JACS-2010-132-6622-Protonated Titanate Nanotubes as Solid Acid Catalyst
- 格式:pdf
- 大小:186.93 KB
- 文档页数:2

异丙基二油酸酰氧基(二辛基磷酸酰氧基)钛酸酯一、化学名: 异丙基二油酸酰氧基(二辛基磷酸酰氧基)钛酸酯二、英文名: Isopropyl dioleic(dioctylphosphate) titanate三、CAS 编号: 61417-49-0四、分子式: C55H111O9Ti五、结构式:六、分子量: 963.0七、物化性质: 本品为无毒无腐蚀性液体,外观为酒红色粘稠液体。
组成复合型单烷氧基类钛酸酯技术指标密度≥0.950 g/c;粘度90±15%m/s;折光率N 1.478±0.005:闪点(开口)≥65℃; PH值4.5±0.5分解温度>240℃(与填料处理后分解温度300℃以上)。
UP-101与弱极性材料兼容性好,因此适用于非极性或弱极性聚合物,如:PE、PP等,以提高复合材料的机械强度及其它性能。
可溶于有机溶剂(如:异丙醇、二甲苯、甲苯、DOP、矿物油),遇水水解。
八、用途: 本品主要用于处理碳酸钙、滑石粉等无机填料,改善无机填料与树脂的兼容性,从而改善非极性或弱极性聚合物,如:PE、PP等复合材料制品的机械性能、加工性能,可提高复合材料的热稳定性,实现高填充。
用本品处理过的无机填料用于涂料中,可降低体系粘度、提高无机填料填充量。
用于磁记录材料和橡塑磁性材料,磁粉经它处理后,可改善其在基材上的分散以及对聚合物的粘合,使磁记录材料有较好的流动性、可涂性、高剪切强度、不易脱落,且韧性好。
九、注意事项:填料预处理后,若出料存放,应注意散热(搅拌热)以免填料受热性能下降。
本品非螯合型,不可与水接触,否则失效。
但填料中游离水份无影响。
十、包装: 25KG或200KG塑料桶装。
十一、贮存: 密封储存于阴凉、干燥通风处,避光、隔热。
异丙基三(二辛基磷酸酰氧基)钛酸酯英文名:Isopropyl tri(dioctylphosphate) titanateCAS 编号:65345-34-8分子式:C51H109O13P3Ti结构式:分子量: 1070.0物化性质: 本品为米黄色粘稠液体,密度(ρ20℃)1.01g/㎝3,可溶于异丙醇、苯、甲苯、二甲苯等有机溶剂,易水解,与增塑剂DOP反应,分解温度260℃。

Supporting InformationSynthesis and Reactivity of Iron Acyl Complexes Modeling the Active Site of [Fe]-HydrogenaseDafa Chen, Rosario Scopelliti, and Xile Hu*Laboratory of Inorganic Synthesis and Catalysis, Institute of Chemical Sciences and Engineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), SB-ISIC-LSCI, BCH 3305, Lausanne 1015, Switzerland.E-mail: xile.hu@epfl.chExperimental SectionA. Chemicals and ReagentsAll manipulations were carried out under an inert N2(g) atmosphere using glovebox techniques. Solvents were purified using a two-column solid-state purification system (Innovative Technology, NJ, USA) and transferred to the glove box without exposure to air. Deuterated solvents were purchased from Cambridge Isotope Laboratories, Inc., and were degassed and stored over activated 3 Å molecular sieves. All other reagents were purchased from commercial sources. Liquid compounds were degassed by standard freeze-pump-thaw procedures prior to use. Complex [PPN][Fe(COCH3)(CO4)] (1) was prepared according to literature method,1 but using [Li][Fe(COCH3)(CO4)]2 rather than [Na][Fe(COCH3)(CO4)]. Complexes [PPN]CN3 and [PPN][Fe(COCH3)(CO)3I2] (2)4 were prepared as described previously.B. Physical methodsThe 1H, 31P and 13C NMR spectra were recorded at 293 K on a Bruker Avance 400 spectrometer. 1H NMR chemical shifts were referenced to residual solvent as determined relative to Me4Si (δ = 0 ppm). The 31P{1H} chemical shifts were reported in ppm relative to external 85% H3PO4. The 13C{1H} chemical shifts were reported in ppm relative to the carbon resonance of CDCl3 (77.0 ppm). IR spectra were recorded on solid samples on a Varian 800 FT-IR spectrometer using ATR sampling techniques. Elemental analyses were performed on a Carlo Erba EA 1110 CHN instrument at EPFL. X-ray diffraction studies were carried out in the EPFL Crystallographic Facility. Data collections were performed at low temperature using four-circle kappa diffractometers equipped with CCD detectors. Data were reduced and then corrected for absorption.5 Solution, refinement and geometrical calculations for all crystal structures were performed by SHELXTL.6C. Synthetic methodsSynthesis of [PPN][Fe(COCH3)(CO4)] (1)CH3Li in ether (1.6 M, 5 mL, 8.0 mmol) was added into Fe(CO)5 (1.57 g, 8.0 mmol) in 30 mL ether at -60 °C. After being warmed to room temperature slowly (about 4 h), the solution was added into a solution of [PPN]Cl (4.59 g, 8.0 mmol) in 30 mL CH2Cl2. The resulting mixturewas stirred for 1 h and then the solvent was evaporated. The residual solid was extracted withCH2Cl2 (30 mL). After removing about half of the solvent, 20 mL ether was added to precipitate the product [PPN][Fe(COCH3)(CO4)] (1) (5.63 g, 7.52 mmol, 94%) as white solid.Synthesis of [Fe(COCH3)(CO)2(MePyS)]2 (MePySH = 6-methyl-2-mercaptopyridine) (3) MePySNa (180 mg, 1.22 mmol) was added into a solution of [PPN][Fe(COCH3)(CO)3I2] (1.19 g, 1.22 mmol) (152 mg, 1.22 mmol) in ether (5 mL) under stirring. The resulting solution was stirred for 1.5 h and then the solvent was evaporated in vacuum. The residue solid was extracted with benzene (10 mL). After removing the solvent of the filtrate, the residue was recrystallized from dichlomethane/ether at -30 °C to afford 3 (290 mg, 0.52 mmol, 85%) as orange crystals.1H NMR (400.13 MHz, CDCl): 7.13 (t, J = 7.2 Hz, 2H), 6.65 (d, J = 7.2 Hz, 2H), 6.31 (d, J3= 7.2 Hz, 2H), 2.68 (s, 6H), 2.45 ppm (s, 6H). 13C NMR (100.62 MHz, CDCl3): 255.9 (C OCH3), 214.4 (terminal C O), 208.5 (terminal C O), 167.3, 159.3, 137.1, 125.2, 121.1, 46.5, 22.5 ppm. IR (νCO, cm-1): 2019 (s), 1996 (s), 1955 (s), 1934 (s), 1661 (s, CO CH3), 1612 (s, CO CH3). Anal. Calcd for C20H18Fe2N2O6S2: C, 43.03; H, 3.25; N, 5.02. Found: C, 42.95; H, 3.48, N, 4.92.Synthesis of [Fe(COCH3)(CO)2(2,6-Me2C6H3NC)(MePyS)] (4)2,6-dimethylphenyl isocyanide (2,6-Me2C6H3NC) (17.2 mg, 0.13 mmol) was added into a solution of 3 (36.6 mg, 0.066 mmol) in CH2Cl2 (2 mL). After 2 minutes, pentane (5 mL) was added into this solution. The resulting solution was put into a freezer at -30 °C to afford 4 (17.0 mg, 0.041 mmol, 32%) as yellow crystals.1H NMR (400.13 MHz, CDCl): 7.26-7.05 (m, 2H), 7.08 (d, J = 7.2 Hz, 2H), 6.67 (d, J = 8.03Hz, 1H), 6.59 (d, J = 8.0 Hz, 1H), 2.53 (s, 3H), 2.29 (s, 6H), 2.28 ppm (s, 3H). IR (νCO/NC, cm-1): 2164 (s, NC), 2016 (s, terminal CO), 1968 (s, terminal CO), 1620 (s, CO CH3). Anal. Calcd for C19H18FeN2O3S: C, 55.62; H, 4.42; N, 6.83. Found: C, 55.20; H, 4.72, N, 6.49.Synthesis of [PPN][Fe(COCH3)(CO)2(CN)(MePyS)] (5)Using a similar procedure as described above. [PPN]CN (91.6 mg, 0.16 mmol) was added into a solution of 3 (45.3 mg, 0.081 mmol) in CH2Cl2 (2 mL). After 2 minutes, pentane (10 mL) was added into this solution. The oily precipitate was isolated and dried in vacuum to afford 5 (128 mg, 0.15 mmol, 94%) as yellow solid.1H NMR (400.13 MHz, CDCl): 7.79-7.40 (m, 30H), 7.02 (t, J = 8.0 Hz, 1H), 6.53 (d, J = 8.03Hz, 1H), 6.39 (d, J = 8.0 Hz, 1H), 2.60 (s, 3H), 2.26 ppm (s, 3H). 31P NMR (162 MHz, CDCl3): 21.7 ppm. 13C NMR (100.62 MHz, CDCl3): 270.1 (C OCH3), 214.6 (terminal C O), 211.6 (terminal C O), 176.6, 158.1, 153.9, 134.6, 134.0, 132.2, 132.1, 132.0, 129.8, 129.7, 129.6, 127.5, 126.4, 121.6, 115.3, 46.2, 22.8 ppm. IR (νCO/CN, cm-1): 2109 (w, CN), 1998 (s, terminal CO), 1932 (s, terminal CO), 1607 (s, CO CH3). ESI-MS: m/z 304.9; calcd for (C11H9FeN2O3S)-: 305.0. Anal. Calcd for C47H39FeN3O3P2S: C, 66.91; H, 4.66; N, 4.98. Found: C, 66.08; H, 5.00, N, 4.80. Synthesis of [Et4N][Fe(COCH3)(CO)2(CN)(MePyS)] (5’)Using a similar procedure as described above. [Et4N]CN (85.0 mg, 0.54 mmol) was added into a solution of 3 (45.3 mg, 0.27 mmol) in CH2Cl2 (2 mL). After 2 minutes, ether (10 mL) was added into this solution. The oily precipitate was isolated and dried in vacuum to afford 5’ (210 mg, 0.48 mmol, 89%) as yellow oil.1H NMR (400.13 MHz, CDCl): 7.09 (t, J = 6.8 Hz, 1H), 6.53 (d, J = 6.8 Hz, 1H), 6.47 (d, J3= 6.8 Hz, 1H), 3.22 (br s, 8H), 2.58 (s, 3H), 2.34 (s, 3H), 1.25 ppm (br s, 12H). 13C NMR (100.62 MHz, CDCl3): 267.5 (C OCH3), 215.1 (terminal C O), 211.2 (terminal C O), 175.7, 158.5, 154.4, 135.1, 121.4, 115.8, 53.0, 46.5, 22.9, 8.1 ppm. IR (νCO/CN, cm-1): 2107 (w, CN), 1998 (s, terminal CO), 1930 (s, terminal CO), 1605 (s, CO CH3). ESI-MS: m/z 304.9; calcd for (C11H9FeN2O3S)-: 305.0. Anal. Calcd for C19H29FeN3O3S: C, 52.42; H, 6.71; N, 9.65. Found: C, 51.20; H, 6.82, N, 9.41.Synthesis of [Fe(COCH3)(CO)2(PPh3)(MePyS)] (6)PPh3 (38.3 mg, 0.15 mmol) was added into a solution of 3 (40.8 mg, 0.073 mmol) in CH2Cl2 (2 mL). After 2 minutes, the solvent was evaporated in vacuum. The residue was recrystallized from dichlomethane/pentane at -30 °C to afford 6 (73.2 mg, 0.14 mmol, 93%) as orange crystals.1H NMR (400.13 MHz, CDCl): 7.52-7.17 (m, 15H), 6.91 (t, J = 7.6 Hz, 1H), 6.39 (d, J = 7.63Hz, 1H), 5.98 (d, J = 7.6 Hz, 1H), 2.86 (s, 3H), 2.23 ppm (s, 3H). 31P NMR (162 MHz, CDCl3): 36.0 ppm. 13C NMR (100.62 MHz, CDCl3): 256.3 (C OCH3), 214.7 (terminal C O), 211.7 (terminal C O), 176.0, 158.3, 135.6, 133.4, 133.3, 129.8, 128.2, 128.1, 123.5, 117.5, 46.5, 22.6 ppm. IR (νCO, cm-1): 2003 (s), 1931 (s), 1636 (s, CO CH3). Anal. Calcd for C28H24FeNO3PS: C, 62.12; H, 4.47; N, 2.59. Found: C, 61.68; H, 4.89, N, 2.47.Reaction of 3 with CO: Equilibrium between [Fe(COCH3)(CO)3(MePyS)] (7A+7B) and 3A solution of 3 (10 mg, 0.018 mmol) in 0.5 mL CDCl3 was added to a J Young NMR tube. The solution was frozen and the tube was evacuated under vacuum. 1 atm of CO was added and the tube was sealed. The solution was thawed and a 1H NMR spectrum was obtained within 2 min. The 1H NMR spectrum showed two isomers in a ratio of about 3:1. The tube was then opened in glove box and there was a little precipitate in the tube because of decomposition. After filtration, the filtrate was evaporated in vacuum and 3 (8.0 mg, 0.014 mmol, 80%) was recovered.Synthesis of [Fe(13COCH3)(13CO)3(MePyS)] (7A+7B) and [Fe(13COCH3)(13CO)2(MePyS)]2 (3*)A solution of 3 (10 mg, 0.018 mmol) in 0.5 mL CDCl3 was added to a J Young NMR tube. The solution was frozen and the tube was evacuated under vacuum. 1 atm of 13CO was added and the tube was sealed. 1H and 13C NMR spectra were obtained within 2 min after the solution was thawed, which showed two isomers in a ratio of about 3:1 (fac- : mer-), suggesting the formation of [Fe(13COCH3)(13CO)3(MePyS)] (7A+7B).The tube was opened in glove box, then filled with 13CO and opened in glove box again to make sure the complex 3 was totally turned into [Fe(13COCH3)(13CO)2(MePyS)]2 (3*) (7.0 mg, 0.013 mmol, 70%).3* can be converted back to 3 by two similar cycles of 12CO charge/discharge.Major isomer (7A): 1H NMR (400.13 MHz, CDCl3): 7.33 (m, 1H), 6.69 (m, 2H), 2.81 (s, 3H), 2.40 (s, 3H). 13C NMR (100.62 MHz, CDCl3): 248.8 (C OCH3), 208.5 (terminal C O), 206.0 (terminal C O), 204.8 (terminal C O).Minor isomer (7B): 1H NMR (400.13 MHz, CDCl3): 7.33 (m, 1H), 6.58 (m, 2H), 2.49 (s, 3H), 2.24 (s, 3H). 13C NMR (100.62 MHz, CDCl3): 251.2 (C OCH3), 214.4 (terminal C O), 207.3 (terminal C O).3*: IR (νCO, cm-1): 1975 (s), 1952 (s), 1911 (s), 1892 (s), 1623 (s, CO CH3), 1578 (s, CO CH3). Attempts for H2 activation with 3.The reactivity of complex 3 with H2 was investigated. No reaction occurred under up to 50 bar of H2, and no Fe-H2 adduct could be detected by high-pressure NMR. Addition of a base such as 2,6-dimethylpyridine did not lead to H2 activation. Likewise, no hydrogenation of unsaturated molecules such as styrene and PhCH=NCH2Ph took place in the presence of 3.D. Crystallographic Details for [Fe(COCH3)(CO)2(MePyS)]2 (3)A total of 13819 reflections (-13 < h < 14, -13 < k < 14, -21 < l < 22) were collected at T = 140(2) K in the range of 3.26 to 27.87o of which 5001 were unique (R int = 0.0295); Mo Kαradiation (λ= 0.71073 Å). The structure was solved by the Direct method. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated idealized positions. The residual peak and hole electron densities were 0.320 and –0.244 eA-3, respectively. The absorption coefficient was 1.506 mm-1. The least squares refinement converged normally with residuals of R(F) = 0.0266, wR(F2) = 0.0468 and a GOF = 0.973 (I>2σ(I)). C20H18Fe2N2O6S2, Mw = 558.18, space group P32, Trigonal, a = 10.7960(2), b = 10.7960(2), c = 16.8101(5) Å, V = 1696.78(7) Å3, Z = 3, ρcalcd = 1.639 Mg/m3.E. Crystallographic Details for [Fe(COCH3)(CO)2(PPh3)(MePyS)] (6)A total of 19385 reflections (-14 < h < 14, -21 < k < 21, -16 < l < 15) were collected at T = 140(2) K in the range of 2.85 to 26.37o of which 5112 were unique (R int = 0.0412); Mo Kαradiation (λ= 0.71073 Å). The structure was solved by the Direct method. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were placed in calculated idealized positions. The residual peak and hole electron densities were 0.472 and –0.300 eA-3, respectively. The absorption coefficient was 0.775 mm-1. The least squares refinement converged normally with residuals of R(F) = 0.0388, wR(F2) = 0.0839 and a GOF = 1.038 (I>2σ(I)). C28H24FeNO3PS, Mw = 541.36, space group P21/n, Monoclinic, a = 11.4641(5), b = 17.3380(8), c = 13.0286(6) Å, β = 103.027(5)°, V = 2523.0(2) Å3, Z = 4, ρcalcd = 1.425 Mg/m3.Table S1. Selected IR data for complexes 3-6.Complex νCO(cm-1)νacyl(cm-1)νCN(cm-1)32019,19961955,19341661,16124 2016,1968 16205 1998,1932 160721096 2003,1931 1636hmd a2011,1944Extractedcofactor a2031,1972CN--inhibited hmd a 2020,1956 20913* 1975,19521911,18921623,1578a from reference 5.25024023022021020013C Chemical Shifts / ppmFigure S1. 13C NMR spectrum of the 13CO adducts of 3; the scan duration was 2 minutes.Figure S2. Crystal structure of complex 3.Selected bond lengths (Å): Fe(1)-C(15): 1.769(3); Fe(1)-C(16): 1.771(3); Fe(1)-N(1):1.9859(19); Fe(1)-C(13): 1.991(2); Fe(1)-S(1):2.3584(7); Fe(1)-S(2): 2.4786(6); Fe(2)-C(20):1.770(3); Fe(2)-C(17): 1.777(3); Fe(2)-C(18): 1.990(2); Fe(2)-N(2):2.0044(18); Fe(2)-S(2):2.3699(7); Fe(2)-S(1): 2.4507(7); O(2)-C(15): 1.149(3); O(3)-C(16): 1.149(3); O(4)-C(17): 1.138(3); O(6)-C(20): 1.147(3).Bond angle (o):Fe(1)-S(1)-Fe(2): 93.82(2); Fe(2)-S(2)-Fe(1): 92.83(2); C(20)-Fe(2)-C(17):87.03(11); C(15)-Fe(1)-C(16): 87.87(12).Figure S3. Crystal structure of complex 6.Selected bond lengths (Å): Fe(1)-C(25): 1.753(3); Fe(1)-C(26): 1.793(3); Fe(1)-N(1):2.0111(19); Fe(1)-C(27): 2.016(2); Fe(1)-S(1): 2.3441(7); Fe(1)-P(1): 2.3513(6); O(1)-C(25) : 1.151(3); O(2)-C(26): 1.116(3).Bond angle (o):C(25)-Fe(1)-C(26): 92.24(11).Figure S4. IR spectrum of Fe(COCH3)(CO)2(MePyS)]2 (3) in the solid state.Figure S5. IR spectrum of [Fe(COCH3)(CO)2(2,6-Me2C6H3NC)(MePyS)] (4) in the solidstate.Figure S6. IR spectrum of [PPN][Fe(COCH3)(CO)2(CN)(MePyS)] (5) in the solid state.Figure S7. IR spectrum of [Et4N][Fe(COCH3)(CO)2(CN)(MePyS)] (5’) in the solid state.Figure S8. IR spectrum of [Fe(COCH3)(CO)2(PPh3)(MePyS)] (6) in the solid state.Figure S9. IR spectrum of [Fe(13COCH3)(13CO)3(MePyS)] (3*) in the solid state.Figure S10. 1H NMR spectrum of [Fe(COCH 3)(CO)3(MePyS)] (7A+7B ) in CDCl 3.50000100000150000255.926214.350208.493ppm (f1)501001502002500500256.187214.275208.498Figure S11. 13C NMR spectra of [Fe(COCH 3)(CO)3(MePyS)] (3) (top) and[Fe(13COCH 3)(13CO)3(MePyS)] (3*) (bottom) in CDCl 3.References(1) Alper, H.; Tanaka, M. J. Am. Chem. Soc.1979, 101, 4245-4249.(2) Brunet, J. J.; Chauvin, R.; Donnadieu, B. ; Thepaut, E. J. Organomet. Chem.1999, 579,198-205.(3) Martinsen, A.; Songstad, S. Acta Chem. Scand., Ser. A1977, 31, 645-650.(4) Mitsudo, T.; Ishlhara, A.; Suzuki, T.; Watanabe, Y.; Masuda, H. Organometallics 1990,9, 1357-1358.Acta Crystallogr. A 1995, 51, 33-38.(5) Blessing,H.R.(6) Sheldrick, G. M. SHELXTL release 6.1.4 ed.; Bruker AXS Inc.: Madison, Wisconsin,53719, USA, 2003.。

专利名称:反式-9,10-脱氢埃坡霉素C和D,其类似物以及制备这些化合物的方法
专利类型:发明专利
发明人:李勇,K·森德曼,唐莉,D·迈尔斯
申请号:CN200380103451.8
申请日:20031107
公开号:CN1711259A
公开日:
20051221
专利内容由知识产权出版社提供
摘要:本发明提供新的由反式-9,10-脱氢埃坡霉素C和反式-9,10-脱氢埃坡霉素D为基础衍生的化合物、组合物和体外抑制细胞超增殖和/或稳定微管的方法,以及体内治疗超增殖疾病的方法。
还公开了这些化合物的制备方法。
申请人:科桑生物科学公司
地址:美国加利福尼亚
国籍:US
代理机构:中国国际贸易促进委员会专利商标事务所
代理人:顾颂逦
更多信息请下载全文后查看。
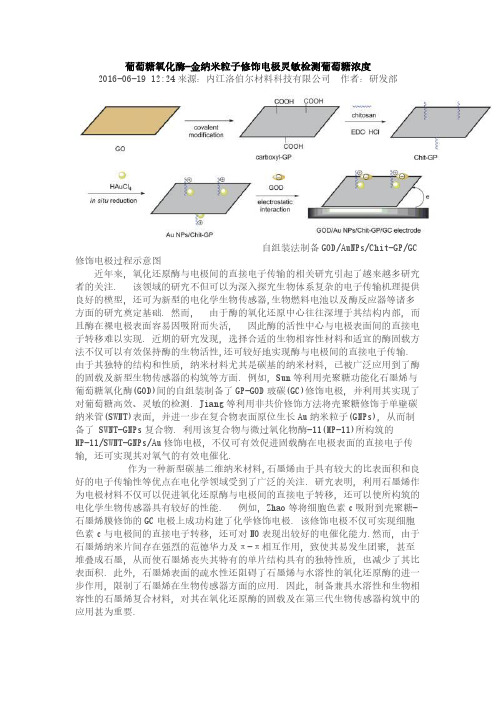
葡萄糖氧化酶-金纳米粒子修饰电极灵敏检测葡萄糖浓度2016-06-19 12:24来源:内江洛伯尔材料科技有限公司作者:研发部自组装法制备GOD/AuNPs/Chit-GP/GC 修饰电极过程示意图近年来, 氧化还原酶与电极间的直接电子传输的相关研究引起了越来越多研究者的关注. 该领域的研究不但可以为深入探究生物体系复杂的电子传输机理提供良好的模型, 还可为新型的电化学生物传感器,生物燃料电池以及酶反应器等诸多方面的研究奠定基础. 然而, 由于酶的氧化还原中心往往深埋于其结构内部, 而且酶在裸电极表面容易因吸附而失活, 因此酶的活性中心与电极表面间的直接电子转移难以实现. 近期的研究发现, 选择合适的生物相容性材料和适宜的酶固载方法不仅可以有效保持酶的生物活性,还可较好地实现酶与电极间的直接电子传输. 由于其独特的结构和性质, 纳米材料尤其是碳基的纳米材料, 已被广泛应用到了酶的固载及新型生物传感器的构筑等方面. 例如, Sun等利用壳聚糖功能化石墨烯与葡萄糖氧化酶(GOD)间的自组装制备了GP-GOD玻碳(GC)修饰电极, 并利用其实现了对葡萄糖高效、灵敏的检测. Jiang等利用非共价修饰方法将壳聚糖修饰于单壁碳纳米管(SWNT)表面, 并进一步在复合物表面原位生长Au纳米粒子(GNPs), 从而制备了 SWNT-GNPs复合物. 利用该复合物与微过氧化物酶-11(MP-11)所构筑的MP-11/SWNT-GNPs/Au修饰电极, 不仅可有效促进固载酶在电极表面的直接电子传输, 还可实现其对氧气的有效电催化.作为一种新型碳基二维纳米材料,石墨烯由于具有较大的比表面积和良好的电子传输性等优点在电化学领域受到了广泛的关注. 研究表明, 利用石墨烯作为电极材料不仅可以促进氧化还原酶与电极间的直接电子转移, 还可以使所构筑的电化学生物传感器具有较好的性能. 例如, Zhao等将细胞色素c吸附到壳聚糖-石墨烯膜修饰的GC电极上成功构建了化学修饰电极. 该修饰电极不仅可实现细胞色素c与电极间的直接电子转移, 还可对NO表现出较好的电催化能力.然而, 由于石墨烯纳米片间存在强烈的范德华力及π-π相互作用, 致使其易发生团聚, 甚至堆叠成石墨, 从而使石墨烯丧失其特有的单片结构具有的独特性质, 也减少了其比表面积. 此外, 石墨烯表面的疏水性还阻碍了石墨烯与水溶性的氧化还原酶的进一步作用, 限制了石墨烯在生物传感器方面的应用. 因此, 制备兼具水溶性和生物相容性的石墨烯复合材料, 对其在氧化还原酶的固载及在第三代生物传感器构筑中的应用甚为重要.辽宁大学绿色合成与先进材料制备化学辽宁省重点实验室张谦等人通过共价键作用和原位还原法制备了金纳米粒子/壳聚糖-石墨烯纳米复合材料(AuNPs/Chit-GP). 利用FT-IR, UV-vis, TEM 以及XRD对所合成的纳米复合物的结构和形貌进行了表征. AuNPs/Chit-GP呈现明显的正电荷, 因此可通过静电相互作用固载葡萄糖氧化酶(GOD),并构建GOD/AuNPs/Chit-GP/GC修饰电极. 该修饰电极不仅可成功地实现GOD与电极间的直接电子转移, 还对葡萄糖表现出良好的催化性能. 实验结果表明, 其催化的线性范围为2.1~5.7μmol/L, 检出限为0.7μmol/L, 灵敏度为 79.71 mA•cm-2•mM-1.这种集金属纳米粒子、生物相容性高分子以及石墨烯为一体的纳米复合物的构筑为无媒介体的电化学生物传感器的研究提供了一个良好的平台.。
拓扑替康结构式-回复拓扑替康(Tofacitinib)是一种属于JAK抑制剂的药物,用于治疗关节炎、银屑病和溃疡性结肠炎等自身免疫性疾病。
该药物的结构式如下所示:[拓扑替康结构式]拓扑替康属于一种小分子化合物,其化学名称为“3-{(3R,4R)-4-[(4-{(1E)-2-(5-氯-2-氧代苯基)乙-1-烯基}-1-苯基环己基)氨基]-3-甲基环己基}丙酸甲酯”。
它的化学式为C16H20N6O,分子量为312.37g/mol。
拓扑替康为无色结晶状固体,可溶于有机溶剂或水。
作用机制:拓扑替康通过抑制Janus激酶(JAK)的活性来发挥其治疗作用。
JAK是一类细胞内酪氨酸激酶,对细胞信号转导和调控起着重要作用。
通过抑制JAK的活性,拓扑替康可以干扰多个细胞因子途径的信号传递,从而调控免疫系统,减轻相关疾病的症状。
药物应用:拓扑替康已被美国食品药品监督管理局(FDA)批准用于治疗成人类风湿性关节炎、银屑病性关节炎、中度至重度溃疡性结肠炎以及活动性类风湿性关节炎。
它通常在其他治疗方法无效或无法耐受的情况下使用。
治疗效果:在临床试验中,拓扑替康已被证明能有效减少关节炎相关的关节疼痛、关节肿胀和关节运动受限等症状。
在银屑病和溃疡性结肠炎的治疗中,使用拓扑替康也能减少病情的恶化和复发。
注意事项:1. 在使用拓扑替康之前,患者应告知医生有关其过敏史、其他药物的使用情况以及存在的其他疾病,以便医生进行全面评估。
2. 拓扑替康可能会增加感染的风险。
在使用期间,患者应密切注意任何感染的症状,并及时向医生报告。
3. 服用拓扑替康可能导致一些不良反应,如头痛、腹泻、恶心、呕吐等。
如有不适,应立即告知医生。
总结:拓扑替康是一种抗关节炎和免疫性疾病药物,通过抑制JAK的活性,调控免疫系统,从而减轻相关疾病的症状。
尽管其可以有效改善患者的症状,但患者在使用该药物时需要密切关注可能产生的不良反应,并向医生报告任何异样症状。
通过科学的用药指导和临床监测,拓扑替康可以为患者提供更好的治疗效果。
Mesoporous Titania Spheres with Tunable Chamber Stucture and EnhancedPhotocatalytic ActivityHexing Li,*,†Zhenfeng Bian,†Jian Zhu,†Dieqing Zhang,†Guisheng Li,†Yuning Huo,†Hui Li,†andYunfeng Lu*,‡Department of Chemistry,Shanghai Normal Uni V ersity,Shanghai200234,China,and Chemical and Biomolecular Engineering Department,Uni V ersity of California,Los Angeles,California90095Received March28,2007;E-mail:hexing-li@;luucla@Many natural materials,like seashell and lotus leaf,are composedonly with ordinary composition but exhibit fascinating propertyowing to their unique structure.1Such intricate natural designs haveinspired extensive research in synthesizing materials with controlledstructure and morphology,with expectations of achieving novel orenhanced properties.2,3This work,for the first time,reports thesynthesis of hollow titania spheres with tunable interior structureand urchinlike morphology and examines their photocatalyticactivity enhanced by such unique structure.Hollow spheres,generally,are of great interest for a largespectrum of applications,such as adsorbents,delivery carriers,catalysts,and biomedical uses.4-6Their synthesis often relies ontemplating approaches,in which hard(e.g.,inorganic,metal,andpolymer particles)or soft sacrificial templates(e.g.,supramolecularassemblies of surfactant and polymer)were used to create a hollowstructure.7-12The template approach can be easily implemented;however,the capability of constructing complicated structure islimited by the availability of a template.As an alternative,template-free approaches based on different mechanisms were also developedto synthesize hollow spheres with more complicated structure.13-16To synthesize the hollow titania spheres with such complicatedstructure and morphology,we adapted a template-free approach,in which a titania precursor,TiOSO4,was solvothermally reactedin glycerol,alcohol,and ethyl ether.Judicious choice of the alcoholmolecules(e.g.,methanol,ethanol,and propanol)and reaction timeaffords the synthesis of spheres with adjustable morphology,size,and interior structure that is tunable from solid,sphere-in-sphere,to hollow(see Supporting Information).Figure1shows electron micrographs depicting the structuralevolution with reaction time.Uniform,smooth,solid spherical particles(a)were obtained after1-hour reaction.Small surface platelets(b)were formed after12h and further grow with time, forming an urchinlike prickly surface(c-f).It was also found that the platelets formed shells on the solid cores,creating a core-shell structure(c)after1-day reaction.Interestingly,these cores shrink with time,forming a unique sphere-in-sphere structure with continuously reducing innersphere size(d-f).The innerspheres finally diminished after14-days of reaction and created a hollow spiny structure(f).Accompanying this interesting structural evolu-tion is the growth of the outside-sphere diameter from1.6to3.1µm(Table S1).The outside-sphere diameter can also be tuned by choosing the alcohol molecules.For example,the spheres synthe-sized with methanol,ethanol,and propanol under similar conditions for2days display a similar sphere-in-sphere structure and urchinlike morphology,while their outside-sphere diameter systematically increases from2.0and2.4to5.2µm(Figure S1).Although the as-synthesized spheres are amorphous,they turn to be highly crystalline(anatase)after calcination at550°C for3h while maintaining their sphere-in-sphere structure,as confirmed by X-ray diffraction(XRD),high-resolution TEM,and selected area electron diffraction studies(Figures S2-3).Such a spiny structure possesses mesoporous networks evidenced by the nitrogen sorption experiment(Figure S4).Surface area and pore volume of the uncalcined spheres increase from46to167 m2/g and0.11to0.32cm3/g,respectively,when reaction time was increased from0.5to2days,and remain similar or slightly decrease for a longer reaction time(Table S1).Such porous structure provides efficient transport pathways to their interior voids,which is critical for catalyst,delivery,and other applications.More importantly,the unique sphere-in-sphere structure allows multireflections of elec-tromagnetic waves,such as ultraviolet and visible light,within their interior cavities,endowing these spheres with greatly enhanced properties.To demonstrate this intricate structure-function correction,we examined photocatalytic activity of the calcined spheres using phenol degradation as a probe reaction(Figure2).The solid spheres (12h,also see Figure1a)show the lowest activity of55%while other spheres show significantly higher activities.In particular,the spheres with sphere-in-sphere structure(2day,denote as P2,also†Shanghai Normal University.‡University ofCalifornia.Figure1.SEM and TEM(insets)images of the titania spheres synthesizedfor(a)1/24,(b)0.5,(c)1,(d)2,(e)7,and(f)14days,showing transitinginterior structure from dense,to sphere-in-sphere,to hollow and surfacemorphology from smooth toprickly.Published on Web06/16/200784069J.AM.CHEM.SOC.2007,129,8406-840710.1021/ja072191c CCC:$37.00©2007American Chemical Societysee Figure 1d)show an activity as high as 93%.Since all these spheres possess similar crystalline structure (Figure S2)and surface area (e.g.,32m 2/g for the solid spheres and 62m 2/g for P 2,see Table S1),such dramatic activity enhancement is raised from the unique sphere-in-sphere structure.In fact,destroying the sphere-in-sphere structure by grinding the spheres slightly increases the surface area from 62to 68m 2/g;however,the crushed sample showed a dramatically decreased activity of 57%,similar to that of the solid spheres.As schematically illustrated in the inset,we believe that a sphere-in-sphere structure with an appropriate innersphere diameter allows multiple reflections of UV light within the interior cavity,allowing more efficient use of the light source and therefore offering an improved catalytic activity (Figure S7).The spheres synthesized with 7-day reaction (Figure 1e)display a lower activity (87%)than P 2,possibly because of their smaller innerspheres that reduce reflection within their interior cavity.Consistently,the hollow spheres (14day,also see Figure 1f)show even lower activity (83%)because of the even less reflections.The above results unambiguously suggest that such unique structure provides titania with greatly enhanced photocatalytic activity.More importantly,this multiple-reflection concept may be generalized to the design and fabrication of novel materials with enhanced pro-perties for microelectronics,optoelectronics,and other applications.The formation of such spheres may involve aggregation of titania building clusters into spheres and their subsequent reaction,dissolution,and re-deposition process.During the solvothermal condition,etherifying reactions between alcohol and glycerol produce water continuously.Titania building clusters may be generated through alcoholysis reaction or hydrolysis -condensation reactions of TiOSO 4.As-formed clusters aggregate and react forming solid spheres that contain a large number of hydrolyzable ligands (e.g.,-SO 4,glyceroxy,and ethoxy groups)possibly due to slow reaction kinetics.With increasing reaction time,water is produced continuously through the etherifying reactions and reacts with the spheres,leading to the dissolution and rearrangement of the surface building clusters.As a result from this process,surface plates (Figure 1b)were initially formed and grown into a thin shell layer (Figure 1c).Continuation of this process gradually dissolves the core spheres,sequentially creating the observed sphere-in-sphere and hollow structure.The occurrence of etherifying reactions was clearly proved by GC-MAS and NMR studies,evidenced from the formation of ethyl ether and the ethers from ethanol and glycerol (Figure S5).It is worth pointing out that reacting TiOSO 4with glycerol in the absence of ethanol could not lead to any precipitant,possibly because of a steric effect that hinders the etherifying or alcoholysis reactions.Similarly,the presence of hydrolyzable ligands within the spheres was also confirmed by TGA -DTA analysis.As shown in Figure S6,the spheres after 1-hour reaction show multiple exothermicpeaks and a weight loss of 60%;while the spheres after 2-day reaction only show a single exothermic peak and a lower weight lost of 49%.Consistent with the TGA analysis,energy dispersive X-ray spectroscopy (EDXS)analysis suggests an increasing Ti and decreasing carbon composition with increasing reaction time (Table S2).The presence of these hydrolyzable moieties allows redisso-lution and re-deposition of the building clusters upon further reactions.Therefore,the formation of larger spheres is expected from building clusters containing more and larger removal ligands.Indeed,as shown in Figure S1,increasing the carbon-chain length of the alcohol slows the alcoholysis and etherifying reactions and produces building clusters with more removal ligands,which leads to the formation of larger spheres.It is also worth mentioning that replacing TiOSO 4with other titanium sources,such as tetrachloride,tetrabutyl titanate,and tetraisopropyl titanate,only resulted in solid particles.This is possibly attributed to the sulfuric acid produced from the alcoholysis reaction or the sulfuric group itself that catalyzes the etherifying reactions.Meanwhile,we discovered that use of ethyl ether as a cosolvent is essential to ensure the formation of such a unique structure.The optimum molar ratio of ethyl ether/TiOSO 4was around 11,and only dense particles were obtained for an ethyl ether/TiOSO 4ratio higher than 20or less than 5.More detailed investigation is under way.In summary,we have demonstrated the synthesis of photocata-lytic hollow titania spheres with unique urchinlike morphology and tunable interior structure.As-created sphere-in-sphere structure endows the spheres with greatly enhanced photocatalytic activity possibly attributed to multiple reflections of UV light within the sphere interior voids.This work provides a novel pathway to the synthesis of hollow spheres with complicated structure,offering a new material platform for catalyst,microelectronic,and other applications.Acknowledgment.This work is supported by the National Natural Science Foundation of China (20377031),Science and Technology Ministry of China (2005CCA01100),Shanghai Leading Academic Discipline Project (No.T0402),National Science Foundation (CAREER),and Sandia National Laboratories.Supporting Information Available:Detailed information on the sphere synthesis,activity characterization,XRD,sorption,GC-MAS,NMR,TEM,TGA,and EDS studies.This material is available free of charge via the Internet at .References(1)Douglas,T.Science 2003,299,1192.(2)Lu,Y.F.;Fan,H.Y.;Stump,A.;Ward,T.L.;Rieker,T.;Brinker,C.J.Nature 1999,398,223.(3)Cha,J.N.;Stucky,G.D.;Morse,D.E.;Deming,T.J.Nature 2000,403,289.(4)Nakashima,T.;Kimizuka,N.J.Am.Chem.Soc.2003,125,6386.(5)Caruso,F.;Caruso,R.A.;Mo ¨hwald,H.Science 1998,282,1111.(6)Yin,Y.D.;Rioux,R.M.;Erdonmez,C.K.;Hughes,S.;Somorjai,G.A.;Alivisatos,A.P.Science 2004,304,711.(7)Mitchell,D.T.;Lee,S.B.;Trofin,L.;Li,N.C.;Nevanen,T.K.;Soderlund,H.;Martin,C.R.J.Am.Chem.Soc.2002,124,11864.(8)Dinsmore,A.D.;Hsu,M.F.;Nikolaides,M.G.;Marquez,M.;Bausch,A.R.;Weitz,D.A.Science 2002,298,1006.(9)Caruso,R.A.;Schattka,J.H.;Greiner,A.Ad V .Mater.2001,13,1577.(10)Valtchev,V.Chem.Mater.2002,14,956.(11)Qi,L.M.;Li,J.;Ma,J.M.Ad V .Mater.2002,14,300.(12)Peng,Q.;Dong,Y.;Li,Y.Angew.Chem.,Int.Ed.2003,42,3027.(13)Guo,C.W.;Cao,Y.;Xie,S.H.;Dai,W.L.;Fan,mun.2003,700.(14)Afanasiev,P.;Bezverkhy,I.J.Phys.Chem.B 2003,107,2678.(15)Yin,Y.;Rioux,R.M.;Erdonmez,C.K.;Hughes,S.;Somorjai,G.A.;Alivisatos,A.P.Science 2004,304,711.(16)(a)Yang,H.G.;Zeng,H.C.J.Phys.Chem.B 2004,108,3492.(b)Chang,Y.;Teo,J.J.;Zeng,ngmuir 2005,21,1074.(c)Liu,B.;Zeng,H.C.J.Am.Chem.Soc.2004,126,16744.(d)Teo,J.J.;Chang,Y.;Zeng,ngmuir 2006,22,7369.JA072191CFigure parison of photocatalytic activities of the titania spheres with solid,sphere-in-sphere,and hollow structure.Inset shows a schematic illustration of multireflections within the sphere-in-sphere structure.C O M M U N I C A T I O N SJ.AM.CHEM.SOC.9VOL.129,NO.27,20078407。
Nanotubes as Solid Acid CatalystMasaaki Kitano,†,|Kiyotaka Nakajima,†Junko N.Kondo,‡Shigenobu Hayashi,§andMichikazu Hara*,†,|Materials and Structures Laboratory,Tokyo Institute of Technology,4259Nagatsuta,Midori-ku,Yokohama 226-8503,Japan,Chemical Resources Laboratory,Tokyo Institute of Technology,4259Nagatsuta,Midori-ku,Yokohama 226-8503,Japan,Research Institute of Instrumentation Frontier,National Institute of Ad V anced Industrial Science and Technology (AIST),Central 5,1-1-1Higashi,Tsukuba,Ibaraki 305-8565,Japan,andKanagawa Academy of Science and Technology,3-2-1Sakado,Takatsu-ku,Kawasaki 213-0012,JapanReceived January 18,2010;E-mail:mhara@msl.titech.ac.jpLewis acid catalysts such as AlCl 3and BF 3are essential in many industrially important reactions,such as the synthesis of ethylbenzene,linear alkyl benzene,cumene,and aromatic ketones.Although such homogeneous acid catalysts are highly active,they have serious drawbacks,such as the production of waste,separation from the product,and corrosion of equipment.From the viewpoint of the environmentally benign chemical process,the use of solid acid catalysts has been required to minimize the emission of toxic byproducts.1Metal oxides are utilized for both their acid -base and redox properties and constitute the largest family of catalysts in heterogeneous catalysis.2We have focused on titanium oxide as a solid acid catalyst,because titanium dioxide contains Lewis acid sites and titanium is the second most abundant transition metal (ninth of all elements)in the Earth’s crust.Among titanium oxide materials,titanate nanotubes have attracted much attention due to their unique physicochemical properties and unusual morphology.3Titanate nanotubes are synthesized by the hydrothermal treatment of TiO 2in a basic aqueous solution,followed by H +-exchange,a simple method requiring neither expensive ap-paratus nor special chemicals.The scrolling of an exfoliated layered titanate nanosheet results in the nanotube structure during hydrothermal treatment.Titanate nanotubes have been extensively investigated in areas such as catalysis,photocatalysis,electrocatalysis,sensors,and lithium batteries.3In the field of catalysis,most of the studies for the material are directed toward the utilization of the material as a support with a large surface area.3However,the acid catalytic properties of the titanate nanotube itself have not yet been investigated in detail.In this study,protonated titanate nanotubes were studied as a solid acid catalyst for potential application to the environmentally benign production of chemicals.The material exhibits remarkable catalytic performance as a Lewis acid catalyst with active Brønsted acid sites.Figure 1a shows a high resolution transmission electron microscopy (HRTEM)image of the resulting material.The tube structures of ca.10nm in outer diameter and ca.5nm in inner diameter are observed.The interlayer spacing of protonated titanate nanotubes is ca .0.7nm (Figure 1b),which is close to the value reported in the literature.3,4The nitrogen adsorption -desorption analysis revealed that protonated titanate nanotubes have a high density of mesopores with a narrow distribution (2-10nm)(Figure S1).The mesopore sizes are ap-proximately equal to the pore diameter observed by HRTEM.The specific surface area calculated from the isotherm was approximately 400m 2g -1,which is larger than that of the starting material (300m 2g -1).The catalytic performance of the protonated titanate nanotubes was examined through the Friedel -Crafts (FC)alkylation of toluene withbenzylchloride,a liquid phase test reaction.Figure S2shows the time courses of benzyltoluene production over protonated titanate nanotubes at 373K.For comparison,the results for the conventional solid acid catalysts are also shown.The formation of o -benzyltoluene and p -benzyltoluene was observed in all catalysts (Table S1).In the case of ion-exchange resins,the yields of benzyltoluene after 1h do not even reach 5%.On the other hand,protonated titanate nanotubes,TiO 2,Nb 2O 5·n H 2O,SO 42-/ZrO 2,and zeolites all exhibited superior activity to that of ion-exchange resins.These inorganic solid acids possess Lewis acid sites 1that play an important role in determining the catalytic activity for this reaction,because FC alkylation of aromatic rings and alkyl halides proceed effectively over a Lewis acid catalyst rather than a Brønsted acid catalyst.5Figure 2shows the catalytic activity of each catalyst at near room temperature (300K).At 300K,almost all of the conventional catalysts displayed negligible catalytic activity,and even the catalytic activities of SO 42-/ZrO 2and H were moderate.In contrast to the conventional catalysts,the titanate nanotubes exhibit remarkable catalytic performance for the reaction at 300K;the yield of benzyl-toluene reached 90%at 180min.Assuming that the reaction is catalyzed only on Lewis acid sites,the turnover number at 180min†Materials and Structures Laboratory,Tokyo Institute of Technology.‡Chemical Resources Laboratory,Tokyo Institute of Technology.§AIST.|Kanagawa Academy of Science and Technology.Figure 1.(a)HRTEM image of the protonated titanate nanotubes.(b)Enlarged HRTEM image of (a).Figure 2.Time courses of benzyltoluene formation using various solidacid catalysts.Reaction conditions:catalyst (0.2g),toluene (0.1mol),benzylchloride (0.02mol),reaction temperature 300K.Published on Web 04/28/201010.1021/ja100435w 2010American Chemical Society66229J.AM.CHEM.SOC.2010,132,6622–6623was estimated to be ca.320(see below).No byproducts were observed in the solution after the reaction using titanate nanotubes.The titanate nanotubes were recoverable by filtration and washing,and the recovered solid acid was confirmed to be reusable without a significant decrease in catalytic activity (Figure S3).Although HCl is formed during the FC reaction,it was confirmed that HCl and H 2SO 4cannot catalyze the FC reaction at 300K (Figure S4).AlCl 3is a highly active homogeneous Lewis acid that has been used for the production of industrially important chemicals but is not reusable and has a much higher catalytic activity for the reaction than titanate nanotubes,with the conversion of benzylchloride reaching ca.100%within 1h at 300K.However,the benzyltoluene yield cannot exceed 70%,due to the formation of byproducts from the further alkylation of reactants and products.The difference FT-IR spectra of pyridine-adsorbed TiO 2and protonated titanate nanotubes (298K)are shown in Figure S5.The bands observed at ca.1450cm -1in both spectra of TiO 2and the titanate nanotubes are assignable to pyridine coordinatively bound to Lewis acid sites.6The band at ca.1540cm -1,due to pyridinium ions formed by Brønsted acid sites,6was not observed for TiO 2,but only for titanate nanotubes,which indicates that protonated titanate nano-tubes possess both Brønsted and Lewis acid sites.The concentrations of Brønsted and Lewis acid sites on the titanate nanotubes were estimated to be 0.10and 0.25mmol g -1,respectively (see the Supporting Information).The results suggest that the efficient reaction on protonated titanate nanotubes is due to not only the Lewis acid sites but also the active Brønsted acid sites.The reaction was further investigated using Na +-exchanged titanate nanotubes prepared by the cation exchange of protonated titanate nanotubes in an aqueous NaOH solution.This material only with Lewis acid sites (Figure S6)showed high catalytic performance at 373K as shown in Table S2.However,it cannot function at 300K.Apparently,the alkylation by the Lewis acid sites at room temperature does not proceed without the Brønsted acid sites.Such synergy between Brønsted and Lewis acid sites has been reported to enhance the catalytic activity in isomerization,cracking,and FC alkylation.7In many cases,it is expected that the synergy facilitates carbocation formation by acidity enhancement,resulting in high catalytic activity.7The Brønsted acid sites may also promote carbocation formation with the formation of HCl at room temperature although the details are currently under investigation.It should be noted that titanate nanosheets,such as H 2Ti 3O 7which has a similar crystal structure of titanate nanotubes,4do not have high catalytic activity for the FC reaction,although protonated titanate nanotubes are formed from these titanate nanosheets.3The catalytic performance of layered H 2Ti 3O 7,H 2Ti 3O 7nanosheets,and titanate nanotubes for the FC alkylation of toluene with benzylchloride at 300K is summarized in Table 1.In the case of layered H 2Ti 3O 7,the reactants cannot approach the Ti -OH groups in narrow interlayer spaces,resulting in no catalytic activity.Although many Ti -OH groups can be exposed to the reactants in aggregated H 2Ti 3O 7nanosheets prepared by the aggregation of exfoliated H 2Ti 3O 7nanosheets from layered H 2Ti 3O 7,8this FC alkylation does not proceed efficiently on the nanosheet material.Figure S7shows the results for the formationreactions than the tested conventional solid acids,including resins,zeolites,and H 2Ti 3O 7nanosheets.Because both reactions proceed through the dehydration of fructose,Brønsted acids promote these reactions,indicating that titanate nanotubes have more effective Brønsted acid sites than the other solid acids.These results indicate that the amount of effective Brønsted acid sites on H 2Ti 3O 7nanosheets is much smaller than that of titanate nanotubes,which suggests that scrolling of the exfoliated titanate nanosheets forms Brønsted acid sites available for the FC reaction and HMF formation.Silanol groups (Si -OH)are typically neutral functional groups in many SiO 2materials.However,Si -OH groups in some SiO 2materials with mesopores,such as FSM-16and MCM-41,do function as relatively strong Brønsted acid sites,11which can be attributed to distortion of the lattice.11Therefore,lattice distortion due to the scrolling of titanate nanosheets may form effective Brønsted acid sites.In summary,protonated titanate nanotubes function as a highly active heterogeneous Lewis acid catalyst,due to effective Brønsted acid sites on the scrolled titanate nanosheets,large amounts of Lewis acid sites,and large pores.Acknowledgment.This work was supported by the Ministry of the Environment,Japan,the Research and Development in a New Interdisciplinary Field Based on Nanotechnology and Materials Science programs of the Ministry of Education,Culture,Sports,Science and Technology (MEXT)of Japan,and a Grant-in-Aid for Young Scientists (B)(No.20760533)from the Japan Society for the Promotion of Science (JSPS).Supporting Information Available:Experimental section,Table S1-S2,and Figures S1-S7.This material is available free of charge via the Internet at .References(1)(a)Anastas,P.T.;Kirchhoff,M.M.Acc.Chem.Res.2002,35,686.(b)Corma,A.Chem.Re V .1995,95,559.(2)(a)Kung,H.H.Stud.Surf.Sci.Catal.1989,45,1.(b)Tanabe,K.;Hattori,H.In Handbook of Heterogeneous Catalysis ;Wiley-VCH:Weinheim,1997;Vol.1,p 404.(3)(a)Kasuga,T.;Hiramatsu,M.;Hoson,A.;Sekino,T.;Niihara,ngmuir1998,14,3160;Ad V .Mater.1999,11,1307.(b)Bavykin,D.V.;Friedrich,J.M.;Walsh,F.C.Ad V .Mater.2006,18,2807.(4)(a)Chen,Q.;Zhou,W.;Du,G.H.;Peng,L.M.Ad V .Mater.2002,14,1208.(b)Tsai,C.C.;Teng,H.S.Chem.Mater.2006,18,367.(c)Yang,H.G.;Zeng,H.C.J.Am.Chem.Soc.2005,127,270.(5)Bandini,M.;Melloni,A.;Umani-Ronchi,A.Angew.Chem.,Int.Ed.2004,43,550.(6)Zaki,M.I.;Hasan,M.A.;Al-Sagheer,F.A.;Pasupulety,L.Colloids Surf.,A 2001,190,261.(7)(a)Corma,A.;Fornes,V.;Rey,F.Appl.Catal.1990,59,267.(b)Li,S.H.;Zheng,A.M.;Su,Y.C.;Zhang,H.L.;Chen,L.;Yang,J.;Ye,C.H.;Deng,F.J.Am.Chem.Soc.2007,129,11161.(c)Telalovic,S.;Ng,J.F.;Maheswari,R.;Ramanathan, A.;Chuah,G.K.;Hanefeld,mun.2008,4631.(8)(a)Sasaki,T.;Watanabe,M.;Michiue,Y.;Komatsu,Y.;Izumi, F.;Takenouchi,S.Chem.Mater.1995,7,1001.(b)Takagaki,A.;Sugisawa,M.;Lu,D.;Kondo,J.N.;Hara,M.;Domen,K.;Hayashi,S.J.Am.Chem.Soc.2003,125,5479.(9)Chheda,J.N.;Huber,G.W.;Dumesic,J.A.Angew.Chem.,Int.Ed.2007,46,7164.(10)(a)Roma ´n-Leshkov,Y.;Chheda,J.N.;Dumesic,J.A.Science 2006,312,1933.(b)Watanabe,M.;Aizawa,Y.;Iida,T.;Nishimura,R.;Inomata,H.Appl.Catal.,A 2005,295,150.(11)(a)Hartmann,M.;Poppl,A.;Kevan,L.J.Phys.Chem.1996,100,9906.(b)Yamamoto,T.;Tanaka,T.;Funabiki,T.;Yoshida,S.J.Phys.Chem.B 1998,102,5830.(c)Iwamoto,M.;Tanaka,Y.;Sawamura,N.;Namba,S.J.Am.Chem.Soc.2003,125,13032.JA100435Wchloride (0.02mol),reaction temperature 300K.b Total pore volume.cYield of benzyltoluene.J.AM.CHEM.SOC.9VOL.132,NO.19,20106623。