材料科学基础课后习题答案(部分)_第2版_西安交通大学_石德珂主编演示教学
- 格式:ppt
- 大小:362.00 KB
- 文档页数:13

第一章8. 计算下列晶体的离于键与共价键的相对比例(1) NaF(2) CaO ⑶Z nS解:1、查表得:X Na =0.93,X F =3.981(0.93 3.98) 2根据鲍林公式可得 NaF 中离子键比例为:[1 e 4 ] 100% 90.2%共价键比例为:1-90.2%=9.8%2(1.00 3.44) 22、 同理,CaO 中离子键比例为:[1 e 4] 100% 77.4%共价键比例为:1-77.4%=22.6%23、 ZnS 中离子键比例为:ZnS 中离子键含量[1 e 1/4(2.58 1.65) ] 100% 19.44%共价键比例为:1-19.44%=80.56%10说明结构转变的热力学条件与动力学条件的意义•说明稳态结构与亚稳态结构之间的关系。
答:结构转变的热力学条件决定转变是否可行,是结构转变的推动力,是转变的必要条件;动力学条件决定转变速度 的大小,反映转变过程中阻力的大小。
稳态结构与亚稳态结构之间的关系:两种状态都是物质存在的状态,材料得到的结构是稳态或亚稳态,取决于转交过程的推动力和阻力(即热力学条件和动力学条件),阻力小时得到稳态结构,阻力很大时则得到亚稳态结构。
稳态结 构能量最低,热力学上最稳定,亚稳态结构能量高,热力学上不稳定,但向稳定结构转变速度慢,能保持相对稳定甚 至长期存在。
但在一定条件下,亚稳态结构向稳态结构转变。
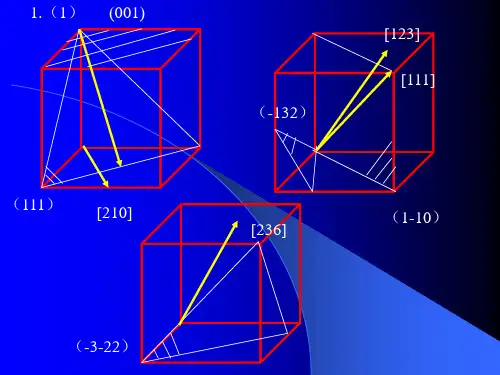
第二章1. 回答下列问题:(1) 在立方晶系的晶胞内画出具有下列密勒指数的晶面和晶向:(001 )与[210] , (111)与[112] , (110)与[111], (132)与[123], (322)与[236: (2) 在立方晶系的一个晶胞中画出(111)和(112)晶面,并写出两晶面交线的晶向指数。
(3) 在立方晶系的一个晶胞中画出同时位于(101). (011)和(112)晶面上的[111]晶向。
解:1、2 .有一正交点阵的 a=b, c=a/2。

材料科学基础课后作业及答案(分章节)第一章8.计算下列晶体的离于键与共价键的相对比例(1)NaF (2)CaO (3)ZnS 解:1、查表得:XNa=,XF= 根据鲍林公式可得NaF中离子键比例为:[1?e共价键比例为:%=% 2、同理,CaO中离子键比例为:[1?e共价键比例为:%=% 12?(?)412?(?)4]?100%?% ]?100%? % 23、ZnS中离子键比例为:ZnS 中离子键含量?[1?e?1/4(?)]?100%?% 共价键比例为:%=% 10说明结构转变的热力学条件与动力学条件的意义.说明稳态结构与亚稳态结构之间的关系。
答:结构转变的热力学条件决定转变是否可行,是结构转变的推动力,是转变的必要条件;动力学条件决定转变速度的大小,反映转变过程中阻力的大小。
稳态结构与亚稳态结构之间的关系:两种状态都是物质存在的状态,材料得到的结构是稳态或亚稳态,取决于转交过程的推动力和阻力(即热力学条件和动力学条件),阻力小时得到稳态结构,阻力很大时则得到亚稳态结构。
稳态结构能量最低,热力学上最稳定,亚稳态结构能量高,热力学上不稳定,但向稳定结构转变速度慢,能保持相对稳定甚至长期存在。
但在一定条件下,亚稳态结构向稳态结构转变。
第二章1.回答下列问题:(1)在立方晶系的晶胞内画出具有下列密勒指数的晶面和晶向:(001)与[210],(111)与[112],(110)与[111],(132)与[123],(322)与[236](2)在立方晶系的一个晶胞中画出晶面族各包括多少晶面?写出它们的密勒指数。
[1101]4.写出六方晶系的{1012}晶面族中所有晶面的密勒指数,在六方晶胞中画出[1120]、晶向和(1012)晶面,并确定(1012)晶面与六方晶胞交线的晶向指数。
5.根据刚性球模型回答下列问题:(1)以点阵常数为单位,计算体心立方、面心立方和密排六方晶体中的原子半径及四面体和八面体的间隙半径。

第一章晶体几何基础1-1 解释概念:等同点:晶体结构中,在同一取向上几何环境和物质环境皆相同的点。
空间点阵:概括地表示晶体结构中等同点排列规律的几何图形。
结点:空间点阵中的点称为结点。
晶体:内部质点在三维空间呈周期性重复排列的固体。
对称:物体相同部分作有规律的重复。
对称型:晶体结构中所有点对称要素(对称面、对称中心、对称轴和旋转反伸轴)的集合为对称型,也称点群。
晶类:将对称型相同的晶体归为一类,称为晶类。
晶体定向:为了用数字表示晶体中点、线、面的相对位置,在晶体中引入一个坐标系统的过程。
空间群:是指一个晶体结构中所有对称要素的集合。
布拉菲格子:是指法国学者 A.布拉菲根据晶体结构的最高点群和平移群对称及空间格子的平行六面体原则,将所有晶体结构的空间点阵划分成14种类型的空间格子。
晶胞:能够反应晶体结构特征的最小单位。
晶胞参数:表示晶胞的形状和大小的6个参数(a、b、c、α 、β、γ ).1-2 晶体结构的两个基本特征是什么?哪种几何图形可表示晶体的基本特征?解答:⑴晶体结构的基本特征:①晶体是内部质点在三维空间作周期性重复排列的固体。
②晶体的内部质点呈对称分布,即晶体具有对称性。
⑵14种布拉菲格子的平行六面体单位格子可以表示晶体的基本特征。
1-3 晶体中有哪些对称要素,用国际符号表示。
解答:对称面—m,对称中心—1,n次对称轴—n,n次旋转反伸轴—n螺旋轴—ns ,滑移面—a、b、c、d1-5 一个四方晶系的晶面,其上的截距分别为3a、4a、6c,求该晶面的晶面指数。
解答:在X、Y、Z轴上的截距系数:3、4、6。
截距系数的倒数比为:1/3:1/4:1/6=4:3:2晶面指数为:(432)补充:晶体的基本性质是什么?与其内部结构有什么关系?解答:①自限性:晶体的多面体形态是其格子构造在外形上的反映。
②均一性和异向性:均一性是由于内部质点周期性重复排列,晶体中的任何一部分在结构上是相同的。
异向性是由于同一晶体中的不同方向上,质点排列一般是不同的,因而表现出不同的性质。

《材料科学基础》课后习题答案第一章材料结构的基本知识4. 简述一次键和二次键区别答:根据结合力的强弱可把结合键分成一次键和二次键两大类。
其中一次键的结合力较强,包括离子键、共价键和金属键。
一次键的三种结合方式都是依靠外壳层电子转移或共享以形成稳定的电子壳层,从而使原子间相互结合起来。
二次键的结合力较弱,包括范德瓦耳斯键和氢键。
二次键是一种在原子和分子之间,由诱导或永久电偶相互作用而产生的一种副键。
6. 为什么金属键结合的固体材料的密度比离子键或共价键固体为高?答:材料的密度与结合键类型有关。
一般金属键结合的固体材料的高密度有两个原因:(1)金属元素有较高的相对原子质量;(2)金属键的结合方式没有方向性,因此金属原子总是趋于密集排列。
相反,对于离子键或共价键结合的材料,原子排列不可能很致密。
共价键结合时,相邻原子的个数要受到共价键数目的限制;离子键结合时,则要满足正、负离子间电荷平衡的要求,它们的相邻原子数都不如金属多,因此离子键或共价键结合的材料密度较低。
9. 什么是单相组织?什么是两相组织?以它们为例说明显微组织的含义以及显微组织对性能的影响。
答:单相组织,顾名思义是具有单一相的组织。
即所有晶粒的化学组成相同,晶体结构也相同。
两相组织是指具有两相的组织。
单相组织特征的主要有晶粒尺寸及形状。
晶粒尺寸对材料性能有重要的影响,细化晶粒可以明显地提高材料的强度,改善材料的塑性和韧性。
单相组织中,根据各方向生长条件的不同,会生成等轴晶和柱状晶。
等轴晶的材料各方向上性能接近,而柱状晶则在各个方向上表现出性能的差异。
对于两相组织,如果两个相的晶粒尺度相当,两者均匀地交替分布,此时合金的力学性能取决于两个相或者两种相或两种组织组成物的相对量及各自的性能。
如果两个相的晶粒尺度相差甚远,其中尺寸较细的相以球状、点状、片状或针状等形态弥散地分布于另一相晶粒的基体内。
如果弥散相的硬度明显高于基体相,则将显著提高材料的强度,同时降低材料的塑韧性。

第四部分模拟试题石德珂《材料科学基础》(第2版)配套模拟试题及详解(一)一、选择题(每题3分,共15分)1.在非化学计量化合物ZrO2-x中存在的晶格缺陷是()。
A.阴离子空位B.阳离子空位C.阴离子填隙D.阳离子填隙【答案】A【解析】非化学计量化合物ZrO2-x中Zr为正四价,那么可见氧离子不足,于是产生氧离子空位,也就是阴离子空位。
2.在烧结过程中,只使坯体的强度逐渐增加,而坯体不发生收缩的传质方式是()。
A.晶格扩散B.流动传质C.蒸发-凝聚D.溶解-沉淀【答案】C【解析】晶格扩散指原子在晶体内部的扩散过程,其主要机制是空位扩散。
对流传质是指发生在相际之间的非流向传质,即当流体流经与其浓度不同的异相表面时,发生在两相之间的传质现象。
溶解-沉淀的实质是沉淀溶解平衡的移动。
蒸发-凝聚是制备高性能金属及合金超微粉末的有效方法,可用于烧结过程。
3.可以用同一个标准投影图的晶体有()。
A.立方和菱方晶体B.四方晶体和斜方晶体C.立方和四方晶体D.立方晶体【答案】D4.六方晶系的[100]晶向指数,若改用四坐标轴的密勒指数标定,可表示为()。
A.[1120]B.[2110]C.[2110]D.[1210]【答案】B5.硅酸盐晶体结构中的基本结构单元是()。
A.由硅和氧组成的硅氧多面体B.由硅和氧组成的6[SiO]-六面体6C.由硅和氧组成的4[SiO]-四面体4D.由二氧化硅组成的8[Si O]-八面体28【答案】C二、填空题(每题5分,共15分)1.固态烧结的主要传质方式有______、______,而液相烧结的主要传质方式有______和______。
这四种传质过程的△L/L与烧结时间的关系依次为______、______、______和______。
【答案】蒸发-凝聚传质;扩散传质;流动传质;溶解-沉淀传质;【解析】晶格扩散指原子在晶体内部的扩散过程,其主要机制是空位扩散。
对流传质是指发生在相际之间的非流向传质,即当流体流经与其浓度不同的异相表面时,发生在两相之间的传质现象。


第一章晶体几何基础1-1 解释概念:等同点:晶体结构中,在同一取向上几何环境和物质环境皆相同的点。
空间点阵:概括地表示晶体结构中等同点排列规律的几何图形。
结点:空间点阵中的点称为结点。
晶体:内部质点在三维空间呈周期性重复排列的固体。
对称:物体相同部分作有规律的重复。
对称型:晶体结构中所有点对称要素(对称面、对称中心、对称轴和旋转反伸轴)的集合为对称型,也称点群。
晶类:将对称型相同的晶体归为一类,称为晶类。
晶体定向:为了用数字表示晶体中点、线、面的相对位置,在晶体中引入一个坐标系统的过程。
空间群:是指一个晶体结构中所有对称要素的集合。
布拉菲格子:是指法国学者 A.布拉菲根据晶体结构的最高点群和平移群对称及空间格子的平行六面体原则,将所有晶体结构的空间点阵划分成14种类型的空间格子。
晶胞:能够反应晶体结构特征的最小单位。
晶胞参数:表示晶胞的形状和大小的6个参数(a、b、c、α 、β、γ ).1-2 晶体结构的两个基本特征是什么?哪种几何图形可表示晶体的基本特征?解答:⑴晶体结构的基本特征:①晶体是内部质点在三维空间作周期性重复排列的固体。
②晶体的内部质点呈对称分布,即晶体具有对称性。
⑵14种布拉菲格子的平行六面体单位格子可以表示晶体的基本特征。
1-3 晶体中有哪些对称要素,用国际符号表示。
解答:对称面—m,对称中心—1,n次对称轴—n,n次旋转反伸轴—n螺旋轴—ns ,滑移面—a、b、c、d1-5 一个四方晶系的晶面,其上的截距分别为3a、4a、6c,求该晶面的晶面指数。
解答:在X、Y、Z轴上的截距系数:3、4、6。
截距系数的倒数比为:1/3:1/4:1/6=4:3:2晶面指数为:(432)补充:晶体的基本性质是什么?与其内部结构有什么关系?解答:①自限性:晶体的多面体形态是其格子构造在外形上的反映。
②均一性和异向性:均一性是由于内部质点周期性重复排列,晶体中的任何一部分在结构上是相同的。
异向性是由于同一晶体中的不同方向上,质点排列一般是不同的,因而表现出不同的性质。



材料科学基础第二版答案材料科学基础是材料科学与工程专业的入门课程,它为学生提供了材料科学的基本概念、原理和知识体系。
本文档将为您提供材料科学基础第二版的答案,希望能够对您的学习和教学有所帮助。
第一章,材料科学基础概论。
1. 什么是材料科学?材料科学是研究材料的结构、性能、制备和应用的学科,它涉及金属、陶瓷、高分子材料等各种材料的研究和开发。
2. 材料的分类有哪些?材料可以分为金属材料、无机非金属材料和有机高分子材料三大类,每一类又可以进一步细分。
3. 材料的性能指标有哪些?材料的性能指标包括力学性能、物理性能、化学性能、热学性能等多个方面。
第二章,晶体结构。
1. 什么是晶体?晶体是由原子或分子按一定的规则排列而成的固体,具有规则的几何形状和周期性的结构。
2. 晶体结构的分类有哪些?晶体结构可以分为离子晶体、共价晶体、金属晶体和分子晶体四种类型,每一种类型都有其特定的结构特点和性质。
3. 晶体缺陷对材料性能有何影响?晶体缺陷会对材料的机械性能、热学性能、电学性能等产生影响,了解晶体缺陷对材料设计和制备具有重要意义。
第三章,材料的物理性能。
1. 材料的密度如何影响其性能?材料的密度直接影响其质量和体积,对材料的力学性能、热学性能等有重要影响。
2. 材料的热膨胀系数是什么?材料的热膨胀系数是材料在温度变化时长度变化的比例,对材料的热胀冷缩性能有重要影响。
3. 材料的导热性能和电导率有何关系?材料的导热性能和电导率都与材料内部的电子、原子结构密切相关,了解二者之间的关系对材料的应用具有指导意义。
第四章,材料的力学性能。
1. 材料的弹性模量是什么?材料的弹性模量是材料在受力时表现出的弹性变形能力,是衡量材料刚度的重要参数。
2. 材料的屈服强度和抗拉强度有何区别?材料的屈服强度是材料在受力时开始产生塑性变形的应力值,而抗拉强度是材料在拉伸断裂时所承受的最大应力值。
3. 材料的硬度测试方法有哪些?材料的硬度测试方法包括布氏硬度、洛氏硬度、维氏硬度等多种方法,每种方法都有其适用的范围和特点。
《材料科学基础》课后习题答案第一章材料结构的基本知识4. 简述一次键和二次键区别答:根据结合力的强弱可把结合键分成一次键和二次键两大类。
其中一次键的结合力较强,包括离子键、共价键和金属键。
一次键的三种结合方式都是依靠外壳层电子转移或共享以形成稳定的电子壳层,从而使原子间相互结合起来。
二次键的结合力较弱,包括范德瓦耳斯键和氢键。
二次键是一种在原子和分子之间,由诱导或永久电偶相互作用而产生的一种副键。
6. 为什么金属键结合的固体材料的密度比离子键或共价键固体为高?答:材料的密度与结合键类型有关。
一般金属键结合的固体材料的高密度有两个原因:(1)金属元素有较高的相对原子质量;(2)金属键的结合方式没有方向性,因此金属原子总是趋于密集排列。
相反,对于离子键或共价键结合的材料,原子排列不可能很致密。
共价键结合时,相邻原子的个数要受到共价键数目的限制;离子键结合时,则要满足正、负离子间电荷平衡的要求,它们的相邻原子数都不如金属多,因此离子键或共价键结合的材料密度较低。
9. 什么是单相组织?什么是两相组织?以它们为例说明显微组织的含义以及显微组织对性能的影响。
答:单相组织,顾名思义是具有单一相的组织。
即所有晶粒的化学组成相同,晶体结构也相同。
两相组织是指具有两相的组织。
单相组织特征的主要有晶粒尺寸及形状。
晶粒尺寸对材料性能有重要的影响,细化晶粒可以明显地提高材料的强度,改善材料的塑性和韧性。
单相组织中,根据各方向生长条件的不同,会生成等轴晶和柱状晶。
等轴晶的材料各方向上性能接近,而柱状晶则在各个方向上表现出性能的差异。
对于两相组织,如果两个相的晶粒尺度相当,两者均匀地交替分布,此时合金的力学性能取决于两个相或者两种相或两种组织组成物的相对量及各自的性能。
如果两个相的晶粒尺度相差甚远,其中尺寸较细的相以球状、点状、片状或针状等形态弥散地分布于另一相晶粒的基体内。
如果弥散相的硬度明显高于基体相,则将显著提高材料的强度,同时降低材料的塑韧性。
Solutions for Chapter 21. Find: Number of valence electrons in Group IIIB and GroupVB elements.Data: Periodic Table in Appendix A.Solution: By definition and/or by examination of Appendix B, Group IIIB elements contain 3 valence electronswhile Group VB elements contain 5 valence electrons.2. Find: The electron configuration of the element that comesnext in the series Si, Ge, ?Data: Periodic Table in Appendix A.Solution: Examination of the Periodic Table shows that Si and Ge are both Group IVB elements. As shown inExample Problem 2.2-1, have a valence electronconfiguration of the form xs2xp2 when x=3 for Si andx=4 for Ge. The next group IVB element in the seriesis Sn with an electron configuration (from the PeriodicTable) of [1s22s22p63s23p63d104s24p64d10]5s25p2. Note thatthe valence electron configuration for Sn is also ofthe form xs2xp2 with x=5.Comments: The similarity of their valence electronconfigurations suggests that Sn should displayproperties similar to those of Si and Ge. This istrue over a limited temperature range but thereare other factors (to be discussed in the nextchapter) that explain why Sn also has someproperties that differ from those of the other twoelements.3. Find: Should Ca and Zn exhibit similar properties?Data: Periodic Table in Appendix A.Solution: Examination of the Periodic Table shows that Ca has the electron configuration [1s22s22p63s23p64s2]while Zn has configuration [1s22s22p63s23p63d10]4s2.Since both elements have two valence electrons (4s2)we should expect these elements to display somesimilar properties.Comments: Although Zn and Ca have similar valence electron configurations, they have other structuraldifferences that result in differences inproperties.4. Find: Suggest some consequences of electron energies notbeing quantized.Solution: Although there are many possible answers to this question, one of the more important results mightbe a breakdown in the periodic arrangement of theelements. The Periodic Table owes its existenceto the quantization of energy. If quantization ofenergy did not exist, we would lose the ability tounderstand and predict properties based on valenceelectron configuration.5. FIND: How many electrons, protons, and neutrons are in Cu?DATA: From Appendix A, the atomic number of Cu is 29 and the atomic mass is 63.54 g/mole.SOLUTION: Cu has 29 electrons and 29 protons, each proton weighing about 1 g/mole. The balance of the atomic mass is from neutrons.COMMENTS: Elements can have different masses, from having different numbers of neutrons. They are called isotopes.6. FIND: What is the electronic structure of C?DATA: From Appendix A, the atomic number of C isSOLUTION: Carbon is capable of 4 covalent bonds of equal strength. Just think of some hydrocarbons that you know, such as methane, CH4. Four H bond to a central C. The bonding of Cmight be expected to be 1s22s22p2, again from Appendix A. What occurs in practice is called hybridization. The four electronsin the 2s and 2p levels hybridize, giving four electrons of equal bond strength capability. The bonds are as far apart as possible in space (tetrahedral bond angles).7. FIND: Describe the desirable environmental stability of a "gold standard".ASSUMPTIONS: You want the standard's critical properties to be invariant with timeSOLUTION: Gold is one of the few metals whose pure metallic state is more thermodynamically table than its oxide. Hence,gold does not oxidize. If it did, then it might gain or lose weight with time of exposure to air.COMMENTS: This is why gold is found in nature as nuggets, whereas, for example, iron and aluminum are found as oxides or sulfides.8. Find: Can pure water exist at -1o C?Solution: Yes, if the pressure of the system is raisedabove one atmosphere, water can exist at -1o C.Comments: Since most of our daily experiences occur at (or near) atmospheric pressure, we tend to forgetabout pressure as an important system variable.There are, however, many important engineeringprocesses that occur at either substantiallyhigher or lower pressures.9. Find: Change in flow rate when molasses is heated from 10o Cto 25o C.Given: Activation energy, Q, for Arrhenius Process is50 kJ/mole.Data: R= 8.314 J/mol-K, K=o C+273Assumptions: Flow rate at temperature T is given byF(T)= F o exp(-Q/RT)Solution: The ratio of the flow rates at any twotemperatures is:F(T1) = F o exp(-Q/RT1) = exp(-Q/R(1/T1-1/T2))F(T2) F o exp(-Q/RT2)F(25︒C) = exp(-50,000J/mol/8.134J/mol-K)(1/298K- 1/283K)) = 2.91 F(10o C)A temperature increase of 15o C results in almost afactor of three increase in the flow rate ofmolasses.10.Find: Change in polymerization rate when temperatureincreases by 10o C.Given: Activation energy, Q, for Arrhenius Process is80 kJ/mole.Data: R= 8.314 J/mole-K, K= o C+273Assumption: Polymerization rate at temperature T is given by P(T)= P o exp(-Q/RT)Solution: The ratio of the polymerization rates at any two temperatures is:P(T1) = P o exp(-Q/RT1) = exp(-Q/R(1/T1 - 1/T2))P(T2) P o exp(-Q/RT2)P(T1) = exp[-Q/R((T2-T1)/T1T2))] = exp[-Q/R(∆T/T1T2)]P(T2)This form of the expression shows that the problemcan not be solved with the information given. Aknowledge of ∆T is not sufficient. We must alsoknow the two temperatures.Comments: When the temperature increases from 10o C to20o C, the rate increases by a factor of 3.19. Incontrast, a temperature increase from 40o C to 50o Cresults in a rate increase of 2.59. This exampleillustrates the general result that a fixed changein temperature has a greater influence on thereaction rate if the average temperature is low. 11.FIND: Why are high quality electronic cable ends or contact points gold-coated?SOLUTION: You do not want the resistance of theconnection to increase with time. Oxides are generally good electrical insulators. Steel points rust and the oxide prevents them from working. Car points, for example, need to be changed frequently.COMMENTS: In some electronic devices a slight impedance increase due to oxide formation can cause a circuit to fail catastrophically, destroying a number of components.12.Find: Explanation for man's ability to convert aluminum oxide to aluminum.Given: Oxide represents a lower energy state than pure Al. Solution: Thermodynamics describes the direction ofspontaneous change. That is, balls roll down hilland Al will transform to Al2O3 if the kinetics arefavorable and no other factors are acting on thesystem. We know, however, that a ball can be moveduphill if energy is supplied to the system (i.e.if it is carried uphill). Furthermore, it mayremain at a higher elevation if there are activa-tion barriers associated with its return to thelowest energy position. Similar logic applies tothe reduction of Al2O3 to 2Al+1.5O2. If mansupplies (thermal) energy, the metal can beextracted from its ore and will remain in ametastable state. However, the metal will returnto its more stable oxide at a later time ifconditions permit.13.Find: Characteristics of primary bonds.Solution: Provided in the form of a table.+----------------------------------------------------------+|primary | types of | bonding | if sharing || bond | atoms | electrons | occurs is it|| type | usually | shared or | localized or|| | involved | transferred | delocalized|+----------+-----------------+--------------+--------------|| ionic | electroneg. and | transferred | --------- || | electropositive | | |+----------+-----------------+--------------+--------------|| covalent | electronegative | shared | localized || |(usually w N VE>3)+----------+-----------------+--------------+--------------|| metallic | electropositive | shared | delocalized|| |(usually w N VE 3)+----------------------------------------------------------+14.Find: Primary bond type in each of a series of compounds. Data: Electronegativities and numbers of valence electronsfor each element can be obtained from thePeriodic Table in Appendix A.Element | Electronegativity | No. of valence electrons--------+-------------------+-------------------------O | 3.44 | 6Na | 0.93 | 1F | 3.98 | 7In | 1.78 | 3P | 2.19 | 5Ge | 2.01 | 4Mg | 1.31 | 2Ca | 1.00 | 2Si | 1.90 | 4C | 2.55 | 4H | 2.20 | 1| |Assumptions: Percent ionic character of a bond is afunction of the difference in the electro-negativities of the elements involved (SeeAppendix A for conversion table). In a metal,the average number of valence electrons isgenerally ≤ 3. In a covalent solid the averagenumber of valence electrons is generally > 3.Solution:• O2, ∆EN=0 so that the bond is not ionic. Since O is an electronegative element and the average number ofvalence electrons is 6, we predict a covalentbond.• NaF, ∆EN = EN(F)-EN(Na) = 3.98-0.93 = 3.05. This ∆ENcorresponds to a bond that is approximately90% ionic.• InP, ∆EN = EN(P)-EN(In) = 2.19-1.78 = 0.41. Since thisbond is only about 4% ionic, we must examine the average number of electrons, N VE. Since N VE =(3+5)/2 = 4, we predict that the bond will becovalent.• Ge, ∆EN=0 so the bond is not ionic. Ge is neitherstrongly electropositive or electronegative, but it does have N VE=4. Thus we predict covalentbonding.• Mg, ∆EN=0 so the bond is not ionic. Mg is an electro-positive element with N VE =2. It will havemetallic bonds.• CaF2, ∆EN = EN(F)-EN(Ca) = 3.98-1.0 = 2.98. This ∆ENcorresponds to a bond that is appproximately 89% ionic.• SiC, ∆EN = EN(C)-EN(Si) = 2.55-1.90 = 0.65. Since thiscorresponds to a bond that is only ≈10% ionic, we must consider N VE. The average number ofvalence electrons is (4+4)/2 = 4 so the bond ispredicted to be covalent.• (CH2), ∆EN = 2.55-2.20 = 0.35 so the bond is not ionic.Although the average N VE in this compound is(4+1)/2 = 2.5, the bond is predicted to becovalent because H is a recognized exception tothe trend and is known to favor the formation ofcovalent bonds.• MgO, ∆EN = EN9O)-EN(Mg) = 3.44-1.31 = 2.13 whichcorresponds to a bond that is ≈68% ionic.• CaO, ∆EN = EN(O)-EN(Ca) = 3.44-1.0 = 2.44 whichcorresponds to a bond that is 77% ionic.Comments: All of these predictions are in agreement with experimental observations.15.FIND: Show the octet rule in C.DATA: Carbon forms four equal bonds.SKETCH: Shown is methane, simply as an example:HHHCHSOLUTION: The 8 dots represent the electrons: 4 come from the central carbon and 1 from each of the 4 hydrogen. The electrons are localized between the atoms sharing the electrons.16.Find: Characteristics of the Coulombic Force.Data: F a(x) is given in Equation 2.4-1 and sketched inFigure 2.4-2(a).Solution: The Coulombic Force varies inversely with thesquare of the separation distance between the charge centers. Another familiar example of aninverse square law is the force of gravity. Afunction of this form has no local maxima orminima because the derivative of equation 2.4-1has a nontrivial values for which it is equal tozero and the maximum value occurs as x approachesinfinity (for the sign convention adopted inFigure 2.4-2(a)).17.Find: Definitions of ionization potential and electronaffinity.Solution: Ionization potential is the energy required toremove an electron from an isolated neutralatom. The energy released when an isolatedneutral electronegative atom gains an electron isits electron affinity.Comments: The significance of these variables is that thedifference between the ionization potentialand the electron affinity is the amount of workthat must be done to create a pair of isolatedmonovalent ions. This quantity is important inthe determination of the total bond energy in anionic bond.18.Find: Reason why covalent bonds are restricted to electro-negative elements.Assumptions: During bonding, atoms seek to obtain a filledvalence electron shell.Solution: Electronegative elements generally have nearlyfilled valence shells and are seeking a fewadditional electrons. If electropositive atomsare nearby, they can transfer electrons to theelectronegative atoms and an ionic bond is formed.If, however, only electronegative atoms arepresent then the only way they can all acquireextra electrons is to share them. This is thedefinition of a covalent bond.Comments: Hydrogen also forms covalent bonds.19.Find: Is the Si-O bond covalent?Given: O forms covalent bonds with itself and Si alsoforms covalent bonds with other Si atoms.Data: From Appendix B: EN(Si)=1.90 and EN(O)=3.44.Assumptions: The percent ionic character of a bond isgiven by the table in Appendix A.Solution: It is impossible to form ionic bonds in any pureelement since there will be no difference inEN values for identical atoms. In the case ofSi-O, however, the difference inelectronegativity is 1.54. This corresponds to abond that is approximately 45% ionic and 55%covalent.Comments: This type of primary bond is often described asa mixed ionic/covalent bond.20.Find: Can an ionic solid be a good electrical conductor?Assumptions: High electrical conductivity requires a highdensity of mobile charge carriers.Solution: In ionic solids there are usually few, if any,free electrons. Charge transport requiresthe motion of comparatively large ions which isgenerally a more difficult process than electronmotion. If, however, an ionic solid had either ahigh free electron density or extremely mobileions, then it would be a good electricalconductor.Comments: We will find some examples of materials withthese characteristics in Chapter 10.21.Find: Physical significance of p<q in Equation 2.4-7.Data: Equation 2.4-7 states: F A+F R=0=A'/x p-B'/x q.Solution: This equation describes the competing forces ofattraction and repulsion in a covalent bond.Since the repulsive force is known to dominate atsmall separation distances (x→0), we requireB'/x q >> A'/x p as x→0. This is equivalent to requiring that x q << x p (for x→0) which issatisfied if and only if q>p.22.Find: Types of information available from the bond-energycurve.Solution: The depth of the bond-energy curve describes thestrength of the bond (i.e. the bond energy)and also provides information about thevaporization temperature since it is anindication of the amount of energy that must besupplied to move atoms to an infinite separationdistance. The value of x for which the bondenergy is a minimum corresponds to theequilibrium separation distance. It is, however,not possible to gain information about theprimary bond type from the general shape of thebond energy curve.23.FIND: Identify which material shown in Fig.2.5-1 has a higher melting temperature.GIVEN: The slope of material A at zero force is greater than that of B.SOLUTION: The ease of separating atoms or molecules with heat is similar to the ease of separating atoms with force. Hence, A has a higher melting temperature than does B.24.Find: Relationship between x th and melting temperature.Data: x th(Al)=25x10-6︒C-1 and x th(SiC)=4.3x10-6︒C-1.Assumptions: Melting temperature is related to the depthof the bond energy curve and thermalexpansion coefficient is related to the asymmetryof the curve.Solution: Since "deep" energy wells tend to be moresymmetric, materials with high meltingtempera-tures tend to have low expansioncoefficients. Thus, we expect SiC to have ahigher T m than Al.Comments: This prediction is consistent with experiment -T m(SiC) = 2700︒C and T m(Al) = 660︒C.25.Find: Relationship between stiffness and thermal expansion. Assumptions: Stiffness is related to the curvature (secondderivative) of the bond energy curve at itsminimum and expansion coefficient is related toasymmetry of the curve.Solution: Bond energy curves that are sharply curved havelarge second derivatives and high stiffness.However, such curves will also be relativelysymmetric so they will exhibit low coefficientsof thermal expansion. In order to obtain a stiffmaterial with a high expansion coefficient itwould require a tightly curved but asymmetricshape. This combination is difficult to achieve.26.Find: Explanation for the similarity of the moduli ofoxide ceramics.Solution: Elastic modulus is one of the properties thatcan be determined from the bond energy curve.If several materials have similar bondcharacter-istics then we should expect thosematerials to display similar modulus values.This is the case with many oxide ceramics andother ionic solids.27.Find: Estimate relative deflections of oxide and polymerglasses.Given: E(oxide)=10 E(polymer)Data: Deflection is inversely proportional to modulus(stiffness).Solution: The material with the higher modulus willdeflect less under the same load. Therefore, the plastic will display 10 times as muchdeflection as the oxide.28.Find: Sketch bond curves for a material with x th <0.Solution: Sketch|||↑ ||E |+-----------------------| x|||| |||↑ ||F |+-----------------------| x||||Comments: The bond energy curve is steeper to the right ofx︒ than to the left. This means that themidpoints of the constant energy line segmentsshift to the left as energy (temperature)increases.29.FIND: Calculate the atomic separation of pure Ti and Cu at 625︒C.GIVEN: The atomic separation at room temperature (25︒C) for Ti is 2.94 A and the thermal expansion coefficient is 9 x 10-6 / ︒C.DATA: The atomic separation of Cu is 1.28 A x 2 = 2.56 A according to Appendix C. The thermal expansioncoefficient of Cu is 17 x 10-6 / ︒C, according to Table 2.5-1.SOLUTION: The atomic spacing of Ti at 625︒C is:2.94 A + 600︒C x (9 x 10-6 / ︒C) = 2.94 A + 0.0054 A = 2.9454 A. Similarly, the atomic spacing of Cu at 625︒C is:2.56 A + 600︒C x (17 x 10-6 / ︒C) = 2.94 A + 0.0102 A = 2.57 A.COMMENTS: There is a problem with significant digits in thisproblem. The atomic spacing at 25︒C was provided only to 2 decimal places. Better accuracy is required in the atomic spacings if better accuracy in the calculated results are required.30.Find: Methods for measuring Young's modulus and coefficient of thermal expansion in the lab.Sketch:Solution: Young's Modulus relates the stiffness ordeflection of a material to the magnitude of the force or load causing that deflection. Themodulus of a material could be measured usingeither of the methods sketched above, that is, bymeasuring the deflection of a cantilever beam orthe change in length of an axially loaded rod.Coefficient of thermal expansion is measuredby observing the change in length of asample resulting from a temperature change(see Equation 2.5-4).31.Find: CNs for ions in CaF2Given: r(Ca)=0.197nm, r(Ca2+)=0.106nm, r(F)=0.06nm,r(F-) = 0.133nmData: r/R ranges for various CNs given in Table 2.6-1.Solution: Since this is an ionic compound user(Ca2+)/R(F-) = 0.106nm/0.133nm = 0.797From Table 2.6-1, this corresponds to CN(Ca2+)=8.Since the anion:cation ratio is 2:1, theCN(F-) is given by:CN(F-)= 1/2[CN(Ca2+)] = 4.Comments: This prediction is consistent with experiment. 32.Find: CNs of cations in NiO, ZnS, and CsI.Data: Radii in Appendix B→r(Ni2+)=0.078, r(O2-)=0.132,r(Zn2+)=0.083, r(S2-)=0.174, r(Cs+)=0.165, r(I-)=0.220Assumptions: Ionic bonding in all three compounds. Thiscould be checked by investigating ∆EN values.Solution: For NiO: r(Ni2+)/R(O2-)=0.105/0.132=0.795, so thatfrom Table 2.6-1 we find CN(Ni2+)=6. For ZnS: r(Zn2+)/R(S2-)=0.083/0.174=0.477, so that fromTable 2.6-1 we find CN(Zn2+)=6. For CsI:r(Cs+)/R(I-)=0.165/0.220=0.750, so that from Table2.6-1 we find CN(Cs+)=8.33.FIND: Sketch the structure of methane andO as in Fig. 2.6-5. SKETCH: Methane is a covalently-bonded material.SOLUTION:COMMENTS: In methane the C is in the center ofthe cube and the H are centered on the appropriate cube corners. The bond angles are 109.5︒ and the bond lengths are all the same. In the ketone the C is located within an equilateral triangle. The O double bond and the other two single bonds are planar, essentially 120︒ apart. 34. Find: Relationship between bond type and density. Solution: Density depends on the mass and radius of the atoms in the solid and on the efficiency with which the atoms are packed together. The later factor is a function of bond type through its influence on coordination numbers. Metals tend to have high CNs, typically 8 or 12. Ionic solids typically have CNs ranging from 3 to 8. Covalent solids typically have CNs ranging from 2 to 4. Therefore, if all other factors are roughly equal, covalent solids will display the lowest densities and metals will have the highest densities.Comments: For covalent solids CN= 8-N VE and N VE ≥ 4. This combination implies that CN(covalent) ≤ 4. 35. Find: When do you use r/R to predict CN in a covalent compound? Solution: Since in covalent compounds CN is determined by the 8-N VE rule, the r/R rule is never used to predict CN in covalent compounds. 36. Find: Find CN in pure Ge. Given: Ge is a Group IV covalently bonded compound. Solution: In a covalent compound CN=8-N VE . For Group IV Ge N VE =4. Therefore, CN(Ge)=8-4=4. Comment: This prediction is consistent with experiment.37.Find: Radius range for CN=4.Assumption: Assume ionic bondingSketches:Solution: The appropriate figures are sketched above andin Table 2.6-1. The minimum r/R ratio isfound using the sketch on the left. For thisgeometry:r+R=a√3/2 [anions touch cations along 1/2 a body diagonal]and R+R=a√2 [anions touch each other alongface diagonals]Dividing the first equation by the second gives:(r+R)/2R = 3/2√2orr/R = (√3/√2)-1 = 0.225The maximum r/R for CN=4 in the minimum valuefor CN=6. Using the geometry on the right:(r+R) = a/2and(R+R) = a√2/2Dividing the first equation by the second gives:r/R = (2/√2) -1 = 0.414Therefore, the radius ratio range for CN=4 is0.225 ≤ (r/R) < 0.414.38.Find: Characteristics of an ionic bond.Given: A2B compound with r(A)=0.12nm, r(B)=0.15nm,r(B+)=0.14nmSolution:A. Anions are generally larger than their neutralcounterparts because the added electronsincrease electron-electron mutual repulsion anddecrease the relative magnitude of the nuclearcharge. Therefore, r(A-) > r(A). Using theinverse argument we predict r(B+) < r(B).B. If the compound is ionic we must use an r/R ratioto predict the CN of the smaller ion (in this case the anion).r(A-)/R(B+)=0.13/0.14=0.929From Table 2.6-1, this implies CN(A-)=8. Sincethe anion:cation ratio is 2:1, thecoordination number for the cation is predictedto be CN(B+)= 2[CN(A-)]= 16. A CN of 16, howeveris not possible. Therefore, the most likelyvalues are CN(B+)=12 and CN(A-)=6. Recall thatlower CN values are always possible but aregenerally not energetically favorable.39.Find: Relative size of atoms if CN(A)=CN(B)=12.Solution: A coordination number of 12 for all atoms/ionsin a compound suggests that all of theatoms/ions are the same size. That is r/R=1.40.Find: Bond characteristics of Si and C.Solution: Both Si and C are Group IV elements and incovalent compounds they will each have CN=4.Thus we should expect some similarity between C-based (organic) structures and Si-basedstructures.Comments: These similarities will be investigated inChapter 6.41.Find: Structure of C2H6Assumptions: This is a covalent compoundSolution/Sketch:H H| || |H ----- C ------ C ------ H| || |H HComments: CN(C)=4 and CN(H)=1.42.Find: Explanation for no solids with CN = 5, 7 or 9.Solution: It is geometrically impossible to fill threedimensional space with the polyhedrons (solid geometrical figures) that result from CNs of 5, 7,or 9.Comment: To get a "feeling" for this statement you mighttry to "fill" two dimensional space with aseries of equal sized (regular) pentagons orheptagons.43.Find: Location of tacks in a room to maximize separationdistance.Sketch/Solution:The "tacks" or atoms should be placedin four of the eight corners of thecube (room) such that no two adjacentcorners are occupied. The separationis a√2.44.Find: Bond angle H-N-H in NH3Assumptions: Covalent bonding with CN(N)=3 and CN(H)=1Sketch:Solution: The bond angle is close to the tetrahedral angleof 109.5︒ (see Figure 2.6-4(a) and associated text).Comments: See Example Problem 2.6-4.45.Find: Type of bond between (NH4)+ and Cl-.Solution: The bonding in (NH4)+ essentially results infour exposed protons. These H+ ions can form hydrogen bridges between the electronegative N and Cl ions resulting in comparatively strong secondary bonds.46.Find: Predict which material has the higher T m.Assumptions: For compounds with secondary bonds, theimportant factors are the relative strengthof the bonds and the size of the molecules (sincelarger molecules have a greater surface area overwhich secondary bonds can occur).Solutions:A. H F | || | ----- C ------ C ------ | | | | H F will have a higher T m thanF F| || |----- C ------ C ------| || |F Fbecause the former is a permanent dipole while thelatter is too symmetric to have strongsecondary bonds.B. H | | H ----- C ------ H | | Hwill have a lower T V thanH | | H ----- N ------ H |⋅|⋅← (nonbonding electron pair)because the latter is permanent dipole while theformer is too symmetric to have strongsecondary bonds.C. Although their structures are very similar, C14H30will have a higher T m than C5H12 because it isa larger molecule with more surface for secondarybond formation.47.FIND: Suggest whether natural polymers - amides and cellulose - are moisture sensitive.GIVEN: Amides contain the group Handcellulose contains - OH's.SOLUTION: N contains a lone pair of electrons. Hence,the N tends to be partially negative and the H partially positive. O has two lone pairs of electrons, so O is partially negativeand the H is partially positive. Hence, both groups are dipolar. Water is also a dipole. Hence, water and NH's and OH's are attracted to one another. Cellulose and amides are moisture sensitive. Their properties - volume, strength, mass, etc. - depend on relative humidity.COMMENTS: This is in part why cotton (cellulose) and wool (a polyamide) are such comfortable fibers.48.FIND: Describe what binds the molecules in a。
绪论1、仔细观察一下白炽灯泡,会发现有多少种不同的材料?每种材料需要何种热学、电学性质?2、为什么金属具有良好的导电性和导热性?3、为什么陶瓷、聚合物通常是绝缘体?4、铝原子的质量是多少?若铝的密度为2.7g/cm3,计算1mm3中有多少原子?5、为了防止碰撞造成纽折,汽车的挡板可有装甲制造,但实际应用中为何不如此设计?说出至少三种理由。
6、描述不同材料常用的加工方法。
7、叙述金属材料的类型及其分类依据。
8、试将下列材料按金属、陶瓷、聚合物或复合材料进行分类:黄铜钢筋混凝土橡胶氯化钠铅-锡焊料沥青环氧树脂镁合金碳化硅混凝土石墨玻璃钢9、 Al2O3陶瓷既牢固又坚硬且耐磨,为什么不用Al2O3制造铁锤?晶体结构1、解释下列概念晶系、晶胞、晶胞参数、空间点阵、米勒指数(晶面指数)、离子晶体的晶格能、原子半径与离子半径、配位数、离子极化、同质多晶与类质同晶、正尖晶石与反正尖晶石、反萤石结构、铁电效应、压电效应.2、(1)一晶面在x、y、z轴上的截距分别为2a、3b、6c,求出该晶面的米勒指数;(2)一晶面在x、y、z轴上的截距分别为a/3、b/2、c,求出该晶面的米勒指数。
3、在立方晶系的晶胞中画出下列米勒指数的晶面和晶向:(001)与[210],(111)与[112],(110)与[111],(322)与[236],(257)与[111],(123)与[121],(102),(112),(213),[110],[111],[120],[321]4、写出面心立方格子的单位平行六面体上所有结点的坐标。
5、已知Mg2+半径为0.072nm,O2-半径为0.140nm,计算MgO晶体结构的堆积系数与密度。
6、计算体心立方、面心立方、密排六方晶胞中的原子数、配位数、堆积系数。
7、从理论计算公式计算NaC1与MgO的晶格能。
MgO的熔点为2800℃,NaC1为80l℃, 请说明这种差别的原因。
8、根据最密堆积原理,空间利用率越高,结构越稳定,金钢石结构的空间利用率很低(只有34.01%),为什么它也很稳定?9、证明等径圆球面心立方最密堆积的空隙率为25.9%;10、金属镁原子作六方密堆积,测得它的密度为1.74克/厘米3,求它的晶胞体积。
第一章8.计算下列晶体的离于键与共价键的相对比例(1)NaF(2)CaO(3)ZnS解:1、查表得:X Na =0、93,X F =3、98根据鲍林公式可得NaF 中离子键比例为:21(0.93 3.98)4[1]100%90.2%e---⨯= 共价键比例为:1-90、2%=9、8%2、同理,CaO 中离子键比例为:21(1.00 3.44)4[1]100%77.4%e---⨯=共价键比例为:1-77、4%=22、6% 3、ZnS 中离子键比例为:21/4(2.581.65)[1]100%19.44%ZnS e --=-⨯=中离子键含量共价键比例为:1-19、44%=80、56%10说明结构转变的热力学条件与动力学条件的意义.说明稳态结构与亚稳态结构之间的关系。
答:结构转变的热力学条件决定转变就是否可行,就是结构转变的推动力,就是转变的必要条件;动力学条件决定转变速度的大小,反映转变过程中阻力的大小。
稳态结构与亚稳态结构之间的关系:两种状态都就是物质存在的状态,材料得到的结构就是稳态或亚稳态,取决于转交过程的推动力与阻力(即热力学条件与动力学条件),阻力小时得到稳态结构,阻力很大时则得到亚稳态结构。
稳态结构能量最低,热力学上最稳定,亚稳态结构能量高,热力学上不稳定,但向稳定结构转变速度慢,能保持相对稳定甚至长期存在。
但在一定条件下,亚稳态结构向稳态结构转变。
第二章1.回答下列问题:(1)在立方晶系的晶胞内画出具有下列密勒指数的晶面与晶向:(001)与[210],(111)与[112],(110)与 [111],(132)与[123],(322)与[236](2)在立方晶系的一个晶胞中画出(111)与 (112)晶面,并写出两晶面交线的晶向指数。
(3)在立方晶系的一个晶胞中画出同时位于(101)、 (011)与(112)晶面上的[111]晶向。
解:1、2.有一正交点阵的 a=b, c=a/2。
某晶面在三个晶轴上的截距分别为 6个、2个与4个原子间距,求该晶面的密勒指数。
材料科学基础课后答案材料科学基础是一门探索材料结构与性能之间关系的学科,它为我们提供了一种更深入了解不同材料特性与应用的途径。
在本篇文章中,我将根据材料科学基础课后题目,对每个问题给出具体答案。
问题1:什么是材料科学基础?答案:材料科学基础是研究材料的物理和化学特性,以及这些特性与材料结构之间相互关系的学科。
它涉及材料的性能、制备、加工、表征和应用等方面的知识。
问题2:材料的结构对其性能有何影响?答案:材料的结构与性能之间存在着密切的关系。
材料的结构包括原子、晶格、晶界、位错等组成部分,而这些组成部分的排列和类型对材料的性能产生直接影响。
例如,晶体结构的不同可以导致材料的硬度、强度、导电性和热导率等性能的差异。
问题3:什么是晶体结构?答案:晶体结构是指材料中原子、分子或离子排列形成的有序结构。
晶体结构可以用晶格参数和原子坐标来描述。
晶体结构的类型包括立方晶系、正交晶系、单斜晶系、斜方晶系、菱方晶系和三斜晶系。
问题4:什么是非晶体?答案:非晶体是指材料中原子或分子呈无序排列的结构。
与晶体不同,非晶体中没有规则的晶体结构。
非晶体具有无定形、随机性等特点。
非晶态材料常见的有非晶合金、非晶聚合物和非晶硅等。
问题5:材料的热处理对其性能有何影响?答案:材料的热处理是通过控制材料的加热和冷却过程,改变材料的结构和性能。
热处理可以提高材料的强度、硬度、韧性和耐腐蚀性等性能,同时也可以改善材料的加工性能和电磁性能等。
问题6:材料的表征方法有哪些?答案:材料的表征方法用于研究和分析材料的结构和性能。
常见的材料表征方法包括显微镜、X射线衍射、扫描电子显微镜、电子探针微区分析、拉伸实验和硬度测试等。
问题7:什么是位错?答案:位错是材料中存在的晶格缺陷。
位错由晶格中原子的错位或者间隙引起。
位错对材料的性能有重要影响,例如可以显著影响材料的塑性变形和织构等特性。
问题8:为什么要进行材料的成分分析?答案:进行材料的成分分析可以确定材料的组成、控制材料的成分含量,以及评估材料的质量。