噬菌体抗体淘筛方法
- 格式:doc
- 大小:103.00 KB
- 文档页数:8

噬菌体抗体库几种筛选方法的比较
王刚;刘玉峰;王琰;化冰
【期刊名称】《第四军医大学学报》
【年(卷),期】2001(22)16
【摘要】目的对多种不同的噬菌体抗体库筛选方法进行比较研究. 方法应用抗原固相化吸附筛选法、生物素化抗原液相筛选法和解离速率筛选法对半合成噬菌体抗体库或轻链替换库进行抗角蛋白噬菌体抗体的筛选,比较各自的筛选效率和优缺点. 结果 3种方法筛选抗角蛋白抗体均获成功,抗原固相化吸附筛选法效果可靠,所获抗体特异性高但抗原消耗量大;生物素化抗原液相筛选法方法敏感且节省抗原,可以根据实验目的和抗体库性质灵活调节筛选体系,其不足是筛选获得的克隆中容易出现非特异性结合的克隆;而解离速率筛选是选择性获得高亲和力抗体的有效手段. 结论不同的筛选策略各具优势,在实际应用中可根据不同的实验目的选择相应的筛选方法.【总页数】3页(P1482-1484)
【作者】王刚;刘玉峰;王琰;化冰
【作者单位】第四军医大学西京医院皮肤科,;第四军医大学西京医院皮肤科,;海军总医院中心实验科,;海军总医院中心实验科,
【正文语种】中文
【中图分类】R392.11
【相关文献】
1.两种不同噬菌体筛选方法所获的卫氏并殖吸虫抗原模拟表位抗原性比较 [J], 雷家慧;姜昌富;李天群
2.抗人B型钠尿肽噬菌体抗体库的构建及噬菌体抗体的筛选 [J], 刘世明;李民友;钟赟;吴楚财
3.噬菌体抗体库分类与筛选方法的研究 [J], 张媛;常思源
4.噬菌体抗体库筛选方法的研究进展 [J], 张青;郝晓柯;苏明权
5.从人源性噬菌体抗体库中筛选人抗HBsAg的Fab噬菌体抗体(摘要) [J], 王志毅;刘杞;万泽生;张定凤
因版权原因,仅展示原文概要,查看原文内容请购买。


抗体筛选鉴定推荐流程抗体筛选鉴定推荐流程:1.包被免疫管:将100ug 抗体加入到2mL PBS中并加入到免疫管中,4度过夜孵育。
2.封闭:将扩增和纯化噬菌体文库后的噬菌体500ul 加入到1mL 3% BSA中,室温旋转孵育2h。
同时往包被好的免疫管中加入2-3mL 3% BSA,室温旋转孵育2h。
3.抗原和噬菌体孵育:将封闭后的免疫管用含有0.01%吐温的PBS洗3次,每次5分钟。
将封闭后的噬菌体文库加入到封闭后的免疫管中,添加PBS直至2-3mL,室温旋转孵育1h。
4.清洗:将抗原和噬菌体孵育后的免疫管用含有0.01%吐温的PBS洗20次,每次5分钟。
5.洗脱:往免疫管中加入1mL 100mM Trimethymime,室温孵育10分钟,加入1M Tris-HCl中和Trimethymime,将最后1.5mL 的洗脱噬菌体转移到新的离心管中。
将洗脱的噬菌体按照扩增和纯化噬菌体文库扩增后再重复筛选过程2次,逐次减半包被免疫管的抗体量,得到3次筛选后的洗脱噬菌体。
6.ELISA鉴定:将上一步筛选得到的噬菌体稀释10^6倍后,取100ul加入到OD600为0.5的SS320菌液中,37度培养30分钟后涂布含有四环素和氨苄霉素的2X YT培养板上,37度过夜培养第二天得到单克隆菌落。
挑选96个单克隆菌落到含有四环素和氨苄霉素的2X YT培养液的96孔细胞培养板上,37度培养3-4小时后往培养孔中加入卡那霉素和20:1的辅助噬菌体,30度过夜培养。
第二天将过夜培养后的细胞液离心,获得上清液。
将过夜包被抗原和用3%BSA封闭过后的96孔ELISA板中加入上一步获得的噬菌体上清液,室温孵育1h。
用含有0.05%吐温的PBS清洗3次后,用噬菌体抗体抗体作为一抗,用相应的二抗TMB显色后在波长450读取每个孔的吸光值。
选取吸光值读数最高的SS320菌落送去测序,得到抗体的基因序列。
抗体筛选技巧:1. 合适量的抗原包被和抗原的分子量大小、疏水亲水性质、结构有关,也和包被缓冲液和包被介质的选择有关,合理的包被是成功筛选的基础,如果有必要可以进行预实验确定包被的条件。


这两个文库来自于单个人的V H和Vκ的基本结构,具有和那些已知结构的抗原结合位点的侧链多样性,并且在成熟的体系中中具有很高的多样性。
由这个框架来编码的标准结构是目前人抗体技术体系中最普通的。
在能够形成抗原结合表面的前提下重链的CDR3设计的尽可能短。
这两个文库都可以在未知筛选的克隆的序列的情况下进行筛选和免疫亲和力成熟。
这两个文库是噬菌粒/单链片段可变区格式的,并且都经过与蛋白A和蛋白L的结合筛选,所以未筛选的克隆的大多数都是具有功能的。
TomlinsonI构建在plT2载体((HIS myc 标签),多样化的侧链主要是来自于原始体系中多样性的位点(一共18个残基,H50, H52, H52a, H53, H55, H56, H58, H95, H96, H97, H98, L50, L53, L91, L92, L93, L94 and L96)此文库经过筛选后与体细胞突变的多样性的共同作用使其成熟。
我们保证上述信息在没有另外通知之前是严格可信的。
使用此文库之前请认真阅读以下材料确认收到以上材料2. 确认收到的文库未溶解,且使用之前-70摄氏度保存。
3. 参照方案G制备KM134. 在使用文库前清仔细阅读这个方案:在含有100 μg/ml氨苄青霉素和1 %葡萄糖的TYE平板上将对照划线培养,置于培养箱37℃过夜培养,挑单菌落置于摇床,接种在5ml含有100 μg/ml氨苄青霉素和1 %葡萄糖的2倍TY培养基内,37℃过夜培养。
分别制备阳性和阴性的噬菌体对照(第一天到第十天过夜用500微升)。
用阳性对照和阴性对照产生的噬菌体按照1:100的比例混合,进行一轮的筛选,用100 μg/ml的遍在蛋白在PBS中包被。
通过单克隆噬菌体ELISA检验遍在蛋白的富集程度(经过第一轮筛选后应该超过50%)5. 尽可能的用专用的移液管和一次性的手套,由于噬菌体会非特异的吸附到其他的塑料上,建议使用聚丙烯管。
6. 为提高感染效率,大肠杆菌应该在37℃培养至对数生长期(600nm的OD值应该在0.4)(1)、从一个小型的培养板上转移一个细菌克隆至5ml的2倍TY培养基(不加抗生素和葡萄糖),37℃震荡过夜培养(2)、次日稀释100倍至新鲜的2倍TY培养基内,震荡培养至培养至对数生长期600nm的OD值应该在0.4。
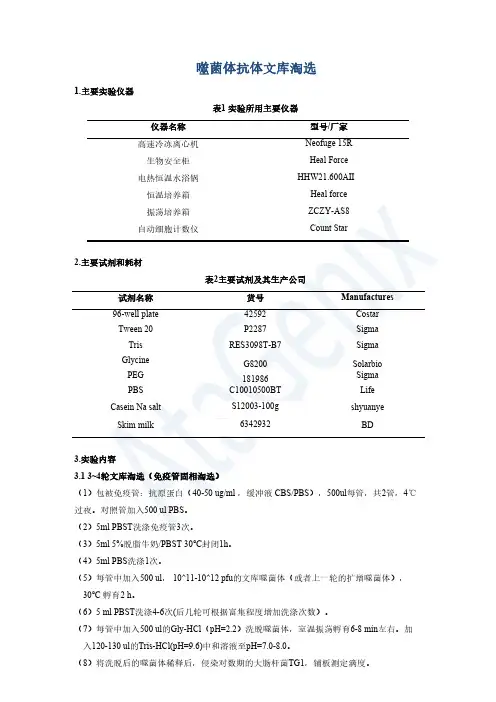
噬菌体抗体文库淘选1.主要实验仪器表1实验所用主要仪器仪器名称型号/厂家高速冷冻离心机Neofuge15R生物安全柜Heal Force电热恒温水浴锅HHW21.600AⅡ恒温培养箱Heal force(3)5ml5%脱脂牛奶/PBST30℃封闭1h。
(4)5ml PBS洗涤1次。
(5)每管中加入500ul,10^11-10^12pfu的文库噬菌体(或者上一轮的扩增噬菌体),30℃孵育2h。
(6)5ml PBST洗涤4-6次(后几轮可根据富集程度增加洗涤次数)。
(7)每管中加入500ul的Gly-HCl(pH=2.2)洗脱噬菌体,室温振荡孵育6-8min左右。
加入120-130ul的Tris-HCl(pH=9.6)中和溶液至pH=7.0-8.0。
(8)将洗脱后的噬菌体稀释后,侵染对数期的大肠杆菌TG1,铺板测定滴度。
3.2洗脱噬菌体的扩增(1)吸取洗脱后的噬菌体,加入到对数期的大肠杆菌TG1菌液中,37℃静置30min后,220rpm培养30min-1h。
(2)培养基中加入抗生素Amp,37℃,220rpm培养至菌液OD=0.4-0.6左右。
(3)在菌液中加入辅助噬菌体。
37℃静置30min后,220rpm培养45min-1h。
(4)3000-5000rpm离心菌液,弃上清。
用同等体积2YT-Amp-Kan培养基重悬菌体。
30℃,220rpm培养过夜。
(5)次日,8000rpm,4℃,20min离心菌液,将上清转入新的离心管中。
加入1/4体积的(4)用PBS稀释每一轮扩增后的噬菌体,稀释倍数3倍递增。
初始浓度为10^12pfu/ml。
每孔中加入100ul稀释后的扩增噬菌体。
30℃孵育1h,300ul PBST洗涤4-6次。
(5)加入100ul二抗(抗噬菌体M13)稀释液,30℃孵育1h,300ul PBST洗涤4-6次。
(6)加入100ul显色液TMB避光显色3-8min,加入100ul2M HCl终止反应,酶标仪读数(450nm-620nm)。

抗体库筛选技术介绍导读自从噬菌体展示技术于1985年创立以来,细胞生物学、免疫学、蛋白质工程以及医药行业等领域深受影响。
它从根本上了改变了传统的单抗制备流程(杂交瘤技间接术),宣告在体外改良抗体的特异性以及进行亲和力成熟。
随着该技术的不断发展,继而出现了核糖体展示、mRNA展示、细菌展示和酵母展示等多种展示技术.这篇文章主要以噬菌体展示抗体库为例,来介绍抗体库的筛选技术。
抗体库的筛选是指从抗体库中筛选出针对某一抗原的特异性抗体,是获得高亲和力抗体过程中的关键环节。
那什么是抗体库呢?通过PCR和DNA重组技术克隆人类或者动物体内全套抗体可变区基因(关于抗体的具体结构详见抗体的基本结构),并通过展示技术进行表达,得到的全套抗体基因表达文库即为抗体库.图1、抗体库克隆的抗体基因片段(SCFV)图2、噬菌体展示抗体库构建流程由于单抗性质的千差万别,抗体库的筛选需要根据不同的单抗制定严格的筛选条件,优化筛选方法,因此抗体库的筛选技术一直处于发展和改进的状态,根据出现时间的先后,主要分为经典筛选法和新型筛选法。
1、经典筛选法经典筛选法主要包括固相筛选法和液相筛选法,适合针对性质明确并且可纯化的抗原进行抗体筛选。
固相筛选法是通过包被在酶标板或者免疫试管等固相介质上的抗原富集高亲和性的噬菌体;液相筛选法是将生物素化的抗原包被在与亲和素偶联的磁珠或琼脂糖上,通过磁珠富集能与抗原特异性结合的噬菌体抗体,再通过洗涤、洗脱、回收等步骤。
如此反复筛选数次,可得到高亲和性的噬菌体.这两种方法可通过添加脱脂牛奶或者BSA来减少非特异性结合.2、新型筛选法对于抗原无法提纯或者性质不明确的情况(如癌细胞表面受体),或者经典筛选过程可能造成抗原失活的情况,需要开发新的筛选方法。
目前的新型筛选法主要有细胞筛选法、组织切片或体内筛选法、选择感染筛选法和蛋白质芯片筛选法等。
细胞筛选法:细胞筛选能维持抗原和抗体的天然构象,因此在对肿瘤细胞筛选方面应用较多,该技术还适合于细胞表面受体筛选和抗原鉴定等。

噬菌体抗体淘筛方法一、噬菌体展示技术原理噬菌体展示技术基于噬菌体颗粒表面的基因插入法。
通过将抗体片段的基因插入到噬菌体基因组中,使噬菌体能够在其表面展示抗体。
随后,将含有目标抗原的库经过一系列的筛选步骤,如淘洗、洗涤和分离,以筛选出与目标抗原特异性结合的抗体。
噬菌体核心蛋白质pIII等表面蛋白链通过基因插入的方法与外源基因连接,实现抗原展示。
二、噬菌体抗体淘筛方法的流程1.抗原制备:首先,需要制备目标抗原。
可以通过多种方法制备抗原,如重组蛋白表达系统、细胞和细胞溶解物、组织和分离物等。
2.抗体库构建:构建包含大量抗体片段的抗体库。
一般使用转录组或基因组DNA作为起始材料,使用PCR扩增抗体基因片段,并将其插入合适的载体中。
3.噬菌体包装:将构建好的抗体库与噬菌体粒子一起包装成噬菌体颗粒。
4.抗原吸附:将噬菌体抗体库与目标抗原进行反应,使抗体与抗原结合。
5.淘洗分离:用洗涤缓冲液对混合物进行混洗,以除去非特异性结合的噬菌体。
6.噬菌体放大:将经过淘洗分离的噬菌体在培养基中进行放大培养。
7.ELISA筛选:使用ELISA检测噬菌体是否与目标抗原的特异结合。
将阴性和阳性对照样品和待测样品加入到蛋白质包被的酶标板孔中,通过检测酶标物质的生成或反应物颜色的变化,判断噬菌体是否与目标抗原结合。
8.质粒DNA提取和测序:选择特异性结合抗原的噬菌体进行质粒DNA 提取和测序,以获取抗体的DNA序列。
9.后续鉴定和分析:鉴定筛选出的抗体的亲和力、特异性、敏感性等性质,以及进行进一步的功能分析。
三、噬菌体抗体淘筛方法的注意事项1.抗原的选择:选择合适的抗原非常关键,抗原应具有特异性且容易从培养基或生物样品中提取。
2.抗体库的构建:构建抗体库时,要确保插入的抗体片段多样性和覆盖性。
3.抗原吸附条件的优化:抗原吸附条件的优化对淘洗分离步骤的效果具有重要影响。
4.筛选条件的优化:在ELISA筛选过程中,需要对反应温度、时间、缓冲液浓度等条件进行优化,以提高筛选效果。

噬菌体抗体库筛选操作流程tomlinsonijtheLibrariesGeneral Introduction toOver the past 10 years Greg Winter’s lab at the MRC Laboratory of Molecular Biology and the MRC Centre for Protein Engineering (Cambridge, UK) has created a number of artificial libraries of antibodies that can be used to derive binders to almost any target molecule using phage display and selection. These binders can be used for all the same applications as conventional monoclonal antibodies (ELISA, Western blotting, FACS, immunohistochemistry etc) but can be isolated in a fraction of the time and without the need for animal immunisation. To date these so called “na?ve” or “single pot” phage-antibody libraries have been used successfully in hundreds of molecular biology labs world-wide to derive highly specific antibody reagents to a wide range of different proteins, peptides or small molecule compounds.The latest libraries (Tomlinson I and J) that are being distributed by the MRC HGMP Resource Centre each comprise over 100 million different scFv fragments cloned in an ampicillin resistant phagemid vector and transformed into TG1 E. Coli cells (scFv fragments comprise a single polypeptide with the VH and VL domains attached to one another by a flexible Glycine-Serine linker). By carefully following the protocol provided, large numbers of phagemids can be produced and used to select specific binders to target molecules that are attached to the surface of a tube or biotinylated and captured by streptavidin coated beads (so called “panning”). After each round of panning, the non-binders are washed away and the phagemids bound to the target molecule/s are eluted and amplified by infection into fresh TG1 cells. After producing new phagemids from the previous round of panning, the process can be repeated. Typically two or three rounds of panning are required to ensure that more than half the different scFvs in the selected population bind to the target molecule. The monoclonal scFvs can then be screened for binding (using a simple ELISA based protocol) and then used for further analysis of the target molecule. Since all the functional scFvs in the Tomlinson I and J libraries bind Proteins A and L, either of these secondary reagents can be used for detection, purification or immobilisation. Alternatively, secondary reagents that bind the attached myc or HIS6 tags can be used, although in our experience it is better to use the Protein A or L reagents.Finally, we would like to emphasise that these libraries represent a valuable resource. Whether you are familiar with phage display or not we recommend that you perform test selections and subsequent ELISA screening using the anti-bovine serum albumin and anti-bovine ubiquitin controls provided. Only when these experiments have been successfully carried out should you defrost the libraries and start preparing library phage.Derivation of librariesBoth libraries are based on a single human framework for V H (V3-23/DP-47 and J H 4b) and V κ (O12/O2/DPK9 and J κ1) with side chain diversity incorporated at positions in the antigen binding site that make contacts to antigen in known structures and are highly diverse in the mature repertoire. The canonical structure (V H : 1-3, V κ: 2-1-1) encoded by this framework is by far the most common in the human antibody repertoire. The CDR3 of the heavy chain was designed to be as short as possible yet still able to form an antigen binding surface. Both libraries can be selected and affinity matured without knowing the sequences of selected clones. Libraries are in phagemid/scFv format and have been pre-screened for binding to Protein A and Protein L so that the majority of clones in the unselected libraries are functional.Constructed in pIT2 (HIS myc tag). Diversified (DVT) side chains based mainly on those positions which are diverse in the primary repertoire (total of 18 residues - H50, H52, H52a, H53, H55, H56, H58, H95, H96, H97, H98, L50, L53, L91, L92, L93, L94 and L96). After selection, can be matured by incorporating additional diversity based on somatic mutation. Library size (with insert): 1.47 x 108 Percentage insert: 96%As above but with NNK side chains. Library size (with insert): 1.37 x 108 Percentage insert: 88%1. Check that you have received:a tube of Library I (~500 µl)a tube of Library J (~500 µl)a glycerol stock of a positive control anti-ubiquitin ScFv in bacterial strain TG1 (labeled TG1-antiubi)a glycerol stock of a positive control anti-BSA ScFv in bacterial strain TG1 (labeled 13CG2)a glycerol stock of T-phage resistant E. Coli. TG1 for propagation of phage (labeled TG1Tr)(lac-proAB) supE thi hsdD5/F' traD36 proA+B lacI q lacZM15)(K12a glycerol stock of E. Coli. HB2151 for expression of antibody fragmentsara ?(lac-proAB) thi/F' proA+B lacI q lacZ?M15)(K12KM133 (~100 µl with 107 pfu/ml)Phage2. Check the library is still frozen and make sure you keep it frozen at -70°C until needed.3. Make stock of KM13 according to Protocol G.4. Run through the protocols using the control clones before you use the library: Streak the controlson TYE plates containing 100 µg/ml ampicillin and 1 % glucose. After overnight growth at 37°C in an incubator pick a single colony from each and grow these overnight (shaking at 37°C) in 5 ml 2xTY1 containing 100 µg/ml ampicillin and 1 % glucose. Make phage for the positive and negative controls separately (use 500 µl of overnight in D1-D10). Use a 1:100 mixture of phage produced from the positive and the negative controls and perform one round of selection (C1-C11) using 100 µg/ml of ubiquitin2 in PBS for coating. Check for enrichment of ubiquitin binders (should be over 50% after one round of selection) by monoclonal phage ELISA (E9-E14).5. Wherever possible use devoted pipettes and disposable plastic ware. The use of polypropylenetubes is recommended as phage may adsorb non-specifically to other plastics.6. For efficient infection of phage, E. coli must be grown at 37°C and be in log phase (OD at 600 nmof 0.4). To prepare this:i. Transfer a bacterial colony from a minimal media plate into 5 ml of 2xTY medium (no antibioticsor glucose). Grow shaking overnight at 37°C.ii. Next day dilute overnight 1:100 into fresh 2xTY medium. Grow shaking at 37°C until OD 600 is0.4 (1.5-2 hrs)7. All centrifugations, except those performed in a micro centrifuge, are performed at 4°C.8.Libraries I and J must be used separately and preferably in parallel. This will ensureselecting the most antigen binding clones.In advanceGather all equipment and reagents (product details are given in the notes at the end of all the protocols). Make sure you have all the necessary media and plates for bacterial growth (the large Bio-Assay plates need to be air-dried in a sterile environment for 2 hrs before use).Plan your time - most of the daily procedures can be performed simultaneously, so read through each protocol carefully before starting.StepsProcedureDay 1 (5 hrs) A1-A6 Grow libraries I and J and make phageB1-B3 Make secondary stock of librariesDay 2 (6 hrs) A7-A12 Grow libraries I and J and make phage (cont.)C1 Coat immunotubes for 1st round of selectionDay 3 (6.5 hrs) C2-C11 1st round of selectionDay 4 (3 hrs) D1-D6 Make phage from 1st round of selectionC1 Coat immunotubes for 2nd round of selectionDay 5 (6.5 hrs) D7-D11 Make phage from 1st round of selection (cont.)C2-C11 2nd round of selectionDay 6 (3 hrs) D1-D6 Make phage from 2nd round of selectionC1 Coat immunotubes for 3nd round of selectionDay 7 (6.5 hrs) D7-D11 Make phage from 2nd round of selection (cont.)C2-C11 3rd round of selectionDay 8 (3 hrs) D1-D6 Make phage from 3rd round of selectionE1 Coat 96 well plate for polyclonal phage ELISADay 9 (6.5 hrs) D7-D11 Make phage from 3rd round of selection (cont.)E2-E8 Polyclonal phage ELISAFurther characterisation of individual clones can be performed by monoclonal phage ELISA (protocol E), monoclonal ELISA using soluble scFv fragments (protocol F), PCR screening (to check for insert, protocol H) and sequencing (protocol I).1. Add the library stock to 200 ml pre-warmed 2xTY containing 100 µg/ml ampicillin and 1 %glucose.2. Grow shaking at 37°C until the OD 600 is 0.4 (1-2 hrs).3. Take 50 ml of this and add 2x1011 KM13 helper phage3. (Use the remaining 150 ml to make asecondary bacterial stock of the library by following protocol B).4. Incubate without shaking in a 37°C water bath for 30 min.5. Spin at 3,000 g for 10 min (3,600 rpm in Centra 8 or equivalent). Resuspend in 100 ml of 2xTYcontaining 100 µg/ml ampicillin, 50 µg/ml kanamycin and 0.1% glucose.6. Incubate shaking at 30°C overnight.7. Spin the overnight culture at 3,300 g for 30 min (4,000 rpm in Centra 8 or equivalent).8. Add 20 ml PEG/NaCl (20 % Polyethylene glycol 6000, 2.5 M NaCl)to 80 ml supernatant. Mix welland leave for 1 hr on ice.9. Spin 3,300 g for 30 min (4,000 rpm in Centra 8 or equivalent). Pour away PEG/NaCl. Respinbriefly and aspirate any remaining dregs of PEG/NaCl.10 Resuspend the pellet in 4 ml PBS and spin at 11,600 g for 10 min in a micro centrifuge to removeany remaining bacterial debris.11. Store the phage at 4°C for short term storage or in PBS with 15 % glycerol for longer termstorage at -70°C.12. To titre the phage stock dilute 1µl phage in 100µl PBS, 1µl of this in 100µl PBS and so on untilthere are 6 dilutions in total. Add 900µl of TG1 at an OD 600 of 0.4 to each tube and incubate at 37°C in a waterbath for 30 mins. Spot 10 µl of each dilution on a TYE5 plate containing 100 µg/ml ampicillin and 1 % glucose and grow overnight at 37°C. Phage stock should be 1012-1013/ml, enough for at least 10 selections.1. Grow the remaining 150 ml from A3 for a further 2 hr shaking at 37°C.2. Spin down the cells at 10,800 g for 10 min. Resuspend in 10 ml of 2xTY containing 15 % glycerol.3. Store this secondary stock in 20x 500 µl aliquots at -70°C. Use one aliquot for each phagepreparation according to protocol A - this will only be necessary if you wish to do more than 10 selections.(Alternatively, phage can be selected using biotinylated antigen in solution or affinity chromatography. For details see Winter et al. (1994) Annu. Rev. Immunol.12, 433)immunotube6 overnight with 4 ml of the required antigen. The efficiency of coating can1. Coatdepend on the antigen concentration, the buffer and the temperature. Usually 10-100 µg/mlantigen in PBS is used.2. Next day wash tube 3 times with PBS (pour into the tube and then pour it immediately out again).3. Fill tube to brim with 2 % MPBS (2 % Marvel milk powder7 in PBS). Incubate at rt. Standing onthe bench for 2 hr to block.4. Wash tube 3 times with PBS.1012 to 1013 phage from A11 in 4 ml of 2 % MPBS. Incubate for 60 min at rt. rotating using 5. Addan under-and-over turntable and then stand for a further 60 min at rt. Throw away supernatant.6. Wash tubes 10 (round 1)-20 (rounds 2 and 3) times with PBS containing 0.1 % Tween 20.7. Shake out the excess PBS and elute phage by adding 500 µl of trypsin-PBS (50 µl of 10mg/mltrypsin stock solution8 added to 450 µl PBS) and rotating for 10 min at rt using an under-and-over turntable.8. Take 1.75 ml of TG1 at an OD 600 of 0.4 and add 250 µl of the eluted phage (the remaining 250µl should be stored at 4°C). Incubate for 30 min at 37°C in a water bath without shaking.µl, 10 µl of a 1:102 dilution and 10 µl of a 1:104 dilution on TYE plates containing 1009. Spot10µg/ml ampicillin and 1 % glucose and grow overnight at 37°C to titre the phage.10. In round 1 if using a complex antigen (eg cells, cell lysates etc): take the remaining TG1 cultureand spin at 11,600 g in a micro centrifuge for 5 min. Resuspend the pelleted bacteria in 1 ml of 2xTY and plate on a large square Bio-Assay dish9 containing TYE, 100 µg/ml ampicillin and 1 % glucose.In round 1 if using a single hapten, carbohydrate or protein antigen and in subsequent rounds for all antigens: take the remaining TG1 culture and spin at 11,600 g in a micro centrifuge for 5 min.Resuspend the pelleted bacteria in 50 µl of 2xTY and plate on a regular TYE plate containing 100 µg/ml ampicillin and 1 % glucose.11. Grow plates at 37°C overnight.The first round of selection is the most important. Any errors made at this point will be amplified in subsequent rounds of selection. After each round you should get back at least 100 infective phage. If you get less than this it is probable that a mistake has occurred. If so, try repeating the infection with a freshly grown TG1 culture (from a new overnight) at an OD 600 of 0.4 using the remaining 250 µl of eluted phage from C7. If this still yields less than 100 phage, repeat the selection starting at C1.1. After overnight growth add 7 ml of 2xTY 15 % glycerol to the large square Bio-Assay dish or 2mlsto the regular plates and loosen the cells with a glass spreader, mixing them thoroughly. After inoculating 50 µl of the scraped bacteria to 50 ml of 2xTY containing 100 µg/ml ampicillin and 1 % glucose, store 1 ml of the remaining bacteria at -70°C in 15% glycerol.2. Grow shaking at 37°C until the OD 600 is 0.4 (1-2 hrs).3. Take 10 ml of this culture and add 5x1010 helper phage.4 Incubate without shaking in a 37°C water bath for 30 min.5. Spin at 3,000 g for 10 min. Resuspend in 50 ml of 2xTY containing 100 µg/ml ampicillin, 50 µg/mlkanamycin and 0.1% glucose.6. Incubate shaking at 30°C overnight.7. Spin the overnight culture at 3,300 g for 15 min.8. Add 10 ml PEG/NaCl (20 % Polyethylene glycol 6000, 2.5 M NaCl)to 40 ml supernatant. Mix welland leave for 1 hr on ice.9. Spin 3,300 g for 30 min. Pour away PEG/NaCl. Respin briefly and aspirate any remaining dregsof PEG/NaCl.10. Resuspend the pellet in 2 ml PBS and spin at 11,600 g for 10 min in a micro centrifuge to removethe remaining bacterial debris.11. Use 1 ml of this phage for the next round of selection (protocols C and D) and store 1 ml at 4°C.12. Repeat selection for another 2 rounds.Populations of phage produced at each round of selection can be screened for binding by ELISA to identify "polyclonal" phage antibodies. Phage from single colonies can then be screened by ELISA to identify "monoclonal" phage antibodies. Alternatively, after a polyclonal phage ELISA you could proceed directly to making monoclonal soluble antibody fragments, see protocol F. In general, we have found that 2% Marvel in PBS is best for blocking during phage ELISA whereas 3% BSA in PBS is best for blocking during scFv ELISA.1. Coat a 96 well flexible assay plate10 overnight with 100 µl per well of antigen in the same bufferand at the same concentration as used for selection.2. Wash wells 3 times with PBS. Plates can be immersed in a shallow bath containing PBS but youshould check that all wells fill with wash solution (if they do not you may create false positivesduring later washes). Discard liquid by flipping plate over and then shaking it. Add 200 µl per well of 2 % MPBS (2 % Marvel in PBS) or 3% BSA-PBS (3% bovine serum albumin in PBS) to block and incubate for 2 hr at rt.3. Wash wells 3 times with PBS. Add 10 µl PEG precipitated phage from the end of each round ofselection in 100 µl 2 % MPBS (or 3 % BSA-PBS).4. Incubate for 1 hr at rt. Discard phage solution and wash 3 times with PBS-0.1 % Tween 20.5. Add 1 in 5000 dilution of HRP-anti-M1311 in 2 % MPBS (or 3 % BSA-PBS). Incubate for 1 hr atrt., wash 3 times with PBS-0.1 % Tween 20.µl of substrate solution (100 µg/ml TMB12 in 100 mM sodium acetate, pH 6.0. with10 µl 1006. Addof 30 % hydrogen peroxide added to 50 ml of this solution directly before use) to each well and leave at rt. for 2-15 min. A blue colour should develop.7. Stop the reaction by adding 50 µl 1 M sulphuric acid. The blue colour should turn yellow.9. Inoculate individual colonies from the titration plates from each round of selection (see C11) into100 µl 2xTY containing 100 µg/ml ampicillin and 1 % glucose in 96 cell-well plates13. Growshaking (250 rpm) overnight at 37°C.10. Use a 96 well transfer device14 to transfer a small inoculum (about 2 µl) from this plate to asecond 96 cell-well plate containing 200 µl of 2xTY with 100 µg/ml ampicillin and 1 % glucose per well. Grow shaking (250 rpm) at 37°C for 2 hr. (Make glycerol stocks of the original 96-well plate, by adding glycerol to a final concentration of 15 %, and then storing the plates at -70°C).11. After 2 hr incubation (of the second plate) add 25 µl 2xTY containing 100 µg/ml ampicillin, 1 %glucose and 109 helper phage.12. Shake (250 rpm) at 37°C for 1 hr. Spin 1,800 g for 10 min, then aspirate off the supernatant.13. Resuspend pellet in 200 µl 2xTY containing 100 µg/ml ampicillin and 50 µg/ml kanamycin. Growshaking (250 rpm) overnight at 30°C.14. Spin at 1,800 g for 10 min and use 50 µl of the supernatant in phage ELISA as detailed above.Individual colonies picked from the various rounds of selection (as plated on the dilution series) can be induced in TG-1 to produce soluble scFv (F2-F6). This will ensure the expression of all selected clones including those in which the scFvs contain TAG stop codons (TG-1 as able to suppress termination and introduce a glutamate residue at these positions). Unfortunately, since the TAG stop codon between the scFv and the gIII gene is also suppressed this leads to co-expression of the scFv-pIII fusion which tends to lower the overall levels of scFv expression, even in clones where there are no TAG stop codons in the scFv itself. To circumvent this problem, the selected phage can be used to infect HB2151 (a non-suppresor strain) which is then induced to give soluble expression of antibody fragments (scFv genes that do not contain TAG stop codons will now yield higher levels of soluble scFv than in TG-1, but those that contain TAG stop codons will not produce any soluble scFv) (F1-F6). The expressed scFvs can then be used in ELISA. Detection of bound scFv can be performed using either Protein A-HRP15 or Protein L-HRP16 conjugates.1. From each selection take 10 µl of eluted phage and infect 200 µl exponentially growing HB2151bacteria (OD 600 of 0.4) for 30 min at 37°C in a water bath. Plate 50 µl, 50 µl of a 1:102 dilution,50 µl of a 1:104 dilution and 50 µl of a 1:106 dilution on TYE plates containing 100 µg/mlampicillin and 1 % glucose and grow overnight at 37°C.2. Pick individual colonies into 100 µl 2xTY 100 µg/ml ampicillin and 1 % glucose in 96 cell-wellplates and grow shaking (250 rpm) overnight at 37°C./doc/d1ca22bb960590c69ec376eb.html e a 96 well transfer device14 to transfer a small inocula (about 2 µl) from this plate to a second96 cell-well plate containing 200 µl 2xTY containing 100 µg/ml ampicillin and 0.1 % glucose perwell. Grow shaking (250 rpm) at 37°C until the OD 600 is approximately 0.9 (about 3 hr). (A stock can be made of the first plate, by adding glycerol to a final concentration of 15 % and storing at -70°C).4. Once OD 0.9 is reached (wells look quite cloudy) add 25 µl 2xTY containing 100 µg/ml ampicillinand 9 mM IPTG (isopropyl β-D-thiogalactoside, final concentration 1 mM IPTG). Continueshaking (250 rpm) at 30°C overnight.5. Coat a 96 well flexible assay plate overnight with 100 µl per well of antigen in the same buffer andat the same concentration as used for selection.6. Spin the plate from step F4 at 1,800 g for 10 min and use 50 µl of the supernatant (take care notto transfer any bacteria) for ELISA in 3% BSA-PBS (final concentration) (protocol E) except usinga 1:5000 dilution of Protein A-HRP15 or Protein L-HRP16 to detect binding in step E5.µl TG1 at an OD 600 of 0.4 with 10 µl of 100-fold serial dilutions of KM13 helper 2001. Infectphage3 (in order to get well separated plaques) in a 37°C water bath (without shaking) for 30 min.Add to 3 ml molten H-top agar (42°C) and pour onto warm TYE plates (no antibiotics). Allow to set and incubate overnight at 37°C.2. Pick a small plaque into 5 ml of fresh TG1 at an OD 600 of 0.4. Grow for about 2 hr shaking at37°C.3. Add to 500 ml 2xTY in a 2 litre flask and grow shaking at 37°C for 1 hr. Add kanamycin to a finalconcentration of 50 µg/ml (no glucose). Grow overnight shaking at 30°C.4. Spin overnight culture at 10,800 g for 15 min. Add 100 ml PEG/NaCl (20 % polyethylene glycol6000, 2.5 M NaCl) to 400 ml supernatent and leave for 1 hr on ice.5. Spin 10,800 g for 30 min. Pour away PEG/NaCl.6. Resuspend the pellet in 8 ml PBS and add 2 ml PEG/NaCl. Mix well and leave for 20 minutes onice.7. Spin 3,300 g for 30 min. Respin briefly and aspirate any remaining dregs of PEG/NaCl.8. Resuspend pellet in 5 ml PBS and spin at 11,600 g for 10 min in a micro centrifuge to remove anyremaining bacterial debris.9. Store the helper phage at 4°C for short term storage or in PBS with 15 % glycerol for longer termstorage at -70°C.10. To titre the helper phage take 45µl phage and add 5µl trypsin stock solution. Incubate for 30 minsat 37 °C. Dilute 1µl of trypsin treated phage in 1ml PBS and make five 100 fold serial dilutions of this in 1ml aliquots of PBS. Add 50µl of the six dilutions to six separate tube containing 1ml of TG1 at an OD 600 of 0.4. Mix, add 3 ml molten H-Top and pour evenly onto TYE plates.Perform the same dilution series using 1µl of non-trypsin treated phage. The titre of the trypsin treated phage should be 105-108 lower than for the non-trypsin treated phage. If not, pickanother plaque and repeat helper phage preparation.Once the libraries have been selected you may wish to check individual clones for the presence of full length VH and Vκinsert. All PCRs are at annealing temperature of 55°C. 1 min extension for V H or Vκon their own, 2 min extension for V H and Vκ together.For V H only use LMB3: CAG GAA ACA GCT ATG AClink seq new: CGA CCC GCC ACC GCC GCT Gwith insert = 527 bpwithout insert = 227 bpFor Vκ only use DPK9 FR1 seq: CAT CTG TAG GAG ACA GAG TCpHEN seq: CTA TGC GGC CCC ATT CAwith insert = 368 bpwithout insert = no bandFor V H and Vκ use LMB3: CAG GAA ACA GCT ATG ACpHEN seq: CTA TGC GGC CCC ATT CAwith insert = 935 bpwithout insert = 329 bpFor sequencing of selected clones the following primers are recommended.For V H use link seq new CGA CCC GCC ACC GCC GCT GFor Vκ use pHEN seq CTA TGC GGC CCC ATT CAXhoI NotIRBS CAGGAAACAGCTATGACCATGATTACGCCAAGCTTGCATGCAAATTCTATTTCAAGGAGACAGTCATA ATG AAA TAC CTA ----------------> M K Y L LMB3SfiI __NcoI__TTG CCT ACG GCA GCC GCT GGA TTG TTA TTA CTC GCG GCC CAG CCG GCC ATG GCC GAG GTG TTT L P T A A A G L L L L A A Q P A M A E V F XhoI linkerGAC TAC TGG GGC CAG GGA ACC CTG GTC ACC GTC TCG AGC GGT GGA GGC GGT TCA GGC GGA GGTD Y W G Q G T L V T V S S G G G G S G G GSalI NotI HIS-tag GGC AGC GGC GGT GGC GGG TCG ACG GAC ATC CAG ATG ACC CAG GCG GCC GCA CAT CAT CAT CACG S G G G G S T D I Q M T Q A A A H H H H<------------------------link seq newmyc-tagCAT CAC GGG GCC GCA GAA CAA AAA CTC ATC TCA GAA GAG GAT CTG AAT GGG GCC GCA TAG ACTH H G A A E Q K L I S E E D L N G A A * T<---------------------pHEN seq1. 2xTY is 16g Tryptone, 10g Yeast Extract and 5g NaCl in 1 litre.2. Bovine ubiquitin (5 mg) is available from Fluka, Chemika-BioChemika, Industriestrasse 25, CH-9470 Buchs, Switzerland, Tel +41 81 755 2511, Fax +41 81 756 5449.3. KM13 is the protease cleavable helper phage described i n Kristensen and Winter, Folding andDesign3, 321-328 (1998).4. PBS is5.84 g NaCl, 4.72 g Na2HPO4 and 2.64 g NaH2PO4.2H20, pH 7.2, in 1 litre.5. TYE is 15g Bacto-Agar, 8g NaCl, 10g Tryptone, 5g Yeast Extract in 1 litre.6. Nunc Maxisorp immuno test tubes (Cat. No. 4-44202) are available from Gibco BRL, LifeTechnologies Ltd., P. O. Box 35, Trident House, Washington Road, Paisley, PA3 4EF, Scotland, U.K, Tel +44 141 814 6100, Fax +44 141 887 1167.7. 'Marvel' is dried skimmed milk powder.8. Trypsin (T-1426 Type XIII from Bovine Pancreas - Sigma Chemical Company Ltd., Fancy Rd.,Dorset, BH17 7NH, U.K, Tel +44 1202 733114; Fax +44 1202 715460) made up in 50mM Tris-HCl pH7.4, 1mM CaCl2 and stored at -20°C9. Nunc Bio-Assay dish is available from Gibco BRL (see note 6).10. Falcon MicroTest III flexible 96 well flat bottomed assay plates are available from BectonDickinson Labware, Becton Dickinson and Co., 2 Bridgewater Lane, Lincoln Park, NJ 07035,USA.11. HRP-anti-M13 is available from Amersham International plc, Amersham Place, Little Chalfont,Buckinghamshire, HP7 9NA, UK. Tel: +44 01494 544000; Fax: +44 01494 542929.12. TMB is 3,3',5,5'-tetramethylbenzidine and is available from Sigma (see Note 8). A 10 mg/ml stocksolution can be made by dissolving the TMB in DMSO.13. Corning 'Cell Wells' flat-bottomed multiple well tissue culture treated plates are available fromCorning Glass Works, Corning N.Y. 14831. USA.14. The 96 well transfer device is a piece of wood the size of a microtitre plate with a handle on oneside and 96 metal pins (each 7 cm long with a concave end) on the other. This can be sterilised between bacterial transfer by immersion in a bath of ethanol and then by flaming (hold well away from your body and any flamable objects). If you haven't got one of these (or something similar) you will have to make one yourself or alternatively use a multichannel pipette for bacterialtransfer.15. Horse Radish Peroxidase conjugated Protein A is available from Amersham International plc (seenote 11)14. Horse Radish Peroxidase conjugated Protein L is available from Actigen Ltd, 5 Signet Court,Swanns Road, Cambridge, CB5 8LA, UK. Tel: +44 01223 319101; Fax: +44 01233 316443.。

用不同方法从大容量噬菌体抗体库中筛选抗完整食管癌细胞的
单链抗
噬菌体抗体库是一种非常强大的生物技术工具,可以用于筛选出针对特定疾病、特定抗原的单链抗体。
在搜索抗完整食管癌细胞的单链抗体时,可以采用以下几种方法。
第一种方法是基于表面展示技术的筛选。
此方法利用噬菌体表面的展示蛋白,将特定单链抗体的DNA序列插入其中,并让
噬菌体体表面展示这种单链抗体分子。
接下来,将经过筛选的噬菌体与完整食管癌细胞接触,将其在体外培养。
通过重复筛选和挑选,可以得到经过筛选的却对完整食管癌细胞具有很强抗体效果的单链抗体。
第二种方法是利用串联抗体的筛选。
此方法先将多个单链抗体连接在一起,形成一个可变区域长的串联单链抗体,再将其插入噬菌体表面展示蛋白中。
同样的,将经过重复筛选和挑选,可以得到抗完整食管癌细胞效果很好的串联单链抗体。
第三种方法是利用功能筛选法。
此方法首先需要将完整食管癌细胞切成小碎片,然后使用这些碎片投放到噬菌体抗体库中,让这些碎片吸附在噬菌体表面,以模拟完整食管癌细胞在人体内的生存环境。
接下来,通过加入某些化合物或特定条件,可以筛选出对完整食管癌细胞有特异性的单链抗体。
综上所述,虽然每种筛选方法都各有优缺点,但我们可以选择最适合的筛选方法,以此从大容量的噬菌体抗体库中筛选出一种高效抗完整食管癌细胞的单链抗体,以提高食管癌治疗效果。

亲和层析法用于噬菌体抗体库的筛选陈瑛炜,罗文新,王明桥,王晋,李利峰,袁权,张军,夏宁邵(福建省医学分子病毒学研究中心,厦门大学细胞生物学与肿瘤细胞工程教育部重点实验室,厦门 361005)摘要:本研究报道一种基于固定化金属亲和层析(IMAC )的噬菌体抗体库液相筛选方法。
将纯化的带有His 标签的抗原与噬菌体抗体库混合,噬菌体抗体与抗原充分结合后再加入亲和介质,使噬菌体抗体抗原复合物通过His 标签与介质结合,然后通过充分洗涤去除非特异性噬菌体抗体,最后将特异性噬菌体抗体洗脱下来,感染TG 1,进行下一轮筛选。
整个筛选过程中抗原与抗体的结合在液相中完成,不仅消除了固相介质对抗原表位的影响,也更有利于噬菌体抗体与抗原的充分作用。
将此方法应用于HEV N E2蛋白特异性人源噬菌体抗体的筛选,抗原竞争EL ISA ,阳性血清阻断,可溶性单链抗体表达检测及测序结果表明,最终获得2个特异性人源抗体。
关键词:噬菌体抗体库;固定化金属亲和层析;液相筛选中图分类号:R373;Q78 文献标识码:A 文章编号:1000-8721(2006)01-0044-06收稿日期:2005-04-27;修回日期:2005-07-12基金项目:福建省科技重大项目(编号:2002F013);国家十五创新药物博士基金(编号:2003AA2Z3539);福建省自然科学基金(编号:C0310005);厦门市科技计划项目(编号:3502Z20055002)。
作者简介:陈瑛炜(1982-),女,硕士,从事基因工程抗体研究。
通讯作者:罗文新,副教授,Tel 186259222184113;Fax :86259222181258;E 2mail :wxluo @jingxian 1xmu 1edu 1cn 噬菌体抗体库技术已经成为目前获得人源抗体的主要途径之一。
从大容量天然人源噬菌体抗体库中,理论上可以获得针对任何抗原的人源单抗。
噬菌体抗体库筛选方法包括纯抗原筛选,非纯抗原筛选,功能性筛选,选择性感染筛选等[1]。
从噬菌体展示随机肽库中淘选多肽药物Selection of Peptide drugs from Phage-displayedRandom Peptide Library生命科学学院99级沈抒殚摘要以凝血酶为靶分子,利用噬菌体展示和亲和淘选技术,从随机十五肽库中筛选到结合凝血酶的3个特异结合肽克隆,并对其中结合活性最强的短肽进行了序列测定。
ELISA鉴定结果表明,3个克隆对于凝血酶都有一定的结合能力,且都与凝血酶的天然抑制剂水蛭素产生竞争。
关键词:噬菌体展示,随机肽库,淘选,凝血酶AbstractThrombin as target molecule, and with the new bio-technique of phage display and biopanning, 3 special binding peptide clones were selected out from a phage-displayed random 15-peptide library. The sequence of the clone, which had the highest affinity with thrombin, has been determined. Data of ELISA showed that all the three clones could be combined to thrombin to some extent. And all of them could compete with hirudin, which is the natural inhibitor of thrombin.Key words: phage display, random peptide library, biopanning, thrombin一、前言噬菌体展示随机多肽库噬菌体展示随机多肽库技术是以噬菌体展示技术(phage display)为基础的。
综述・讲座噬菌体抗体库筛选技术及在肿瘤研究中的应用薛国柱, 吕勇刚, 窦科峰第四军医大学西京医院肝胆外科,陕西西安710032Selection technology of phage antibody library and its application in tumor re searchX U E Guo2z hu,L U・・Yong2gang,DOU Ke2f engDepartment of Hepato2biliary S urgery,Xi jing Hospital,Fourth Military Medical University,Xi’an710032,P.R.China【摘要】 噬菌体抗体是指表达在噬菌体表面的抗体分子Fab或scFv,这些分子的群体称为抗体库。
经特定抗原或细胞筛选后可以获得针对该抗原或细胞表面标志物的特异性抗体。
经典的筛选方法为固相或液相抗原识别,前提条件是能得到抗原纯品,对于抗原无法提纯或抗原性质不确定的情况则不能适用,所以又出现全细胞筛选,直接利用肿瘤细胞或组织细胞进行筛选,无需纯抗原,但目标抗原一定要有较高的表达水平,对于表达较低的情况,一种策略是先利用正常细胞筛选,去掉无关抗原后,再利用肿瘤细胞筛选;另一种策略是细胞内化筛选,特异性抗体被内化入细胞,然后用酸性洗脱液洗脱掉细胞膜上非特异结合的噬菌体抗体后裂解细胞,获得特异性抗体。
其余筛选方法还有切片组织筛选、体内筛选等,适用面较窄,未获推广。
筛选效率主要通过以下几项的检测:转化数,即被感染的噬菌体数,它随筛选轮数的增加而增加;抗体基因插入载体的频率,高比例的丢失意味着筛选的低效率;抗体亲和性。
抗体库技术避免了杂交瘤技术中的许多难点,如细胞融合、动物免疫等,目前该技术已逐渐在肿瘤诊断和肿瘤治疗中发挥重要作用。
肿瘤防治杂志,2005,12(23):1829-1832[ABSTRACT] Phage antibody means those Fab or scFv, which display in the membrane of phages.The repertoires of these antibodies are antibody library.Biopinned by antigen or cell,relevant antibody can be got.Classical biopanning tech2 nique is antigen(solidified or liquefied)recognition by purified antigen,when the purified antigen can not be obtained,the classical technique can not be applied.So f ull cell biopanning has appeared,which needs not purify antigen,but the target antigen must be highly expressed,otherwise,some strategy can be used.One is wiping off the irrespective antigen by nor2 mal cells,then tumor cell antigen is relatively high,another strategy is internalizing biopanning:special antibody is inter2 nalized by certain technique,then the irrelative antibody is washed off,special antibody is got by splitting the cells.Oth2 er techniques such as in tissue sections selection and in vivo selection were narrowly applied.The biopanning efficiency is detected by following aspects:transforming unit,enhanced with the round of biopanning;the f requency of gene f using, high deletion of antibody gene meaning low efficiency;the af2 finity of antibody.The antibody library technique can avoid many problems of hybridoma such as cell f usion and animal immunity.It has been used in tumor diagnosis and treatment.Chin J Cancer Prev T reat,2005,12(23):1829-1832【关键词】 肿瘤/免疫学;噬菌体/免疫学;抗体,细菌;综述文献[KE YWOR DS] neoplasms/immunology;bacteriophages/immunology;antibodies,bacterial;review literature 【中图分类号】 R730.3 【文献标识码】 A 【文章编号】 1009-4571(2005)23-1829-04 噬菌体抗体是指表达在噬菌体表面的抗体分子Fas或单链抗体ScFv。
噬菌体库的筛选原理
噬菌体库(phage library)是一种用于筛选特定目标的噬菌体(phage)的方法。
其筛选原理如下:
1. 构建噬菌体库:从已知的DNA或RNA样品中,随机将目标序列片段插入噬菌体的基因组中,并将这些基因组片段包裹在噬菌体的外壳中。
构建好的噬菌体库含有许多噬菌体,每个噬菌体携带着不同的目标序列片段。
2. 筛选目标:将噬菌体库与特定靶点进行反应,例如与靶蛋白质结合。
未结合的噬菌体可以被洗去,而与靶点结合的噬菌体则保留下来。
3. 提取目标噬菌体:将与靶点结合的噬菌体进行扩增,使其数量增加。
通常使用大肠杆菌等宿主细胞进行扩增。
4. 再次筛选:将扩增后的目标噬菌体再次与靶点进行反应和洗脱,以进一步筛选出特异性更高的噬菌体。
5. 验证筛选结果:对所得的单个目标噬菌体进行验证,确认其与靶点的结合能力和特异性。
噬菌体库筛选原理的核心是利用噬菌体随机插入外源基因的特点,通过与目标分子结合的能力将其筛选出来。
这种方法被广泛应用于蛋白质相互作用、抗体产生
和蛋白质工程等领域。
噬菌体筛选过程
噬菌体筛选过程是指通过一系列实验方法和结果的分析,找出具有高效寄生能力和选择性的噬菌体。
该过程通常包括以下几个步骤: 1. 菌株的选择:首先需要选择一个目标菌株,并确定其生长条件和培养基。
这些条件应该能够满足噬菌体的生长和寄生需求。
2. 噬菌体的初步筛选:在满足菌株生长条件的基础上,使用不同的噬菌体悬液进行初步筛选。
评价指标通常包括噬菌效率、寄生速度和选择性等。
3. 噬菌体的进一步筛选:根据初步筛选结果,选择效果较好的噬菌体进行进一步筛选。
该阶段通常包括寄生速度、选择性、感染范围等指标的评估。
4. 噬菌体的鉴定和纯化:在得到优良噬菌体后,需要进行鉴定和纯化工作,以保证筛选得到的噬菌体纯度和有效性。
5. 噬菌体的应用:最后,筛选得到的噬菌体可以应用在医疗、农业等领域,例如治疗细菌感染、控制农业有害菌等。
- 1 -。
噬菌斑分析1、将合适的宿主菌接种到M9TB培养基上,37℃摇至0D600=1.0;2、使用前,可将宿主菌保存在4℃,保存超过48h的菌不可使用;3、熔化足够量的顶层培养基,每个平板需5ml,将熔化后的培养基放在45-55℃的水浴锅中;4、使用无菌LB培养基制备一系列稀释度的样品。
通常选择103-106的重组噬菌体稀释度。
空白对照则选择107;10-210-310-710μL样品100μL …………990μL 900μL5、准备4mL的无菌管,加入250μL的宿主菌。
从最高稀释度开始加起,每管加入100μL噬菌体稀释液。
注意换枪头;6、在每管中加入3mL的顶层培养基,混合后倒在无抗LB/LB carb+/LB carb+amp+平板上,轻摇使其迅速铺平;7、平板静置几分钟后,翻转,37℃培养3-4h或室温过夜;8、数噬菌斑数并计算噬菌体滴度。
如:稀释度为10-6的平板上有200个噬菌斑,则滴度为200×106×10=2×109pfu/mL样品中噬菌体总数由滴度×样品总量决定。
如:1μg的载体DNA被包装,最终的包装反应体积为0.3mL(25μL包装提取物+5μL连接物+270μL无菌培养基)。
在包装反应中pfu总数为2×109pfu/mL×0,3mL=6×108pfu。
另外,因为使用了1μg的载体,效率是6×108pfu。
文库扩增当原始重组体总数少于5×106,选择平板裂解法;当需要大量扩增时,选择液体裂解法。
平板裂解扩增1、接种1mL过夜培养的宿主菌到50mL TB培养基,37℃摇至OD600=0.6-1.0,如果使用5615菌,当OD600=0.5时,添加IPTG至终浓度1mM,然后继续摇30min;2、如果包装反应保存在氯仿里,轻微离心分离出氯仿,将噬菌体吸出;3、计算需要的细胞和噬菌体量。
每10mL细胞含1×106,将噬菌体和细胞混合,加入50mL培养基,这些混合物需在20min内倒入平板。
这两个文库来自于单个人的V H和Vκ的基本结构,具有和那些已知结构的抗原结合位点的侧链多样性,并且在成熟的体系中中具有很高的多样性。
由这个框架来编码的标准结构是目前人抗体技术体系中最普通的。
在能够形成抗原结合表面的前提下重链的CDR3设计的尽可能短。
这两个文库都可以在未知筛选的克隆的序列的情况下进行筛选和免疫亲和力成熟。
这两个文库是噬菌粒/单链片段可变区格式的,并且都经过与蛋白A和蛋白L的结合筛选,所以未筛选的克隆的大多数都是具有功能的。
TomlinsonI构建在plT2载体((HIS myc 标签),多样化的侧链主要是来自于原始体系中多样性的位点(一共18个残基,H50, H52, H52a, H53, H55, H56, H58, H95, H96, H97, H98, L50, L53, L91, L92, L93, L94 and L96)此文库经过筛选后与体细胞突变的多样性的共同作用使其成熟。
我们保证上述信息在没有另外通知之前是严格可信的。
使用此文库之前请认真阅读以下材料确认收到以上材料2. 确认收到的文库未溶解,且使用之前-70摄氏度保存。
3. 参照方案G制备KM134. 在使用文库前清仔细阅读这个方案:在含有100 μg/ml氨苄青霉素和1 %葡萄糖的TYE平板上将对照划线培养,置于培养箱37℃过夜培养,挑单菌落置于摇床,接种在5ml含有100 μg/ml氨苄青霉素和1 %葡萄糖的2倍TY培养基内,37℃过夜培养。
分别制备阳性和阴性的噬菌体对照(第一天到第十天过夜用500微升)。
用阳性对照和阴性对照产生的噬菌体按照1:100的比例混合,进行一轮的筛选,用100 μg/ml的遍在蛋白在PBS中包被。
通过单克隆噬菌体ELISA检验遍在蛋白的富集程度(经过第一轮筛选后应该超过50%)5. 尽可能的用专用的移液管和一次性的手套,由于噬菌体会非特异的吸附到其他的塑料上,建议使用聚丙烯管。
6. 为提高感染效率,大肠杆菌应该在37℃培养至对数生长期(600nm的OD值应该在0.4)(1)、从一个小型的培养板上转移一个细菌克隆至5ml的2倍TY培养基(不加抗生素和葡萄糖),37℃震荡过夜培养(2)、次日稀释100倍至新鲜的2倍TY培养基内,震荡培养至培养至对数生长期600nm的OD值应该在0.4。
(1.5-2小时)7. 所有的离心,除了在微型离心机中操作的之外,都是在4℃下进行。
8.两个文库最好是同时使用,以保证筛选得到最多的抗原结合克隆。
In advance准备好用到的仪器和试剂(详见方案后注解),确保有足够的用于细菌增殖的培养基(大的生物分析的培养皿在使用前进行灭菌和干燥处理)在实验之前仔细阅读这个方案,以便安排你的时间同时进行操作Steps ProcedureDay 1 (5 hrs) A1-A6 Grow libraries I and J and make phageB1-B3 Make secondary stock of libraries第一天A1-A6 增殖文库I和J,并且制备噬菌体B1和B3 制作文库的第二原种Day 2 (6 hrs) A7-A12 Grow libraries I and J and make phage (cont.)C1 Coat immunotubes for 1st round of selection第二天A7-A12 增殖文库I和J,并且制备噬菌体C1 包被第一轮筛选用免疫管Day 3 (6.5 hrs) C2-C11 1st round of selection第三天C2-C11 第一轮筛选Day 4 (3 hrs) D1-D6 Make phage from 1st round of selectionC1 Coat immunotubes for 2nd round of selection第四天D1-D6 从第一轮筛选的结果中制备噬菌体C1 包被第二轮筛选用免疫管Day 5 (6.5 hrs) D7-D11 Make phage from 1st round of selection (cont.)C2-C11 2nd round of selection第五天D7-D11 从第一轮筛选的结果中制备噬菌体(继续)C2-C11 第二轮筛选Day 6 (3 hrs) D1-D6 Make phage from 2nd round of selectionC1 Coat immunotubes for 3nd round of selection第六天D1-D6 从第二轮筛选的结果中制备噬菌体(继续)C1 包被第二轮筛选用免疫管Day 7 (6.5 hrs) D7-D11 Make phage from 2nd round of selection (cont.)C2-C11 3rd round of selection第七天D7-D11 从第二轮筛选的结果中制备噬菌体(继续)C2-C11 第三轮筛选Day 8 (3 hrs) D1-D6 Make phage from 3rd round of selectionE1 Coat 96 well plate for polyclonal phage ELISA第八天D1-D6 从第三轮筛选的结果中制备噬菌体E1 包被多克隆噬菌体ELISA用的96孔板Day 9 (6.5 hrs) D7-D11 Make phage from 3rd round of selection (cont.)E2-E8 Polyclonal phage ELISA第九天D7-D11 从第三轮筛选的结果中制备噬菌体(继续)E2-E8 多克隆噬菌体ELISA每个克隆的进一步的描述可以通过单克隆噬菌体ELISA(方案E),单克隆噬菌体ELISA用水溶性的scFv 片段(方案F)。
PCR检测(检测插入,方案H)和测序(方案I)1. 将文库的加入到200ml预热的含有100 μg/ml氨苄青霉素和1 %葡萄糖的2xTY培养基上2. 37°C振荡培养至OD 600为0.43. 从中取50ml,加入2x1011 KM13辅助噬菌体(剩余的150ml在方案B中用来制备文库的第二细菌储存液)4. 37°C水中温浴30分钟5. 3000g,离心10min,用100ml含有100 μg/ml氨苄青霉素、50 μg/ml的卡那霉素和1 %葡萄糖的2xTY培养基6. 30度振荡过夜培养7. 将过夜产物在3300g离心30分钟8. 取上层液体80ml,加入20mlPEG/NACL(含20 % PEG,2.5 M NaCl)溶液9. 3300g离心30分钟,倒掉PEG/NACL溶液,重新离心,吸去剩余的PEG/NACL溶液10 用4mlPBS缓冲液悬浮沉淀,11600g离心10分钟,去掉任何剩余的细菌残渣11. 短期使用的话可以保存在4℃,长期保存的话置于含有15%甘油的PBS中,-70℃保存。
12. 将噬菌体稀释100倍,然后继续稀释至6个浓度,加入900μl OD 600 为0.4的TG1至每一个管中,37°C水浴30min,取每个稀释浓度的10μl加入到TYE培养基上,其中包含100 μg/ml氨苄青霉素和1 %葡萄糖,37摄氏度过夜培养。
浓度在1012-1013/ml,至少可以进行10次筛选1.将A3步骤中剩余的150ml液体,37摄氏度下振荡培养2小时2、10800g离心10分钟,用10ml含有15%甘油的2xTY悬浮3、每管500微升,分装20管,保存于-70℃。
每次制备的时候用其中一管。
这个仅限于你打算做超过10次筛选的时候。
或者,噬菌体可以用生物素酰化的抗原或者亲和层析来完成1、用目的抗原4ml过夜来包被免疫管。
包被的效率取决于抗原的浓度,温度和缓冲液,通常用抗原浓度10-100 μg/ml的PBS缓冲液。
2. 第二天用PBS缓冲液洗三次(倒入管内,然后马上倒出来)3. 用含有2%的脱脂奶粉的磷酸缓冲液注满管子,室温下孵化,置于工作台上两个小时候停止4. 用PBS缓冲液洗管3次5. 将1012- 1013噬菌体键入到4ml的含有2%的脱脂奶粉的磷酸缓冲液。
室温下旋转培养孵育60分钟,然后静止培养60分钟,弃掉上清液6. 用含有0.1%的吐温20的磷酸缓冲液洗管子10-20遍7. 甩干多余的PBS缓冲液,用含有50 μl of 10mg/ml胰蛋白酶的磷酸缓冲液500微升稀释噬菌体,用可翻滚的摇床室温培养10min.8. 取1.5mlOD值为0.4的大肠杆菌TG1,加入250 μl稀释后的噬菌体(剩余的4°C保存),37℃水浴30min..9. 吸取液体,及稀释100倍的,和稀释10000倍的溶液各10微升,加到含有100 μg/ml氨苄青霉素、和1 %葡萄糖的TYE培养基上,37度过夜培养来测定噬菌体10、第一轮筛选时如果用复合抗原,取剩余的TG1培养物,11600g离心5min。
将底部的细菌用1ml的2xTY,涂于大的圆形的Bio-Assay dish,包含100 μg/ml氨苄青霉素、和1 %葡萄糖的TYE培养基上。
如果用的是单个的半抗原,糖类或者蛋白质抗原及所有抗原的下面的步骤:取剩余的TG1培养物,11600g离心5min。
将底部的细菌用1ml的2xTY,涂于大的圆形的Bio-Assay dish,包含100 μg/ml 氨苄青霉素、和1 %葡萄糖的TYE培养基上。
11. 37°C过夜培养第一轮的筛选是最重要的。
任何的错误都会导致以后的错误。
每轮结束后最少能够得到100个火星的噬菌体,如果少于这个数量,说明可能出现了错误。
所以,试着用新鲜的TG1感染剩余的250ml噬菌体,如果这一步仍然少于100噬菌体,从C1开始重复筛选。
1. 过夜增殖以后,加7ml含有15%甘油的2 xTY到large square Bio-Assay dish或者加2ml到常规的培养皿,然后用玻璃涂布器松散细胞,彻底混匀。
将得到的50 μl细菌接种到50 ml包含100 μg/ml 氨苄青霉素、和1 %葡萄糖的2xTY中。
剩余的1ml保存在15%的甘油中,-70°C保存。
2. 振荡培养至OD600为0.43. 取10ml培养物,加入5x1010辅助噬菌体4 37°C水浴30min5. 3000g离心10分钟。
用含有50 ml包含100 μg/ml氨苄青霉素、和0.1 %葡萄糖的2xTY悬浮6. 30°C振荡培养过夜7. 将过夜产物3300g离心10分钟8. 加入10ml PEG/NaCl (20 % PEG 6000, 2.5 M NaCl)至40ml上清液。