无菌空气的制备
- 格式:ppt
- 大小:18.96 MB
- 文档页数:131



无菌空气制备工艺流程介绍下载温馨提示:该文档是我店铺精心编制而成,希望大家下载以后,能够帮助大家解决实际的问题。
文档下载后可定制随意修改,请根据实际需要进行相应的调整和使用,谢谢!并且,本店铺为大家提供各种各样类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,如想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by theeditor. I hope that after you download them,they can help yousolve practical problems. The document can be customized andmodified after downloading,please adjust and use it according toactual needs, thank you!In addition, our shop provides you with various types ofpractical materials,such as educational essays, diaryappreciation,sentence excerpts,ancient poems,classic articles,topic composition,work summary,word parsing,copy excerpts,other materials and so on,want to know different data formats andwriting methods,please pay attention!无菌空气制备工艺流程是在实验室、制药、食品加工等行业中保证产品质量和安全的重要环节。


第1篇一、实验目的1. 了解无菌空气制备的原理和方法。
2. 掌握无菌空气制备过程中的操作步骤。
3. 评估无菌空气制备的效果。
二、实验原理无菌空气制备是通过过滤、灭菌等手段,将空气中的微生物含量降低到极低水平,以防止微生物对实验样品、培养基等产生污染。
常用的无菌空气制备方法包括:空气过滤器、紫外线照射、高压蒸汽灭菌等。
三、实验材料1. 空气过滤器2. 紫外线照射装置3. 高压蒸汽灭菌器4. 实验室无菌操作台5. 实验室无菌操作器材6. 实验样品7. 计时器四、实验步骤1. 准备实验器材:将空气过滤器、紫外线照射装置、高压蒸汽灭菌器等实验器材准备齐全。
2. 空气过滤:将空气过滤器安装在实验室无菌操作台上,打开空气过滤器,使空气通过过滤器。
计时器开始计时,记录过滤时间。
3. 紫外线照射:将紫外线照射装置打开,对空气进行照射。
照射时间根据实验要求设定。
4. 高压蒸汽灭菌:将高压蒸汽灭菌器打开,将实验样品放入灭菌器内,进行灭菌处理。
灭菌时间根据实验要求设定。
5. 无菌操作:在无菌操作台上,穿戴无菌操作服、手套等,进行无菌操作。
将经过灭菌处理的实验样品取出,放入无菌容器中。
6. 无菌空气制备效果评估:将制备的无菌空气通过无菌操作台上的空气过滤器,观察过滤器是否出现污染现象。
若过滤器未出现污染,则说明无菌空气制备效果良好。
五、实验结果与分析1. 空气过滤:实验过程中,空气过滤器未出现污染现象,说明空气过滤效果良好。
2. 紫外线照射:实验过程中,紫外线照射装置对空气进行照射,未出现明显污染现象,说明紫外线照射效果良好。
3. 高压蒸汽灭菌:实验过程中,高压蒸汽灭菌器对实验样品进行灭菌处理,未出现污染现象,说明高压蒸汽灭菌效果良好。
4. 无菌空气制备效果评估:实验过程中,制备的无菌空气通过空气过滤器,过滤器未出现污染现象,说明无菌空气制备效果良好。
六、实验结论本次实验通过空气过滤、紫外线照射、高压蒸汽灭菌等方法,成功制备了无菌空气。



标准无菌空气制备流程各环节的作用英文回答:The standard aseptic air preparation process consists of several steps, each with its own purpose and role. Here, I will explain each step and provide examples to illustrate their importance.1. Filtration: This is the initial step where the air is passed through a high-efficiency particulate air (HEPA) filter to remove any particles or contaminants. The HEPA filter is designed to trap particles as small as 0.3 micrometers, ensuring that the air is clean and free from any potential sources of contamination. For example, in a pharmaceutical manufacturing facility, the air used in the cleanroom is filtered to remove dust, bacteria, and other contaminants that could compromise the quality of the products being produced.2. Sterilization: After filtration, the air issubjected to a sterilization process to eliminate any remaining microorganisms. This is typically achieved through the use of ultraviolet (UV) radiation or heat. UV radiation damages the DNA of microorganisms, rendering them unable to reproduce and causing their death. Heat sterilization, on the other hand, involves exposing the air to high temperatures to kill any microorganisms present. For instance, in a hospital operating room, the air is sterilized to reduce the risk of surgical site infections and ensure a sterile environment for the surgical team.3. Pressurization: Once the air has been filtered and sterilized, it is pressurized to create a positive pressure environment. This helps to prevent the entry of contaminants from outside the controlled area. For example, in a cleanroom used for semiconductor manufacturing, the positive pressure prevents outside air from entering and contaminating the sensitive electronic components being produced.4. Monitoring: Throughout the process, the quality of the aseptic air is continuously monitored to ensure that itmeets the required standards. This is done by regularly sampling the air and testing it for the presence of microorganisms or other contaminants. If any deviations from the acceptable limits are detected, corrective actions can be taken to maintain the integrity of the aseptic environment. For instance, in a biotechnology laboratory, air samples may be collected and analyzed to ensure that the environment is free from any microbial contamination that could affect the experiments being conducted.In summary, the various steps in the standard aseptic air preparation process work together to ensure that theair used in controlled environments is clean, sterile, and free from any potential sources of contamination.Filtration removes particles and contaminants,sterilization eliminates microorganisms, pressurization prevents the entry of outside contaminants, and monitoring ensures the ongoing quality of the aseptic air.中文回答:标准无菌空气制备流程包括几个环节,每个环节都有其特定的作用和角色。
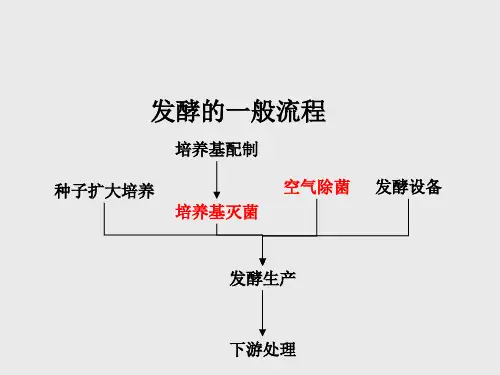

制备无菌空气的流程Preparing sterile air is a critical process in various industries, including pharmaceuticals, food processing, and healthcare. 通过消除空气中的微生物和有害粒子来制备无菌空气对于确保产品质量至关重要。
There are several steps involved in the preparation of sterile air, which require careful attention to detail and adherence to strict protocols.The first step in preparing sterile air is to clean the air intake to remove any dust, dirt, and other contaminants. 空气进入系统之前的清洁至关重要,以确保在空气进入处理过程之前从根本上减少微生物和颗粒污染。
This may involve the use of filters, air purifiers, and other air cleaning technologies to ensure that the incoming air is free from any potential contaminants.After the air intake has been cleaned, the next step is to sterilize the air using methods such as UV radiation, chemical disinfection, or heat treatment. 无菌化的方法可能包括使用紫外线辐射杀菌、化学消毒剂或热处理等。