Chmielewski,2001,Response of tree phenology to climate change across Europe
- 格式:pdf
- 大小:418.28 KB
- 文档页数:12

依泽替米贝文献综述一. 前言1.1背景随着我国人民生活水平的不断提高,饮食结构的改变导致人类疾病结构的改变,高脂血症已呈高发病趋势。
自上世纪末以来,美国每年有120万患急性心肌梗死,死亡总人口中,1/3死于冠心病。
据统计,我国成人血脂异常患病率为 18.6%,年发病率为560/10万,全国血脂异常现患人数为1.6亿。
而高血脂又是冠心病、动脉硬化、心肌梗死的重要病因之一,并能够加重高血压、糖尿病、脂肪肝、肝硬化、胰腺炎等疾病的病情恶化,严重威胁着人们的生命安全,成为威胁人类健康的劲敌。
降血脂药物进展研究和发展新型降血脂药物具有重大的社会效益和经济效益,因此降血脂药物的开发和应用一直是医学界的热点领域1.2依替米贝概述依泽替米贝是由默克(Merck)和先灵葆雅公司共同开发的一种新型的抑制胆固醇吸收的降血脂药物。
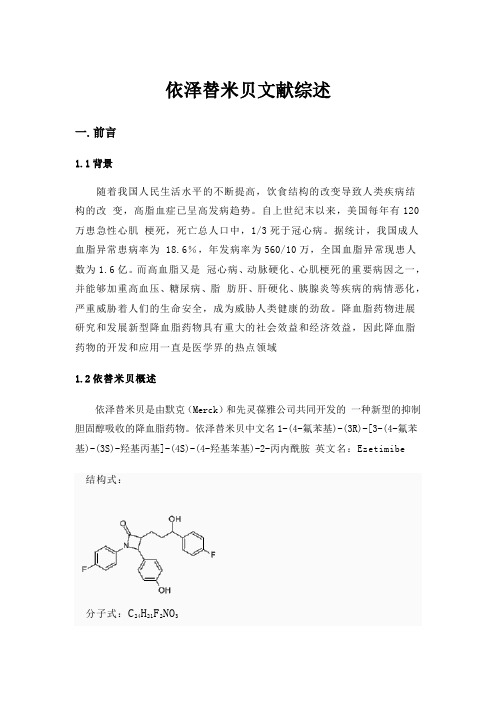
依泽替米贝中文名1-(4-氟苯基)-(3R)-[3-(4-氟苯基)-(3S)-羟基丙基]-(4S)-(4-羟基苯基)-2-丙内酰胺英文名:Ezetimibe结构式:分子式:C24H21F2NO3分子量:409.43CAS:163222-33-1对眼睛、呼吸道和皮肤有刺激作用依泽替米贝为白色结晶性粉末,极易溶于乙醇、甲醇和丙酮,不溶于水,熔点约为163℃,在常温下稳定。
依替米贝能够通过抑制小肠刷状缘TC吸收而降低血Te,它本身也具有微弱的调血脂作用。
口服吸收后,与葡萄糖醛酸苷结合生成活性物质一依泽替米贝一葡萄糖醛酸苷,经胆汁和肾排泄。
口服后在4~12h 内达血药峰浓度,Cmax为3.4~5.5 mg/l,生物利用度在35%~60%之间,T1/2约为22h。
依替米贝及依替米贝一葡萄糖醛酸苷与血浆蛋白结合率高于90%。
依泽替米贝是一类新型的选择性胆固醇吸收抑制剂,通过与小肠刷状缘膜小囊泡上膜蛋白结合后,抑制小肠对饮食中和经胆汁输送到肠道中的胆固醇的吸收,降低血清和肝脏中的胆固醇含量,降低血浆低密度脂蛋白分数。

爱尔兰/英国科学家报道了在与肺炎、脑膜炎以及败血病斗争
中的突破性进展
无
【期刊名称】《国外药讯》
【年(卷),期】2010(000)012
【摘要】科学家最近报告了一项发现,这一发现被认为将使我们理解机体免疫系统应答肺炎链球菌感染的方式产生引人注目的转变,并将为生产更有效的疫苗铺平道路。
【总页数】2页(P23-24)
【作者】无
【作者单位】不详
【正文语种】中文
【中图分类】R378.12
【相关文献】
1.英国科学家研究皮肤癌取得突破性进展 [J],
2.英国证据法研究的突破性进展--评齐树洁教授主编的《英国证据法》 [J],
3.英国科学家在爱尔兰北部海底发现最长寿动物——一只405岁的蛤 [J],
4.我国科学家在控制水稻分蘖的新激素独角金内酯信号转导研究中取得突破性进展[J], 本刊讯
5.英国等欧洲多国科学家发现脑膜炎易感基因 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。

美国就基因治疗事故展开调查
毓勤
【期刊名称】《国外医学情报》
【年(卷),期】2001(022)003
【摘要】@@ 美国的生物医学研究围绕着Jesse Gelsinger的死亡事件消耗了大量的精力,这个亚利桑那州的青少年在宾夕法尼亚大学的试验中成为死于基因治疗的第一人.
【总页数】2页(P25-26)
【作者】毓勤
【作者单位】无
【正文语种】中文
【中图分类】R394
【相关文献】
1.日本东京电力公司隐瞒事故隐患,核能安全部门展开调查 [J], 辛文
2.美国对华反补贴诉讼中有关人民币汇率的法律问题分析——兼评美国Nucor公司告美国商务部拒绝就人民币汇率补贴展开调查案 [J], 龚柏华;尤浩
3.事故分析与报道:美国Equilon炼油厂火灾事故调查 [J], 无
4.美国国际贸易委员会对中国等国家纺织品出口展开“332条款”调查——2005年配额保护期结束后美国对有关国家可能采取贸易救济措施研究 [J], 王屹
5.美国国际贸易委员会对中国等国家纺织品出口展开"332条款"调查 2005年配额保护期结束后美国对有关国家可能采取贸易救济措施研究 [J],
因版权原因,仅展示原文概要,查看原文内容请购买。

膜蒸馏技术膜蒸馏(Membrane Distillation, MD)是一种采用疏水微孔膜以膜两侧蒸汽压力差为传质驱动力的膜分离过程。
虽然早在20世纪60年代就开始了较系统的膜蒸馏研究,但当时由于受到技术条件的限制,膜蒸馏的效率不高,直到20世纪80年代初由于高分子材料和制膜工艺方面迅速发展,膜蒸馏显示出其实用潜力。
本文就膜蒸馏的原理、特征及应用情况作一总结和评述。
1 膜蒸馏技术的简介MD是膜技术与蒸发过程相结合的膜分离过程,以膜两侧蒸汽温度差为传质驱动力,它是热量和质量同时传递的过程,膜孔内的传质过程是分子扩散和努森扩散的综合结果。
1.1 膜蒸馏过程区别于其他膜过程的特征所用的膜为微孔膜;膜不能被所处理的液体润湿;在膜孔内没有毛细管冷凝现象发生,只有蒸汽能通过膜孔传质;所用膜不能改变所处理液体中所有组分的气液平衡;膜至少有一面与所处理的液体接触;对于任何组分该膜过程的推动力是该组分在气相中的分压差。
[1]1.2 膜蒸馏的优缺点膜蒸馏的优点有很多:蒸馏过程几乎是在常压下进行,设备简单、操作方便,可以采用非金属设备;在非挥发性溶质水溶液的MD过程中,只有水蒸气能透过膜孔,蒸馏十分纯净,有望成为大规模、低成本制备超纯水的手段;可以处理极高浓度的水溶液,是目前唯一能从溶液中直接分离出结晶产物的膜过程;MD 组件很容易设计成潜热回收形式,并具有以高效的小型膜组件构成大规模生产体系的灵活性;膜两侧只需维持适当的温差即可进行操作,有望利用太阳能、地热、温泉和工厂的余热等廉价能源。
同时膜蒸馏也有一定缺点:MD是一个有相变的膜过程,汽化潜热降低了热能的利用率。
MD与制备纯水的其他膜过程相比通量较小,目前尚未实现在工业生产中应用,MD用膜的材料和制备工艺选择方面有限。
MD过程中的膜污染是其实现工业应用的主要障碍。
[2]1.3膜蒸馏的分类及原理根据膜下游侧冷凝方式的不同,MD可分为4种形式:直接接触膜蒸馏(DCMD)、气隙式膜蒸馏(AGMD)、吹扫气膜蒸馏(SGMD)和真空膜蒸馏(VMD,又名减压膜蒸馏)。

植物学报Chinese Bulletin of Botan ydoi:10.37241SP.J.1259.2012.00217·研究报告·H202介导的H2S产生参与干旱诱导的拟南芥气孔关闭王兰香,侯智慧,侯丽霞,赵方贵,刘新。
青岛农业大学生命科学学院,山东省高校植物生物技术重点实验室。
青岛266109摘要以野生型拟南芥(Arabidopsis tha/iana)及其突变体(a trbo hD、at rbohF、atr bohD IF、at/-cdes、atd-cd es)和过表达株系(O EL.C De s、O ED.CO e s)材料,利用药理学实验,结合分光光度法和激光共聚焦显微技术,探讨硫化氢(h yd ro g en sulfide,H2S)在干旱诱导的拟南芥气孔关闭中的作用及其与过氧化氢(hydrogen peroxide,H202)雕]关系。
结果表明,H2S清除剂次牛磺酸(hypotaurine,HT)及合成抑制剂氨氧基乙酸(aminooxy acetic acid,AOA)、羟胺(hydroxylamine。
NH20H)和丙酮酸钾(potasium pyruvate,C3H3K03)+氨水(ammonia,NH3)均可不同程度抑制干旱诱导的气孔关闭;干旱对OEL-CDes和0ED.cDes植株气孔关闭的诱导作用明显.而atl-cdes和atd-cdes叶片气孔对干旱胁迫反应的敏感性下降:干旱胁迫能明显增加拟南芥保卫细胞CH202水平及叶片dPH2S含量,提高D-/L-半胱氨酸脱巯基酶活性及基因表达量,而对突变体atrbohD、 atrbohF和atrbohD,F没有显著影响。
清除H202可减弱干旱胁迫对H2S含量和D-/L一半胱氨酸脱巯基酶活性的诱导效应。
研究结果表MR2S位于H202下游参与干旱诱导拟南芥气孔关闭的信号转导过程。
关键词拟南芥,干旱胁迫,过氧化氢。
Example analysis of biodiversity survey data with R packagegradientForestC.Roland Pitcher,Nick Ellis,Stephen J.SmithMarch21,2011Contents1Introduction1 2Gradient Forest basics22.1Data (2)2.2gradientForest analysis (2)2.3gradientForest plots (3)3Gradient Forest predictions83.1Transforming predictors (9)3.2Biplot of the biological space (9)3.3Mapping in geographic space (12)3.4A clustered version (12)4Session information15 References16 1IntroductionR package gradientForest[Ellis et˜al.,2010]developsflexible non-parametric functions to quantify multi-species compositional turnover along environmental gradients.Theflexibility comes from the method’s origins in Random Forests[Breiman,2001];specifically R package randomForest[Liaw and Wiener,2002].This document provides an example to demonstrate the use of gradientForest for ecological analysis of biodiversity survey data.A multi-regional application is provided by[Pitcher et˜al.,2010].The document assumes some familiarity both with R and with community analysis.The example has some analogues with constrained ordi-nation methods and with Generalized Dissimilarity Modelling[Ferrier et˜al.,2007],which are both complementary.Package randomForest includes functions for plotting non-linear responses in compositional along environmental gradients,and for using these responses to transform en-vironmental data layers to biological scales.The transformed multi-dimensional biological space can be represented as a biplot and can be mapped in geographic space.This example demonstrates typical scripts for a gradient forest analysis and provides in the package a sites-by-species(row-by-columns)matrix and a matching sites-by-environment(row-by-columns)dataframe.The number of rows and their order must match between these two data objects.The data should not include NAs.It is assumed that users will be familiar with the data-processing steps necessary to produce such data objects.12Gradient Forest basics2.1DataThe example data provided in package gradientForest are real species data from a cross-shelf survey in the far northern Great Barrier Reef of northeast Australia[Poiner et˜al.,1998, Burridge et˜al.,2006].Of>1,000species observed,a subset of110are included from197of the sites sampled.The environmental data include28predictors,either measured at each site or attributed to each site by interpolation[Pitcher et˜al.,2002].>require(gradientForest)>load("GZ.sps.mat.Rdata")>dim(Sp_mat)[1]197110>load("GZ.phys.site.Rdata")>dim(Phys_site)[1]197282.2gradientForest analysisThe function gradientForest is a wrapper function that calls extendedForest,a modified version of randomForest,and collates its output across all the species in the data matrix. The key modification in extendedForest extracts the numerous tree split values along each predictor gradient and their associatedfit improvement,for each predictor in each tree,for the forests and returns that information to gradientForest.Like randomForest,extendedForest assesses the importance of each variable for predic-tion accuracy;information that is further collated and processed by gradientForest.Often, predictor variables are correlated however.The standard approach in random forests assesses marginal importance of predictor by randomly permuting each predictor in turn,across all sites in the dataset,and calculating the degradation prediction performance of each tree.Package extendedForest can account for correlated predictors by implementing conditional permutation [Ellis et˜al.,2010],following the strategy outlined by Strobl et˜al.[2008].In conditional per-mutation,the predictor to be assessed is permuted only within blocks of the dataset defined by splits in the given tree on any other predictors correlated above a certain threshold(e.g. r=0.5)and up to a maximum number of splits set by the maxLevel option(if required).>nSites<-dim(Sp_mat)[1]>nSpecs<-dim(Sp_mat)[2]>lev<-floor(log2(nSites*0.368/2))>lev[1]5The gradientForest may take several minutes to run.Other options that can be set include the number of trees typically500,whether the splits should be compact into bins(advising to prevent memory problems for large datasets)and the number of bins,and the correlation threshold for conditional permutation.The summary shows the number of species with positive R2ie.those species that could be predicted to any extent by the available predictor.The returned object is a list containing the data,predictor importances,species R2’s and other information described in the html help pages under Value.>gf<-gradientForest(cbind(Phys_site,Sp_mat),+predictor.vars=colnames(Phys_site),response.vars=colnames(Sp_mat),+ntree=500,transform=NULL,compact=T,+nbin=201,maxLevel=lev,corr.threshold=0.5)>gfA forest of500regression trees for each of90speciesCall:gradientForest(data=cbind(Phys_site,Sp_mat),predictor.vars=colnames(Phys_site), response.vars=colnames(Sp_mat),ntree=500,transform=NULL,maxLevel=lev,corr.threshold=0.5,compact=T,nbin=201)Important variables:[1]BSTRESS MUD S_AV Si_AV CHLA_AV>names(gf)[1]"X""Y""result"[4]"overall.imp""overall.imp2""ntree"[7]"imp.rsq""species.pos.rsq""ranForest.type"[10]"res""res.u""dens"[13]"call"2.3gradientForest plotsSeveral types of plots are available for the gradientForest object.Thefirst is the predictor overall importance plot.This show the mean accuracy importance and the mean importance weighted by species R2.in this example,both are conditional importance.Seabed stress and sediment mud fraction are clearly the most important variables across these89species.>plot(gf,plot.type="O")PO4_SRASPECT SLOPE O2_SR O2_AV PO4_AV CHLA_SR S_SR K490_SRSi_SR SST_SR BIR_AV BIR_SR GRAVEL BA THY NO3_SR T_AV T_SR SST_AV CRBNT NO3_AV Si_AV SAND K490_AV CHLA_AV S_AV MUD BSTRESSAccuracy importance0.000.040.08PO4_SRASPECT O2_SR SLOPE O2_AV CHLA_SR PO4_AV Si_SR S_SR BIR_AV BIR_SR K490_SR SST_SR NO3_SR BA THY T_SR GRAVEL SST_AV NO3_AV T_AV CRBNT K490_AV SAND CHLA_AVSi_AV S_AV MUD BSTRESSR 2weighted importance0.0000.0100.020The predictor gradient plots are best presented in order of importance;in this example the top 25predictors are presented in 5by 5panels.>most_important <-names(importance(gf))[1:25]>par(mgp =c(2,0.75,0))The second plot is the splits density plot (plot.type="S"),which shows binned split impor-tance and location on each gradient (spikes),kernel density of splits (black lines ),of observations (red lines )and of splits standardised by observations density (blue lines ).Each distribution in-tegrates to predictor importance.These show where important changes in the abundance of multiple species are occurring along the gradient;they indicate a composition change rate.Many of the usual plot options can be set in the call.>plot(gf,plot.type ="S",imp.vars =most_important,+leg.posn ="topright",cex.legend =0.4,cex.axis =0.6,+b =0.7,line.ylab =0.9,par.args =list(mgp =c(1.5,+0.5,0),mar =c(3.1,1.5,0.1,1)))0.10.30.50.000.040.080.12BSTRESS02040600e +004e −048e −04MUD 34.835.20.000.040.080.12S_AV 1.5 2.5 3.50.0000.0060.012Si_AV0.51.0 1.52.00.000.020.04CHLA_AV020*******.000000.00015SAND0.060.100.140.00.20.40.6K490_AV4060801000.00000.00100.0020CRBNT27.528.028.529.00.0000.010T_AV0.20.30.40.50.000.050.100.15NO3_AV26.726.927.10.000.040.08SST_AV020*******.000000.00015GRAVEL1.02.03.00.0000.0060.012T_SR−50−40−30−20−100e +003e −046e −04BATHY 0.10.20.30.40.50.000.020.040.06NO3_SR4.2 4.65.0 5.40.0000.0060.012SST_SR 0.050.100.150.200.000.100.20K490_SR0.050.150.250.000.020.04BIR_SR0.00.20.40.60.80.0000.0150.030BIR_AV 1.02.03.00.0000.0020.004S_SR1234560.00000.0010Si_SR 0.140.180.000.10PO4_AV 123450.0000.0040.008CHLA_SR 4.254.35 4.450.0000.0100.020O2_AV 0.0 1.0 2.00.0000.0020.0040.006SLOPED e n s i t yThe third plot is the species cumulative plot (plot.type="C",show.overall=F ),which for each species shows cumulative importance distributions of splits improvement scaled by R 2weighted importance,and standardised by density of observations.These show cumulative change in abundance of individual species,where changes occur on the gradient,and the species changing most on each gradient.Again many of the usual plot options can be set in the call;in this example the legend identifies the top 5most responsive species for each predictor >plot(gf,plot.type ="C",imp.vars =most_important,+show.overall =F,legend =T,leg.posn ="topleft",+leg.nspecies =5,b =0.7,cex.legend =0.4,+cex.axis =0.6,line.ylab =0.9,par.args =list(mgp =c(1.5,+0.5,0),mar =c(2.5,1,0.1,0.5),omi =c(0,+0.3,0,0)))0.10.20.30.40.50.000.100.20BSTRESS01030500.000.020.040.06MUD34.835.035.235.40.000.040.08S_AV1.52.53.50.000.040.080.12Si_AV0.5 1.0 1.5 2.00.000.020.04CHLA_AV0204060801000.000.040.08SAND0.060.100.140.000.020.040.06K490_AV4060801000.000.020.04CRBNT27.528.028.529.00.000.020.040.06T_AV0.20.30.40.50.000.020.04NO3_AV26.726.826.927.027.10.000.020.04SST_AV0204060801000.000.020.04GRAVEL1.0 1.52.0 2.53.0 3.50.000.020.04T_SR−50−40−30−20−100.000.020.04BATHY0.10.20.30.40.50.000.020.04NO3_SR4.2 4.65.0 5.40.0000.0100.020SST_SR0.050.100.150.200.000.020.040.06K490_SR0.050.150.250.0000.0100.020BIR_SR0.00.20.40.60.80.0000.0100.020BIR_AV1.02.03.00.000.020.04S_SR1234560.000.020.04Si_SR 0.140.160.180.200.0000.0150.030PO4_AV 123450.0000.0100.0200.030CHLA_SR 4.254.354.450.0000.0040.0080.012O2_AV 0.00.5 1.0 1.5 2.0 2.50.0000.0100.0200.030SLOPEC u m u l a t i v e i m p o r t a n c eThe fourth plot is the predictor cumulative plot (plot.type="C",show.species=F ),which for each predictor shows cumulative importance distributions of splits improvement scaled by R 2weighted importance,and standardised by density of observations,averaged over all species.These show cumulative change in overall composition of the community,where changes occur on the gradient.Again many of the usual plot options can be set in the call;in this example com-mon.scale=T ensures that plots for all predictors have the same y-scale as the most important predictor.>plot(gf,plot.type ="C",imp.vars =most_important,+show.species =F,common.scale =T,cex.axis =0.6,+b =0.7,line.ylab =0.9,par.args =list(mgp =c(1.5,+0.5,0),mar =c(2.5,1,0.1,0.5),omi =c(0,+0.3,0,0)))0.10.20.30.40.50.0000.0100.020BSTRESS01030500.0000.0100.020MUD34.835.035.235.40.0000.0100.020S_AV1.52.53.50.0000.0100.020Si_AV0.51.0 1.52.00.0000.0100.020CHLA_AV0204060801000.0000.0100.020SAND0.060.100.140.0000.0100.020K490_AV4060801000.0000.0100.020CRBNT27.528.028.529.00.0000.0100.020T_AV0.20.30.40.50.0000.0100.020NO3_AV26.726.826.927.027.10.0000.0100.020SST_AV0204060801000.0000.0100.020GRAVEL1.0 1.52.0 2.53.0 3.50.0000.0100.020T_SR−50−40−30−20−100.0000.0100.020BATHY0.10.20.30.40.50.0000.0100.020NO3_SR4.2 4.65.0 5.40.0000.0100.020SST_SR0.050.100.150.200.0000.0100.020K490_SR0.050.150.250.0000.0100.020BIR_SR0.00.20.40.60.80.0000.0100.020BIR_AV1.02.03.00.0000.0100.020S_SR1234560.0000.0100.020Si_SR 0.140.160.180.200.0000.0100.020PO4_AV 123450.0000.0100.020CHLA_SR 4.25 4.35 4.450.0000.0100.020O2_AV0.00.5 1.0 1.5 2.0 2.50.0000.0100.020SLOPEC u m u l a t i v e i m p o r t a n c eThe fifth plot shows the R 2measure of the fit of the random forest model for each species,ordered in various ways.>plot(gf,plot.type ="P",s =F,horizontal =F,+cex.axis =1,bels =0.7,line =2.5)qq q q qq q q qq q q q qqq q q q qq q q qq q q q q q q q qq q q q q q q q q q q q q q q q q q qq q q q q q q q q qq q q q q qq q q q q q q q qq q q q q q q q q q q q q1234567891011121314151617181920212223242526272829303132333435363738394041424344454647484950515253545556575859606162636465666768697071727374757677787980818283848586878889900.00.10.20.30.40.50.6Species performance rankR 2Overall performance of random forests over speciesSeveral other alternative formats of the R 2fit performance plot are available,e.g.:>plot(gf,plot.type ="P",s =T,horizontal =F,+cex.axis =1,bels =0.7,line =2.5)>plot(gf,plot.type ="P",s =F,horizontal =T,+cex.axis =1,bels =0.6,line =2.5)>plot(gf,plot.type ="P",s =T,horizontal =T,+cex.axis =1,bels =0.6,line =2.5)3Gradient Forest predictionsIn addition to examining compositional change along environmental gradients,the predictor cumulative functions can also be used to transform grid data layers of environmental variables to a common biological importance scale.This transformation of the multi-dimensional en-vironment space is to a biological space in which coordinate position represents compositionassociated with the predictors.These inferred compositional patterns can be mapped in bio-logical space and geographic space in a manner analogous to ordination,but takes into account the non-linear and sometimes threshold changes that occur along gradients.3.1Transforming predictorsThe example provided includes gridded environmental variables for a roughly10,000km2area of the far northern Great Barrier Reef where the biological surveys were conducted.The data include North and East coordinates plus28predictors at8,682grid cells.The grid data must include the same predictors with the same names as sites included in the gradientForest call.>load("GZ.phys.grid.Rdata")>dim(Phys_grid)[1]868230>names(Phys_grid)[1]"NORTH""EAST""BATHY""SLOPE""ASPECT" [6]"BSTRESS""CRBNT""GRAVEL""SAND""MUD"[11]"NO3_AV""NO3_SR""PO4_AV""PO4_SR""O2_AV"[16]"O2_SR""S_AV""S_SR""T_AV""T_SR"[21]"Si_AV""Si_SR""CHLA_AV""CHLA_SR""K490_AV"[26]"K490_SR""SST_AV""SST_SR""BIR_AV""BIR_SR"The grid variables are transformed using the gradientForest predict function.>imp.vars<-names(importance(gf))>Trns_grid<-cbind(Phys_grid[,c("EAST","NORTH")],+predict(gf,Phys_grid[,imp.vars]))It is useful to also transform the site environmental predictors,which are available from gf$X.>Trns_site<-predict(gf)3.2Biplot of the biological spaceThe multi-dimensional biological space can most effectively be represented by taking the prin-ciple components of the transformed grid and presenting thefirst two dimensions in a biplot. It must be acknowledged that while most variation in patterns is captured by thefirst dimen-sions,additional compositional pattern contained in the higher dimensions is not shown.A user defined RGB colour palette is set up based on thefirst3dimensions.>PCs<-prcomp(Trns_grid[,imp.vars])>a1<-PCs$x[,1]>a2<-PCs$x[,2]>a3<-PCs$x[,3]>r<-a1+a2>g<--a2>b<-a3+a2-a1>r<-(r-min(r))/(max(r)-min(r))*255>g<-(g-min(g))/(max(g)-min(g))*255>b<-(b-min(b))/(max(b)-min(b))*255The environmental variables may be shown as vectors,perhaps limited to the most important predictors—in this example,variables to show as vectors are selected.>nvs<-dim(PCs$rotation)[1]>vec<-c("BSTRESS","MUD","SST_AV","T_AV","CHLA_AV", +"SAND","CRBNT","GRAVEL")>lv<-length(vec)>vind<-rownames(PCs$rotation)%in%vec>scal<-40>xrng<-range(PCs$x[,1],PCs$rotation[,1]/scal)*+ 1.1>yrng<-range(PCs$x[,2],PCs$rotation[,2]/scal)*+ 1.1>plot((PCs$x[,1:2]),xlim=xrng,ylim=yrng,+pch=".",cex=4,col=rgb(r,g,b,max=255),+asp=1)>points(PCs$rotation[!vind,1:2]/scal,pch="+")>arrows(rep(0,lv),rep(0,lv),PCs$rotation[vec,+1]/scal,PCs$rotation[vec,2]/scal,length=0.0625)>jit<-0.0015>text(PCs$rotation[vec,1]/scal+jit*sign(PCs$rotation[vec,+1]),PCs$rotation[vec,2]/scal+jit*sign(PCs$rotation[vec,+2]),labels=vec)Different coordinate positions in the biplot represent differing compositions,as associated with the predictors.Further information may be added to the biplot including the location of sites in biological space,the weight mean location of species,and selected species may be identified interactively.−0.02−0.010.000.010.02−0.02−0.010.000.010.02PC1P C 2>PCsites <-predict(PCs,Trns_site[,imp.vars])>points(PCsites[,1:2])>SpsWtd <-sweep(gf$Y,2,apply(gf$Y,2,min),+"-")>SpsWtdPCs <-(t(SpsWtd)%*%(PCsites[,1:2]))/colSums(SpsWtd)>points(SpsWtdPCs,col ="red",pch ="+")If required,the abundance of any given species may be plotted on the biplot.For example the first species from gf$Y =A1010102,an alga from the family Caulerpaceae that appears to prefer carbonate gravelly sand area with moderate bedstress and lower temperature.>sp <-colnames(SpsWtd)[1]>points(PCsites[,1:2],col ="blue",cex =SpsWtd[,+sp]/2)Alternatively,specifically named examples could be plotted:e.g.E4030373a Fungiid coral;M2020101a Strombid mollusc;or S1010671a Perophorid ascidian to name a few.3.3Mapping in geographic spaceThe biplot and the colour palette in the previous section can be used as a key to visualise com-positional patterns mapped in geographic space.The following map plots predicted PC scores in geographic coordinates,using the same colour palette as above,and represents continuous changes in inferred compositional patterns associated with the predictors.>plot(Trns_grid[,c("EAST","NORTH")],pch =".",+cex =3,asp =1,col =rgb(r,g,b,max =255))−0.6−0.4−0.20.00.20.40.6−0.6−0.4−0.20.00.20.4EASTN O R T H3.4A clustered versionSome applications may require a hard clustered output,representing inferred assemblages,rather than a continuous representation of biodiversity composition.The following example uses clara to make 8clusters.This is a fast claustering algorithm suitable for large data sets.The medoids are labelled and the colour key takes the value for each medoid.Other clustering methods maybe used(for example,pam would take several minutes)as alternatives,and their various cluster diagnostics may provide a guide to the appropriate numbers of clusters.>require(cluster)>ncl<-8>clPCs<-clara(PCs$x,ncl,sampsize=1000)>medcolR<-r[clPCs$i.med]>medcolG<-g[clPCs$i.med]>medcolB<-b[clPCs$i.med]>plot((PCs$x[,1:2]),xlim=xrng,ylim=yrng,+pch=".",cex=4,col=rgb(medcolR[clPCs$clustering],+medcolG[clPCs$clustering],medcolB[clPCs$clustering],+max=255),asp=1)>points(PCs$rotation[!vind,1:2]/scal,pch="+")>arrows(rep(0,lv),rep(0,lv),PCs$rotation[vec,+1]/scal,PCs$rotation[vec,2]/scal,length=0.0625)>text(PCs$rotation[vec,1]/scal+jit*sign(PCs$rotation[vec,+1]),PCs$rotation[vec,2]/scal+jit*sign(PCs$rotation[vec,+2]),labels=vec)>text(clPCs$medoids[,1:2],labels=seq(1,ncl))>legend("bottomleft",as.character(seq(1,ncl)),+pch=15,cex=1,col=rgb(medcolR,medcolG,+medcolB,max=255))−0.02−0.010.000.010.02−0.02−0.010.000.010.02PC1P C 2>plot(Trns_grid[,c("EAST","NORTH")],pch =".",+cex =3,asp =1,col =rgb(medcolR[clPCs$clustering],+medcolG[clPCs$clustering],medcolB[clPCs$clustering],+max =255))>points(Trns_grid[clPCs$i.med,c("EAST","NORTH")],+pch =as.character(seq(1,ncl)))>legend("bottomleft",as.character(seq(1,ncl)),+pch =15,cex =1,col =rgb(medcolR,medcolG,+medcolB,max =255))−0.6−0.4−0.20.00.20.40.6−0.6−0.4−0.20.00.20.4EASTN O R T H4Session informationThe simulation and output in this document were generated in the following computing envi-ronment.•R version 2.12.2Patched (2011-03-18r54866),x86_64-unknown-linux-gnu•Locale:LC_CTYPE=en_US.UTF-8,LC_NUMERIC=C ,LC_TIME=en_US.UTF-8,LC_COLLATE=C ,LC_MONETARY=C ,LC_MESSAGES=en_US.UTF-8,LC_PAPER=en_US.UTF-8,LC_NAME=C ,LC_ADDRESS=C ,LC_TELEPHONE=C ,LC_MEASUREMENT=en_US.UTF-8,LC_IDENTIFICATION=C•Base packages:base,datasets,grDevices,graphics,methods,stats,utils •Other packages:cluster˜1.13.3,extendedForest˜1.4,gradientForest˜0.1-11,lattice˜0.19-17•Loaded via a namespace(and not attached):grid˜2.12.2,tools˜2.12.2ReferencesL.˜Breiman.Random Forests.Machine Learning,45(1):5–32,2001.C.Y.Burridge,C.R.Pitcher,B.J.Hill,T.J.Wassenberg,and I.R.Poiner.A comparison of demersal communities in an area closed to trawling with those in adjacent areas open to trawling:a study in the Great Barrier Reef Marine Park,Australia.Fisheries Research,79: 64–74,2006.N.˜Ellis,S.J.Smith,and C.R.Pitcher.Gradient forests:calculating importance gradients on physical predictors.submitted manuscript.2010.S.˜Ferrier,G.˜Manion,J.˜Elith,and K.˜ing generalized dissimilarity modelling to analyse and predict patterns of beta diversity in regional biodiversity assessment.Diversity and Distributions,13(3):252–264,2007.Andy Liaw and Matthew Wiener.Classification and regression by randomforest.R News,2(3): 18–22,2002.URL /doc/Rnews/.C.R.Pitcher,W.˜Venables,N.˜Ellis,I.˜McLeod,M.˜Cappo, F.˜Pantus,M.˜Austin, P.˜Doherty,and N.˜Gribble.Gbr seabed biodiversity mapping project:Phase1 report to crc-reef.Technical report,CSIRO/AIMS/QDPI Report,2002.URL .au/resprogram/programC/seabed/Seabedphase1rpt.htm.C.R.Pitcher,P.˜Lawton,N.˜Ellis,S.J.Smith,L.S.Incze,C-L.Wei,M.E.Greenlaw,N.H.Wolff, J.˜Sameoto,and P.V.R.Snelgrove.The role of physical environmental variables in shaping patterns of biodiversity composition in seabed assemblages.submitted manuscript.2010.IR˜Poiner,J.˜Glaister,CR˜Pitcher, C.˜Burridge,T.˜Wassenberg,N.˜Gribble, B.˜Hill,SJM Blaber,DA˜Milton, D.˜Brewer,et˜al.The environmental ef-fects of prawn trawling in the far northern section of the Great Barrier Reef Marine Park:1991–1996.Final Report to GBRMPA and FRDC,1998.URL http://www.publish.csiro.au/books/bookpage.cfm?PID=2419.C.˜Strobl,A.L.Boulesteix,T.˜Kneib,T.˜Augustin,and A.˜Zeileis.Conditional variable im-portance for random forests.BMC Bioinformatics,9(1):307,2008.。
文献综述植被是生物地球化学循环如碳循环、氮循环、水循环等地表过程中的重要控制因子,通过植被遥感提供的植被宏观变化信息,可为地表系统过程模拟提供关键参数,所以对于植被的遥感研究极为重要。
在近年的遥感研究中,在利用多波段、多时相、高光谱的遥感数据来提高遥感对地物识别能力的同时,越来越多的学者将针对地物双向反射及它的孪生姊妹偏振反射的测量作为新型遥感手段来进行研究,努力使定性遥感走向定量遥感。
通过这些手段得到的信息在资源调查农作物估产、农林牧业发展、军事目标识别、地质勘探、找矿、土壤分析、环境监测、灾害估计、海洋开发利用、遥感数据订正等方面有着特殊意义(乔延利等,2001;杨之文,2004;张绪国等,2008;麻金继等,2009)。
20 世纪 70 年代以来,遥感对地和大气的观测主要采取垂直收集数据的方式,利用目标地物的辐射强度来推求目标的表面状态、温度、物质组成及其他一些物理化学特性,以获得地面的二维信息(宋开山等,2004)。
遥感解译的主要依据是根据不同的地物具有不同的吸收、反射和发射电磁波的能力来分辨地球表层的地物分布,并假定目标地物的反射光谱在2π空间内分布是一致的,即所谓的朗伯体(赵云升等,2000)。
但实践证明,这种假定引发的结论与实际相差较大,不尽合理。
另外,随着遥感应用的深入研究,传统的解译模式已经不能满足实际的需要,现实表明人们不仅需要地面目标的平面信息,如位置和大小,而且还需要了解更高层的信息,即目标的三维信息(赵云升等,2005)。
因此,在20世纪70年代末,美国Suit G.,Goel N. S., Strahler A. H.及中国的李小文等一小批学者首先开始了分布函数BRDF (Bidirectional Reflectance Distribution Function ,即双向反射分布函数)的研究(Li Xiaowen et al.,1985;Lucht W et al.,2000;Wang Jun-Fa et al.,2001)。
百令片对维持性血液透析患者外周血T淋巴细胞的影响杨涛;张任;赵黎;李正东;陈华茜【摘要】目的探讨百令片对维持性血透患者T淋巴细胞的影响.方法以在我院血液净化中心维持性血液透析患者为研究对象,用回顾性分析的方法,接受百令片治疗者为治疗组,未接受百令片治疗者为对照组,检测各组患者外周血淋巴细胞表达频率、T细胞及亚群表达比例;回顾分析两组患者近1年因肺部感染住院频次及住院时间.结果两组间基本资料血清肌酐、尿素、年龄、血清白蛋白、血红蛋白、透析时间、透析频率等方面两组无显著性差异.两组患者外周血淋巴细胞均较正常值下降,但服用百令片组淋巴细胞绝对值(1.46±0.12)G/L、T淋巴细胞比例均较未服用组高[(1.46±0.12)G/L vs(1.16±0.08)G/L;(72.34±2.86)%vs(62.74±3.45)%,P<0.05];两组患者T淋巴细胞亚群比例(CD4+/CD8+T)无显著差异.服用百令片组患者近1年因肺部感染住院频率、住院时间显著低于对照组[0.07 vs 0.23;(6.67±0.33)dvs(9.57±0.72)d,P<0.05].结论百令片能改善血透患者的低淋巴细胞血症,部分纠正患者T淋巴细胞比例,调节患者的免疫功能,减少患者因肺部感染住院频次.【期刊名称】《中国医药科学》【年(卷),期】2017(007)015【总页数】4页(P178-181)【关键词】维持性透析;淋巴细胞;CD3;CD4;CD8【作者】杨涛;张任;赵黎;李正东;陈华茜【作者单位】湖北医药学院附属东风总医院,湖北十堰442000;湖北医药学院附属东风总医院,湖北十堰442000;湖北医药学院附属东风总医院,湖北十堰442000;湖北医药学院附属东风总医院,湖北十堰442000;湖北医药学院附属东风总医院,湖北十堰442000【正文语种】中文【中图分类】R587.2维持性血液透析是终末期肾脏病(ESRD)患者维系生命的重要手段,其全因死亡率是普通人群的10~20倍以上[1],其中感染占第2位[2]。
Agricultural and Forest Meteorology108(2001)101–112Response of tree phenology to climate change across EuropeFrank-M.Chmielewski∗,Thomas RötzerHumboldt-University of Berlin,Faculty of Agriculture and Horticulture,Institute of Crop Sciences,Subdivision of Agricultural Meteorology,Albrecht-Thaer-Weg5,D-14195Berlin-Dahlem,GermanyReceived25October2000;received in revised form5February2001;accepted19February2001AbstractTo investigate the impact of recent climatic changes on the plant development in Europe,this study uses phenological data of the International Phenological Gardens for the period1969–1998.For this study,the leafing dates of four tree species (Betula pubescens,Prunus avium,Sorbus aucuparia and Ribes alpinum)were combined in an annual leaf unfolding index to define the beginning of growing season.The end of growing season was defined using the average leaf fall of B.pubescens, P.avium,Salix smithiana and R.alpinum.A nearly Europe-wide warming in the early spring(February–April)over the last 30years(1969–1998)led to an earlier beginning of growing season by8days.The observed trends in the onset of spring corresponded well with changes in air temperature and circulation(North Atlantic Oscillation Index(NAO-index))across Europe.In late winter and early spring,the positive phase of NAO increased clearly,leading to prevailing westerly winds and thus to higher temperatures in the period February–April.Since the end of the1980s the changes in circulation,air temperature and the beginning of spring time were striking.The investigation showed that a warming in the early spring(February–April) by1◦C causes an advance in the beginning of growing season of7days.The observed extension of growing season was mainly the result of an earlier onset of spring.An increase of mean annual air temperature by1◦C led to an extension of5 days.©2001Elsevier Science B.V.All rights reserved.Keywords:Phenology;Growing season;Climate change;NAO;Temperature1.IntroductionPhenological observations are some of the most sensitive data in identifying how plant species respond to regional climate conditions and to climatic changes. Therefore,phenology has emerged recently as an important focus for ecological research(Schwartz, 1999).In mid-latitudes the seasonal timing of spring events such as budding,leafing orflowering of plants depends highly on air temperature.With increasing ∗Corresponding author.Tel.:+49-30-314-71210;fax:+49-30-314-71211.E-mail address:chmielew@agrar.hu-berlin.de(F.-M.Chmielewski).temperatures plant development in spring starts earlier within a year.A lot of recent phenological studies were reported on earlier spring events in recent decades.Depending on the species and the investigated period,the results vary to a certain extent.Beaubien and Freeland(2000)reported on a long-term trend(1900–1997)in timing offirst bloom of Populus tremuloides(aspen poplar)of−2.7days/ decade at Edmonton/Alberta(Canada).Mainly since 1973the negative deviations from the long-term mean prevail.The springflowering index—mean of thefirst flowering dates of P.tremuloides,Amelanchier alnifo-lia(saskatoon)and Prunus virginiana(chokecherry)—had also advanced by 1.3days/decade in the0168-1923/01/$–see front matter©2001Elsevier Science B.V.All rights reserved. PII:S0168-1923(01)00233-7102F .-M.Chmielewski,T.Rötzer /Agricultural and Forest Meteorology 108(2001)101–1121936–1996period in the Edmonton area.Menzel (2000)investigated the trends of individual species of the International Phenological Gardens (IPGs)in Eu-rope for the period 1959–1996.She found an average trend of −2.1days/decade for all springtime phases (leaf unfolding,May-shoot and flowering of different species)and a mean trend of +1.6days/decade for the autumn phases (leaf colouring and leaf fall).The data point to an extension of growing season by 3.6days within 10years.Timings of spring and summer species have been mostly related to air temperatures.Fitter et al.(1995)found a relationship between first flowering date in England and air temperature of −4days/◦C.Likewise for the British Isles,Sparks et al.(2000)detected a response of flowering times of different spring and midseason species to warming of 2–10days/◦C.For Hungary,Walkovszky (1998)reported that a rise of temperature by 1◦C causes an advanced flowering of Robinia pseudoacacia (locust tree)by 7days.Generally,higher temperatures in the late winter and early spring promote earlier flowering and leafing ofplants.Fig.1.Locations of the IPGs in Europe.Active and already closed stations with observations for more than 10years are shown.This study attempts to explain the trends observed in the phenological data of the IPGs in Europe,with the aim of showing whether the detected regional or Europe-wide trends in the beginning of growing season correspond with climatic trends on the same spatial scale.2.Materials and methods 2.1.Phenological dataThe IPGs are a phenological network in Europe which was founded by F.Schnelle and E.V olkert in 1957(http://www.agrar.hu-berlin.de/pflanzenbau/agrarmet/ipg.html).At present the network is co-ordinated by the Humboldt-University of Berlin,Institute of Crop Sciences (Chmielewski,1996).The idea of this network was to obtain comparable pheno-logical data across Europe (among others beginning of leaf unfolding,flowering,autumn colouring,leaf fall)from plants which are not influenced by dif-ferent genetic conditions.For this reason,vegetativeF .-M.Chmielewski,T.Rötzer /Agricultural and Forest Meteorology 108(2001)101–112103propagated species of trees and shrubs were planted at different sites in Europe.In 1959,the first IPG started its phenological observations.Today,about 50IPGs across Europe record phenological data from 23species (Fig.1).For this study,the leafing dates of four species (Betula pubescens ,Prunus avium ,Sorbus aucuparia and Ribes alpinum )were combined in an annual leaf unfolding index to define the beginning of growing season (BGS).For the end of the growing season (EGS)the average timing of leaf fall of B.pubescens ,P .avium ,Salix smithiana and R.alpinum were used.The difference between the end and the beginning of the growing season was defined as the length of grow-ing season (LGS).For the selection of species used to calculate BGS and EGS,the quantity of observations between 1969and 1998was decisive.For the species above the largest amount of data for leafing and leaf fall wasavailable.Fig.2.Definition of NR in Europe (NR01–NR12).The most important precondition of obtaining comparable observation values is the exact defini-tion of the phenological phases which are observed.Beginning of leaf unfolding is registered when the first regular surfaces of leaves become visible in several places (about 3–4)on the observed plant.The first leaf of a plant has pushed out of the bud up to its leaf-stalk.The leaf fall is defined if more than half of the leaves of the observed plant have fallen.Phenological data from single sites are often ‘noisy’because the quality of data depends on the skills and precision of the observers (Schnelle,1955;Sparks et al.,2000).Special microclimates can also lead to an advanced or delayed timing of spring.A good method to reduce the ‘noise’in the data is to average the obser-vations across several sites.For this reason,12natural regions (NRs)across Europe were defined (Fig.2),considering the classification of Wagner (1971).The104F.-M.Chmielewski,T.Rötzer/Agricultural and Forest Meteorology108(2001)101–112 Table1Classification of the IPGs to NRs(NR01–NR12),average of latitude,longitude and altitude of the NRsNo.NR Averagelatitude(◦)Averagelongitude(◦)Averagealtitude(m)No.ofIPGs aNo.ofIPGs b01British Isles/Channel Coast51.6−3.85074 02North Sea/Central European Lowlands52.910.14553 03Baltic Sea Region57.215.25387 04North Atlantic Mountain Region61.98.13822 05North Scandinavia67.126.714821 06Northern Central European Highlands50.48.730766 07Southern Central European Highlands48.28.259187 08North Alpine Foreland47.810.960375 09Bav.-Bohemian Highlands/Carpathian Mountains48.615.561188 10Great Hungarian Lowlands/Danube-Save-Region46.019.615254 11Dinaric Mountain Region/Dalmatia42.920.360184 12Portugal41.3−8.53010a Total number of IPGs in each NR.b Number of IPGs used for the calculation of length of growing season.IPGs were associated to the regions(Table1),so that up to eight phenological gardens belonged to an area. NR04,NR05and NR12are represented by only one or two stations.For Portugal where only one IPG is available the national boundary was used as NR.2.2.Climatic dataIn order to investigate trends in phenology in relation to climatic changes,gridded near surface temperatures(NCEP/NCAR reanalysis data,Kalnay et al.,1996)and the North Atlantic Oscillation In-dex(NAO-index)(NAO,Hurrel,1995)for the period 1969–1998were used.The horizontal resolution of NCEP data is about210km,a region extending from70◦N to40◦N and from10◦W to25◦E was selected.The used NAO-index is the difference of normalized sea level pressure between Ponta Delgada (Azores)and Stykkisholmur/Reykjavik(Iceland).A positive phase of the NAO reflects below-normal pressure in the northern North Atlantic(Iceland)and above-normal pressure over the central North At-lantic(Azores).This usually leads to strong westerly winds which are associated with warm and moist air masses across the North European continent in winter (December–March).In this case,in southern Europe and the Middle East often below-normal temperatures are observed(Hurrel,1995).A negative phase reflects an opposite pattern in circulation and air temperature across Europe.3.Results3.1.Average growing season in Europe(1969–1998) The length of growing season is an important measure in forestry,agriculture and horticulture.Its variability is mainly caused by temperature-induced variations in the timing of spring events(budding, leafing,andflowering).The autumn phases(leaf colouring and leaf fall)usually show smaller annual variations.On an average the beginning of growing season in Europe starts on23April(Table2).In south-west (NR12)and south-east Europe(NR10)as well as in the thermal favoured region NR01,growing season starts before15April.In Portugal,it already starts at the end of March(25March).The latest onset of spring can be observed in the cold areas of Europe (NR04:7May;NR05:23May).On an average the green wave in Europe moves annually with44km/day from south to north,with200km/day from west to east and with32m/day with increasing altitude(Rötzer and Chmielewski,2001).The end of growing season generally shows a smaller variability across Europe.On an average leaf fall starts on28October.An early EGS can be observed in the high latitudes(NR05:9October; NR04:26October)and in the highlands(NR09:23 October).In the maritime region(NR03)the EGS occurred relatively late(5November).F .-M.Chmielewski,T.Rötzer /Agricultural and Forest Meteorology 108(2001)101–112105Table 2Beginning (B),end (E)and length (L)of growing season in Europe (EU)and in different NRs (NR01–NR12),1969–1998,s:standard deviation;DOY:day of the year NRB (DOY)s (within NR)(days)E (DOY)s (within NR)(days)L (days)s (within NR)(days)EU (NR01–NR11)113(23April) 5.8301(28October) 2.4188 5.5011018.2301 3.42008.1021049.4302 4.81989.9031207.3309 3.01897.004127 6.2299 6.71728.005143 6.328210.01399.2061068.4305 5.21998.4071089.1305 3.21979.2081107.9303 3.81937.209119 6.8296 5.11777.810101 6.3303 5.6202 6.9111097.4300 6.21918.9128410.7No data No data s (NR01–NR11)12.87.018.6The average length of growing season in Europe (EGS–BGS)lasts 188days and depends highly on the mean annual air temperature (Fig.3).The regression equation indicates that 1◦C increase in mean air tem-perature is associated with an extension of growing season by about 5days.The shortest duration was observed in North Scan-dinavia (NR05)with only 139days (4.5months)and in NR04with 172days.NR05has the most conti-nental climate of all regions with positivemonthlyFig.3.Relationship between mean annual air temperature (T Y )and length of growing season (L)in different NRs of Europe.air temperatures only between May and September.NR04is slightly thermal favoured because of the North Atlantic gulf pared with NR05,the LGS is 1month longer.A long duration of growing season of about 6.5months was calculated for the natural regions 01,02,06,07.The longest period,with an average of 202days,occurred in the ‘Great Hungarian Lowlands/Danube-Save-Region’(NR10).3.2.Trends of growing season in Europe (1969–1998)In the last 30years,the beginning of growing season in Europe has advanced altogether by 8days,this corresponds to a significant trend (p <0.05)of 2.7days/decade (Fig.4).Mainly,since the end of the 1980s,early dates prevail.Between 1989and 1998,8out of 10years had an advanced onset of spring.In 1989and mainly in 1990,the BGS was extremely pared to the long-term mean,in 1990leaf-ing in Europe started 14days earlier (9April).Because of the long and strong winter in 1995/1996,the BGS in 1996was again relatively late.However,with 10days above-normal (3May)the latest date was observed in 1970,a year with strong negative temperature anoma-lies of up to −3.5◦C between February and April in central and northern Europe.Compared to the BGS,the end of growing sea-son shows smaller annual variations (Fig.4).The106F .-M.Chmielewski,T.Rötzer /Agricultural and Forest Meteorology 108(2001)101–112Fig.4.Trends in the average beginning (B),end (E)and length (L)of growing season in Europe,1969–1998,Y:year;DOY:day of the year;trend with *p <0.05,**p <0.01.difference between the extreme years was only 10days whereas for BGS it was 24days.The trend to a later end of about 1day/decade is also relatively small.Mainly influenced by the BGS,the length of grow-ing season had advanced for the period 1969–1998by 10.5days,corresponding to a significant trend of 3.5days/decade (p <0.01).Because of the very early on-set in 1990,this year had the longest growing season (200days).Table 3Long-term trends of the beginning (B),the end (E)and the length (L)of growing season in Europe (EU)and in different NRs (NR01–NR12)for the period 1969–1998(significant trends are printed in italics)No.NRB (days/decade)E (days/decade)L (days/decade)EU (NR01–NR11)−2.7∗∗+0.9∗+3.5∗∗∗01British Isles/Channel Coast−5.7∗∗∗−0.4+5.3∗∗∗02North Sea/Central European Lowlands −5.0∗∗∗+0.4+5.9∗∗∗03Baltic Sea Region−4.3∗∗∗−0.1+4.5∗∗∗04North Atlantic Mountain Region −0.9+0.2+0.605North Scandinavia−1.9+2.4+4.3∗∗06Northern Central European Highlands −4.5∗∗∗+0.5+4.9∗∗∗07Southern Central European Highlands −5.0∗∗∗+1.5∗∗+6.3∗∗∗08North Alpine Foreland−3.1∗+0.5+3.5∗∗09Bav.-Bohemian Highlands/Carpathian Mountains −0.8+0.9+1.610Great Hungarian Lowlands/Danube-Save-Region −0.1+1.0+1.111Dinaric Mountain Region/Dalmatia +2.4+3.4∗∗∗+0.912Portugal−4.7∗∗No dataNo data∗Significant at p <0.1.∗∗Significant at p <0.05.∗∗∗Significant at p <0.01.Most of the European regions showed significant negative trends in BGS,which range between 3and 6days/decade (Table 3).The strongest trends were observed in central Europe:in the NRs ‘British Isles/Channel Coast’,‘North Sea and Central Euro-pean Lowlands’,‘Baltic Sea Region’,‘Northern and Southern Central European Highlands’as well as in the ‘North Alpine Foreland’.Also for the IPG in Portugal (NR12)a trend of −4.7days/decade wasF.-M.Chmielewski,T.Rötzer/Agricultural and Forest Meteorology108(2001)101–112107 Table4Correlation between monthly air temperature from January(T1)to May(T5)and the beginning of growing season(B)in Europe(EU) and in different NRs(NR01–NR12)for the period1969–1998(significant coefficients(p<0.05)are printed in italics)NR B(average date)T1T2T3T4T5T24a EU23April−0.54−0.65−0.72−0.43−0.19−0.830111April−0.24−0.51−0.77−0.45−0.40−0.75 0214April−0.26−0.63−0.55−0.32−0.12−0.75 0330April−0.60−0.62−0.59−0.70−0.18−0.75 047May−0.56−0.47−0.54−0.44−0.23−0.66 0523May−0.15−0.35−0.01−0.53−0.33−0.60b 0616April−0.20−0.50−0.69−0.21−0.13−0.77 0718April−0.09−0.52−0.68−0.14−0.30−0.81 0820April−0.10−0.43−0.60−0.25−0.16−0.71 0929April−0.34−0.49−0.51−0.210.03−0.66 1011April0.03−0.29−0.75−0.310.15−0.70 1119April0.06−0.36−0.68−0.120.15−0.64 1225March0.24−0.41−0.35−0.21−0.09−0.45ca T24:average air temperature from February to April.b T45:April–May.c T23:February–March.calculated.Weak trends were found in northern Scan-dinavia and in south-east Europe.The latter region even had a positive trend(NR11:Dinaric Mountain Region/Dalmatia)which,however,was not significant. The regional trends of the end of growing season tend to a latter timing,but in most areas no significant trend was found.The significant trends in the length of growing sea-son range between4days/decade(NR08)and6days/ decade(NR02,NR07).Altogether seven out of11NRs showed an extended growing season in Europe.3.3.Relations to air temperatureThe annual timing of leaf unfolding is to a great extent a temperature response.Thus the beginning of growing season(in our case an average leaf unfolding index of four species)should reflect the thermal regime in Europe.Table4shows that the BGS is mainly influenced by the air temperature in March.Also in February and partially in April significant correlation coefficients between temperature and BGS were found.In these 3months all coefficients are negative,meaning that higher temperatures in the late winter and early spring promote earlier leaf unfolding.As it can be seen,in the last column of Table4all correlation coefficients between the average air temperature from February to April(T24)and BGS are significant(p<0.05).Thismeans that the temperature in this period is decisivefor the annual timing of spring in Europe.For the moreextreme climatic regions(NR05,NR12)differentperiods,which correspond more closely to the onsetof spring in this area,were used to calculate the meantemperature.The correlation coefficient between the BGS inEurope(EU)and air temperature(T24)is−0.83 (p<0.05).The relationship between average tem-perature variations from February to April and BGSin Europe was strong(Fig.5).The extreme years inphenology(late:1970;early:1989,1990)correspondwell with the deviations in air temperature.Bothtime-series show a significant trend.According tothe regression equation,a warming in Europe of1◦Cleads to an advanced beginning of growing season by6.7days.In order to investigate the causes of regional trendsin the BGS in a more detailed way,a trend analysisof air temperature for all NRs was done.The gridpoints of air temperature were associated to the NRsto calculate the trends(Table5).In most European regions,we found positive trendsin air temperature for the last30years,which ex-plain well the observed phenological trends in spring(e.g.in NR01,NR02,NR03,NR06,NR07,NR12).The highest positive trend in temperature was found108F .-M.Chmielewski,T.Rötzer /Agricultural and Forest Meteorology 108(2001)101–112Fig.5.Trends in air temperature (thin line)from February to April (T 24)and in the beginning of growing season (B)in Europe (bold line),1969–1998(left).Correlation between T 24and B,1969–1998(right),DOY:day of the year.Table 5Long-term trends of the beginning of growing season (B)and of mean air temperature from February to April (NR05:April–May;NR12:February–March)in Europe and in different NRs (NR01–NR12)for the period 1969–1998(significant trends are printed in italics)No.NRTrend in B (days/decade)Trend in air temperature (◦C/decade)EU (NR01–NR11)−2.7∗∗+0.23+01British Isles/Channel Coast−5.7∗∗∗+0.34∗∗02North Sea/Central European Lowlands −5.0∗∗∗+0.51∗03Baltic Sea Region−4.3∗∗∗+0.47+04North Atlantic Mountain Region −0.9+0.2105North Scandinavia−1.9−0.0106Northern Central European Highlands −4.5∗∗∗+0.38+07Southern Central European Highlands −5.0∗∗∗+0.25+08North Alpine Foreland−3.1∗+0.0409Bav.-Bohemian Highlands/Carpathian Mountains −0.8+0.0210Great Hungarian Lowlands/Danube-Save-Region −0.1−0.1311Dinaric Mountain Region/Dalmatia +2.4−0.40+12Portugal−4.7∗∗+0.59∗∗∗+Significant at p <0.2.∗Significant at p <0.1.∗∗Significant at p <0.05.∗∗∗Significant at p <0.01.in NR12.As a result,BGS in NR12advanced by 4.7days/decade.Negative trends in air temperature were only found in south-east Europe (NR10,NR11).The decreasing mean temperatures from February to April in NR11corresponded well to the delayed beginning Table 6Correlation coefficients (r )between NAO-index and mean air temperature in Europe for different months,1969–1998(correlation coefficients >0.36are significant with p <0.05)Month January February March April February–April r0.690.750.540.450.73of growing season.Strong positive trends in air tem-perature were observed for central Europe,where the most striking changes in the BGS occurred.Leaf fall in autumn is a more complex process,which is also induced by lack of light and chilliness.F.-M.Chmielewski,T.Rötzer/Agricultural and Forest Meteorology108(2001)101–112109Fig.6.Mean NAO-index(thin line)from February to April(N24)and average beginning of growing season(B)in Europe(bold line), 1969–1998(left).Correlation between N24and B,1969–1998(right),DOY:day of the year.Fig.7.Correlation between average NAO-index(N24)and average air temperature(T24)from February to April as well as correlation coefficients r between N24and beginning of growing season(B)in different NRs(NR01–NR11).110F.-M.Chmielewski,T.Rötzer/Agricultural and Forest Meteorology108(2001)101–112It shows no strong relationship to air temperature likeleafing in spring.For this reason,it is not possible toexplain the beginning of leaf fall only by temperature.Here more detailed studies are necessary.3.4.Relations to circulationWinter and early spring temperatures in Europe aremainly influenced by the prevailing circulation.TheNAO is a suitable and often used index to describethe circulation over Europe(Hurrel,1995).Positivephases of NAO from January to April tended to beassociated with above-normal temperatures in Europein these months(Table6).The highest correlation (r=0.75)between NAO and air temperature exists in February.For the period February–April,averageNAO-index and air temperature in Europe are wellcorrelated(r=0.73).This gives the possibility to in-vestigate how the annual variability of NAO influencesthe timing of spring in Europe.The very early beginning of growing season in1989and1990corresponds well with the high positive in-dices of NAO in both years(Fig.6).Similar to theBGS the NAO showed strong positive values since1989as well.The relatively late spring in1996wasalso well reflected in the time-series of NAO.Thecorrelation coefficient between NAO(February–Aprilaverage)and the BGS in Europe was−0.70for theperiod1989–1998.The highest regional correlation coefficients bet-ween NAO and BGS were calculated for central andnorthern Europe where the correlation between airtemperature and NAO was high as well(Fig.7).Inthese regions,the strongest negative trends of BGSwere detected.In south-east Europe(NR10,NR11)the relationshipbetween NAO and air temperature was weak,so thatin this region no tendency to an earlier beginning ofgrowing season was found.Here,in last few yearsslightly decreasing temperatures were observed.4.DiscussionThe obtained results concerning the Europe-wideand regional trends in the beginning and end of grow-ing season agreed with those of Menzel and Fabian(1999)as well as of Menzel(2000).In addition to these results,the climatic causes for the observed trends in the beginning of leafing could now be presented.The Europe-wide trend as well as the regional trends in the beginning of growing season correspond well with changes in circulation and in air temperature of the early spring.The analysed trends in temperature are in accordance with the results of Schönwiese and Rapp (1997)and Rapp(2000),who found increasing tem-peratures in central Europe and a tendency to declin-ing temperatures in parts of south-east Europe as well. The strong positive trend in the Iberian peninsula is in accordance with IPCC(Watson et al.,1998).The increased positive phase of NAO since1989led to milder temperatures in late winter and early spring because of prevailing westerly winds.This resulted in an advanced beginning of spring in Europe and thus in an extension of growing season by3.5days/ decade.The results of this paper confirmfindings of other authors,concerning the influence of air temperature on the timing of spring events.In most recent studies, an advanced timing of spring events such as budding, leafing andflowering between2and4days per degree was found(e.g.Beaubien and Freeland,2000;Kramer et al.,2000;Sparks et al.,1997,2000).The result that an increase in mean annual air temperature of1◦C is associated with an extension of growing season by5 days in Europe coincide exactly with thefindings of White et al.(1999)for US stations.There is no doubt that a global warming will lead to changes in the length of growing season within certain limits.This investigation showed that the extension of growing season was mainly influenced by an earlier beginning.The end of growing season showed a lower variability in all regions of Europe.Generally,the impact of global warming on the ex-tension of growing season will depend on what extent the timing of leaf unfolding and leaf fall will change in future.A linear extrapolation of the statistical trends,found in this or in other studies,is of course not possible.Forest growth models must be used for a better understanding of the responses of trees to cli-matic changes.Investigations by Kramer et al.(2000), Linkosalo(2000)and Chuine et al.(1999)showed that the phenological response of trees to an increase in temperature depends on the plant species.Both an extension as well as non-extension or a reduced length of growing season are possible(Kramer et al.,F.-M.Chmielewski,T.Rötzer/Agricultural and Forest Meteorology108(2001)101–1121112000).In the latter case,the date of leaf fall advanced more than the date of leaf unfolding did.5.Concluding remarksThis study presented a climatic approach to explain the observed changes in the timing of phenological events.It was possible to confirm that phenology is a good indicator of global warming.The most important results of this study can be summed up as follows:1.In the last30years,the average beginning of grow-ing season in Europe has advanced by8days, whereby the earliest dates were observed since the end of the1980s.2.In almost all NRs a trend of an earlier onset ofspring was observed.Tendencies to a later begin-ning were only found in SE-Europe(NR10,NR11), where the average spring air temperature decreased over the30-year period.3.The strongest trends were noticed for centralEurope(NRs:01,02,03,06,07,08),where the average spring air temperature increased over the 30-year period.4.A warming in the early spring(February–April)of1◦C leads to an advanced beginning of growing season by approximately7days.5.The end of growing season showed in nearly allNRs a tendency for a slight delay.6.The length of growing season was mainly influ-enced by its beginning through which in seven out of11NRs a significant trend towards an extension of the growing season was observed.7.The average growing season in Europe(period:1969–1998)lasts188days(BGS:23April;EGS: 28October).It extends by5days per1◦C increa-sing mean annual air temperature.8.The observed Europe-wide and regional trends inthe beginning of growing season correspond well with the changes in air temperature in early spring (February–April)and with the increased positive phases of NAO-index.AcknowledgementsThe authors thank all observers of the International Phenological Gardens in Europe for the honorary and valuable work in the last40years.We are also grateful to Carola Krischker,who assisted us within the project. This study wasfinancially supported by the BMBF in Germany(Project:Climate variability and phenology in Europe,01LA98501).ReferencesBeaubien,E.G.,Freeland,H.J.,2000.Spring phenology trends in Alberta,Canada:links to ocean temperature.Int.J.Biometeorol.44,53–59.Chmielewski,F.-M.,1996.The International Phenological Gardens across Europe.Present state and perspectives.Phenol.Seasonality1,19–23.Chuine,I.,Cour,P.,Rousseau,D.D.,1999.Selecting models to predict the timing offlowering of temperate trees:implication for tree phenology modelling.Plant Cell Environ.22,1–13. Fitter,A.H.,Fitter,R.S.R.,Harris,I.T.B.,Williamson,M.H.,1995.Relationships betweenfirstflowering date and temperature in theflora of a locality in central England.Funct.Ecol.9,55–60. Hurrel,J.W.,1995.Decadal trends in the North Atlantic Osci-llation:regional temperatures and precipitation.Science269, 676–679.Kalnay,E.,Kanamitsu,M.,Kistler,R.,Collins,W.,Deaven,D., Gandin,L.,Iredell,M.,Saha,S.,White,G.,Woollen,J.,Zhu, Y.,Chelliah,M.,Ebisuzaki,W.,Higgins,W.,Janowiak,J., Mo,C.K.,Ropelewski,C.,Wang,J.,Leetmaa,A.,Reynolds, R.,Jenne,R.,Dennis,J.,1996.The NCEP/NCAR40-year Reanalysis Project.Bulletin of the American Meteorological Society,pp.437–471.Kramer,K.,Leinonen,I.,Loustau, D.,2000.The importance of phenology for the evaluation of impact of climate change on growth of boreal,temperate and Mediterranean forest ecosystems:an overview.Int.J.Biometeorol.44,67–75. Linkosalo,T.,2000.Analyses of the spring phenology of boreal trees and its responses to climate change.Dissertation.Faculty of Agriculture and Forestry,University of Helsinki.Department of Forest Ecology Publications No.22,55pp.Menzel, A.,2000.Trends in phenological phases in Europe between1951and1996.Int.J.Biometeorol.44,76–81. Menzel,A.,Fabian,P.,1999.Growing season extended in Europe.Nature397,659.Rapp,J.,2000.Konzeption,Problematik und Ergebnisse klimato-logischer Trendanalysen für Europa und Deutschland,Berichte DWD,212,145S.Rötzer,T.,Chmielewski, F.-M.,2001.Phenological maps of Europe.Clim.Res.,in press.Schnelle,F.,1955.Pflanzen-Phänologie.Geest und Portig,Leipzig. Schönwiese, C.D.,Rapp,J.,1997.Climate Trend Atlas of Europe Based on Observations1891–1990.Kluwer Academic Publishers,Dordrecht,The Netherlands,228pp. Schwartz,M.D.,1999.Advancing to full bloom:planning phenological research for the21st century.Int.J.Biometeorol.42,113–118.Sparks,T.H.,Carey,P.D.,Combes,J.,1997.First leafing dates of trees in Surrey between1947and1996.Lond.Nat.76,15–20.。