Synthesis of Water Soluble Gold Nanoparticles with Control_over_Size_and_Surface_Functionalities
- 格式:pdf
- 大小:1.14 MB
- 文档页数:10


[Communication ]物理化学学报(Wuli Huaxue Xuebao )Acta Phys.-Chim.Sin.2017,33(3),458-463Marchdoi:10.3866/PKU.WHXB201701041Received:November 25,2016;Revised:January 3,2017;Published online:January 4,2017.*Corresponding author s.CHEN Xin,Email:xinchen73@;Tel:+86-21-64253582.JIN Chuan-Hong,Email:chhjin@;Tel:+86-571-87953700.The project was supported by the Shanghai Leading Academic Discipline Project,China (B502),Shanghai Key Laboratory Project,China (08DZ2230500),Shanghai Committee of Science and Technology Support Project,China (11nm0507000),State Key Laboratory of Functional Materials for Informatics Open Project,China (SKL201306)and National Natural Science Foundation of China (51222202).上海市重点学科项目(B502),上海市重点实验室项目(08DZ2230500),上海市科学技术委员会项目(11nm0507000),信息功能材料国家重点实验室开放课题(SKL201306)及国家自然科学基金(51222202)资助©Editorial office of Acta Physico -Chimica Sinica用原位液体池透射电镜技术表征金属钯在球形金纳米颗粒表面的异质沉积周晓琴1,3张辉3张泽3陈新1,2,*金传洪3,*(1华东理工大学材料科学与工程学院,超细材料制备与应用教育部重点实验室,上海市先进聚合物材料重点实验室,上海200237;2中国科学院上海微系统与信息技术研究所,信息功能材料国家重点实验室,上海200050;3浙江大学材料科学与工程学院,硅材料国家重点实验室,杭州310027)摘要:采用原位液体池透射电镜技术,在扫描透射电子显微镜(STEM)中,实时观察溶液中金属钯(Pd)在金(Au)纳米颗粒及团簇周围的异质沉积过程。

聚乙烯吡咯烷酮碱性水溶液中金纳米花的简易合成任月萍;徐程程;方云【摘要】Three-dimensional (3D) gold nanoflowers of 60-80 nm in diameter were successfully synthesized using polyvinyl pyrrolidone (PVP) as both a protecting agent and a reducing agent in alkaline aqueous solutions.Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) images revealed that many antennae of 10-15 nm existed on their surfaces.X-ray diffraction (XRD) pattern suggested face-centered cubic (fcc) structures for these gold nanoflowers.The selected area electron diffraction (SAED) pattern of a single gold nanoflower indicated polycrystal characteristics.We found that there were three key stages in the growth of the gold nanoflowers: primary nanocrystals agglomerated to form multipod-like nanoparticles, and then the multipod-like nanoparticles aggregated into loose flower-like nanoparticles that ultimately grew into compact gold nanoflowers through Ostwald ripening.During the synthesis of gold nanoflowers, the molar ratios of PVP/HAuCl4 at fixed HAuCl4 and NaOH concentrations mostly influenced the morphologies of the final products.Therefore, a proper molar ratio of PVP/HAuCl4 and a suitable NaOH concentration were essential for the synthesis of typical gold nanoflowers with controlled sizes and antenna architectures.%以聚乙烯吡咯烷酮PVP)兼作保护剂和还原剂在碱性水溶液中直接还原HAuCl4制备出了60-80 nm的三维(3D)金纳米花.产物的透射电子显微镜(TEM)和扫描电子显微镜(SEM)图像显示,金纳米花表面布满10-15 nm左右的纳米触角,X射线衍射(XRD)表征揭示产物为金的面心立方晶体,选区电子衍射(SAED)花样说明金纳米花为多晶结构.金纳米花的生长经历了三个关键步骤,即初级纳米晶聚集成多脚状纳米粒子,随后在合适的PVP/HAuCl4浓度比及NaOH浓度下,多脚状纳米粒子进一步聚集形成疏松的花状粒子,最终经过Ostwald熟化形成致密的花状产物.一定HAuCl4浓度下PVP/HAuCl4浓度比和NaOH浓度对产物的形貌影响显著,因此通过同时调控合适的PVP/HAuCl4浓度比和NaOH浓度,就能得到适应各种应用需求的尺度可控和纳米触角形貌可控的金纳米花.【期刊名称】《物理化学学报》【年(卷),期】2011(027)005【总页数】5页(P1244-1248)【关键词】金;纳米花;纳米触角;聚乙烯吡咯烷酮;氢氧化钠【作者】任月萍;徐程程;方云【作者单位】江南大学化学与材料工程学院,江苏,无锡,214122;江南大学化学与材料工程学院,江苏,无锡,214122;江南大学化学与材料工程学院,江苏,无锡,214122【正文语种】中文【中图分类】O648贵金属纳米粒子的催化性质,1光电性质2,3及其在生物/化学传感,4,5非线性光学6和表面增强的拉曼散射(SERS)7等方面潜在的应用使其受到了越来越多的关注.纳米粒子的性质很大程度上取决于它们的形状和大小,目前人们在一维(1D)和二维(2D)Au/ Ag/Pt纳米粒子的可控合成方面已经取得了很大的进展.最近,表面粗糙的三维(3D)花状(或枝状)纳米粒子的研究受到了广泛的关注,这些纳米粒子具有密实的核和粗糙的具有5-10 nm触角的表面.8这些纳米粒子的粗糙表面赋予其独特的性能,一方面纳米花具有较大的比表面积,是理想的催化剂材料,如Mohanty等9研究发现Pt和Pd纳米花对碘基苯与苯基硼酸或苯乙烯的C-C偶联反应具有显著的催化作用;另一方面纳米花表面的纳米触角使得在同一个纳米粒子表面具有很多个热点,研究发现Au或Ag纳米花的SERS增强因子可达到107-108量级.10而Au纳米花的显著SERS增强效应加之良好的生物相容性使其成为拉曼光谱原位检测的理想选择, Xie等11将罗丹明B(RhB)包覆在金纳米花的表面,然后以牛血清白蛋白层稳定RhB制备出Au@ RhB@BSA的SERS标签,实现了拉曼光谱在肝细胞中的原位检测.目前花状纳米粒子的合成主要是通过晶种法,即将小的纳米晶粘结到晶种表面来构造花状形貌,12,13如Zhao等14以25 nm的Au纳米粒子为种子,羟胺为还原剂制备出了50 nm左右的金纳米花;Lu等15以Ag纳米粒子为种子在HAuCl4/K2CO3/明胶生长溶液中制备出了金纳米花.最近,Jena和Raj16以羟乙基哌嗪乙硫磺酸(HEPES)为保护剂和还原剂直接在水溶液中还原HAuCl4也制备出了3D金纳米花,这无疑使金纳米花的制备更趋简单化,本文在更简易模板PVP水溶液中仅仅通过调控碱度便可得到使用晶种法或复杂保护剂才能获得的结果,使得金纳米花的制备方法更趋实用性.2.1 材料与仪器聚乙烯吡咯烷酮(PVP K30,Mw=49000),美国ISP公司;HAuCl4·4H2O,NaOH均为AR级,国药集团化学试剂有限公司;超纯水(电阻率18.2 MΩ· cm),美国Millipore Synergy UV超纯水系统.D8 Advance型X射线衍射(XRD)仪,Cu靶,λ= 0.15406 nm,德国Bruker公司;S-4800型扫描电子显微镜(SEM),日本日立公司;JEOL JEM-2100型透射电子显微镜(TEM),日本电子株式会社.2.2 金纳米花的制备及表征在恒温40°C的PVP水溶液中加入HAuCl4水溶液迅速混匀,加入NaOH水溶液再混匀并在40°C恒温条件下避光反应1 h.最终体系中PVP,HAuCl4和NaOH的浓度分别为0.8、0.2和2 mmol·L-1,其中PVP与HAuCl4的摩尔浓度比为4:1.将得到的金溶胶离心(8000 r·min-1)分离,沉淀物用超纯水洗涤后进行XRD物相分析,用SEM及TEM观察产物形貌,并进行选区电子衍射(SAED)花样和高分辨透射电子显微镜(HRTEM)图像分析.3.1 金纳米花的表征图1a为产物的XRD衍射图,可见产物粒子为面心立方结构,晶体空间群为Fm3m(225).2θ角在30°-85°范围内的5个衍射峰分别指标化为(111), (200),(220),(311)和(222)晶面衍射峰.(200)与(111)晶面衍射峰强度的比值为0.24,明显低于标准卡片(JCPDS)中两者的比值0.52,说明(111)晶面在产物粒子的结构中占主导地位.图1b表明产物为60-80 nm左右的3D花状金纳米粒子,图1c的SEM图像显示这些金纳米花由许多15 nm左右的纳米晶聚集形成,图1d进一步说明这些纳米花表面布满10-15 nm的纳米触角构造.图1e的HRTEM图像显示金纳米花表面的纳米触角具有一致的晶格取向,因而为单晶结构,而图1f的SAED花样却表明金纳米花为多晶结构,说明金纳米花可能是由很多纳米晶以随机的方式聚集而成的.金纳米花粗糙表面的纳米触角使得单个纳米粒子上同时具有多个“热点”,这将显著增强其SERS效应.17另外金纳米花较高的比表面积使得其在催化上具有潜在的应用价值.183.2 金纳米花的生长过程了解金纳米花的形貌演变过程对于推断其生长机制非常重要,图2为不同反应时间产物的TEM图像.反应进行到2 min时,产物为10-20 nm的无规则初级纳米晶(见图2a).5 min时,产物为20-40 nm的多脚状纳米粒子,这些纳米粒子由少数几个初级纳米晶聚集而成(见图2b).反应进行到10 min时,粒子的花状轮廓(60-70 nm)基本形成,但是结构还比较疏松,并且图2c中仍然可见正在聚集的粒子(图中方块标识).反应到1 h时,产物为60-80 nm结构较为致密的3D金纳米花(见图2d).基于上述实验现象,推测金纳米花的生长应该经历了图3所示的三个关键步骤:在PVP的缓慢还原作用和保护作用下,初级纳米晶首先聚集成多脚状纳米粒子,随后在合适的PVP 及NaOH浓度下进一步聚集形成疏松的花状粒子,最后经过Ostwald熟化过程形成表面布满15 nm左右纳米触角构造的致密金纳米花.113.3 PVP与HAuCl4的初始摩尔浓度比对产物形貌的影响由于PVP在金纳米粒子的生长过程中同时充当还原剂和保护剂,因此体系中PVP 与HAuCl4的初始摩尔浓度比([PVP]/[HAuCl4])对产物的形貌影响显著.图4为不同[PVP]/HAuCl4]下产物的TEM图像,其中[HAuCl4]固定为0.2 mmol·L-1.[PVP]/ HAuCl4]为6:1时,产物为50-60 nm的金纳米花(见图4a),然而其表面的纳米触角个数明显少于[PVP]/ [HAuCl4]为4:1时体系制备所得金纳米花(见图4b).这可能是由于高[PVP]/[HAuCl4]时,PVP在纳米粒子的生长过程中提供了过强的保护作用,限制了纳米晶聚集形成多脚状和疏松花状纳米粒子,经过Ostwald熟化过程后长成了表面纳米触角凸起较低的纳米花.11将[PVP]/[HAuCl4]降低到3:1后,得到了40-50 nm的金纳米花,这些纳米花表面具有更多纳米触角构造(见图4c),同时纳米花尺寸有所缩小.这可能是降低[PVP]/[HAuCl4]不仅削弱了PVP对纳米粒子的保护作用,还降低了反应体系的还原速度,因此反应初期形成的初级纳米晶浓度降低,从而由它们聚集形成的纳米花尺寸明显变小.继续降低[PVP]/[HAuCl4]到2:1时,产物为30 nm左右的纳米花(见图4d),这可能是由于体系的还原作用相对更弱,因此反应初期形成的初级纳米晶的浓度未达到体系饱和浓度因此未能彼此聚集,经过Ostwald熟化过程后形成了更小的纳米花.由于金纳米粒子的表面形貌和大小对其SERS效应和催化性能具有显著的影响,9,17因此本方法通过调节[PVP]/[HAuCl4]就能根据应用需要制备尺度可控,纳米触角形貌特异的金纳米花.3.4 NaOH浓度对产物形貌的影响文献19,20已经披露NaOH的加入有助于枝状金纳米粒子的形成,这可能是水溶液中Au3+的配合物会随着pH值的增加转化为羟基化配合物(AuCl4-x(OH)-x).21如图5a所示,未添加NaOH时产物为100 nm左右的无规粒子和500 nm左右的纳米片的混合物.当向体系中加入2 mmol·L-1的NaOH后, Au3+的配合物变为还原电势较低的AuCl4-x(OH)-x,与相比的反应活性降低,21这将减缓初级纳米晶本身的生长速度,使其能够在PVP的作用下相互聚集形成多脚状粒子,并进一步演化成60-80 nm的金纳米花(见图1).随着NaOH浓度的增大,金配合物的反应活性将进一步降低,因此增大NaOH浓度到3 mmol·L-1时,产物为40-60 nm的花状金纳米粒子,这些花状粒子与图2c中粒子相似(见图5b),说明NaOH的加入确实降低了反应速率.继续增大NaOH浓度到4 mmol·L-1时,产物为15-32 nm的多脚状金纳米粒子,即使将反应时间延长到3 h产物仍然是多脚状金纳米粒子(见图5c),并没有形成纳米花.这可能是由于溶液中还原反应速率过低,反应初期形成的初级纳米晶的浓度未达到体系饱和浓度,未能彼此聚集.上述实验现象说明NaOH在金纳米花的形成过程中同样起到了非常关键的作用.以PVP兼作还原剂和保护剂在简约水相体系,常温常压的温和条件下制备出了60-80 nm的3D金纳米花.金纳米花表面具有丰富的10-15 nm左右的纳米触角构造,这种纳米级的表面粗糙结构使得金纳米花在催化和SERS方面具有潜在的应用前景.实验结果表明,金纳米花的生长经历了三个关键步骤,首先是初级纳米晶聚集成多脚状纳米粒子,随后在合适的PVP/HAuCl4浓度比及NaOH浓度下进一步聚集形成疏松的花状粒子,最后经过Ostwald熟化过程形成致密的金纳米花.由此可见PVP 浓度和NaOH浓度对于产物的尺度和形貌影响显著,因此在一定HAuCl4浓度下,通过同时控制合适的PVP/HAuCl4浓度比和NaOH浓度就有可能得到适应各种应用需求的尺度可控和纳米触角形貌可控的金纳米花.目前,利用PVP兼作还原剂和保护剂在低温水溶液中制备3D金纳米花尚未见报道,本方法反应条件温和,环境友好,操作简便,无需外加还原剂便可得到使用晶种法或复杂保护剂才能获得的结果,使得金纳米花的制备方法更趋实用性.【相关文献】(1)Hashmi,S.K.;Rudolph,M.Chem.Soc.Rev.2008,37,1766.(2) Sun,Y.G.;Xia,Y.N.Analyst 2003,128,686.(3) Xiang,C.X.;Güell,A.G.;Brown,M.A.;Kim,J.Y.; Hemminger,J.C.;Penner,R.M.NanoLett.2008,8,3017.(4)Fan,M.;Thompson,M.;Andrade,M.L.;Brolo,A.G.Anal. Chem.2010,82,6350.(5)Lin,C.Y.;Yu,C.J.;Lin,Y.H.;Tseng,W.L.Anal.Chem.2010, 82,6830.(6) Lippitz,M.;Dijk,M.A.;Orrit,M.Nano Lett.2005,5,799.(7) Deckert-Gaudig,T.;Deckert,V.Small 2009,5,432.(8) Sharma,J.;Tai,Y.;Imae,T.J.Phys.Chem.C 2008,112,17033.(9) Mohanty,A.;Garg,N.;Jin,R.Angew.Chem.Int.Edit.2010,49, 4962.(10)Liang,H.Y.;Li,Z.P.;Wang,W.Z.;Wu,Y.S.;Xu,H.X.Adv. Mater.2009,21,4614.(11) Xie,J.P.;Zhang,Q.B.;Lee,J.Y.;Wang,D.I.C.ACS Nano 2008,2,2473.(12) Kou,X.S.;Sun,Z.H.;Yang,Z.;Chen,H.J.;Wang,J.F. Langmuir 2009,25,1692.(13) Joseph,D.;Geckeler,ngmuir 2009,25,13224.(14) Zhao,L.L.;Ji,X.H.;Sun,X.J.;Li,J.;Yang,W.S.;Peng,X.G. J.Phys.Chem.C 2009,113,16645.(15) Lu,L.H.;Ai,K.L.;Ozaki,ngmuir 2008,24,1058.(16) Jena,B.K.;Raj,ngmuir 2007,23,4064.(17)Xu,D.;Gu,J.J.;Wang,W.N.;Yu,X.H.;Xi,K.;Jia,X.D. Nanotechnology 2010,21,375101.(18) Liao,H.G.;Jiang,Y.X.;Zhou,Z.Y.;Chen,S.P.;Sun,S.G. Angew.Chem.Int.Edit.2008,47,9100.(19) Chen,S.H.;Wang,Z.L.;Ballato,J.;Foulger,S.H.;Carroll,D. L.J.Am.Chem.Soc.2003,125,16186.(20)Wu,H.Y.;Liu,M.;Huang,M.H.J.Phys.Chem.B 2006,110, 19291.(21) Goia,D.V.;Matijević,E.Colloids and Surf.APhysicochemical and Engineering Aspects 1999,146,139.。
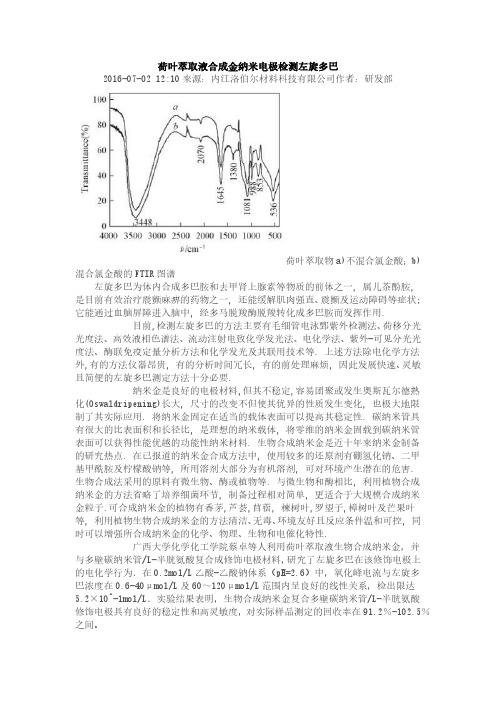
荷叶萃取液合成金纳米电极检测左旋多巴2016-07-02 12:10来源:内江洛伯尔材料科技有限公司作者:研发部荷叶萃取物a)不混合氯金酸;b)混合氯金酸的FTIR图谱左旋多巴为体内合成多巴胺和去甲肾上腺素等物质的前体之一, 属儿茶酚胺, 是目前有效治疗震颤麻痹的药物之一, 还能缓解肌肉强直、震颤及运动障碍等症状; 它能通过血脑屏障进入脑中, 经多马脱羧酶脱羧转化成多巴胺而发挥作用.目前,检测左旋多巴的方法主要有毛细管电泳鄄紫外检测法、荷移分光光度法、高效液相色谱法、流动注射电致化学发光法、电化学法、紫外-可见分光光度法、酶联免疫定量分析方法和化学发光及其联用技术等. 上述方法除电化学方法外,有的方法仪器昂贵, 有的分析时间冗长, 有的前处理麻烦, 因此发展快速、灵敏且简便的左旋多巴测定方法十分必要.纳米金是良好的电极材料,但其不稳定,容易团聚或发生奥斯瓦尔德熟化(Oswaldripening)长大, 尺寸的改变不但使其优异的性质发生变化, 也极大地限制了其实际应用. 将纳米金固定在适当的载体表面可以提高其稳定性. 碳纳米管具有很大的比表面积和长径比, 是理想的纳米载体, 将零维的纳米金固载到碳纳米管表面可以获得性能优越的功能性纳米材料. 生物合成纳米金是近十年来纳米金制备的研究热点. 在已报道的纳米金合成方法中, 使用较多的还原剂有硼氢化钠、二甲基甲酰胺及柠檬酸钠等, 所用溶剂大部分为有机溶剂, 可对环境产生潜在的危害. 生物合成法采用的原料有微生物、酶或植物等. 与微生物和酶相比, 利用植物合成纳米金的方法省略了培养细菌环节, 制备过程相对简单, 更适合于大规模合成纳米金粒子.可合成纳米金的植物有香茅,芦荟,苜蓿, 楝树叶,罗望子,樟树叶及芒果叶等, 利用植物生物合成纳米金的方法清洁、无毒、环境友好且反应条件温和可控, 同时可以增强所合成纳米金的化学、物理、生物和电催化特性.广西大学化学化工学院蔡卓等人利用荷叶萃取液生物合成纳米金,并与多壁碳纳米管/L-半胱氨酸复合成修饰电极材料,研究了左旋多巴在该修饰电极上的电化学行为.在0.2mol/L乙酸-乙酸钠体系(pH=2.6)中,氧化峰电流与左旋多巴浓度在0.6-40μmol/L及60~120μmol/L范围内呈良好的线性关系,检出限达5.2×10^-1mol/L.实验结果表明,生物合成纳米金复合多壁碳纳米管/L-半胱氨酸修饰电极具有良好的稳定性和高灵敏度,对实际样品测定的回收率在91.2%-102.5%之间。


抗坏血酸还原法制备金纳米花的机理杨爽;纪小会;杨文胜【摘要】The mechanism in the preparation of gold nanoflowers using the mixture of HAuCl4 and ascorbic acid ( AA ) as growth solution was investigated. It was identified that the AA concentration affected the attachment of the small gold particles on the seeds and the intraparticle ripening of the gold nanoflowers, thus resulting in the formation of the flowers with different morphologies and optical properties. It is effective to tune morphology and optical property of the gold nanoflowers by optimizing the AA concentration and pH of the growth solution simultaneously. Surface enhanced Raman scattering( SERS) results indicated the as⁃prepared gold nanoflowers showed good SERS activity to Rhodamine 6G, suggesting the clean surface character of the flowers prepared by AA reduction.%研究了以抗坏血酸和氯金酸为生长溶液制备金纳米花的反应机理。
胶体金(纳米金Gold Nanoparticles)的详细制备步骤和注意事项胶体金的制备一般采用还原法,常用的还原剂有柠檬酸钠、鞣酸、抗坏血酸、白磷、硼氢化钠等。
下面介绍最常用的制备方法及注意事项。
1、玻璃容器的清洁:玻璃表面少量的污染会干扰胶体金颗粒的生成,一切玻璃容器应绝对清洁,用前经过酸洗、硅化。
硅化过程一般是将玻璃容器浸泡于5%二氯二甲硅烷的氯仿溶液中1分钟,室温干燥后蒸馏水冲洗,再干燥备用。
专用的清洁器皿以第一次生成的胶体金稳定其表面,弃去后以双蒸馏水淋洗,可代替硅化处理。
2、试剂、水质和环境:氯金酸极易吸潮,对金属有强烈的腐蚀性,不能使用金属药匙,避免接触天平称盘。
其1%水溶液在4℃可稳定数月不变。
实验用水一般用双蒸馏水。
实验室中的尘粒要尽量减少,否则实验的结果将缺乏重复性。
金颗粒容易吸附于电极上使之堵塞,故不能用pH电极测定金溶液的pH值。
为了使溶液pH值不发生改变,应选用缓冲容量足够大的缓冲系统,一般采用柠檬酸磷酸盐(pH3~5.8)、Tris-HCL (pH5.8~8.3)和硼酸氢氧化钠(pH8.5~10.3)等缓冲系统。
但应注意不应使缓冲液浓度过高而使金溶胶自凝。
3、柠檬酸三钠还原法制备金溶胶:取0.01%氯金酸水溶液100ml 加热至沸,搅动下准确加入1%柠檬酸三钠水溶液0.7ml,金黄色的氯金酸水溶液在2分钟内变为紫红色,继续煮沸15分钟,冷却后以蒸馏水恢复到原体积,如此制备的金溶胶其可见光区最高吸收峰在535nm,A1cm/535=1.12。
金溶胶的光散射性与溶胶颗粒的大小密切相关,一旦颗粒大小发生变化,光散射也随之发生变异,产生肉眼可见的显著的颜色变化,这就是金溶胶用于免疫沉淀或称免疫凝集试验的基础。
金溶胶颗粒的直径和制备时加入的柠檬酸三钠量是密切相关的,保持其他条件恒定,仅改变加入的柠檬酸三钠量,可制得不同颜色的金溶胶,也就是不同粒径的金溶胶,见附表。
附表100 ml 氯金酸中柠檬酸三钠的加入量对金溶胶粒径的影响1%柠檬酸三钠ml 0.30 0.45 0.70 1.00 1.50 2.00金溶胶颜色蓝灰紫灰紫红红橙红橙吸收峰(nm) 220 240 535 525 522 518径粒(nm) 147 97.5 71.5 41 24.5 154、柠檬酸三钠-鞣酸混合还原剂:用此混合还原剂可以得到比较满意的金溶胶,操作方法如下:取4ml1%柠檬酸三钠(Na3C6H5O7.2H2O),加入0~5ml1%鞣酸,0~5ml 25mmo/L K2CO2(体积与鞣酸加入量相等),以双蒸馏水补至溶液最终体积为20ml,加热至60℃取1ml1%的HAuCl4,加于79ml双蒸馏水中,水浴加热至60℃,然后迅速将上述柠檬酸-鞣酸溶液加入,于此温度下保持一定时间,待溶液颜色变成深红色(约需0.5~1小时)后,将溶液加热至沸腾,保持沸腾5分钟即可。
聚离子液体固载金纳米粒子的制备及其催化性能彭建兵1*,丁艳2,谭蓉2,银董红2,喻宁亚2*(1顺德职业技术学院,中国顺德528300;2湖南师范大学精细催化合成研究所,中国长沙410081) 摘要:合成了氨基功能化乙烯基咪唑离子液体(AIL),利用氨基固定金纳米粒子以及双键的聚合作用,获得了聚离子液体固载金纳米粒子的催化剂(GNPs-P-AIL)。
采用红外光谱、紫外-可见光谱和透射电子显微镜等方法对GNPs-P-AIL进行了表征。
结果表明,AIL在固定金纳米粒子并聚合后仍然保持着离子液体的基本结构,金纳米粒子分布均匀,粒径为6~8 nm。
GNPs-P-AIL催化剂对苯乙烯环氧化具有较好的催化活性,以双氧水为氧化剂,在60 o C下反应6 h时,苯乙烯的转化率和环氧苯乙烷的选择性分别可达81.5%和88.3%。
关键词:氨基功能化离子液体;金纳米粒子;聚合;苯乙烯;环氧化中图分类号:文献标识码:文章编号:Preparation and Catalytic Performance of Gold Nanoparticles Stabilized by Ionic CopolymerPENG Jian-Bin1*, DING Yan2, TAN Rong2, YIN Donghong2, YU Ningya2*(1Shunde Polytechnic, Shunde 528300, China; 2Insititute of Fine Catalysis and Synthesis, Hunan Normal University, Changsha 410081, China)Abstract: An amino-functionalized ionic liquid of N-(3-aminopropyl), N(3)-(vinyl)-imidazolium bromide (AIL) was synthesized and used to stabilize gold nanoparticles (GNPs). Further polymerization of the resultant GNP-containing AIL led to a composite catalyst (GNPs-P-AIL) in which GNPs were supported by ionic copolymer. The composite catalyst was characterized by means of Fourier transform infrared spectroscopy, ultraviolet-visible spectroscopy, and transmission electron microscopy. It was found that the structure of ionic liquid moieties in GNPs-P-AIL remained intact after the GNP loading and successive polymerization processes and that the GNPs in the composite catalyst dispersed uniformly with the particle size of 6~8 nm. GNPs-P-AIL showed good catalytic performance in the epoxidation of styrene using H2O2 as the oxidant. The conversion of styrene and the selectivity for styrene oxide were as high as 81.5% and 88.3%, respectively, provided that the epoxidation reaction was performed at 60 o C for 6 h.Key words: Amino-functionalized ionic liquid; gold nanoparticles; Polymerization; styrene; epoxidation*通讯作者,E-mail: sdpjb2005@; yuningya@第一作者简介:彭建兵(1968-),男,湖南双峰人,副教授,主要从事新型材料催化合成与应用研究。
/Langmuir ©2010American Chemical SocietyOne-Phase Synthesis of Water-Soluble Gold Nanoparticles with Control overSize and Surface FunctionalitiesEunkeu Oh,†Kimihiro Susumu,†Ramasis Goswami,‡and Hedi Mattoussi*,†,§†Division of Optical Sciences,Code 5611and ‡Materials Science Division,Code 6355,U.S.Naval Research Laboratory,Washington,D.C.20375.§Present address:Department of Chemistry and Biochemistry,Florida State University,4006Chemical Sciences Building,Tallahassee,Florida 32306.Received November 23,2009.Revised Manuscript Received January 7,2010We report a simple and efficient synthetic method to prepare gold nanoparticles (AuNPs)in aqueous phase using HAuCl 4and poly(ethylene glycol)(PEG)ligands appended with bidentate anchoring groups.Our approach provides narrow size distribution nanocrystals over the size range between 1.5and 18nm;this range is much wider than those achieved using other small molecules and polymer ligands.The NP size was simply controlled by varying the molar ratio of Au-to-PEG ligand precursors.Further passivation of the as-prepared AuNPs permitted in situ functionalization of the NP surface with the desired functional groups.The prepared AuNPs exhibit remarkable stability in the presence of high salt concentrations,over a wide range of pHs (2-13),and a strong resistance to competition from dithiothreitol (DTT).These results are a clear manifestation of the advantages offered by our synthetic approach to prepare biocompatible AuNPs,where modular,multifunctional ligands presenting strong anchoring groups and hydrophilic PEG chains are used.IntroductionGold nanoparticles (AuNPs)along with an array of other metallic and semiconducting nanocrystals with their size-depen-dent physical and chemical properties have generated a tremen-dous interest in the past decade.This interest has also been driven by the great potential for applications in optical and electronic devices and more recently in biological sensing and imaging.1-3AuNPs with size larger than 2nm (in diameter)exhibit size-and shape-dependent surface plasmon absorption band (SPB);AuNPs smaller than 2nm,in comparison,exhibit photoinduced emission.4The SPB is also sensitive to proximity-driven NP-to-NP interactions and to the surrounding environment,including surface ligands,solvent,and temperature.Thus,red shift of the SPB coupled with broadening of the peak width has been observed in the presence of NP aggregation,which can be triggered by interactions with a particular target or in response to an external stimulus (changes in pH or excess ion con-centrations).Larger size AuNPs and nanorods can also trans-form laser excitation to local thermal heating.These unique properties have provided researchers in biology with powerful methods to develop sensing techniques that include (1)colori-metric assays to detect NP assemblies,1,5,6(2)molecular rulers based on plasmon coupling to detect bridging between NP pairs(e.g.,DNA hybridization),7,8(3)surface-enhanced Raman scat-tering (SERS)nanoparticle tags for in vivo spectroscopy,9,10and (4)photoinduced heating of Au nanoparticles and nanorods for localized photothermal therapy.11,12Recently,the size-depen-dent uptake of AuNPs by live cells was studied for sizes ranging from 2to 100nm.13-15AuNPs are also efficient quenchers of dyes and luminescent quantum dots (via resonance energy transfer)when brought in close proximity.16,17These applica-tions often require that the AuNPs are (1)available in a broad size range,(2)stable over a broad range of conditions,and (3)easy to surface functionalize with specific reactive groups for further coupling to target molecules.Since the classic citrate reduction of aurate to prepare citrate-stabilized AuNPs was pioneered by the groups of Turkevich and Frens,18,19there has been a sustained effort aimed at develop-ing new chemical routes to prepare AuNPs that are stable and easily dispersible in water.These efforts have also focused on designing synthetic schemes to functionalize the AuNP surfaces with specific target ligands.20Some of the most common methods*To whom correspondence should be addressed.E-mail:mattoussi@.(1)Daniel,M.C.;Astruc,D.Chem.Rev.2004,104,293–346.(2)Zheng,J.;Nicovich,P.R.;Dickson,R.M.Annu.Rev.Phys.Chem.2007,58,409–431.(3)De,M.;Ghosh,P.S.;Rotello,V.M.Adv.Mater.2008,20,4225–4241.(4)Schaeffer,N.;Tan,B.;Dickinson,C.;Rosseinsky,M.J.;Laromaine,A.;McComb,D.W.;Stevens,M.M.;Wang,Y.Q.;Petit,L.;Barentin,C.;Spiller,D.G.;Cooper,A.I.;Levy,mun.2008,3986–3988.(5)Mirkin,C.A.;Letsinger,R.L.;Mucic,R.C.;Storhoff,J.J.Nature 1996,382,607–609.(6)Lee,J.S.;Ulmann,P.A.;Han,M.S.;Mirkin,C.A.Nano Lett.2008,8,529–533.(7)Sonnichsen,C.;Reinhard,B.M.;Liphardt,J.;Alivisatos,A.P.Nat.Biotechnol.2005,23,741–745.(8)Reinhard,B.M.;Siu,M.;Agarwal,H.;Alivisatos,A.P.;Liphardt,J.Nano Lett.2005,5,2246–2252.(9)Qian,X.M.;Peng,X.H.;Ansari,D.O.;Yin-Goen,Q.;Chen,G.Z.;Shin,D.M.;Yang,L.;Young,A.N.;Wang,M.D.;Nie,S.M.Nat.Biotechnol.2008,26,83–90.(10)Qian,X.M.;Nie,S.M.Chem.Soc.Rev.2008,37,912–920.(11)Huang,X.H.;Jain,P.K.;El-Sayed,I.H.;El-Sayed,sers Med.Sci.2008,23,217–228.(12)Jain,P.K.;Huang,X.H.;El-Sayed,I.H.;El-Sayed,M.A.Acc.Chem.Res.2008,41,1578–1586.(13)Jiang,W.;Kim,B.Y.S.;Rutka,J.T.;Chan,W.C.W.Nat.Nanotechnol.2008,3,145–150.(14)Wang,S.G.;Lu,W.T.;Tovmachenko,O.;Rai,U.S.;Yu,H.T.;Ray,P.C.Chem.Phys.Lett.2008,463,145–149.(15)Perrault,S.D.;Walkey,C.;Jennings,T.;Fischer,H.C.;Chan,W.C.W.Nano Lett.2009,9,1909–1915.(16)Dulkeith,E.;Morteani,A.C.;Niedereichholz,T.;Klar,T.A.;Feldmann,J.;Levi,S.A.;van Veggel,F.C.J.M.;Reinhoudt,D.N.;Moller,M.;Gittins,D.I.Phys.Rev.Lett.2002,89,203002.(17)Oh,E.;Hong,M.Y.;Lee,D.;Nam,S.H.;Yoon,H.C.;Kim,H.S.J.Am.Chem.Soc.2005,127,3270–3271.(18)Turkevich,J.;Stevenson,P.C.;Hillier,J.Discuss.Faraday Soc.1951,55–.(19)Frens,G.Nat.Phys.1973,241,20–22.(20)Weisbecker,C.S.;Merritt,M.V.;Whitesides,ngmuir 1996,12,3763–3772.Oh et al.Articleinclude the following:(1)Growth of1.5-5.2nm AuNPs surface-functionalized with alkanethiol using two-phase toluene/water reaction developed by Brust and co-workers;21,22this route produced hydrophobic nanoparticles.(2)Use of poly(ethylene glycol)oligomer(PEG)containing ligands combined with the two-phase reaction developed by Brust and co-workers,to pre-pare Au clusters first reported by Murray et al.23and then later by Brust et al.24The resulting NPs(water dispersible)are capped with either monothiol-terminated PEG(MW5000)oligomers,23 or tetraethylene glycol ligands.24(3)Use of dialkyl disulfide stabilizers to prepare 1.4-3.8nm diameter AuNPs.25,26(4) Synthesis of1.5-8nm size of AuNPs using water-soluble alkyl thioether-and thiol-functionalized poly(methacrylic acid).27,28(5) Preparation of phosphine-stabilized1.4nm AuNPs;291.4nm AuNPs surface-functionalized with maleimide are commercially available(Nanoprobes,Yaphank,NY).These preparation meth-ods reflect the tremendous progress made in the past decade.They provided researchers with an array of AuNPs which have been employed for developing a variety of applications.Nonetheless, each of these synthetic schemes has encountered some limitations. These include(1)reduced stability against excess salts and changes in solution pH(e.g.,citrate-stabilized NPs);(2)the inability to prepare nanocrystals over a wide size regime(citrate reduction usually produces AuNPs smaller than10nm,but larger sizes require additional refluxing in the presence of sodium citrate);(3)most NPs prepared using thiol-alkyl-Au interac-tions are hydrophobic,and the transfer to aqueous media further requires postsynthetic processing via ligand exchange,which can be tedious and requires large amounts of ligands.Murphy and co-workers combined seeding,growth,and temperature treatment in the presence of cetyltrimethylammonium bromide(CTAB)to synthesize larger size AuNPs and Au nanorods(5-40nm range).30These findings combined clearly indicate that,despite the tremendous progress,there is still a need to develop new simple synthetic methods to prepare AuNPs over a wide range of sizes that exhibit enhanced stability in buffer media(often rich in ionic complexes)and which can be surface-functionalized.We have designed and synthesized an array of modular ligands made of a tunable length PEG segment appended with either a thioctic acid(TA,which has a terminal disulfide)or a dihydro-lipoic acid(DHLA,formed by reducing the terminal disulfide to dithiol)at one end and a potentially reactive group at another end (see Figure1).31-34The synthetic procedures we have developed are simple to implement and provide multigram scale of materials.We have also shown that both TA-PEG-and DHLA-PEG-basedligands can be used to cap-exchange citrate-stabilized NPs.Inaddition,we have demonstrated that DHLA-PEG ligands pro-vide effective surface functionalization of semiconductor quan-tum dots and promote their transfer to buffer media.Further-more,the bidentate nature of the anchoring group provides betterstability to both types of nanocrystals compared with theirmonothiol analogues.32,33Here,we build on the synthetic rationales reported for thepreparation of thiol stabilized AuNPs combined with the natureof our TA-PEG ligands to develop a simple one-phase(aqueous)growth and passivation method to prepare a series of hydro-philic AuNPs.This new route provides nanocrystals with abroad range of sizes(1.5-18nm in diameter),which can alsobe readily functionalized with reactive end groups for furthercoupling to target bioreceptors.Since these NPs are stabilizedwith disulfide anchoring groups,they exhibit remarkable stabi-lity in the presence of excess counterions,against competi-tion with dithiothreitol(DTT),and to changes in solutionpH,similar to what was reported for Au nanoparticles cap-exchanged with TA-PEG ligands(starting with citrate-coatedNPs)or prepared in the presence of thiol-functionalized poly-mers.28,35We describe the synthetic details developed anddiscuss optical and structural characterization of the preparedAuNPs using UV-vis spectroscopy,high-resolution transmis-sion electron microcopy(HRTEM),and dynamic light scatter-ing(DLS).Results and DiscussionControl of Au Nanocrystal Growth.For this study,wesynthesized TA-PEG-OCH3ligands with average poly(ethyleneglycol)methyl ether molecular weight(MW)of550or750.Ligands with a PEG MW∼550(TA-PEG550-OCH3)were usedthroughout this report,unless otherwise noted.For in situfunctionalization,we used TA-PEG600-COOH(PEG MW∼600)ligand mixed with TA-PEG-OCH3during AuNP synthesis.Synthesis of TA-PEG-OCH3and TA-PEG600-COOH wascarried out following the procedures detailed in our previousreports.32-34For control experiments,we used mercaptohexa-noic-acid-appended PEG(HS-PEG550-OCH3,monothiol ap-pended ligand).The synthetic details are provided in theExperimental Section.The AuNPs were prepared using a three-step reaction consisting of(1)precursor formation by reacting theligands with tetrachloroauric(III)acid(HAuCl433H2O);(2) growth of the AuNP cores triggered by addition of NaBH4reducing agent;and(3)further passivation and functionalizationof the cores by adding extra free ligands,as schematicallydiagrammed in Figure1.Tetrachloroauric acid and the ligandswere first mixed in water to promote the formation of Au-TA-PEG-OCH3metal-ligand precursors.This precursor formationmanifests in a rapid color change of the original yellow solution tored,yellow,and finally colorless.Addition of NaBH4initiatesreduction of the Au ions and rapid growth of the Au nanocrystals.We varied the molar ratio of Au-to-ligand as a means ofcontrolling the size of the resulting metal core.Once the growthwas complete(usually associated with a saturation in the UV-visabsorption spectrum),free ligands were further added to thesolution(to a final Au/ligand molar ratio of∼1:1).This last stepprovided additional passivation and functionalization(whendesired)of the AuNPs by filling unoccupied surface sites.None-theless,when NPs were grown under initial low Au-to-ligand(21)Brust,M.;Walker,M.;Bethell,D.;Schiffrin,D.J.;Whyman,R.J.Chem. Soc.,mun.1994,801–802.(22)Brust,M.;Fink,J.;Bethell,D.;Schiffrin,D.J.;Kiely,C.J.Chem.Soc., mun.1995,1655–1656.(23)Wuelfing,W.P.;Gross,S.M.;Miles,D.T.;Murray,R.W.J.Am.Chem. Soc.1998,120,12696–12697.(24)Kanaras,A.G.;Kamounah,F.S.;Schaumburg,K.;Kiely,C.J.;Brust,M. mun.2002,2294–2295.(25)Yonezawa,T.;Yasui,K.;Kimizuka,ngmuir2001,17,271–273.(26)Shon,Y.S.;Mazzitelli,C.;Murray,ngmuir2001,17,7735–7741.(27)Hussain,I.;Graham,S.;Wang,Z.X.;Tan,B.;Sherrington,D.C.; Rannard,S.P.;Cooper,A.I.;Brust,M.J.Am.Chem.Soc.2005,127,16398–16399.(28)Wang,Z.X.;Tan,B.E.;Hussain,I.;Schaeffer,N.;Wyatt,M.F.;Brust,M.; Cooper,ngmuir2007,23,885–895.(29)Weare,W.W.;Reed,S.M.;Warner,M.G.;Hutchison,J.E.J.Am.Chem. Soc.2000,122,12890–12891.(30)Jana,N.R.;Gearheart,L.;Murphy,ngmuir2001,17,6782–6786.(31)Uyeda,H.T.;Medintz,I.L.;Jaiswal,J.K.;Simon,S.M.;Mattoussi,H. J.Am.Chem.Soc.2005,127,3870–3878.(32)Susumu,K.;Uyeda,H.T.;Medintz,I.L.;Pons,T.;Delehanty,J.B.; Mattoussi,H.J.Am.Chem.Soc.2007,129,13987–13996.(33)Mei,B.C.;Susumu,K.;Medintz,I.L.;Delehanty,J.B.;Mountziaris,T.J.; Mattoussi,H.J.Mater.Chem.2008,18,4949–4958.(34)Susumu,K.;Mei,B.C.;Mattoussi,H.Nat.Protoc.2009,4,424–436.(35)Mei,B.C.;Oh,E.;Susumu,K.;Farrell,D.;Mountziaris,T.J.;Mattoussi,ngmuir2009,25,10604–10611.Article Oh et al.molar ratio (smaller than 1:1),no extra free ligands were added to the final sample;the initial molar concentration of the ligands was high enough to provide sufficient passivation of the nanoparticles in this case.In a typical reaction,the following conditions were used to prepare our AuNPs.An amount of 156μL (7.92Â10-6mol)of 50.8mM tetrachloroauric(III)acid (HAuCl 433H 2O)stock solu-tion and the desired molar concentration of TA-PEG550-OCH 3were dissolved in 25mL of deionized water;the mixture was then stirred at room temperature for 1h.The stock solution of (HAuCl 433H 2O)was prepared by mixing 100mg (2.54Â10-4mol)of AuCl 4in 5mL of H 2O.Stirring for 1h was based on the anticipated minimum time necessary to promote precursor for-mation (see below).An amount of 72μL (6.3Â10-5mol)of 880mM sodium borohydride (NaBH 4)stock solution in deio-nized water was added (in aliquots of 18μL)over 30min withvigorous stirring.To prepare different size nanoparticles,we fixed the molar concentration of HAuCl 4and varied the amount of the ligands used.Following addition of the reducing agent,the color of the reaction mixture immediately changed to brown or red depending on size.The mixture was then left stirring for at least 3h,as it gradually progressed to its final appearance (when the absorption spectrum reached saturation).The AuNP dispersions (as-prepared)were characterized using UV -vis spectroscopy,TEM,and DLS.For extra passivation (or passivation and functionalization),TA-PEG550-OCH 3or a mixture of either TA-PEG550-OCH 3/TA-PEG600-COOH or TA-PEG550-OCH 3/TA-PEG600-NH 2(final ratio of Au to ligand =1:1)and 18μL (1.6Â10-5mol)of 880mM NaBH 4were further added to the AuNP solution,and left stirring for an additional 3h.The dispersion was then purified from free ligands by three cycles of centrifugation using a membrane filtration device (Millipore).Figure 1.(A)Schematic representation of the one-phase growth method using TA-PEG-OCH 3ligands.(B)Normalized UV -vis absorption spectra for several Au/ligand molar ratios used for the growth (of AuNPs with different sizes),along with a few representative TEM images collected for selected subsets of these nanocrystals.(inset)Image collected from a series of AuNP dispersions in deionized water.Changes in solution color from light to dark brown and to red (from left to right)reflect increase in AuNPsize.Oh et al.Article These AuNPs were then characterized and compared to thosenot subjected to the extra passivation/functionalization step.Inour experimental protocol,we used10Âtotal molar excess ofthe reducing agent during the growth and passivation steps(i.e.,total NaBH4-to-Au molar ratio∼10).This value wasbased on those used in previous studies and provided the mosteffective preparation conditions including those reported usingthe Brust method.21Reducing this ratio to9or8does not changethe results.Lower values(e.g.,∼1Â),however,do not providegrowth of homogeneous AuNP dispersions.As controls,wesynthesized thioctic acid(TA)-AuNPs,mercaptohexanoic acid(MHA)-AuNPs,and HS-PEG-OCH3-AuNPs using the samemethod but mixing either TA,MHA,or monothiol-appendedPEG ligands instead of TA-PEG-OCH3(Figure1A).Characterization of the AuNPs. 1.UV-Vis Absorp-tion Spectroscopy.Figure1B shows the absorption spectracollected from a set of AuNP dispersions prepared using anincreased molar ratio of Au to TA-PEG-OCH3;some represen-tative TEM images collected from these dispersions are alsoshown.The absorption spectra show that at low Au-to-ligandmolar ratio the characteristic SPB located at520nm is veryweak(not discernible for the smallest size NPs).However,thepeak becomes sharper and more defined at Au/ligand molarratios above6:1.The well-defined and narrow SPB peaks for thesedispersions(in particular for those prepared with high Au-to-ligand molar ratios)indicate that narrow size distribution char-acterizes these NPs.The inset shows an image of several NPdispersions in deionized water.The dispersion color changedfrom dark yellow to brown and then to red as the Au-to-TA-PEG-OCH3ligand ratio increased,indicating an increase in theNP size.Data clearly indicate that our NP growth method iscapable of providing high quality nanocrystals over a broad sizerange,where effective control over the NP dimensions has simplybeen achieved by varying the Au/ligand ratio used during growth.The growth reaction carried out in the presence of TA,MHAand HS-PEG-OCH3yielded only smaller size AuNPs(diameter<10nm).In addition,dispersions prepared using high Au/ligandratios easily precipitated when monothiol-appended ligands wereused(see Figure S1in the Supporting Information).For instance,dynamic light scattering experiments indicated the presence ofaggregate buildup(∼190nm size)for the dispersions of HS-PEG-OCH3-AuNPs(see Figure S2in the Supporting Information).The rest of the structural characterization will focus on nano-crystals prepared using TA-PEG-OCH3ligands.2.Transmission Electron Microscopy(TEM).We relied onthe use of high-resolution TEM to determine nanocrystal sizesand assess their crystalline quality;only data collected fromTA-PEG-OCH3-AuNPs are shown.Figure2shows the TEMimages collected from six representative nanocrystal dispersionsprepared using a Au/TA-PEG-OCH3ratio that varied from1:10to4000:1.The images show that the size of the NPs progressivelyincreased with increasing Au-to-ligand molar ratio,as anticipatedfrom the absorption properties shown in Figure1B.The averagediameter extracted from these TEM images together with theAu/ligand ratios are listed in Table1.Typically,the measureddiameters were1.5(0.3nm(for Au/ligand=1:10),2.2(0.3nm(for Au/ligand=1.4:1),4.6(0.4nm(Au/ligand=30:1),10(1.4nm(Au/ligand=600:1),12(1.7nm(Au/ligand=2000:1),and18(3.3nm(Au/ligand=4000:1),respectively.These images do not show any sign of nanocrystal clumping on the TEM grid, further confirming the colloidal stability of these dispersions.The TEM images also show that spherical shapes dominate the disper-sions of small size nanocrystals.However,slight inhomogeneities in nanoparticle shape are observed for the larger size NPs(see panel6in Figure2A).Higher resolution images for a few representative nanocrystal sizes with different crystal orientations are shown in Figure2B together with the corresponding fast Fourier transfor-mation(FFT)and inversed FFT(IFFT).The lattice spacing along the(111)planes measured for our nanocrystals was2.4A,in agreement with what has been previously reported(2.36A)for Figure2.(A)TEM images of several different size AuNPs(scale bar=20nm).Average size and standard deviation are reported for each sample.Samples for TEM were prepared by spreading a drop of the AuNP dispersions onto the TEM grid(made of a holey carbon film on a400mesh Cu grid).(B)IFFT of the TEM image of 2.2nm TA-PEG-OCH3-AuNPs(inset,upper left)and FFT of 12nm TA-PEG-OCH3-AuNPs(inset,lower right).T indicates the twin plane in the lower right ttice spacing is about0.24nm for both.(C)High-resolution image of a larger size NP showing multiple twinning,which is characteristic of FCCstructure.ArticleOh et al.face-centered cubic (FCC)AuNPs.36The TEM images also show that twinning defects characteristic of FCC crystalline structures are observed primarily for the larger size nanocrystals (see Figure 2C).A plot summarizing the progression of particle size (extracted from TEM)versus Au-to-ligand ratio for the full set of nanocrystals prepared using our growth method is shown in Figure 3A.To further test the quality of our AuNPs,we plotted the progression of the extinction coefficient at 520nm versus diameter (based on TEM measurements)together with those measured for commercially available citrate-stabilized AuNPs (Ted Pella Inc.);see Figure 3B.The data are essentially identical for both sets of NPs.The overall trend for the nanocrystal size versus Au-to-ligand ratio compiled for our materials indicates a rapid size increase at low ratios (Au/ligand e 10:1,diameter e 3nm).This dependence becomes moderate for the medium size range (diameter ∼3-10nm,Au/ligand ratio ∼10-600)and eventually very weak for large size NPs (diameter >10nm,at Au-to-ligand ratio exceeding ∼600).The rapid size change measured at low ratios reflects the pronounced effects of surface-to-volume ratios for small size NPs;this ratio is sensitive to the amounts of ligands used.As the NP size increases and the relative surface-to-volume ratios decreases,effects of ligand concentration become less dominant,which translates into a weaker change in NP size at rather large Au/ligand ratios.We should emphasize that the size range described here is not exclusive,as slightly smaller NPs (∼1-1.4nm)and larger size nanocrystals (∼20nm)can be made.We were not able to collect high quality TEM images for NPs smaller than 1.5nm,due to weaker electronic contrast and potential interference from the ligands;we also limited our char-acterization of the largest size to 18nm e of thiol-terminated poly(methacrylic acid)(as ligand)for the synthesis of water-soluble AuNPs has been reported by Brust and co-workers.27,28The authors also explored the effects of changing the exact polymer structure as well as hydrophobicity and/or “denticity”of the end groups on the size and distribution of prepared NPs.In particular,they showed that AuNPs with sizes ranging from 1.5to 8nm can be made.283.Dynamic Light Scattering (DLS ).Dynamic light scatter-ing (DLS)is a powerful technique that has widely been used toprobe the stability of colloidal dispersions and polymeric solutions.37-39Due to the extreme sensitivity of the scatteredTable 1.Sizes Extracted from High-Resolution TEM and DLS Experiments Shown Together with the Corresponding Au-to-LigandRatios Used throughout the StudyAu/ligand ratio aTEM (nm)DLS (nm)bDLS (nm)c1:10 1.5(0.31:1 1.7(0.21.4:1 2.2(0.33:1 2.4(0.310:1 3.0(0.520:1 4.3(0.630:1 4.6(0.411(2.413(2.7100:1 5.0(0.7250:17(1.213(2.819(4.6300:18(0.9600:110(1.416(3.621(3.82000:112(1.721(4.025(5.34000:118(3.327(8.433(13aPrecursor ratios used during core synthesis of AuNPs.b Sizes measured for as-prepared AuNP dispersions.c Sizes measured after the extra passivation step with additional ligands.Figure 3.(A)Plot of the AuNP size measured by TEM versus Au-to-TA-PEG-OCH 3molar ratio.Inset shows a blow up of the region near the origin (small ratios).(B)Plot of the extinction coefficients of AuNPs,calculated from UV -vis absorbance at 520nm,versus diameter extracted from TEM.Inset shows a blow up of the section correspond-ing to small ratios.(C)Intensity versus hydrodynamic diameter profiles extracted from DLS for TA-PEG-OCH 3-AuNPs (as-prepared)with different Au-to-ligand molar ratios:2000:1,600:1,250:1,and 30:1.The corresponding hydrodynamic diameters are 21(4.0nm,16(3.6,13(2.8,and 11(2.4,respectively.(D)Intensity versus hydrodynamic diameter profiles for the same series after the extra passivation.(36)Kuo,C.H.;Chiang,T.F.;Chen,L.J.;Huang,M.ngmuir 2004,20,7820–7824.(37)Berne,B.J.;Pecora,R.Dynamic light scattering:with applications to chemistry,biology,and physics ;Dover Publications:Mineola,NY,2000.(38)Brown,W.Dynamic light scattering:the method and some applications ;Clarendon Press:Oxford,New York,1993.(39)Pons,T.;Uyeda,H.T.;Medintz,I.L.;Mattoussi,H.J.Phys.Chem.B 2006,110,20308–20316.Oh et al.Articlesignal to changes in the radius (R )of the scattering objects (scattered intensity µR 6),DLS can detect even small fractions of aggregate buildup in NP dispersions and polymer solutions.DLS also provides a measure for the hydrodynamic diameter 2R H of individual nanocrystals (with R H being the nanocrystal hydrodynamic radius),which accounts for subtle effects of sur-face ligand charges on the hydrodynamic interactions.38R H is extracted from the autocorrelation function of the scattered signal and is often provided as a number or intensity distribution to account for size dispersity/ditsribution.37,39Figure 3C shows the experimental plots of the intensity versus hydrodynamic diameter for four representative TA-PEG-OCH 3-AuNPs dispersions pre-pared using different Au-to-ligand molar ratios.Single narrow peaks were measured for all four samples,indicating narrow size distributions in these dispersions (i.e.,unimodal distributions and absence of aggregate formation;see Figure 3C).Table 1shows the average values of the hydrodynamic diameter measured for these samples side-by-side with those extracted from TEM;only hydrodynamic diameters for the larger size are provided as weak scattering prevented the collection of reliable DLS data for smaller size NPs (2R TEM <5nm).A ratio R TEM /R H of ∼0.55was measured for the various samples.The larger hydrodynamic sizes were consistently measured for the various dispersions due to contributions from the ligand shell (TA-PEG-OCH 3)and hydro-dynamic interactions.37-39This confirms our previous DLS measurements applied to CdSe and CdSe-ZnS quantum dots,where the sizes extracted from DLS were also consistently larger than those derived from TEM;a ratio of 0.5was measured for those materials.39The difference between R TEM and R H cannot be simply accounted for by a geometrical superposition of the metallic core plus the TA-PEG-OCH 3ligands as discussed above.DLS and TEM were combined to understand the effects of adding extra free ligands following complete growth of the nanocrystals (e.g.,effects of extra passivation of the metal cores).TEM measurements showed that there were no differences between sizes measured for the as-prepared nanocrystals and for those subject to extra passivation.The intensity distribution data extracted from DLS showed single peaks,which indicates absence of potential etching or aggregation buildup after theaddition of extra free ligands (Figure 3D).However,we consis-tently observed a small increase in the measured hydrodynamic size following extra passivation (compare data in Figure 3C and D);the increase was more pronounced for the larger size nanocrystals.For example,the hydrodynamic diameter increased from 21nm for the sample prepared using a Au/ligand ratio of 2000:1to ∼25nm for the same one following extra passivation (see Table 1).In comparison,the diameter measured by TEM was ∼12nm for both samples (Table 1).We infer from this change that addition of extra ligands essentially increases the density of the surface ligands on the nanocrystals (resulting in a more homo-geneous surface coverage),which slightly changes the contribution of the hydrodynamic interactions to the measured size.The slightly larger increase in hydrodynamic diameter for larger size NPs (prepared using higher Au/ligand ratio)can be attributed to a lower ligand coverage of the nanocrystals during the initial growth.The ligand density is higher for their smaller size counterparts because higher molar concentrations of the ligands are used during the growth.Colloidal Stability Tests. 1.Stability to Excess Salts,pH Changes,and Refluxing at Higher Temperature.We first explored the effects of added excess NaCl on the colloidal stability of AuNPs 40prepared using the various ligands introduced above,namely,TA-PEG-OCH 3and TA (both are disulfide-terminated)along with HS-PEG-OCH 3and MHA;the last three constitute control samples.For consistent comparison,we used similar size nanoparticles.Figure 4A shows side-by-side images of ∼5nm AuNPs grown using TA,HS-PEG-OCH 3,and TA-PEG-OCH 3dispersed in deionized water with and without 2M NaCl;data were collected after 30min and 4days of storage.We found that aggregation buildup combined with a clear color transformation took place for TA-and MHA-capped NPs (data for MHA -AuNPs are not shown);the dispersions changed color from red to violet immediately after salt addition and precipitated within 1h.In comparison,dispersions of TA-PEG-OCH 3-AuNPs and HS-PEG-OCH 3-AuNPs were stable and aggregate-freeFigure 4.Images of AuNP dispersions under various conditions:(A)TA-PEG-OCH 3-AuNP dispersions without (-)and with (þ)2MNaCl after 30min and 4days at room temperature;(B)images of NPs subjected to refluxing at 100°C for 30min;and (C)NP dispersions mixed with 0.5M DTT at pH 8and kept at room temperature for 1day.(D)pH stability test of AuNPs synthesized with the various ligands after 9months of storage at room temperature.(40)Levy,R.;Thanh,N.T.K.;Doty,R.C.;Hussain,I.;Nichols,R.J.;Schiffrin,D.J.;Brust,M.;Fernig,D.G.J.Am.Chem.Soc.2004,126,10076–10084.。