Genomics revolution on andrology: genetic testing for male infertility
- 格式:pdf
- 大小:98.32 KB
- 文档页数:2

Gene OntologyGO分析Gene Ontology可分为分子功能Molecula r Function生物过程b iological proce ss和细胞组成cellular component三个部分。
蛋白质或者基因可以通过ID 对应或者序列注释的方法找到与之对应的GO号而GO号可对于到Ter m即功能类别或者细胞定位。
参考网站http://www.g eneontology.org功能富集分析功能富集需要有一个参考数据集通过该项分析可以找出在统计上显著富集的GO T erm。
功能或者定位有可能与研究的目前有关。
图1. 基于G O的蛋白质富集分析图谱GO功能分类GO功能分类是在某一功能层次上统计蛋白或者基因的数目或组成往往是在GO的第二层次。
此外也有研究都挑选一些Term而后统计直接对应到该Term的基因或蛋白数。
结果一般以柱状图或者饼图表示。
1.GO分析根据挑选出的差异基因计算这些差异基因同GO 分类中某几个特定的分支的超几何分布关系GO 分析会对每个有差异基因存在的GO返回一个p-value小的p值表示差异基因在该GO 中出现了富集。
GO 分析对实验结果有提示的作用通过差异基因的G O 分析可以找到富集差异基因的GO分类条目寻找不同样品的差异基因可能和哪些基因功能的改变有关。
2.Pathway分析根据挑选出的差异基因计算这些差异基因同Pathway 的超几何分布关系Pathway 分析会对每个有差异基因存在的pat hway 返回一个p-valu e小的p 值表示差异基因在该p athway 中出现了富集。
Pathway 分析对实验结果有提示的作用通过差异基因的Pa thway 分析可以找到富集差异基因的Pathway 条目寻找不同样品的差异基因可能和哪些细胞通路的改变有关。
与GO 分析不同pathway 分析的结果更显得间接这是因为pathw ay 是蛋白质之间的相互作用p athway 的变化可以由参与这条pathway 途径的蛋白的表达量或者蛋白的活性改变而引起。

问:基因组学、转录组学、蛋白质组学、结构基因组学、功能基因组学、比较基因组学研究有哪些特点?答:人类基因组计划完成后生物科学进入了人类后基因组时代,即大规模开展基因组生物学功能研究和应用研究的时代。
在这个时代,生命科学的主要研究对象是功能基因组学,包括结构基因组研究和蛋白质组研究等。
以功能基因组学为代表的后基因组时代主要为利用基因组学提供的信息。
基因组研究应该包括两方面的内容:以全基因组测序为目标的结构基因组学(struc tural genomics)和以基因功能鉴定为目标的功能基因组学(functional genomics)。
结构基因组学代表基因组分析的早期阶段,以建立生物体高分辨率遗传、物理和转录图谱为主。
功能基因组学代表基因分析的新阶段,是利用结构基因组学提供的信息系统地研究基因功能,它以高通量、大规模实验方法以及统计与计算机分析为特征。
功能基因组学(functional genomics)又往往被称为后基因组学(postgenomics),它利用结构基因组所提供的信息和产物,发展和应用新的实验手段,通过在基因组或系统水平上全面分析基因的功能,使得生物学研究从对单一基因或蛋白质的研究转向多个基因或蛋白质同时进行系统的研究。
这是在基因组静态的碱基序列弄清楚之后转入基因组动态的生物学功能学研究。
研究内容包括基因功能发现、基因表达分析及突变检测。
基因的功能包括:生物学功能,如作为蛋白质激酶对特异蛋白质进行磷酸化修饰;细胞学功能,如参与细胞间和细胞内信号传递途径;发育上功能,如参与形态建成等采用的手段包括经典的减法杂交,差示筛选,cDNA代表差异分析以及mRNA差异显示等,但这些技术不能对基因进行全面系统的分析。
新的技术应运而生,包括基因表达的系统分析,cDNA微阵列,DNA芯片等。
鉴定基因功能最有效的方法是观察基因表达被阻断或增加后在细胞和整体水平所产生的表型变异,因此需要建立模式生物体。
功能基因组学中文名称:功能基因组学英文名称: Functional Genomics学科分类:遗传学注释:运用遗传技术,通过识别其在一个或多个生物模型中的作用来认识新发现基因的功能。
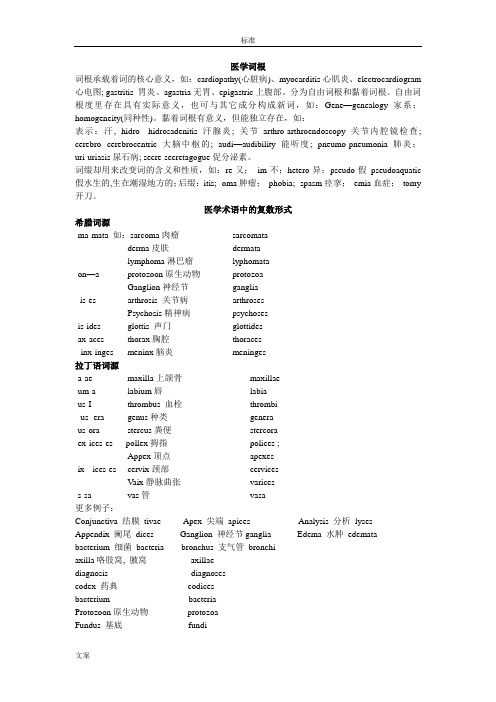
医学词根词根承载着词的核心意义,如:cardiopathy(心脏病)、myocarditis心肌炎、electrocardiogram 心电图; gastritis 胃炎、agastria无胃、epigastric上腹部。
分为自由词根和黏着词根。
自由词根度里存在具有实际意义,也可与其它成分构成新词,如:Gene—genealogy家系;homogeneity(同种性)。
黏着词根有意义,但能独立存在,如:表示:汗, hidro---hidrosadenitis汗腺炎; 关节arthro-arthroendoscopy关节内腔镜检查; cerebro--cerebrocentric大脑中枢的; audi—audibility 能听度; pneumo-pneumonia肺炎;uri-uriasis尿石病; secre-secretagogue促分泌素。
词缀却用来改变词的含义和性质,如:re-又;im-不;hetero-异;pseudo-假pseudoaquatic 假水生的,生在潮湿地方的;后缀:-itis; -oma肿瘤;-phobia; -spasm痉挛;-emia血症;-tomy 开刀。
医学术语中的复数形式希腊词源-ma-mata 如:sarcoma肉瘤sarcomataderma皮肤dermatalymphoma淋巴瘤lyphomata-on—a protozoon原生动物protozoaGanglion神经节ganglia- is-es arthrosis 关节病arthrosesPsychosis精神病psychoses-is-ides glottis 声门glottides-ax-aces thorax胸腔thoraces--inx-inges meninx脑炎meninges拉丁语词源-a-ae maxilla上颌骨maxillae-um-a labium唇labia-us-I thrombus 血栓thrombi--us- era genus种类genera-us-ora stercus粪便stercora-ex-ices-es pollex拇指polices ;Appex顶点apexes-ix- ices-es cervix颈部cervicesVaix静脉曲张varices-s-sa vas管vasa更多例子:Conjunctiva 结膜tivae Apex 尖端apices Analysis 分析lysesAppendix 阑尾dices Ganglion 神经节ganglia Edema 水肿edemata bacterium 细菌bacteria bronchus 支气管bronchiaxilla咯肢窝, 腋窝axillaediagnosis diagnosescodex 药典codicesbacterium bacteriaProtozoon原生动物protozoaFundus 基底fundiFungus 菌类, 蘑菇fungi同型类Species speciesTempo速度tempiCorps 尸体corps(古)同现Corpse另:corpus尸体corpora缩略词1、词首+词尾Abortion+mortuary----abortuary流产胎儿存放室;metabolism+genomics----metanomics代谢组织;nutrition+ pharmaceutical营养物质;2、词首+整词Medical+ care---medicare; radiation therapy—radiotherapy3、截短Influenza-flu; poliomyelitis---polio; morning—mng, tuberculosis- TB; Hemoglobin-Hb血红蛋白;intravenous- i.v. 静脉内的;subcutaneous- SC皮下的4、首字母BP-blood pressure; GH-growth hormone; MRI- magnetic resonance imaging; DNA-deoxyriboneuclsic acid; MeSH- Medical subject headings; RC-conditioned response; ID- diabetic index两栖词汇Dressing 敷料;discharged 出院;entity病种;intervention.治疗;conception 妊娠;culture medium 培养基;trace elements 微量元素;acid fast抗酸;identical twin同卵;labor分娩;Acute 急性的;murmur有杂音;tender触痛感;secondary bacterial pneumonia.继发性肺炎;Cardiac arrest 心脏骤停;pulmonary consolidation.(肺实变征象);term(预产期)医学术语的词源一、以医生或者患者为术语,如:Parkinson’s Dissease,振颤麻痹,hippocratic face 死后面相,以医生为名。

外刊每日精读 | The Human genome Project at 20文章脉络【1】人类基因组计划的历史与成就【2】基因组学从另一个层面研究生命和进化【3】基因测序使得生物学成为一门数据科学【4】&【5】基因组学能够广泛用于医疗活动【6】应该进一步降低基因测序的成本,提高可及性【7】有关基因组之间以及基因与自然环境的关系需要进一步研究经济学人原文The Human genome Project at 20:Epic ambitionThe genomics revolution has transformed biology. Its work is not over yet【1】TWENTY YEARS ago the Human genome Project (HGP) unveiled a mostly complete sequence of the roughly 3bn base pairs of DNA found in every set of human chromosomes. The project was chock-full of ego and hype, but also heralded the rapid improvements and dramatically lower costs of sequencing. This fed the success of the burgeoning field of genomics, which has transformed biology and medicine—and still holds plenty of promise.【2】genomics has added a new dimension to the study of life and evolution. It has helped scientists understand genes and proteins, and how they govern the growth and function of cells. CRISPR gene editing—a way to precisely modify the DNA in cells—gives researchers a handle on cellular function and dysfunction. The first treatments based on gene editing could be approved within a year. Plant scientists have acquired ways to create disease- and heat-resistant crops.【3】The era of cheap genome sequencing opened the doors to biology as a data science. The data and findings from the HGP came close tobeing hidden behind patents. Instead they were opened up to the public, which proved crucial—a useful lesson for other big projects. Biologists’databases now hold the sequences of millions of people and other organisms. This has helped draw links between genes, traits and diseases and also enhanced scientists’ understandingof evolution.【4】Most of the revolution’s tangible effects have been in medicine. Screening for serious but treatable genetic diseases is already possible. Cancer is largely the result of genetics gone awry. sequencing the genome has become a routine part of treating many tumours. Doing so allows doctors to work out which mutations the cancer has and therefore which course of treatment is likely to work best.【5】genomics will increasingly inform doctors’ decisions. Starting later this year, 100,000 babies in England will have their genomes sequenced and screened for around 200 conditions. Each disease is rare; together, they affect nearly onein 200 children. Early detection means early treatment, and a higher chance of a better outcome. The hope is that, in time, the precise variations in many hundredsof locations on a person’s genome will guide doctors. They seem likely to become a factor in assessments of whether a patient is likely to develop conditions suchas cardiovascular disease and type-2diabetes.【6】Yet for the genomics revolution to realise its potential, plenty more can be done. sequencing has fallen in cost from over $50m a genome at the end of the HGPto a few hundred dollars today, but making it even cheaper andmore convenient would allow it to be more widely available.People’s genetic sequences need to be integrated into their medical records, requiring data infrastructure, digitised records, and the setting of robust securityand privacy standards. Scientists must also continue to collect more diverse data, beyond those of patients in the rich world. That will help themunderstand variations in the genome. Some projects, such as the Three Million African genomes and GenomeAsia 100k, are already under way.【7】The science will need to progress further, too. Researchers now have a decent understanding of diseases that are affected by single genes. But they do not yet have a good grasp of how genes interact with each other. And much is unclear aboutthe interplay between groups of genes and people’s environments. Natureversus nurture was once a popular debate in genetics but these days is largely seenas a false dichotomy. With genomes as with much successful research, the more you find out, the more you realise that you do not have the whole story.长难句:1.原文:Doing so allows doctors to work out which mutations the cancer has and therefore which course of treatment is likely to work best.2.分析:主句是主谓宾结构Doing so(动名词作主语)allows(谓语动词)doctors(宾语)。

gene是DNA分子中含有特定遗传信息的一段核苷酸序列,是遗传物质的最小功能单位。
基因组(genome):生物所具有的携带遗传信息的遗传物质的总和,是指生物细胞中所有的DNA,包括所有的基因和基因间区域。
genomics以整个基因组为研究对象,而不是以单个基因为单位作为研究对象。
指以分子生物学技术、计算机技术和信息网络技术为研究手段,以生物体内全部基因为研究对象,在全基因背景下和整体水平上探索生命活动的内在规律及其内外环境影响机制的科学。
基因组学包括3个不同的亚领域结构基因组学(structural genomics) 通过基因组作图、核苷酸序列分析,研究基因组结构,确定基因组成、基因定位的科学。
功能基因组学(functional genomics)详尽分析序列,描述基因组所有基因的功能,包括研究基因的表达及其调控模式,这就是功能基因组学。
比较基因组学(comparative genomics) 研究不同物种之间在基因组结构和功能方面的亲源关系及其内在联系的学科,以便深入理解每个基因组的功能和进化关系。
基因组学的意义生物学研究医学生物技术制药工业社会经济生物进化尤其是人类疾病基因的研究C 值悖理(矛盾)(C-value paradox):在结构、功能很相似的同一类生物中,甚至在亲缘关系十分接近的物种之间,它们的C值可以相差数10倍乃至上百倍。
基因组的序列组成高度重复序列中度重复序列单一序列基因主要位于单一序列DNA基因家族同一物种或不同物种中来自一个共同的祖先,因加倍和趋异而产生的一组具有相似序列组成或略不同的基因成员。
超基因家族起源于共同祖先,由相似DNA序列组成的许多基因亚家族或相似的基因成员构成的群体,具有相似的功能。
反义基因:与已知基因编码序列互补的的负链编码基因,参与基因的表达调控,可以干扰靶基因mRNA转录与翻译。
假基因: 来源于功能基因但已失去活性的DNA序列.真核生物基因组的特征结构松弛大量重复顺序由线性DNA与蛋白质组成染色体结构含有细胞器基因组原核生物基因组的特征1) 结构紧凑2) 大小在5 Mb以下3) 缺少重复顺序4) 很少非编码顺序为何要绘制遗传图与物理图1)基因组太大,必需分散测序,然后将分散的顺序按原来位置组装,需要图谱进行指导。

⼀键完成microRNA定量PCR引物设计尽管microRNA芯⽚和microRNA测序检测⽅法已经普遍使⽤,但qRT-PCR依旧是检验microRNA表达定量的⾦标准。
由于microRNA的结构特殊,长度只有18-25个碱基,⽆法直接采⽤常规的PCR技术扩增,因此RT-qPCR的引物设计对很多刚刚接触microRNA的同学都是⼀个难题。
在针对microRNA的PCR技术中,设计其引物的理念是基于延长待测microRNA的长度,构建出⼀个⾜够长的PCR模板,才能进⼀步应⽤PCR技术来定量分析。
最常⽤的microRNA 反转录PCR⽅法就是茎环法(stem-loop)和加尾法(poly-A tail)。
由于茎环法反转录的引物设计原理限制,加尾法的检测通量⽐茎环法更⾼,因此加尾法也被实验室普遍使⽤。
今天⼩编就给⼤家介绍⼀个批量设计microRNA加尾法反转录PCR引物的软件miRprimer,重⼀键完成哦!点是⼀键完成miRprimer 官⽅推荐下载⽹站:https:///projects/miRprimer/⽹站后台⽂件直接下载miRprimer地址:https:///project/miRprimer/miRprimer2_installer.zipmiRprimer2_installer.zip,整个软件的压缩包只有2.68M (2848kb)⼤⼩。
提醒:提醒1. 软件⽀持在Windows XP或更⾼系统中运⾏,还没在苹果电脑Mac OS系统测试(原因是⼩编的钱包羞涩~~)2. 经⼩编测试,最新版本miRprimer顺利运⾏,不需在电脑中安装Ruby脚本环境。
第⼀步miRprimer2_installer.zip压缩包2.68M⼤⼩,下载后解压缩⽣成同名⽂件夹,内有三个⽂件:input_miRs.txt、miRprimer2.exe、README.txt。
特别提⽰:不要更改这些⽂件的⽂件名。
特别提⽰:第⼆步input_miRs.txt,fasta格式储存的是需要设计引物的microRNA名字和序列。
转录组基因家族
转录组(Transcriptome)和基因家族(Gene family)是基因组学(Genomics)研究中的两个重要概念。
它们分别关注基因的转录表达和基因间的进化关系。
1. 转录组
转录组是指一个细胞或组织在某一特定时刻转录产生的所有RNA分子,包括mRNA、tRNA、rRNA和其他非编码RNA。
通过对转录组的研究,科学家可以了解基因的表达模式、调控机制以及不同细胞类型和生理状态下基因的活性。
转录组学(Transcriptomics)是研究转录组的一门学科,主要利用高通量测序技术(如RNA-seq)来获取和分析RNA 序列数据。
2. 基因家族
基因家族是指在基因组中具有相似结构、起源和功能的一组基因。
基因家族成员通常具有较高的序列相似性和相似的调控元件,它们可能起源于一个共同的祖先基因,通过基因重复和演化形成了多个拷贝。
基因家族在生物进化过程中起着重要作用,如新功能的产生、基因剂量效应以及基因的亚细胞定位等。
总结:
转录组关注的是基因在特定时间和环境下的转录表达,而基因家族则强调基因间的进化关系和功能相似性。
在基因组学研究中,这两个概念都具有重要的意义,它们共同揭示了生物体基因表达调控和进化过程中的复杂性和多样性。
The genomics of probiotic intestinal microorganismsSeppo Salminen1 , Jussi Nurmi2 and Miguel Gueimonde1(1) Functional Foods Forum, University of Turku, FIN-20014 Turku, Finland(2) Department of Biotechnology, University of Turku, FIN-20014 Turku, FinlandSeppo SalminenEmail: *********************Published online: 29 June 2005AbstractAn intestinal population of beneficial commensal microorganisms helps maintain human health, and some of these bacteria have been found to significantly reduce the risk of gut-associated disease and to alleviate disease symptoms. The genomic characterization of probiotic bacteria and other commensal intestinal bacteria that is now under way will help to deepen our understanding of their beneficial effects.While the sequencing of the human genome [1, 2] has increased ourunderstanding of the role of genetic factors in health and disease, each human being harbors many more genes than those in their own genome. These belong to our commensal and symbiotic intestinal microorganisms - our intestinal 'microbiome' - which play an important role in maintaining human health and well-being. A more appropriate image of ourselves would be drawn if the genomes of our intestinal microbiota were taken into account. The microbiome may contain more than 100 times the number of genes in the human genome [3] and provides many functions that humans have thus not needed to develop themselves. The indigenous intestinal microbiota provides a barrier against pathogenic bacteria and other harmful food components [4–6]. It has also been shown to have a direct impact on the morphology of the gut [7], and many intestinal diseases can be linked to disturbances in the intestinal microbial population [8].The indigenous microbiota of an infant's gastrointestinal tract is originally created through contact with the diverse microbiota of the parents and the immediate environment. During breast feeding, initial microbial colonization is enhanced by galacto-oligosaccharides in breast milk and contact with the skin microbiota of the mother. This early colonization process directs the microbial succession until weaning and forms the basis for a healthy microbiota. The viable microbes in the adultintestine outnumber the cells in the human body tenfold, and the composition of this microbial population throughout life is unique to each human being. During adulthood and aging the composition and diversity of the microbiota can vary as a result of disease and the genetic background of the individual.Current research into the intestinal microbiome is focused on obtaining genomic data from important intestinal commensals and from probiotics, microorganisms that appear to actively promote health. This genomic information indicates that gut commensals not only derive food and other growth factors from the intestinal contents but also influence their human hosts by providing maturational signals for the developing infant and child, as well as providing signals that can lead to an alteration in the barrier mechanisms of the gut. It has been reported that colonization by particular bacteria has a major role in rapidly providing humans with energy from their food [9]. For example, the intestinal commensal Bacteroides thetaiotaomicron has been shown to have a major role in this process, and whole-genome transcriptional profiling of the bacterium has shown that specific diets can be associated with selective upregulation of bacterial genes that facilitate delivery of products of carbohydrate breakdown to the host's energy metabolism [10, 11]. Key microbial groups in the intestinal microbiota are highly flexible in adapting to changes in diet, and thus detailed prediction of their actions and effects may be difficult. Although genomic studies have revealed important details about the impact of the intestinal microbiota on specific processes [3, 11–14], the effects of species composition and microbial diversity and their potential compensatory functions are still not understood.Probiotics and healthA probiotic has been defined by a working group of the International Life Sciences Institute Europe (ILSI Europe) as "a viable microbial food supplement which beneficially influences the health of the host" [15]. Probiotics are usually members of the healthy gut microbiota and their addition can assist in returning a disturbed microbiota to its normal beneficial composition. The ILSI definition implies that safety and efficacy must be scientifically demonstrated for each new probiotic strain and product. Criteria for selecting probiotics that are specific for a desired target have been developed, but general criteria that must be satisfied include the ability to adhere to intestinal mucosa and tolerance of acid and bile. Such criteria have proved useful but cumbersome in current selection processes, as there are several adherence mechanisms and they influence gene upregulation differently in the host. Therefore, two different adhesion studies need to be conducted on each strain and theirpredictive value for specific functions is not always good or optimal. Demonstration of the effects of probiotics on health includes research on mechanisms and clinical intervention studies with human subjects belonging to target groups.The revelation of the human genome sequence has increased our understanding of the genetic deviations that lead to or predispose to gastrointestinal disease as well as to diseases associated with the gut, such as food allergies. In 1995, the first genome of a free-living organism, the bacterium Haemophilus influenzae, was sequenced [16]. Since then, over 200 bacterial genome sequences, mainly of pathogenic microorganisms, have been completed. The first genome of a mammalian lactic-acid bacterium, that of Lactococcus lactis, a microorganism of great industrial interest, was completed in 2001 [17]. More recently, the genomes of numerous other lactic-acid bacteria [18], bifidobacteria [12] and other intestinal microorganisms [13, 19, 20] have been sequenced, and others are under way [21]. Table 1lists the probiotic bacteria that have been sequenced. These great breakthroughs have demonstrated that evolution has adapted both microbes and humans to their current state of cohabitation, or even symbiosis, which is beneficial to both parties and facilitates a healthy and relatively stable but adaptable gut environment.Table 1Lessons from genomesLactic-acid bacteria and bifidobacteria can act as biomarkers of gut health by giving early warning of aberrations that represent a risk of specific gut diseases. Only a few members of the genera Lactobacillus and Bifidobacterium, two genera that provide many probiotics, have been completely sequenced. The key issue for the microbiota, for probiotics, and for their human hosts is the flexibility of the microorganisms in coping with a changeable local environment and microenvironments.This flexibility is emphasized in the completed genomes of intestinal and probiotic microorganisms. The complete genome sequence of the probiotic Lactobacillus acidophilus NCFM has recently been published by Altermann et al. [22]. The genome is relatively small and the bacterium appears to be unable to synthesize several amino acids, vitamins and cofactors. Italso encodes a number of permeases, glycolases and peptidases for rapid uptake and utilization of sugars and amino acids from the human intestine, especially the upper gastrointestinal tract. The authors also report a number of cell-surface proteins, such as mucus- and fibronectin-binding proteins, that enable this strain to adhere to the intestinal epithelium and to exchange signals with the intestinal immune system. Flexibility is guaranteed by a number of regulatory systems, including several transcriptional regulators, six PurR-type repressors and ninetwo-component systems, and by a variety of sugar transporters. The genome of another probiotic, Lactobacillus johnsonii [23], also lacks some genes involved in the synthesis of amino acids, purine nucleotides and numerous cofactors, but contains numerous peptidases, amino-acid permeases and other transporters, indicating a strong dependence on the host.The presence of bile-salt hydrolases and transporters in these bacteria indicates an adaptation to the upper gastrointestinal tract [23], enabling the bacteria to survive the acidic and bile-rich environments of the stomach and small intestine. In this regard, bile-salt hydrolases have been found in most of the sequenced genomes of bifidobacteria and lactic-acid bacteria [24], and these enzymes can have a significant impact on bacterial survival. Another lactic-acid bacterium, Lactobacillus plantarum WCFS1, also contains a large number of genes related to carbohydrate transport and utilization, and has genes for the production of exopolysaccharides and antimicrobial agents [18], indicating a good adaptation to a variety of environments, including the human small intestine [14]. In general, flexibility and adaptability are reflected by a large number of regulatory and transport functions.Microorganisms that inhabit the human colon, such as B. thetaiotaomicron and Bifidobacterium longum [12], have a great number of genes devoted to oligosaccharide transport and metabolism, indicating adaptation to life in the large intestine and differentiating them from, for example, L. johnsonii [23]. Genomic research has also provided initial information on the relationship between components of the diet and intestinal microorganisms. The genome of B. longum [12] suggests the ability to scan for nutrient availability in the lower gastrointestinal tract in human infants. This strain is adapted to utilizing the oligosaccharides in human milk along with intestinal mucins that are available in the colon of breast-fed infants. On the other hand, the genome of L. acidophilus has a gene cluster related to the metabolism of fructo-oligosaccharides, carbohydrates that are commonly used as prebiotics, or substrates to肠道微生物益生菌的基因组学塞波萨米宁,尤西鲁米和米格尔哥尔摩得(1)功能性食品论坛,图尔库大学,FIN-20014芬兰图尔库(2)土尔库大学生物技术系,FIN-20014芬兰图尔库塞波萨米宁电子邮件:seppo.salminen utu.fi线上发表于2005年6月29日摘要肠道有益的共生微生物有助于维护人体健康,一些这些细菌被发现显着降低肠道疾病的风险和减轻疾病的症状。