以上表格方式列出专用溶剂的处方。
(3)说明产品所使用的包装材料及容器。
3.2.P.2 产品开发说明产品开发目标。
说明原研药上市情况。
详细提供包括原研药的质量概况在内的相关研究资料或文献资料来论证本品的剂型、处方组成、生产工艺、包装材料选择和确定的合理性。
3.2.P.2.1处方组成3.2.P.2.1.1原料药参照《化学药物制剂研究的技术指导原则》,提供资料说明原料药和辅料的相容性,分析原料药的关键理化特性(如BCS分类、晶型、溶解性、粒度分布等)与制剂生产及制剂性能的相关性,并提供相关的研究资料与文献。
3.2.P.2.1.2辅料说明辅料是否适合所用的给药途径结合辅料在处方中的作用分析辅料的哪些性质会影响制剂特性,提供相关的研究资料与文献。
3.2.P.2.2 制剂研究3.2.P.2.2.1处方开发过程参照《化学药物制剂研究的技术指导原则》,提供处方的研究开发过程和确定依据,包括文献信息(如对照药品的处方信息)、研究信息(包括处方设计,处方筛选和优化、处方确定等研究内容)、辅料种类和用量选择的依据、分析辅料用量是否在常规用量范围内,以及自制样品与原研药的质量特性对比研究结果(需说明原研药的来源、批次和有效期,自研样品批次,对比项目、采用方法),并重点说明在药品开发阶段中处方组成的主要变更、原因以及支持变化的验证研究。
如生产中存在过量投料的问题,应提供过量投料的必要性和合理性的相关研究资料。
3.2.P.2.2.2制剂相关特性对与制剂性能相关的理化性质,如pH、离子强度、溶出度、再分散性、复溶、粒径分布、聚合、多晶型、流变学等进行分析。

TECHNOLOGY AND INFORMATION医疗与信息化科学与信息化2020年7月中 133阿托伐他汀钙片剂制剂及质量控制概述刘磊哈药集团技术中心 黑龙江 哈尔滨 150025摘 要 阿托伐他汀钙是新一代他汀类降血脂药,它不仅能降低胆固醇,而且也能降低甘油三酯,临床作用显著。
本文对其有关物质进行了系统的研究,制定出完善的制剂质量标准草案,从而使本品制剂的安全性、有效性和产品的均一性得到保证。
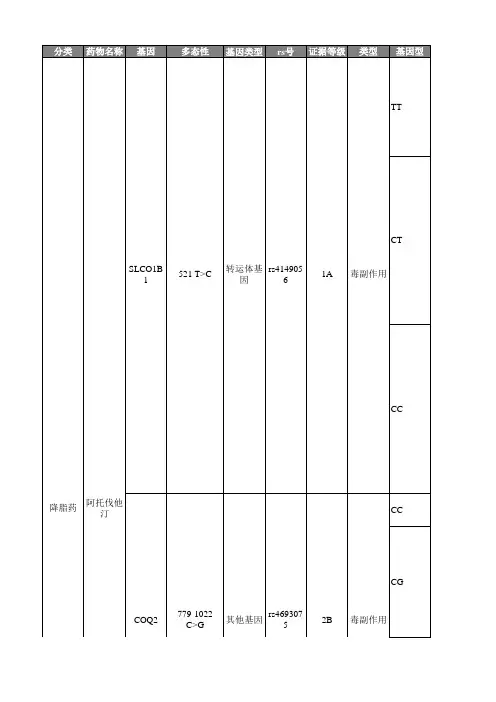
关键词 阿托伐他汀钙片剂;制剂;质量1 阿托伐他汀钙研究现状在阿托伐他汀钙片质量标准研究中,最容易忽略的是对产品中非对映光学异构体和对映光学异构体的研究。
在大多厂家提供的有关物质研究资料中,有关物质检查的色谱条件并不能很好的分离他汀类药物的对映光学异构体(某些色谱条件仅能分离非对映光学异构体),因此需要采用手性色谱条件进行研究。
在制定质量标准时需要注意有关物质、非对映光学异构体和对映光学异构体应单独设定质控限度[1]。
2 阿托伐他汀钙片剂制剂工艺研究阿托伐他汀钙原研厂家(美国辉瑞)的说明书显示对照药品使用的辅料有碳酸钙、微晶纤维素、一水乳糖、交联羧甲基纤维素钠、吐温-80、羟丙基纤维素、硬脂酸镁,参照对照药品的处方并结合自身的处方工艺研究,最终确定的处方中所使用的辅料:碳酸钙、微晶纤维素、乳糖、交联羧甲基纤维素钠、吐温-80、羟丙基纤维素、硬脂酸镁,辅料与原研厂家一致。
参照阿托伐他汀钙市售品处方,确定其工艺如下:(1)原辅料准备:阿托伐他汀钙过120目筛备用;碳酸钙、微晶纤维素、一水乳糖、交联羧甲基纤维素钠,过80目筛备用。
(2)混料:称取处方量的阿托伐他汀钙、碳酸钙、微晶纤维素、一水乳糖及1/2处方量的交联羧甲基纤维素钠,混匀。
(3)黏合剂制备:称取处方量的吐温-80,加入到60mL 水中,50℃加热溶解后将加入处方量的羟丙基纤维素,搅拌混合后放置4小时左右,制成含羟丙基纤维素5%的水溶液。
(4)制粒:用上述溶液制软材,过24目筛制粒。

【药物名称】阿托伐他汀钙 atorvastatin calcium【药物类别】降血脂药【药物别名】立普妥 Lipitor【制剂规格】片剂:10mg ,20mg,40mg 。
【批准文号】辉瑞,国药准字(2000)J-25(1)号(10mg ),国药准字(2000)J-25(2)号(20mg),国药准字(2000)J-25(3)号(30mg)。
本品为白色或类白色结晶性粉末,不溶于pH≤4的水溶液,微溶于蒸馏水、pH7.4的磷酸盐缓冲液、乙腈,轻度溶于乙醇,易溶于甲醇。
【药理毒理】本品为HMG-CoA 还原酶选择性抑制剂,通过抑制HMG-CoA 还原酶和胆固醇在肝脏的生物合成而降低血浆胆固醇和脂蛋白水平,并能通过增加肝细胞表面低密度脂蛋白(LDL )受体数目而增加LDL 的摄取和分解代谢。
本品也能减少LDL 的生成和其颗粒数。
本品还能降低某些纯合子型家族性高胆固醇血症(FH )患者的低密度脂蛋白胆固醇(LDL-C )水平,而这一类型的人群对其他类型的降脂药物治疗很少有应答。
本品能降低纯合子和杂合子家族性高胆固醇血症、非家族性高胆固醇血症以及混合性脂类代谢障碍患者的血浆总胆固醇(TC )、LDL-C 和载脂蛋白B (ApoB ),还能降低极低密度脂蛋白胆固醇(VLDL-C )和三酰甘油(TG )的水平,并能不同程度地提高血浆高密度脂蛋白胆固醇(HDL-C )和载脂蛋白A 1(ApoA 1)的水平。
【临床评价】据资料,对杂合子家族性或非家族性高胆固醇血症和混合性高脂血症患者,在2个多中心、安慰剂对照试验中,安慰剂和本品10,20,40,80mg·d -1组分别为21,22,20,21和23例,6周后,各组与基线水平比较变化的平均百分数(%)如下:TC 分别为4和-29,-33,-37,-45,LDL-C 分别为4和-39,-43,-50,-60,ApoB 分别为3和-32,-35,-42,-50,TG 分别为10和-19,-26,-29,-37,HDL-C 分别为-3和6,9,6,-5,非HDL-C/HDL-C 分别为7和-34,-41,-45,-53,表明本品可显著降低TC ,LDL-C ,VLDL-C ,ApoB 和TG 。
制剂CTD格式申报资料4、类制剂剂型及产品组成1〕说明具体的剂型,并以表格的方式列出单位剂量产品的处方组成,列明各成分在处方中的作用,执行的标准。
如有过量参加的情况需给予说明。
对于处方中用到但最终需去除的溶剂也应列出。
表1〔注:表格依次编号,下同〕:处方成分用量过量参加作用执行标准工艺中使用到并最终去除的溶剂〔2〕如附带专用溶剂,参照以上表格方式列出专用溶剂的处方。
〔3〕说明产品所使用的包装材料及容器。
产品开发说明产品开发目标。
说明原研药上市情况。
详细提供包括原研药的质量概况在内的相关研究资料或文献资料来论证本品的剂型、处方组成、生产工艺、包装材料选择和确定的合理性。
处方组成原料药参照?化学药物制剂研究的技术指导原那么?,提供资料说明原料药和辅料的相容性,分析原料药的关键理化特性〔如BCS分类、晶型、溶解性、粒度分布等〕与制剂生产及制剂性能的相关性,并提供相关的研究资料与文献。
辅料说明辅料是否适合所用的给药途径结合辅料在处方中的作用分析辅料的哪些性质会影响制剂特性,提供相关的研究资料与文献。
制剂研究处方开发过程参照?化学药物制剂研究的技术指导原那么?,提供处方的研究开发过程和确定依据,包括文献信息〔如对照药品的处方信息〕、研究信息〔包括处方设计,处方筛选和优化、处方确定等研究内容〕、辅料种类和用量选择的依据、分析辅料用量是否在常规用量范围内,以及自制样品与原研药的质量特性比照研究结果〔需说明原研药的来源、批次和有效期,自研样品批次,比照工程、采用方法〕,并重点说明在药品开发阶段中处方组成的主要变更、原因以及支持变化的验证研究。
如生产中存在过量投料的问题,应提供过量投料的必要性和合理性的相关研究资料。
制剂相关特性对与制剂性能相关的理化性质,如pH、离子强度、溶出度、再分散性、复溶、粒径分布、聚合、多晶型、流变学等进行分析。
提供自研产品与原研药品的理化性质、质量特性比照研究结果,例如有关物质等。
如为口服固体制剂,需提供详细的自研产品与原研药在不同溶出条件下的溶出曲线比拟研究结果,推荐采用f2相似因子的比拟方式。
阿托伐他汀钙Atnrvastatin Calcium【适应证】①各型高胆固醇血症和混合型高脂血症。
②冠心病和脑中风的防治。
③心肌梗死后不稳定性心绞痛及血管重建术后;对急性冠脉综合征可显著减少心血管事件、心绞痛、脑卒中的危险性。
【药理】(1)药效学为一种选择性、羟甲戊二酰辅酶 A (HMG- CoA)还原酶抑制药,作用同洛伐他汀。
与安慰药对照,本品每日10-80mg.使总胆固醇下降30% -46% ;LDL-C下降41%~61%;载脂蛋白β下降34%~50%;也使TG下降14%~33%,和HDL-C增加6%~9%。
在他汀类中。
阿托伐他汀调脂作用较强。
近年的研究证实。
他汀类除调脂作用外还具有抗炎症、抗氧化、减少内皮素生成、减少组织因子表达、抑制血小板集聚、稳定斑块、抗血栓等多方面的抗动脉粥样硬化作用。
因此可用于动脉粥样硬化和冠心病、脑卒中的防治。
(2)药动学口服后迅速被吸收,血药浓度达峰值时间为l~2小时。
绝对生物利用度12%。
血浆蛋白结合率98%以上。
本品在肝脏经细胞色素P450 3A4代谢。
原药半衰期约14小时;因其代谢产物也具活性,对HMG-CoA还原酶抑制的半衰期可长达20-39小时。
本品及其代谢产物主要由胆管排泄。
经尿排除的不到2%。
本品可分泌至人乳。
【不良反应】通常耐受良好。
不良反应常为轻度和一过性。
发生率约1%。
(1)最常见便秘、胃肠胀气、消化不良、腹痛、头痛、恶心、肌痛、无力、腹泻和失眠。
也有报道血清氨基转移酶升高和血清磷酸肌酸激酶(CPK)升高。
(2)罕见肌炎、肌病、横纹肌溶解、感觉异常、周围性神经病变、胰腺炎、肝炎、胆汁淤积性黄疽、厌食、呕吐、脱发、搔痒、皮疹、阳痿、高血糖症、低血糖症、胸痛、头晕、血小板减少症和过敏反应(包括血管神经性水肿)。
并非所有列出的不良事件都与本品治疗相关。
【禁忌证】对本品所含的任何成分过敏者、活动性肝病患者、血清氨基转移酶持续超过正常上限3倍且原因不明者、肌病、孕期、哺乳期及任何未采取适当避孕措施的育龄妇女禁用。
CTD格式8号申报资料主要研究信息
(药学部分:制剂)
3.2.P.1 剂型选择依据及产品组成
(1)本品为普通片剂,规格为:10mg
每片XXXXX片的处方组成见表3.2.P.1。
附表3.2.P.1 XXXXX片药物组成表
(2)本品在制备过程中没有使用专用的溶剂。
(3)XXXXX片的内包装材料为铝塑板,有避光和防潮的作用,与XXXXX 的内包装材质相同。
内包材的生产厂家具有内包材注册证,执行国家标准。
3.2.P.2 产品开发
XXXXX是HMG-CoA还原酶的一选择性、竞争性抑制剂,可以显著降低胆固醇水平,并降低心肌梗死或脑卒中的发病危险。
临床试验已经证实XXXXX
降低胆固醇的临床疗效明显优于其它汀类药物,对原发性高胆固醇血症、包括家族性高胆固醇血症或混合型高脂血症患者以及纯合子家族性高胆固醇血症者有明显疗效。
XXXXX(商品名)作为目前世界上顶级降血脂药物,由美国华纳-兰伯特公司研制开发。
1997年上市,之后并入辉瑞公司。
自1998年以来取得了优异的业绩,成为当今世界增长最快的药品,连续三年名列全球畅销处方药第一位,2003年全球销售92.3亿美元,2004年高达108.6亿美元。
华纳-兰伯特公司的XXXXX 在1999年9月获准中国申请药品行政保护,在国内由大连辉瑞生产销售。
目前,国内原北京红惠制药(现更名北京嘉林药业)已获得XXXXX及片剂产品的生产批文,天方药业研制开发XXXXX胶囊,于2005年9月29日获得新药证书和药品注册批件。
我公司立项仿制XXXXX片,规格与辉瑞制药(大连)有限公司的XXXXX 相同,为:10mg/片。
3.2.P.2.1 处方组成
3.2.P.2.1.1 原料药
性状:白色至类白色结晶性粉末。
溶解性:XXXXX不溶于PH≤4的水溶液,能微溶于蒸馏水、PH为7.4的磷酸盐缓冲液、乙腈,轻度微溶于乙醇,易溶于甲醇。
贮藏:遮光,密封保存。
有效期:48个月
分子式:XXXXX
分子量:XXXX
化学名:XXXXXXXXXXXX
英文名:Atorvastatin Calcium
化学结构式:
XXXXXXXXXXXX。