奥瑞那集团产品说明书
- 格式:doc
- 大小:56.00 KB
- 文档页数:9

Ordering Information Cat. No. Product ***********MagNA Lyser Instrument (230 Volt)***********MagNA Lyser Instrument (110 Volt)(Instruments supplied with rotor and rotor cooling block)***********MagNA Lyser Green Beads (100 tubes)Related Products Cat. No. Product***********MagNA Pure LC DNA Isolation Kit II (Tissue)***********MagNA Pure LC mRNA Isolation Kit II (Tissue)03 330 591 001MagNA Pure LC RNA Isolation Kit III (Tissue)***********MagNA Pure LC DNA Isolation Kit III (Bacteria, Fungi)***********MagNA Pure LC RNA Isolation Tissue Lysis Buffer – Refill (70 ml)System DescriptionHomogenize up to 16 samples in just a few seconds.Save valuable lab space with a small benchtop instrument.Reduce hands-on time by replacing the mortar and pestle and other manual methods.Integrate your workflow with the automated nucleic acid isolation of the MagNA Pure LC Instrument.Perform consistent and reproducible sample disruption.Process many different sample types.Prevent nucleic acid degradation with the benchtop cooling unit.Ease your setup with a removable rotor and prefilled disposable vials.Automate with an easy-to-use instrumentVersatile, efficient, and rapid pre-preparationFigure 71. Add your sample and lysis buffer to the MagNA Lyser Green Beads.2. Homogenize with the MagNA Lyser Instrument.3. Centrifuge to pellet the debris.4. Proceeed with the supernatant to prepare nucleic acids or proteins.For detailed information,visit or contact your local representative.Trademarks:MagNA Pure, MagNA Lyser, LightCycler, and the MagNA Pure Logo are trademarks of a member of the Roche Group.The technology used for the LightCycler System is licensed from Idaho Technology Inc., Salt Lake City, UT, USA.Fully automated sample preparationon the PCR Workflow SystemRoche Diagnostics GmbH Roche Applied Science Nonnenwald 282372 Penzberg Germany0000Roche Applied Science Part of Roche DiagnosticsMagNA Lyser InstrumentStart the Ball Rollingwith Automated Tissue HomogenizationᕤᕣᕢᕡFigure 6Components of the system.The MagNA Lyser InstrumentAutomated tissue homogenizationProcessing conditionsRefer to the following tables for guidelines on setting up your homogenizationSample material(10 mg)*Time settings(seconds)Cooling(between the runs)Speed Average yield(µg)***Average purity(OD 280/260 nm)***Spleen 2 x 25 906,00030–40 1.9Liver 25-6,00016–18 1.8Lung 2 x 25906,00025 1.8Kidney25-6,000201.8Maize leaves **20-5,00010n.d.Maize polenta **20-5,0008n.d.Tortilla chips **20-5,0001n.d.*Aliqout containing 10 mg sample material (here mouse and food samples) was taken for the DNA purificationusing the MagNA Pure LC DNA Isolation Kit II (Tissue), (see pack insert)**Centrifugation after the homogenization for 5 minutes at 2,200 x g*** Yield and purity strongly depend on the condition of the sample material n.d.not determinedData kindly provided by Dr. Peterhänsel, RWTH Aachen, GermanyFigure 1Gel electrophoresis from genomic DNA isolated from tissue homogenized with the MagNA LyserInstrument, using the MagNA Pure LC DNA Kit II (Tissue).Marker: DNA Marker III*Aliquot containing 10 mg sample material (here mouse and human research samples) was taken to purify RNAeither with the MagNA Pure LC RNA Isolation Kit III (Tissue) or the MagNA Pure LC mRNA Isolation Kit II (Tissue) homogenized with the MagNA Lyser Instrument.** Yield and purity strongly depend on the condition of the sample material. The yield for mRNA was not determined.Sample material(10 mg)*Time settings(cycles/seconds)Cooling(between/afterthe runs in seconds)SpeedAverage yield (mg)(total RNA)**Average purity(OD 280/260 nm)**RNA/mRNARarely expressed targets in small numbers of target cells,as seen in experiments about minimalresidual diseases,are difficult to detect.Increasing the cell number can improve sensitivity and lead to accurate results.Without the MagNA Lyser pre-processing,the MagNA Pure mRNA HS Kit can efficiently obtain mRNA from a maximum of 1 x 107white blood cells (WBCs),as shown in research studies with human samples.However,using greater cell numbers results in a saturation effect with quantitative assays (Figure 3).Homogenization of the lysate with the MagNA Lyser Instrument prior to the purification eliminatesthe amplification saturation at 1 x 107cells and allows the use of up to 2.5 x 107WBCs (Figure 4 and 5),enhancing the analytical sensitivity of the assay.Eliminate sensitivity barriers with increased sample inputFigure 3mRNA was purified from different amounts of human white blood cells with the MagNA Pure mRNA HS Kit. G6PDH was amplified using the LightCycler t(9;22) Quantification Kit (see text beside).Figure 4mRNA was purified from different amounts of human white blood cells with the MagNA Pure mRNA HS Kit. The lysates from 2.5 x 107cells and 5 x 107cells were homogenized with the MagNA Lyser Instrument (2x50 seconds with 90 seconds cooling in between) prior to the mRNA purification. G6PDH was amplified using the LightCycler t(9;22) Quantification Kit (see text beside).Figure 5Scalability from 1 x 106cells to 2.5 x 107cells is represented in the graph and the table of the relationship between crossing points and cell numbers. The limitation of cell input is indicated by no change in crossing point with increased cell number (see text beside).Cell number 5 x 1072.5 x 1071 x 1075 x 1061 x 106Log (cell number)7.77.47.06.76.0Crossing point 20.320.321.822.424.4crossingpointLog(cell number)252423222120195.86.36.87.37.8Figure 2Gel electrophoresis from total RNA isolated from tissue homogenized with the MagNA Lyser Instrument, using the MagNA Pure LC RNA Kit III (Tissue).Ma r k e rS p l e e nL i v e rL u n gK i d n e yM a r k e rMa i z e l e a v e sMa i z e l e a v e sS p l e e nL i v e r11 kb5 kb5 kb28 S rRNA 18 S rRNASpleen 2 x 50 90 6,500–7,000 30–40 1.9Liver 50 - 6,500–7,000 13–17 2.0Thymoid tissue60906,500n.d.n.d.Heart 60 90 6,500 n.d. n.d.Abdominal fat 60 90 6,500 n.d. n.d.Aorta 60 90 6,500 n.d. n.d. Other samples1+n x 50 90 6,500–7,000- -1 x 105 x 101 x 10- 5 x 101 x 105 x 105 x 10- 5 x 102.5 x 10 5 x 10。
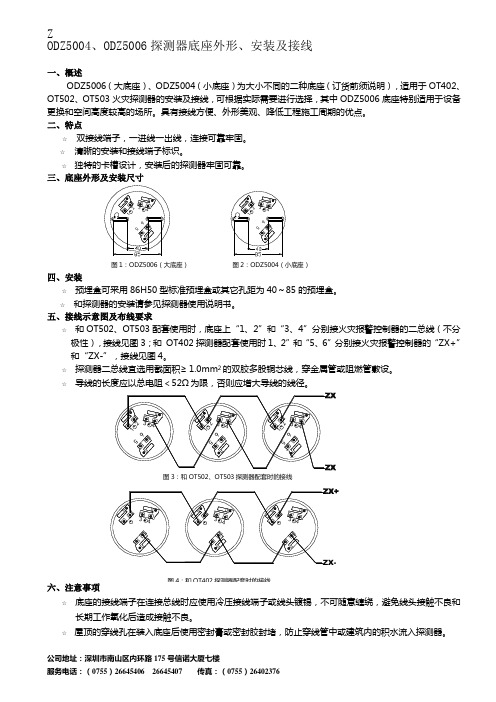
Z ODZ5004、ODZ5006探测器底座外形、安装及接线一、概述ODZ5006(大底座)、ODZ5004(小底座)为大小不同的二种底座(订货前须说明),适用于OT402、OT502、OT503火灾探测器的安装及接线,可根据实际需要进行选择,其中ODZ5006底座特别适用于设备更换和空间高度较高的场所。
具有接线方便、外形美观、降低工程施工周期的优点。
二、特点☆ 双接线端子,一进线一出线,连接可靠牢固。
☆ 清晰的安装和接线端子标识。
☆独特的卡槽设计,安装后的探测器牢固可靠。
图1:ODZ5006(大底座) 图2:ODZ5004(小底座)四、安装☆ 预埋盒可采用86H50型标准预埋盒或其它孔距为40~85的预埋盒。
☆ 和探测器的安装请参见探测器使用说明书。
五、接线示意图及布线要求☆ 和OT502、OT503配套使用时,底座上“1、2”和“3、4”分别接火灾报警控制器的二总线(不分极性),接线见图3;和 OT402探测器配套使用时1、2”和“5、6”分别接火灾报警控制器的“ZX+”和“ZX-”,接线见图4。
☆探测器二总线宜选用截面积≥1.0mm 2的双胶多股铜芯线,穿金属管或阻燃管敷设。
☆ 导线的长度应以总电阻<52Ω为限,否则应增大导线的线径。
六、注意事项 ☆ 底座的接线端子在连接总线时应使用冷压接线端子或线头镀锡,不可随意缠绕,避免线头接触不良和 长期工作氧化后造成接触不良。
☆ 屋顶的穿线孔在装入底座后使用密封膏或密封胶封堵,防止穿线管中或建筑内的积水流入探测器。
图3:和OT502、OT503探测器配套时的接线 图4:和OT402探测器配套时的接线。

ORENA火灾报警及消防联动控制系统产品设计应用手册彩页:产品技术特点和优势控制器大容量,全系列控制器单机容量从64点、128点、192点、384点、768点、1536点、2304点……直至11520点,可多台控制器联网通讯,完全满足各种工程的需要,具有较高的性能价格比。
双向分布智能回路器件(探测器、模块、手报按钮)采用MCU微处理器,具有独立的智能分析和判断能力,内含智能软件,可根据现场环境的变化(温度、湿度、灰尘污染)自动调整报警阈值、滤除干扰,并与控制器双向传输信息,大大降低了误报率,加快了报警响应时间,最大限度地保证了报警的准确性。
真正的全总线系统结构系统内所有的各种探测器、模块、报警按钮、楼层火灾显示盘、回路扩展单元等外部设备全部挂接在总线上,不需额外的信号线。
系统结构简洁,节省大量线材和布线工时。
数字化信号传输二总线电流量脉宽数字化信号传输技术,通讯可靠,抗干扰性能强。
先进的自动编址功能无需人工设定地址,所有外部设备在线自动识别,自动编址,节省大量安装调试时间,提高了可靠性。
安装调试简便无极性两总线,避免了由于接线不当而引起的系统损坏。
可T形/环形任意布线,任意分支,节省大量线材和布线工时。
控制器上可显示回路器件的供电电压,方便系统调试。
大容量事件记录事件记录簿的容量为4096条,为用户日常使用管理和物业管理部门对使用情况的监督以及万一发生火灾后事故成因的分析都提供一个超长时间的可靠数据记录。
系统结构图前言奥瑞那公司成立于1995年,一直专业从事火灾自动报警设备的研发、生产、销售和服务。
200余名高素质员工、5000m2现代化厂房、众多自动化生产检测设备,严谨完善的质量管理体系,充分保证了产品的先进性和可靠性。
先进的技术和设备、严格的质量控制、优良的售后服务,是奥瑞那向客户提供可靠产品并让客户放心满意的有力保证。
奥瑞那的产品具有外形美观、质量可靠、服务优良的特点,主导产品火灾报警及消防联动控制系统已成功应用于数千项工程,在广东省消防指挥中心、广东省政协办公楼、甘肃省政府办公楼、江西省政协大厦、天健世纪花园、天然居(6栋32层住宅楼)、绿景蓝湾畔岛(8栋33层商住楼)、天鸿安柏丽晶、万科东海岸等许多重大工程中发挥着重要作用。

J B-Q B-O Z H200火灾报警控制器使用说明书(V1.0)-CAL-FENGHAI.-(YICAI)-Company One1目录第一章产品介绍 (2)概述 (2)主要技术指标 (2)产品功能特点 (2)第二章安装与接线.......................................................................................... 错误!未定义书签。
设备外观与机箱安装.. (3)部件组成 (3)电源连接 (3)端子说明 (4)第三章键盘及主界面说明.............................................................................. 错误!未定义书签。
键盘说明. (4)主界面说明 (5)第四章菜单界面.............................................................................................. 错误!未定义书签。
设置菜单. (5)操作菜单 (6)编程菜单 (6)查询菜单 (7)编码菜单 (7)帮助菜单 (7)第五章部件类型对照表.................................................................................. 错误!未定义书签。
第六章常见故障排除方法.............................................................................. 错误!未定义书签。
第七章联系方式.............................................................................................. 错误!未定义书签。

1.4 火灾报警控制器概述OZH4800系列火灾报警控制器是消防报警及联动系统的核心设备,它负责检测各探测器、模块等部件的状态,及时将回路总线上各部件的火警信息以声、光形式反应出来,并按预先编制的联动关系以总线形式联动相应设备,完成报警及联动灭火任务。
奥瑞那公司的OZH4800系列火灾报警控制器具有强大的功能和可靠的长期稳定性,完全符合国家技术标准规定和用户的实际要求。
产品特点及功能z容量:单机最大容量11520地址,可以1~8台控制器联网组成超大系统;每回路总线监控容量 192地址;每块回路扩展卡配置4个回路,共768地址;单机基本配置1块回路扩展卡,可最多扩展到15块回路扩展卡。
z总线:1)探测器、手报:2总线,无极性,允许枝状布线,总线长度可达1500m;2)总线联动、楼层显示器:4总线,除与探测器、手报按钮共享信号2总线 外,另加两根电源线(24V,0V);3) 多线联动:N+1线制z地址:由控制器自动编址,探测器和总线联动模块统一编址;火灾显示盘独立编址,不占用整机容量空间。
z显示器:1)主显示器:320X240点阵液晶屏,显示汉字、表格和图形;2)时钟显示器:VFD真空荧光数码管。
z事件记录:可循环记录4096条事件,如:开机、关机、火警、故障、反馈、编程操作、复位等;记录信息掉电或脱机可保存10年;为分析事件提供证据。
z高速微打:可选配热敏或针式微型汉字打印机。
z操作灵活:可测试探测器等部件的电压、电流,反应烟温信息的各种参数。
z自动编址:通过登记操作,对探测器等部件自动编址。
z 外形:壁挂式、立柜式和琴台式三种外型结构,符合不同场所的空间要求。
配壁挂式、柜式和琴台式照片主要技术参数主电源 AC187~242V 50Hz备电源 DC24V 7Ah功耗 监视状态:2.2W 报警状态 :10W巡检周期 3.5秒外形尺寸 壁挂式:700mm(高)×520mm(宽)×140mm立柜式:500mm(长)×610mm(宽)×1860mm(高)标准单琴台式:1040mm(长)×610mm(宽)×1115mm(高)标准单琴台式:1040mm(长)×610mm(宽)×1115mm(高)技术标准 GB4717-93、GB16806-1997配置参考z JB-QB-OZH5800/64 智能火灾报警控制器(联动型) 壁挂64点z JB-QB-OZH5800/128 智能火灾报警控制器(联动型)壁挂128点z JB-QB-OZH5800/192 智能火灾报警控制器(联动型)壁挂192点z JB-QB-OZH5800/384 智能火灾报警控制器(联动型)壁挂384点z JB-QB-OZH5800/768 智能火灾报警控制器(联动型)壁挂768点z JB-QG/T-OZH5800/768 智能火灾报警控制器(联动型) 柜式/台式768点z JB-QG/T-OZH5800/1536 智能火灾报警控制器(联动型) 柜式/台式1536点 z JB-QG/T-OZH5800/2304 智能火灾报警控制器(联动型) 柜式/台式2304点 z JB-QG/T-OZH5800/3072 智能火灾报警控制器(联动型) 柜式/台式3072点 z JB-QG/T-OZH5800/3840 智能火灾报警控制器(联动型) 柜式/台式3840点 每增加一块回路扩展卡,容量增加768点。

OX520消火栓按钮使用说明书V1.01.0 概述1.1 OX520消火栓按钮(以下简称按钮),是人工发送报警信号、启动水泵及显示水泵运行指示的部件。
1.2 该按钮具有控制器自动编址和电子编码器编址两种编址方式、自动检测消火栓按钮两端电压的功能。
与我公司生产的OZH4800型火灾报警控制器配合使用。
自动编址方式参见控制器使用说明书,电子编码器编址方式参见电子编码器使用说明书。
2.0 结构特征及工作原理2.1壳体材料和颜色采用ABS 复合塑胶设计成型,外观红色、安装方便。
2.2工作原理、工作特性该按钮采用MCU 单片机微处器。
SMT 贴片技术和二线制无极性输入方式设计,具有控制器自动编址和电子编码器编址两种编址方式、自动检测按钮两端电压的功能。
2.3 主要部件该消火栓按钮是由按钮开关、电话插孔、线路板,外壳主要部件组成。
3.0 技术特征3.1 工作电压:DC20V ~ 28V3.2 监视电流: ≤250uA报警电流:≤2mA3.3 线制:二总线,无极性3.4 地址设定范围:1~192号3.5 触点容量:DC24V/1A3.6 环境条件:温 度 -10℃~+50℃。
相对湿度 ≤95%(40±2℃)3.7尺寸及重量长:85mm 宽:85mm 高度:38.5mm 重量:约129g4.0 安装4.1 安装基础、条件及要求4.1.2 安装前消火栓按钮应保存在防尘、防潮、防霉的地方。
4.2 消火栓按钮的布线4.2.1 要求用截面积1mm 2以上的铜质双绞线,每米绞合节数为20~30,导线总电阻低于60Ω,总分布电容低于0.2uF 。
在总线负载较重的情况下,宜根据实际最大负载电流计算导线压降,以保证现场单元得到20V 以上的总线电压。
4.2.2 屋顶的穿线管在装入底座后应使用密封膏或密封胶封堵,防止积水进入消火栓按钮。
4.2.3 接线时导线应使用冷压接线端子或做镀锡处理,不可随意缠绕。
避免线头接触不良。
OM515 隔离模块
使用说明书
编号________OM515-SS___________ 拟制___________ 日期___________ 审核___________ 日期___________ 批准___________ 日期___________
深圳奥瑞那光子技术有限公司
OM515隔离模块
使用说明书V1.0
一.概述:
OM515隔离模块用于二总线火灾报警控制器的输入回路,安装在每个分支回路的前端,当该分支回路发生短路时,隔离模块可将该部分回路与总线隔离,保证其余部分正常工作。
当故障排除后,隔离模块自动恢复正常。
二.技术指标:
1. 工作电压: DC8V ~30V
2. 保护方式:输出端短路
3.使用环境:
温度: -10℃~+50℃
相对湿度:≤95% (40℃±2℃)
4.线制:二总线
5.外型尺寸: 96 mm×65 mm×37mm(长×宽×厚)
三.安装使用与接线
1.安装:
它采用的底座为我公司OMDZ51型模块底座,为插拔结构安装,OMDZ51模块底座安
装完毕并接好线后,插入即可。
2.接线图:
输入端接线:
9端
10端接输入总线“IN-”
输出端接线:
7端为输出总线“UT-”
8端为输出总线“UT+”。
SEE MORE AT Request QuoteAs a responsible manufacturer of pressure, differential pressure, level, temperature and vacuum instruments, SOR Inc. has long recognized its accountability for the reliability and integrity of its products. As processes become more intricate and less tolerant, built-in safety features and rigorous in-house and third party testing is legally and morally mandated. International and USA standards such as ANSI/ISA 84 and other agency listings accompany most SOR ® instruments as a part ofits continuing excellence program. Now the IEC 61508 Functional Safety Standard has been added to this impressive offering.SOR pressure, differential pressure, level, temperature and vacuum instruments meet the rigorous, international Safety Integrity Level (SIL) standards. SILcertification identifies all process hazards, estimates the risk of failure anddetermines if a failure occurs, the product will “fail safely.” Every component in the instrument must be examined and verified in light of this critical criteria in order to comply with the SIL constraints. The purpose of SIS is to maintain the safety of one or more Safety InstrumentedFunctions (SIF). It is related to a specific hazardous event in which a process may be compromised. Some SIF applications include:• Shutdown in a hazardous process• Open excess pressure relief valve• On/off tank overflow control• Add coolant to arrest exothermic runaway• Automatic shutdown when manual control is not present or available• Close a feed valve to prevent tank overflow• Fire suppressant release• Evacuation alarm alertThe International Electro T echnical Commission (IEC) provides the standardsfor “Functional Safety of Electrical/Electronic/Programmable Electronic Safety Related Systems”. Its aim is to provide design standards for SIS to an acceptable level by following hardware and software safety life-cycle procedures and tomaintain detailed documentation. IEC 61508 is the international standard used by safety equipment manufacturers to verify and document that their products are suitable for use in SIL rated systems. SIL Suitable SwitchesSafety Instrumented Systems (SIS)IEC 61508 Guidelines & ObjectivesSafety Integrity Level QUICK GUIDE4 SIL Levels A Safety Integrity Level is determined via a procedure called Risk/Process Hazard Analysis (PHA). It identifies all the hazards of a process and estimates the risks inherently involved and determines if that risk is tolerable/acceptable. This safety procedure must verify that each instrument utilized in the system (SIS) as well as each instrument’s parts such as sensors, logic solvers and integral components will work safely to achieve the Safety Instrumented Functions (SIF) in compliance with the confines required by SIL.For each instrument used within a SIS, the analysis team must concentrate their evaluation on the design and performance of its Safety Failure Fraction (SFF) and Probability of Failure on Demand (PFDavg). Every device in the SIF must meet or surpass the safety standards in both categories required by SIL to achieve SIL recognition.SIL standards allow for a manufacturer’s proven, in-use data that demonstrates reliability as well as fully assessed, third party analysis. Although SIL is a powerful, peace-of-mind benefit, it is always the end-user’s responsibility to verify and document all calculations for the entire SIS safety loop. All instruments/devices in a SIF loop must work together to meet the SIL requirement.SIL certification is a tool to measure the amount of risk reduction provided by a Safety Instrumented Function. It assesses the tolerable/acceptable failure rate of an individual device. This is important when installing or retrofitting an instrument into a SIS. The SIL level number is based on the amount of risk reduction needed to maintain an acceptable rate of failure. Each of the 4 levels of SIL represents an order of magnitude of risk reduction – the higher the level, the greater the impact a failure (and thereby, the lower the acceptable failure rate). SIL 4 has the highest level of safety – Level 1 the lowest.With the use of the Safe Failure Fraction and Probability of Failure on Demand values calculated during the product design and evaluation, SIL levels are determined using charts within the IEC 61508 standard. SOR evaluated their switches with T ype A and low demand constraints.SIL ExplainedSafe Failure Fraction* A hardware fault toleranceof N means that N + 1 faultscould cause a loss of thesafety function.Probability of Failure on Demand* SFF and PFD values noted are worst case for the product grouping listed above.For calculated values applicable to a specific product, please visit .T o determine the risk reduction needed in your process operation as required by IEC 61508, a Failure Modes, Effects and Diagnostic Analysis (FMEDA) report serves as a systematic tool to methodically assess the effects of all probable andknown failure modes. The report includes on-line monitoring and error-checking of SIS components. An FMEDA report aids you in evaluating the estimated failure rates, failure modes and diagnostic capabilities of an instrument/device to fit your process demands. It provides detailed circuit and performance data specific to the instrument/device and its components.SOR has the following SIL Level Certification on these T ype A Device - Low Demand Mode Use products.Assessing your SIL Level needsSIL LevelsThe SOR Tradition of Continuing Excellence Manufacturing integrity, rugged construction and reliability has long been the foundation of SOR pressure, differential pressure, level, temperature and vacuum switches. It is what has made it the brand of choice for customers throughout the world for more than 60 years. Whether your instrumentation needs call for new orreplacement or primary or redundant switches, you can be confident in your choice to go with SOR. The addition of the SIL rating just makes your decision easier.T o learn more about SOR instruments for the process industry, contact your local SOR Representative or visit .Proof Tests T o maintain the necessary SIL Level in any SIS, the system (and each componentwithin the system) must be functionally tested for correct operation. The method to perform this functional testing is called “Proof T esting”. This testing is to be performed on the system and each product at intervals not greater than the period mandated by each device manufacturer. This “Proof T est Interval” is applied when calculating the PFD and determining a SIL level, thus it may be different for each device in the SIS and the entire system should be evaluated to determine the overall test interval.The “Proof T est Interval” used for the calculation of all SOR SIL ratings is once per year.T o allow for future auditing and review of the performance of any SIS, the results of all Proof T esting should be recorded and retained for future evaluation.SOR switches are simple devices with snap acting “on/off” operation.Confirmation of required function only involves increasing or decreasing the process with which the device is installed to measure (pressure, temperature, level, flow).For an increasing set point, start at a process lower than set point and increase until the device changes state. Next, decrease process until the device resets. For a decreasing set point, start at a process higher than set point and decrease until the device changes state. Next, increase process until the device resets.If the found calibration of set point is as required, the proof test is complete; record data and move on to the next device. If calibration is found out of tolerance, recalibrate following the directions provided within the General Instructions document for the device.Note: Testing may be performed as installed or the switch may be required to be removed from service and tested on a calibration bench. The method used will be determined by the limitations of the SIS.Proof Testing of SOR Products。
Arzerra® (ofatumumab)(Intravenous)Document Number: IC‐0208 Last Review Date: 04/04/2022Date of Origin: 08/26/2014Dates Reviewed: 03/2015, 05/2015, 08/2015, 11/2015, 02/2016, 05/2016, 08/2016, 11/2016, 02/2017,05/2017, 08/2017, 11/2017, 02/2018, 05/2018, 04/2019, 04/2020, 04/2021, 04/2022I.Length of Authorization 1,10Coverage will be provided for 6 months with renewal subject to the following:∙CLL/SLL (first-line) may be renewed to allow for a total of 12 cycles∙CLL/SLL (relapsed) may not be renewed (unless the provisions for extended treatment have been met)∙CLL/SLL (single agent subsequent therapy) may not be renewed (unless the provisions for extended treatment have been met)∙CLL/SLL (extended treatment) may be renewed to provide for a total of 2 years of therapy ∙Waldenström’s Macroglobulinemia/Lymphoplasmacytic lymphoma may be renewed to allow for up to a total of 3 cyclesII.Dosing LimitsA.Quantity Limit (max daily dose) [NDC Unit]:∙Arzerra 100 mg/5 mL single-use vial: 3 vials Day 1∙Arzerra 1000 mg/50 mL single-use vial: 2 vials weekly x 7 doses, then 2 vials every 4 weeks, then 1 vial every 8 weeks for up to 24 monthsB.Max Units (per dose and over time) [HCPCS Unit]:CLL/SLL First‐Line▪30 billable units on day 1 and 100 billable units on day 8; then▪100 billable units every 28 days for up to 11 dosesSingle agent subsequent therapy▪30 billable units on day 1; then▪200 billable units weekly x 7 doses; then▪200 billable units every 28 days x 4 dosesRelapsed▪30 billable units on day 1 and 100 billable units on day 8; then▪100 billable units every 28 days for up to 5 dosesExtended Treatment▪30 billable units on day 1 and 100 billable units on day 8; then▪100 billable units 7 weeks later and every 8 weeks thereafterWaldenström’s Macroglobulinemia / Lymphoplasmacytic Lymphoma ▪30 billable units on day 1; then▪200 billable units every 7 days x 4 dosesIII.Initial Approval Criteria 1Coverage is provided in the following conditions:∙Patient is at least 18 years of age; ANDUniversal Criteria 1∙Patient has been screened for the presence of hepatitis B (HBV) infection (i.e., HBsAg and anti-HBc) prior to initiating therapy and patients with evidence of current or prior HBVinfection will be monitored for HBV reactivation during treatment; AND∙Must not be administered concurrently with live vaccines; ANDChronic Lymphocytic Leukemia (CLL)/Small Lymphocytic Lymphoma (SLL) † Ф1-3∙Used as first-line therapy; ANDo Used in combination with chlorambucil in patients considered inappropriate for fludarabine-based therapy (Note: only applies to CLL);ORo Used in combination with bendamustine ‡; AND▪Patient does not have del(17p)/TP53 mutation (patients ≥ 65 years, or younger patients with or without significant comorbidities; excluding use in frail patients[i.e., creatine clearance (CrCl) <70 mL/min]); OR∙Used as subsequent therapy; ANDo Used as a single agent; ORo Used in combination with fludarabine and cyclophosphamide (FC) for relapsed disease (Note: only applies to CLL);OR∙Used as extended treatment in patients with complete or partial response after at least 2 lines of therapy for recurrent or progressive disease (Note: only applies to CLL) Waldenström’s Macroglobulinemia/Lymphoplasmacytic Lymphoma ‡ 2,4∙Used as a single agent OR as part of combination therapy; AND∙Patient is intolerant to rituximab; ANDo Patient has previously failed primary therapy; ORo Patient has progressive or relapsed disease† FDA Approved Indication(s); ‡ Compendia Recommended Indication(s); Ф Orphan Drug IV.Renewal Criteria 1Coverage may be renewed based on the following criteria:∙Patient continues to meet universal and other indication-specific relevant criteria such as concomitant therapy requirements (not including prerequisite therapy), performancestatus, etc.identified in section III; AND∙Disease response with treatment as defined by stabilization of disease or decrease in size of tumor or tumor spread; AND∙Absence of unacceptable toxicity from the drug. Examples of unacceptable toxicity include: Hepatitis B virus reactivation/infection, progressive multifocal leukoencephalopathy, severe infusion reactions, tumor lysis syndrome, cytopenias (neutropenia, anemia, andthrombocytopenia), etc.V.Dosage/Administration 1,10CLL/SLL (First-line)Administer 300 mg on Day 1, then 1,000 mg on Day 8, followed by 1,000 mg on Day 1 of subsequent 28-day cycles for a minimum of 3 cycles until best response or amaximum of 12 cyclesCLL/SLL (Single agent subsequent therapy)Administer 300 mg on Day 1, followed 1 week later by 2,000 mg given weekly x 7 doses (infusions 2 through 8), followed 4 weeks later by 2,000 mg every 4 weeks for 4 doses (infusions 9 through 12) for a total of 12 dosesCLL/SLL (Relapsed) Administer 300 mg on Day 1, then 1,000 mg on Day 8, followed by 1,000 mg on Day 1 of subsequent 28-day cycles for a maximum of 6 cyclesCLL/SLL (Extended treatment)Administer 300 mg on Day 1, then 1,000 mg on Day 8, followed by 1,000 mg 7 weeks later and every 8 weeks thereafter for up to a maximum of 2 yearsWaldenström’s/ Lymphoplasmacytic lymphoma Cycle 1:∙Administer 300 mg on day 1, then 1,000 mg weekly for weeks 2 through 4; OR∙Administer 300 mg on day 1, then 2,000 mg weekly for weeks 2 through 5Cycle 2-3:∙Patients with stable disease or a minor response at week 16 of cycle 1 are eligible to receive a re-dosing cycle of 300 mg on day 1, then 2,000 mg for weeks 2 through5.Patients responding to cycle 1 or the redosing cycle who developed diseaseprogression within 36 months can receive treatment with 300 mg on day 1, then2,000 mg for weeks 2 through 5.VI.Billing Code/Availability InformationHCPCS Code:•J9302 – Injection, ofatumumab, 10 mg; 1 billable unit = 10 mgNDC:•Arzerra 1000 mg/50 mL single-use vial: 00078-0690-xx•Arzerra 100 mg/5 mL single-use vial: 00078-0669-xxVII.References1.Arzerra [package insert]. East Hanover, NJ; Novartis Pharmaceuticals Corporation, August2016. Accessed March 2022.2.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) ofatumumab. National Comprehensive Cancer Network, 2022. The NCCNCompendium® is a derivative work of the NCCN Guidelines®. NATIONALCOMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCN GUIDELINES® aretrademarks owned by the National Comprehensive Cancer Network, Inc. To view the mostrecent and complete version of the Compendium, go online to . Accessed March2022.3.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma. Version2.2022. National Comprehensive Cancer Network, 2022. The NCCN Compendium® is aderivative work of the NCCN Guidelines®. NATIONAL COMPREHENSIVE CANCERNETWORK®, NCCN®, and NCCN GUIDELINES® are trademarks owned by the NationalComprehensive Cancer Network, Inc. To view the most recent and complete version of theCompendium, go online to . Accessed March 2022.4.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) Waldenstrom’s Macroglobulinemia/Lymphoplasmacytic Lymphoma. Version2.2022. National Comprehensive Cancer Network, 2022. The NCCN Compendium® is aderivative work of the NCCN Guidelines®. NATIONAL COMPREHENSIVE CANCERNETWORK®, NCCN®, and NCCN GUIDELINES® are trademarks owned by the NationalComprehensive Cancer Network, Inc. To view the most recent and complete version of theCompendium, go online to . Accessed March 2022.5.Furman RR, Eradat H, DiRienzo CG, et al. A phase II trial of ofatumumab in subjects withWaldenstrom's macroglobulinemia. Blood. 2011;118:37016.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapyin fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010;28:1749-17557.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) B-Cell Lymphomas. Version 1.2022. National Comprehensive CancerNetwork, 2022. The NCCN Compendium® is a derivative work of the NCCN Guidelines®.2NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCNGUIDELINES® are trademarks owned by the National Comprehensive Cancer Network,Inc. To view the most recent and complete version of the Compendium, go online to. Accessed March 2022.8.Rosenbaum CA, Jung SH, Pitcher B, et al. Phase 2 multicentre study of single-agentofatumumab in previously untreated follicular lymphoma: CALGB 50901 (Alliance). Br JHaematol. 2019 Feb 5.9.Van Imhoff GW, McMillan A, Matasar MJ et al. Ofatumumab Versus Rituximab SalvageChemoimmunotherapy in Relapsed or Refractory Diffuse Large B-Cell Lymphoma: TheORCHARRD Study. J Clin Oncol 2017;35 (5):544-551.10.Furman RR, Eradat HA, DiRienzo CG, et al. Once-weekly ofatumumab in untreated orrelapsed Waldenström's macroglobulinaemia: an open-label, single-arm, phase 2 study.Lancet Haematol. 2017 Jan;4(1):e24-e34. doi: 10.1016/S2352-3026(16)30166-1. Epub 2016Dec 1.11.Hillmen P, Robak T, Janssens A, et al. Chlorambucil plus ofatumumab versus chlorambucilalone in previously untreated patients with chronic lymphocytic leukaemia(COMPLEMENT 1): a randomised, multicentre, open-label phase 3 trial. Lancet. 2015 May 9;385(9980):1873-83. doi: 10.1016/S0140-6736(15)60027-7. Epub 2015 Apr 14.12.Robak T, Warzocha K, Govind Babu K, et al. Ofatumumab plus fludarabine andcyclophosphamide in relapsed chronic lymphocytic leukemia: results from theCOMPLEMENT 2 trial. Leuk Lymphoma. 2017 May;58(5):1084-1093. doi:10.1080/10428194.2016.1233536. Epub 2016 Oct 12.13.van Oers MH, Kuliczkowski K, Smolej L, et al. Ofatumumab maintenance versusobservation in relapsed chronic lymphocytic leukaemia (PROLONG): an open-label,multicentre, randomised phase 3 study. Lancet Oncol. 2015 Oct;16(13):1370-9. doi:10.1016/S1470-2045(15)00143-6. Epub 2015 Sep 13.14.Lemery SJ, Zhang J, Rothmann MD, et al. U.S. Food and Drug Administration Approval:Ofatumumab for the Treatment of Patients with Chronic Lymphocytic Leukemia Refractory to Fludarabine and Alemtuzumab. 10.1158/R-10-0570 Published September2010.15.Chen L, Shah R, Cwynarski K. et al. Ofatumumab is a feasible alternative anti-CD20therapy in patients intolerant of rituximab. Br J Haematol. 2019 Feb;184(3):462-465.doi: 10.1111/bjh.15110. Epub 2018 Jan 24.Appendix 1 – Covered Diagnosis Codes1010C83.00 Small cell B-cell lymphoma, unspecified siteC83.01 Small cell B-cell lymphoma, lymph nodes of head, face and neckC83.02 Small cell B-cell lymphoma, intrathoracic lymph nodes1010C83.03 Small cell B-cell lymphoma, intra-abdominal lymph nodesC83.04 Small cell B-cell lymphoma, lymph nodes of axilla and upper limbC83.05 Small cell B-cell lymphoma, lymph nodes of inguinal region and lower limbC83.06 Small cell B-cell lymphoma, intrapelvic lymph nodesC83.07 Small cell B-cell lymphoma, spleenC83.08 Small cell B-cell lymphoma, lymph nodes of multiple sitesC83.09 Small cell B-cell lymphoma, extranodal and solid organ sitesC88.0 Waldenström macroglobulinemiaC91.10 Chronic lymphocytic leukemia of B-cell type not having achieved remissionC91.12 Chronic lymphocytic leukemia of B-cell type in relapseAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD), Local Coverage Determinations (LCDs), and Local Coverage Article (LCAs) may exist and compliance with these policies is required where applicable. They can be found at: https:///medicare-coverage-database/search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCA/LCD): N/AJurisdiction Applicable State/US Territory ContractorE (1) CA, HI, NV, AS, GU, CNMI Noridian Healthcare Solutions, LLCF (2 & 3) AK, WA, OR, ID, ND, SD, MT, WY, UT, AZ Noridian Healthcare Solutions, LLC5 KS, NE, IA, MO Wisconsin Physicians Service Insurance Corp (WPS)6 MN, WI, IL National Government Services, Inc. (NGS)H (4 & 7) LA, AR, MS, TX, OK, CO, NM Novitas Solutions, Inc.8 MI, IN Wisconsin Physicians Service Insurance Corp (WPS) N (9) FL, PR, VI First Coast Service Options, Inc.J (10) TN, GA, AL Palmetto GBA, LLCM (11) NC, SC, WV, VA (excluding below) Palmetto GBA, LLCNovitas Solutions, Inc.L (12) DE, MD, PA, NJ, DC (includes Arlington &Fairfax counties and the city of Alexandria in VA)K (13 & 14) NY, CT, MA, RI, VT, ME, NH National Government Services, Inc. (NGS)15 KY, OH CGS Administrators, LLC。
目录一、OZH280火灾报警控制器容量、配置、线制及设置 (1)二、OZH280火灾报警控制器操作方法 (3)三、附A: OZH280火灾报警控制器字符区位码对照表 (14)四、附B: OZH280火灾报警控制器汉字区位码对照表 (15)OZH280火灾报警控制器容量、配置、线制及设置一、主要技术指标1、交流输入电压:220V±10%, 50Hz±1%。
2、备用电源:24V 4Ah, 全密封免维护蓄电池。
3、使用环境:温度:0℃~+50℃;相对湿度:≤95% ( 40℃±2℃)。
4、容量:共2回路总线,每回路总线带192地址,整机最大容量为384个地址点。
1回路地址范围1~192;2回路地址范围201~392。
二、配置该设备由主机、电源和多线联动控制部分组成。
1、主机部分:(1)主机面板:液晶屏,键盘,指示灯,扬声器等。
(2)主机主板:(主机板包括回路部分)。
(3)主机电源:(电源板)。
三、线制(1)报警单元:两总线(无极性)(2)接口模块:四总线,两根信号线(无极性),两根电源线(24V,0V)。
(3)复示盘:四总线,两根信号线(无极性),两根电源线(24V,0V)。
(4)线制要求:a. 信号二总线(无极性),采用RVS双绞线,截面积≥1.0mm2。
根据工程具体情况配置。
b. DC24V输出线采用BV线,截面积≥2.5mm2。
c.信号二总线的主干线≥1.5mm2d. 控制器每路总线的往返电阻<20Ω.四、设置(1)系统刷新:首次使用应进行刷新操作,将以前的信息、记录、安装位置、联动编程、综合编程等全部清除;然后再进行登记操作、安装位置编程、联动编程、综合编程、多线联动设置等操作。
操作密码为:11111111。
(2)登记操作:首次加电或总线上部件变更时,需进行登记操作。
登记过程是自动完成的。
登记是按回路号进行的,登记后显示总数信息。
操作密码为:44441111。
(3)主机板上8位拔码开关的设置1~4位:设置地址,固定为1。
5位:键盘控制位,ON------锁键盘,OFF开键盘。
6位:ON 不检测电源;OFF检测电源。
7位、8位:未用五、引线标注1交流电源输入端子标示注: L :交流零线N :交流火线FG :地线2 24V 电压输出及回路总线接线端子标示注:ZX1+、 ZX2+: 回路1、2总线正极; ZX1、 ZX2-: 回路1、2总线负极;+24V 、GND :控制器由此端子输出24V 电压,供其他设备使用。
3 24V 电压输入接线标示注:控制器由此端子输入24V 电压,供回路巡检使用。
4 系统电源状态检测输入接线标示注:控制器由此端子输入主电、备电、充电等电源状态信号,供MCU 系统使用。
5 24V 电压输入接线标示注:控制器由此端子输入+5V 电压,供MCU 系统使用。
OZH280火灾报警控制器操作方法一、键盘说明主机面板上设有25只按键,其中数字键10只,功能键15只。
1、【消音】当有火警或故障时,扬声器发出火警或故障声,按下【消音】键,声音停止,同时点亮消音指示灯(黄色)。
2、【复位】键在菜单中,按下【复位】键,菜单向后退一级。
在厂标画面中,按下【复位】键,经确认后(按下【确认】键)执行复位操作。
3、【光标】【←】【→】用于移动光标或在综合界面中可切换当前显示项。
【↑】【↓】用于移动当前显示行。
4、【*】用于回路切换或编程组加1。
5、【#】用于回路切换或编程组减1。
6、【启动】【停止】用于启动、停止声光报警器。
7、【主菜单】用于进入主菜单画面。
8、【确认】用于执行所选功能。
9、【F2】用于自检操作,此功能是对控制器的所有发光二极管、声响器件,LCD显示器件进行检测。
10、【F1】----未用二、正常画面开机后,主机首先进行初始化,初始化完成后,自动进入厂标画面。
在此画面下,按【复位】键并确认后进行复位操作,按【主菜单】键进入主菜单画面。
三、异常画面异常画面中包括三个显示区,首火警区、声光报警器区、异常信息显示区。
当有异常情况发生都会出现如下画面,相应信息出现在相应的显示区内。
第一行:是首火警行。
“190”表示首火警的地址号是1回路190号。
表示此点是烟感,类型设置可在综合编程中修改;“2006/07/15 15:23”表示事件发生的日期和时间;第二行:是声光报警器行。
“208”表示声光报警器的地址号是2回路008号。
“2006/07/15 14:21”表示事件发生的日期和时间;“屏蔽”表示声光报警器的当前状态。
左边显示火警、屏蔽、监管、故障、启动、反馈的总数,右边显示信息的具体内容,显示格式是完全一样的,每类信息同时只显示一条。
通过*、#键可在这六项中切换当前显示的信息种类,通过上下键进行当前项内容的切换。
45秒无键盘输入时显示内容自动滚屏刷新。
显示内容的具体意义和首火警的说明是一样的。
四、主菜单注:打开键盘锁后,(使拨码第5位处于OFF位置)才能进行键盘操作。
按【主菜单】键进入主菜单。
主菜单主要包括八项一级菜单:信息查询、事件记录、通讯状态、系统设置、测试操作、启动操作、屏蔽操作、登记操作。
如下图所示,在此画面中可用光标键选择不同的功能项,按【确认】键执行相应的功能项。
除通讯状态和登记操作外其他每个菜单中又包含二级菜单。
通过上下光标键进行选择,按确认键即可进入相应功能菜单。
按复位键即可从下一级返回到上一级菜单。
(一)信息查询信息查询功能可查询火警、屏蔽、监管、故障、启动、反馈等异常信息(具体显示格式同异常画面);同时还可对部件信息和总线进行查询,其中部件信息查询包含三级菜单,可查询整机信息、各路信息、某路ID、某路电流、部件类型等信息。
(二)事件记录显示系统已经发生的火警、故障、启动、反馈、监管、操作、屏蔽和监管故障记录。
以上图火警记录为例,右上角为信息总页数和当前页数,每页4条记录。
每条记录所表示的意义如下:[001]15:28 奥瑞那公司生产部06年07月13日NO:001[001]探测器地址。
15:28火灾发生的时间。
06年07月13日火警发生的日期。
奥瑞那公司生产部报警部件安装位置描述,可在安装位置编程中修改。
表示火警触发部件的类型即感烟探测器。
类型设置可在综合编程中修改。
本系统用的部件类型共24种,可查阅部件信息中的部件类型。
NO:001 记录发生的顺序号。
(三)通讯状态(四)系统设置系统设置包括:时间设置、安装位置编程、联动与I、联动与II、多线联动设置、综合编程、联动或组、系统刷新八项功能。
1、时间设置:选择此菜单后按【确认】键,即可设置当前系统的日期和时间,完成设置后按【确认】键保存。
2、安装位置编程:描述部件具体的现场安装位置,用中文或数字表示,最多8个字。
具体内容是由实际安装位置决定,先输入部件的地址号,再采用区位码方式输入安装位置,编辑时,输入汉字或字母的区位码即可。
可查阅附表《区位码对照表》。
区码、位码都小于10时输入的是数字。
3、联动与I:如下图所示,联动与I共32组,每组输入条件1、条件2的表达式各为0~12个,每组输出模块数量为0~20个。
输入无误按【确认】键保存。
按#、*键可以切换浏览其他组的编程情况。
001~009:表示一回路的地址为1~9号的9个部件,205~213:表示二回路的地址为5~13号的9个部件,105~105:表示一回路的地址为105号的1个部件。
当条件1集合的任意一个或多个与条件2集合的任意一个或多个同时成立时,联动输出。
4、联动与II、联动或组:如下图所示,联动或组、联动与II共32组,每组输入条件的表达式各为0~12个,每组输出模块数量为0~30个。
输入无误按【确认】键保存。
按#、*键可以切换浏览其他组的编程情况。
001~009:表示一回路的地址为1~9号的9个部件,253~321:表示二回路的地址为53~121号的68个部件,105~105:表示一回路的地址为105号的1个部件。
当联动或组的联动条件集合的任意一个或多个成立时,联动输出。
当联动与II的联动条件集合的任意二个或多个成立时,联动输出。
5、多线联动设置:(待扩展)多线联动控制的设备类型在此界面进行编程,输入相应设备类型的代码即可;设备的安装位置在安装位置编程中进行,1~5号多线对应的安装位置编程地址为401~405号。
正确输入设备类型的代码后按【确认】键保存设置。
6、综合编程:当前行移到此行后按【确认】键,出现要求输入地址号界面,输入002号后再按【确认】键。
如下图第一行表示对1路123号编程。
类型:表示该点的属性。
此例001代表是烟感。
(具体某数所对应的是什么类型请见附表)。
安装位置:描述具体的现场安装位置,用八位汉字表示。
具体内容是由安装位置编程设定的。
显示盘:对于LED显示盘进行的编程,1201表示12号显示盘的01号灯。
对于LCD显示盘不需要编程。
注: A. 如连续编程,按【确认】键保存当前编程后地址号自动加1.B. 如果没有意外情况,类型和阈值一般不需要进行综合编程。
7.系统刷新:系统在初次使用时进行次操作,目的是清除FLASH内的所有记录、信息和各种编程信息,对存储器进行格式化,以便使用。
用光标选择相应的子菜单,按【确认】键进入要测试的画面,输入正确的测试部件地址号,按【确认】键即自动进行测试,并用曲线显示。
(六)启动操作用光标选择相应的子菜单,按【确认】键进入相应的子菜单的画面,输入正确的部件地址号,按【确认】键即自动进行启动/停止操作或进行模拟火警测试操作。
(七)屏蔽操作主要功能包括:当前故障屏蔽、选择地址屏蔽、解除所有屏蔽、选择地址解除四项操作。
1、当前故障屏蔽当某一或多个部件发生故障需隔离处理时,通过光标选择此项,按【确认】,待此项后出现“成功”时,故障隔离完成。
隔离后的部件不会再次发出火警、故障及联动信号。
2、选择地址屏蔽对某特定部件(故障或无故障状态)进行的选择性隔离操作。
若要对某一部件进行隔离,可用光标选择此项,按【确认】键进入子菜单,在选择地址隔离的画面中输入此部件的地址号XXX 号后按【确认】键即可。
退出画面后部件被隔离。
隔离后的部件不会发出火警、故障及联动信号。
3、解除所有屏蔽对当前所有隔离部件的一种解除操作,若要解除当前所有隔离,可通过光标选择此项,按【确认】键,待此项后出现“成功”字样时,当前所有被隔离部件被解除。
解除后的部件恢复到原有的状态。
4、选择地址解除对某特定隔离部件进行的选择性解除隔离操作。
若要对某一部件进行解除隔离,可用光标选择此项,在选择地址隔离的画面中输入此部件的地址号XXX号后按【确认】键,待此项后出现“成功”字样时,当前地址号部件被解除隔离。
解除后的部件恢复到原有的状态。
登记是在系统初始调试或系统内部位点有变化时需要进行的一种操作,它对系统内的部位点进行确认。