cochrane纳入的RCT文献质量评价中文版
- 格式:doc
- 大小:103.00 KB
- 文档页数:7

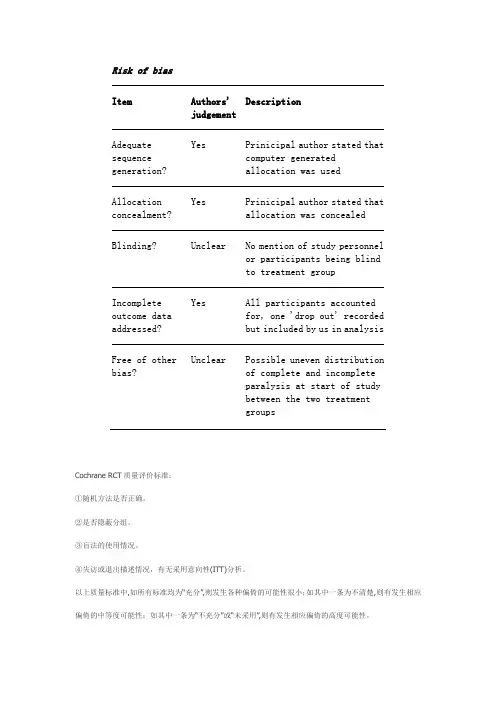
Risk of biasItem Authors'judgementDescriptionAdequate sequence generation? Yes Prinicipal author stated thatcomputer generatedallocation was usedAllocation concealment? Yes Prinicipal author stated thatallocation was concealedBlinding? Unclear No mention of study personnelor participants being blindto treatment groupIncomplete outcome data addressed? Yes All participants accountedfor, one 'drop out' recordedbut included by us in analysisFree of other bias? Unclear Possible uneven distributionof complete and incompleteparalysis at start of studybetween the two treatmentgroupsCochrane RCT质量评价标准:①随机方法是否正确。
②是否隐蔽分组。
③盲法的使用情况。
④失访或退出描述情况,有无采用意向性(ITT)分析。
以上质量标准中,如所有标准均为“充分”,则发生各种偏倚的可能性很小;如其中一条为不清楚,则有发生相应偏倚的中等度可能性;如其中一条为“不充分”或“未采用”,则有发生相应偏倚的高度可能性。
可参见:RCT的质量评价标准选择总结/bbs/topic/18137535?tpg=1&age=-1Quality assessmentThe quality of the trials was assessed and graded independently by two authors according to the criteria described in The Cochrane Handbook 4.2.6 (Higgins 2006). Gradings were compared and any inconsistencies between the authors in the interpretation of inclusion criteria and their significance to the selected study were discussed and resolved.The selected study was assessed for the following characteristics:1. The adequacy of the randomisation process (possible selection bias). Adequate randomisation includes any one of the following methods: computer generated or table of random numbers, drawing of lots, coin-toss, shuffling cards or throw of a dice. Inadequate methods of randomisation include the following: case record number, date of birth or alternate numbers.2. The adequacy of the allocation concealment (possible selection bias). Adequate methods of allocation concealment include either central randomisation (i.e. separate to other aspects of trial administration) or sequentially numbered sealed opaque envelopes. Inadequate concealment means an open allocation sequence in which either participants or trialists were able to foresee the upcoming assignment.3. The blinding of outcome assessors (i.e. whether the persons assessing the outcome of care were aware of which treatment the participant had received - possible performance bias).4. The extent and handling of losses to follow up (possible attrition bias). Adequate handling of losses to follow up involves a clear description and explanation being given of any significant difference between the losses of the intervention groups. An unacceptable loss in any one intervention group was considered to be loss greater than 20%.Study gradings A, B or C were employed for overall quality as follows.A: Minimisation of bias in all four categories above: i.e. adequate randomisation, few losses to follow up and intention-to-treat analysis, blinding of outcome assessors, high quality outcome assessment;B: Each of the criteria in A partially met;C: One or more of the criteria in A not met.Risk of bias in included studiesWe classified this study as grade C because of the uncertainty about blinding. The possibility of an uneven distribution of complete and incomplete palsies between the two groups is another potential source of bias and we conclude overall that this is a low quality study.Table 8.5.a: The Cochrane Collaboration’s tool for assessi ng risk of biasTable 8.5.c: Criteria for judging risk of bias in the ‘Risk of bias’ assessment toolFigure 8.6.a: Example of a ‘Risk of bias’ table for a single study (fictional)Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies。


文献质量评估
各纳入RCT的方法学质量评价采用Cochrane5.1手册推荐的简单评估法[9],评价的关键指标:①随机方法是否正确;②是否做到分配隐藏,分配方法是否正确;③是否实施盲法;
④是否报告失访和退出情况;⑤基线是否可比。
对于分配隐藏,将试验评为A(完全隐藏)、B(不清楚是否隐藏)、C(隐藏不充分)和D(没有使用隐藏)4个等级。
在其他方面将试验评为A(是)、B(不清楚)、C(否)三级。
如各评价条目均为A级,则为低度偏倚,发生各种偏倚的可能性最小,质量评为A级;若有一条目或多个条目为B,则该试验有发生相应偏倚的中等度可能性,质量评为B级;如其中有一条目或多个条目为C,则该试验有发生相应偏倚的高度可能性,质量评为C级。


Cochrane文献评价手册在医学和健康领域,Cochrane文献评价手册是一个备受推崇的参考资料。
它是一个致力于提供高质量、广度和深度兼具的文献评价和系统评价信息的权威评台。
通过对临床试验和其他医学研究进行全面评估和分析,Cochrane文献评价手册为医生、医学研究者和患者提供了宝贵的参考意见。
在Cochrane文献评价手册中,我们可以查找到各种主题的文献评价和系统评价,涵盖了从疾病预防到治疗、康复的各个方面。
无论是针对特定疾病、特定干预措施或特定人群的研究,Cochrane文献评价手册都提供了可靠的信息和意见,帮助人们做出更明智的医疗决策。
对于医学研究者来说,Cochrane文献评价手册提供了一个值得借鉴和学习的标准。
他们可以通过查阅Cochrane文献评价手册,了解到如何进行高质量的文献评价和系统评价,以及如何准确地汇总和分析研究结果。
这有助于提高他们的学术水平,促进医学研究的发展和进步。
对于医生和医疗工作者来说,Cochrane文献评价手册则是一个宝贵的临床参考工具。
他们可以在这里找到与自己临床实践相关的最新研究成果和最可靠的证据,以指导自己的临床决策。
这有助于提高临床实践的质量,保证患者获得更好的医疗服务。
对于患者和公众来说,Cochrane文献评价手册提供了一个可靠的信息来源。
他们可以在这里了解到关于自己健康问题的最新研究成果和治疗建议,从而更好地了解自身疾病,做出更明智的健康决策。
Cochrane文献评价手册是一个对医学研究、临床实践和健康决策都具有重要意义的权威评台。
它以其高质量、深度和广度兼具的文献评价和系统评价信息,为医学领域的各个参与者提供了宝贵的帮助和指导。
相信随着医学研究的不断进步和发展,Cochrane文献评价手册将为我们带来更多的惊喜和启发。
Cochrane文献评价手册作为权威评台,其对医学领域具有重要意义的确无庸置疑。
在现代医学研究中,由于信息的爆炸性增长和研究成果的不断涌现,医生和研究者需要一个可靠的参考工具来指导他们的决策和实践。
中文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool研究者描述随机序列产生过程譬如:参考随机数字表使用计算机随机数字生成器扔硬币洗牌的卡片和信封掷骰子抽签最小化*最小化,可实现无随机元素,被认为相当于是随机的。
研究者描述序列的产生使用的是非随机的方法。
通常是系统的非随机方法,例如:通过奇偶或出生日期产生序列通过入院日期产生序列通过类似住院号或门诊号产生序列相对于上面提到的系统方法,其它非随机的方法少见的多,也更明显。
通常包括对参与者进行判断或非随机的方法,例如:临床医生判断如何分配参与者判断如何分配基于实验室检查或系列测试的结果分配基于干预的可获取性进行分配中心分配(包括电话,网络,药房控制随机)相同外形的顺序编号的药物容器;顺序编号、不透明、密封的信封参与者以及纳入参与者的研究者可能事先知道分配,因而引入选择偏倚,譬如基于如下方法的分配:使用摊开的随机分配表(如随机序列清单)分发信封但没有合适的安全保障(如透明、非密封、非顺序编号)交替或循环出生日期病历号其它明确的非隐藏过程任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无缺失数据缺失数据的产生不大可能与真实结局相关(对于生存数据,删失不大可能引入偏倚)缺失数据的数目在各干预组相当,且各组缺失原因类似对二分类变量,与观察事件的发生风险相比,缺失比例不足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)不会大到影响观察的效应规模;缺失的数据用合适的方法进行估算任何如下标准:缺失数据的产生很大可能与真实结局相关, 缺失数据的数目及缺失原因在各干预组相差较大对二分类变量,与观察事件的发生风险相比,缺失比例足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)足以影响观察的效应规模;意向治疗分析中存在实际干预措施与随机分配的干预相违背的情况对缺失数据进行简单的不合适的估算任何如下标准:没有报道缺失或排除的情况,无法判断高风险或低风险(如未说明随机的数量,未提供数据缺失的原因)研究未描述此情况任何如下标准:实验的计划书可获取,系统评价感兴趣的所有首要或次要结局均按计划书预先说明的方式报道实验计划书不可得,但很明显发表的报告包括所有的结局,包括预先说明的结局(这种性质的有说服力的文字可能少见)任何如下标准:不是所有的预先说明的首要结局均被报道一个或多个首要结局为采用预先说明的测量方法、分析方法或数据子集来报道系统评价感兴趣的一个或多个首要结局报道不全,以至于不能纳入meta分析研究未报道此研究应当包含的主要关键结局具有与特殊试验设计相关的潜在偏倚来源或被指欺诈或其它问题可能存在偏倚风险,但存在以下两种中的一种没有足够信息评估是否存在其它重要的偏倚风险没有足够的证据认为发现的问题会引入偏倚Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies英文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment toolprocess such as:Referring to a random number table;Using a computer random number generator;Coin tossing;Shuffling cards or envelopes;Throwing dice;Drawing of lots;Minimization*.*Minimization may be implemented without a random element, and this isconsidered to be equivalent to being random.judgement The investigators describe a non-random component in the sequence generation process. Usually, the description would involve somesystematic, non-random approach, for example:Sequence generated by odd or even date of birth;Sequence generated by some rule based on date (or day) of admission;Sequence generated by some rule based on hospital or clinic recordnumber.Other non-random approaches happen much less frequently than thesystematic approaches mentioned above and tend to be obvious. Theyusually involve judgement or some method of non-random categorization ofparticipants, for example:Allocation by judgement of the clinician;Allocation by preference of the participant;Allocation based on the results of a laboratory test or a seriesof tests;Allocation by availability of the intervention.Criteria for a judgement Participants and investigators enrolling participants could not foreseeassignment because one of the following, or an equivalent method, was usedto conceal allocation:Central allocation (including telephone, web-based andpharmacy-controlled randomization);Sequentially numbered drug containers of identical appearance;Sequentially numbered, opaque, sealed envelopes.judgement Participants or investigators enrolling participants could possiblyforesee assignments and thus introduce selection bias, such as allocationbased on:Using an open random allocation schedule (e.g. a list of randomnumbers);Assignment envelopes were used without appropriate safeguards(e.g. if envelopes were unsealed or nonopaque or not sequentiallynumbered);Alternation or rotation;Date of birth;Case record number;Any other explicitly unconcealed procedure.Criteria for a judgement Any one of the following:No blinding or incomplete blinding, but the review authors judgethat the outcome is not likely to be influenced by lack of blinding;Blinding of participants and key study personnel ensured, andunlikely that the blinding could have been broken.judgementAny one of the following:No blinding or incomplete blinding, and the outcome is likely tobe influenced by lack of blinding;Blinding of key study participants and personnel attempted, butlikely that the blinding could have been broken, and the outcomeis likely to be influenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No blinding of outcome assessment, but the review authors judge thatthe outcome measurement is not likely to be influenced by lack ofblinding;Blinding of outcome assessment ensured, and unlikely that theblinding could have been broken.judgementAny one of the following:No blinding of outcome assessment, and the outcome measurement islikely to be influenced by lack of blinding;Blinding of outcome assessment, but likely that the blinding couldhave been broken, and the outcome measurement is likely to beinfluenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No missing outcome data;Reasons for missing outcome data unlikely to be related to trueoutcome (for survival data, censoring unlikely to be introducingbias);Missing outcome data balanced in numbers across interventiongroups, with similar reasons for missing data across groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk not enough to have a clinicallyrelevant impact on the intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesnot enough to have a clinically relevant impact on observed effectsize;Missing data have been imputed using appropriate methods.judgement Any one of the following:Reason for missing outcome data likely to be related to trueoutcome, with either imbalance in numbers or reasons for missingdata across intervention groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk enough to induce clinicallyrelevant bias in intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesenough to induce clinically relevant bias in observed effect size;‘As-treated’ analysis done with substantial departure of theintervention received from that assigned at randomization;Potentially inappropriate application of simple imputation.judgement ‘Unclear risk’ ofAny one of the following:Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number ran domized not stated,no reasons for missing data provided);The study did not address this outcome.Criteria for a judgement Any of the following:The study p rotocol is available and all of the study’spre-specified (primary and secondary) outcomes that are of interestin the review have been reported in the pre-specified way;The study protocol is not available but it is clear that thepublished reports include all expected outcomes, including thosethat were pre-specified (convincing text of this nature may beuncommon).judgementAny one of the following:Not all of the study’s pre -specified primary outcomes have beenreported;One or more primary outcomes is reported using measurements,analysis methods or subsets of the data (e.g. subscales) that werenot pre-specified;One or more reported primary outcomes were not pre-specified(unless clear justification for their reporting is provided, suchas an unexpected adverse effect);One or more outcomes of interest in the review are reportedincompletely so that they cannot be entered in a meta-analysis;The study report fails to include results for a key outcome thatwould be expected to have been reported for such a study.judgementThere is at least one important risk of bias. For example, the study: Had a potential source of bias related to the specific study designused; orHas been claimed to have been fraudulent; orHad some other problem.judgement ‘Unclear risk’ ofThere may be a risk of bias, but there is either:Insufficient information to assess whether an important risk of bias exists; orInsufficient rationale or evidence that an identified problem willintroduce bias.Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies。

rct文献质量评价表
rct(随机对照试验)文献质量评价表通常包括以下几
个方面的评价内容:
1.随机方法:评价随机方法是否正确,是否能够保证试验组和对照组的基线一致性。
2.对照设置:评价对照组的设置是否合理,是否能够反映实际临床情况,以及对照组是否与试验组具有可比性。
3.盲法实施:评价试验实施过程中是否采用盲法,是否能够减少主观偏倚的影响。
4.样本量:评价样本量是否足够大,以确保结果的稳定性和可靠性。
5.数据完整性:评价数据是否完整,是否有缺失或异常值,以及是否进行了合理的处理。
6.基线情况:评价试验组和对照组的基线情况是否相似,以确保试验结果的公正性和客观性。
7.疗效评价:评价疗效评价标准是否科学、客观、可重复,以及是否进行了合理的统计学分析。
8.安全性评价:评价安全性评价方法是否科学、客观、可重复,以及是否进行了合理的统计学分析。
9.试验流程:评价试验流程是否规范、合理,以及是否符合伦理要求。
10.结论可靠性:综合以上各点,对试验结论的可靠性进行评价。
在具体应用中,可以根据实际情况对rct文献质量评价表进行适当调整和增删。
cochrane文献质量评价分级医学文献的定义及类别“文献”一词出现在中国已有2000多年的历史,从春秋战国时期就有关于文献的记载,最早见于《论语八佾》。
随着人类文明的发展,“文献”的概念也发生了巨大的变化,人们对文献的研究一直持续至今。
文献的具体定义尚缺乏统一的定论,《现代汉语词典》对文献的定义是“有历史价值或参考价值的资料”[1];《中华人民共和国国家标准—文献著录总则》将文献定义为“记载有知识的一切载体”[2]。
有学者将文献定义为“文献就是将知识、信息用文字、符号、图像、音频等记录在一定物质载体上的结合体”[3]。
有历史价值和研究价值的知识、一定的载体、一定的方法和手段、一定的意义表达和记录体系这4个方面构成文献的基本要素。
医学文献(medicalliterature)就是与医学有关的有参考价值的资料。
按照文献的研究类型分为:系统评价、随机对照临床试验、队列研究、病例对照研究、病例系列研究、病例报告和专家经验总结等。
决定文献质量的关键部分:研究有首创性或提供了新证据、研究对象的选择合理、科研设计合理、偏倚得到有效控制、研究样本量足够大和研究的时间足够长。
然而医学文献是如何发挥作用的,前提就是该文献必须有一定的价值,而且有可靠的等级评价体系。
医学文献的价值就是根据其文献内在及外在真实性及临床意义的重要性去评判,是根据医学研究的方向不同确定评价的原则和方法[4],其中内在真实性是文献评价的重点。
2西医文献评价体系2.1证据分级20世纪60年代,首次提出证据分级概念,将随机对照研究的质量定为最高,并引入内部真实性和外部真实性的概念。
证据分级为西医识别文献可靠性的准则。
最初3级标准:1级:设计良好的随机对照试验(RCT);2级:1级和3级中间的类型;3级:专家经验。
老5级标准:1级:收集所有质量可靠的RCT后做出的系统评价或Meta分析结果,大样本多中心随机对照试验;2级:单个大样本的RCT结果;3级:设有对照但未用随机方法分组的研究病例对照研究和队列研究;4级:无对照的系列病例观察;5级:专家意见、描述性研究和病例报告。
cochrane文献评价手册【原创实用版】目录1.Cochrane 文献评价手册的概述2.手册的目的和适用范围3.手册的主要内容4.手册的评价标准和方法5.手册的优点和不足6.对我国相关领域的影响和启示正文1.Cochrane 文献评价手册的概述Cochrane 文献评价手册是由 Cochrane 协作组织编写的一本关于如何评价和分析医学文献的指南。
Cochrane 协作组织是一个国际性的、非营利的医学研究组织,致力于通过系统评价和荟萃分析的方法,为临床决策提供高质量的证据。
2.手册的目的和适用范围本手册的主要目的是为系统评价和荟萃分析提供一种标准和方法,以便为临床决策提供可靠的证据。
它适用于所有从事医学研究、临床实践和卫生政策制定的人员。
3.手册的主要内容手册主要包括以下几个方面:(1)文献筛选:包括文献的检索、筛选和纳入;(2)文献的质量评估:包括随机对照试验、队列研究、病例对照研究等不同类型文献的质量评估;(3)数据提取和分析:包括数据的提取、整理和分析;(4)结果的报告和解释:包括结果的报告方式、如何解释结果等。
4.手册的评价标准和方法手册提供了一套详细的评价标准和方法,包括风险偏倚、质量评估、数据提取和分析等。
这些标准和方法被广泛接受和应用,是进行系统评价和荟萃分析的基础。
5.手册的优点和不足手册的优点在于提供了一套系统、全面、实用的评价方法和标准,对于提高研究的可靠性和质量具有重要的指导作用。
然而,手册也存在一些不足,例如部分内容较为复杂,对研究者的统计学和医学知识要求较高。
6.对我国相关领域的影响和启示Cochrane 文献评价手册对我国的医学研究、临床实践和卫生政策制定产生了深远的影响。
它提供了一种科学的、可靠的评价方法,有助于提高我国医学研究的质量和水平。
·综述·集束化护理模式对肿瘤患者骨髓抑制期有效性的Meta 分析李成成 殷利 向明芳 张萱 吕俭霞Meta-analysis of the eff ectiveness of the cluster nursing model on myelosuppression in cancer patients LI Chengcheng, YIN Li, XIANG Mingfang, ZHANG Xuan, LV Jianxia Sichuan Cancer Hospital, Chengdu 610041, China【Abstract 】 Objective To systematically evaluate the eff ect of cluster nursing on myelosuppression in cancer patients. Methods We searched Chinese and English databases at home and abroad, included randomized controlled trials (RCT) on the eff ect of cluster nursing intervention on myelosuppression, and evaluated the quality of the literature. This study used RevMan 5.4 statistical software for data analysis. Results A total of 25 RCT were included, including 23 Chinese and 2 English, with a total of 2,072 patients. The Meta-analysis results showed that in the experimental group (cluster nursing measures), the incidence rate of infection was reduced by RR =0.43, 95% CI : 0.36~0.50, P <0.00001; the incidence rate of bleeding was RR =0.33, 95% CI : 0.24~0.46, P <0.00001; the incidence rate of fever was RR =0.54, 95% CI : 0.44~0.67, P <0.00001; the patient's psychological state, anxiety level, MD=-2.88, 95% CI : -3.65~-2.11, P <0.00001, and depression level, MD=- 1.49, 95% CI : -12.06~-0.92, P <0.00001, and improved patient satisfaction RR =1.25, 95% CI : 1.15~1.35, P <0.00001, both higher than the control group, and the diff erences between the groups were statistically signifi cant(P <0.05). Conclusion Cluster nursing can prevent the occurrence of complications in the myelosuppression phase of cancer patients, while improving the patient's psychological state and improving patient satisfaction.【Key words 】 Tumor; Myelosuppression; Cluster nursing; Effectiveness; Meta-analysis 四川省肿瘤医院/四川省癌症防治中心/电子科技大学医学院(四川省成都市,610041)【摘要】 目的 系统评价集束化护理对肿瘤患者骨髓抑制的效果。
循证护理文献质量评价工具
循证护理文献质量评价工具是用于对医学或护理学科领域中的文献进
行质量评价的工具,其目的是确定文献的可信度和可靠性,以便提供客观、证据化的医学或护理实践建议。
以下是一些常见的循证护理文献质量评价工具:
1. Cochrane协作网络的风险偏倚工具(Risk of Bias Tool):用
于评价随机对照试验(RCTs)的质量,包括研究设计、随机化、盲法、缺
失数据等方面的风险偏倚。
2. Newcastle-Ottawa量表(NOS):用于评价病例对照研究和队列
研究的质量,包括选择对照组、病例和对照组间的匹配、病例和对照组间
的比较、评价因素是否对结果造成影响等方面。
3. Jadad量表:用于评价RCTs的质量,包括随机化的方法、盲法、
隐瞒分组等方面。
4.GRADE系统:用于评价循证医学研究证据的质量和可靠性,包括研
究设计、风险偏倚、一致性、精度、重要性等方面。
以上工具都可以通过评分的方式,对文献的各个方面进行客观评价和
分析,从而确定其在临床实践中的应用价值和可靠性,进一步提高医学和
护理的水平和效果。
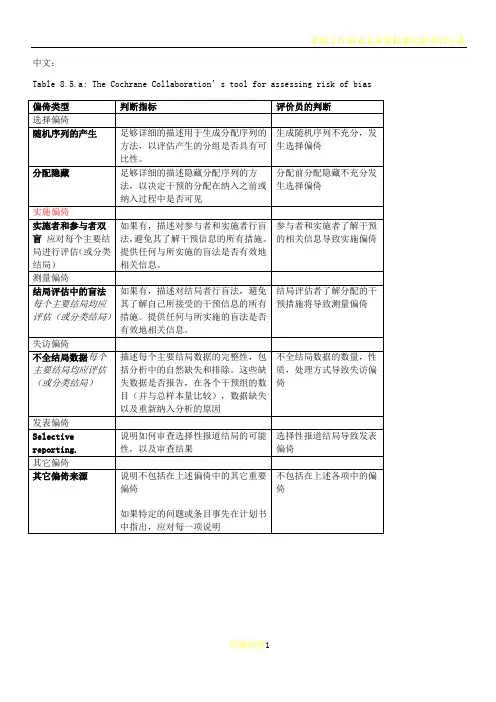
cochrane纳入的RCT文献质量评价中文版
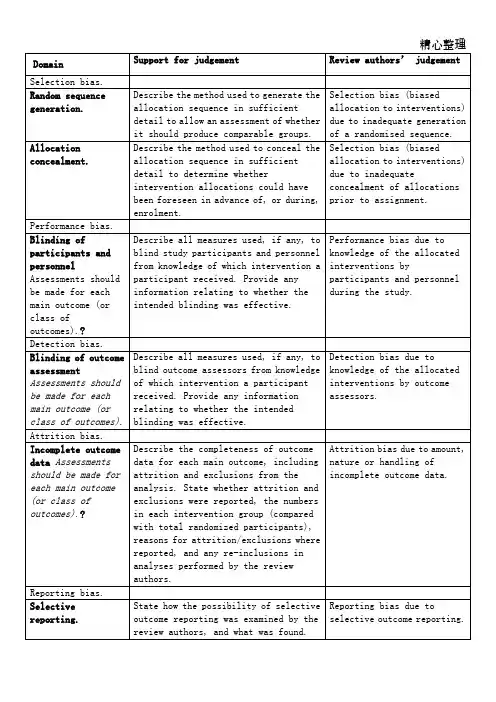
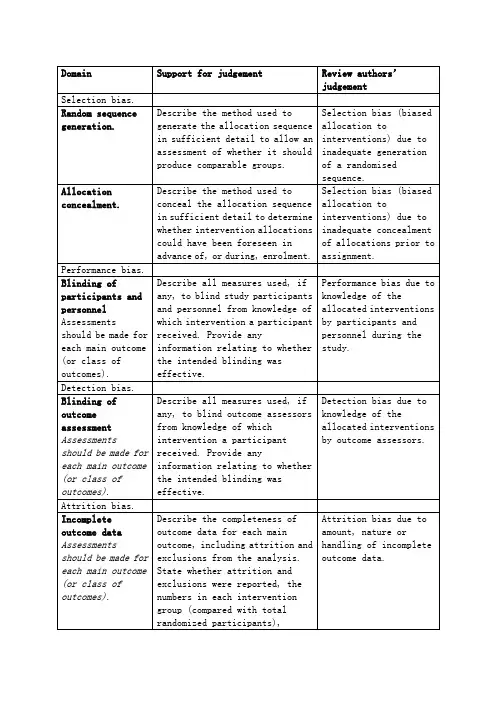
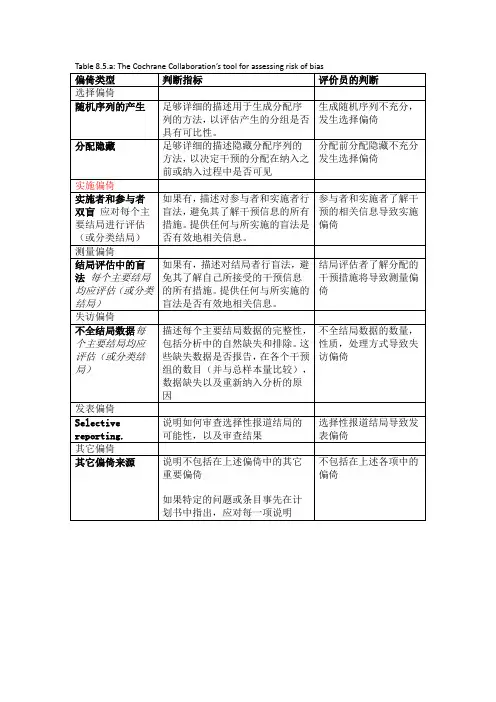
Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of bias
偏倚类型判断指标评价员的判断
选择偏倚
随机序列的产生足够详细的描述用于生成分配序
列的方法,以评估产生的分组是否
具有可比性。
生成随机序列不充分,发生选择偏倚
分配隐藏足够详细的描述隐藏分配序列的
方法,以决定干预的分配在纳入之
前或纳入过程中是否可见分配前分配隐藏不充分发生选择偏倚
实施偏倚
实施者和参与者双盲应对每个主要结局进行评估(或分类结局)如果有,描述对参与者和实施者行
盲法,避免其了解干预信息的所有
措施。
提供任何与所实施的盲法是
否有效地相关信息。
参与者和实施者了解干
预的相关信息导致实施
偏倚
测量偏倚
结局评估中的盲法每个主要结局均应评估(或分类结局)如果有,描述对结局者行盲法,避
免其了解自己所接受的干预信息
的所有措施。
提供任何与所实施的
盲法是否有效地相关信息。
结局评估者了解分配的
干预措施将导致测量偏
倚
失访偏倚
不全结局数据每个主要结局均应评估(或分类结局)描述每个主要结局数据的完整性,
包括分析中的自然缺失和排除。
这
些缺失数据是否报告,在各个干预
组的数目(并与总样本量比较),
数据缺失以及重新纳入分析的原
因
不全结局数据的数量,
性质,处理方式导致失
访偏倚
发表偏倚
Selective reporting. 说明如何审查选择性报道结局的
可能性,以及审查结果
选择性报道结局导致发
表偏倚
其它偏倚
其它偏倚来源说明不包括在上述偏倚中的其它
重要偏倚
如果特定的问题或条目事先在计
划书中指出,应对每一项说明不包括在上述各项中的偏倚
Table 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool
随机序列的产生
随机序列产生不充分导致选择偏倚
判断为低风险的标准研究者描述随机序列产生过程譬如:
•参考随机数字表
•使用计算机随机数字生成器
•扔硬币
•洗牌的卡片和信封
•掷骰子
•抽签
•最小化
*最小化,可实现无随机元素,被认为相当于是随机的。
判断为高风险的标准研究者描述序列的产生使用的是非随机的方法。
通常是
系统的非随机方法,例如:
•通过奇偶或出生日期产生序列
•通过入院日期产生序列
•通过类似住院号或门诊号产生序列
相对于上面提到的系统方法,其它非随机的方法少见的
多,也更明显。
通常包括对参与者进行判断或非随机的
方法,例如:
•临床医生判断如何分配
•参与者判断如何分配
•基于实验室检查或系列测试的结果分配
•基于干预的可获取性进行分配
偏倚风险不清楚的判断标准没有足够的信息判断随机序列的产生存在高风险或低风险
分配隐藏
分配前不充足的分配隐藏导致选择偏倚
低风险判断标准参与者以及纳入参与者的研究者因以下掩盖分配的方法
或相当的方法,事先不了解分配情况
•中心分配(包括电话,网络,药房控制随机)
•相同外形的顺序编号的药物容器;
•顺序编号、不透明、密封的信封
高风险判断标准参与者以及纳入参与者的研究者可能事先知道分配,因
而引入选择偏倚,譬如基于如下方法的分配:
•使用摊开的随机分配表(如随机序列清单)
•分发信封但没有合适的安全保障(如透明、非密
封、非顺序编号)
•交替或循环
•出生日期
•病历号
•其它明确的非隐藏过程
风险未知没有足够信息判断为低风险或高风险。
通常因分配隐藏
的方法未描述或描述不充分。
例如描述为使用信封分配,
但为描述信封是否透明?密封?顺序编号?
对参与者和实施者的盲法
因参与者和实施者了解干预情况而导致实施偏倚
偏倚低风险标准任何如下标准:
•无盲法或盲法不充分,但系统评价员判断结局不
太可能受到缺乏盲法的影响
•参与者和主要实施者均实施可靠的盲法,且盲法
不太可能被打破
偏倚高风险标准任何如下标准:
•无盲法或盲法不充分,但系统评价员判断结局很
可能受到缺乏盲法的影响
•尝试对关键的参与者和实施者行盲法,但盲法很
可能被打破,结局很可能受到缺乏盲法的影响
风险未知任何如下标准:
•没有足够信息判断为低风险或高风险
•研究未描述此情况
对结局评价实施盲法
结局评价者了解干预分配信息将导致测量偏倚
偏倚低风险标准任何如下标准:
•无盲法或盲法不充分,但系统评价员判断结局不
太可能受到缺乏盲法的影响
•参与者和主要实施者均实施可靠的盲法,且盲法
不太可能被打破
高风险判断标准任何如下标准:
•无盲法或盲法不充分,但系统评价员判断结局很
可能受到缺乏盲法的影响
•尝试对关键的参与者和实施者行盲法,但盲法很
可能被打破,结局很可能受到缺乏盲法的影响
风险未知任何如下标准:
•没有足够信息判断为低风险或高风险
•研究未描述此情况
结局数据不完整
不全结局数据的数量,性质,处理方式导致失访偏倚
偏倚低风险标准任何如下标准:
•无缺失数据
•缺失数据的产生不大可能与真实结局相关(对于
生存数据,删失不大可能引入偏倚)
•缺失数据的数目在各干预组相当,且各组缺失原
因类似
•对二分类变量,与观察事件的发生风险相比,缺
失比例不足以影响预估的干预效应
•对连续性结局数据,缺失数据的合理效应规模(均
数差或标准均数差)不会大到影响观察的效应规
模;
•缺失的数据用合适的方法进行估算
高风险判断标准任何如下标准:
•缺失数据的产生很大可能与真实结局相关, 缺失
数据的数目及缺失原因在各干预组相差较大
•对二分类变量,与观察事件的发生风险相比,缺
失比例足以影响预估的干预效应
•对连续性结局数据,缺失数据的合理效应规模(均
数差或标准均数差)足以影响观察的效应规模;
•意向治疗分析中存在实际干预措施与随机分配
的干预相违背的情况
•对缺失数据进行简单的不合适的估算
风险未知任何如下标准:
•没有报道缺失或排除的情况,无法判断高风险或
低风险(如未说明随机的数量,未提供数据缺失
的原因)
•研究未描述此情况
选择性发表
选择性发表导致发表偏倚
偏倚低风险标准任何如下标准:
•实验的计划书可获取,系统评价感兴趣的所有首
要或次要结局均按计划书预先说明的方式报道
•实验计划书不可得,但很明显发表的报告包括所
有的结局,包括预先说明的结局(这种性质的有
说服力的文字可能少见)
高风险判断标准任何如下标准:
•不是所有的预先说明的首要结局均被报道
•一个或多个首要结局为采用预先说明的测量方
法、分析方法或数据子集来报道
•系统评价感兴趣的一个或多个首要结局报道不
全,以至于不能纳入meta分析
•研究未报道此研究应当包含的主要关键结局
风险未知没有足够信息判断高风险或低风险,貌似大部分研究会
被分为此类
OTHER BIAS
不包括在以上五种的其它偏倚
偏倚低风险标准研究应未引入其它来源的偏倚
高风险判断标准至少有一种重要的偏倚风险,例如:
•具有与特殊试验设计相关的潜在偏倚来源
•或被指欺诈
•或其它问题
风险未知可能存在偏倚风险,但存在以下两种中的一种
•没有足够信息评估是否存在其它重要的偏倚风险
•没有足够的证据认为发现的问题会引入偏倚
Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies
Risk of bias 解释对单个研究对多个研究整体
Low risk of 合理的偏倚不太每一类偏倚均为绝大多数信息均来
bias. 可能严重改变结
果低风险自偏倚低风险的研
究
Unclear risk of bias. 合理的偏倚会对
结果产生一定的
怀疑
一类或多类偏倚
风险未知
绝大多数信息均来
自偏倚低风险或风
险未知的研究
High risk of bias. 偏倚严重削弱结
果的可信度
一类或多类偏倚
为高风险
来自高偏倚风险研
究的信息比例足以
影响结果的解释。