FTOC Protocol 小鼠胚胎胸腺培养
- 格式:doc
- 大小:73.00 KB
- 文档页数:8

小鼠胚胎早期发育过程中OCT4作用的研究进展引言小鼠胚胎早期发育是一个复杂的过程,其中涉及到许多基因的调控和表达。
其中一个关键基因是OCT4,是一种转录因子,对于胚胎干细胞的自我更新和分化具有重要作用。
在过去的几十年里,科学家们对OCT4在小鼠胚胎早期发育中的作用展开了大量研究,取得了一系列重要的进展。
本文将综述这些研究成果,探讨OCT4在小鼠胚胎早期发育过程中的作用及其潜在机制。
OCT4的功能和表达调控OCT4是一个关键的转录因子,它在胚胎干细胞中的作用被广泛研究。
OCT4对于维持胚胎干细胞的干性、自我更新和多能性具有重要作用。
在小鼠早期胚胎发育中,OCT4的表达模式也备受关注。
研究显示,OCT4在小鼠受精卵后期的囊胚阶段开始表达,并且在早期内细胞(ICM)中高度表达。
随着胚胎发育的进展,OCT4的表达范围逐渐限制在胚胎内细胞中,而在外细胞中逐渐减少。
这种表达模式的调控与胚胎干细胞的定向分化密切相关。
OCT4调控基因表达的机制OCT4通过调控多个基因的表达来维持胚胎干细胞的干性和多能性。
在早期的研究中发现,OCT4可以与其他转录因子(如SOX2、NANOG等)形成复合物,共同调控多个靶基因的表达。
近期的研究进展显示,OCT4还可以通过与表观遗传修饰因子相互作用,影响染色质结构和可及性,从而调控基因的表达。
OCT4还可以通过miRNA的调控网络影响胚胎干细胞的功能。
这些新的研究成果为我们深入理解OCT4的作用机制提供了新的思路。
OCT4在转基因技术中的应用随着对OCT4功能的深入研究,人们发现OCT4在胚胎干细胞和再生医学领域具有广阔的应用前景。
利用OCT4转基因技术可以实现胚胎干细胞的高效自我更新和多能性维持,并且为再生医学提供了丰富的细胞来源。
OCT4还可以通过调控其下游的基因网络,促进器官再生和损伤修复。
OCT4在转基因技术中的应用潜力巨大,将为再生医学领域带来革命性的进展。
结论OCT4在小鼠胚胎早期发育过程中发挥着重要作用。

项目名称:胸腺的起源、发生、维持与退化首席科学家:张毓北京大学起止年限:2010.9至2015.9依托部门:教育部二、预期目标1.总体目标通过该项目的实施,整合我国胸腺发育研究的主要力量,形成一个有竞争力的研究团队,协同解决该领域的一些重大问题,在胸腺的物种和胚胎起源,胸腺上皮细胞发育分化及其表观遗传调控机制,发育中TEC和胸腺细胞间的相互作用,胸腺退化的意义和发生机制等方向上取得一批原创性的成果,并力争在表观遗传和器官退化理论方面有所创新,努力使我国成为国际上这一前沿领域的学术研究中心之一。
通过该项目的实施,产生针对胸腺功能低下相关病理状态进行医学干预的新思路,发现一些具有自主知识产权和药物开发前景的分子靶标,为应对即将到来的老龄化社会所要面临的人口健康和社会经济发展方面的挑战提供理论和技术储备。
2.五年预期目标1)建立和完善胸腺器官发育研究技术和方法,重点发展能够支持胸腺上皮细胞前体发育的体外培养体系和单个前体细胞胸腺内移植技术。
2)分析鉴定文昌鱼中存在的原始胸腺上皮细胞和原始胸腺样细胞,揭示原始胸腺的胚胎起源,提供无脊椎动物原始胸腺发生的证据。
3)阐明Mpc2及Bcl-3调控基因表达和胸腺上皮细胞发育分化的表观遗传机制。
4)揭示LT-LTβR信号对TEC发育分化及其所介导的阴性选择的影响,确立Netrin和Slit在胸腺细胞定向迁移中的作用。
5)分析胸腺退化对外周T细胞库重塑及对固有免疫应答和记忆免疫应答的影响,揭示Mpc2、LTβ、Bcl-3和Aire在胸腺上皮细胞前体维持中可能发挥的作用。
6)发现和鉴定出10个以上与胸腺发育或退化相关的新分子(mRNA、miRNA或蛋白质),阐明它们调控胸腺发育或退化的机理,并确定它们是否具有临床应用前景。
7)发表高水平论文(影响因子>5)20-30篇,包括2-4篇顶级期刊论文,力争在1-2个方向上取得有影响力的、突破性成果。
8)培养博士研究生40-60名,产生长江学者、国家杰出青年基金获得者、中科院“百人计划”级别的人才3-5名,造就一支在胸腺发育研究领域敬业乐业、团结协作、善于攻关的高水平研究队伍。

体外皮肤变态反应U937细胞激活试验方法U937Cell Line Activation Test1范围本方法规定了体外皮肤变态反应U937细胞激活试验的基本原则、要求和方法。
本方法适用于化妆品用化学原料潜在致敏性的评价。
2试验目的本试验用于检测体外培养的人组织细胞淋巴瘤细胞表面标记物CD86的表达变化,以评价受试物引起皮肤变态反应的可能性。
3定义3.170%细胞存活率浓度值70%cell viability(CV70)受试物染毒后,细胞存活率为70%时对应的受试物浓度值。
3.2刺激指数stimulation index(S.I.)与溶剂对照相比,扣除同型对照后流式细胞仪测定的受试物阳性细胞相对强度值。
3.3CD86有效作用浓度值Effective Concentration150(EC150)CD86的相对荧光强度值达到150时对应的受试物浓度值。
4试验的基本原则当致敏物质接触皮肤后,树突状细胞在移动到淋巴器官的过程中分化成熟,并上调一系列表面分子的表达。
体外培养类树突状细胞:人组织细胞淋巴瘤细胞,并和受试物共暴露48h后,使用荧光抗体染料对细胞表面分子CD86染色并用流式细胞仪测定,从而评价受试物是否具有致敏性。
5试剂5.1细胞选用人组织细胞淋巴瘤细胞(The human histiocytic lymphoma cell line,U937细胞)。
细胞使用前应进行稳定性检测。
推荐使用clone CRL1593.2细胞株。
5.2培养基RPMI1640基础培养液中加入10%胎牛血清、适量抗生素,配制成1640完全培养基。
5.3染色缓冲液磷酸盐缓冲液中加入5%的胎牛血清。
5.4染料和抗体类物质7-氨基放线素菌-D染料(7-AAD)或碘化丙啶(propidium iodide,PI)FITC标记的小鼠单克隆CD86抗体(FITC-CD86)FITC标记的小鼠IgG1(FITC-IgG1)6试验方法6.1细胞准备6.1.1细胞培养U937悬浮细胞使用1640完全培养基,于37℃、5%CO2培养箱内培养,倒置显微镜下观察细胞状态。

八子补肾胶囊延缓SAMP6小鼠增龄性胸腺退化的初探李兆栋;陈银潇;龚博炀;徐喆;于智先;石悦暄;彭雁飞;边育红;侯云龙;王相玲;赵舒武【期刊名称】《中国药理学通报》【年(卷),期】2024(40)6【摘要】目的探讨八子补肾胶囊对SAMP6小鼠胸腺退化的改善作用及其可能机制。
方法20只12周龄雄性SAMP6小鼠随机分为模型组(SAMP6)和八子补肾治疗组(SAMP6+BZBS),10只SAMR1小鼠设为同源对照组(SAMR1)。
SAMP6+BZBS组每日灌胃八子补肾胶囊混悬液(2.8 g·kg^(-1)),其余两组每日灌胃等量蒸馏水,给药9周后观察各组胸腺形态并计算胸腺指数;HE染色观察胸腺组织结构变化;SA-β-gal染色检测胸腺衰老情况;流式细胞术检测各组胸腺CD3+T细胞比例;Western blot检测胸腺中p16、Bax、Bcl-2、cleaved caspase-3蛋白水平;免疫荧光检测各组皮质胸腺上皮细胞比例;ELISA检测胸腺中IL-7水平。
结果与SAMP6组小鼠比较,SAMP6+BZBS组胸腺指数明显提高(P<0.05);紊乱的胸腺结构得到明显改善;SA-β-gal染色阳性比例明显降低(P<0.01);CD3+T细胞比例明显提高(P<0.05);p16蛋白水平明显降低(P<0.05);Bcl-2蛋白水平明显增加(P<0.05),而cleaved caspase-3蛋白水平明显减少(P<0.05);皮质胸腺上皮细胞比例明显提高;IL-7水平明显升高(P<0.01)。
结论八子补肾胶囊能够延缓SAMP6小鼠的胸腺退化、抑制胸腺中细胞凋亡、有助于胸腺细胞发育,可能与其提高皮质胸腺上皮细胞比例,促进IL-7分泌相关。
【总页数】7页(P1186-1192)【作者】李兆栋;陈银潇;龚博炀;徐喆;于智先;石悦暄;彭雁飞;边育红;侯云龙;王相玲;赵舒武【作者单位】天津中医药大学中西医结合学院;天津中医药大学医学技术学院;络病研究与创新中药国家重点实验室【正文语种】中文【中图分类】R-332;R285.5;R322.53;R339.38;R583.02【相关文献】1.补肾活血液延缓雄性大鼠增龄性骨质疏松的研究2.Foxn1在小鼠胸腺增龄性萎缩中的作用3.补肾中药复方延缓老年雄性大鼠股骨皮质骨、松质骨骨量增龄性丢失的实验研究4.小鼠胸腺形态结构的增龄性变化5.八子补肾胶囊对衰老小鼠骨质量的保护作用及其对SIRT6/NF-κB/cathepsin K通路的影响因版权原因,仅展示原文概要,查看原文内容请购买。

小鼠胚胎早期发育过程中OCT4作用的研究进展OCT4是一种高度保守的Pou家族转录因子,主要在早期胚胎和胚胎干细胞中表达。
其在胚胎发育中的功能得到了广泛的研究,研究显示OCT4在小鼠胚胎干细胞的形成和维持中具有重要作用。
OCT4还参与调控胚胎干细胞的自我更新和分化,对胚胎干细胞的干细胞特性维持起着至关重要的作用。
OCT4在小鼠胚胎早期发育过程中的作用备受关注。
在小鼠胚胎早期发育过程中,OCT4的作用主要体现在三个方面:细胞命运决定、胚胎干细胞的形成和自我更新以及干细胞特性维持。
OCT4通过调控其下游基因的表达,影响胚胎干细胞的细胞命运决定。
研究表明,OCT4可以调控其他干细胞基因的表达,如Nanog、Sox2等,从而影响干细胞的命运。
OCT4在胚胎干细胞的形成和自我更新中发挥重要作用。
OCT4蛋白通过与其他转录因子和辅助蛋白结合,参与形成复合物,调控胚胎干细胞的基因表达,从而维持胚胎干细胞的自我更新能力。
OCT4对干细胞特性的维持也具有重要作用。
OCT4的下调或过表达都会导致干细胞特性的丧失,从而影响胚胎干细胞的干细胞性能和分化潜能。
除了以上提到的方面,最新研究还发现,OCT4在小鼠胚胎早期发育过程中可能还涉及到其他一些新的作用。
一些研究表明OCT4参与了胚胎干细胞的细胞周期调控,通过调控细胞周期相关蛋白的表达,影响胚胎干细胞的增殖和分化。
OCT4还可能参与胚胎干细胞的代谢调控,调控能量代谢和相关信号通路的活化。
这些新的研究进展为我们更全面地了解OCT4在小鼠胚胎早期发育中的作用提供了新的视角和机会。
在研究中,科学家们通过使用基因编辑技术、分子生物学方法、细胞生物学技术等手段,对OCT4在小鼠胚胎早期发育中的作用进行了深入研究。
通过构建OCT4的敲除小鼠模型,科学家们发现OCT4缺失会导致胚胎停滞和胚胎干细胞的缺失,从而进一步验证了OCT4在小鼠胚胎早期发育中的重要作用。
科学家们还通过对OCT4下游基因的功能和与OCT4相互作用的分子的研究,进一步揭示了OCT4参与调控胚胎干细胞自我更新和分化的分子机制。
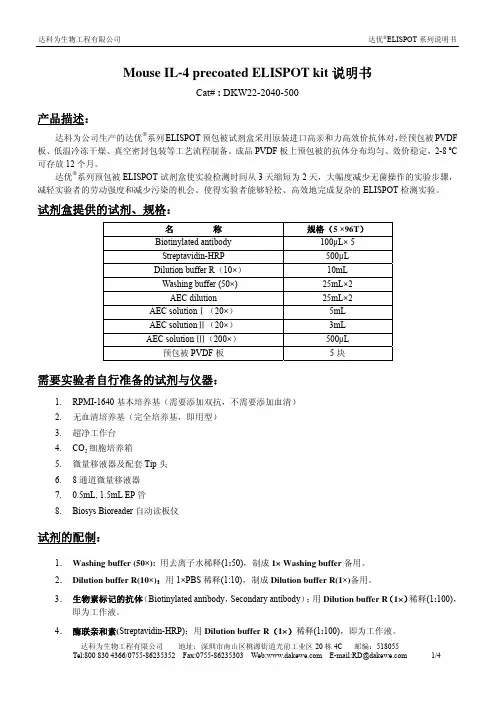
Mouse IL-4 precoated ELISPOT kit说明书Cat# : DKW22-2040-500产品描述:达科为公司生产的达优®系列ELISPOT预包被试剂盒采用原装进口高亲和力高效价抗体对,经预包被PVDF 板、低温冷冻干燥、真空密封包装等工艺流程制备。
成品PVDF板上预包被的抗体分布均匀、效价稳定,2-8 ºC 可存放12个月。
达优®系列预包被ELISPOT试剂盒使实验检测时间从3天缩短为2天,大幅度减少无菌操作的实验步骤,减轻实验者的劳动强度和减少污染的机会。
使得实验者能够轻松、高效地完成复杂的ELISPOT检测实验。
试剂盒提供的试剂、规格:名称规格(5 ×96T)Biotinylated antibody 100μL× 5Streptavidin-HRP 500μLDilution buffer R(10×) 10mLWashing buffer (50×) 25mL×2AEC dilution 25mL×2AEC solutionⅠ(20×) 5mLAEC solutionⅡ(20×) 3mLAEC solution Ⅲ(200×) 500μL预包被PVDF板5块需要实验者自行准备的试剂与仪器:1.RPMI-1640基本培养基(需要添加双抗,不需要添加血清)2.无血清培养基(完全培养基,即用型)3.超净工作台4.CO2细胞培养箱5.微量移液器及配套Tip头6.8通道微量移液器7.0.5mL, 1.5mL EP管8.Biosys Bioreader自动读板仪试剂的配制:1.Washing buffer (50×):用去离子水稀释(1:50),制成1× Washing buffer备用。
2.Dilution buffer R(10×):用1×PBS稀释(1:10),制成Dilution buffer R(1×)备用。


小鼠胚胎干细胞培养实验步骤一般培养-保持胚胎干细胞处于未分化状态培养基细胞复苏冻存细胞明胶包被细胞传代体外分化培养基包被有多聚鸟氨酸/纤维结合蛋白的培养板(使用或不使用盖玻片)体外分化方法注:以下培养针对于小鼠的R1胚胎干细胞系,其它胚胎干细胞的培养可以参考。
不过人的胚胎干细胞培养不可以采用下面的protocol,需要用专用的protocol 和培养基。
一般培养--维持ES细胞处于未分化状态ES细胞培养用含有LIF(白血病抑制因子)和Feed细胞的培养基(高糖)来阻止细胞的分化。
为细胞提供包被有0.1%明胶的平板作为粘附细胞的基质。
建议每2-3天从达到80%-90%融合的平板按1:8的比率传代细胞一次,细胞传代以后,在将细胞接种在0.1%明胶包被的培养皿之前,通过预先将细胞接种在没有经过包被的组织培养板2个小时,使分化细胞粘附,从而将分化和未分化细胞分开。
将细胞全程置于37℃,5%CO2,100%湿度条件下培养。
如果在Feed 细胞,那么就需要采用MMC进行处理,抑制Feed细胞增殖,但仍然能保持其分泌LIF因子的活性。
下文中暂不提及Feed细胞。
Feed细胞可以来源于STO细胞或原代胚胎成纤维细胞。
培养基ES:配制一20×不含DMEM,HS,LIF的溶液(该溶液也能用于EB培养基--见下文)。
分装在50ml 离心管中,(稀释为2×,每管42ml),贮存在-20℃。
通过将21ml 该溶液,HS和LIF加入450ml DMEM中制备培养基,0.22 μm滤膜过滤。
贮存于4℃,时间不要超过2周。
贮存液DMEM(高糖)马血清(HS)L-谷氨酰胺(200mM)MEM NEAA(10mM)HEPES(1M)β-巯基乙醇(55Mm)PESTLIF复苏细胞细胞被冻存在10%二甲基亚砜(DMSO)中防止结晶的形成,结晶的形成会损害细胞。
然而,二甲基亚砜对细胞有毒性,快速的进行细胞复苏是很重要的。

小鼠乳腺上皮细胞体外原代培养方法研究进展摘要综述了有关于小鼠乳腺上皮细胞体外原代培养方法的研究进展,阐述了乳腺细胞培养的意义,介绍了乳腺组织原代培养的过程,并分析了所取得的研究成果。
关键词小鼠;乳腺上皮细胞;原代培养;细胞工程原代培养即直接从有机体摘取细胞、组织或器官,使其在体外维持与生长。
合适的培养条件对体外培养细胞的生存和增殖都十分重要。
一般而言,不同动物、不同组织器官来源的细胞对培养条件的要求存在一定差异,因此,需要使体外环境下的培养条件尽可能地模拟体内环境。
体内乳腺组织的增殖、分化和组织结构重塑受内环境(如类固醇、生长因子、结合蛋白及细胞外基质间复杂的相互作用等)因素的影响和作用,这些因素同样也影响体外培养乳腺上皮细胞的增殖分化和功能表现。
对于某种动物特定组织细胞的体外培养,对培养液、添加因子、附着底物及培养温度、pH值等都需进行仔细的选择,乳腺上皮细胞的体外培养建立在乳腺上皮原代培养细胞的基础上,针对不同的研究目的应采取不同的培养方法,以对细胞进行分化诱导。
张以涛比较了不同pH值、不同血清浓度配比的生长培养液对小鼠乳腺上皮细胞生长的影响,结果表明, pH值7. 4含10%血清的培养基为最佳生长液。
现代研究普遍认为,内分泌激素在乳腺上皮细胞发育各个阶段都发挥影响作用,不同时期乳腺组织的形态、组成及功能均有差异,这对选择乳腺上皮细胞体外培养的取材时间有指导意义。
张以涛比较了取自不同时期乳腺上皮细胞的生物学特性,得出妊娠期细胞生存、增殖能力均强于泌乳期细胞,妊娠期小鼠乳腺为获得细胞的最佳取材时期。
乳腺上皮细胞的原代培养,首先是尽可能地分离出乳腺上皮细胞,同时减少分离过程对细胞的损伤和浪费,以及通过采用一定的方法,使最终得到富集的乳腺上皮细胞及杂质细胞尽可能少甚至被完全清除。
1乳腺细胞培养的意义将动物细胞或组织在体外进行培养,能有效排除神经体液等因素对其造成的影响,可直接进行药物对细胞的活动代谢、毒性和杀伤作用等检测,如秦君等以王不留行为试验材料,对其3类主要成分(皂甙类、黄酮甙类及香豆素类)进行提取,分别以不同浓度添加到细胞培养液中,寻找王不留行促进泌乳的有效成分。

小鼠胚胎干细胞培养实验步骤以下是小鼠胚胎干细胞的培养实验步骤:1.准备培养基和细胞培养器材:-将培养基预热至37摄氏度。
-准备含有培养基的无菌试管、离心管和细胞培养板。
-洗手并戴上合适的培养操作手套。
2.预处理培养皿:-用无菌PBS(磷酸盐缓冲液)将细胞培养板彻底洗涤,去除残留的培养基和细胞残留物。
-用0.1%胰蛋白酶溶液或0.25%胰蛋白酶-EDTA溶液将细胞培养板上的细胞进行脱落。
-将细胞均匀地分布在预处理的培养皿中,使其形成单层细胞。
3.检查细胞数目和品质:-用显微镜检查细胞的形态和活力。
-用细胞计数计算细胞的数目。
-计算细胞的分裂倍增时间。
4.细胞传代:-按照需要的细胞密度将细胞从一个培养皿转移到另一个培养皿。
-用0.05%胰蛋白酶-EDTA将细胞从培养皿上脱落。
-投入适当比例的培养基到新的培养皿中,使其形成单层细胞。
5.制备小鼠胚胎纤维母细胞:-从小鼠胚胎体内收集纤维母细胞。
- 将纤维母细胞转移到含有DMEM(Dulbecco's Modified Eagle Medium)和10%胎牛血清的细胞培养皿中。
-培养纤维母细胞在37摄氏度、5%二氧化碳的培养箱中直至细胞接触起离皿表面。
6.制备和维持小鼠胚胎干细胞:-转接分裂期的小鼠胚胎干细胞到新的培养皿中。
-用无菌吸管或离心管的尖端轻轻挑取细胞克隆。
-将细胞克隆转移到含有mESC培养基(如DMEM、15%胎牛血清、2mML-谷氨酰胺和1×LIF(小鼠白细胞介素-6)的细胞培养板中。
-在体外细胞培养中维持小鼠胚胎干细胞的自我更新和增殖。
7.细胞培养的常规维护:-定期更换新鲜的培养基。
-检查细胞的形态和活力。
-定期传代细胞,以保持其细胞数目和活力。
-控制培养基和细胞的污染。
以上是小鼠胚胎干细胞培养的一般步骤,实验过程可能因研究目的和实验室的要求而有所不同。
为了保证实验的准确性和可重复性,建议在实验过程中随时记录和保留实验数据,并使用正确的实验操作和生物安全规范。

细胞的原代培养点击次数:540 作者:佚名发表于:2009-03-06 16:26转载请注明来自丁香园一、原代细胞培养原理原代细胞培养是将机体内的某组织取出,分散成单细胞,在人工条件下培养使其生存并不断生长、繁殖的方法。
借助这种方法可以观察细胞的分裂繁殖、细胞的接触抑制以及细胞的衰老、死亡等生命现象。
• 幼稚状态的组织和细胞,如:动物的胚胎、幼仔的脏器等更容易进行原代培养• 掌握无菌操作技术• 了解小鼠解剖操作技术• 了解原代细胞培养的一般方法与步骤•了解培养细胞的消化分散• 了解倒置显微镜的使用二、实验材料• 实验动物:孕鼠或新生小鼠• 液体:细胞生长液(内含20%小牛血清)0.25%胰蛋白酶平衡盐溶液70%乙醇•器材:灭菌镊子、剪刀若干把灭菌培养皿、细胞培养瓶、小瓶、烧杯若干个吸管若干支酒精灯原代细胞培养方法三、胰酶消化法(1)胰酶消化法操作步骤——取材a. 用颈椎脱位法使孕鼠迅速死亡。
b. 把整个孕鼠浸入盛有75%乙醇的烧杯中数秒钟消毒,取出后放在大平皿中携入超净台。
c. 用无菌的镊子和剪子在前腿下作一腹部水平切口,用无菌镊子将皮肤扯向后腿。
d. 用另一无菌的剪刀和镊子切开腹部,取出含有胚胎的子宫,置于无菌的培养皿上。
e. 剔除胚胎周围的包膜(若胚胎较大,应剪去头、爪),将胚胎放于无菌的含有平衡盐溶液的培养皿中。
f. 漂洗胚胎,去掉平衡盐溶液。
继续用平衡盐溶液漂洗胚胎直至清洗液清亮为止。
(2)胰酶消化法操作步骤——切割a. 将部分胚胎转移至一个无菌小瓶中,用平衡盐溶液漂洗。
b. 然后用眼科手术剪刀小心地绞碎胚胎,直到成1mm3左右的小块,再用平衡盐溶液清洗,洗到组织块发白为止。
c. 静置,使组织块自然沉淀到管底,弃去上清。
(3)胰酶消化法操作步骤——消化、接种培养a. 视组织块量加入5-6倍的0.25%胰酶液,37℃中消化20-40分钟,每隔5分钟振荡一次,或用吸管吹打一次,使细胞分离。
b. 加入3-5ml细胞生长液以终止胰酶消化作用(或加入胰酶抑制剂)。
胸腺素治疗肝癌的实验观察
方天祥;王兴友
【期刊名称】《山东医药》
【年(卷),期】1990(30)11
【摘要】用胸腺素治疗肝癌尚未见报道。
我们对胸腺索治疗肝癌进行了动物实验观察,以期探讨其临床应用价值。
一、材料与方法1.实验动物及肝癌模型复制:实验动物为山东省医学科学研究院动物室饲养的Wistar品系大白鼠,30~37日龄,体重80~106克。
诱癌剂采用二乙基亚硝胺(DENA),
【总页数】2页(P1-2)
【作者】方天祥;王兴友
【作者单位】不详;不详
【正文语种】中文
【中图分类】R735.7
【相关文献】
1.电针抗吗啡戒断大鼠胸腺细胞凋亡的实验观察 [J], 张荣军;宋小鸽;唐照亮;侯晓蓉;陈全珠;许冠荪;陈亦宜
2.胚胎胸腺移植配合蛇毒抗癌素治疗晚期肝癌 [J], 吴启才;仉周勇
3.异基因小鼠胚胎胸腺移植治疗红斑狼疮的实验观察 [J], 虞春华;黄笑燕
4.异基因小鼠胸腺细胞微囊移植治疗SLE的实验观察 [J], 徐高四;张建国;吴江锋;付承英;黎家华;韩莉
5.异基因小鼠胚胎胸腺移植治疗系统性红斑狼疮模型鼠的实验观察 [J], 徐高四;刘巧云;曹会生;李丽影;秦旭军;虞春华;万青峰
因版权原因,仅展示原文概要,查看原文内容请购买。
小鼠鞘氨醇激酶(SPHK)ELISA检测试剂盒使用说明书小鼠鞘氨醇激酶(SPHK)ELISA检测试剂盒说明书检测原理试剂盒采纳双抗体一步夹心法酶联免疫吸附试验(ELISA)。
往预先包被鞘氨醇激酶(SPHK)抗体的包被微孔中,依次加入标本、标准品、HRP标记的检测抗体,经过温育并彻di洗涤。
用底物TMB 显色,TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最后的黄色。
颜色的深浅和样品中的鞘氨醇激酶(SPHK)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),计算样品活性。
样品收集、处理及保存方法1.血清:使用不含热原和内毒素的试管,操作过程中避开任何细胞刺激,收集血液后,3000转离心10分钟将血清和红细胞快速当心地分别。
2.血浆:EDTA、柠檬酸盐或肝素抗凝。
3000转离心30分钟取上清。
3.细胞上清液:3000转离心10分钟去除颗粒和聚合物。
4.组织匀浆:将组织加入适量生理盐水捣碎。
3000转离心10分钟取上清。
5.保存:假如样本收集后不适时检测,请按一次用量分装,冻存于20℃,避开反复冻融,在室温下解冻并确保样品均匀地充分解冻。
自备物品1.酶标仪(450nm)2.高精度加样器及枪头:0.510uL、220uL、20200uL、2001000uL3.37℃恒温箱操作注意事项试剂盒保存在28℃,使用前室温平衡20分钟。
从冰箱取出的浓缩洗涤液会有结晶,这属于正常现象,水浴加热使结晶wan全溶解后再使用。
试验中不用的板条应立刻放回自封袋中,密封(低温干燥)保存。
浓度为0的S0号标准品即可视为阴性对比或者空白;依照说明书操作时样本已经稀释5倍,最结束果乘以5才是样本实际浓度。
严格依照说明书中标明的时间、加液量及次序进行温育操作。
全部液体组分使用前充分摇匀。
试剂盒构成名称96孔配置48孔配置备注微孔酶标板12孔×8条12孔×4条无标准品0.3mL*6管0.3mL*6管无样本稀释液6mL3mL无检测抗体HRP10mL5mL无20×洗涤缓冲液25mL15mL按说明书进行稀释底物A6mL3mL无底物B6mL3mL无停止液6mL3mL无封板膜2张2张无说明书1份1份无自封袋1个1个无注:标准品(S0S5)浓度依次为:0、1、2、4、8、16U/mL试剂的准备20×洗涤缓冲液的稀释:蒸馏水按1:20稀释,即1份的20×洗涤缓冲液加19份的蒸馏水。
75~801张星, 庄临之, 李树民, 等1 穿心莲抗生育作用的研究, I 对体外培养的人绒毛滋养层细胞激素分泌功能的影响[J ]1 动物学报, 1985, 31 (1) : 52~581孙敏之, 吴江声, 张兰芬, 等1 穿心莲、雪莲对人早期妊娠胎盘绒毛的影响——组织学和组织化学观察[J ] 1北京医学院学报, 1979, 11 (2) : 79~821曹路敏, 毛惠益, 肖邦宙, 等1 紫草抗人绒毛膜促性腺激素及抗早孕作用的实验观察[J ]1 武汉医学院学报, 1985, 14 (4) : 280~2821姚寓晨, 姚石安1 中医药避育节育研究及其进展( 综述) [J ]1 江苏中医杂志, 1981, 2 (5) : 61~641倪志疆, 沈霞萍, 邵晓恂, 等1 中药紫草等对药物流产临床效果的影响[J ] 1中国计划生育杂志, 1999, 7 ( 1) : 37~381饶伟文1 癌胚共性与抗生育中草药的抗肿瘤作用[J ] 1中草药, 1997, 28 (3) : 178~1801李希新, 张恒齐1 论妊娠禁忌药在妊娠期的应用[J ] 1山东中医杂志, 1999, 18 (8) : 368~3701睢大笕, 吕忠福, 李淑惠1 九里香化学及抗生育作用研究概况[J ]1 中草药, 1994, 25 (8) : 435~4371柳红芳, 高学敏1 蒲黄水煎液对小鼠妊娠影响的实验研究[J ]1 中药药理与临床, 1994, 10 (2) : 26~291王建军, 李成林1 几种孕妇禁忌中药对早孕绒毛的组织化学及超微结构的影响[J ]1 中国优生与遗传杂志, 1993 (4) : 19~211杨守业, 皮晓霞, 何民, 等1 妊娠禁忌中药的研究思路和方法[J ]1 中医药信息, 1991, 8 (2) : 36~381刘静, 谭亚非1 昆明山海棠抗生育作用研究进展[J ] 1中医药学报, 1997, (6) : 331李培全, 张建国1 芫花萜膜及其临床应用[M ] 1河南: 河南科学技术出版社, 1992, 871束怀德, 魏湘, 吴白云, 等1 注射用天花粉对早孕兔子宫收缩的作用[J ]1 生理学报, 1979, 31 (1) : 21~281高恩缘, 李维智, 罗淑宜, 等1 天花粉蛋白对体外无血清培养的人早期绒毛细胞滋养层细胞H C G、孕酮分泌的影响[J ]1 生殖与避孕, 1996, 16 (6) : 418~4211李英娟, 素晓芹, 付国庆, 等1 结晶天花粉应用于早、中期流产的临床分析[J ]1 中医药学报, 1996, (2) : 411中药生化汤配合米非司酮、米索前列醇抗早孕临床效果分析[J ]1 中草药, 1998, 29 (8) : 547~5481王青, 赵红, 王右麟, 等1 排胚汤协同米非司酮抗早孕流产物超微结构改变的研究[J ] 1中医杂志, 2000, 41 ( 10) : 612~61313 124 13145156 167 178 189 1910 2011T 细胞在胸腺内的分化发育宋芳王建军李俊平(包头医学院组胚教研室, 内蒙古包头014010)中图分类号R 32912+1 文献标识码A文章编号1006- 740X (2001) 01- 0075- 03T 细胞即胸腺依赖淋巴细胞( t h y m u s dep e n d en t lym p h o c y t e) , 是在胸腺内分化并发育成熟的。
小鼠esc培养方案一、细胞来源与获取小鼠胚胎干细胞(ESC)可来源于受精后3.5\~5.5天的胚胎,这些胚胎可通过显微操作技术从囊胚中剥离。
剥离的胚胎干细胞需要在含有血清和饲养层细胞的培养基中进行培养。
二、培养基选择与配制培养基是小鼠胚胎干细胞(ESC)生长的必要条件,常用的培养基包括Glasgow MEM、Ham F12、ES Cell Medium等。
在配制培养基时,需要添加适量的血清(一般为胎牛血清)和抗生素等成分,以维持细胞的生长和繁殖。
三、饲养层细胞准备饲养层细胞可以为胚胎干细胞提供必要的生长因子和细胞因子,常用的饲养层细胞包括STO、STO-derived fibroblasts等。
在准备饲养层细胞时,需要将细胞接种在消毒后的培养皿或培养瓶中,待细胞生长至适宜密度后,再进行后续操作。
四、细胞接种与培养将剥离的胚胎干细胞接种在饲养层细胞上,通常情况下,接种密度为1\~5万个/cm²。
在培养过程中,需要保持适宜的温度和湿度,并定期更换培养基,以维持细胞的生长状态。
一般情况下,小鼠胚胎干细胞(ESC)会在接种后2\~3天开始贴壁生长。
五、细胞传代与扩增当小鼠胚胎干细胞(ESC)生长至适宜密度时,需要进行传代和扩增。
通常情况下,传代时的细胞密度为1\~2万个/cm²。
在传代过程中,需要将细胞从培养皿或培养瓶中吹散,并接种到新的培养皿或培养瓶中。
扩增过程需要定期更换培养基,并保持适宜的温度和湿度。
六、细胞冻存与复苏为了长期保存小鼠胚胎干细胞(ESC),需要进行细胞冻存。
将处于对数生长期的细胞进行离心和清洗后,加入适量的冷冻保护剂(一般为二甲基亚砜),然后放入液氮中进行保存。
在需要使用时,将冻存的细胞取出,进行复苏。
胸腺实验报告做引言胸腺是一种位于胸腔内的脏器,是免疫系统的重要组成部分之一。
胸腺在胚胎发育过程中发挥着重要的作用,对免疫系统的发育和功能起着关键性的调控作用。
通过对胸腺的研究,我们可以更好地了解免疫系统的工作原理以及相关疾病的发生机制。
本实验旨在观察胸腺的组织结构并探究其功能。
材料与方法材料- 实验动物:小鼠- 实验仪器:显微镜、玻片、盖玻、刀片、PBS缓冲液等方法1. 实验动物准备- 饲养小鼠并确保其健康状态- 麻醉小鼠以减少疼痛感2. 取出胸腺- 将小鼠背部剖开- 将胸腺取出并放入PBS缓冲液中冲洗去除血液3. 制备组织切片- 将胸腺用刀片切割成薄片- 将切割好的组织片放入PBS缓冲液中浸泡4. 准备玻片- 取出玻片并清洗- 将玻片放入盖玻中- 将浸泡在PBS缓冲液中的组织片放在玻片上5. 组织染色- 取出染色剂,并按照说明书的要求稀释- 将稀释好的染色剂滴在组织片上- 将组织片置于染色剂中静置一段时间6. 显微镜观察- 取出染好色的组织片- 将组织片放在显微镜下- 调节显微镜的放大倍数和焦距,观察胸腺组织的细胞结构和细胞数量7. 数据分析- 对观察到的胸腺组织进行计数和分析- 统计不同区域的细胞数量以及细胞形态的差异结果与讨论通过使用显微镜观察染色的胸腺组织切片,我们可以看到胸腺由许多小梁和囊丛组成,其中包含大量的淋巴细胞和上皮细胞。
这些细胞密集地分布在胸腺组织中,形成了一个高度有序的结构。
通过计数胸腺组织中的细胞数量,我们可以发现不同区域的细胞密度存在差异,这可能与不同区域细胞的功能有关。
据研究表明,胸腺对免疫系统功能的发挥起着重要作用。
通过胸腺,免疫细胞得以发育和分化为具有特定功能的细胞,从而参与免疫应答和免疫调节。
胸腺还参与胸腺肽激素的合成和释放,这些激素对免疫细胞的分化和活化起着重要的调控作用。
在本实验中,我们对胸腺组织进行了初步的观察和分析。
然而,由于实验条件的限制,我们只能对胸腺组织的组织结构进行观察,并未对其功能进行深入研究。
ProtocolFetal Thymus Organ CultureINTRODUCTIONThe generation of functionally competent T-cells from their progenitors involves a series of developmental events including proliferation, differentiation, and survival. T-cell development is a non-cell-autonomous event, and requires interactions with thymic stromal cells. Fetal thymus organ cultures provide an in vitro system in which isolated embryonic thymus lobes can be maintained in culture, allowing the study of T-cell development as well as thymic stromal cell function. This system remains the only in vitro system that supports a complete program of T-cell development, including positive and negative selection of the developing T-cell receptor repertoire. Modifications of the basic fetal thymus organ culture system, such as hanging drop cultures and reaggregate thymus organ cultures, provide useful systems to analyze thymus colonization and thymic stromal cell function, respectively.MATERIALSReagentsAll media should be prewarmed to 37°C before use.10% CO2 in air, contained in gas cylinder2'-deoxyguanosine (dGuo) (Sigma-Aldrich, D0901)Prepare a 9 mM stock in 1X PBS. It takes ~1 h at 37ºC for the dGuo to dissolve.Mix the solution well during this period.Dilute the dGuo stock to a final concentration of 1.35 mM in1X PBS (see Step 3).Dulbecco’s modified Eagle’s medium (DMEM) for thymus organ culture70% ethanolMice, pregnant female (gestational age E14-E16)1X PBS (Ca++/Mg++-free)RF10-H medium10X trypsin (Sigma-Aldrich,T4674)Prepare a 1X trypsin solution by diluting the 10X stock in Ca++/Mg++-free PBS containing 0.02% EDTA.EquipmentArtiwrap sponges, 1 cm2 (Medipost Ltd.)Aspirator tube assembly (Sigma-Aldrich, A5177)Boxes (rectangular, plastic) with fitted lids (Watkins and Doncaster,E6052)Bunsen burner, fish-tailFilters (isopore membrane) with 0.8-µm pore size (Millipore,ATTP01300)Forceps, watchmaker, Dumont #5 (TAAB)Incubator, preset to 37°CMicrocentrifugeMicrocentrifuge tubes, 1.5 mLMicropipette, 1 mLMicroscope (stereo-dissecting) with magnification range 0.8X-5X(e.g., Zeiss, Stemi SV)Petri dishes, 90-mm diameter, sterile bacteriological grade(Sterilin)Plate inserts for multiwell plates (e.g., for a six-well plate,use Millicell 0.4-µm plate inserts; Millipore, PICM03050)(optional; see Step 2.i)Plates, Terasaki (Sterilin)Scissors, surgicalTapeTubing, glass, to make capillary pipettes (Fisher Scientific UK, FB51460)Vortex mixerMETHODThe standard fetal thymus organ culture (FTOC) method described in Steps 1 and 2 can be modified to study thymus colonization using hanging drop cultures (Steps 3-5; Jenkinson et al. 1982).In addition, reaggregate thymus organ cultures (RTOC) can be used, in which three-dimensional organ cultures are generated from defined mixtures of thymic stromal cells and thymocytes(Steps 6-16). This latter method is particularly useful in studying thymic stromal cell function and the development of an individual cohort of thymocytes at a defined developmental stage.Fetal Thymus Organ Culture (FTOC)1. To dissect fetal thymus lobes:i. Swab the abdominal region of a sacrificed female mouse using70% ethanol.Reflect the skin using scissors and forceps, and cut through the abdominalwall to reveal the uterus. Separate the uterus from its attachments at the two uterine horns laterally,and from the bladder anteriorly.ii. Transfer the uterus to a 90-mm Petri dish. Using scissors and forceps, cut open the uterus lengthwise to remove the embryos.iii. Place the embryos in a Petri dish containing 1X PBS to wash off any blood.Use forceps to remove the placenta and membranes from each embryo.iv. Place the embryos in a Petri dish containing RF10-H medium.Decapitate the embryos by pinching them with forceps just below the chin.Place each embryo on its back,and open the anterior surface of the chest wall by placing the tips of a closed pair of forceps into the chest cavity and allowing the forceps to open.v. Under the dissecting microscope,remove the entire thoracic tree--heart, lungs, trachea, thymus lobes--by grasping below the heart.Place the thoracic trees in a Petri dish containing fresh RF10-H medium.Problem 1: When removing the thoracic tree from the embryos, thymuslobes are left in the chest cavity.[Step 1.v]Solution:It is important to grasp the heart (a red, apple-shaped organ) firmly. Sharp straight forceps also help; if forceps are blunt or bent, fine dissection is difficult.vi. Check each tree for the presence of the two thymus lobes.Remove the thymus lobes from the thoracic tree using forceps.Thymus lobes appear as oval encapsulated organs on either side of the trachea (Fig. 1 ).Problem2:Instruments become sticky with congealed blood and tissue during the preparation of large numbers of embryos, making it difficult to manipulate dissected lobes.[Step 1.vi] Solution:Wash the instruments regularly with 70% alcohol and allow them to air-dry before use.i. Place 4 mL of prewarmed DMEM in a 90-mm Petri dish, and use forceps to place two 1-cm2 Artiwrap sponges and two 0.8-µm filters into the dish.Make sure that the medium wets the filters from below.See Troubleshooting. As an alternative to the sponge-and-filter method, multiwell plate inserts with membranes of the appropriate pore size can be used.Problem3: Large areas of the bottom of the Petri dish are not covered by medium.[Step 2.i]Solution: Make sure the volume of medium is accurate; insufficient medium in the dishes can adversely affect organ cultures. This may also be due to sponge supports that are too large and consequently soak up the medium.Problem4: Filters slip off the sponge supports and sink.[Step 2.i]Solution: Adding too much medium to the Petri dish increases the depth of the medium, which can cause filters to float off their support and sink. Make sure the volume of medium is accurate.Also, use sponge supports that are larger in area than the filters.ii. Prepare a finely drawn glass pipette by heating the glass tubing in a Bunsen flame, and allow the pipette to cool before attaching to the aspirator tube.ing a mouth-controlled glass pipette from Step 2.ii,pick up the thymus lobes individually and transfer them to the surface of the filters.See Troubleshooting. As an alternative approach to the glass pipette method, fine forceps can be used.Problem5: A large volume of medium is transferred to the filter during the placement of lobes on the filter.[Step 2.iii]Solution:Use a fine mouth-controlled glass pipette to transfer the lobes. The diameter of the glass pipette should be approximately half the size of the thymus lobe. If forceps are used, medium is easily transferred to the filter, which can submerge the lobes.iv.Place up to three 90-mm Petri dishes into a plastic rectangular box containing H2O and a Petri dish lid as a support. Gas the boxes for 10 min witha mixture of 10%CO2 in air, seal with tape, and incubate at 37ºC.The length of incubation depends on the experiment; it is typically5-10 d.See Troubleshooting.Hanging Drop Cultures3. Isolate and set up in organ culture E14-E15 fetal thymus lobes asdescribed in Steps 1 and 2. To eliminate endogenous thymocytes from the lobes, grow organ cultures in the presence of 1.35 mM 2'-deoxyguanosine (dGuo) at 37°C for 5-7 d (Jenkinson et al. 1982).4. Resuspend the thymocyte population of interest (e.g., fetal liver T-cellprogenitors, CD4–8– thymocyte subsets)in 20 µL of prewarmed DMEM, and transfer the cell(s) to a single Terasaki well. The number of the transferred cells at the outset of culture can be as few as one per well, but is typically around 2000. Place a single dGuo-treated lobe (?)in each well, and invert the plate by turning it over, so that the medium forms a hanging drop in the plate.5. Culture overnight,as for FTOC (see Step 2.iv). Colonized lobes can then beremoved from the Terasaki plate and analyzed immediately to study thymus colonization, or transferred to normal FTOC to reveal the developmental potential of the introduced cells.Reaggregate Thymus Organ Cultures (RTOC)6. Prepare dGuo-treated FTOC (see Step 3), and transfer the lobes to a dishof prewarmed RF10-H medium.7. Using a 1-mL micropipette, transfer the lobes to a 1.5-mL microcentrifugetube with no more than 20 lobes per tube, and allow them to sink to the bottom.8. Remove the medium, and wash the lobes three times in 1-mL volumes ofprewarmed 1X PBS.9. Resuspend the lobes in 600 µL of prewarmed 1X trypsin solution,andincubate the lobes at 37ºC for 10 min.10. Pipette the lobes using a 1-mL micropipette, and return them to theincubator for an additional 5 min.11. Pipette the lobes again until they completely disperse.Neutralize thetrypsin by adding400 µL of RF10-H medium.Centrifuge and resuspend the cells in 1 mL of RF-10H medium.Count the cells.Problem6: dGuo-treated thymus lobes do not digest completely in trypsin.[Step 11]Solution:It is important to digest the lobes in small batches (typically 20 lobes) rather than as one large batch. If they are not digested completely after 15 min, transfer the lobes back to 37ºC for an additional 5 min.12. Mix together appropriate numbers of thymocytes and stromal cells in a1.5-mL microcentrifuge tube, and centrifuge.To prepare RTOC, thymic stromal cells obtained as above need to be mixed with the desired thymocyte population (Jenkinson et al. 1992).In a typical experiment, ~7.5 x 105 thymic stromal cells can be mixed with 7.5 x 105 CD4+8+thymocytes.13. Remove the supernatant from the cell pellet.It is essential that all of the liquid is removed from the cell pellet prior to reaggregation.14. Vortex the cell pellet for 5 sec.This disrupts the pellet and forms a thick cell slurry.15. Using a mouth-controlled glass pipette (from Step 2.ii),transfer theslurry to the center of a 0.8-µm filter in a Petri dish.Where mouth pipetting is not possible, the cell slurry can be drawn up into a small pipette tip using a hand-controlled micropipette,and gently expelled onto the filter surface. See Troubleshooting.Problem7:When making RTOC, the cell slurry spreads out on the filter surface and intact lobes fail to form.[Step 15]Solution: If even a small volume (2 µL) of liquid is left on the cell pellet prior to reaggregation, the slurry is too thin and will spread out. During Step 13, remove the supernatant in stages using a micropipette set at 200 µL.16.Culture as for standard FTOC (see Step 2.iv).DISCUSSIONThe use of isolated thymus lobes between E14 and E16 is optimal for fetalthymus organ culture. Thymus lobes after E16 of gestation are large, a factor that often can lead to considerable necrosis in the explanted tissue.Maintaining the organ cultures in a4-mL volume of culture medium allows thecultures to be kept for several weeks without the need for replacement of the spent medium. While cultures can be maintained in CO2 incubators,the use of sealed tissue culture boxes that are individually gassed results in cultures that are unaffected by repeated openings of incubator doors. Humidity is also maintained, which helps prevent evaporation of the medium in longer-term cultures; thus,fetal thymus organ cultures can be maintained in these culture conditions for several weeks. As a general rule, if E15 fetal lobes are explanted, maturation from the CD4–8–to the CD4+8+ stage is readily detectable after 4 d in culture.Cohorts of CD4+8– and CD4–8+ cells first appear around days 7-8 of culture, and can be used to study selection events operating on CD4+8+ thymocytes. For reaggregate thymus organ culture, the number of dGuo FTOC lobes used depends upon the experiment being performed. Typically, in order to study the positive selection of a cohort of CD4+8+ thymocytes, we would use 10-15 E15 dGuo FTOCs to generate a singe RTOC, and harvest after 5 d of culture, when cohorts of CD4+8– and CD4–8+ cells are detectable.Through the use of mouse fetal thymus organ culture (FTOC) techniques, Robinson and Owen (9) showed that functionally competent T cells could be generated within explanted thymic tissue under controlled conditions in vitro, paving the way for the study of mechanisms regulating T cell development.By adopting approaches that had previously been described and used to study embryonic organ development, including thymus (10–12), Robinson and Owen described a method in which embryonic mice at embryonic days 14 and 15 of gestation were used as a source of thymus lobes, which at this developmental stage contained only large blast-like cells thought to represent lymphocyte precursors. After explantation into organ culture on the surface of floating membrane filters, thymic cultures were shown to undergo significant increases in size, correlating with an increase in lymphocyte recovery of up to 14-fold by day 7 of culture. Such culture conditions were also shown to support the emergence of lymphocytes with a size typical of peripheral lymphocytes, as well as the acquisition of the surface marker Thy-1. Importantly, B cells were found to beabsent from thymus organ cultures, an important indication that the specialization of the thymus for the production of T cells in vivo could also be recapitulated in vitro. Perhaps most significantly, Robinson and Owen also treated lymphocytes generated in FTOC with the T cell mitogens PHA and Con A, together with 125 I-labeled iododeoxyuridine. This experiment was important for two reasons. Firstly, it showed that function-ally competent T cells capable of undergoing proliferation in response to stimulation could be generated in vitro in the context of the thymic microenvironment. Secondly, it also showed the ease with which the FTOCs and the T cells generated within them could be manipulated by the addition of exogenous reagents that could enable the study of stages in Tcell development. For example, the authors went on to show that FTOC also generated T cells that responded to alloantigens in mixed leukocyte culture (13) and that tolerance to alloantigens could be induced by co culturing thymus with fragments of histoincompatible spleen (14).Since its initial description, the FTOC system has been used extensively to study the mechanisms regulating key events that occur during intra thymic T cell development. For example, FTOC proved to be a key system in unraveling the role of MHC-bound peptides in the positive and negative selection of the developing TCR repertoire by enabling study of the effects of individual peptides on a relatively synchronous wave of thymocyte development in the absence of effects on peripheral T cells (15–16). In addition, the “standard operating procedure”for FTOC has undergone several revisions that have allowed study of the role of the thymic microenvironment in T cell production, including thymic epithelial cell development and function. The ability to generate a lymphoid FTOCs by exposure to 2-deoxyguanosine(17) and the production of reaggregate thymus organ cultures from defined stromal components (18) are notable in this context, the latter currently being used as a model to study thymocyte migration and development in real time using two-photon microscopy (19).In summary, the description by Robinson and Owen (9) of an in vitro system to study T cell development has proved to be a cornerstone for our understanding of thymic function and the stages and events that occur during T cell development. That this system still remains the only in vitro system capable of supporting a full program of T cell development, from progenitor recruitment to MHC restriction and clonal deletion is a testament to the use of simple experimental approaches to study complex biological events.。