CNOOC Appendix.doc
- 格式:doc
- 大小:247.50 KB
- 文档页数:6

目录网站首页登陆 (1)一、学生工作室首页及功能模块 (2)1.1学生工作室首页 (2)1.2模块拆解说明 (3)二、课程学习模块 (5)2.1我的课程 (5)2.2毕业论文 (10)2.3网考辅导 (12)2.4学位英语 (12)三、事务办理 (14)3.1选课 (14)3.2课程办理 (15)3.3学籍异动 (18)3.4毕业生登记表、学位申请表 (19)四、综合查询 (20)五、应用模块 (21)5.1个人信息维护 (21)5.2学习日历的使用 (22)5.3其他应用 (23)六、视频答疑和答辩系统应用 (24)6.1 登录 (24)6.2 文字交互 (26)网站首页登陆打开浏览器,在地址栏内输入中国石油大学(北京)远程教育学院网址:http://,在左上方“教学平台登录”窗口输入自己的用户名(学号)及密码。
学生在第一次缴费注册后得到登录平台的学号和初始密码,请在第一次登录后及时修改密码。
注:浏览器尽量使用IE、360、遨游,以避免造成无法登陆。
1一、学生工作室首页及功能模块1.1学生工作室首页新学生工作室首页新学生工作室首页包括五大功能模块区,即功能导航区、温馨提示区、课程学习区、个人信息区、功能辅助区。
功 能 导 航 区温 馨 提 示 区课 程 学 习 区个人 信息区 功 能 辅 助 区1.2模块拆解说明1.2.1功能导航区按功能不同分为课程学习、事务办理、综合查询、特色资源四个大项、十六个小项,用于满足同学们日常网络学习中可能遇到的各项需求。
后面的章节将对每项功能进行详细讲解。
1.2.2温馨提示区温馨提示区包括学生学习过程中的所有环节的提示,提示会在相应的时间内由系统统一发出。
以本科生为例,提示区包括:选课提示—作业提示—考试提示—统考提示—论文提示等;学生可随时查看自己的未完成操作。
如是否未做作业,是否未选课等。
便于学生们更加直观地了解自己的学习动态,更主动地进行学习。
31.2.3课程学习区本模块分在学课程、已修课程、未修课程三个部分,分别显示当前学习课程、已经完成学习课程、教学计划中未修课程。

国开电大 C++编程实践:第七课——打造用户管理功能1. 课程概述本课程将指导您如何使用C++编程语言打造一个用户管理功能。
通过本课程的学习,您将能够理解并实现用户注册、登录、查看个人信息、修改密码等基本功能。
2. 课程准备在开始本课程之前,建议您已完成国开电大C++编程的前六的课程,并具备一定的C++编程基础。
3. 课程内容3.1 用户管理功能需求分析在开始编写代码之前,我们需要对用户管理功能进行需求分析。
主要包括以下几个方面:- 用户注册:新用户可以创建一个新的账户,需要提供用户名、密码、邮箱等基本信息。
- 用户登录:已注册的用户可以通过用户名和密码登录系统。
- 查看个人信息:用户可以查看自己的基本信息,如用户名、密码、邮箱等。
- 修改密码:用户可以修改自己的密码。
3.2 数据结构设计为了实现用户管理功能,我们需要设计一个合适的数据结构来存储用户信息。
在这里,我们可以使用结构体(struct)来定义一个用户(User)类,其中包括用户名(username)、密码(password)、邮箱(email)等基本信息。
struct User {std::string username;std::string password;std::string email;};3.3 用户注册功能实现用户注册功能的实现主要包括以下几个步骤:1. 创建一个空的用户列表,用于存储注册的用户信息。
2. 提示用户输入用户名、密码、邮箱等基本信息。
3. 将用户信息添加到用户列表中。
void registerUser(std::vector<User>& users) {std::string username, password, email;std::cout << "请输入用户名:" << std::endl;std::cin >> username;std::cout << "请输入密码:" << std::endl;std::cin >> password;std::cout << "请输入邮箱:" << std::endl;std::cin >> email;User newUser = {username, password, email};users.push_back(newUser);}3.4 用户登录功能实现用户登录功能的实现主要包括以下几个步骤:1. 创建一个空的用户列表,用于存储已注册的用户信息。

深海教育网校使用说明V How long is forever? Who can tell me网校使用手册V3.0目录网校使用手册一、简介本手册包含学员使用时涉及到的所有操作;包含学员注册、登录、购买课程、学习课程等步骤;以及需要注册的地方.请学员在使用前认证阅读;注册后;请首先前往个人信息处设置自己的“VIP”信息;以方便网校为你推荐适合的课程..二、基础操作:1.学员登录点击右上方登录按钮;输入账号以及密码;点击登录按钮;即可完成登录.“手机号码”学员报名时填写的手机号码.“密码”学员报名手机号码后6位.“登录”以上信息填写好之后;点击登录按钮;即可完成登录.“忘记密码”学员忘记密码时;可点击登录按钮下方的“忘记密码”进行密码找回.2.找回密码学员点击登录按钮后;可点击下方的“忘记密码”进行密码找回.“手机号”学员注册时使用的手机号码.“验证码”学员根据图示填写即可;可点击”换一张”更换新的验证码.“短信验证”点击后方的免费获取验证码 ;接收短信验证码.“下一步”点击下一步按钮;进入重置密码页面.“新密码”填写新密码.学员自行设置.“确认密码”再次输入新密码;确保两次密码输入一致.“确定”以上信息填写完成之后;点击确定按钮;即可完成密码重置.三、学员使用学员登陆后;可将鼠标悬浮于右上角自己的手机号码或昵称;下方会出现包含“个人中心”“个人设置”“退出”三项内容的下拉菜单.“个人中心”个人中心中包含对于课程的所有设置;可在个人中心进行上课;查看自己购买课程的订单;还可以在此处找到自己收藏的课程;查看自己记录的笔记等.“个人设置”学员可在此处设置自己的昵称;昵称会在右上方进行显示;并且会在直播课程进行显示.还可以完善自己的真是信息;方便网校为你推荐适合的课程.“退出”退出登录.1.个人中心个人中心包含“我的课程”“我的订单”“我的收藏”“我的笔记”“我的题库”“我的问答”以及“我的消息”;下面会分别介绍以上功能的使用.“我的课程”学员购买的所有课程;都会保存在“我的课程”内.点击课程封面;会进入到课程详情页面;可通过此进行上课.“我的订单”学员购买课程的订单记录;都会保存在“我的订单”内;对于没有支付的课程;也可在此处找到;并且继续支付.“我的收藏”学员在网校页面收藏的课程;都会保存在“我的收藏”内;学员可通过此处快速找到自己收藏的课程进行购买.“我的笔记”学员观看录播课程所记录的笔记;都会在此处显示.并且可以直接点击;定位到填写笔记时的视频处.“我的题库”学员的做题记录;都会在“我的题库”内.“我的消息”学员收到的站内信;全部在“我的消息”内;学员也可点击右上方的小信封图标;快速进入到”我的信息” .2.个人设置个人设置包含“账号信息”“VIP信息”“设置头像”“修改密码”;下面会分别介绍以上功能.“账号信息”学员可在此处设置昵称;邮箱;签名.昵称会显示在直播课的学员列表内.“VIP信息”学员的真是信息;学员需如实填写;以便网校为你推荐适合的课程.“设置头像”学员的头像;可自行设置.“修改密码”学员可通过此功能进行密码的重置.3.学员上课在个人中心-我的课程在”我的课程”内点击某一课程的封面;会跳转到课程详情页面.“二维码”课程名称后方;使用微信扫码;即可通过手机观看此教学视频.“分享”可将课程分享到微信;以及微博.“课程进度”课程进度会根据你学习的多少按照百分比进行显示.“课程目录”点击课程目录之后;点击具体课程进行上课.录播课录播视频界面如下“章节”点击章节;视频界面右侧会出现所有课程的章节;学员可以快速切换视频课程;并且可以一览自己学习的进度.后方圆形;空则未学习过.半圆则学习了部分.全满则已学习完.“笔记”学员可在看录播课程时;点击笔记按钮;输入自己需要记录的笔记;并点击完成按钮;学员可通过笔记上方的时间按钮快速把视频调节回到记录笔记的时间点.“评论”通过评论对课程进行评价;仅可评价一次;可修改.“下载”点击下方的下载按钮;可以下载相关的课程资料.。

LINUX应用编程函数自学手册(学生用模板)国嵌编著嵌入式LINUX培训专用版权申明该资料版权归属成都国嵌信息技术有限公司(简称“国嵌”)所有, 并保留一切权力。
非经国嵌同意(书面形式),任何单位和个人不得擅自摘录本手册部分或全部,违者我们将追究其法律责任。
目录第1类时间编程类 (5)1.1 获取日历时间 (5)1.2 获取格林威治时间 (5)1.3 获取本地时间 (6)1.4 以字符串方式显示时间 (6)1.5 获取高精度时间 (6)第2类系统调用文件编程类 (8)2.1 打开文件 (8)2.2 创建文件 (8)2.3 关闭文件 (9)2.4 读文件 (9)2.5 写文件 (10)2.6 定位文件 (10)2.7 复制文件描述符 (11)第3类库函数文件编程类 (12)3.1 打开文件 (12)3.2 关闭文件 (12)3.3 读文件 (13)3.4 写文件 (13)3.5 定位文件 (14)第4类多进程编程类 (15)4.1 创建进程 (15)4.2 创建进程 (15)4.3 进程等待 (16)4.4 执行程序 (16)第5类管道通讯编程类 (17)5.1 创建无名管道 (17)5.2 创建有名管道 (17)5.3 删除有名管道 (18)第6类信号通讯编程类 (19)6.1 发送信号 (19)6.2 处理信号 (19)第8类信号量编程类 (20)8.1 创建/打开信号量集合 (20)8.2 操作信号量 (20)第1类时间编程类1.1 获取日历时间1.1.1 函数名1.1.2 函数原形1.1.3 函数功能1.1.4 所属头文件1.1.5 返回值1.1.6 参数说明1.2 获取格林威治时间1.2.1 函数名1.2.2 函数原形1.2.3 函数功能1.2.4 所属头文件1.2.5 返回值1.2.6 参数说明1.3 获取本地时间1.3.1 函数名1.3.2 函数原形1.3.3 函数功能1.3.4 所属头文件1.3.5 返回值1.3.6 参数说明1.4 以字符串方式显示时间1.4.1 函数名1.4.2 函数原形1.4.3 函数功能1.4.4 所属头文件1.4.5 返回值1.4.6 参数说明1.5 获取高精度时间1.5.1 函数名1.5.3 函数功能1.5.4 所属头文件1.5.5 返回值1.5.6 参数说明第2类系统调用文件编程类2.1 打开文件2.1.1 函数名2.1.3 函数功能2.1.4 所属头文件2.1.5 返回值2.1.6 参数说明2.2 创建文件2.1.1 函数名2.1.2 函数原形2.1.3 函数功能2.1.4 所属头文件2.1.5 返回值2.1.6 参数说明2.3 关闭文件2.3.1 函数名2.3.2 函数原形2.3.3 函数功能2.3.4 所属头文件2.3.5 返回值2.4 读文件2.4.1 函数名2.4.2 函数原形2.4.3 函数功能2.4.4 所属头文件2.4.5 返回值2.4.6 参数说明2.5 写文件2.5.1 函数名2.5.2 函数原形2.5.3 函数功能2.5.4 所属头文件2.5.5 返回值2.5.6 参数说明2.6 定位文件2.6.1 函数名2.6.2 函数原形2.6.3 函数功能2.6.4 所属头文件2.6.5 返回值2.6.6 参数说明2.7 复制文件描述符2.7.1 函数名2.7.2 函数原形2.7.3 函数功能2.7.4 所属头文件2.7.5 返回值2.7.6 参数说明第3类库函数文件编程类3.1 打开文件3.1.2 函数原形3.1.3 函数功能3.1.4 所属头文件3.1.5 返回值3.1.6 参数说明3.2 关闭文件3.2.1 函数名3.2.2 函数原形3.2.3 函数功能3.2.4 所属头文件3.2.5 返回值3.2.6 参数说明3.3 读文件3.3.1 函数名3.3.2 函数原形3.3.3 函数功能3.3.4 所属头文件3.3.5 返回值3.3.6 参数说明3.4 写文件3.4.1 函数名3.4.2 函数原形3.4.3 函数功能3.4.4 所属头文件3.4.5 返回值3.4.6 参数说明3.5 定位文件3.5.1 函数名3.5.2 函数原形3.5.3 函数功能3.5.4 所属头文件3.5.5 返回值3.5.6 参数说明第4类多进程编程类4.1 创建进程4.1.1 函数名4.1.2 函数原形4.1.3 函数功能4.1.4 所属头文件4.1.5 返回值4.1.6 参数说明4.2 创建进程4.2.1 函数名4.2.2 函数原形4.2.3 函数功能4.2.4 所属头文件4.2.5 返回值4.2.6 参数说明4.3 进程等待4.3.1 函数名4.3.2 函数原形4.3.3 函数功能4.3.4 所属头文件4.3.5 返回值4.3.6 参数说明4.4 执行程序4.4.1 函数名4.4.2 函数原形4.4.3 函数功能4.4.4 所属头文件4.4.5 返回值4.4.6 参数说明第5类管道通讯编程类5.1 创建无名管道5.1.1 函数名5.1.2 函数原形5.1.3 函数功能5.1.4 所属头文件5.1.5 返回值5.1.6 参数说明5.2 创建有名管道5.2.1 函数名5.2.2 函数原形5.2.3 函数功能5.2.4 所属头文件5.2.5 返回值5.2.6 参数说明5.3 删除有名管道5.3.1 函数名5.3.2 函数原形5.3.3 函数功能5.3.4 所属头文件5.3.5 返回值5.3.6 参数说明第6类信号通讯编程类6.1 发送信号6.1.1 函数名6.1.2 函数原形6.1.3 函数功能6.1.4 所属头文件6.1.5 返回值6.1.6 参数说明6.2 处理信号6.2.1 函数名6.2.2 函数原形6.2.3 函数功能6.2.4 所属头文件6.2.5 返回值6.2.6 参数说明第8类信号量编程类8.1 创建/打开信号量集合8.1.1 函数名8.1.2 函数原形8.1.3 函数功能8.1.4 所属头文件8.1.5 返回值8.1.6 参数说明8.1.7 范例代码8.2 操作信号量8.2.1 函数名8.2.2 函数原形8.2.3 函数功能8.2.4 所属头文件8.2.5 返回值8.2.6 参数说明. .。

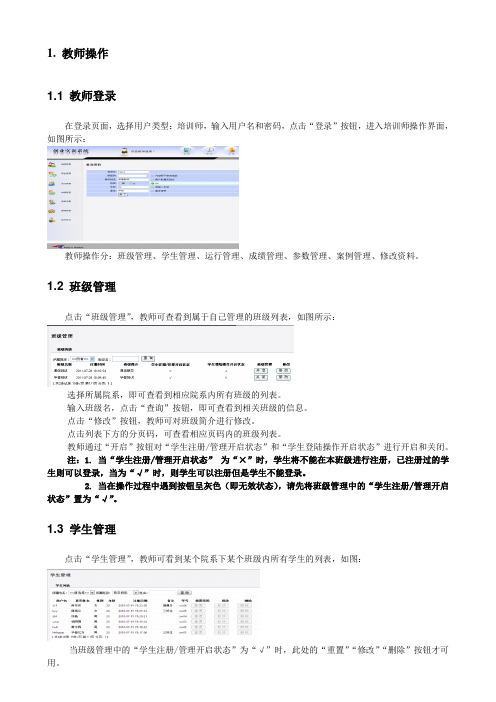
1. 教师操作1.1 教师登录在登录页面,选择用户类型:培训师,输入用户名和密码,点击“登录”按钮,进入培训师操作界面,如图所示:教师操作分:班级管理、学生管理、运行管理、成绩管理、参数管理、案例管理、修改资料。
1.2 班级管理点击“班级管理”,教师可查看到属于自己管理的班级列表,如图所示:选择所属院系,即可查看到相应院系内所有班级的列表。
输入班级名,点击“查询”按钮,即可查看到相关班级的信息。
点击“修改”按钮,教师可对班级简介进行修改。
点击列表下方的分页码,可查看相应页码内的班级列表。
教师通过“开启”按钮对“学生注册/管理开启状态”和“学生登陆操作开启状态”进行开启和关闭。
注:1. 当“学生注册/管理开启状态”为“×”时,学生将不能在本班级进行注册,已注册过的学生则可以登录,当为“√”时,则学生可以注册但是学生不能登录。
2. 当在操作过程中遇到按钮呈灰色(即无效状态),请先将班级管理中的“学生注册/管理开启状态”置为“√”。
1.3 学生管理点击“学生管理”,教师可看到某个院系下某个班级内所有学生的列表,如图:当班级管理中的“学生注册/管理开启状态”为“√”时,此处的“重置”“修改”“删除”按钮才可用。
设置查询条件,点击“查询”按钮,可查看相关的学生列表。
点击“重置”按钮,可重置学生密码,系统默认重置后的密码为“stu123”。
点击“修改”按钮,可修改相关信息。
点击“删除”按钮,教师可删除学生。
点击列表下方的分页码,可查看到相应分页中的学生列表。
1.4 运行管理点击“运行管理”,系统自动显示运行管理中的企业核心管理,如图:运行管理操作分:企业经营管理、创业测评、创立企业。
1.4.1 企业经营管理点击“企业经营实训”图标,如图所示:企业经营管理操作分:轮次管理和破产设置。
1.点击“轮次管理”,教师即可查看所带班级所有行业的轮次开始状况,如图:选择“选择班级”,可查看相应班级的轮次状况。
选择“请选择行业”,可查看相应行业的轮次状况。
中国海洋大学本科生课程大纲课程属性:工作技能,课程性质:选修一、课程介绍1.课程描述:本课程在高等数学、概率统计等数理课程的基础上,结合环境科学专业的特点与需求,面向环境科学、环境工程及相近专业的本科生讲授环境数据分析课程,使学生掌握环境数据分析的基本原理,重点讲述环境数据的处理与分析技术,讲解不同统计分析方法的特点与应用情景,掌握各种分析方法的软件实现及结果解读,为今后从事环境科学研究和环境事业奠定定量分析能力的基础。
课程有着较强的实践性,有利于培养学生独立分析和解决问题的能力,养成实事求是、严肃认真的科学态度,以及敢于创新的开拓精神。
学生应具备数理统计、计算机应用、环境生物学与环境化学等基本专业知识和基本技能。
2.设计思路:环境系统中存在许多不确定性现象,并且有大量的数据需要进行统计分析和处理,对数据分析的理论和方法提出了更高的要求。
在自然、社会与环境关系的基础上,用统计方法对环境问题予以量化描述和分析已成为环境研究的迫切需要。
本课程通过阐述统计假设与检验、方差分析与试验设计、环境一元线性回归分析、环境多元线性回归分析、环境系统聚类分析、环境判别分析、环境主成分分析、环境- 6 -因子分析、环境空间统计分析、数据可视化技术的基本原理,重点结合具体应用,通过分析案例,培养学生利用数理统计方法处理或解决环境中的不确定性问题,寻找变量之间的定量关系、从数据中发现环境趋势、探索环境系统变化规律的能力。
3. 课程与其他课程的关系:先修课程:概率统计,并行课程数据处理应用。
二、课程目标掌握环境统计的基本原理、方法、模型,学会对环境统计量进行量化分析与建模,掌握使用专业软件对环境实验数据进行处理、转化与分析的方法,培养学生综合运用所学知识解决实际问题的能力及创新意识。
通过本课程的学习,可以提高学生数据分析能力,有助于培养分析解决问题的创新意识,为今后从事科研工作以及深造打下坚固的基础。
三、学习要求掌握统计假设与检验、方差分析与试验设计、环境一元线性回归分析、环境多元线性回归分析、环境系统聚类分析、环境判别分析、环境主成分分析、环境因子分析、环境空间统计分析、数据可视化技术的基本原理,会使用相应的软件工具完成数据分析并解释基本结果。
BOSS管理员使用手册北京邮电大学计算机科学与技术学院目录目录 (1)1. 引言 (2)1.1.概述 (2)1.2.功能简介 (2)1.3.系统特点 (2)1.4.系统要求(待定) (2)1.5.常用术语 (2)1.6.参考资料 (4)2. 系统概述 (4)2.1.系统结构 (5)2.2.系统管理 (5)2.2.1. 部门管理 (6)2.2.2. 用户管理 (8)2.2.3. 角色管理 (1)2.2.4. 功能管理 (7)2.2.5. 动作管理 (9)2.2.6. 日志管理 (11)2.2.7. 在线统计 (16)1.引言1.1.概述为了实现加电网络的智能化运作和高度集中管理,为用户提供便捷的迅速的服务,确保加电站运营商的事业管理职能,必须建立电动汽车加电站网络运营管理系统,提供客户信息管理、车辆信息管理、电池信息管理、加电站信息管理、电池配送管理等功能。
进而规范业务运营流程、提高业务运营效率、节省业务运营成本、完善职能决策,帮助运营商实现集约化管理。
在每个加电站内部,为了提供流畅高效的充换电服务,必须保障站内各种设备的正常运行,站内信息管理必不可少。
加电站站内信息管理系统是对加电站内部所有设备运行信息进行监控管理的系统,能够及时发现设备故障,保障网络的运行效率。
1.2.功能简介该系统由以下6个模块构成:系统管理:负责对部门、用户和系统权限进行管理;电池信息管理:运营业务管理:车辆信息管理:客户信息管理:电池配送管理:1.3.系统特点系统采用B/S架构,服务器端采用开源J2EE服务器JBoss,数据库服务器采用SqlServer2000。
方便用户通过web使用系统。
1.4.系统要求(待定)1.5.常用术语加电站:配送中心:充电桩:充电桩集群:电池:针对城市电动车专用的电池组,不同的型号对应不同的车辆。
客户:在普天海油开户,享受相关服务的实体,分为单位客户、个人客户,单位客户下可以增加子客户,车辆入网时选择的车主必须是在网客户;电池计划:当运营了一段时间后,每个站点仓库就能基本确定自己每天库存电池的型号及其额定数量,来保证客户服务和每天的运营。
APPENDIX 4. TOXICOLOGICAL DATA FOR CLASS 1 SOLVENTS 2BENZENE23Category: Human carcinogen (IARC 1)4Not teratogenic56Toxic Effects:7Benzene causes central nervous system depression and destroys bone marrow, leading to8injury in the hematopoietic system.910Carcinogenesis:11There is sufficient evidence to establish that benzene is a human carcinogen (lymphatic and 12hematopoietic cancers). In animal studies, Zymbal gland tumors, preputial gland tumors, skin carcinomas, mammary gland tumors and leukemia are observed.131415Genotoxicity:Chromosomal aberration and DNA adducts tests are positive but other mutagenicity tests are 1617negative.1819Assessment:From the data of human leukemia and exposure concentrations of benzene, it was calculated 2021that a daily intake of 0.02 mg was associated with a lifetime excess cancer risk of 10-5 (IRIS). 22The guideline value for benzene is 0.02 mg per day (2 ppm).References2324Reviews: IARC Monographs 93 (1982)25Toxicological Profile ATSDR/TP 92/03Pharmacopieal Forum (1991) Jan-Feb2627Integrated Risk Information System (IRIS). US EPA, 1990.CARBON TETRACHLORIDE23Category4Possible human carcinogen (IARC 2B).56Genotoxicity7Not mutagenic with or without metabolic activation in bacterial (Ames) test with S.8typhimurium or E. coli.9Refs. McCann J and Ames BN Proc. Natl Acad. Sci. 1976 73 950-954Barber ED et al., Mutat. Res. 1981 90 31-481011Uehleke H et al., Mutat. Res. 1976 38 11412Uehleke H et al., Xenobiotica 1977 7 393-40013De Flora S, Carcinogenesis 1981 2 283-29814De Flora S et al., Mutat. Res. 1984 133 161-19815Negative for induction of umu gene expression in S. typhimurium TA1535/pSK1002 when 16tested at up to 5.3 mg/mL.17Ref. Nakamura S et al., Mutat. Res. 1987 192 239-24618Induced DNA repair in E. coli strains, in the absence of metabolic activation.19Ref. De Flora S et al., Mutat. Res. 1984 133 161-19820De Flora S et al., Mutat. Res. 1984 134 159-165Induced gene convertants, recombinants and revertants at high concentrations in S. cerevisiae 2122without microsomal activation (not tested with S9).23Ref. Callen DF et al., Mutat. Res. 1980 77 55-6324Positive for lambda prophage induction endpoint of Microscreen assay in presence of25metabolic activation.26Ref. Rossman TG et al., Mutat. Res. 1991 260 349-36727Caused DNA single strand breaks in alkaline elution/rat hepatocyte assay at 3 mM (viability 28approximately 45%).Ref. Sina JF et al., Mutat. Res. 1983 113 357-39112Positive in DNA strand break test in mouse lymphoma cells at ≥ 6.55 x 10-3 M.3Ref. Garberg P et al., Mutat. Res. 1988 203 155-176Positive at low rate in 1 of 2 media in SHE transformation assay.45Ref. Amacher DE and Zelljadt I Carcinogenesis 1983 4 291-2956Negative for SCE and chromosome aberrations in rat liver cell line RL1 or CHO cells, with or 7without microsomal activation.8Refs. Dean BJ and Hodson-Walker G Mutat. Res. 1979 64 329-3379Loveday K et al., Environ. Mol. Mutagen. 1990 16 272-30310Negative in chromosome aberration test in bone marrow in vivo.Ref. Lil'p IG Soviet Genet. 1983 18 1467-14721112Negative in mouse lymphoma TK+/- assay, in presence of metabolic activation (not carried 13out without S9).14Ref. Wangenheim J and Bolcsfoldi G Mutagenesis 1988 3 193-20515Negative in rat hepatocyte UDS assay in vivo at up to 400 mg/kg.16Ref. Mirsalis JC and Butterworth BE Carcinogenesis 1980 1 621-62517Bermudez E et al., Environ. Mol. Mutagen. 1982 4 667-67918Binds to calf thymus DNA in vitro following activation by microsomes from phenobarbitone-pretreated rats.1920Ref. DiRenzo AB et al., Toxicol. Lett. 1982 11 243-252Apparently binds in vivo to hepatic DNA (mouse) and RNA (rat) if animals are pretreated2122with 3-methylcholanthrene.23Ref. Rocchi P et al., Int. J. Cancer 1973 11 419-4252425Overall, there is no convincing evidence for genotoxicity.262728Carcinogenicity1Mice Strain A mice were given 0.16, 0.32, 0.64, 1.28 or 2.5 g/kg orally (1-5 days between 2doses for 30 doses), and the animals examined at 150 days. There were no hepatomas in3animals given 30 doses of 2.5 g/kg over 30 days, but a significant number in all groups that 4received 0.16 g/kg or more over a period of 90 days or more.5Ref. Eschenbrenner AB and Miller E J. Natl. Cancer Inst. 1944 4 385-38867PDE160 x 5012 x 10 x 1 x 10 x100.67 mg/day ==8 9Limit0.67 x 10001067 ppm ==1011Strain A mice were given approximately 40, 80, 160 or 320 mg/kg (30 doses at 4-day12intervals) or 10, 20, 40 or 80 mg/kg (120 daily doses) orally. The mice were 3 months old 13when first dosed, and were examined for the presence of hepatomas at 8 months of age. 14Hepatomas were present in all groups except at 10 mg/kg/day.15Ref. Eschenbrenner AB and Miller E J. Natl. Cancer Inst. 1946 6 325-3411617PDE10 x 5012 x 10 x 10 x 10 x10.04 mg/day ==18 19Limit (ppm)0.04 x 1000104 ppm ==2021B6C3F1 mice received 1250 or 2500 mg/kg orally, 5 days/week for 78 weeks, and were22killed 12-14 weeks later. The incidence of hepatocellular carcinomas and adrenal tumours was 23significantly increased at both doses.24Ref. Weisburger EK Environ. Health Perspect. 1977 21 7-1625267 2PDE893 x 5012 x 10 x 1 x 10 x103.7 mg/day ==3 4Limit3.7 x 100010370 ppm ==56Rats Osborne-Mendel rats received 47 or 94 (males) or 80 or 160 (females) mg/kg orally, 5 7days/week for 78 weeks, and were killed 32 weeks later. There was a small increase in8incidence of hepatocellular carcinoma, and a greater increase in the incidence of neoplastic 9nodules, without dose-relationship.10Ref. Weisburger EK Environ. Health Perspect. 1977 21 7-161112For continuous exposure47 x 57= 33.6 mg/kg =13 14PDE33.6 x 505 x 10 x 1 x 10 x100.34 mg/day ==15 16Limit0.34 x 10001034 ppm ==1718Wistar, Osborne-Mendel, Japanese, Black and Sprague-Dawley rats were given 1.3 mL/kg (2 19g/kg) by subcutaneous injection twice weekly. Black and Sprague-Dawley animals died with 20severe cirrhosis at between 5 and 18 weeks. There was a significant increase in incidence of 21hepatocellular carcinoma in Wistar, Osborne-Mendel and Japanese rats surviving for 6822weeks or more.23Ref. Reuber MD and Glover EL J. Natl. Cancer Inst. 1970 44 419-42724257 2PDE571 x 505 x 10 x 1 x 10 x105.7 mg/day ==3 4Limit5.7 x 100010570 ppm ==56Several other earlier and/or grossly inadequately designed oral, inhalation or subcutaneous7carcinogenicity studies in mouse, hamster and trout have been carried out. Note that in no8study conducted to a currently acceptable design has an entirely convincing no-effect dose for 9tumorigenesis been determined. The studies reported by Weisburger are of adequate length, 10and of generally sufficient design, but the lowest doses used were 1250 mg/kg/day in mice, 11and 47 mg/kg/day in rats. The investigations of Eschenbrenner and Miller are relatively short, 12and only hepatocellular tumours were scored.1314Hamsters Syrian golden hamsters given approximately 200 mg/kg once weekly for 7 weeks, 15followed by approximately 100 mg/kg for 30 weeks, and survivors killed 25 weeks later.16There were liver cell carcinomas in animals dying or being killed from week 43 onwards.17Total numbers used in this study were low, and it appears that no concurrent controls were 18employed. Ref. Della Porta G et al., J. Natl. Cancer Inst. 1961 26 855-8631920For continuous exposure100 x 17= 14.3 mg/kg =21 22PDE14.3 x 5010 x 10 x 1 x 10 x100.07 mg/day ==23 24Limit0.07 x 1000107 ppm ==2526Reproductive Toxicity 27Sprague-Dawley rats exposed by inhalation to 300 or 1000 ppm, 7h/day on days 6 through 15 1of gestation. Foetal body weight and crown-rump length were significantly reduced at both2concentrations, and probably associated with reduced maternal food consumption and body 3weight gain. The incidence of sternebral anomalies was claimed to be increased at 1000 ppm, 4but in the control group exposed to air concurrently with the 300 ppm group the incidence5was as high as in the group exposed to 1000 ppm. LOEL (foetotoxicity) = 300 ppm. Ref.6Schwetz BA et al., Toxicol. Appl. Pharmacol. 1974 28 452-46478300 ppm300 x 153.8424.451888 mg/m= 1.89 mg/L3==9 10For continuous exposure1.89 x 724= 0.55 mg/L =11 12Daily dose0.55 x 2900.330= 483 mg/kg =13 14PDE483 x 505 x 10 x 1 x 1 x1048.3 mg/day ==15 16Limit48.3 x 1000104830 ppm ==1718This appears to be the only satisfactory teratogenicity study to have been conducted. Other 19studies suggest that very large doses result in foetal death, i.e. that carbon tetrachloride is20foetotoxic, but not teratogenic.2122Rats given 80 or 200 ppm in the diet (carbon tetrachloride intake up to 10-18 mg/kg/day), 23commencing two weeks after weaning. Females mated for 5 successive pregnancies (once to 24control, 4 times to treated males), beginning at 3 months of age. No effects on pregnancy rate 25or litter parameters. Worst case NOEL = 10 mg/kg/day.26Ref. Alumot E et al., Food Cosmet. Toxicol. 1976 14 105-110271PDE10 x 505 x 10 x 1 x 1 x110 mg/day ==2 3Limit10 x 1000101000 ppm ==45Large doses of carbon tetrachloride cause testicular (seminiferous tubule and interstitial cell) 6damage and affect the oestrous cycle in females, but the significance of the changes is7impossible to assess, some evidence is contradictory, and the effects of low doses have not 8been explored.910Toxicity11Oral LD50 in mice 8.26 g/kg.12Ref. Wenzel DG and Gibson RD J. Pharm. Pharmacol. 1951 3 169-17613Oral LD50 in rats 2.81 g/kg.14Ref. Smyth HF et al., Toxicol. Appl. Pharmacol. 1970 17 498-50315Oral LD50 in dogs 2.3 g/kg.16Ref. Klaasen CD and Plaa GL Toxicol. Appl. Pharmacol. 1967 10 119-13117Dermal LD50 in rabbits and guinea pigs > 14 g/kg.18Ref. Roudabush RL et al., Toxicol. Appl. Pharmacol. 1965 7 559-56519Intraperitoneal LD50 in mice 4.675 g/kg.20Ref. Gehring PJ Toxicol. Appl. Pharmacol. 1968 13 287-29821Subcutaneous LD50 in mice 31 g/kg.22Ref. Plaa GL et al., J. Pharmacol. Exp. Ther. 1958 123 224-2292324There is a vast literature on the toxicity of carbon tetrachloride in animals, largely dealing 25with the characteristics and mechanism of liver damage. Low hepatotoxic doses of carbon 26tetrachloride produce characteristic fatty livers. Higher exposures result in centrilobular27necrosis; cirrhosis and hepatic tumours may develop after prolonged administration.28Hepatotoxicity is dependent on activation by cytochrome P450, and agents that induce1monooxygenase activity (including ethanol and barbiturates) markedly increase the2hepatotoxicity of carbon tetrachloride.3Refs. e.g. Recknagel RO and Glende EA CRC Crit. Rev. Toxicol. 1973 2 263-2974Glende EA et al., Biochem. Pharmacol. 1976 25 2163-21705Kalf GF et al., Annu. Rev. Pharmacol. Toxicol. 1987 27 399-42767Other target organs include kidney, testes and lung.8Refs. e.g. Chen W-J et al., Lab. Invest. 1977 36 388-3949New PS et al., J. Am. Med. Assoc. 1962 181 903-9061011Many papers report the outcome of administration of one or a few doses of carbon12tetrachloride. The following comprise a large proportion of those involving administration for 1310 days or more that have been reported during the last 50 years.1415Mice CD-1 mice treated orally for 90 days at 12, 120, 540 or 1200 mg/kg/day. Dose-related 16altered serum parameters of liver damage and histopathological changes (including necrosis 17and fatty degeneration) at 12 mg/kg/day and above. LOEL = 12 mg/kg/day.18Ref. Hayes JR et al., Fund. Appl. Toxicol. 1986 7 454-4631920PDE12 x 5012 x 10 x 5 x 1 x100.10 mg/day ==21 22Limit0.10 x 10001010 ppm ==2324CD-1 mice given 1.2, 12 or 120 mg/kg orally, 5 days/week, for 90 days. Dose-related altered 25serum parameters of liver damage and histopathological changes at 12 mg/kg/day and above. 26Minimal necrosis in single animal at 1.2 mg/kg/day. Virtual NOEL = 1.2 mg/kg/day.27Ref. Condie LW et al., Fund. Appl. Toxicol. 1986 7 199-206281For continuous exposure1.2 x 57= 0.857 mg/kg =2 3PDE0.857 x 5012 x 10 x 5 x 1 x10.071 mg/day ==4 5Limit0.071 x 1000107.1 ppm ==67Rats Wistar rats exposed by inhalation to 5, 10, 25, 50, 100, 200 or 400 ppm, 7h/day on 127-8146 occasions during a period of 173-205 days. Fatty degeneration of the liver at 10 ppm or 9more; cirrhosis at 50 ppm or more; evidence of increased mortality at 100 ppm or more.10Biochemical changes were present above 5 ppm. NOEL = 5 ppm (145 exposures in 20511days). Ref. Adams EM et al., AMA Arch. Ind. Hyg. 1952 6 50-6612135 ppm5 x 153.8424.4531.5 mg/m= 0.0315 mg/L3==14 15For continuous exposure0.0315 x 7 x 14524 x 205= 0.0065 mg/L =16 17Daily dose0.0065 x 2900.425= 4.44 mg/kg =18 19PDE4.44 x 505 x 10 x 2 x 1 x12.2 mg/day ==20 21Limit2.2 x 100010220 ppm ==22 23Long-Evans or Sprague-Dawley rats exposed continuously for 90 days to atmospheres1containing 61 or 6.1 mg/m3. Hepatic damage at 61 mg/m3, NOEL 6.1 mg/m3 = 0.0061mg/L 2Ref. Prendergast JA Toxicol. Appl. Pharmacol. 1967 10 270-28934Daily dose0.0061 x 2900.425= 4.16 mg/kg =5 6PDE4.16 x 505 x 10 x 5 x 1 x10.8 mg/day ==7 8Limit0.8 x 10001080 ppm ==910Male F344 rats given 5, 10, 20 or 40 mg/kg/day for 10 days. Increased AST and ALT at 20 11and 40 mg/kg/day, at least minimal hepatic vacuolar degeneration at all doses, hepatic12necrosis at 10 mg/kg/day and more. No consistent changes in parameters of immune function. 13LOEL = 5 mg/kg/day.14Ref. Smialowicz RJ et al., Fund. Appl. Toxicol. 1991 17 186-1961516PDE5 x 505 x 10 x 10 x 1 x50.10 mg/day ==17 18Limit0.10 x 10001010 ppm ==1920Male F344 rats given 20 or 40 mg/kg orally, 5 days/week for 12 weeks. Dose-related 21retardation of growth, alterations in serum parameters of liver damage, hepatic necrosis, 22vacuolar degeneration and cirrhosis at both doses. LOEL = 20 mg/kg/day.23Ref. Allis JW et al., Fund. Appl. Toxicol. 1990 15 558-5702425For continuous exposure20 x 57= 14.3 mg/kg =1 2PDE14.3 x 505 x 10 x 5 x 1 x100.28 mg/day ==3 4Limit0.28 x 10001028 ppm ==56Male Sprague-Dawley rats given 1, 10 or 33 mg/kg orally, 5 days/week for 12 weeks.7Retarded growth at 33 mg/kg, and dose-related alterations in serum parameters of liver8damage at 10 and 33 mg/kg. Hepatic centrilobular vacuolisation at 10 mg/kg, and extensive 9degenerative lesions and hyperplastic nodules at 33 mg/kg. NOEL = 1 mg/kg.10Ref. Bruckner JV et al., Fund. Appl. Toxicol. 1986 6 16-341112For continuous exposure1 x 57= 0.714 mg/kg =13 14PDE0.714 x 505 x 10 x 5 x 1 x10.14 mg/day ==15 16Limit0.14 x 10001014 ppm ==1718Guinea Pigs of heterogeneous origin exposed by inhalation to 5, 10, 25, 50, 100, 200 or 400 19ppm, 7h/day on 93-184 occasions during a period of 126-258 days. Fatty degeneration of the 20liver at 10 ppm or more; cirrhosis at 25 ppm or more; renal tubular degeneration at 200 ppm 21and more; increased mortality at 100 ppm or more. Biochemical changes were present above 225 ppm. NOEL = 5 ppm (143 exposures in 203 days).23Ref. Adams EM et al., AMA Arch. Ind. Hyg. 1952 6 50-6624255 ppm5 x 153.8424.4531.5 mg/m= 0.0315 mg/L3==1 2For continuous exposure0.0315 x 7 x 14324 x 203= 0.0065 mg/L =3 4Daily dose0.0065 x 4300.500= 5.6 mg/kg =5 6PDE5.6 x 5010 x 10 x 2 x 1 x11.4 mg/day ==7 8Limit1.4 x 100010140 ppm ==910Hartley guinea pigs exposed continuously for 90 days to atmospheres containing 61 or 6.1 11mg/m3. Hepatic damage and some deaths at 61 mg/m3, slight reduction in body weight gain 12at 6.1 mg/m3. LOEL 6.1 mg/m3 = 0.0061mg/L.13Ref. Prendergast JA Toxicol. Appl. Pharmacol. 1967 10 270-2891415Daily dose0.0061 x 4300.500= 5.25 mg/kg =16 17PDE5.25 x 5010 x 10 x 5 x 1 x50.1 mg/day ==18 19Limit0.1 x 10001010 ppm ==2021Rabbits White rabbits exposed by inhalation to 10, 25, 50 or 100 ppm, 7h/day on 139-178 22occasions during a period of 197-248 days. Fatty degeneration and cirrhosis of the liver at 25 23ppm or more; significant depression of growth at 100 ppm. NOEL = 10 ppm (139 exposures 1in 197 days). Ref. Adams EM et al., AMA Arch. Ind. Hyg. 1952 6 50-662310 ppm10 x 153.8424.4562.9 mg/m= 0.0629 mg/L3==4 5For continuous exposure0.0629 x 7 x 13924 x 197= 0.0129 mg/L =6 7Daily dose0.0129 x 14404= 4.64 mg/kg =8 9PDE4.64 x 502.5 x 10 x 2 x 1 x14.6 mg/day ==10 11Limit4.6 x 100010460 ppm ==1213New Zealand white rabbits exposed continuously for 90 days to atmospheres containing 61 or 146.1 mg/m3. Hepatic damage at 61 mg/m3, reduced body weight gain at 6.1 mg/m3. LOEL 6.1 15mg/m3 = 0.0061 mg/L Ref. Prendergast JA Toxicol. Appl. Pharmacol. 1967 10 270-2891617Daily dose0.0061 x 14404= 2.2 mg/kg =18 19PDE2.2 x 502.5 x 10 x 5 x 1 x50.18 mg/day ==20 21Limit0.18 x 10001018 ppm ==22 23Dogs Beagle dogs exposed continuously for 90 days to atmospheres containing 61 or 6.1 1mg/m3. Hepatic damage at 61 mg/m3, some evidence of reduced body weight gain at 6.1 2mg/m3. LOEL 6.1 mg/m3 = 0.0061 mg/L3Ref. Prendergast JA Toxicol. Appl. Pharmacol. 1967 10 270-28945Daily dose0.0061 x 900011.5= 4.77 mg/kg =6 7PDE4.77 x 502 x 10 x 5 x 1 x50.48 mg/day ==8 9Limit0.48 x 10001048 ppm ==1011Monkeys Rhesus monkeys exposed by inhalation to 25, 50 or 100 ppm, 7h/day on 148-198 12occasions during a period of 212-277 days. Of two monkeys exposed to 100 ppm, slight13growth depression in both, some cloudy swelling in the liver of one, and slight fatty14degeneration throughout the liver of the other. NOEL = 50 ppm (198 exposures in 277 days). 15Ref. Adams EM et al., AMA Arch. Ind. Hyg. 1952 6 50-66161750 ppm50 x 153.8424.45315 mg/m= 0.315 mg/L3==18 19For continuous exposure0.315 x 7 x 19824 x 277= 0.0657 mg/L =20 21Daily dose0.0657 x 11502.5= 30.2 mg/kg =22 23PDE30.2 x 5010 x 10 x 2 x 1 x17.6 mg/day ==24 25Limit7.6 x 100010760 ppm ==12Human3Carbon tetrachloride is extremely lipophilic; it is readily absorbed in animals and, apparently, 4in humans after oral ingestion. Fatal human poisonings by carbon tetrachloride have been5reported since 1909, and deaths continue to occur occasionally following either inhalation or 6ingestion. Toxicity is exacerbated by alcoholism or concurrent exposure to alcohol and carbon 7tetrachloride. Liver and renal damage are the most common effects.8Refs. Veley VH 1909 Lancet 1162-11639Hardin BL 1954 Ind. Med. Surg. 23 93-1051011The genotoxicity of carbon tetrachloride is unconvincing, and liver tumorigenesis in animal 12species may be related to chronic damage and regenerative cell proliferation. This standpoint 13generally has been taken in setting occupational exposure limits for carbon tetrachloride.14There are only a few anecdotal cases in which exposure has been linked with hepatic tumours 15in man. Limited epidemiological studies indicate an excess of some cancers in communities 16exposed to chlorinated hydrocarbons, but the general limitations of the studies and mixed17solvent exposure do not allow firm conclusions to be drawn regarding the carcinogenic18potential of carbon tetrachloride in man.19Refs. e.g. Tracey JP and Sherlock P N.Y. State J. Med. 1968 8 2202-220420Simler M et al., Strasbourg Med. 1964 15 910-91721Blair A et al., Am. J. Pub. Health 1979 69 508-51122Capurro PU Clin. Toxicol. 1979 14 285-2942324Carbon tetrachloride is classed by IARC in Group 2B (possibly carcinogenic in humans), by 25NTP in Group 2 (reasonably anticipated to be a carcinogen), by ACGIH as A2 (suspected26human carcinogen) and by NIOSH and OSHA as a carcinogen, without further classification. 272829301Environmental Impact2Under the revised Montreal Protocol, production and use of carbon tetrachloride are34scheduled to be phased out by the year 2000 by ratifying parties (excluding 10-yearderogations for developing nations), because of its contribution to atmospheric ozone56depletion (ozone-depleting potential 0.9, similar to that of fully chlorinated CFCs).78Conclusion9Possible human carcinogen. Animal carcinogen (balance of evidence suggests probably by 1011non-genotoxic mechanism). Hepatotoxic at low doses in man and laboratory species.12Production scheduled to be phased out in 2000 under Montreal Protocol.1314The guideline value for carbon tetrachloride is 0.04 mg/day (4 ppm).1,2-DICHLOROETHANE234Category: Possible human carcinogen (IARC 2B). Not teratogenic56Toxic Effects:7Repeated exposure induces anorexia, nausea, abdominal pain, irritation of mucous8membranes, dysfunction of liver and kidney and neurological disorders. Depression of9leukocyte, antibody-forming cell and cellular immunity was found in mice; necrosis of10cerebellum and hyperplasia and inflammation of forestomach were observed in male rats after 11oral administration.1213Carcinogenesis:14There is no evidence of carcinogenicity in humans. Forestomach cancer, hemangiosarcoma, 15breast cancer, uterine cancer and respiratory tract cancer were found in rats or mice after16gavage treatment.1718Genotoxicity:19The balance of evidence indicates 1,2-dichloroethane is potentially genotoxic.2021Assessment:22Excess cancer risk at 10-5 is 0.05mg/day for 50 kg human based on hemangiosarcoma using a 23linearized multistage model without body surface correction.24The guideline value for 1,2-dichloroethane is 0.05 mg per day (5 ppm).25References26Reviews; Environmental Health Criteria 62 (1987)27IARC Monographs 20 (1979)28NCI (1978) TR-55.1,1-DICHLOROETHENE2Genotoxicity3Some positive in vitro results in Ames test and mouse lymphoma, results being enhanced in4presence of liver microsomal samples. Negative results in in vitro SCE and chromosome5abberation studies and in CHE cells. Negative results in vivo in micronucleus test, UDS assay 6and dominant lethal assay.7Refs. Mortelmans K et al., Environ. Mutagen 1986 8 1-119.8Greim H et al., Biochem. Pharmacol. 1975 24 2013-17.9Bronzetti G et al., Mut. Res. 1981 89 179-85.10McGregor D et al., Environ. Mol. Mutagen. 1991 17 (2) 122-9.11Drevon C and Kuroki T. Mut. Res. 1979 67 (2) 173-82.12Sawanda M et al., Mut. Res. 1987 187 (3) 157-63.13Reitz RH et al., Toxicol. Appl. Pharmacol. 1980 52 (3) 357-70.14Anderson D et al., Biochem. Pharmacol. 1977 21 71-8.15Carcinogenicity16Positive results have been reported after inhalation exposure; however, no increase in tumour 17incidence is noted following oral administration.18Swiss mice exposed to 25 ppm 4 h/day, 5 days/week for 52 weeks and retained until 9819weeks showed an increased incidence of renal adenocarcinomas, mainly in males.20Ref. Maltoni C. Environ. Health Perspect 1977 21 1-5. LOEL = 25 ppm212225 ppm25 x 96.9424.4599.1 mg/m 0.099 mg/L3===23 24For continuous dosing0.099 x 4 x 524 x 7= 0.012 mg/L =25 26Daily dose0.012 x 430.02818.1 mg/kg ==1 2PDE18.1 x 5012 x 10 x 1 x 10 x 100.08 mg/day ==3 4Limit0.08 x 1000108 ppm ==56Sprague-Dawley rats given 100 ppm 4-7 h/day, 5 days/week for 2 years. Others were7exposed in utero and then for 2 years following birth and showed an increased incidence of 8leukaemia.9Ref. Cotti G et al., Ann. NY Acad. Sci. 1988 534 160-681011100 ppm100 x 96.9424.45396 mg/m 0.4 mg/L3===12 13For continuous dosing =0.4 x 4 x 524 x 70.047 mg/L=14 15Daily dose =0.047 x 2900.425= 32 mg/kg16 17PDE =32 x 505 x 10 x 10 x 10 x 1= 0.32 mg/day18 19Limit =0.32 x 100010= 32 ppm2021B6C3F1 mice given 2 and 10 mg/kg by gavage 5 days/week for 2 years showed no increase in 22tumour incidence (except leukaemia which was discounted because it only occurred in low 23dose females).24Ref. NTP Programme Tech. Report 228 1982. NEL 10 mg/kg. 12For continuous dosing =10 x 57= 7.14 mg/kg3 4PDE =7.14 x 5012 x 10 x 1 x 1 x 1= 2.98 mg/day5 6Limit =2.98 x 100010= 298 ppm78Sprague-Dawley rats given time-weighted average of 7, 10 and 20 mg/kg (males) and 9, 14 9and 30 mg/kg (females) for 2 years in drinking water. No increase in tumour incidence was 10noted. Ref. Quast JF et al., Fund. Appl. Toxicol. 1983 3 55-62. NOEL = 20 mg/kg1112PDE =20 x 505 x 10 x 1 x 1 x 120 mg/day=13 14Limit =20 x 100010= 2000 ppm1516Reproductive toxicity17Rats given 200 mg/L in drinking water days 6-15 showed no adverse effects and offspring 18were normal.19Ref. Norris JM in Proceedings of Technical Association of Pulp and Paper Industries20Conference, Chicago 1977. NEL 200 mg/L=2122Rat drinks 30 mg/dayDaily consumption =200 x 3010006 mg/day=231Dose =60.33= 18.2 mg/kg2 3PDE =18.2 x 505 x 10 x 1 x 1 x 118.2 mg/day=4 5Limit18.2 x 100010= 1820 ppm =67Rats given 20-160 ppm by inhalation 7 h/day days 6-15. Embryo and foetal toxicity 8associated with maternal toxicity but no teratogenic effects.9Ref. Norris JM in Proceedings of Technical Association of Pulp and Paper Industries 10Conference, Chicago 1977.111220 ppm =20 x 96.9424.45= 79 mg/m 0.08 mg/L3=13 14For continuous dosing =0.08 x 724= 0.023 mg/L15 16Daily dose =0.023 x 2900.33= 20.2 mg/kg17 18PDE =20.2 x 505 x 10 x 1 x 1 x 10= 2.02 mg/day19 20Limit =2.02 x 100010= 202 ppm2122Rabbits dosed at 20-160 ppm by inhalation 7 h/day days, 6-18 showed embryo and foetal 23toxicity associated with maternal toxicity but no teratogenic effects.24。
广州大学WEB课件制作工具使用手册南京乔木科技有限公司2009-2目录1总体介绍 (3)2课件 (6)2.1 新建课件 (6)2.2 打开课件 (8)2.3 保存课件 (10)2.4 导出课件 (10)2.5 上报课件 (10)2.6 关闭课件 (10)3课程内容 (10)3.1 课程章节设置 (11)3.2 添加章节 (11)3.3 管理章节页面 (12)3.4 查看与修改章节信息 (12)3.5 删除章节 (13)3.6 调整章节位置 (14)4栏目设置 (14)4.1 栏目设置 (14)4.2 添加栏目 (15)4.3 编辑栏目页面 (16)4.4 设置栏目属性 (17)4.5 删除栏目 (18)4.6 调整栏目位置 (18)5编辑栏目、章节内容 (18)5.1 普通栏目、章节 (18)5.2 自测与练习栏目 (19)5.3 内容导入 (20)6图文混排编辑器 (21)7界面定制 (23)7.1 课件框架界面 (23)7.2 欢迎页 (23)8课件信息 (24)9管理模板 (25)9.1 模板管理 (25)9.2 添加模板 (25)1总体介绍本系统是一个制作Web 形式的课程课件的平台。
课件按课程的章节为内容划分依据,包含HTML 、视频以及其它多媒体。
具体说来,包括:1. 课件以HTML 页面作为框架,根据课程的目录结构进行浏览,支持常规的HTML 以及csf ,asf 等其它媒体资源。
2. 课件的栏目包括课程负责人、课程建设规划、自我评价、教学录象、网络课程、申报表、教学队伍、课程描述等内容。
3. 支持刻录成光盘或者上传至Web 服务器上发布成网站,支持录制功能。
Web 课件的结构如下:··· ··· ··· ···图1-1整个Web 课程由栏目和课程内容构成,课程内容下由若干个章节构成。
Appendix 1IntroductionChina National Offshore Oil Corp. (CNOOC) is a State oil company incorporated on February 15,1982,with approval from State Council. Fully authorized by the "regulations of the People's Republic China on Exploitation Offshore Petroleum Resources in Cooperation with Foreign Enterprises" which was promulgated by the State Council, CNOOC is assumed with the overall responsibilities for the exploitation of offshore petroleum and natural gas resources in the People's Republic of China in cooperation with foreign enterprises. Headquartered in Beijing, CNOOC registered with a capital of 20 billion RMB and at present employs 21 thousand personnel in active service. It has majority stake in CNOOC Ltd., a listing independent oil/gas company primarily engaged in E&P sector, owns China Offshore Oil Research Center, one chemicals company, eight specialized services companies and five logistic companies and, runs a joint venture petrochemicals company with Shell. In addition, it operates four overseas representative offices in Houston, Jakarta, Tokyo and Singapore. The main business strain of CNOOC is to organize the exploration for, development and production of offshore petroleum and natural gas resources, refining, petro-chemical, and natural gas processing and utilization, to market the petroleum and natural gas, oil and gas processed products, petro-chemical products, and the produced and processed products by its subordinate companies, and to provide the clients with services for petroleum and natural gas exploration, development, production and marketing and general purpose services.Appendix 2Business StatusCNOOC has experienced a prime time of fast and sound development in the past 19 years, and has become a petroleum company with fast growth and high competitive edge worldwide.From 1982 to 2000, CNOOC scarcely received direct investment from state government, while its oil and gas production rushed from 90,000 tons of barrels of oil equivalent (BOE) to 22.35 million tons of BOE, its assets increased from 2.8 billion RMB to 53.3 billion RMB, with net assets increasing from 2.2 billion RMB to 41.55 billion RMB, and its productivity was improved from 10,000 RMB per capita to 778,000 RMB per capita. Besides, CNOOC has generated a total tax contribution of 14.1 billion RMB, 8.55 times of state investment. In 2000, CNOOC realized a net profit of 9.87 billion RMB.Appendix 3CNOOC Rated Baa2 and BBB (2001-9-29)Moody’s Investors Service and Standard & Poor’s have assigned a Baa2 an d BBB issuer rating to China National Offshore Oil Corporation (CNOOC) respectively. It is the highest rating ever received by Chinese companies and the first by SOEs.Moody’s Investors Service said the Baa2 rating reflected the strategic significance of the Chinese domestic oil and gas sector and the inherent strength of CNOOC’s E&P business and its strong cash flow.Standard & Poor’s assigned a BBB rating to CNOOC, same as China’ sovereign rating. S&P recognized the substantial net reserves of oil and gas, promising growth potentials, competitive cost operation and stable and healthy financial management of CNOOC, as well as its successful international cooperation and joint ventures in downstream expansion. CNOOC’s rating success told a story that wi th a healthy management practice, credited operation results and promising growth potentials, the unlisted parent company can be well recognized by international rating agencies.Appendix 4Human ResourceSince CNOOC was incorporated, though the production increased from less than 100,000 tons of crude oil in 1982 to 16.17 millions of crude oil and 4.39 BCM of natural gas in 1999, yet the total number of employees has down to 21,000 in 1999 compared with that of 27,215 in 1982. Meanwhile, CNOOC has been dedicated in the personnel structure adjustments and improvements of staff qualification. As a result, the CNOOC employees with college and university graduation certificates have increased to 56.2% now in 1999 from that of 26% in 1982. The percentage of staff with college and university certificates in CNOOC Ltd. is as high as 94.6%. Therefore an offshore oil workforce has been well established which is withall subjects, complete professions and capability of operating according to international practice, and has the ability of conducting independent exploration, development, field construction and management of modern offshore oil/gas fields. And such ability is matching with 1990s world standard.Appendix 5NewsFoundation Ceremony for CNOOC Fertilizer Project(2001-5-27)CNOOC lawfully took over the equities of Hainan Fudao Fertilizer Plant on January 13 earlier this year. The foundation ceremony initiated Phase II development of Fudao. Wei Liucheng, president of China National Offshore Oil Corporation, delivered a speech in the ceremony. He said, “CNOOC will put a direct investment of more than 10 billion RMB in Hainan Province in the next three years, not only to complete the 450,000-ton synthetic ammonia and 800,000-ton urea project laid foundation today, but also to develop Dongfang 1-1 gas field and forge a 600,000-ton compound fertilizer project coincidently. It will help Dongfang, where the project is sited, develop into the No.1 fertilizer base and one of the largest natural gas and chemicals bases in China and possess considerable competitiveness at home and worldwide, with a yearly turnout capacity of two million ton by that time.”Du Qinglin, secretary of CPC Hainan Committee and various government officials presented the foundation ceremony.CNOOC Join the Planning of Shandong Natural Gas Pipeline Network(2001-10-11)CNOOC announced that it had signed with the Development and Planning Commission of Shandong Province and Shandong International Trust Investment Company to participate in the planning of Shandong natural gas pipeline network and LNG import.Due to the rapid economic growth and the severe unbalanced energy mix, Shandong province has been hungry for clean energy like natural gas. To maintain the sustainable development of its economy, society and environment, Shandong province has been actively exploring the utilization of natural gas.CNOOC has been developing the natural gas reserves in Bohai Bay and planning to supply gas from Bonan area to Yantai and Qingdao via Longkou in Shandong province. In addition, as the leading coordinator of the first LNG project in China, CNOOC has experiences in LNG project and the knowledge of domestic market. Therefore, the Development and Planning Commission of Shandong Province has decided to cooperate with CNOOC in the planning of Shandong natural gas pipeline network (from 2001 to 2015) and the investigation of LNG import.CNOOC Limited has the option to take its parent’s working interest in potential investment projects that may be generated.CNOOC Signed with Husky Energy the Petroleum Contract for Block 39/05 (2001-7-27)On July 26, 2001, China National Offshore Oil Corporation (CNOOC) signed with Husky Oil China Ltd the petroleum contract for Block 39/05 in the Pearl River Mouth Basin of South China Sea. Husky Oil China Ltd is a subsidiary of Canadian Husky Energy Inc.It is the second PSC CNOOC signed with foreign oil companies this year. CNOOC so far has signed 148 petroleum contracts with 70 companies from 18 countries and regions worldwide.CNOOC Signed the first petroleum contract for WC 13-1 and WC 13-2 with Husky Energy in October of 2000.The contract block covers a total acreage of 5,700 square kilometers, located in the Pearl River Mouth Basin of South China Sea, eastern 100 kilometers offshore to Hainan Island. Husky Oil China Ltd has a 100 percent participating interest in the block during exploration phase.美文欣赏1、走过春的田野,趟过夏的激流,来到秋天就是安静祥和的世界。