化学官能团相互转换大全(part2)
- 格式:doc
- 大小:491.00 KB
- 文档页数:13

有机合成中的官能团互变与官能团保护官能团互变和官能团保护是有机合成中常用的两种策略,用于在合成过程中对官能团进行转化或保护,以达到所需的目的。
本文将具体介绍官能团互变和官能团保护的原理和应用。
一、官能团互变官能团互变是指将一个官能团转化为另一个官能团,通常通过一系列的反应步骤实现。
这种方法在有机合成中广泛应用,可以有效地构建复杂有机分子。
下面以几种常见的官能团互变反应为例进行介绍。
1. 羰基互变反应羰基互变反应是一种常见的官能团互变方法,通常通过氧化、还原或加成等反应来实现。
例如,醛和酮可以通过还原反应互变,常用的还原剂有金属碱金属催化剂或氧化铝。
此外,也可以通过氧化反应将醇氧化为醛或酮。
2. 环化反应环化反应是官能团互变的重要手段之一,可以将直链分子转化为环状分子。
常见的环化反应有氧化环化和还原环化。
例如,氧化还原反应可以将烯烃转化为环烃,通常采用氢化反应或氧化反应。
3. 置换反应置换反应是官能团互变的一种常见方法,通过官能团之间的取代反应来实现。
例如,烷基取代反应可以将一个烷基官能团替换为另一个烷基官能团,常用的取代试剂有碱金属、烷基卤化物等。
二、官能团保护官能团保护是在有机合成中常用的一种策略,用于保护特定的官能团,以防止其在反应过程中发生不需要的反应。
在合成过程中,有时需要对某些官能团进行保护,以确保其他官能团能够正常进行反应。
下面以几种常见的官能团保护反应为例进行介绍。
1. 羟基保护反应羟基保护反应是一种常见的官能团保护方法,通常通过酯化或硅醚化反应来实现。
酯化反应将羟基转化为酯基,以保护羟基。
硅醚化反应则将羟基转化为硅醚基,以保护羟基。
这些保护基在需要时可以通过去保护反应去除。
2. 氨基保护反应氨基保护反应是一种常见的官能团保护方法,通常通过酰化或巯基化反应来实现。
酰化反应将氨基转化为酰基,以保护氨基。
巯基化反应则将氨基转化为巯基,以保护氨基。
这些保护基在需要时可以通过去保护反应去除。

有机化学中的碳氢键官能团转化方法有机化学是研究碳基化合物的科学,其中碳氢键官能团的转化是有机合成中至关重要的一步。
官能团的转化可以改变分子的性质和功能,为有机化学研究和应用提供了广阔的空间。
本文将介绍有机化学中常用的碳氢键官能团转化方法。
一、氧化还原反应氧化还原反应是有机化学中常用的转化方法之一。
在碳氢键官能团的转化中,常用的氧化剂包括酸性高锰酸钾、过氧化苯甲酸等;常用的还原剂包括亚铁氯化物、氢气等。
例如,苯可以通过酸性高锰酸钾氧化为苯酚:C6H6 + KMnO4 + H2SO4 → C6H5OH + MnSO4 + H2O二、酸碱反应酸碱反应也是有机化学中常用的转化方法。
碳氢键上的氢原子可以被酸基或碱基取代,从而引入新的官能团。
例如,甲烷可以和氢氟酸反应生成氟甲烷:CH4 + HF → CH3F + H2O三、卤代反应卤代反应是将碳氢键官能团转化为卤素取代物的常用方法。
常用的卤化剂包括氯化亚铁、溴代甲烷等。
例如,乙烷可以通过氯化亚铁反应生成氯乙烷:C2H6 + FeCl2 → C2H5Cl + HCl四、还原反应还原反应是将碳氢键上的官能团还原成烷烃的方法。
常用的还原剂包括氢气和金属催化剂。
例如,乙醛可以通过氢气还原为乙烷:CH3CHO + H2 → CH3CH3 + H2O五、亲电取代反应亲电取代反应是将碳氢键上的氢原子被亲电试剂取代的常用方法。
常用的亲电试剂包括卤素、酸酐、酰卤等。
例如,甲烷可以被溴取代为溴甲烷:CH4 + Br2 → CH3Br + HBr六、核苷取代反应核苷取代反应是一类特殊的碳氢键官能团转化方法,常用于催化剂的存在下进行。
这种反应可以将一个核苷取代为另一个核苷,引入新的官能团。
例如,氯乙烷可以和氢氧化钾反应生成乙醇:C2H5Cl + KOH → C2H5OH + KCl总结:有机化学中的碳氢键官能团转化方法包括氧化还原反应、酸碱反应、卤代反应、还原反应、亲电取代反应和核苷取代反应。
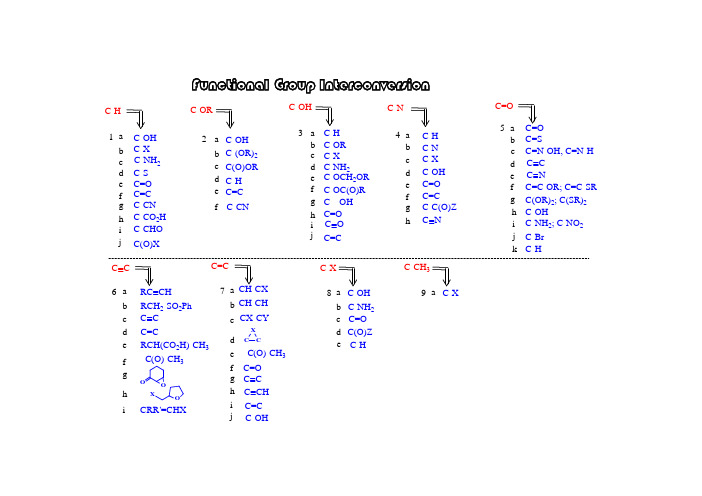

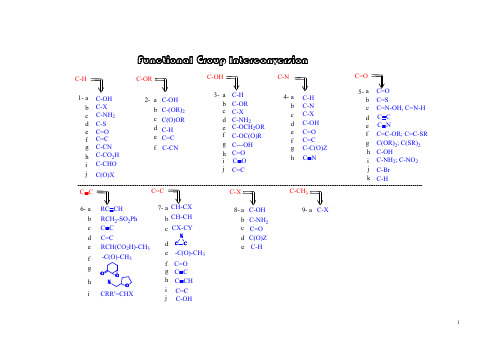
i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX F u n c t i o n a l G r o u p I n t e r c o n v e r s i o nC=CC C C=CC C RCH 2-SO 2Ph RC CHC C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Z d c b a e d c b a f C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:LiAlH 4(1). for 1', 2' alcohol:1-i h g f e dc b a C-CHO C-CO 2HC-CN C=C C=O C-SC-NH 2C-X C-OH C-H CH 3CH3CH 3H n -Bu SnH C S O PhClRCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3PhPhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 2via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.CHCl 2rt, 3 hr1-bBu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)H OJOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOH Zn; Fe; Sn; Mg(3). metal reduction(2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu 3SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973, 654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney NiBuLi1-d1-c 4RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -2NaH 2(3).Ar-NH 2Ar-H1-e(2). thioketal:(3). Wolff-Kishner reduction:(6). enol derivatives:SHSH/ BFTf2similar:(4). Pd-C / HCO2NH4(7). Et3SiH / CF3COOH1-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me 2N)3P=O 1-g (1). K / Al 2O 3 K / HMPA (2). Na / NH 31-h JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me 2N)3P=O yes for white mouse, uncertain for humanmodified to: NN O1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROO RR OROR RCH 2 OCH 2CH 2OH(1). h ν / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2O RC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t Bu RC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with base t -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl (5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X e d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3RaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-eC-OH C-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2RC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH 232Oi. HOAc: weak acid: good leaving groupii. H 2i. F - : HF, Py-H + F -; n -SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+3-b triphenylmethylorganic base: TMG3-c(1). O H -(2). K O 2 / D M S O 3-d not practically useful: R -O H cheaper than R -XJO C , 1975, 40, 1678.(2). N a 2[F e(C N )5(N O )] / K 2C O 3 / H 2O3-e(1). S ym m etry:ketal: use H 3O +acetal: use H 3O +(2). unsym etry:R O -M O M R O -M E M R O -M T M R O -T H Pi. H 3O +; ii. H C l / M eO H p -T sO H / M eO Hi. H 3O +; ii. Z nB r 2 / C H 2C l 2H gC l 2 / C H 3C N (aq.)actually, a c e t a l e x c h a n g e (3). A g 2O / H 2OT H L , 1975, 3183.JO C , 1986, 51, 3913.R O 2C(C H 2)3H RN H 2R O 2C(C H 2)3H RO H2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversionSynthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOHacetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOH3)266hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.。

化学官能团相互转换⼤全(part3)1N H+2C r 2O 7-2N H C l.C rO 3A g 2O :1. a m ild oxidizing agent2. m ust be freshly prepared : N aO H into A gN O 3 (aq)3. m ay involve surface change, react w ith C O 2, lightS w ern oxidation: (D M S O , ox alyl chloride, E t 3N )draw back: react at low TC ollins reagent: (C rO 3 - 2 P y)1. draw back: use 6 equivalent, a m essy reaction2. m ust b e very dry, fire easily; purify by C aH 23. an old ox idizing m aterial, isolated by C ollin. i. P C Cii. P D Civ. M v. S w vi. D D Q vii. M viii. O ix. K 2C RO Haldehyde1st alcohol2nd alcohol1st alcoholRO HORRORHO 5-hi. P C C ii. P D Ciii. C P D C (pyridinium dicho rm ate) (H 2C r 2O 7 + 2 P y) P C C (pyridinium chlorochrom ate) (P y-H C l-C rO 3)m ost w idely useduse 1 - 1.2 eq.60 %JO C , 1977, 42, 1991.S ynthesis, 1981, 165.O I OO A c O A cO2pH 6: w eak acid buffer, avoid interfere w ith ketal groupM cM urray reactioni. C orey approach: subtituted-quinone // H 3O +ii. W att approacha. PhC H O // M C PB A // H 3O +b. A rPhO // M C PB A // H 3O +c. N B S // K O H // H 3O +5-iOPhPhOPhN H 2Ph PhN H 2NCO H// H 3O +O O5-i.15-i.2i. Et 3N // H 3O+ii. TiC l 3 / pH 1 or 6iii. SiO 2 / N aO H // H 3O +JA C S, 1977, 99, 3861.iv. LD A / M oO 5-Py -v. N aO H // C H 3O H OOH 3O +vi. K M nO 4 / K O H C hem. R ev. 1955, 55, 137.35-j5-kIOOO H O (3 eq.)JA C S, 2001, 123, 3183.C H 3C H O2. D D Q / TFA.Synthesis, 1979, 537.JC S, 1932, 1875.Ph-F / D M SO 3.1. SeO 2a select oxidantindrect: change to R C -O H follow ed by oxidation direct: 1. D M A PO / D B U / C H 3C NF 4RB r RD M SO / A gB F 4- M e 2SB ull Soc. Jpn., 1981, 54, 2221. TH L, 1974, 917.2. N aIO 4 / D M FO B r84 %150 oC , 40 m inN aIO 4 / D M F a new m ethod 3. D M SO reagents:ii. D M SO / ZnSR CH B rM eR C (O )M eD M SO B rO H OO HZ nSJA C S, 2003, 68, 2480.RO44T H L , 1975, 4467.R C C H C C R R 'R C C HR C C ArR C C HC CRPhR C C Hsteric base, prevent N u attackn -B uL i: not M eL i, or t -B uL i, fire easily R X : R -B r, R -T O S , R C H O , R C (O )R n -B uL i / R 'C H O // A c 2O // K O t B uC lO M eN L iiii.ii. (Ph 3P)2PdC l 2, C uI, E t 2N H / PhIi. n-B uL i / R X6-b 6-a b c d e g 6-aCCC CCsulfonic acid: PhSO 3H ; sulfinic acid: PhSO 2H ; sulfenic acid: P hS O H iv. C uI, N aI, N a 2C O 3, RC C C H 2C lRCCHC lC H 2C C R 'RCCC H 2CCR 'Synthesis, 2000, 691.R C H 2-SO 2Ph R C C Hh fiR C H (C O 2H )-C H3-C(O )-C H 3OOOXC R R '=C H X5in fact: convert to C =C firstlyii. protect - deprotecti. m ove to term inal 6-c N H 2N H Kuse: K A P Ause: C o (C O )8 // F e(N O 3)3, E tO HJA C S , 1975, 97, 891.6-d use: i. B r 2 / C C l 4 // K O t B u6-euse: P b(O A c)4, L iC l // K O t B u // B r 2/C C l 4 // K O t B F e(N O 3)3: w eak oxidizing agent ii. B r 2 // K O HJAC S, 1941, 63, 1180.P hP hP hP h66-f, B r 2, K O tB uii. N H 2O H , N aN O 2 / H 2S O 4 // A c 2O / D M A Piii. L D A , C lP O (O E t)2ON NOD M A P :4-N ,N -D i m ethyl a m inop y ridinem ixture ofA c 2O / D M A P :N NC H 3O6-guse: T sN H N H 2 / E tO H , heatT H L , 1967, 3943.3(l)G erm an invention, as acylating agentL D A : L i N (iP r)2, ignored a long tim e, re-introduced by M ichigan S tate U. becam e fam ous, appeared every w eek 7LiN H 2 / N H 3 (l)R Xuse: LiN H 2 / N H 3 (l) / R -XO C l6-h6-i.JAC S, 1958, 80, 4599.JAC S, 1955, 77, 3293.M ePhHO SO 2C F 3B uLiM e C C Phvia:M e CPhJO C , 1978, 43, 364.A rA r'HB r A rC C A r'N aO Etvia:A r A r'B ri. N aO E t (w hen X = B r)ii. B uL i (w hen X = -O SO 2C F 3) 8R C H =C H 2:P B uR C H 2C H 2-O -P B u 3R C H 2C H 2-O HP h-S e-P B u 3P h-S e-C Nm echanism :O A c C O 2M eO A cM eOM eO 2CN 2S eNO A cC O 2M eO A c M eO M eO 2Capplied for reactions: w ithout rearrangem ent; no regiosiom er C CHO HD ean-S tark(C O 2H )2 / benzeneOC l C lC l C lOC l C lN C N COiii. P d-C ; or N i; P t, R hii. chloronaili. D D Q use base: D B Ni. C H 3I / A g 2O ii. H C H O / H C O 2H use: heatuse: heatuse base: i. R O N b7-i. p-T S O H.H 2O or C S Aii. w eak acid: H O A c; H C O 2H ; H 2C 2O 4iii strong acid: H iv. A rS eC N , P B use: C C H I C C HN H 2C CO C (S )S M e C C A c C C M s C C HO Ha7-i hg CCX C CC C CC HC O C C f e dcb a7--C(O )-C H 3C H -C HC H -C X9N aI / Zn (C u)i. Zn / acetone i. C SC l 2/C CO M s O M s C C B rB rC C O H O Hc7-C C O HIii. C SC l 2 / P(O M e)3P NN PO C l 3 / py // Snvia:C C IIapplication: i. protect alkene: via B r 2 // ZnC C C C C 36 o C C C C C =C 31 o C C C C C C l lC C B rO A cZn / H O A cOA cO A cOA cOB rO A cZn OO A c O A cO A cJO C , 1978, 43, 364.HO A c JC S.C C , 1998, 2113.ii. In / M eO Hii. purify com pound10d7-e7-i. W C l 6 / R L i ii. L iP Ph 2 / C H 3I product retention product inversion N aR C C HC H 2C H 2C H 2O H OC lRiii. N a(special structure):7-d.7-d.SR 1R 2R 1R 2(E tO )3Puse: (E tO )3PSynthesis , 1977, 1134.117-f.2not for W ittig, ylid unstableJO C , 1978, 43, 3253.JA C S, 1974, 96, 4706.C hem. Lett, 1973, 1041.T iC l 3-L iA lH 4 / T H F T iC l 3 / M g T iC l 4 / Z n T iC l 4 / K ii. M cM urry C ouplingi. use: N 2H 4 / H 2S / Pb(O A c)4B A SF, 1973, 2147.via:Z n-C uP(O E t)1. H 2S2. Pb(O A c)43. H 3O 1. H 2S2. Pb(O A c)4OON SN N NOSN NSNONNNSNN NOOSO ONOOO OOT iC l 3N 2H 412via : betaine, oxaphosphetane (N M R )expensivedifficult to prepareOE tC NPPh 3CNPPh 3HO PPh 3OC O 2M e+notPh 3PC H E tH COC O 2M enotPh 3P C H C O 2M eE tH O ++++stable ylid gives trans (E )unstable ylid gives cis (Z )w ater soluble, rem oved by extraction(com parison: O =PPh 3 highly soluble in organic solvent)use:L i Ph S ONM eCH 2// A l (H g)M e 3SiC H R -L i +Ph 3SiC H 2-L i +=== Ph 3SiC H 2B r + n-B uL i (exchange)M e 3SiC -H -M gB r === M e 3SiC H 2C l + M g (m etal reduction)Ph 3SiC -H C H 2Ph === Ph 3SiC H =C H 2 + PhL M e 3SiC -H C O 2E t === M e 3SiC H 2C O 2E t + L i (m etalation)M e 3SiC H =PPh 3 === M e 3SiC H 2PPh 3+ X - + K HR O = M eO -, E tO -use: (R O )2PO -C H R 'use: Ph 3P-C H R 'vi. Sulfoxim ide (Johnson C.)iii. Silyl W ittig R eaction (Peterson R eaction)ii. Phosphonate W ittig R eaction (H orner-Em m ons M odification)i. W ittig R eaction7-f7-f.Synthesis, 1984, 384.TH L, 1981, 2751.JO C , 1968, 33, 780.iv. C H 2(ZnI)2C hem. Lett, 1995, 259.Synlett, 1988, 12, 1369.H 2C H 2(ZnI)2v. C H 2C H B r 2, Sm , SnI 2 / C rC l 3, TH F R O Rvii. G rignard reagent:1. T M SC H M gC l use: TM SC H 2M gC l TH L, 1973, 3497.TH L, 1988, 4339.2. N aO A c, A cO Hm ethylenationR R 'e 3H advantages over the W ittig:1. by-products are m ore easily rem oved,2. reaction suffers less from steric effects. via:(olefination reaction)1953 discoverg 7-form trans alkene: form cis alkene:i. Li / N H3; or other IA m etalsii. Li / EtN H2iii. L iA lH4 / TH Fi. H2 / N i2B (P-2 catalyst)ii. H2 / Pd-C aC O3 (Lindlar catalyst)iii. H2 / Pd-B aSO4iv. B2H6 / H O A c (D iborane)v. N2H2vi. H C H O / Pd-C / E t3Nnot use H2 / Pt: m ight convert to alkaneh7-all form trans alkene:i. R2B H / B r-C N (hydroboration)C C HRHHii. D IB A L / n-B uLi / C H3I (hydroalum ination) iii. C p2ZrC lH / R X (hydrozirconation)13application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=CC=C C=CC=CC CC CH O Hnot for double bond m ight m ove M nO2 / Ph3P C H3B r- / M TB DNNNvia diradicalJAC S, 1998, 100, 877. Ph Ph Ph7-i7-j148-a8-a.28-a.38-a.41. H I3. T sC l / C62. P I3JC S, 1905, 87, 1592.C H O H C H IP I38-a.12. F3S-N E t2SNSFO OOOC h em. R ev., 1996, 96, 1737.C H C l2FC H3SOOO HO NC H C l2C H31. C F C H F C F N E t2. H O A c / i P rO H$ 65 / 500 g$ 80 / 50 gP B r3P I3$ 35 / 1000 gP B r3$ 500 / 125 gJO C, 1993, 58, 3800.8-C-XC-O H C(O)ZdcaC-N H2C=O2. P P h3 / I2e C-H15168-d8-d.1R C OO H1. A gN O 3/K O H2. B r 2R B r B er. 1942, 75, 296.8-d.2C lOClR hC l(PPh 3)38-bN aN O 2 / H C l / H B F 4 /for arom C hem R ev., 1956, 56, 219. 8-cC F 2B r 2 / ZnFF 58 %JC S.PT I, 1993, 335.178-e8-e.1I86 %I 2 / H N O 3JA C S,1917, 39, 437.8-e.3I 2 / H N O 3PhC H 2C (O )C H 3PhC H C (O )C H 3F N S FOOF +C hem. R ev., 1996, 96, 1737.F-TEDA-B F4 JC S.C C , 1994, 149.F 2-N 2 / C FC l 3-C H C l 3JO C , 1988, 53, 2803.90 %ada m anta ne1. regioselective fluorination at the m ore substituted positions2. electrophilic in natureF -N C FCl 3-C HCl 3OO NXR 1R 3OOR 2HM g(C lO 4)2R 1R 3O OR 2XN B X / M g(C lO 4)2JO C , 2002, 67, 7429.8-e.2X = C l, B r, IX = C l, B r, IN B XN B X :i.ii.iii.iv.RRF R = C H 3C O , C O C F 3, C C l 3, N O 2 H F / electrolysis1.4-1.6 Valready industrilizedN F 3O / TB A H / C H 3C Nv.T B H A : Tetrabutylam m onium hydroxideTH L , 2003, 44, 2799.189-a9-C -C H 3C -Xa (C H 3)3AlM e 3A l98 %O rganom et. C hem. R ev., 1996, 4, 47.C H 2C l 2bridgehead m ethylation。
JA C S, 1972, 94, 7159.L A H ------------ alm ost all: ald, ketone, acie, ester, acyl X, anhydrideN aB H4 --------------- not for acid, ester (but L iB H4 w ork for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:L iA lH4; N aB H4; N a / N H3A l (O i Pr)3 / i PrO H ----------- M eerw ein-Pondorf-V erley rxnIrC l4 / i P rO H / P(O M e)3 ------ H enbest rxnL iB H(sec Bu)3 ------------------ H. C. B row nfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). H C H O reagent:M e C H O M eO HH C H OJA C S, 1935, 511, 903.C H3C H O C(C H2O H)42O rg.Syn, 1925, 4, 53.H C H O / K O HH C H O / C a(O H)2S ynthesis, 1994, 1007.PhN O2OPhN O2H O HB H / SM eJO C, 2001, 66, 7514.JO C, 2003, 68, 2030.OB H3 / T H F99.5 % transsolvent: T H F, S M e23-iR3B, H O C H2C H2O H // H2O2 // N aO HJO C, 1986, 51, 4925.C O RRR3BRRRRR3C B OH O C H2C H2O HR3C BOOH2O2O HR3HO BOOR3CH2OR3C O Hp ra c tic e3-k OO HHO HO HOO HO HHO HO HJO C , 1967, 32, 3452.H 2O 2: dangerous,skin w hiten, m etal decom poseH g (O A c)2: toxic, hard to rem ove (3). B 2H 6, H 2O 2 / O H -, H 2O(2). H g(O A c)2, H 2O // N aB H 4(1). H 3O +3-j3-j.13-j.2hydration:(1). K M nO 4 / N aO H (2). O sO 4(3). H 2O 2/H C O 2H (4). N a / E tO HH Hcis tran cis +trancisM e 2NNNC H 3HC lH 3N C H 3H H N C H 3CH H+NC H 3C l N H H C H OCO O HA C H 21. L A H R 3C N H 2RCN R 2R C N H R R 3CO HR 2C O H R C O H R C N H 2tertiarysecondary prim ary C om pare nom enclature class:not a very useful reactionC -NC -H C -N C -X C -O H C =OC =C 4-abc d ef g 4-aSO 2N H 2Ph IO A c O A cS O ON H S OON I P h Fe (T PP )C lS O ON H 2(insertion)T P PN NNNP h2. N aN 3N C O1. SO 2C l 2O 2CCO O h iC N C (O )X C -C (O )XN H 2H 2R C N O 2R C N H 2i ii4-b C F 3C O 3H // F e / H O A c1. m an y red u cin g ag en ts4-b.14-b.21.2.3.4.F e 3(C O )12 / C H 3O HJO C , 1972, 37, 930.N aB H 4 / P d -C N a 2S S n / H C l V o g el's 12.57V o g el's 12.58V o g el's 12.595.H 2 / P t (S )-CJA C S , 1965, 87, 2767.su lfid ed p latiu mn o t affect: aro m atic rin g s, k eto n es, h alid es, n itriles, am id e, eastersJA CS, 1933, 55, 4579.2H CH O N M e 2CO 2EtN H 2CO 2EtRC N CC NRCCRC N H 2iC N R N N+-C N R R'ii 1. H CH O / H CO 2H 1. RBCl 2 / base1. H C(O Et)3 // N aBH 4;2. R 2CO // N aBH 3CN N H 2NCH 3CH 3H CH O H CO O HN 3N HBCl 2N H 2C O O HN CO O HHCH 3H C(O Et)N aBH 4b.3 2. H CH O // H 2 / Pd-CN 3N O 2M eO 2CN aBH 4CoCl 26H 2O (cat)rtN H 2N O 2M eO 2CSynthesis, 1979,537.m ild conditionhigh yieldnot affect:: N O 2, C=C, CN , CO O R, CO O H2. N aBH 4 / CoCl 2-6H 2Ono t g o od , u su ally co n tain p o lyalk ylatio n p ro d u cts2. D elep in e3. N aN 3 / R E D4-d4-c 5. U n p o lu n g4. N aN 3 / R E D3. D elep in e2. G ab riel:1. N H 3N OK N 2H 42Oi. L A H , N aB H 4ii. H 2 / catiii Z n / H C l; A l (H g )i. M g // N H 2C lii. M g // P h S C H 2-N 3co m m ercial av ailab le, tetram er o f M e 3N24. C B r 4, P P h 3, N aN 3, D M F // P P h 3 / T H FJO C , 2000, 65, 7110.u ro tro p in e (乌洛托品)m eth en am in e (六甲烯胺)h ex am eth ylen etetram in e (环六亚甲基四胺)内服后遇酸分解出 H C H O ,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2N O SO 3HB 2H 6 / H 2N OC H 3C N / H 3O +B 2H 6 / N H 2C l C =CC -C -N H C O C H 3C =C C -C -N H 24-f4-e5. P 4S 10 // R aN i4. E t 3O + B F 4- // N aB H 43. B 2H 62. N aB H 3(O C O R )1. L iA lH 46. L aw esson's reagent // R 4-h4-g4-g.a4-g.b RC N H 2R C N H 2R 'formformA lH 3 / T H FB rCNB r N H 2JO C , 2000, 65, 8152.A lH T H FT H , 1989, 30, 5137.JO C , 1987, 52, 3901.R 'L i // N aB H 4R 'M gX // N aB H 4R 'M gX // L i/N H 3(l)R '2C uL i // N aB H 4T H , 1989, 30, 5139.JO C , 1993, 58, 4313.RCNR CN H 2R 'R 'M // H4-iN H 2ON HO C H 3O PhI(O A c)23JO C , 1993, 58, 2478.RCON H 2RCONIPhO A c RNCOR N HCOO C H 3C H 3O HPhI, O A ccPhI(O A c)4-i.2CN H 2R C H 2PhI(O A c)2 // K O H / C H 3O HC(O R)2C(S R)2hC-N H2C-N O2C NC C5-agfdcba5-C=C-O RC=C-S RC-O HC=N-O HC=N-HC=SC=OC=Ov. via: epox ysilan eR COC RR COC H2R242H3O+C O2H3OOO2-4OO3H3O+Z nT sN H N H2M eL i T M S C l M C P B A L A H24C H2ORRC H2ORRaq C H3C N/C H2CORRi. via:α-C O2Hii. via: α-haloketon eiii. via: ald ol p ro cessiv. via: thioen ol etherROC H2Rd raw back: req uire sim ple stru cture, use m an y p ow erful ag ents: M eL i, L A H, M C P B Aeij C-B rk C-Hii. M C P B Ai. h yd ro lysis 5-b5-c C =N -O HC =N -Hi. R aN i ii. T iC l 3iii. K M n O 4 / A l 2O 3H 3O+5-dH g 2+ / H 2OJO C , 1972, 37, 2138.JO C , 1970, 35, 858.H g S O 4 / H 2O / H 2O5-c.15-c.2T H L , 2001, 42, 4775.1. D IB A L / H 3O +5-eS tenphen reductionm ostly forJ.O rg.S yn , 1925, 3, 1874.2. H C l./ S nC l 2 / E t 2O 5-e.1R -CH 2-CN5-e.25-e.3-C H 2-C OHR -C H -C OH R 'R -C H -C OR "R 'R 'X / n -B uL i C H 3I R ''M gB r H 3O+3.O HO HH 3O +O HO H5-fH 3O +H g 2+ / C H 3C N (aq)C =C -O RC =C -SRO C H 3OH 3O +SC H 3OH g 2+3H 3O H g2+3H 3O+OO O SSS S SR SR O O O R O R 5-gH g 2+/ H 3O+H 3O + / solv (aq)H 3O + / solv (aq)H g 2+ / H 3O +O R O RO H O HH 3O +/ solv (aq)acid catalysta very com m on protecting group, deprotect back to ketoneHCO E t O E tO E tR M gX / H 3O +HCO E t O E tO E tR M gXRCHO。