化工原理课程(全英文)教学课件 9
- 格式:pdf
- 大小:2.72 MB
- 文档页数:56

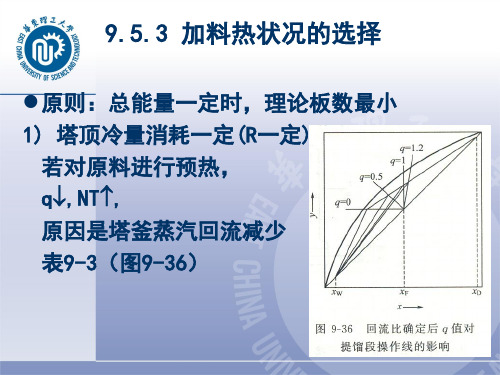
Distillation-for separation of components in a liquid mixture9.1 IntroductionWhen a liquid mixture is partially vaporized, the compositions in the liquid (x) and the vapor (y) are different.If component A is more volatileBAB A x x y y >(9-1) 1. Distillation:--- a method of separation based on the volatility difference between components in a liquid mixture.✧ More volatile fraction ✧ Less volatile2. Flash or equilibrium distillation--- a continuous process at steady stateThe superheated liquid vaporizes partially, in which the heat of evaporation is from the sensible heat of the liquid.3. Simple distillation---a batch process, unsteady state9.2 Vapor Liquid Equilibrium of Two-component Systems9.2.1 Ideal systems✧ The liquid is an ideal solution, which obeys Raoult ’s law;✧ The vapor is ideal gas, which obeys pV=nRT or Dalton ’s law of partial pressure. The intermolecular interactions between various components are the same.1. According to Raoult ’s law, the equilibrium vapor pressure of a solution isx p x p p oA A o A A ==)1(x p x p p oA B o B B -== (9-2)Where p 0 is the vapor pressure of pure liquid.With Dalton ’s law, the relationship between p A and the total pressure p isp A =py A p B =py B (9-3)The liquid mixture is boiling whenB A p p p += (9-4)From above equations, the equilibrium composition of an ideal vapor-liquid system isA oB o A o B P P p p x --=(9-5)px p y o A A = (9-6)2. The relationship between p 0 and boiling point t is from✧ Phase equilibrium data✧ Empirical formulas, such as the Antoine equationct BA p o +-=log (9-7) Calculations for equilibrium composition and boiling point t : ✧ Given p 0=f (t )→ p A 0, p B 0 → x A , y A ;✧ Given x A , y A→ boiling point t (by trial and error method)3. Phase equilibrium4. Volatility and relative volatilityThe volatilities of components in a solution isB B B A A A x p v x p v ==,The relative volatility is defined as B B A A B A x p x p v v == αFor ideal gas, p i = py iBA BA x x y y =αFor multi-component systems, A and B are two of the key components. If α = 1, for two-component systems,A A A A x xy y -=-11 i.e., y A = x ASeparation by conventional distillation is not possible.Phase equilibrium equation (ideal gas)B A B A x x y y = αFor binary systems, () 11AAA x x y -+=αα (9-14)For ideal solution,o Bo Ap p =αα depends on the properties of pure component, and varies with temperature.Relative volatility of mixtures of benzene and toluene:α rises as t fall so that it is sometimes worthwhile to reduce the boiling point by operating at a reduced pressure.When Eq. (9-14) is used, an average value of α must be taken over the whole column.9.2.2 Non-ideal systems--- resulting from different intermolecular interactions between compoents. In equilibrium, the fugacity of components in two phase are identical,py i φi = f i L x i γiφi ----- fugacity coefficient in gas phase γi ------ activity coefficient in liquid phase f i L ----- fugacity of pure liquid9.3 Flash Distillation and Simple Distillation9.3.1 Flash or equilibrium distillationIt is a continuous process at steady state.F--- flowrate of feed D--- flowrate of distillateW---- flowrate of residue or bottomsUnit: kmol/per unit time1. Material balanceThe total material balance isF=D+Wand the material balance on the more volatile component isFx F = Dy + WxElimination of W givesF x DF x D F y +-=)1( ➢ q = W/F---- ratio of liquefaction , 0< q <1;➢ D/F = 1-q---- ratio of vaporization ;→ W = qF , D = (1-qF )The equation may be written as11---=q x q qx y FThis is an operating relation,✧ reflecting the relationship of composition in two phases ✧ determined by material balance2. Heat balanceWhen the pressure is reduced, the sensible heat released from the liquid is equal to the latent heat required by vaporizing part of liquid,()()Fr q t T Fc e p m -=-1,where t e is the equilibrium temperature , c m, p and r are the average values of heat capacity and latent heat of liquid mixture, respectively. The feed is heated to()pm e c r q t T ,1-+=and the heat load is()F p m t T Fc Q -=,3. Phase equilibrium relationship Phase equilibrium equation()x f y =The equilibrium temperature t e and composition x satisfy the boiling point equation ()x t e φ=Both of them reflect the feature of process.4. Calculation: (graphic or analytical method) given F, x F , t F , and (1-q), find y, x, and t e .Phase equilibrium: ()x f y = Operating Eq.: 11---=q xq qx y F Boiling point: ()x t e φ=9.3.2 Simple distillation------ batchwise, unsteady state. At time τ:amount of liquid Wcomposition x in the still In time d τ:vaporized amount dWcomposition in the vapor phase y The material balance is()() ydW dx x dW W W x +-⋅-=Neglecting dWdx ,→ x y dxW dW -=The integration isxy dxW W x x -=⎰1221ln The equilibrium relation is presented by a curve or table, integration by graphical or numerical method.9.4 Rectification9.4.1 Fractionating process1. PrinciplePartial vaporization and partial condensationIn a continuous fractionating column, successive vaporization and condensation are accomplished with reflux .Distillation column: ➢ Rectification section--- above the feed point ➢ Stripping section--- below the feed point2. Material balance for the column➢ Flowrates: F, D, W and L (kmol/per unit time) ➢ Compositions: x F , x D , and x W➢ Total material balance:W D F +=➢ Material balance on the more volatile component: W D F W x Dx Fx +=→ WD WF x -x x x F D -=WD FD1x x x x F D F W --=-=3. Reflux ratio and energy consumptionThe reflux ratio isR=L/D ➢ R ↑, degree of separation ↑;➢ But V ↑, the heat load in the reboiler Q ↑, energy consumption ↑ ➢ The reflux ratio should be appropriate.9.4.2 Mathematical description of distillation process1. Equipment:➢ stagewise contacting plate column ➢ differential contacting packed column2. Basic equations:➢ material balance ➢ heat balance➢ for the features of process3. Method:Differential contacting equipment:➢ obtain the basic equations for a differential length➢ integrate the equation for H or calculate it by length-to-length methodStagewise equipment:✧ Obtain the basic equations for a plate (stage) ✧ Solve the problemby stage-to-stage method or by solving a set of equations9.4.3 Mathematical description for the process on a plate1. Theoretical plateTheoretical plate✧ The vapor and liquid phases are intimately mixed; ✧ Heat and mass transfer takes place rapidly; ✧ Two phases are in equilibrium;✧ y n and x n are in equilibrium✧Two phases have the same temperature no mater what y n+1(t n+1) and x n-1(t n-1) are.2. Plate efficiencyThe plate efficiency expressed in vapor terms is given by1*1mv ++--=n n n n y y y y E *n y is the vapor composition in equilibrium with x n , and y n is actual composition in equilibriumwhen the vapor passes through the plate.E mv is referred to as the Murphree plate efficiency.A problem of distillation may be considered from two aspects: ✧ Vapor-liquid phase equilibrium y n ~ x n ; ✧ Operating relation y n+1 ~ x n ;3. Assumption of constant molar flow✧ The molar flowrate of vapor V from each plate is the same in rectification or stripping section,but V in the two sections may be unequal.✧ The molar flowrate of liquid L from each plate is the same in rectification or stripping section,but L in the two sections may be unequal.The condition for constant molar flow:➢ The molar latent heat of components is the same;➢ The sensible heat transferred between vapor and liquid with different temperatureis negligible;➢ The heat loss in the equipment may be neglected.It is an equimolecular counter diffusion between phases.4. Material balance across a plateThe material balance for the more volatile component is V y n+1 + L x n-1 = V y + L x n where()n n x f y =For a theoretical plate with constant molar flow, the heat balance is not needed.5. Analysis for feed plate(1) Theoretical feed plate✧ y n and x n are in equilibrium✧ two phases have the same temperature no mater what y n+1(t n+1), x n-1(t n-1) and x F (t F ) are.(2) The nature of the feed✧ Cold feed as liquor (supercooled liquid) ✧ Feed at boiling point (saturated liquid) ✧ Feed partly vapor (vapor-liquid mixture) ✧ Saturated vapor ✧ Superheated vaporThe flowrates of two phases in two sections depend on the thermal condition of the feed.(3) Flowrates in distillation and stripping sectioni – enthalpy of saturated liquid I – enthalpy of saturated vapor✧ Material balance:L V L V F +=++✧ Heat balance:L V L V F i L Vi Li i V Fi +=++→i I i I F L L F--=- Let iI i I F L L q --=-'=F=heat to vaporize 1 mol of feed/molar latent heat of the feed q is the parameter of thermal condition of the feed.The relationship of flowrates of liquid in rectification and stripping section is qF L L += For the flowrate of vapor,F q V V )1(--=Comparison: in the flash distillation✧ q : ratio of liquefaction ✧ (1-q ): ratio of vaporization.✧ (a) cold feed as liquor q >1 ✧ (b) feed at boiling point q =1 ✧ (c) vapor-liquid mixture 0<q <1 ✧ (d) saturated vapor q =0 ✧ (e) superheated vapor q <0qF L L += F q V V )1(--=9.4.4 Molar flowrate in distillation columnCondition:✧ Total condenser at the top of column ✧ Reflux at boiling point ✧ Reflux ratio RRectification section:RD L =D R D L V )1(+=+=Srtripping section:qF L L +='F q V V )1(--='The thermal load:✧ Total condenser Q c = Vr c ✧ Reboiler b b Vr Q =where r is the mean latent heat of liquid mixture.In the reboiler and partial condenser, the vapor and liquid are in equilibrium, so that they are considered as a theoretical plate.9.4.5 Equation of operating line✧ Vapor-liquid phase equilibrium y n ~ x n ✧ Operating relation y n+1 ~ x n1. Operating line in rectification section From the plate n to the condenser: ✧ Total material balance V = L + D✧ Material balance on the light component Vy n+1 = Lx n + Dx DEquation of operating line in rectification section:11D D 1+++=+=+R x x R R x V D x V L y n n n2. Operating line in stripping sectionFrom plate n (below the feed point) to the reboiler:✧ Total material balance:W V L +=✧ Material balance on the light component:w n n W x y V x L +=+1Equation of operating line in stripping section:W n n x VW x V L y -=+1 The material balance on the light component for the column gives:F D W Fx Dx W x -=- → VFx Dx x V L y FDn n -+=+13. Rectification StrippingD 1x V D x V L y n n +=+ W n n x VW x V L y -=+1 RD L = qF RD L +=)1(+=R V F q D R V )1()1(--+=W D F W x Dx Fx +=9.4.6 Calculation for distillation process⏹ Solve the problem by stage-to-stage method✧ For design----- determine the number of plates N✧ For operation (trial and error method) ⏹ Solve the set of equations simultaneously✧ For operation (N is given)----- (2N equations for 2N unknowns)9.5 Calculation in Design of Distillation Process for Binary SystemsAccording to the specification for separation,✧ choose the operating condition , and ✧ calculate the number of theoretical plates N T9.5.1 Calculation for the number of theoretical plates✧ Given F , x F✧ Specify the degree of separation such as x D and x W , or the recoveryF D Fx Dx =η✧ Choose operating pressure,thermal condition of feed (→ q ), and reflux ratio R .1. Graphical method (1) Operating lines Rectification section:11D 1+++=+R x x R Ry n n The operating line passes through point a(x D , x D ) and point b(0, 1+R x D)Stripping section:The operating line passes through point c(x W , x W ) and the point of intersection of the operating lines d(?, ?)The coordinates of the point of intersection d satisfy the two equations of operating lines,qR qx Rx y DFq ++= qR x q x R x DF q +-++=)1()1(Eliminating x D , we have:Equation of q-line or feed line11---=q x x q q y F q qwhich passes through point f(x F , x F ), with the slope of q /(q-1) and the intercept1-q x F.Drawing of operating lines:✓ Operating line in rectification section:------ pass through points a, b;✓ q-line:------- pass through f, with slope q/(q-1); ✓ operating line in stripping section ------- pass through points c, d.(2) Graphical method for N T With a total condenser,y 1=x D (point a)✓ Equilibrium relation and operating relation are applied in turn. ✓ N T is the number of steps.✓ The feed point is at the plate passing point d.(3) Location of feed pointThe appropriate position of feed point is to use less N T .2. Method of stage-to-stageIt is the same as the graphical method.Starting from x D , apply the equilibrium relation and operating relation in turn for the compositions in vapor and liquid phases, until x n ≤ x W .The result is more accurate compared with the graphic method, but the equation for equilibrium relationship is needed.9.5.2 Selection of reflux ratioRectification section: R ↑, 1+=R R V L ↑, the slope of operating line ↑. Stripping section:qF RD DF LV +--=1 ↑, the slope of operating line ↓. R ↑:N T ↓, but the proportion of product ↓, cost of operation ↑.The reflux ratio R=L/D varies in the range of R min ~ ∞ (total reflux). ✓ R<R minIt is impossible to obtain the desired enrichment, however many plates are used. ✓ total refuxThe minimum number of plates is required for a given separation.1. Total reflux and the minimum N TWhen D=0, F=W=0 in general.Dn n x R x R R y 1111+++=+As R →∞, slope = 1, intercept =0, so that the operating lines coincide with the line y=x .∴ total reflux ~ N min .The number of plates at total reflux: ----- Fenske ’s methodFor an ideal system, we may use the method of stage-to-stage. According to the definition,B A B A x x yy = αFor plate n, the equilibrium relationship is n B A nB A y y x x ⎪⎪⎭⎫⎝⎛=⎪⎪⎭⎫ ⎝⎛11α (A)Operating line for total reflux:y n+1 = x n (B)Equations (A) and (B) are applied in turn to obtain the compositions of vapor and liquid on each plate.When a total condenser is used, y 1=x Di.e., DB A B A x xy y ⎪⎪⎭⎫⎝⎛=⎪⎪⎭⎫⎝⎛1 from the equilibrium relationship (A),DB A B A B A x x y y x x ⎪⎪⎭⎫⎝⎛=⎪⎪⎭⎫ ⎝⎛=⎪⎪⎭⎫ ⎝⎛αα11111On the N (reboiler), the composition of liquid is DB A NN B A x x x x ⎪⎪⎭⎫ ⎝⎛=⎪⎪⎭⎫ ⎝⎛ααα 211Define N N αααα 21=→ DB A N NB A x x x x ⎪⎪⎭⎫⎝⎛=⎪⎪⎭⎫ ⎝⎛α1 When the change in α is not large, we may use N ααα1=The Fenske equation: αlog ]log[min WBBD B A x x x x N ⎪⎪⎭⎫ ⎝⎛⎪⎪⎭⎫ ⎝⎛=, which is applicable to multi-componentdistillation.For a binary system, αlog )]1)(1log[(min WW D D x x x x N --=.2. Minimum reflux ratio R minAs R →R min , the intersection point of operating lines d lies at the equilibrium curve, so that no enrichment occurs between the feed plate and the adjacent plate. The compositions are said to be “pinched ”.R min depends on the thermal condition of feed.Calculation of R min(1) Graphical methodFor ideal mixtures, find the intersection point of operating lines at the equilibrium curve d (x q , x q )qD q D x x y x R R --=+1min mini.e., qq q D x y y x R --=minnon-ideal mixtures:(2) Analytical methodFor ideal systems, the intersection point d lies at the equilibrium curve, qqq x x y )1(1-+=αα→ ⎥⎥⎦⎤⎢⎢⎣⎡----=q D q D x x x x R 1)1(11min ααx q depends on the thermal condition of feed.3. Selection of economic reflux ratioR ↑:✓ The operating costs rise;✓ The capital cost initially falls since N fall rapidly;✓ The capital cost rises at high R, since the diameter of column,size of reboiler and the condenser increase for greater V . The economic reflux ratio is R opt = (1.2~2)R min4. short-cut method for calculating the number of platesFor estimation of N T(1) calculate R min and N T ;(2) select a value of R for operation;(3) find N T using empirical relations (correlation or figure)9.5.3 Selection of the nature of the feedThe slope of the q-line is governed by the nature of feed, which affects the operating line in stripping line in stripping section.11---=q x x q q y F q qFeed q slope q /(q-1)Feed cold liquid >1 +Feed saturated liquid 1 ∞ feed at bubble point Feed partially vaporized 0<q<1 -Feed saturated vapor 0 0 feed at dew point Feed superheated vapor <0 +q ↓, N T ↑, reboiler Q ↓.Example 1The feeding at dew point is fed to a continuous fractionating column ,and the equilibrium relations are given by equations: y=0.723x + 0.263 ( in rectifying column); y=1.25x – 0.0187 (in stripping column) .Calculate(a) the compositions of feeding, overhead product and bottom product , respectively(b) the reflux ratio Solution: The slope of the operating line in rectifying column is0.7231RR =+We can get :R=2.61The intercept of the rectifying line on y axis is 0.26310.263(2.611)0.95DD X R X =+∴=+= The intersection of the stripping line and the diagonal is Y=X=Xwso Xw=0.0748Form the intersection of these two operating lines, gives:0.7230.263 1.250.01870.5350.7230.53350.2630.65X X X Y +=-==⨯+= Because the feed at dew point ,so the feed line is horizon ,and the composition of feed is0.65F X Y ==Example 2The equilibrium data for the alcohol —water are given in example 1-7. A continuous fractionating column is to used to separate a feeding contains 15 mole percent alcohol and 85 mole percent water ,the feeding is saturated liquid. Overhead product containing 80 mole percent at least and a bottom product containing 2 mole percent alcohol .required. .A reflux ratio is 2. If get the product which is saturated liquid on the certain board place of rectifying column,then the mole flow equals to half that of overhead product ,calculate (a) how many ideal plates are needed(b) the positions of the feeding plate and the lateral line outlet .Solution: Because rectification have lateral line outlet, so the rectification divided into the upper and the low section, The products operation line equation of outlet the above of lateral line is11111D n n R XY X R R +=+++ ()The operating line equation in the low section should be calculated and published by the overall balance. Do the overall balance according to a dotted line range offigure of subject under discussion, have121112222)3s S D D V L D D V Y L X D X D X L L D +''''=++''''++''=- (= () (4)Uniting vertical 2, the formula 3 and formula 4 have:2112211112112211///511D D S S D D S S L D D X D X Y X L D L D R L D R D D X X D D Y X R R ++-+=+++=-+⋅∴=+++ ()U nite it is vertical 1 and 5s,solve two line point of intersect coordinates isx=x 2D ,as the intersect of two operating lines of subject under discussion g shows. The operating line of stripping column do not changeCalculate the number of the ideal plates with graphic method, and the steps are omittedThe intercept of operating line in the upper section of rectification: 10.80.267121D X R ==++The intercept of operating line in the low section of rectification:122110.80.6/20.367121D D X X D D R +⨯+⋅==++ 12 ideal plates are needed (besides reboiler ),the lateral line outlet at the fifthideal plate from the top of column ,and the feeding is introduced on the tenth plate from the top of column.Example 3It is desired to produce an overhead product containing 95.7 mole percent A form a ideal mixture of 44 mole A and 50 mole percent B feeding into a continuous fractionating column .The average relative volatility equals to 2.5 ,and a minimum reflux ratio is 1.63.Explain the heat state of feeding and calculate the value of q .Solution: Form equilibrium equation: 2.511(1)1 1.5X XY X Xαα==+-+ ()Form operating equation :1.630.95711 1.631 1.6310.620.364D R X Y X X R R X =+=+++++=+ Substituting in eq.1), gives: X=0.365 Y=0.59Form defines of the minimum reflux ratio ,the intersection of the two upper equations is also that of the equilibrium curve and the feed line.,F F X X Y X <> so the feed is a mixture of liquid and vapor.Form the feed equation (feed line), gives:(1)0.44(1)0.590.365F X q Y qXq q =-+=-⨯+⨯Solve and have:20.6673q ==9.5.4 Other distillation process for binary systems1. Heated by steam directlyThe liquid mixture to be separated is an aqueous solution, in which water is the less volatile component. Steam is passed directly into the liquid in the still.2. Multiple feedsThe feeds with different composition are added into the column at appropriate feed points.For i feed points, there are i +1 operating lines.3. Distillation with side-stream drawoffSide-stream drawoff is defined as any product stream other than the overhead product and residue.Side-stream drawoffs are most often removed with multi-component systems, but they can be used with binary mixtures.4. Recovery columnIt is used to recover the light component in dilute solutions. It has stripping section only.9.5.5 Calculation of N T for linear equilibrium relationIn very dilute binary solutions, the equilibrium relation is nearly a straight line.By the stage-to-stage method, Eq. (9-90) is obtained for the number of plates.9.6 Calculation for operation of distillation column for binarysystems9.6.1 CalculationsGiven: equipment and operating condition e.g., N T, feed plate m, equilibrium relationship, x F, D/F, q, RDetermine: x D, x WTrial and error method(1)assume a value of x W (or x D)(2)calculate x D (or x W) by the material balance(3)starting from x D, calculate x W by graphical or stage-to-stage method;(4)compare the result with that in (1) or (2)The location of feed point may not be the optimum position.✓Example: continuous distillation for benzene-toluene systemThe reflux ratio R=5 is changed to R=8 with other condition unchanged.Determine the composition of overhead and bottom products.9.6.2 The temperature profile in a distillation columnThe shape of the temperature profile depends on✓the liquid composition on the plates, and✓pressure difference along the column.The plate on which the temperature changes most rapidly is very sensitive to the disturbances.9.7 Batch distillationThere are many cases where batch distillation is preferred,particularly in the food and pharmaceutical industries. Batchdistillation is used when small amounts of product are made in a pilotplant to provide samples for product sampling or testing.9.7.1 The features(1) Unsteady state✓The composition and temperature in the still change with time.✓The hold-up of liquid affects the process.(2) With rectification only;Calculations:(1)select a basic state and calculate N T as in the calculation for design a distillation column;(2)for the number of plates N T, determine the parameters as in the calculation for operation ofdistillation.Simplification:The effect of hold-up is neglected.Mode of operation:⏹Operation at constant product composition →R increases continuously.⏹Operation at constant reflux ration→ concentration of the light component in theproduct decreases continuously.Advantages: The advantages of batch distillation are that several products can be made form a single unit, and can effectively handle sludges and solids.Disadvantages: For a given product rate, the equipment is larger. It requires more operator attention, uses more energy, and because it is a dynamic process, is harder to control and model.9.8 Special distillationWhen relative volatility near or equal to unity, the separation of components is difficult by conventional distillation, or impossible because of azeotrope formation.Basic principles of azeotropic and extractive distillation: Adding a third component (solvent) to increase the relative volatility of the original components, so that the mixtures with relative volatility near or equal to unity can be separated by conventional distillation.For two types of mixtures, those with small volatility difference or azeotropes, it may be possible to increase the volatility difference by the addition of an extraneous material, which alters the original intermolecular interactions and can be separated later.9.8.1 Azeotropic distillationThe added material [called entrainers(夹带剂)(third component)] forms an azeotrope with one or more of the components of the mixture and in so doing enhances the separability of the original mixture.In this mode, the extraneous material, or azeotropic agent, may leave the column in the distillate (low-boiling azeotrope) or in the residue (high-boiling azeotrope).1. Heterogenous azeotropic distillation for binary systems✓The condensed product is composed of two immiscible liquids.✓The compositions of the two phases deviate fro that of azeotrope so that the extraneous material is not needed.✓Two distillation columns are used for the separation.2. Three-component azeotropic distillationThe azeotropic agent (or entrainer) C is added into the original mixture A+B to form an azetrope AC or ABC, the boiling point of which is significantly different from that of A or B.The azeorope is generally a low-boiling azeotrope, leaving the column in the distillate.Ethanol-water:The azeotrope has a composition of 89 mol per cent of ethanol. 三元恒沸物:苯:0.539 乙醇:0.228 水:0.233 沸点:64.85℃上层苯相苯:0.745 乙醇:0.217少量水下层水相苯:0.0428 乙醇:0.35其余为水Benzene is added to form a ternary low-boiling azetrope, which leaves the column in the distillate and forms two immiscible liquid phases after condensation.The bottom product is the ethanol with high purity.The requirement for the entrainer:(1)formation a low boiling azeotrope with one or more of the componentsIt is better for the component(s) to be in lower concentration, so that the energy required for evaporation is less.(2)easy separation of the azetrope(3)less entrainer in azeotrope for economic operation9.8.2 Extractive distillation✓The third component, termed a solvent or extractive agent, is added, which alters the relative volatility of original constituents.✓The added solvent is of low volatility and leaves the column with the residue.Isooctane-toluene system:The boiling point of isooctane and toluene are 99.3℃and 110.8℃, respectively. Phenol is used as the extractive agent.The requirement for extractive agent:(1)addition of a small amount of solvent may increase the relative volatility of originalcomponents significantly;(2)the solvent is of low volatility, and will not form an azeotrope with the originalconstituents;(3)it can be intimately mixed with the original mixture.Comparison of azeotropic and extractive distillation:In common:Addition of an extraneous material to increase the volatility difference of the components to be separated.In economy:✓Azetropic distillationThe third component leaves in the distillate, so that the energy consumption is higher.✓Extractive distillationThe third component leaves in the residue, so that it is more economic.In operation:✓Azeotropic distillation: applicable to batch and continuous process✓Extractive distillation: for continuous operation。