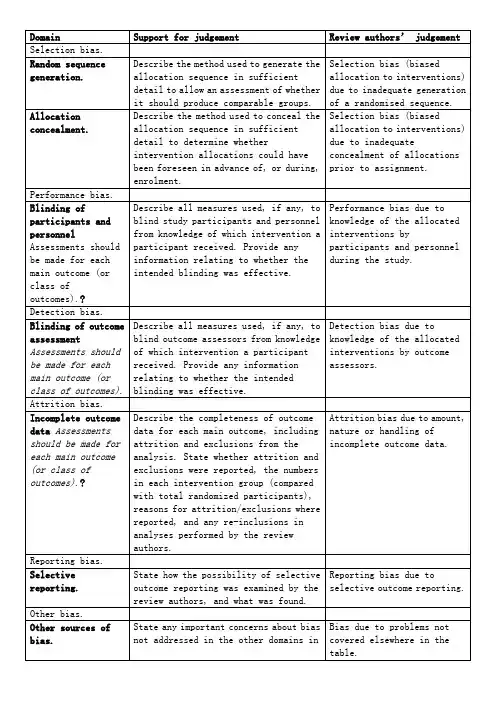
纳入的RCT文献质量评价风险偏倚评估工具中英文对照版
- 格式:doc
- 大小:156.50 KB
- 文档页数:14


完整版)Cochrane协作网的RCT偏倚风险评价工具评估随机对照试验偏倚风险是非常重要的,因为这些偏倚可能会影响试验结果的准确性和可靠性。
表Cochrane协作网提供了一些评价条目,以评估试验的随机序列产生、分配隐藏、施盲和结果数据完整性等方面的偏倚风险。
在随机序列产生方面,低风险的方法包括采用随机数字表、使用计算机随机数字发生器、最小化随机等。
而高风险的方法包括使用开放的随机分配表(如随机数字清单)和交替分配等。
如果关于随机序列产生过程的信息不充分,则无法判断其偏倚风险。
在分配隐藏方面,低风险的方法包括中心化分配(包括电话、基于网络和药房控制的随机化)、同一外观、连续编号的药物和按顺序编号、不透光、密封的信封等。
而高风险的方法包括使用无适当保护措施的分配信封(如信封未密封、透光信封、信封未按顺序编号)和任何其他明显未隐藏的方法。
如果信息不充分,则无法判断其偏倚风险。
在施盲方面,低风险的方法是确保对受试者和主要研究人员施盲,并且不太可能破盲。
而高风险的方法是尝试对受试者和主要研究人员施盲,但很可能破盲,并且结果很可能受缺乏盲法的影响。
如果信息不充分,则无法判断其偏倚风险。
在结果数据完整性方面,低风险的方法是未缺失结果数据,并且缺失的结果数据原因与真实结果不太可能相关。
对于二分类结果数据,缺失结果数据的比例不足以对干预效应估计产生临床相关影响。
对于连续性结果数据,缺失结果数据中似真的效应大小不足以对观测效应大小产生临床相关影响。
而高风险的方法是存在缺失结果数据,并且缺失结果数据的原因可能与真实结果相关。
如果缺失结果数据在各干预组的数量不均衡或组间缺失数据具有不同的原因,则可能会引入偏倚。
如果信息不充分,则无法判断其偏倚风险。
评估随机对照试验的偏倚风险是非常重要的,以确保试验结果的准确性和可靠性。
因此,在进行随机对照试验时,需要采用低风险的随机序列产生、分配隐藏和施盲方法,并确保结果数据的完整性。

循证医学(EBM):有意识地、明确地、审慎地利用现有最好的证据并参酌个人的实践经历和人们的价值取向,进行医学实践。
核心思想:最正确证据、患者的价值取向、具体的医疗环境。
实践循证医学“五部曲〞:①确定拟弄清的临床问题②检索有关的医学文献③严格的文献评价④应用最正确成果于临床决策⑤总结经验与评价能力PICOS原那么:P指特定的患病的人群I干预C对照组或另一种可用于比拟的干预措施O结局S研究设计检索和收集证据的根本步骤:①分析提出的临床问题②选择检索方式与数据库③制定检索策略④判断评估检索到的证据⑤再次检索检索式:①布尔逻辑检索(AND、OR、NOT)②位置运算符(WITH、NEAR、IN) ③范围运算符(=、>、<、<=、>=、-共6个)④截词检索(包括截词符*和通配符?)⑤优先检索(()→NOT→AND→NEAR→WITH→OR)⑥限定字段检索:字段标识符即字段名词即字段释义证据评价的根本要素:真实性的严格评价、临床意义的严格评价、临床适用性的严格评价真实性评价的角度:①研究设计的因素②研究对象的因素③观测结果的因素④资料的收集与整理的因素⑤系统分析的因素评价临床意义的效果指标:1.事件发生率:例如痊愈率,有效率,残疾率,病死率,药物不良反响率,发病率,患病率等等。
这些事件在不同的组别那么分别表示为:①实验组事件发生率(EER)②对照组事件发生率(CER)③预期事件发生率(PEER):即如果患者在不接受任何治疗的情况下,延期事件发生的概率。
2.绝对危险降低率(ARR):即实验组的事件发生率与对照组的事件发生率的绝对差值。
例如:实验组病死率10%,对照组为15%,那么ARR=| 10%-15% | =5%3.相对危险降低率(RRR):即为ARR被CER去除所得的商数值的%例如:RRR=(CER-EER)/CER=(0.15-0.10)/0.15=33%4.预防一例不良事件的发生需要治疗的总例数(NNT)例如:应用溶栓疗法治疗急性心肌梗死患者需要65例,才可取得防止一例死亡的效果,NNT=1/ARR5.绝对危险增高率(ARI):常用于表示试验组与对照组发生药物不良反响或严重事件发生率的绝对差值,AEI=EER-CER(%)6.相对危险增高率(RRI):即为ARI被EER去除所得商值的百分率:RRI =(EER-CER)/EER7.治疗多少例患者才发生一例副作用(NNH),NNH=1/ARI8.相对危险度(RR):用于观测某种危险因素暴露组事件发生率的比值比,其用于前瞻性的对照研究,通常RR≥2方有临床意义9.比值比(OR):用于回忆性病例-对照研究或系统评价中表示暴露组与非暴露组事件发生比值的相比照,其意义与RR相近10.可信区间(CL):为了有助于判断上述指标的真实范围,应用有关的统计学方法,计算相应的95%的可信区间,其分布范围越窄,其精度越高。

中文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool研究者描述随机序列产生过程譬如:参考随机数字表使用计算机随机数字生成器扔硬币洗牌的卡片和信封掷骰子抽签最小化*最小化,可实现无随机元素,被认为相当于是随机的。
研究者描述序列的产生使用的是非随机的方法。
通常是系统的非随机方法,例如:通过奇偶或出生日期产生序列通过入院日期产生序列通过类似住院号或门诊号产生序列相对于上面提到的系统方法,其它非随机的方法少见的多,也更明显。
通常包括对参与者进行判断或非随机的方法,例如:临床医生判断如何分配参与者判断如何分配基于实验室检查或系列测试的结果分配基于干预的可获取性进行分配中心分配(包括电话,网络,药房控制随机)相同外形的顺序编号的药物容器;顺序编号、不透明、密封的信封参与者以及纳入参与者的研究者可能事先知道分配,因而引入选择偏倚,譬如基于如下方法的分配:使用摊开的随机分配表(如随机序列清单)分发信封但没有合适的安全保障(如透明、非密封、非顺序编号)交替或循环出生日期病历号其它明确的非隐藏过程任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无缺失数据缺失数据的产生不大可能与真实结局相关(对于生存数据,删失不大可能引入偏倚)缺失数据的数目在各干预组相当,且各组缺失原因类似对二分类变量,与观察事件的发生风险相比,缺失比例不足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)不会大到影响观察的效应规模;缺失的数据用合适的方法进行估算任何如下标准:缺失数据的产生很大可能与真实结局相关, 缺失数据的数目及缺失原因在各干预组相差较大对二分类变量,与观察事件的发生风险相比,缺失比例足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)足以影响观察的效应规模;意向治疗分析中存在实际干预措施与随机分配的干预相违背的情况对缺失数据进行简单的不合适的估算任何如下标准:没有报道缺失或排除的情况,无法判断高风险或低风险(如未说明随机的数量,未提供数据缺失的原因)研究未描述此情况任何如下标准:实验的计划书可获取,系统评价感兴趣的所有首要或次要结局均按计划书预先说明的方式报道实验计划书不可得,但很明显发表的报告包括所有的结局,包括预先说明的结局(这种性质的有说服力的文字可能少见)任何如下标准:不是所有的预先说明的首要结局均被报道一个或多个首要结局为采用预先说明的测量方法、分析方法或数据子集来报道系统评价感兴趣的一个或多个首要结局报道不全,以至于不能纳入meta分析研究未报道此研究应当包含的主要关键结局具有与特殊试验设计相关的潜在偏倚来源或被指欺诈或其它问题可能存在偏倚风险,但存在以下两种中的一种没有足够信息评估是否存在其它重要的偏倚风险没有足够的证据认为发现的问题会引入偏倚Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies英文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment toolprocess such as:Referring to a random number table;Using a computer random number generator;Coin tossing;Shuffling cards or envelopes;Throwing dice;Drawing of lots;Minimization*.*Minimization may be implemented without a random element, and this isconsidered to be equivalent to being random.judgement The investigators describe a non-random component in the sequence generation process. Usually, the description would involve somesystematic, non-random approach, for example:Sequence generated by odd or even date of birth;Sequence generated by some rule based on date (or day) of admission;Sequence generated by some rule based on hospital or clinic recordnumber.Other non-random approaches happen much less frequently than thesystematic approaches mentioned above and tend to be obvious. Theyusually involve judgement or some method of non-random categorization ofparticipants, for example:Allocation by judgement of the clinician;Allocation by preference of the participant;Allocation based on the results of a laboratory test or a seriesof tests;Allocation by availability of the intervention.Criteria for a judgement Participants and investigators enrolling participants could not foreseeassignment because one of the following, or an equivalent method, was usedto conceal allocation:Central allocation (including telephone, web-based andpharmacy-controlled randomization);Sequentially numbered drug containers of identical appearance;Sequentially numbered, opaque, sealed envelopes.judgement Participants or investigators enrolling participants could possiblyforesee assignments and thus introduce selection bias, such as allocationbased on:Using an open random allocation schedule (e.g. a list of randomnumbers);Assignment envelopes were used without appropriate safeguards(e.g. if envelopes were unsealed or nonopaque or not sequentiallynumbered);Alternation or rotation;Date of birth;Case record number;Any other explicitly unconcealed procedure.Criteria for a judgement Any one of the following:No blinding or incomplete blinding, but the review authors judgethat the outcome is not likely to be influenced by lack of blinding;Blinding of participants and key study personnel ensured, andunlikely that the blinding could have been broken.judgementAny one of the following:No blinding or incomplete blinding, and the outcome is likely tobe influenced by lack of blinding;Blinding of key study participants and personnel attempted, butlikely that the blinding could have been broken, and the outcomeis likely to be influenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No blinding of outcome assessment, but the review authors judge thatthe outcome measurement is not likely to be influenced by lack ofblinding;Blinding of outcome assessment ensured, and unlikely that theblinding could have been broken.judgementAny one of the following:No blinding of outcome assessment, and the outcome measurement islikely to be influenced by lack of blinding;Blinding of outcome assessment, but likely that the blinding couldhave been broken, and the outcome measurement is likely to beinfluenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No missing outcome data;Reasons for missing outcome data unlikely to be related to trueoutcome (for survival data, censoring unlikely to be introducingbias);Missing outcome data balanced in numbers across interventiongroups, with similar reasons for missing data across groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk not enough to have a clinicallyrelevant impact on the intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesnot enough to have a clinically relevant impact on observed effectsize;Missing data have been imputed using appropriate methods.judgement Any one of the following:Reason for missing outcome data likely to be related to trueoutcome, with either imbalance in numbers or reasons for missingdata across intervention groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk enough to induce clinicallyrelevant bias in intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesenough to induce clinically relevant bias in observed effect size;‘As-treated’ analysis done with substantial departure of theintervention received from that assigned at randomization;Potentially inappropriate application of simple imputation.judgement ‘Unclear risk’ ofAny one of the following:Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number ran domized not stated,no reasons for missing data provided);The study did not address this outcome.Criteria for a judgement Any of the following:The study p rotocol is available and all of the study’spre-specified (primary and secondary) outcomes that are of interestin the review have been reported in the pre-specified way;The study protocol is not available but it is clear that thepublished reports include all expected outcomes, including thosethat were pre-specified (convincing text of this nature may beuncommon).judgementAny one of the following:Not all of the study’s pre -specified primary outcomes have beenreported;One or more primary outcomes is reported using measurements,analysis methods or subsets of the data (e.g. subscales) that werenot pre-specified;One or more reported primary outcomes were not pre-specified(unless clear justification for their reporting is provided, suchas an unexpected adverse effect);One or more outcomes of interest in the review are reportedincompletely so that they cannot be entered in a meta-analysis;The study report fails to include results for a key outcome thatwould be expected to have been reported for such a study.judgementThere is at least one important risk of bias. For example, the study: Had a potential source of bias related to the specific study designused; orHas been claimed to have been fraudulent; orHad some other problem.judgement ‘Unclear risk’ ofThere may be a risk of bias, but there is either:Insufficient information to assess whether an important risk of bias exists; orInsufficient rationale or evidence that an identified problem willintroduce bias.Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies。

流行病学常用中英文对照EBM术语中英文对照表Aabsolute benefit increase, ABI绝对效益增高absolute risk reduction, ARR绝对危险度降低absolute risk increase, ARIaccreditation认证accuracy准确度action threshold行动阈值adverse drug reaction,ADR药物不良反应adverse effect不良反应adverse event不良事件allocation concealment分配隐藏alternative hypothesis备择假设applicability实用性arithmetic meanassessment of study quality评价文献质量assembly bias集中性偏倚attrition bias退出偏倚Bbaseline analysis基线分析before-after study自身前后对照研究benefits收益,效益blindness盲法Ccase-control study病例-对照研究case report个例报告case series病案系列CBMdisc中国生物医学文献光盘数据库categorical variable分类变量CD-ROM retrieval光盘检索评价与传播中心数据库center for reviews and disseminationdatabase, CRDDchance机遇因素chance node概率节点choice node选择结citation bias引用偏倚clinical controlled trial database临床试验数据库clinical databases临床资料库clinical decision analysis, CDA临床决策分析clinical economic evaluation临床经济评价clinical epidemiology临床流行病学流行病学常用中英文对照clinical evidence, CE临床证据clinical pathway临床途径clinical practice guideline, CPG临床实践指南clinical research methodology临床科研方法学Cochrane collaboration Cochrane协作网Cochrane controlled trials register Cochrane对照试验注册资料库Cochrane Library Cochrane图书馆coefficient of variation变异系数cognitive dissonance认知无共振cognitive resonance认知共振cohort study队列研究co—intervention干扰collaborative review groups, CRG协作评价组completed review全文评价,全面评价compliance bias依从性偏倚complimentary medicine补充医学concealed random allocation隐藏随机分组confidence intervals, CI可信区间confounders混杂因素临床试验报告指南consolidated standards of reporting trial,CONSORTconstruct validity结构效度contamination沾染content validity内容效度continuous quality improvement, CQI持续质量改进continuous medical education, CME继续医学教育control event rate, CER对照组事件发生率cost-benefit analysis, CBA成本-效益分析cost—effectiveness analysis, CEA成本-效果分析cost-identification analysis, CIA成本—确定分析cost—minimization analysis, CMA最小成本分析cost—utility analysis, CUA成本—效用分析criteria—related validity效标效度critical appraisal严格评价c ronbach’s alphaα系数cross-over study design交叉对照研究cumulative meta-analysis累积Meta-分析Ddatabase of abstracts of reviews of干预疗效评价摘要数据库effectiveness, DARE“dec hallenge-rechallenge”study“脱离接触-再接触”研究decision tree决策树流行病学常用中英文对照descriptive statistics统计描述detection bias测量偏倚diagnostic criteria诊断标准diagnostic test诊断试验direct costs直接成本direct medical costs直接医疗成本direct nonmedical costs直接非医疗成本disease-adjusted life years, DALYs伤残调整生命年doer最佳证据提供者EEBM teaching循证医学教学effect size效应量Embase Datebase Embase数据库event rate事件发生率evidence-based clinical practice循证临床实践evidence—based medicine循证医学evidence-based management循证管理evidence-based medicine reviews, EBMR循证医学评价evidence-based medicine web sites循证医学网站excluding criteria排除(剔除)标准experimental event rate, EER试验组事件发生率exploratory trial探索性临床试验exposure暴露Fface validity表面效度fixed effects model固定效应模型follow up随访foreground questions前景问题funnel plot analysis“倒漏斗"图形分析Ggeneration of the allocation sequence随机序列产生generic quality of the instrument普通生命质量量表gold standard金标准grand rounds大查房Hhealth technology assessment,HTA卫生技术评估health—related quality of life, HRQL健康相关生命质量heterogeneity不齐性,异质性historical control trial历史性对照研究hypothesis testing假设检验Iinception cohort study截距性队列研究流行病学常用中英文对照including criteria纳入标准incremental analysis增量分析indirect costs间接成本individual patient data, IPD单个病人的资料inferential statistcs统计推断intangible costs隐性成本intention—to—treat analysis, ITT意愿治疗分析internet retrieval因特网检索intraclass correlation coefficients, ICC组内相关系数JJadad scale量表journal club文献交流俱乐部KKappa value Kappa值Lleading time bias领先时间偏倚length bias病程长短偏倚lifelong education终身教育受益与危害的似然比,利弊比likelihood of being helped versus harmed,LHHlikelihood ratio, LR似然比language bias语言偏倚locating studies检索文献location biases定位偏倚Mmagnitude强度making decision analysis决策分析matching配对mean difference均差measurement bias测量性偏倚median survival中位生存时间Medical Subject Headings, MeSH医学主题词表Medline-Index Medicus Online医学索引在线MeSH Browse医学主题词浏览器Meta—analysis汇总分析migration bias迁移性偏倚mixed effect model混合效应模型model analysis模型分析multiple publication bias多次发表偏倚multivariable analysis多变量分析NN of 1 trials单病例随机试验流行病学常用中英文对照negative likelihood ratio阴性似然比negative predictive value, NPV阴性预测值non—randomized concurrent control trial非随机同期对照研究null hypothesis无效假设number needed to diagnosis, NND需要诊断的人数number needed to harm, NNH需要多少患者接触致病因素才能产生1例额外的不良反应number needed to screen, NNS需要筛检的病人数number needed to treat, NNT预防1例不良事件的发生,需要治疗的总例数Oodds ratio, OR比数比(比值比)opinion—based practice经验医学outcome research结局研究Pparallel test平行试验patient’s expected event rate (PEER)病人预期事件发生率performance bias实施偏倚Permuted Index医学主题词轮排表per protocol, PP按方案分析pooling合并positive likelihood ratio阳性似然比positive predictive value, PPV阳性预测值post—test probability验后概率practice EBM循证医学实践Practice Guideline实践指南precision精确性,精度pre-clinical students临床前期的医学生predictive value预测值pre—test probability/prevalence验前概率/患病率prevalence患病率prognosis预后prognostic factor预后因素proportion构成比protocol研究方案publication bias发表偏倚publication type文献类型Qqualitative systematic review定性系统评价quality adjusted life years,QALYs质量调整生命年quality of life, QOL生命质量Quality of reporting of Meta-analyses Meta—分析质量评价指南流行病学常用中英文对照statement(QUOROM)quality scales and components质量量表与构成quantitative systematic review定量系统评价quartile四分位数间距Rrandom allocation随机分配random effects model随机效应模型randomization随机化randomized control trial, RCT随机对照试验reaction反应性receiver operator characteristic curve ROC曲线relative benefit increase, RBI相对效益增高(率)relative risk/risk ratio, relative risk, RR相对危险度relative risk increase, RRI相对危险增高(率)relative risk reduction, RRR相对危险度减少(率)reliability信度,可靠性reproducible可重复性restriction限制retrospective回顾性review manager: Revman系统评价管理软件risk difference率差/危险差risks风险Sscale量表screening test筛查试验selecting bias选择性偏倚selecting studies选择文献sensitivity敏感性sensitivity analysis敏感性分析sequential trial序贯试验serial test序列试验severe adverse event, SAE严重不良事件significance test显著性检验size of test检验水准specific quality of life instrument专用生命质量量表specificity特异性split—half reliability折半信度standard deviation标准差stratification分层stratify factor分层因素stratify randomization分层随机法subgroup analysis亚组分析流行病学常用中英文对照subheadings副主题词survival curve生存曲线survival rate生存率systematic review系统综述Ttest—retest reliability重测信度test threshold诊断阈值treatment shreshold治疗阈值time frame时间框架total quality management, TQM全面质量管理Uuser最佳证据应用者utility效用Vvalidity效度,真实性Wweighted mean difference, WMD加权均数差“worst-case"scenario“最差情况”演示分析Zzero time零点。

*基金项目:第三批江苏省名老中医药专家传承工作室建设项目(苏中医科教发[2019]10号)①南京中医药大学附属医院 江苏 南京 210029通信作者:孙子凯麻杏石甘汤治疗慢性阻塞性肺疾病急性加重期的Meta分析*刘莹① 臧莲① 孙子凯①【摘要】 目的:系统评价麻杏石甘汤治疗慢性阻塞性肺疾病急性加重期(AECOPD)的有效性与安全性。
方法:计算机检索维普(VIP)、中国知网(CNKI)、中国生物医学文献数据库(CBM)、万方数据(WanFang Data)和Embase、PubMed、Cochrane Library,收集建库至2022年1月国内外公开发表的关于麻杏石甘汤治疗AECOPD 的临床随机对照试验(RCT)。
运用RevMan 5.4软件行Meta 分析。
结果:最终纳入19个RCT,包括1 698例患者。
Meta 分析示,麻杏石甘汤加减联合西医常规治疗较对照组能显著提高AECOPD 临床有效率[RR =1.14,95%CI (1.10,1.18),P <0.000 01];降低中医症候积分[MD =-4.53,95%CI (-5.98,-3.09),P <0.000 01];改善肺功能,包括第一秒用力呼气量占预计值百分比(FEV 1%pred)[MD =5.89,95%CI (3.96,7.82),P <0.000 01]、第一秒用力呼气量(FEV 1)[MD =0.36,95%CI (0.20,0.52),P <0.000 01]、用力肺活量(FVC)[MD =0.31,95%CI (0.14,0.48),P =0.000 4]和第一秒用力呼气量占用力肺活量百分比(FEV 1/FVC%)[MD =5.67,95%CI (4.50,6.83),P <0.000 01];降低炎症因子水平,包括C 反应蛋白(CRP)[SMD =-1.70,95%CI (-2.62,-0.78),P =0.000 3],白细胞介素-8(IL-8)[SMD =-1.53,95%CI (-2.34,-0.71),P =0.000 2],肿瘤坏死因子-α(TNF-α)[SMD =-2.31,95%CI (-3.58,-1.04),P =0.000 4]。

疏风解毒胶囊辅助治疗慢性支气管炎急性发作的Meta分析作者:储小毛杨勤军李泽庚童佳兵王新汝梁剑晨朱张求来源:《中国民族民间医药·上半月》2022年第03期【摘要】目的:基于疏風解毒胶囊辅助治疗慢性支气管炎急性发作患者的疗效、临床症状及体征、肺功能及实验室指标改善情况进行Meta分析,以期为临床应用和深入研究疏风解毒胶囊提供理论依据。
方法:检索疏风解毒胶囊治疗慢性支气管炎急性发作患者的随机对照试验(RCTs),根据文献纳入标准筛选文献后提取数据,采用Cochrane偏倚风险评估工具评价偏倚风险,应用RevMan 5.3软件进行Meta分析。
结果:共纳入10项研究,956例患者,试验组和对照组各478例,Meta分析结果显示:在常规西药治疗的基础上加用疏风解毒胶囊可提高慢性支气管炎急性发作患者的疗效[RR=1.19,95%CI(1.13,1.26),P【关键词】慢性支气管炎;急性发作;疏风解毒胶囊;临床疗效;Meta分析【中图分类号】R562.2+1 【文献标志码】 A 【文章编号】1007-8517(2022)05-0071-10基金项目:国家中医药管理局重点实验室建设项目(国中药函[2009]95号);国家自然科学基金项目(81874431);中国中医药循证医学中心流感循证能力建设项目(2019XZZX-LG001);安徽省教育厅重点项目(KJ2019A0468)。
作者简介:储小毛(1996-),女,汉族,硕士,执业医师,研究方向为中医药防治呼吸系统疾病。
E-mail:*****************通信作者:童佳兵(1975-),男,汉族,博士,主任医师,研究方向为中医药防治呼吸系统疾病。
E-mail:****************Meta Analysis of Shufeng Jiedu Capsule in the Treatment of Acute Attack of Chronic BronchitisCHU Xiaomao1 YANG Qinjun1 LI Zegeng2,3 TONG Jiabing2,3* WANG Xinru1 LIANG Jianchen1 ZHU Zhangqiu11.Graduate School of Anhui University of Traditional Chinese Medicine. Hefei 230012,China;2.Institute of Respiratory Disease Prevention and treatment of traditional Chinese Medicine,Anhui Academyof traditional Chinese Medicine, Hefei 230012,China;3.The first Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei 230012,ChinaAbstract:Objective To carry out Meta analysis based on the efficacy, clinical symptoms and signs, pulmonary function and laboratory indexes of Shufeng jiedu capsule in the treatment of patients with acute attack of chronic bronchitis, so as to provide theoretical basis for clinical application and further study of Shufeng jiedu capsule. Methods Randomized controlled trial (RCTs), of Shufeng jiedu capsule in the treatment of patients with acute attack of chronic bronchitis was searched. The data were extracted according to the literature inclusion criteria. Cochrane bias risk assessment tool was used to evaluate bias risk, and RevMan5.3 software was used for Meta analysis. Results A total of 10 studies were included, including 478 patients in the experimental group and 478 patients in the control group. Meta analysis showed that Shufeng jiedu capsule could improve the curative effect of patients with acute attack of chronic bronchitis on the basis of routine western medicine treatment [RR=1.19,95%CI(1.13, 1.26),P<0.00001),and reduce the time of clinical symptoms and signs of patients with cough [MD=-1.08,95%CI(-1.6), PKey words:Chronic Bronchitis;Acute Attack;Shufeng Jiedu Capsule;Clinical Efficacy;Meta Analysis慢性支气管炎是发生于气管、支气管及其外周组织的慢性非特异性炎症,起病缓,病程长,临床上主要表现为咳嗽、咳痰、喘息等。
The Joanna Briggs Institute Critical Appraisal toolsfor use in JBl Systematic Reviews澳大利亚JBI循证卫生保健中心对RCT的真实性评价RCT是原始研究中质量最高的证据,对RCT进行文献质量评价的工具有Cochrane协作网的评价工具,澳大利亚JBI循证卫生保健中心的评价工具,英国文献质量评价项目CASP的评价工具等。
澳大利亚JBI循证卫生保健中心(2016)对RCT论文的质量评价工具包含13个评价项目。
评价者需对每个评价项目做出“是”、“否”、“不清楚”、“不适用”的判断,并最终经过小组讨论,决定该研究是纳入、排除,还是需获取进一步的信息。
此评价工具具体条目如下所示。
澳大利亚JBI循证卫生保健中心对RCT的真实性评价评价项目评价结果1. 是否对研究对象真正采用了随机分组的方法?是否不清楚不适用2. 是否做到了分配隐藏?是否不清楚不适用3. 组间基线是否具有可比性?是否不清楚不适用4. 是否对研究对象实施了盲法?是否不清楚不适用5. 是否对干预者实施了盲法?是否不清楚不适用6. 是否对结果测评者实施了盲法?是否不清楚不适用7. 除了要验证的干预措施外,各组接受的其他措施是否相同?是否不清楚不适用8.随访是否完整,如不完整,是否采取措施处理失访?是否不清楚不适用9. 是否将所有随机分配的研究对象纳入结果分析?是否不清楚不适用10. 是否采用相同的方式对各组研究对象的结局指标进行测评?是否不清楚不适用11. 结局指标的测评方法是否可信?是否不清楚不适用12. 资料分析方法是否恰当?是否不清楚不适用13.研究设计是否合理?在实施研究和资料分析过程中是否有不同于标准RCT之处?是否不清楚不适用。
XXX纳入的RCT文献质量评价(风险偏倚评估工具)中英文对照版根据XXX的“偏倚风险评估工具”,表8.5.a列出了评估偏倚类型的指标。
其中,选择偏倚包括评估随机序列的产生,以及对分组可比性的评估。
需要提供足够详细的描述,以便判断分组的产生是否具有可比性。
分配偏倚则需要提供足够详细的描述,以评估分配是否隐藏,并决定干预的分配是否可见。
实施偏倚则需要描述实施者和参与者是否采用了双盲方法,以避免其了解干预信息。
同时,需要提供与实施的盲法是否有效相关的信息。
测量偏倚则需要描述对结局者行盲法,避免其了解所接受的干预信息。
同时,需要提供与实施的盲法是否有效相关的信息。
失访偏差需要描述每个主要结局数据的完整性,以及缺失数据是否报告。
同时,需要说明重新纳入分析的原因。
发表偏差需要说明如何审查选择性报道结局的可能性,以及审查结果。
其他偏差则需要说明不包括在上述偏差中的其他重要偏差。
如果在计划书中指出了特定的问题或条目,需要对每一项进行说明。
表8.5.d列出了评估偏倚风险的标准。
其中,随机序列的产生不充分会导致选择偏差。
对于评判为低风险的标准,研究者需要描述随机序列产生的过程,例如参考随机数字表、使用计算机随机数字生成器、扔硬币、洗牌的卡片和信封、掷骰子、抽签、最小化等方法。
对于评判为高风险的标准,研究者需要描述使用的非随机方法,通常是系统的非随机方法,例如最小化,虽然可实现无随机元素,但被认为相当于是非随机的。
可能被打破,结局很可能受到缺乏盲法的影响风险未知任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况随机化是临床研究中重要的方法之一,但在实施随机化时,存在着一些潜在的风险和偏差。
在随机化的过程中,通常会采用系统方法或非随机的方法来产生序列。
其中,系统方法包括通过奇偶或出生日期、入院日期、住院号或门诊号等方式产生序列;非随机的方法则包括临床医生或参与者的判断、实验室检查或系列测试的结果、干预的可获取性等方式来分配。
rct文献质量评价表
rct(随机对照试验)文献质量评价表通常包括以下几
个方面的评价内容:
1.随机方法:评价随机方法是否正确,是否能够保证试验组和对照组的基线一致性。
2.对照设置:评价对照组的设置是否合理,是否能够反映实际临床情况,以及对照组是否与试验组具有可比性。
3.盲法实施:评价试验实施过程中是否采用盲法,是否能够减少主观偏倚的影响。
4.样本量:评价样本量是否足够大,以确保结果的稳定性和可靠性。
5.数据完整性:评价数据是否完整,是否有缺失或异常值,以及是否进行了合理的处理。
6.基线情况:评价试验组和对照组的基线情况是否相似,以确保试验结果的公正性和客观性。
7.疗效评价:评价疗效评价标准是否科学、客观、可重复,以及是否进行了合理的统计学分析。
8.安全性评价:评价安全性评价方法是否科学、客观、可重复,以及是否进行了合理的统计学分析。
9.试验流程:评价试验流程是否规范、合理,以及是否符合伦理要求。
10.结论可靠性:综合以上各点,对试验结论的可靠性进行评价。
在具体应用中,可以根据实际情况对rct文献质量评价表进行适当调整和增删。
· 共识与指南·中成药治疗溃疡性结肠炎临床应用指南(精简版,2022年)*#&《中成药治疗优势病种临床应用指南》标准化项目组摘要 溃疡性结肠炎(UC )是中医药治疗的优势病种,中成药是中医药的重要组成部分,但其在临床应用方面仍存在大量盲目用药和不规范用药的情况。
《中成药治疗优势病种临床应用指南》标准化项目组召集行业内中、西医临床专家、药学专家,以及指南研究方法学专家共同编制了本指南,分别制定了不同严重程度、治疗目标定位下的中成药口服和(或)灌肠治疗UC 的推荐意见,旨在为临床医师合理使用中成药治疗UC 提供规范性的指导和建议。
关键词 中成药; 结肠炎,溃疡性; 临床应用; 指南Clinical Application Guidelines on Chinese Patent Medicine in Treatment of Ulcerative Colitis (Condensed Edition, 2022) Clinical Application Guidelines for Dominant Diseases Treated With Chinese Patent Medicine Standard ⁃ization Project TeamCorrespondence to: LI Junxiang, Department of Spleen, Stomach, and Hepatobiliary Diseases, Dongfang Hospital, Beijing UniversityofChineseMedicine,Beijing(100078),Email:**********************Abstract Ulcerative colitis (UC) is a dominant disease that can be treated with traditional Chinese medicine, andChinese patent medicine is an important part of traditional Chinese medicine. However, there are still many cases of non⁃standard drug use in the clinical treatment of UC. Clinical Application Guidelines for Dominant Diseases Treated withChinese Patent Medicine Standardization Project Team convened experts on traditional Chinese medicine, Western medicine, pharmacology, and methodology to compile this guideline. This guideline formulated recommendations for oral and (or) clyster treatment of UC in different severities and goals of treatment, in order to provide normative suggestions forclinicians to rationally use Chinese patent medicines in UC.Key words Chinese Patent Medicine; Colitis, Ulcerative; Clinical Application; GuidelinesDOI : 10.3969/j.issn.1008⁃7125.2023.01.004*原文刊载于《中华消化杂志》,经中华医学会和《中华消化杂志》编辑部授权转载#基金项目:国家中医药管理局《中成药治疗优势病种临床应用指南》标准化项目(SATCM⁃2015⁃BZ402⁃040);中医药传承与创新“百千万”人才工程—岐黄学者[国中医药人教函(2018)284号];2018年国家重点研发计划⁃中医药现代化研究重点专项(2018YFC 1705403)&本文通信作者:李军祥,北京中医药大学东方医院脾胃肝胆科(100078),Email:**********************溃疡性结肠炎(ulcerative colitis, UC )是一种以结直肠黏膜连续性、弥漫性炎症改变为特点的慢性非特异性肠道炎症性疾病,其病变大多局限于结直肠黏膜和黏膜下层,属于炎症性肠病范畴[1]。
循证护理文献质量评价工具
循证护理文献质量评价工具是用于对医学或护理学科领域中的文献进
行质量评价的工具,其目的是确定文献的可信度和可靠性,以便提供客观、证据化的医学或护理实践建议。
以下是一些常见的循证护理文献质量评价工具:
1. Cochrane协作网络的风险偏倚工具(Risk of Bias Tool):用
于评价随机对照试验(RCTs)的质量,包括研究设计、随机化、盲法、缺
失数据等方面的风险偏倚。
2. Newcastle-Ottawa量表(NOS):用于评价病例对照研究和队列
研究的质量,包括选择对照组、病例和对照组间的匹配、病例和对照组间
的比较、评价因素是否对结果造成影响等方面。
3. Jadad量表:用于评价RCTs的质量,包括随机化的方法、盲法、
隐瞒分组等方面。
4.GRADE系统:用于评价循证医学研究证据的质量和可靠性,包括研
究设计、风险偏倚、一致性、精度、重要性等方面。
以上工具都可以通过评分的方式,对文献的各个方面进行客观评价和
分析,从而确定其在临床实践中的应用价值和可靠性,进一步提高医学和
护理的水平和效果。