化学化工专业英语(1)
- 格式:ppt
- 大小:573.00 KB
- 文档页数:82

化学工程与工艺专业英语1. Introduction化学工程与工艺是一门涉及化学反应、化学工艺以及工程原理的学科,它在许多工业领域中起着重要的作用。
作为一个化学工程与工艺专业的学生,具备良好的英语沟通能力对于学习和就业都具有重要意义。
本文档将介绍一些化学工程与工艺专业中常用的英语术语和短语,以帮助读者更好地理解和运用这些知识。
2. Basic Terms and Definitions在开始学习化学工程与工艺专业的英语词汇之前,我们需要了解一些基本的术语和定义。
•Chemical Engineering: 化学工程•Process: 过程•Reactor: 反应器•Mass Transfer: 质量传递•Heat Transfer: 热传递•Distillation: 蒸馏•Extraction: 提取•Polymerization: 聚合•Catalysis: 催化•Reaction Kinetics: 反应动力学•Thermodynamics: 热力学•Unit Operation: 单元操作•Unit Process: 单元工艺3. Chemical Engineering Processes化学工程与工艺专业涉及许多不同的化学过程和工艺。
下面是一些常见的过程名称和定义。
3.1 Distillation蒸馏是一种通过利用不同组分的沸点差异进行分离的过程。
在蒸馏过程中,液体混合物被加热,使其沸腾,然后通过冷凝,得到不同组分的纯液体。
蒸馏在石油化工、酒精生产和石油提炼等领域中广泛应用。
3.2 Extraction提取是一种将溶质从溶剂中分离出来的过程。
提取可以通过溶剂选择性地与溶质相互作用,使得溶质从溶剂中转移到新的相中。
提取常用于药物生产、化妆品制造等领域。
3.3 Polymerization聚合是一种将单体分子结合成长链聚合物的过程。
聚合通常需要催化剂和适当的反应条件。
聚合在塑料制造、纤维生产和涂料工业等领域中被广泛应用。




化学化工专业英语电子版课本————————————————————————————————作者:————————————————————————————————日期:ContentPART 1 Introduction to Materials Science &Engineering 1 Unit 1 Materials Science and Engineering 1 Unit 2 Classification of Materials 9 Unit 3 Properties of Materials 17 Unit 4 Materials Science and Engineering: What does the Future Hold? 25 Part ⅡMETALLIC MATERLALS AND ALLOYS 33 Unit 5 An Introduction to Metallic Materials 33 Unit 6 Metal Manufacturing Methods 47 Unit 7 Structure of Metallic Materials 57 Unit 8 Metal-Matrix Composites 68 PartⅢCeramics 81 Unit 9 Introduction to Ceramics 81 Unit 10 Ceramic Structures —Crystalline and Noncrystalline 88 Unit 11 Ceramic Processing Methods 97 Unit 12 Advanced ceramic materials –Functional Ceramics 105 PARTⅣNANOMATERIALS 112 Unit 13 Introduction to Nanostructured Materials 112 Unit14 Preparation of Nanomaterials 117 Unit 15 Recent Scientific Advances 126 Unit 16 The Future of Nanostructure Science and Technology 130 Part ⅤPOLYMERS 136Unit17 A Brief Review in the Development of Synthetic Polymers 136 Unit18 Polymer synthesis: Polyethylene synthesis 146 Unit19 Polymer synthesis:Nylon synthesis 154 Unit 20 Processing and Properties Polymer Materials 165 PART VI POLYMERIC COMPOSITES 172 Unit21 Introduction to Polymeric Composite Materials 172Unit22 Composition, Structure and Morphology of Polymeric Composites 178 Unit23 Manufacture of Polymer Composites 185 Unit24 Epoxy Resin Composites 191 Part 7 Biomaterial 196 Unit 25 Introduction to Biomaterials 196 Unit 26 Biocompatibility 205 Unit 27 Polymers as Biomaterials 213 Unit 28 Future of Biomaterials 224 PARTⅧMaterials and Environment 237 Unit29 Environmental Pollution & Control Related Materials 237 Unit30 Bio-degradable Polymer Materials 241 Unit 31 Environmental Friendly Inorganic Materials 248 Unit 32 A Perspective on the Future: Challenges and Opportunities 256 附录一科技英语构词法263 附录二科技英语语法及翻译简介269 附录三:聚合物英缩写、全名、中文名对照表280 附录四:练习题参考答案284PART 1 Introduction to Materials Science &EngineeringUnit 1Materials Science and Engineering Historical PerspectiveMaterials are probably more deep-seated in our culture than most of us realize. Transportation, housing, clothing, communication, recreation, and food production —virtually every segment of our everyday lives is influenced to one degree or another by materials. Historically, the development and advancement of societies have been intimately tied to the members’ ability to produce and manipulate materi- als to fill their needs. In fact, early civilizations have been designated by the level of their materials development (Stone Age, Bronze Age, Iron Age).The earliest humans had access to only a very limited number of materials, those that occur naturally: stone, wood, clay, skins, and so on. With time they discovered techniques for producing materials that had properties superior to those of the natural ones; these new materials included pottery and various metals. Furthermore, it was discovered that the properties of a material could be altered by heat treatments and by the addition of other substances. At this point, materials utilization was totally a selection process that involved deciding from a given, rather limited set of materials the one best suited for an application by virtue of its characteristics.①It was not until relatively recent times that scientists came to understand the relationships between the structural elements of materials and their properties. This knowledge, acquired over approximately the past 100 years, has empowered them to fashion, to a large degree, the characteristics of materials. Thus, tens of thousands of different materials have evolved with rather specialized charac- teristics that meet the needs of our modern and complex society; these include metals, plastics, glasses, and fibers. deep-seated根深蒂固的, 深层的pottery / ☐♦☯❒♓/ ⏹ 陶器structural elements结构成分;property / ☐❒☐☜♦♓/⏹.性能The development of many technologies that make our existence so comfortable has been intimately associated with the accessibility of suitable materials. An advancement in the understanding of a material type is often the forerunner to the stepwise progression of a technology. For example, automobiles would not havebeen possibl- e without the availability of inexpensive steel or some other comparable substitute. In our contemporary era, sophisticated electronic devices rely on components that are made from what are called semiconducting materials. Materials Science and EngineeringThe discipline of materials science involves investigating the relationships that exist between the structures and properties of materials. In contrast, materials engineering is, on the basis of these structure–property correlations, designing or engineering the structure of a material to produce a predetermined set of properties.“Structure’’ is at this point a nebulous term that deserves some explanation. In brief, the structure of a material usually relates to the arrangement of its internal components. Subatomic structure involves electrons within the individual atoms and interactions with their nuclei. On an atomic level, structure encompasses the organization of atoms or molecules relative to one another. The next larger structural realm, which contains large groups of atoms that are normally agglomerated together, is termed ‘‘microscopic,’’ meaning that which is subject to direct observation using some type of microscope. Finally, structural elements that may be vie wed with the naked eye are termed ‘‘macroscopic.’’The notion of ‘‘property’’ deserves elaboration. While in service use, all materials are exposed to external stimuli that evoke some type of response. For example, a specimen subjected to forces will experience deformation; or a polished metal surface will reflect light. Property is a material trait in terms of the kind and magnitude of response to a specific imposed stimulus. Generally, definitions of properties are made independent of material shape and size.Virtually all important properties of solid materials may be grouped into six different categories: mechanical, electrical, thermal, magnetic, optical, and stepwise / ♦♦♏☐♦♋♓/ ♎逐步的sophisticated/♦☯♐♓♦♦♓ ♏♓♦♓♎/ ♎精制的,复杂的;semiconducting materials 半导体材料nebulous/ ⏹♏♌✞●☯♦/♎ 含糊的,有歧义的subatomic/ ♦✈♌☯♦❍/♎ 亚原子的microscopic/❍♓❒☯♦☐♓/ ♎微观的❍♋♍❒☐♦♍☐☐♓♍/❍✌ ❒☯✞♦☐♓/♎宏观的deteriorative. For each there is a characteristic type of stimulus capable of provokingdifferent responses. Mechanical properties relate deformation to an applied load or force; examples include elastic modulus and strength. For electrical properties, such as electrical conductivity and dielectric constant, the stimulus is an electric field. The thermal behavior of solids can be represented in terms of heat capacity and thermal conductivity. Magnetic properties demonstrate the response of a material to the application of a magnetic field. For optical properties, the stimulus is electro- magnetic or light radiation; index of refraction and reflectivity are representative optical properties. Finally, deteriorative characteristics indicate the chemical reactivity of materials.In addition to structure and properties, two other important components are involved in the science and engineering of materials, viz. ‘‘processing’’ and ‘‘performance.’’ With regard to the relationships of these four components, the structure of a material will depend on how it is processed. Furthermore, a material’s performance will be a function of its properties.Fig. 1.1 Photograph showing the light transmittance of three aluminum oxide specimens. From left to right: single crystal material (sapphire), which is transparent;a polycrystalline and fully dense (nonporous) material, which is translucent; and a polycrystalline material that contains approximately 5% porosity, which is opaque. (Specimen preparation, P. A. Lessing; photography by J. Telford.)We now present an example of these processing-structure-properties-perfor- mance principles with Figure 1.1, a photograph showing three thin disk specimens placed over some printed matter. It is obvious that the optical properties (i.e., the deformation/ ♎♓♐ ❍♏♓☞☯⏹/ ⏹变形deteriorative/♎♓♦♓☯❒♓☯❒♏♓♦♓❖/ ⏹破坏(老化的)elastic modulus 弹性模量strength /♦♦❒♏⏹♑/ ⏹强度;dielectric constant介电常数;heat capacity 热容量refraction/❒♓♐❒✌☞☯⏹/ ⏹折射率;reflectivity/ ❒♓♐●♏ ♦♓❖♓♦♓/ ⏹反射率processing/☐❒☯◆♦♏♦♓☠/ ⏹加工light transmittance) of each of the three materials are different; the one on the left is transparent (i.e., virtually all of the reflected light passes through it), whereas the disks in the center and on the right are, respectively, translucent and opaque.All of these specimens are of the same material, aluminum oxide, but the leftmost one is what we call a single crystal—that is, it is highly perfect—which gives rise to its transparency. The center one is composed of numerous and verysmall single crystals that are all connected; the boundaries between these small crystals scatter a portion of the light reflected from the printed page, which makes this material optically translucent.②And finally, the specimen on the right is composed not only of many small, interconnected crystals, but also of a large number of very small pores or void spaces. These pores also effectively scatter the reflected light and render this material opaque.Thus, the structures of these three specimens are different in terms of crystal boundaries and pores, which affect the optical transmittance properties. Furthermore, each material was produced using a different processing technique. And, of course, if optical transmittance is an important parameter relative to the ultimate in-service application, the performance of each material will be different.Why Study Materials science and Engineering?Why do we study materials? Many an applied scientist or engineer, whether mechanical, civil, chemical, or electrical, will at one time or another be exposed to a design problem involving materials. Examples might include a transmission gear, the superstructure for a building, an oil refinery component, or an integrated circuit chip. Of course, materials scientists and engineers are specialists who are totally involved in the investigation and design of materials.Many times, a materials problem is one of selecting the right material from the many thousands that are available. There are several criteria on which the final decision is normally based. First of all, the in-service conditions must be charac- terized, for these will dictate the properties required of the material. On only rare occasions does a material possess the maximum or ideal combination of properties. transmittance/♦❒✌⏹❍♓♦☜⏹♦/ ⏹. 透射性sapphire /♦✌♐♓☯/ ⏹蓝宝石transparent/♦❒✌⏹♦☐☪☯❒☯⏹♦/ ♎透明的;polycrystalline/ ☐●♓❒♓♦♦☯●♓⏹/ ⏹多晶体;translucent/♦❒✌⏹●✞♦⏹♦/♎ 半透明的;opaque☯✞☐♏♓♎不透明的single crystal 单晶体Thus, it may be necessary to trade off one characteristic for another. The classic example involves strength and ductility; normally, a material having a high strength will have only a limited ductility. In such cases a reasonable compromise between two or more properties may be necessary.A second selection consideration is any deterioration of material properties that may occur during service operation. For example, significant reductions in mecha- nical strength may result from exposure to elevated temperatures or corrosive envir- onments.Finally, probably the overriding consideration is that of economics: What will the finished product cost? A material may be found that has the ideal set of proper- ties but is prohibitively expensive. Here again, some compromise is inevitable.The cost of a finished piece also includes any expense incurred during fabrication to produce the desired shape. The more familiar an engineer or scientist is with the various characteristics and structure–property relationships, as well as processing techniques of materials, the more proficient and confident he or she will be to make judicious materials choices based on these criteria.③Reference:William D. Callister,Materials science and engineering :anintroduction, Press:JohnWiley & Sons, Inc.,2007;2-5 transmission gear传动齿轮dictate/♎♓♦♏♓♦/ ❖ 决定trade off 权衡;折衷ductility♎✈♦♓●♓♦♓⏹延展性overriding/ ☯✞❖☯❒♋♓♎♓☠/♎最主要的judicious/♎✞✞♎♓☞☯♦/♎明智的Notes1.At this point, materials utilization was totally a selection process that involved deciding froma given, rather limited set of materials the one best suited for an application by virtue of itscharacteristics由此看来,材料的使用完全就是一个选择过程,且此过程又是根据材料的性质从许多的而不是非有限的材料中选择一种最适于某种用途的材料。


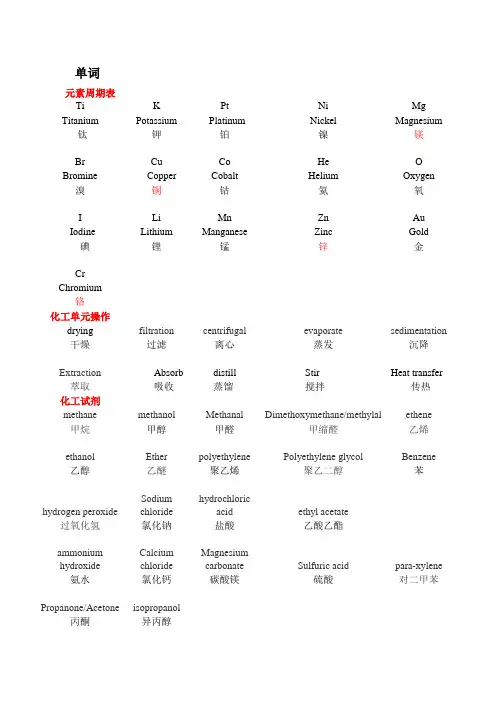
单词元素周期表Ti K Pt Ni Mg Titanium Potassium Platinum Nickel Magnesium 钛钾铂镍镁Br Cu Co He O Bromine Copper Cobalt Helium Oxygen 溴铜钴氦氧I Li Mn Zn AuIodine Lithium Manganese Zinc Gold碘锂锰锌金CrChromium铬化工单元操作drying filtration centrifugal evaporate sedimentation 干燥过滤离心蒸发沉降Extraction Absorb distill Stir Heat transfer 萃取吸收蒸馏搅拌传热化工试剂methane methanol Methanal Dimethoxymethane/methylal ethene 甲烷甲醇甲醛甲缩醛乙烯ethanol Ether polyethylene Polyethylene glycol Benzene 乙醇乙醚聚乙烯聚乙二醇苯hydrogen peroxide Sodiumchloridehydrochloricacid ethyl acetate过氧化氢氯化钠盐酸乙酸乙酯ammonium hydroxide CalciumchlorideMagnesiumcarbonate Sulfuric acid para-xylene氨水氯化钙碳酸镁硫酸对二甲苯Propanone/Acetone isopropanol丙酮异丙醇化工术语calcination Fluid crystallization plate tower packed tower 煅烧流体结晶板式塔填料塔membrane separationChemicalVaporDepositionLiquid-PhaseDeposition supercritical fluid Supercritical膜分离气相沉积液相沉积超临界流体超临界color atlas Sol-gel hydrothermal Muffle furnace freeze 色谱溶胶凝胶水热马弗炉冷冻化工专有名词1、Chlorobromomethane (氯溴甲烷)2、hexachtorocyclohexane (六氯环己烷;六六六)3、hydrodesutfurization (加氢脱硫)4、polytetrafluoroethylene (聚四氟乙烯)数字拉丁或希腊前缀 烷烃-ane alkane烷基-yl alkyl 烯烃-ene Alkene 炔烃-yne alkyne醇-ol alcohol 醛-al aldehyde one mono- meth ane methyl - - methanol methyl aldehyde twodi-; bi-eth aneethylethene, ethylene ethyne; acetylene ethanol; alcohol ethanal; ethyl aldehyde three tri- prop ane propyl propene propyne propanol propyl aldehyde fourtetra- quadri-but anebutylbutenebutyne butanolbutyl aldehydefive pent(a)- pent ane pentyl pentene pentyne pentanol pentanal six hex(a)- hex ane hexyl hexene hexyne hexanol hexanal seven hept(a)- hept ane heptyl heptene heptyne heptanol heptanal eight oct(a)- oct ane octyl octene octyne octanol octyl aldehyde nine non(a)- non ane nonyl nonene nonyne nonanol nonyl aldehyde tendec(a)-dec anedecyldecenedecynedecanoldecyl aldehyde碳酸二甲酯 聚氯乙烯变压吸附句子翻译1、The teacher may be asked questions.可以向老师提一些问题。

化学工程与工艺专业英语课文翻译Unit 1 Chemical Industry化学工业 ..................................................... - 2 - Unit 2 Research and Development研究和开发 .................................. - 7 - Unit 3 Typical Activities of Chemical Engineers化学工程师的例行工作 . -13 -Unit 4 Sources of Chemicals化学资源 .............................................. - 19 - Unit 5 Basic Chemicals基本化学品................................................... - 24 - Unit 6 Chlor-Alkali and Related Processes氯碱及其相关过程.......... - 26 - Unit 7 Ammonia, Nitric Acid and Urea氯、硝酸和尿素 ................... - 31 - Unit 8 Petroleum Processing石油加工 ............................................. - 37 - Unit 9 Polymers 聚合物.................................................................. - 40 - Unit 10 What Is Chemical Engineering?什么是化学工程学 .............. - 45 - Unit 11 Chemical and Process Thermodynamics化工热力学 ........... - 52 - Unit 12 What do we mean by transport phenomena ?如何定义传递现象....................................................................................................... - 57 - Unit 13 Unit Operations in Chemical Engineering化学工程中的单元操作....................................................................................................... - 61 - Unit14 Distillation蒸馏 ..................................................................... - 65 - Unit 15 Solvent Extraction, Leaching and Adsorption溶剂萃取,浸取和吸附................................................................................................... - 71 -Unit 16 Evaporation, Crystallization and Drying蒸发、结晶和干燥.- 76 - Unit 17 Chemical Reaction Engineering化学反应工程 ..................... - 82 - Unit18 Chemical Engineering Modeling化工建模 ............................ - 88 - Unit 19 Introduction to Process Design过程设计简介 ..................... - 92 - Unit 20 Material Science and Chemical Engineer材料科学和化学工程 .. -97 -Unit 21 Chemical Industry and Environment化学工业与环境 ....... - 103 -Unit 1 Chemical Industry化学工业1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。

Elements and Compounds元素与化合物Elements are pure substances that can not be decomposed(分解) into simpler substances by ordinary chemical changes. At present there are 109 known elements. Some common elements that are familiar to you are carbon, oxygen, aluminum, iron, copper, nitrogen, and gold. The elements are the building blocks of matter just as the numerals 0 through 9 are the building blocks for numbers. To the best of1 our knowledge, the elements that have been found on the earth also comprise(包含) the entire universe.元素是单纯的物质,不能通过一般的化学变化分解成为更简单的物质。
目前已知有109个元素。
一些你熟悉的常见元素是碳、氧、铝、铁、氮和金。
元素是组成物质的基本单元,就象0到9的数字是组成数的基本单元一样。
就我们所知,已经在地球上发现的元素也是组成整个宇宙的元素。
About 85% of (85 percent of) the elements can be found in nature , usually combined with other elements in minerals and vegetable matter or in substances like water and carbon dioxide. Copper, silver, gold, and about 20 other elements can be found in highly pure forms. Sixteen elements are not found in nature; theyhave been produced in generally small amounts in nuclear explosions (爆炸)and nuclear research. They are man-made elements.大约有85%的元素可以在大自然的矿物或者植物中,以及如水和二氧化碳这样的物质中找到,通常与别的元素结合。

Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers14.capital intensiveSome Chemicals Used In Our Daily LifeFood artificial fertilizers, pesticide, veterinary products Health antibiotics, β-blockersClothing synthetic fibers (e.g. polyesters, polyamides),synthetic dyesShelter synthetic polymers (e.g. urea-formaldehyde,polyurethanes),plasticsLeisure plastics and polymers (e.g. nylon)Transport additives (e.g. anti-oxidants, viscosity indeximpovements),polymers, plasticsUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9. a emerging case10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam14.cooling water 15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals1.Ethylene2.acetic acid3.Polymerization4.Polyvinyl acetate5.Emulsion paintHigh-volume sector Low-volume sectorProduction scale tens to hundreds of thousandstons per yeartens to a few thousands tonsper yearProducts / a plant single product multi-products Operation manner continuous batch Price or profit fairly cheap very profitable Usage intermediates end-productsChallengesreduced demand, environment pollutionProducts in the sectorsulphuric acid,phosphorus-containingcompounds,nitrogen-containingcompounds,chlor-alkali,petrochemicals,commodity polymersagrochemicals,dyestuffs,pharmaceuticals,speciality polymersUnit 6 Chlor-Alkali and Related Processes1.Ammonia2.ammonia absorber3.NaCl & NH4OH4.Carbon dioxide5.NH4Cl6.Rotary drier7.Light Na2CO38.WaterProduct Raw materialMajor steps orPrincipal reactionsUsesSoda-ashbrine,limestoneammoniating,carbonating,precipitating,filtering,drying,calciningraw material forglassmaking,sodium silicate;as an alkaliChlorine brine2Na+ + 2Cl - +2H2O →NaOH +Cl2 +H2as water purification, bleaching of wood pulp;production of vinyl chloride, solvents, inorganic chlorine-containing productsCaustic soda brine2Na+ + 2Cl - +2H2O →NaOH +Cl2 +H2for paper-making, manufacture of inorganicchemicals, syntheses of organicchemicals, production of aluminaand soapSulfuric acid elemental sulphurS +O2→ SO2SO2 + O2→ SO3SO3 + H2O → H2SO4feedstock for fertilizers;production of ethanol,hydrofluoric acid,aluminum sulphatesUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryCourse Course contentScience and Math.Chemistry, Physics, Biology, Material Science, Mathematics,Computer InstructionChemical EngineeringThermodynamics, Kinetics, Catalysis,Rector Design and Analysis, Unit Operations, Process Control, Chemical Engineering Laboratories, Design / EconomicsOther Engineering Electrical Engineering, Mechanics, Engineering DrawingHumanities and SocialScience Understand the origins of one’s own culture as well as that ofothersUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions9.different plastics10.recycled or disposed11.acidic waste solutions anic components13.membrane technology14.biotechnology15.microorganismsFrontier Research activities or problems facedIn-site processingField tests; Uncertainties of the process, Adverse environment impactsProcess solidsImprove solids fracture processes,Research on the mechanics of pneumatic and slurry transport, Understand the chemical reaction processes,Equipment design and scale-upSeparation processResearch on:membrane separations, chemical selective separation agents, shape-selective porous solids,traditional separation methodsMaterialsFind construction materials, Develop new process-related materials, Develop less energy intensive materialsDesign and scale-up Complexity, Lack of basic data,。
Chemical nomenclature 化学命名法Chemical symbol 化学符号International Union of Pure and Applied Chemists (IUPAC) 国际纯粹与应用化学联合会Commission on the Nomenclature of Inorganic Chemistry (CNIC) 无机化学命名法委员会Carbon ['kɑːb(ə)n] n 碳Sulfur ['sʌlfə] n 硫Iron ['aiən] / ferrum ['ferəm] n 铁Copper ['kɔpə] n 铜Silver ['silvə] / Argentum [ɑː'dʒentəm] n 银Tin [tin] / Stannum ['stænəm] n 锡Platinum ['plætinəm] n 铂Gold [gəuld] / Aurum ['ɔːrəm] n 金Mercury ['mɜːkjəri] /Hydrargyrum [hai'drɑːdʒirəm] n 汞Lead [liːd] / Plumbum ['plʌmbəm] n 铅Chlorine ['klɔːriːn] n 氯Chromium ['krəumiəm] n 铬Rubidium [ru'bidiəm] n 铷Rhodium ['rəudiəm] n 铑Indium ['indiəm] n 铟Iodine ['aiədiːn; -ain; -in] n 碘Caesium ['siːziəm] n 铯Iridium [i'ridiəm; ai-] n 铱Thallium ['θæl iəm] n 铊Hydrogen ['haidrədʒ(ə)n] n 氢Nitrogen ['naitrədʒ(ə)n] n 氮Oxygen ['ɔksidʒ(ə)n] n 氧Phosphorus ['fɔsf(ə)rəs] n 磷Zinc [ziŋk] n 锌Fluorine ['fluəriːn; 'flɔː-] n 氟Bromine ['brəumiːn] n 溴Antimony ['æntiməni] / Stibium ['stibiəm] n 锑Osmium ['ɔzmiəm] n 锇Helium ['hiːliəm] n 氦Selenium [si'liːniəm] n 硒Palladium [pə'leidiəm] n 钯Tellurium [te'ljuəriəm] n碲Cerium ['siəriəm] n铈Uranium [ju'reiniəm] n铀Neptunium [nep'tjuːniəm] n镎Plutonium [pluː'təuniəm] n钚Titanium [tai'teiniəm; ti-] n 钛Vanadium [və'neidiəm] n 钒Cobalt ['kəubɔːlt; -ɔlt] n 钴Nickel ['nik(ə)l] n 镍Arsenic ['ɑːs(ə)n ik] n 砷Niobium [nai'əubiəm] n 铌Promethium [prə'miːθiəm] n钷Tantalum ['tæntələm] n 钽Wolfram ['wulfrəm] / Tungsten ['tʌŋst(ə)n] n 钨Thorium ['θɔːriəm] n钍Curium ['kjuəriəm] n锔Einsteinium [ain'stainiəm] n锿Fermium ['fɜːmiəm] n镄Mendelevium [,mendə'liːviəm; -'leiviəm] n钔Nobelium [nə(u)'biːliəm; -'bel-] n 锘Lawrencium [lɔ'rensiəm] n铹Rutherfordium [,rʌðə'fɔːdiəm] n钅卢,鈩Seaborgium钅喜Bohrium ['bəuəriəm] n 铍Meitnerium ['maitnəriəm] n钅麦Roentgenium钅仑Copernicium 鎶Magnesium [mæg'niːziəm] n 镁Manganese ['mæŋgəniːz] n 锰Strontium ['strɔntiəm; 'strɔnʃ(i)əm] n 锶Cadmium ['kædmiəm] n 铬Yttrium ['itriəm] n 钇Erbium ['ɜːbiəm] n铒Terbium ['tɜːbiəm] n铽Ytterbium [i'tɜːbiəm] n镱Holmium ['həulmiəm] n钬Thulium ['θ(j)uːliəm] n铥Scandium ['skændiəm] n钪Gallium ['gæliəm] n 镓Germanium [dʒɜː'meiniəm] n锗Ruthenium [ru'θiːniəm] n钌Europium [ju(ə)r'əupiəm] n铕Lutetium [luː'tiːʃiəm; -siəm] n镥Hafnium ['hæfniəm] n铪Rhenium ['riːniəm] n铼Polonium [pə'ləuniəm] n钋Francium ['frænsiəm] n钫Americium [,æmə'risjəm] n镅Berkelium [bɜː'kiːliəm; 'bɜːkliəm] n锫Californium [,kæli'fɔːniəm] n锎Dubnium ['duːbniəm] n钅杜Hassium ['hæsiəm] n钅黑Darmstadtium 鐽Beryllium [bə'riliəm] n铍Boron ['bɔːrɔn] n 硼Sodium ['səudiəm] n 钠Aluminium [æl(j)u'miniəm] n 铝Silicon ['silik(ə)n] n 硅Potassium [pə'tæsiəm] n 钾Calcium ['kælsiəm] n 钙Zirconium [zɜː'kəuniəm] n锆Molybdenum [mə'libdənəm] n钼Barium ['beəriəm] n 钡Samarium [sə'meəriəm] n钐Gadolinium [,gædə'liniəm] n 钆Lithium ['liθiəm] n 锂Technetium [tek'niːʃiəm] n 锝Lanthanum ['lænθənəm] n镧Praseodymium [,preiziə(u)'dimiəm] n镨Dysprosium [dis'prəuziəm] n镝Bismuth ['bizməθ] n铋Astatine ['æstətiːn] n砹Radium ['reidiəm] n镭Actinium [æk'tiniəm] n锕Protactinium [,prəutæk'tiniəm] n镤Neon ['niːɔn] n氖Argon ['ɑːgɔn] n 氩Krypton ['kriptɔn] n 氪Xenon ['zenɔn; 'ziː-] n氙Radon ['reidɔn] n氡Unnilquadium [,ju:nil'kwɔdiəm] n 104号元素Unnilpentium [,ju:nil'pentiəm] n 105号元素Unnilhexium [,ju:nil'heksiəm] n 106号元素Unnilseptium [,juːnil'septiəm] n 107号元素Unniloctium [,ju:ni'lɔktiəm] n 108号元素Unnilennium [,ju:ni'leniəm] n 109号元素Ununnilium n 110号元素Unununium n 111号元素Ununbium n 112号元素Ununtrium n 113号元素Ununquadium n 114号元素Ununpentium n 115号元素Ununhexium n 116号元素Ununseptium n 117号元素Ununoctium n 118号元素Alkali metal 碱金属Alkaline earth metal 碱土金属Pnicogen 碳族元素Chalcogen 氧族元素Halogen 卤族元素Noble gas 稀有气体Lanthanoid 镧系元素Actinoid 锕系元素Rare earth element 稀土元素Transition metal 过渡金属Pnictide ['niktaid] n 碳族化合物Chalcogenide ['kælkədʒənaid] n 氧族化合物Halogenide ['hælədʒinaid] / halide ['heilaid] n 卤化物Isotope ['aisətəup] n同位素Deuterium [djuː'tiəriəm] n氘Tritium ['tritiəm] n氚Polyatomic [,pɑliə'tɑmik] n 多原子的Ammonium [ə'məuniəm] n 铵Oxoanion n 含氧阴离子Sulfate ['sʌlfeit] n 硫酸根Sulfite ['sʌlfait] n亚硫酸根Nitrate ['naitreit] n 硝酸根Nitrite ['naitrait] n 亚硝酸根Isopolyacid 同多酸Thioacid 硫代酸Base [beis] 碱Hydrate ['haidreit] 水合物Coordination complex 络合物Ligand ['lig(ə)nd] n 配体Coordination number 配位数Monodentate 单齿配体Bidentate [bai'denteit] n 双齿配体Polydentate 多齿配体chelate ['kiːleit] n 螯合物Linear ['liniə] n 线性的Square planar 平面正方形的Tetrahedral [,tetrə'hiːdrəl] adj 四面体的Octahedral [,ɑktə'hidrəl] adj 八面体的Trigonal bipyramidal 三角双锥的。
1 CHEMISTRY AND CHEMISTWithout chemistry our lives would beunrecognisable, for chemistry is at work all aroundus. Think what life would be like without chemistry- there would be no plastics, no electricity and noprotective paints for our homes. There would be no synthetic fibres to clothe us and no fertilisers to help us produce enough food. We wouldn‟t be able to travel because there would be no metal, rubber or fuel for cars, ships and aeroplane. Our lives would be changed considerably without telephones, radio, television or computers, all of which depend on chemistry for the manufacture of their parts. Life expectancy would be much lower, too, as there would be no drugs to fight disease.Chemistry is at the forefront of scientific adventure, and you could make your own contribution to the rapidly expanding technology we are enjoying. Take some of the recent academic research: computer graphics allow us to predict whether small molecules will fit into or react with larger ones - this could lead to a whole new generation of drugs to control disease; chemists are also studying the use of chemicals to trap the sun‟s energy and to purify sea water; they are also investigating the possibility of using new ceramic materials to replace metals which can corrode.Biotechnology is helping us to develop new sources of food and new ways of producing fuel, as well as producing new remedies for the sick. As the computer helps us to predict and interpret results from the test tube, the speed, accuracy and quality of results is rapidly increasing - all to the benefit of product development.It is the job of chemists to provide us with new materials to take us into the next century, and by pursuing the subject, you could make your positive contribution to society.Here are some good reasons for choosing chemistry as a career.Firstly, if you have an interest in the chemical sciences, you can probably imagine taking some responsibility for the development of new technology. New ideas and materials are constantly being used in technology to improve the society in which we live. You could work in a field where research and innovation are of primary importance to standards of living, so you could see the practical results of your work in every day use.Secondly, chemistry offers many career opportunities, whether working in a public service such as a water treatment plant, or high level research and development in industry. Your chemistry-based skills and experience can be used, not only in many different areas within the chemical industry, but also as the basis for a more general career in business.1 As a qualification, chemistry is highly regarded as a sound basis for employment.You should remember that, as the society we live in becomes more technically advanced, the need for suitably qualified chemists will also increase. Although chemistry stands as a subject in its own right, it acts as the bond between physics and biology. Thus, by entering the world of chemistry you will be equipping yourself to play a leading role in the complex world of tomorrow.Chemistry gives you an excellent training for many jobs, both scientific and non-scientific. To be successful in the subject you need to be able to think logically, and be creative, numerate, and analytical. These skills are much sought after in many walks of life, and would enable you to pursue a career in, say, computing and finance, as well as careers which use your chemistry directly.Here is a brief outline of some of the fields chemists work in:Many are employed in the wealth-creating manufacturing industries - not just oil, chemical and mining companies, but also in ceramics, electronics and fibres. Many others are in consumer based industries such as food, paper and brewing; or in service industriessuch as transport, health and water treatment.In manufacturing and service industries, chemists work in Research and Development to improve and develop new products, or in Quality Control, where they make sure that the public receives products of a consistently high standard.Chemists in the public sector deal with matters of public concern such as food preservation, pollution control, defence, and nuclear energy. The National Health Service also needs chemists, as do the teaching profess ion and the Government‟s research and advisory establishments.Nowadays, chemists are also found in such diverse areas as finance, law and politics, retailing, computing and purchasing. Chemists make good managers, and they can put their specialist knowledge to work as consultants or technical authors. Agricultural scientist, conservationist, doctor, geologist, meteorologist, pharmacist, vet ... the list of jobs where a qualification in chemistry is considered essential is endless. So even if you are unsure about what career you want to follow eventually, you can still study chemistry and know that you‟re keeping your options open.What Do Chemistry Graduates Do?Demand for chemists is high, and over the last decade opportunities for chemistry graduates have been increasing. This is a trend that is likely to continue. Chemistry graduates are increasingly sought after to work in pharmaceutical, oil, chemical, engineering, textile and metal companies, but the range of opportunities also spans the food industry, nuclear fuels, glass and ceramics, optical and photographic industries, hospitals and the automotive industry. Many graduates begin in scientific research, development and design, but over the years, about half change, into fields such as sales, quality control, management, or consultancy. Within the commercial world it is recognised that, because of the general training implicit in a chemistry course, chemistry graduates are particularly adaptable and analytical - making them attractive to a very broad spectrum of employers. There has been a growth of opportunity for good chemistry graduates to move into the financial world, particularly in accountancy, retail stores, and computer software houses.(Summarized from: A brief of the Royal Society of Chemistry,1992)2 NOMENCLATURE OF INORGANICCOMPOUNDSNaming elementsThe term element refers to a pure substance with atoms all of a single kind. At present 107 chemical elements are known. For most elements the symbol is simply the abbreviated form of the English name consisting of one or two letters, for example:oxygen = O nitrogen = N magnesium = MgSome elements, which have been known for a long time, have symbols based on their Latin names, for example:iron = Fe (ferrum) copper = Cu (cuprum) lead = Pb (Plumbum)A few elements have symbols based on the Latin name of one of their compounds, the elements themselves having been discovered only in relatively recent times1, for example: sodium = Na (natrium = sodium carbonate)potassium = K (kalium = potassium carbonate)A listing of some common elements may be found in Table 1.Naming Metal Oxides, Bases and SaltsA compound is a combination of positive and negative ions in the proper ratio to give a balanced charge and the name of the compound follows from names of the ions, for example, NaCl, is sodium chloride; Al(OH)3is aluminium hydroxide; FeBr2is iron (II) bromide or ferrous bromide; Ca(OAc)2is calcium acetate; Cr2(SO4)3is chromium (III) sulphate or chromic sulphate, and so on. Table 3 gives some examples of the naming of metal compounds. The name of the negative ion will need to be obtained from Table 2.Negative ions, anions, may be monatomic or polyatomic. All monatomic anions have names ending with -ide. Two polyatomic anions which also have names ending with -ide are the hydroxide ion, OH-, and the cyanide ion, CN-.Many polyatomic anions contain oxygen in addition to another element. The number of oxygen atoms in such oxyanions is denoted by the use of the suffixes -ite and -ate, meaning fewer and more oxygen atoms, respectively. In cases where it is necessary to denote more than two oxyanions of the same element, the prefixes hypo- and per-, meaning still fewer and still more oxygen atoms, respectively, may be used, for example,hypochlorite ClO-Chlorite ClO2-chlorate ClO3-perchlorate ClO4-Naming Nonmetal OxidesThe older system of naming and one still widely used employs Greek prefixes for both the number of oxygen atoms and that of the other element in the compound 2. The prefixes used are (1) mono-, sometimes reduced to mon-, (2) di-, (3) tri-, (4) tetra-, (5) penta-, (6) hexa-, (7) hepta-, (8) octa-, (9) nona- and (10) deca-. Generally the letter a is omitted from the prefix (from tetra on ) when naming a nonmetal oxide and often mono- is omitted from the name altogether.The Stock system is also used with nonmetal oxides. Here the Roman numeral refers to the oxidation state of the element other than oxygen.In either system, the element other than oxygen is named first, the full name being used, followed by oxide 3. Table 4 shows some examples.Naming AcidsAcid names may be obtained directly from a knowledge of Table 2 by changing the name of the acid ion (the negative ion ) in the Table 2 as follows:The Ion in Table 2Corresponding Acid-ate-ic-ite-ous-ide-icExamples are:Acid Ion Acidacetate acetic acidperchlorate perchloric acidbromide hydrobromic acidcyanide hydrocyanic acidThere are a few cases where the name of the acid is changed slightly from that of the acid radical; for example, H2SO4 is sulphuric acid rather than sulphic acid. Similarly, H3PO4 is phosphoric acid rather than phosphic acid.Naming Acid and Basic Salt and Mixed SaltsA salt containing acidic hydrogen is termed an acid salt.A way of naming these salts is to call Na 2HPO4disodiumhydrogen phosphate and NaH2PO4sodium dihydrogenphosphate. Historically, the prefix bi- has been used innaming some acid salts; in industry, for example, NaHCO3 iscalled sodium bicarbonate and Ca(HSO3)2 calcium bisulphite.Bi(OH)2NO3, a basic salt, would be called bismuthdihydroxynitrate. NaKSO4, a mixed salt, would be calledsodium potassium sulphate.3 NOMENCLATURE OF ORGANIC COMPOUNDSA complete discussion of definitive rules of organic nomenclature would require more space than can be allotted in this text. We will survey some of the more common nomenclature rules, both IUPAC and trivial.AlkanesThe names for the first twenty continuous-chain alkanes are listed in Table 1.Alkenes and AlkynesUnbranched hydrocarbons having one double bond are named in the IUPAC system by replacing the ending -ane of the alkane name with -ene. If there are two or more double bonds, the ending is -adiene, -atriene, etc.Unbranched hydrocarbons having one triple bond are named by replacing the ending -ane of the alkane name with -yne. If there are two or more triple bonds, the ending is -adiyne, -atriyne etc. Table 2 shows names for some alkyl groups, alkanes, alkenes and alkynes.The PrefixesIn the IUPAC system, alkyl and aryl substituents and many functional groups are named as prefixes on the parent (for example, iodomethane). Some common functional groups named as prefixes are listed in Table 3.In simple compounds, the prefixes di-, tri-, tetra-, penta-, hexa-, etc. are used to indicate the number of times a substituent is found in the structure: e.g., dimethylamine for (CH3)2NH or dichloromethane for CH2Cl2.In complex structures, the prefixes bis-, tris-, and tetrakis- are used: bis- means two of a kind; tris-, three of a kind; and tetrakis-, four of a kind. [(CH3)2N]2is bis(dimethylamino) and not di(dimethylamino).Nomenclature Priority of Functional GroupsIn naming a compound, the longest chain containing principal functional group is considered the parent. The parent is numbered from the principal functional group to the other end, the direction being chosen to give the lowest numbers to the substituents. The entire name of the structure is then composed of (1) the numbers of the positions of the substituts (and of the principal functional group, if necessary); (2) the names of the substituts;(3) the name of the parent.The various functional groups are ranked in priority as to which receives the suffix name and the lowest position number1.A list of these priorities is given in Table 4.*-CKetonesIn the systematic names for ketones, the -e of the parent alkane name is dropped and -one is added. A prefix number is used if necessary.In a complex structure, a ketone group my be named in IUPAC system with the prefix oxo-. (The prefix keto- is also sometimes encountered.)AlcoholsThe names of alcohols may be: (1) IUPAC; (2) trivial; or, occasionally, (3) conjunctive. IUPAC names are taken from the name of the alkane with the final -e changed to -ol. In the case of polyols, the prefix di-, tri- etc. is placed just before -ol, with the position numbers placed at the start of the name, if possible, such as, 1,4-cyclohexandiol. Names for some alkyl halides, ketones and alcohols are listed in Table 5.EthersEthers are usually named by using the names of attached alkyl or aryl groups followed by the word ether. (These are trivial names.) For example, diethyl ether.In more complex ethers, an alkoxy- prefix may be used. This is the IUPAC preference, such as 3-methoxyhexane. Sometimes the prefix- oxa- is used.AminesAmines are named in two principal ways: with -amine as the ending and with amino- as a prefix. Names for some ethers and amines can be found in Table 6.Carboxylic AcidsThere are four principal types of names for carboxylic acids: (1) IUPAC; (2)trivial;(3)carboxylic acid; and (4)conjunctive. Trivial names are commonly used.AldehydesAldehydes may be named by the IUPAC system or by trivial aldehyde names. In the IUPAC system, the -oic acid ending of the corresponding carboxylic acid is changed to -al, such as hexanal. In trivial names, the -ic or -oic ending is changed to -aldehyde, such as benzaldehyde. Table 7 gives a list of commonly encountered names for carboxylic acids and aldehydes.Esters and Salts of Carboxylic AcidsEsters and salts of carboxylic acids are named as two words in both systematic and trivial names. The first word of the name is the name of the substituent on the oxygen. The second word of the name is derived from the name of the parent carboxylic acid with the ending changed from -ic acid to -ate.AmidesIn both the IUPAC and trivial systems, an amide is named by dropping the -ic or -oic ending of the corresponding acid name and adding -amide, such as hexanamide (IUPAC) and acetamide (trivial).Acid AnhydridesAcid anhydrides are named from the names of the component acid or acids with the word acid dropped and the word anhydride added, such as benzoic anhydride.The names for some esters, amides and anhydrides are shown in Table 8.Acid HalidesAcid halides are named by changing the ending of the carboxylic acid name from -ic acid to -yl plus the name of the halide, such as acetyl chloride.Some names of aryl compounds and aryls are as follows:benzenephenylbenzylarylbenzoic acid4. Introduction to Chemistry Department of FloridaUniversityProgram of StudyThe Department of Chemistry offers programs of study leading to the M.S. and Ph.D. degrees. Students may elect studies in analytical, inorganic, organic, and physical chemistry. Specialty disciplines, such as chemical physics and quantum, bioorganic, polymer, radiation, and nuclear chemistry, are available within the four major areas.The M.S. and Ph.D. degree requirements include a course of study, attendance at and presentation of a series of seminars, and completion and defense of a research topic worthy of publication1. Candidates for the Ph.D. degree must also demonstrate a reading ability of at least one foreign language and show satisfactory performance on a qualifying examination. The M.S. degree is not a prerequisite for the Ph.D. degree. A nonthesisdegree program leading to the M.S.T. degree is offered for teachers.Students are encouraged to begin their research shortly afterselecting a research director, who is the chairman of the supervisorycommittee that guides the student through a graduate career.Research FacilitiesThe chemistry department occupies 111,000 square feet of space in four buildings: Leigh Hall, the Chemical Research Building, Bryant Hall, and the Nuclear Science Building. Plans for a 65,000-square-foot addition to Leigh Hall are being prepared. A new central science library is located near the chemistry facilities. The University library system holds more than 2.2 million volumes.The major instrumentation includes ultraviolet-visible, infrared, fluorescence, Roman, nuclear magnetic resonance, electron spin resonance, X-ray, ESCA, and mass spectrometers. Many are equipped with temperature-control and Fourier-transform attachments, and some have laser sources. Data-storage and data-acquiring minicomputers are interfaced to some of the instruments, such as the recently constructed quadrupole resonance mass spectrometer. The chemistry department has V AX-11/780 and V AX-11/750 computers as well as multiple terminals connected to IBM machines in the main computer centre on campus.The departmental technical services include two well-equipped stockrooms and glassblowing, electronics, and machine shops to assist in equipment design, fabrication, and maintenance.Financial AidMost graduate students are given financial support in the form of teachingand research assistantships. Stipends range from $9400 - 11,000 for the1986-87 calendar year. State residents and assistantship holders pay in-statefees of about $1400 per calendar year. A limited number of full orsupplemental fellowships are available for superior candidates.Cost of StudyIn 1985-86, in-state students paid a registration fee of $48.62, per credit hour for each semester, out-of-state students paid an additional $ 94.50 ($ 143.12 per credit hour each semester). A small increase in fees is expected for 1986-87.5 ENVIRONMENTAL POLLUTIONWith the coming of the Industrial Revolution the environmentalpollution increased alarmingly. Pollution can be defined as an undesirablechange in the physical, chemical, or biological characteristics of the air, water,or land that can harmfully affect health, survival, or activities of humans orother living organisms. There are four major forms of pollution - waste onland, water pollution (both the sea and inland waters), pollution of the atmosphere and pollution by noise.Land can be polluted by many materials. There are two major types of pollutants: degradable and nondegradable. Examples of degradable pollutantsare DDT and radioactive materials. DDT can decompose slowly buteventually are either broken down completely or reduced to harmless levels. For example, it typically takes about 4 years for DDT in soil to be decomposed to 25 percent of the original level applied. Some radioactive materials that give off harmful radiation, such as iodine-131, decay to harmless pollutants. Others, such as plutonium-239 produced by nuclear power plants, remains at harmful levels for thousands to hundreds of thousands of years.Nondegradable pollutants are not broken down by natural processes. Examples of nondegradable pollutants are mercury, lead and some of their compounds and some plastics. Nondegradable pollutants must be either prevented from entering the air, water, and soil or kept below harmful levels by removal from the environment.Water pollution is found in many forms. It is contamination of water with city sewage and factory wastes; the runoff of fertiliser and manure from farms and feed lots; sudsy streams; sediment washed from the land as a result of storms, farming, construction and mining; radioactive discharge from nuclear power plants; heated water from power and industrial plants; plastic globules floating in the world‟s oceans; and female sex hormones entering water supplies through the urine of women taking birth control pills.Even though scientists have developed highly sensitive measuringinstruments, determining water quality is very difficult. There are a largenumber of interacting chemicals in water, many of them only in trace amounts.About 30,000 chemicals are now in commercial production, and each yearabout 1,000 new chemicals are added. Sooner or later most chemicals end up in rivers, lakes, and oceans. In addition, different organisms have different ranges of tolerance and threshold levels for various pollutants. To complicate matters even further, while some pollutants are either diluted to harmless levels in water or broken down to harmless forms by decomposers and natural processes, others (such as DDT, some radioactive materials, and some mercury compounds) are biologically concentrated in various organisms1.Air pollution is normally defined as air that contains one or more chemicals in high enough concentrations to harm humans, other animals, vegetation, or materials. There are two major types of air pollutants. A primary air pollutant is a chemical added directly to the air that occurs in a harmful concentration. It can be a natural air component, such as carbon dioxide, that rises above its normal concentration, or something not usually found in the air,such as a lead compound. A secondary air pollutant is a harmful chemical formed in the atmosphere through a chemical reaction among air components.We normally associate air pollution with smokestacks and cars, but volcanoes, forest fires, dust storms, marshes, oceans, and plants also add to the air chemicals we consider pollutants. Since these natural inputs are usually widely dispersed throughout the world, they normally don‟t build up to harmful levels. And when they do, as in the case of volcanic eruptions, they are usually taken care of by natural weather and chemical cycles2.As more people live closer together, and as they use machines to produce leisure, they find that their leisure, and even their working hours, become spoilt by a byproduct of their machines – namely, noise,The technical difficulties to control noise often arise from the subjective-objective nature of the problem. You can define the excessive speed of a motor-car in terms of a pointer reading on a speedometer. But can you define excessive noise in the same way? You find that with any existing simple “noise-meter”, vehicles which are judged to be equally noisy may show considerable difference on the meter.Though the ideal cure for noise is to stop it at its source, thismay in many cases be impossible. The next remedy is to absorb iton its way to the ear. It is true that the overwhelming majority ofnoise problems are best resolved by effecting a reduction in thesound pressure level at the receiver. Soft taped music in restaurantstends to mask the clatter of crockery and the conversation at thenext table. Fan noise has been used in telephone booths to maskspeech interference from adjacent booths. Usually, the problem is how to reduce the sound pressure level, either at source or on the transmission path.6 ANALYTICAL INSTRUMENT MARKETThe market for analytical instruments is showing a strength only dreamed about as little as five years ago. Driven by the need for greater chemicalanalysis coming from quality control and government regulation, arobust export market, and new and increasingly sophisticatedtechniques, sales are increasing rapidly1.The analytical instrument business' worldwides sales arenearly double their value of five years ago, reaching $ 4.1 billion in1987. Such growth is in stark contrast to the doldrums of severalyears ago when economic recession held back sales growth to littleor nothing. In recent years, the instrumentation market hasrecovered, growing at nearly 9% per year, and it‟s expected t o continue at this rate at least until the 1990. With sales increases exceeding inflation, the industry has seen the real growth demonstrating the important role of chemical instrumentation in areas such as research and development, manufacturing, defense, and the environment in a technologically advancingworld2.Chromatography is the fastest-growing area, comprising 40%, or $ 1.5billion, in 1987 world sales. Chromatographic methods are used extensively inindustrial labs, which purchase about 70% of the devices made, for separation,purification, and analysis. One of the biggest words in all forms of chromatography is “biocompatibility.” Biocompatible instruments are designed to have chemically inert, corrosion-resistant surfaces in contact with the biological samples.Gas Chromatography sales are growing at about the same rate as the instrument market.Some of the newest innovations in GC technology are the production of more instruments with high-efficiency, high-resolution capillaries and supercritical fluid capability.Despite having only a 3% share of the GC market, supercritical fluid chromatography (SFC) has attracted a great deal of attention since its introduction around 1985 and production of the first commercial instrument around 1986. SFC, which operates using asupercritical fluid as the mobile phase, bridgesthe gap between GC and HPLC. The use ofthese mobile phases allows for higherdiffusion rates and lower viscosities thanliquids, and a greater solvating powerthan gases.Another area showing tremendous growth is ion chromatography (IC). From growth levels of 30% per year in the U.S. and similar levels worldwide, the rate is expected to drop slightly but remain high at 25%. The popularity of IC has been enhanced through extending its applicability from inorganic systems to amino acids and other biological systems by the introduction of biocompatible instruments.Mass spectrometry (MS) sales have been growing about 12% annually. Sales have always been high, especially since MS is the principal detector in a number of hyphenated techniques such as GC-MS, MS-MS, LC-MS, and GC-MS accounts for about 60% of MS sales since it is used widely in drug and environmental testing. Innovations in interface technology such as inductively coupled plasma/MS, SFC/MS, and thermospray or particle beam interfaces for LC-MS have both advanced the technology and expanded the interest in applications. Recent MS instruments with automated sampling and computerized data analysis have added to the attractiveness of the technique for first time users.Spectroscopy accounts for half of all instrument sales and is the largest overall category of instruments, as the Alpert & Suftcliffe study shows. It can be broken down evenly into optical methods and electromagnetic, or nonoptical, spectroscopies. These categories include many individual high-cost items such as MS, nuclear magnetic resonance spectrometers, X-ray equipment, and electron microscopy and spectroscopy setups. Sales of spectroscopic instruments that are growing at or above the market rate include Fourier transform infrared (FTIR), Raman, plasma emission, and energy dispersive X-ray spectrometers. Others have matured and slowed down in growth, but may still hold a large share of the market.The future of analytical instrumentation does not appear to be without its new stars as there continue to be innovations and developments in existing technology. Among these are the introduction of FT Raman, IR dichroism, IR microscopy, and NMR imaging spectrometers. Hyphenated and automated apparatus are also appearing on the market more frequently. New analytical techniques like capillary electrophoresis, gel capillary electrophoresis, scanning tunneling microscopy for the imaging of conducting systems, atomic force microscopy for the imaging of biological systems, and other techniques for surface and materials analysis are already, or may soon be, appearing as commercialized instruments. And, if the chemical industry continues to do well in the next few years, so too will the sales of analytical instrumentation.The effect of alcohol have both medical and medicolegal implications. The estimationof alcohol in the blood or urine is relevant when the physician needs toknow whether it is responsible for the condition of the patient. From themedicolegal standpoint the alcohol level is relevant in cases of suddendeath, accidents while driving, and in cases when drunkenness is thedefense plea. The various factors in determining the time after ingestion showing maximum concentration and the quality of the alcohol are the weight of the subject,。
化学及化工专业词汇英语翻译a-c18 electro n rule 18 电子则abbe refract ometer阿贝折射计abbrevi ated analysi s 简略分析abderha lden's dryer 阿布德尔哈尔登干燥器abderha lden's reactio n 阿布德尔哈尔登反应abegg's rule 阿贝格规则abel closedtester阿贝尔氏密闭实验机abel penskytester阿贝尔彭斯基试验器abel tester阿贝尔试验器abelite阿贝立特aberrat ion 像差abies oil 松节油abietat e 松香酯abietic acid 松香酸abietin松香素abioche mistry无生化学;无机化学ablatio n 消融ablutio n 洗净abnorma l setting反常凝结abnorma lity 反常abradan t 磨料abrader磨损试验机abrasio n 磨耗abrasio n loss 磨损量abrasio n resista nce 耐磨能力abrasio n test 磨耗试验abrasio n testing machine磨损试验机abrasiv e 磨料abrasiv e grain 磨料颗粒abrasiv e industr y 磨料工业abrasiv e paper 砂纸abrasiv eness磨损性abrasiv es 研磨剂abs resin abs 尸abs resinsabs尸abscisi c acid 阿伯喂酸absinth e oil 洋艾油absolut e activit y 绝对活性度absolut e alcohol无水酒精absolut e calibra tion 绝对校准absolut e configu ration绝对构型absolut e dry conditi on 绝对干燥状态absolut e dry weight绝对干重absolut e error 绝对误差absolut e humidit y 绝对湿度absolut e measure ment 绝对测量absolut e reactio n rate 绝对反应速度absolut e sensiti vity 绝对灵敏度absolut e specifi c gravity真比重absolut e tempera ture 绝对温度absolut e unit 绝对单位absolut e value 绝对值absolut e viscosi ty 绝对粘度absolut e zero 绝对零度absorba bility吸收性absorba nce 吸光度absorbe d dose 吸收线量absorbe nt 吸收剂absorbe nt paper 吸收纸absorbe r 吸收器吸收体absorbi ng power 吸收能力absorpt iomete r 吸光测定计absorpt iometr ic analysi s 吸光分析absorpt iometr y 吸收分光光度法absorpt ion 吸收absorpt ion band 吸收带absorpt ion cell 吸收池absorpt ion coeffic ient 吸收系数absorpt ion column吸收塔absorpt ion curve 吸收曲线absorpt ion edge 吸收端absorpt ion factor吸收因子absorpt ion heat 吸收热absorpt ion intensi ty 吸收强度absorpt ion line 吸收线absorpt ion maximum最大吸收absorpt ion method吸收法absorpt ion oil 吸收油absorpt ion pipet 吸收管absorpt ion refrige rator吸收式冷冻器absorpt ion spectro photom etry 吸收分光光度法absorpt ion spectru m 吸收光谱absorpt ion tower 吸收塔absorpt ion tube 吸收管absorpt ive power 吸收能力absorpt ivity吸收率abukuma lite 钇硅磷灰石abysmal deposit深海沉积物abyssal deposit深海沉积物ac polarog raphy交莲谱法acacia阿拉伯屎acarici de 杀螨剂acaroid resin 禾木尸acceler ant 促进剂acceler ated aging 加速老化acceler ated aging test 加速老化试验acceler ated weather ing test 加速风化试验acceler atingagent 促进剂acceler ationglobuli n 促凝血球蛋白acceler ationof gravity重力加速度acceler ator 促进剂accepto r 接受体accesso ry constit uent 副成分accetyl value number乙酰值acciden tal error 偶然误差acclima tizati on 驯化accommo dation适应accommo dation coeffie ient 适应系数accumul ator 蓄电池accumul ator acid 蓄电池酸液accurac y 准确度acenaph thene威杀灵acenaph thenequinone苊醌acenaph thenon e 二氢苊酮acenaph thylen e 萘嵌戊烯acenoco umarol苊香豆醇acephat e 乙酰甲胺磷acetal乙缩醛acetalphospha tide 缩醛磷脂acetalresin 缩醛尸acetald ehydas e 乙醛酶acetald ehyde乙醛acetald ehydeammonia乙醛合氨acetald ehydereducta se 醇脱氢酶acetald ol 3 羟基丁醛acetald oxime乙醛肟acetami de 乙酰胺acetami dine 乙脒acetani lide 乙酰苯胺acetars ol 乙酰胂胺acetate醋酸盐acetate dye 醋酸染料acetate fiber 醋酸纤维acetate film 醋酸纤维胶片acetate rayon 醋酸丝acetazo lamide乙酰唑胺aceticacid 醋酸aceticacid ferment ation乙酸发酵aceticacid glacial冰醋酸aceticaldehyd e 乙醛aceticanhydri de 醋酐aceticbacteri a 醋酸菌aceticester 醋酸酯acetifi cation醋化酌acetime ter 醋酸计acetine醋精acetoac etanil ide 乙酰乙酰替苯胺acetoac etate乙酰醋酸盐acetoac etic acid 乙酰醋酸acetoin醋偶姻acetol丙酮醇acetola ctic acid 乙酰乳酸acetoly sis 乙酸水解acetome roctol醋汞辛酚acetome try 醋酸测定法acetone丙酮acetone alcohol丙酮醇acetone body 酮体acetone butanol ferment ation丙酮丁醇发酵acetone chlorof orm 三氯叔丁醇acetone cyanhyd rin 丙酮合氰化氢acetone dicarbo xylicacid 丙酮二羧酸acetone ferment ation丙酮发酵acetone sugar 丙酮糖acetoni c acid 醋酮酸acetoni trile乙腈acetony l acetone丙酮基丙酮acetoph enetid in n 乙酰乙氧基苯胺acetoph enone苯乙酮acetopu rpurin乙酰替红紫acetoxi me 丙酮肟acetoxy l group 乙酰氧基acetoxy lation乙酸化aceturi c acid 乙酰甘氨酸acetylbromide乙酰溴acetylchlorid e 乙酰氯acetylhydrope roxide过乙酸acetyliodide碘化乙酰acetylketene二酮acetylperoxid e 过氧化乙酰acetylpropion yl 乙酰丙酰acetylvalue 乙酰值acetyla cetone乙酰丙酮acetyla se 乙酰酯酶acetyla ting agent 乙酰剂acetyla tion 乙酰化acetylb enzoyl peroxid e 乙酰过氧化苯甲酰acetylc ellulo se 乙酰纤维素acetylc holine乙酰胆碱acetyle ne 乙炔acetyle ne black 乙炔炭黑acetyle ne burner乙炔燃烧器acetyle ne chemist ry 乙炔化学acetyle ne chlorid e 乙炔基氯acetyle ne complex乙炔络合物acetyle ne generat or 乙炔发生器acetyle ne linkage炔键acetyle ne polymer乙炔聚合物acetyle ne tetrach loride四氯乙炔acetyle ne welding气焊acetyle nic hydroca rbon 乙炔属烃类acetyli de 乙炔化合物acetyli soeuge nol 乙酰异丁子香酚acetylp henylh ydrazi ne 乙酰苯肼acetyls alicyl ic acid 乙酰水杨酸acetylu rea 乙酰脲achiral ity 非手胀achroit e 无色电气石achroma tic lens 消色差透镜aci form 针形acicula r crystal针状结晶acid 酸acid accepto r 受酸体acid albumin酸蛋白acid alizari ne 酸性茜素acid amide 酸胺acid ammoniu m sulfate硫酸氢铵acid ammoniu m tartrat e 酒石酸氢铵acid anhydri de 酸酐acid azid 酰基叠acid azo dye 酸性偶氮染料acid base catalys is 酸碱催化acid base equilib rium 酸碱平衡acid base indicat or 酸碱指示剂acid base pair 酸碱对acid base titrati on 酸碱滴定acid bath 酸浴acid black 酸性黑acid carbona te 酸性碳酸盐acid catalys t 酸催化剂acid chlorid e 酸性氯化物acid content含酸量acid convert er 酸性转炉acid decompo sition酸分解acid dye 酸性染料acid egg 酸蛋acid elevato r 酸蛋acid error 酸误差acid ferment ation酸发酵acid fixingbath 酸性定像浴acid former成酸物质acid fuchsin酸性品红acid green 酸性绿acid group 酸根acid halide酸性卤化物acid hydroly sis 加酸水解酌;酸解acid iodide酰基碘acid mordant dye 酸性媒染料acid number酸值acid of lemon 柠檬酸acid pickle废酸液acid picklin g 酸浸acid potassi um carbona te 酸式碳酸钾acid potassi um sulfate硫酸氢钾acid precipi tation酸雨acid proof alloy 耐酸合金acid proof brick 耐酸砖acid proof enamel防酸搪瓷acid proof paint 耐酸涂料acid proof pump 耐酸泵acid radical酸基acid reactio n 酸性反应acid recover y plant 废酸回收设备acid refract ory 酸性耐火材料acid resista nce 耐酸性acid salt 酸性盐acid sludge废酸acid sodiumcarbona te 酸式碳酸钠acid solutio n 酸溶液acid strengt h 酸强度acid sulfate硫酸氢盐acid treatme nt 酸处理acid value 酸值acidicoil resin 酸性油尸acidicoxide 酸性氧化物acidicreactio n 酸性反应acidictitrant酸性滴定剂acidifi cation酸化acidime ter 酸比重计acidime try 酸量滴定acidity酸度acidity functio n 酸度函数acidoly sis 酸解acidome ter 酸度计acidome try 酸度测定法acidosi s 酸中毒acmite锥辉石aconicacid 乌头酸aconita se 乌头酸酶aconiti c acid 乌头酸aconiti ne 乌头碱acousti cal materia l 音响材料acousto chemic al 声化学的acousto chemis try 声化学acrasin聚集素acridin e 吖啶acridin e dye 吖啶染料acridin e orange吖啶橙acridin e yellow吖啶黄acridon e 吖啶酮acrifla vine 吖啶黄素acrifla vine hydroch loride盐酸氮蒽黄acrinam in 奎纳克林acrolei n 丙烯醛acrolei n dimer 丙烯醛二聚物acrolei n resin 丙烯醛尸acrolei n test 丙烯酸试验acrosin精虫头粒蛋白acrylal dehyde丙烯醛acrylam ide 丙烯酰胺acrylat e 丙烯酸盐acrylic acid 丙烯酸acrylic fiber 丙烯酸纤维acrylic resin 丙烯酸尸acrylic rubber丙烯酸橡胶acrylon itrile丙烯腈actin 肌动蛋白actinic rays 光化射线actinid e 锕类actinid es 锕化物actinis m 光化度actiniu m 锕actiniu m series锕系actinoc hemist ry 光化学actinoc hitin辐几丁质actinol ite 阳起石actinom eter 日光辐射计actinom ycin 放线菌素actinon锕射气actinou ranium锕铀activat ed adsorpt ion 活性吸附activat ed alumina活性矾土activat ed atom 活化原子activat ed carbon活性炭activat ed clay 活性白土activat ed complex活化络合物activat ed molecul e 活化分子activat ed sludge活性污泥activat ed sludgeprocess活性污泥法activat ed state 跃迁态activat ion 活化activat ion analysi s 放射化分析activat ion energy活化能activat or 活化剂activecarbon活性炭activecenter活化中心activecharcoa l 活性炭activedeposit活性沉积物activeearth 漂白土activeearths活性土activefiller活性填料activegroup 活性基activehydroge n 活性氢activemass 有效质量activemateria l 活性物质activenitroge n 活性氮activeoxygen活性氧activepower 有效功率activesolvent活性溶剂activit y 活度activit y coeffic ient 活度系数activit y index 活动指数actomyo sin 肌动球朊acyclic无环的acyclic compoun d 无环化合物acyclic hydroca rbon 无环烃acyl carrier protein酰基载体蛋白acyl chlorid e 酰基氯acyl group 酰基acyl peroxid e 过氧化酰基acylati ng agent 酰化剂acylati on 酰化acylcar bene 酰基聚炔acylnit rene 酰氮烯acyloin偶姻acyloin condens ation偶姻缩合acyloin form 偶姻形acylure a 酰基脲adalin阿达林adamant ane 金刚烷adamkie witz reactio n 阿当凯维奇反应adamsit e 亚当氏毒气adaptab ility适应性adaptat ion 适应adapter接管adaptiv e system自适应系统adatom被吸附原子additio n 附加additio n agent 添加剂additio n compoun d 加成化合物additio n polymer加聚物additio n polymer izatio n 加聚酌additio n product附加产物additio n reactio n 加成反应additio n solid solutio n 加成固溶体additio nal loss 附加损失additiv e 添加剂additiv e color process加色法additiv e mixing加法混合additiv ity 加性adduct加合物adductrubber加合橡胶adenase腺嘌呤酶adenine腺嘌呤adenosi nase 腺苷酶adenosi ne 腺苷adenosi ne diphosp hatase二磷酸腺苷酶adenosi ne monopho sphate腺苷一磷酸adenosi ne triphos phatas e 三磷酸腺苷酶adenosi ne triphos phate三磷酸腺苷adenyli c acid 一磷酸腺苷adeps lanae 羊毛脂adermin抗皮炎素adheren ce 粘合adheren ce test 粘着试验adhesio n 粘合adhesiv e 胶粘的adhesiv e ability粘着能力adhesiv e bond 粘结结合adhesiv e capacit y 粘着能力adhesiv e film 粘附膜adhesiv e power 粘附力adhesiv e tape 粘合带adhesiv eness粘合性adhesiv es 粘着剂adhesiv ity 粘附性adiabat ic calorim eter 绝热量热器adiabat ic change绝热变化adiabat ic compres sibili ty 绝热压缩系数adiabat ic curve 绝热曲线adiabat ic expansi on 绝热膨胀adiabat ic lapse rate 绝热递减率adiabat ic potenti al 绝热电位adiabat ic process绝热过程adiabat ic reactio n 绝热反应adiabat ic reactor绝热反应器adion 吸附离子adipami de 己二酰胺adipate己二酸酯adiphen ine 解痉素adipicacid 己二酸adipoce llulos e 脂纤维素adiponi trile己二腈adjab butter毒雾冰草油admixtu re 掺和物adonito l 阿东醇adrenal cortexhormone肾上腺皮质激素adrenal in 肾上腺素adsorba bility吸附性adsorba te 吸附质adsorbe nt 吸附剂adsorbe r 吸附器adsorpt iomete r 吸附测量表adsorpt iometr y 吸附测量法adsorpt ion 吸附adsorpt ion analysi s 吸附分析adsorpt ion capacit y 吸附能力adsorpt ion chromat ograph y 吸附色层分析法adsorpt ion compoun d 吸附化合物adsorpt ion current吸附电流adsorpt ion equilib rium 吸附平衡adsorpt ion exponen t 吸附指数adsorpt ion heat 吸附热adsorpt ion indicat or 吸附指示剂adsorpt ion isother m 吸附等温线adsorpt ion potenti al 吸附势adsorpt ion site 吸附点adsorpt ion water 吸附水adsorpt ion wave 吸附波aerated concret e 加气混凝土aerated water 充气水aeratio n 吹风aerobe需氧微生物aerobic digesti on 需氧消化aerobic ferment ation需氧发酵aeroche mical空气化学的aeroche mistry空气化学aerocol loid 气溶胶aerodyn amic sound 空气动力声aerogel气凝胶aerophi ly 亲气性aerosil硅胶aerosol气溶胶aerosol propell ant 气溶胶抛射剂aerosol ogical气溶胶的aerosol ology气溶胶学aerothe rmoche mical空气热化学的aerothe rmoche mistry空气热化学aerotro pic 向氧性的aescule tin 七叶亭affinat ion 精制affinit y 亲合力affinit y labelin g 亲和标记aflatox in 黄曲毒素after cure 后硫化after effect后效应after ferment ation后发酵after ripenin g 后熟after vulcani zation后硫化afterco oler 后冷却器afterig nition后点火aftersh rinkag e 后期收缩aftertr eatmen t 后处理agar 琼脂agar agar 琼脂agaricacid 松蕈酸agarici n 松蕈酸agate mortar玛瑙研钵agavose龙舌兰糖age hardeni ng 时效硬化age resiste r 抗老剂agent 试剂agent of oxidati on 氧化剂agglome rate 烧结块agglome ration结块aggluti nant 凝集剂aggluti nation凝集aggluti nation reactio n 凝集反应aggluti nin 凝集素aggluto meter胶粘计aggrega te 聚集体aggrega tion 聚集aging 成熟aging resista nce 抗老化性agitati on 搅拌agitato r 搅拌机aglycon糖苷配基agmatin精胺agricul turalchemist ry 农业化学air bath 空气浴air bubble气泡air cell 空气电池air chamber空气室air classif ier 风力分级器air compres sor 空气压缩机air condens er 空气冷凝器air conditi oning空气第air conditi oningequipme nt 空气第设备air content空气含量air cooler空气冷却器air cure 空气硫化air deficie ncy 空气缺乏air drying空气干燥air elutria tion 风筛air entrain ing agent 加气剂air excess空气过量air filter空气过滤器air fuel ratio 空气燃料比air gas 空气气体air hardeni ng steel 空气淬硬钢air heater空气加热器air humidit y 空气湿度air lift 空气吸扬air liquefi er 空气液化器air liquefy ing apparat us 空气液化装置air oxidati on 空气氧化air polluti on 大气污染air pump 气泵air purifie r 空气净化器air reducti on process空气还原法air reservo ir 储气筒air separat ion 吹气分离air separat or 空气分离器air setting空气硬化air shower空气淋浴air tempera ture 气温airhole风眼ajax powder阿加克斯火药ajmalin e 阿吗灵ajowanoil 香旱芹油alaband ite 硫锰矿alabast er glass 雪花玻璃alanine丙氨酸albariu m 大理石灰albedo反射率alberti te 黑沥青alberto l 阿耳伯特尸albite钠长石albolit e 镁硅塑胶album paper 像簿纸albumin白朊albumin globuli n ratio 白蛋白球蛋白比albumin glue 蛋白胶albumin ate 白蛋白化合物albumin oid 硬朊alchemy炼金术alcianblue 阿尔新蓝alcogel醇凝胶alcohol醇alcohol acid 醇酸alcohol dehydro genase醇脱氢酶alcohol fuel 酒精燃料alcohol lamp 酒精灯alcohol of crystal lizati on 结晶醇alcohol thermom eter 酒精温度计alcohol varnish醇溶清漆alcohol ase 醇酶alcohol ate 烃氧基金属alcohol ic compoun d 醇化合物alcohol ic extract酒精提出物alcohol ic ferment ation酒精发酵alcohol ic potash钾碱醇液alcohol ic solutio n 醇溶液alcohol ism 酒中毒alcohol meter酒精比重计alcohol ometry酒精测定alcohol ysis 醇解alcosol醇溶胶alcoxyl烷氧aldehyd e 醛aldehyd e acid 醛酸aldehyd e alcohol醛醇aldehyd e ammonia醛氨aldehyd e dehydro genase醛脱氢酶aldehyd e resin 聚醛尸aldimin e 亚胺醛aldohep tose 庚醛糖aldohex ose 乙醛糖aldoket ene 醛烯酮aldol 羟醛aldol condens ation醛醇缩合aldol reactio n 醇醛缩合反应aldolas e 醛缩酶aldoliz ation缩醛反应aldonic acid 醛糖酸aldopen tose 戊醛糖aldose醛糖aldoste rone 醛甾酮aldotri ose 丙醛糖aldox process羰醇法aldoxim e 醛肟aldrin艾氏剂alexand rite 翠绿宝石alfin catalys t 阿尔芬催化剂alfin polymer阿尔芬聚合物alfin polymer izatio n 阿尔芬聚合algae 藻类algin 藻酸alginat e 藻蛋白酸盐alginat e fiber 藻酸纤维alginic acid 海藻酸algol blue 阿果蓝algol color 阿果染料algorit hm 算法alicycl ic 脂环族的alicycl ic compoun d 脂环化合物alieste rase 脂族酯酶aliphat ic 无环的aliphat ic acid 脂族酸aliphat ic alcohol脂族醇aliphat ic amine 脂族胺aliphat ic base 脂族碱aliphat ic compoun d 脂族化合物aliphat ic ether 脂族醚aliphat ic hydroca rbon 脂族烃aliphat ic series脂族系aliphat ic unsatur ated carboxy lic acid 脂族不饱羧酸alite 阿里特alizari n 茜素alizari n blue 茜素蓝alizari n brown 茜素棕alizari n dye 茜素染料alizari n lake 茜素色淀alizari n yellow茜黄alizari ne 茜素alkali碱alkaliblue 碱性蓝alkalicellulo se 碱纤维素alkalifusion碱熔融alkaliion diode 碱离子二极管alkalilignin碱木素alkaliliquor碱液alkalimetal 碱金属alkaliresista nce 耐碱性alkalirock 碱性岩alkalisalt 碱金属盐alkalim eter 碱量计alkalim etry 碱量滴定法alkalin e accumul ator 减蓄电池alkalin e bath 碱浴alkalin e cell 碱性电池alkalin e cleaner碱性清洗剂alkalin e earth metal 碱土金属alkalin e earths碱土族alkalin e hydroly sis 加碱水解alkalin e reactio n 碱性反应alkalin e solutio n 碱性溶液alkalin e storage battery减蓄电池alkalin ity 碱度alkaliz ation碱化alkaloi d 生物碱alkaloi d reagent生物碱试剂alkalos is 碱中毒alkamin e 氨基醇类alkane链烷alkanni n 紫草素alkanol amine烷烃醇胺alkansu lfonic acid 链烷磺酸alkene烯烃alkine链炔alkyd paint 醇酸涂料alkyd resin 醇酸尸alkyd resin varnish醇酸清漆alkyl 烷基alkyl cellulo se 烷基纤维素alkyl cyanide烷基氰alkyl group 烷基alkyl halide烷基卤alkyl sulfate烷基硫酸盐alkyl sulfide烷基硫alkyl sulfoni c acid 烷基磺酸alkylar sine 烷基胂alkylar sonicacid 烷基胂酸alkylat e 烷基化产物alkylat ing agent 烷化剂alkylat ion 烷基化alkylbe nzene烷基苯alkylbe nzenesulfona te 烷基苯磺酸盐alkylen e 烷撑alkylid ene 次烷基alkylma gnesiu m halide烷基镁化卤alkylna phthal ene 烷基萘alkyne炔烃alkynol炔醇allanit e 褐帘石allanto ic acid 尿囊酸allanto inase尿囊素酶allanto xanicacid 尿囊毒酸allantu ric acid 尿囊脲酸alleloc hemica l 变异化学的alleloc hemist ry 变异化学allene丙二烯allergy过敏反应allethr in 丙烯拟除虫菊酯allicin蒜辣素alligat or pear oil 鳄梨油allobar bital二烯丙巴比妥allochr omatic crystal羼质色晶体allocin namicacid 别肉桂酸alloiso merism立体异构现象allomer ism 异质同晶allopha ne水铝英石allopha nic acid 脲基甲酸allopre ne 阿洛波林allopur inol 别嘌呤醇allose阿洛糖alloste ric effect别构效应alloste ric enzyme变构酶alloste ric transit ion 变构转变alloste ry 变构性allothr eonine别苏氨酸allotro pe 同素异形体allotro pism 同素异形allotro py 同素异形allowab le error 容许误差allowed transit ion 容许跃迁alloxan阿脲alloxan ic acid 阿脲酸alloxaz ine 咯嗪alloy 合金alloy analysi s 合金分析alloy steel 合金钢allulos e 阿卢糖alluvia l gold 砂金allyl acetate醋酸丙烯酯allyl alcohol烯丙醇allyl amine 烯丙胺allyl bromide烯丙基溴allyl chlorid e 烯丙基氯allyl complex烯丙基络合物allyl compoun d 烯丙基化合物allyl cyanide烯丙基腈allyl iodide烯丙基碘allyl isothio cyanat e 异硫氰酸烯丙酯allyl mercapt an 烯丙硫醇allyl resin 烯丙尸allyl sulfide烯丙基硫allylen e 丙炔allylic rearran gement烯丙重排allylmu stardoil 烯丙基芥子油allylth iourea烯丙基硫脲almandi ne 铁铝榴石almandi te 铁铝榴石almondoil 扁桃仁油aloe 芦荟aloin 芦荟素alpha brass 黄铜alpha cellulo se 纤维素alpha counter粒子计数器alpha iron 铁alpha naphtho l 萘酚alpha positio n 位alpha ray spectro meter射线能谱仪alpha rays 射线alstoni ne 鸡骨常山碱alterna nt hydroca rbon 交替烃alterna ting copolym er 交替共聚物alterna ting current交流alterna ting current polarog raphy交莲谱alterna tion 交替altimet er 测高仪altitud e 高度altrose阿卓糖alum 茂alumel镍基锰合金alumina氧化铝alumina brick 矾土砖alumina bubblebrick 泡沫矾土砖alumina cement矾土水泥alumina fiber 氧化铝纤维alumina gel 铝凝胶alumina silicarefract ory 硅酸铝耐火材料alumina te 铝酸盐alumini um 铝alumini um acetate乙酸铝alumini um alloy 铝合金alumini um ammoniu m sulfate硫酸铝铵alumini um boride硼化铝alumini um bromide溴化铝alumini um bronze铝青铜合金alumini um carbide碳化铝alumini um chlorat e 氯酸铝alumini um chlorid e 氯化铝alumini um ethylat e 乙醇铝alumini um fluorid e 氟化铝alumini um foil 铝箔alumini um hydroxi de 氢氧化铝alumini um nitrate硝酸铝alumini um oleate油酸铝alumini um oxide 氧化铝alumini um plate 铝板alumini um potassi um sulfate硫酸铝钾alumini um powder铝粉alumini um resinat e 尸酸铝alumini um silicat e 硅酸铝alumini um sulfate硫酸铝alumino n 铝试剂alumino silica te 硅铝酸盐alumino thermi t process铝热法alumino thermy铝热法aluminu m 铝alumite茂石alumsto ne 茂石alundum刚铝石alunite茂石amalgam汞齐amalgam cell 汞齐电池amalgam electro de 汞齐电极amalgam ation汞齐化amalgam ationprocess汞齐化过程amaniti n 鹅膏菌素amarant h 蓝光酸性红amatol阿马图amber 琥珀amber glass 琥珀玻璃amberit e 琥珀炸药americi um 镅americy l ion 镅酰离子amethop terin氨甲喋呤amicron次微粒amidase酰胺酶素amidati on 酰胺化amide 酰胺amide chlorid e 二氯代酰胺amidine脒amidine hydroch loride盐酸脒amidohy drolas e 氨基水解酶amidol阿米多amidone美沙酮aminati on 胺化amine 胺amine formald ehyde胺甲醛amine oxidase胺氧化酶amino acid 氨基酸amino acid sequenc e 氨基酸顺序amino compoun d 氨基化合物amino nitroge n 氨基氮amino plastic resin 氨基塑料尸amino resins氨基尸amino sugar 氨基糖amino termina l 氨基末端aminoac etalde hyde 氨基乙醛aminoac etone氨基丙酮aminoal cohol氨基醇aminobe nzoicacid 氨基苯甲酸aminobu tyricacid 氨基丁酸aminoca proicacid 氨基己酸aminodi borane氨基乙硼烷aminogl utaric acid 氨基戊二酸aminogl ycosid e antibio tics 氨基糖苷类抗生物素aminogr am 氨基图aminois ovaler ic acid 氨基异戊酸aminoly sis 氨基分解aminona phthol氨基萘酚aminona phthol sulfoni c acid 氨基萘磺酸aminope ptidas e 氨基胜胨酵素aminoph enol 氨基苯酚aminoph enylar sonicacid 氨基苯胂酸aminoph osphor ylase淀粉磷酸化酶aminoph ylline氨苯碱aminopo lypept idase氨基多胜酵素aminopr otease氨蛋白酶aminopt erin 氨基蝶呤aminopy ridine氨基吡啶aminopy rin 氨基吡啉aminoqu inolin e 氨基喹啉aminosa licyli c acid 氨基水杨酸aminosu ccinic acid 氨基琥珀酸aminosu lfonic acid 氨基磺酸aminoto luene氨基甲苯ammeter电另ammonal阿芒拿ammonia氨ammonia compres sor 氨气压缩机ammonia gas 氨气ammonia poisoni ng 氨中毒ammonia still 氨气塔ammonia synthes is 氨合成ammonia water 氨水ammonia cal brine 氨盐水ammonia cal ferment ation氨发酵ammonia cal latex 氨胶乳ammonia meter氨量计ammonia soda process氨碱法ammonia ted superph osphat e 含铵过磷酸钙ammonia tor 氨化器ammonio metry氨量测定法ammonit e 阿芒炸药ammoniu m 铵ammoniu m acetate乙酸铵ammoniu m alum 铵茂ammoniu m benzoat e 安息香酸铵ammoniu m bifluor ide 氟化氢铵ammoniu m borate硼酸铵ammoniu m carbama te 氨基甲酸铵ammoniu m carbona te 碳酸铵ammoniu m chlorid e 氯化铵ammoniu m chromat e 铬酸铵ammoniu m cyanate氰酸铵ammoniu m dichrom ate 重铬酸铵ammoniu m fluorid e 氟化铵ammoniu m formate甲酸铵ammoniu m hydroge n carbona te 碳酸氢铵ammoniu m hydroxi de 氢氧化铵ammoniu m iodate碘酸铵ammoniu m iron sulfate硫酸铁铵ammoniu m metavan adate偏钒酸铵ammoniu m molybda te 钼酸铵ammoniu m nitrate硝酸铵ammoniu m nitrate explosi ve 硝铵炸药ammoniu m nitrate fertili zer 硝铵肥料ammoniu m oxalate草酸铵ammoniu m perchlo rate 高氯酸铵ammoniu m persulf ate 过硫酸铵ammoniu m phospha te 磷酸铵ammoniu m phosphi te 亚磷酸铵ammoniu m phospho molybd ate 磷钼酸铵ammoniu m picrate苦味酸铵ammoniu m polysul fide 多硫化铵ammoniu m rhodani de 硫氰酸铵ammoniu m salt 铵盐ammoniu m selenat e 硒酸铵ammoniu m stearat e 硬脂酸铵ammoniu m sulfate硫酸铵ammoniu m sulfite亚硫酸铵ammoniu m thiocya nate 硫氰酸铵ammoniu m thiosul fate 硫代硫酸铵ammoniu m uranate铀酸铵ammoniu m vanadat e 钒酸铵ammonob ase 氨基金属ammonol ysis 氨解ammopho s 安福粉amobarb ital 戊巴比妥amodiaq uine 阿莫待喹amorphi sm 无定形amorpho us carbon无定形碳amorpho us graphit e 无定型石墨amorpho us materia l 无定形材料amorpho us metal 无定形金属amorpho us phospho rus 无定形磷amorpho us polymer非晶态聚合物amorpho us state 无定形状态amorpho us sulfur无定形硫ampere安amperem eter 电另amperom etrictitrati on 电廖定amperom etry 电廖定ampheta mine 苯异丙胺amphibo le 闪石amphipa thic molecul e 两亲水脂分子amphiph ilic molecul e 两亲水脂分子ampholy te 两性电解质ampholy tic activeagent 两性表面活性剂ampholy tic surfact ant 两性表面活性剂ampholy toid 两性胶体amphote ric 两性的amphote ric charact er 两性特征amphote ric colloid两性胶体amphote ric compoun d 两性化合物amphote ric ion 两性离子amphote ric oxide 两性氧化物amphote ric resin 两性尸amphote ricele drolyt e 两性电解质amplifi er 放大器ampule安瓿amygdal in 扁桃苷amyl 戊基amyl acetate醋酸戊酯amyl alcohol戊醇amyl bromide戊基溴amyl butyrat e 丁酸戊酯amyl ether 戊醚amyl formate甲酸戊酯amyl mercapt an 戊硫醇amyl nitrite亚硝酸戊酯amyl oleate油酸戊酯amyl propion ate 丙酸戊酯amylami ne 戊胺amylase淀粉酶amylben zene 戊基苯amylene戊烯amylo process淀粉发酵法amylode xtrin淀粉糊精amyloid淀粉状朊amyloly sis 淀粉分解amylope ctin 支链淀粉amylops in 胰淀粉酶amylose直链淀粉amytal戊巴比妥anabasi ne 安纳巴松anaboli sm 同化酌anaerob e 厌氧微生物anaerob ic glycoly sis 无氧糖酵解analcime 方沸石analges ic 镇痛药analogdigital convers ion 模拟数字转换analogsignal模拟信号analogu e 类似analogu e compute r 模拟计算机analysi s 分析analysi s line 分析线analysi s with ion selecti ve electro des 离子选择电极分析法analyte分析物analyti c functio n 解析函数analyti cal balance分析天平analyti cal chemist ry 分析化学analyti cal extract ion 分析抽出analyti cal method分析法analyti cal reactio n 分析反应analyti callypure 分析纯anapait e 斜磷钙铁矿anaphor esis 阴离子电泳anatase octahed rite 锐钛矿anchoragitato r 锚式搅拌器anchorstirrer锚式搅拌器andalus ite 红柱石andesit e 安山岩andreas en pipet 安德烈森型吸管androsi n 雄素androst ane 雄烷androst endion e 雄烯二酮androst erone雄酮andruss ow process安德卢梭法anelast icity滞弹性anemome ter 风速计anemoni n 白头翁脑aneroid baromet er 空盒气压计anesthe sin 氨基苯甲酸乙酯anesthe tic 麻醉剂anethol e 茴香脑aneurin硫胺素angelic a lactone当归内酯angelic a oil 当归油angiote nsin 血管紧张肽angle of polariz ation偏振光角angle of refract ion 折射角angle of repose休止角anglesi te 硫酸铅矿angstro m 埃angular momentu m 角动量anhalon ine 老头掌碱anhydri de 酐anhydri te 硬石膏anhydro ne 无水高氯酸镁anhydro us 无水的anhydro us acid 无水酸anhydro us alcohol无水酒精anhydro us ammonia无水氨anhydro us salt 无水盐anileri dine 氨苄哌替啶anilide酰替苯胺aniline苯胺aniline black 苯胺黑aniline blue 苯胺蓝aniline dye 苯胺染料aniline formald ehyderesin 苯胺甲醛尸aniline hydroch loride盐酸苯胺aniline point 苯胺点aniline red 苯胺红aniline resin 苯胺尸aniline yellow苯胺黄anilol酒精苯胺混合液animalbiochem istry动物生化学animalcharcoa l 骨炭animalchemist ry 动物化学animaldye 动物染料animalfat 动物脂animalfiber 动物纤维animalglue 动物胶animaloil 动物油anime 硬尸anion 阴离子anion activeagent 阴离子表面活性剂anion exchang e 阴离子交换anion exchang e resin 阴离子交换尸anion exchang er 阴离子交换剂anionic polymer izatio n 阴离子聚合anionic surfact ant 阴离子表面活性剂anionoi d reagent类阴离子试剂anionot ropy 阴离子移变现象anisald ehyde茴香醛anise oil 茴香油anisicacid 茴香酸anisicalcohol茴香醇anisidi ne 茴香胺anisole茴香醚anisome tric crystal不等轴晶体anisotr opic body 蛤异性体anisotr opic liquid蛤异性液体anisotr opic membran e 蛤异性膜anisotr opy 蛤异性anisoyl chlorid e 茴香酰氯anisylacetate醋酸茴香酯anisylalcohol茴香醇ankerit e 铁白云石annaber gite 镍华anneali ng 退火anneali ng furnace退火窑anneali ng tempera ture 退火温度annulen e 环轮烯anode 阳极anode effect阳极效应anode process阳极过程anode slime 阳极淀渣anodicoxidati on 阳极氧化anodicpolariz ation阳极极化anodicreactio n 阳极反应anodiza tion 阳极化anodizi ng 阳极化anolyte阳极电解液anomalo us dispers ion 异常弥散anomalo us magneti c moment异常磁矩anomalo us skin effect反常囚效应anomer异头物anone 环己酮anortho clase钠斜微长石antagon ism 拮抗酌antazol ine 安他唑啉anthelm intics驱肠虫剂anthocy an 花青素anthocy anidin花色素anthocy anin 花色素苷anthoph yllite直闪石anthrac ene 蒽anthrac ene oil 蒽油anthrac ite 无烟煤anthrac ite duff 无烟煤粉anthral in 蒽啉anthran il 氨茴内酐anthran ilate邻氨基苯甲酸盐anthran ilic acid 邻氨基苯酸anthran ol 蒽酚anthran one 蒽酮anthrap urpuri n 蒽红紫anthraq uinone蒽醌anthraq uinone dye 蒽醌染料anthrar ufin 蒽绛酚anthrax ylon 结焦素anthron e 蒽酮anti allergi c drug 抗过敏性药anti fouling paint 防污涂料anti tack agent 防粘剂antiaci d 解酸药antiacid additiv e 抗酸添加剂。