10 Reaction Equations要点
- 格式:doc
- 大小:159.50 KB
- 文档页数:12



第2章化学反应的方向、限度与速率第1节化学反应的方向一、反应焓变与反应方向1、ΔH < 0有利于反应自发进行,但自发反应不一定要ΔH < 0。
焓变只是反应能否自发进行的一个因素,但不是唯一因素。
只根据焓变来判断反应方向是不全面的。
二. 反应熵变与反应方向1. 熵:用来度量体系混乱程度的物理量。
2. 符号:S 单位:J·mol1·K13. 体系混乱度越大,熵值越大。
纯物质的熵值的大小与物质的种类、数量、聚集状态以及温度、压强等因素有关。
例如,同一条件下,不同物质的熵不同;同一物质,S(g)>S(l)>S(s)。
4、熵变(ΔS)和熵增原理ΔS = 反应产物的总熵-反应物的总熵对于确定的化学反应,在一定条件下具有确定的熵变。
ΔS > 0 反应体系混乱度增大ΔS < 0 反应体系混乱度减小ΔS>0的反应,其数值越大越有利于反应自发进行,但不绝对,熵减小的反应也有能自发进行的。
注意:(1)与焓变类似,熵变只是反应能否自发进行的一种因素;(2)熵变( ΔS )只取决于体系的始态和终态,与变化的途径无关;(3)熵判据只判断一定条件化学反应能否自发进行,与化学反应速率无关。
总结:自发过程的两大变化趋势:(1)能量趋于减小(焓减)(2)混乱度趋于增大(熵增)三.用焓变与熵变综合判断反应方向研究表明,在等温、等压及除了体积功以外不做其他功的条件下,化学反应的方向可以用反应的焓变和熵变来综合判断,判据为ΔH -TΔS。
ΔH -TΔS < 0 反应正向能自发进行ΔH -TΔS = 0 反应达到平衡状态ΔH -TΔS > 0 反应正向不能自发进行在等温、等压及除了体积功以外不做其他功的条件下,自发反应总是向着ΔH -TΔS < 0 的方向进行,直到达到平衡状态。
该判据指出的是化学反应正向进行的趋势。
大大高小小低小大皆行大小皆停第2节化学反应限度一、化学平衡常数1、化学平衡常数定义:在一定温度时,当一个可逆反应达到平衡状态时,生成物平衡浓度的幂之积与反应物平衡浓度的幂之积的比值是一个常数.简称:平衡常数符号:K单位:(mol·L1) c+dabaA + bB ⇌cC + dD2、化学平衡常数关系式书写规则(1)如果反应中有固体和纯液体参加,它们的浓度不应写在平衡关系式中,因为它们的浓度是固定不变的,化学平衡关系式中只包括气态物质和溶液中各溶质的浓度。

这是同济大学《高等混凝土结构理论》期末考试的复习要点,希望对考博选考3007高等混凝土与钢结构这门课的同学有所帮助。
1.Stress-strain curves of concrete under monotonic, repeated and cyclic uniaxial loadings. 单轴受力时混凝土在单调、重复、反复加载时的应力应变曲线。
2.Creep of concrete (linear and nonlinear) 混凝土的徐变(线性、非线性徐变)3.Components of deformation of concrete 混凝土变形的多元组成4.Process of failure of concrete under uniaxial compression 混凝土在单向受压时破坏的过程。
5.Strength indices of concrete and the relations among them 混凝土的强度指标及其之间关系6.Features of stress-strain envelope curve of concrete under repeated compressive loading. 混凝土单向受压重复加载时的应力应变关系的包络线的特征。
7.The crack contact effect of concrete and its representation in stress-strain diagram. 混凝土的裂面效应及其在应力应变关系图上的表示。
8.The multi-level two-phase system of concrete. 混凝土的多层次二相体系。
9.The rheological model of concrete. 混凝土的流变学模型。
10.Influence of stress gradient on strength of concrete. 应力梯度对混凝土强度的影响。

Limiting ReactantsWhy?Reactants are not always present in the exact amounts required by the balanced chemical reaction equation. In planning any cost-effective production process, it is necessary to recognize which component limits the amount of material that can be produced. Identifying the limiting reactant in a chemical reaction will strengthen your skills in dealing with moles, solution concentrations, and reaction stoichiometry.Learning ObjectivesλDetermine the amounts of material involved in a reaction.λIdentify reactants that limit the extent of a reaction and the amount of product produced.Success CriteriaλQuick identification of the limiting and excess reactants.λAccuracy of calculations of the amounts of material reacting and being produced in chemical reactions.ResourcesOlmsted and Williams (Chemistry 3/e, Wiley, 2002) pp. 148-154.Prerequisitesmoles, chemical compounds, chemical reaction equations, determination ofchemical formulasNew Conceptslimiting reactant, excess reactantVocabularystoichiometryDefinitionsIn your own words, write definitions of the terms in the New Concepts and Vocabulary sections.Limiting ReactantsModel 1: Limiting IngredientA cake recipe calls for2 cups of water4 cups of flour8 squares of chocolate4 cups of sugar8 oz of butter4 eggsIngredients on hand5 cups flour4 cups sugar 12 squares chocolate16 oz butter 6 eggsLimiting Reagents Key Questions1. According to Model 1, how much of each ingredient is necessary to make a cake?water flour chocolate sugar butter eggs 2. If you follow the recipe, using only the ingredients on hand in the model, howmuch of each ingredient will be left over after you have baked the cake?water flour chocolate sugar butter eggs3. Which of the ingredients on hand were in excess for the recipe?4. Which of the ingredients on hand were consumed completely in making the cake?5. Which of the ingredients limit or prevent you from making a second cake?6. What would be a good definition for the term limiting ingredient?7. What would be a good procedure or methodology to use for identifying the limiting component in some manufacturing process? Test your methodology by applying it to the following exercises.Limiting ReactantsExercises1. You want to make 10 dozen standard-size cookies as specified by a recipe that requires 16 oz of butter, 4 eggs, 3 cups of flour, and 4 cups of sugar.In taking inventory of your supplies, you find that you have 16 oz of butter,6 eggs, and 3 cups each of flour and sugar.(a) Which ingredient will limit the number of cookies you can make?(b) How many standard-size cookies can you make?2. You have 100 bolts, 150 nuts, and 150 washers. You assemble a nut/bolt/washer set using the following recipe or equation.2 washers + 1 bolt + 1 nut = 1 set(a) How many sets can you assemble from your supply?(b) Which is the limiting component?3. This reaction of hydrogen with oxygen to produce water is described by the following recipe or reaction equation, which says that 2 molecules of hydrogen react with 1 molecule of oxygen to produce 2 molecules of water.2H2 + O2 = 2H2OYou react 150 H2 molecules with 100 O2 molecules to produce H2O. Which is the limiting reactant, hydrogen or oxygen? How many water molecules can you produce from your supply of hydrogen and oxygen?Limiting Reagents Model 2: Limiting Reactant in MolesThe reaction equation can be interpreted in terms of the number of molecules(see Problem 3 above) or moles reacting (2 moles of hydrogen react with 1 mole of oxygen to produce 2 moles of water). The only difference is that a mole can bedivided into fractions of a mole, a molecule cannot be divided. Each square below represents one mole of hydrogen, oxygen, and water.1 mole of H2 1 mole of O2 1 mole of H2OWhen these react according to the reaction equation,2H2 + O2 = 2H2O,twice as much hydrogen will be used and twice as much water will be producedwhen compared to the amount of oxygen that reacts. The diagram below indicates what happens when 1 mole of hydrogen and 1 mole of oxygen are mixed together and react to produce water.Thinking in terms of moles, we could write the reaction equation asH2 + 1/2 O2 = H2O.Key Questions8. How many moles of hydrogen reacted in the above diagram?9. How many moles of oxygen remained after the reaction?10. Was hydrogen or oxygen the limiting reactant?Limiting ReactantsExercises4. If 6 moles of hydrogen (H2) and 4 moles of oxygen (O2) are mixed and reacted, which is the limiting reactant? How many moles of water would be produced?5. If you had 1.73 moles of hydrogen (H2) and 0.89 moles of oxygen (O2), which is the limiting reactant? How many moles of water can you produce from your supply of hydrogen and oxygen?6. If you had 17.3 g of hydrogen and8.91 g of oxygen, which is the limiting reactant, and how many grams of water could you produce?Problems1. Cisplatin is an antitumor agent. It has the molecular formula Pt(NH3)2Cl2. How many grams of cisplatin can be produced if the limiting reactant is 1 kg of platinum?2. Hydrogen cyanide is used in the production of cyanimid fertilizers. It is produced by the following reaction. (a) How much hydrogen cyanide can be produced starting with 100 kg of each of the reactants? (b) Which is the limiting reactant?2 CH4 + 2 NH3 + 3 O2Æ 2 HCN + 6 H2O。

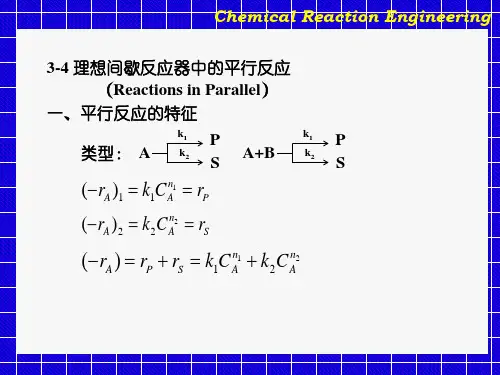
化学反应工程期末总结重点知识点归纳1、化学反应分类:(按相类分类)均相反应,非均相反应,(按操作分类)间歇操作,连续操作,半连续操作。
2、反应器分类:⑴ 管式反应器,一般长径比大于30⑵ 槽式反应器,一般高径比为1—3 ⑶ 塔式反应器,一般高径比在3—30之间;按传热条件分类:等温反应器,绝热反应器,非等温、非绝热反应器3、化学反应速率:4、化学反应动力学方程: 阿累尼乌斯关系:5、反应级数:m ,n :A ,B 组分的反应级数,m +n 为此反应的总级数。
如果反应级数与反应组份的化学计量系数相同,即m =a 并且n =b ,此反应可能是基元反应。
基元反应的总级数一般为1或2,极个别有3,没有大于3级的基元反应。
6、有如下基元反应过程,请写出各组分生成速率与浓度之间的关系。
7、动力学方程的方法:积分法、微分法8、化学反应器的设计基础:按混淆分类:间歇反应器,平推流反13s m mol d d 1--⋅=t V r ξ13B A c A s m mol --⋅=-n m c c k r RT Ek k -=ec0c 222A B C A C DB D E +↔+↔+↔222123422125622212342345656222222A A BC A CD B A B C B DE C A B C A C DD A C D B D EE B D Er k c c k c k c c k c r k c c k c k c c k c r k c c k c k c c k c r k c c k c k c c k c r k c c k c =-+-+=-+-+=--+=--+=-应器(理想置换反应器、活塞流反应器),全混流反应器9、间歇反应器的特点:①由于剧烈搅拌、混合,反应器内有效空间各位置的物料温度、浓度都相同;②由于一次加料,一次出料,反应过程没有加料、出料,所有物料在反应器停留时间相同,不存在不同停留时间物料的混合,即无返混现象;③出料组成与反应器内物料的最终组成相同;④为间歇操作,有辅助生产时间。



化学反应式英文Chemical Reaction Equations。
Chemical reactions are the processes in which substances, known as reactants, are transformed into different substances, known as products. These reactions are described using chemical reaction equations, which provide a concise representation of the reactants and products involved. In this article, we will explore the fundamentals of chemical reaction equations and their importance in understanding and predicting chemical reactions.A chemical reaction equation consists of two main components: the reactants and the products. The reactants are the substances that undergo a chemical change, while the products are the substances that are formed as a result of the reaction. The reactants are written on the left side of the equation, separated by a plus sign (+), and the products are written on the right side, also separated by a plus sign. An arrow pointing from left to right is used to indicate the direction of the reaction.For example, let's consider the combustion of methane, a common hydrocarbon. The chemical reaction equation for this process is:CH4 + 2O2 → CO2 + 2H2O。

化学反应式英文English:A chemical reaction equation is a symbolic representation of a chemical reaction using chemical formulas. It shows the reactants on the left side of the equation and the products on the right side, separated by an arrow indicating the direction of the reaction. The coefficients in the equation represent the relative amounts of each substance involved in the reaction. Additionally, the equation must be balanced to satisfy the law of conservation of mass, meaning that the same number of atoms of each element must be present on both sides of the equation. This balancing is achieved by adjusting the coefficients until the equation is balanced. Chemical reaction equations are essential in understanding and predicting the outcome of chemical reactions, as they provide a concise and specific description of the chemical change that occurs.中文翻译:化学反应式是使用化学式对化学反应进行象征性表示的方式。
化学分析员面试常见问题化学分析员面试常见问题1、鉴别检查在药品质量控制中的意义及一般杂质检查的主要项目是什么? What are the purposes of drug identification and test? What arethe usual items of drug tests?.2、比色比浊操作应遵循的原则是什么? What are the standard operation procedures for the clarity test?3、试计算葡萄糖重金属检查中标准铅溶液的取用量。
How much of the lead standard solution should be taken for thelimit test for heavy metals in this experiment?4、古蔡氏试砷法中所加各试剂的作用与操作注意点是什么? What precautions should be taken for the limit test for arsenic(AppendixVIII J, method 1)? And what is thefunction for each of the test solutions added?5、根据样品取用量、杂质限量及标准砷溶液的浓度,计算标准砷溶液的取用量。
Figure out the amount of the arsenic standard solution that shouldbe taken for the limit test for arsenic(Appendix VIII J, method 1) (0.0001%) inthis experiment with the specified quantity of2.0 gof sample.6、炽灼残渣测定的成败关键是什么?什么是恒重?What is the key step during the determination of residue onignition? What does ‘ignite or dry to constant weight’ mean?7、盐酸普鲁卡因的鉴别原理是什么?What are the principles of the identification of ProcaineHydrochloride.8、盐酸普鲁卡因注射液中为什么要检查对氨基苯甲酸?Why is the limit of 4-aminobenzoic acid tested for ProcaineHydrochloride?9、薄层色谱法检查药物中有关物质的方法通常有哪几种类型?本实验属于哪种?与其它方法有何异同点? How many kinds of thelimit tests for related compounds arethere? What are the differences between them? Which one is used for the limittest of 4-amino-benzoic acid in ProcaineHydrochloride Injection?10、醋酸氢化可的松的鉴别原理是什么?What are the principles of the identification of hydrocortisoneacetate?11、甾体激素中“其它甾体”检查的意义和常用方法是什么?What are the commonly used method for and the significance of thelimit test for other steroids for the steroidal drugs?12、哪类甾体激素可与四氮唑蓝产生反应,是结构中的何种基团参与了反应,反应式是什么?What kind of steroidal drugs can react with the alkalinetetrazolium blue TS? What is the chemicalreaction equation?13、氯贝丁酯的'鉴别原理是什么?What are the principles of the identification of clofibrate?14、氯贝丁酯中为什么要检查对氯酚?其方法及原理是什么?Why is the limit ofp-Chlorophenol tested for clofibrate?What kind of method is employed for the test and what is the principle?15、气相色谱法检查杂质有哪些方法,试比较各种方法的特点?How many types of methods are there for the test of relatedcompounds by the gas chromatography? What are the differences between them?16、抗生素类药物的鉴别和检查有何特点?What are the characteristics for the identification and tests ofantibiotics?17、钠盐的焰色反应应注意什么?What precautions should be taken during the flame reaction ofsodium salts?18、本品吸收度检查的意义是什么?What is the purpose of the light absorption tests for benzylpenicillinsodium?19、药物晶型测定的常用方法有哪些,各有什么特点?What are the commonly used methods for the test of polymorphism?And what are the characteristics of each of them?20、吸收系数测定方法与要求?What are the standard operation procedures for the establishmentof specific absorbance?21、写出异烟肼与溴酸钾的滴定反应式和滴定度的计算过程。
电解反应方程式的书写方法Writing an electrolysis reaction equation can be quite daunting at first, but with practice and understanding, it becomes easier. When writing such an equation, it is essential to remember that electrolysis involves the breaking down of compounds using an electric current. This process results in the formation of new substances at the electrodes. To write the equation, one must first identify the reactants (the compounds being broken down) and the products (the new substances formed).写电解反应方程式起初可能会让人感到困惑,但通过练习和理解,这变得更容易。
在编写这种方程式时,重要的是要记住电解是指使用电流分解化合物的过程。
这个过程会在电极上形成新的物质。
要写出方程式,首先必须确定反应物(被分解的化合物)和生成物(形成的新物质)。
When writing an electrolysis reaction equation, the first step is to identify the reactants and products. For example, in the electrolysis of water, the reactants are water molecules (H2O), and the products are hydrogen gas (H2) and oxygen gas (O2). The next step is to determine which ions are present in the reactants and products. Inthe case of water, it dissociates into hydrogen ions (H+) and hydroxide ions (OH-) when an electric current is passed through it.在编写电解反应方程式时,第一步是确定反应物和生成物。
HSC 5.0 热力学方程的有关计算1) 我想了解甲烷与氧的反应的晗了解甲烷与氧的反应的晗,,吉布斯自由能吉布斯自由能,,以及以及反应能否进行反应能否进行反应能否进行??进入Reaction Equations :输入CH4(g)+O2(g)=CO(g)+H2(g), 点击Balance Equation , 会自动配平 2CH4(g) + O2(g) = 2CO(g) + 4H2(g)点击Calculate,看到delta H, S, G, 以及平衡常数 K在 0 ºC, Delta G<0,反应能进行,但估计是一个很慢的反应(看看动力学)。
800 ºC, Delta G<<0,热力学上反应能进行。
我们实验都是这么做的。
2) 我想知道TiO2与NH3是否是否能能反应生成 TiN 。
可能的配平(抱歉!因为我不知道产物有什么)Calculate :Result: 动力学通不过。
实际上许多文献报道:TiO2与氨反应成TiNxOy ,能做光催化的催化剂。
我做了几个平衡:如Delta G都是大于0,反应不能进行。
请其他人可以试试加入其他的产物和配平,看这个反应是否热力学上能进行。
3) 甲烷甲烷水气水气水气转化转化转化重整重整重整是是如何如何进行进行进行计算计算计算的的。
这个文件是从 Ni+CO 那个文件改的:把Ni,CO,NI(CO)4 简单的换了一下Save as CH4+H2O.igi选Temperature 为x 轴, OK按住 Ctrl ,鼠标点击每个物种, Ok选Temperature, Equilibrium amount如果用Create New Input File (give elements):如果用 Create New Input File (give Species),直接加入反应物,CH4,O2,以及产物 H2和CO。
Amount 自己设的,CH4=1mol, O2=2mol,产物不管。
10. REACTION EQUATIONSClicking the Reaction Equations button in the main menu shows the Reaction EquationsWindow, see Fig. 1. With this calculation option you can calculate the heat capacity,enthalpy, entropy and Gibbs energy values of a single species as well as of specifiedreactions between pure substances.See the reference state definitions, valid notations and abbreviations for the description ofthe chemical formulae in Chapter 28. Databases, chapter 2.10.1 One Chemical SubstanceFig. 1. Reaction Equations Window of HSC Chemistry.Fig. 2.Thermodynamic data of A12O3 (alumina) displayed by the Reaction Equation option of HSC Chemistry.By entering a single chemical formula into the Formula box you will get similar tables ofthermochemical data as presented in many thermochemical data books. HSC will,however, provide the results faster and exactly at those temperatures which you reallywant. Please follow these steps:1.Write a chemical formula into the box, Fig. 1.For example: Fe, Na2SO4, Al2O3, SO4(-a), H(+a) or SO2(g).See the valid notation and syntax of chemical formulae in Chapter 21.2.2.Select the lower limit, upper limit and temperature step.3.Select the Temperature and Energy Units, by clicking the corresponding buttons.4.Select the Format of the results.Normal (Absolute scale):H(species), S(species) and C(species)This format is used for example in the famous I. Barin, O. Knacke, and O.Kubaschewski data compilation1.Delta (Formation functions):∆H = H(species) - ∑H(elements)∆S = S(species) - ∑S(elements)∆G = G(species) - ∑G(elements)∆G = G(ions) - ∑G(elements) + z/2*G(H2(g)) - z*G(H(+a))z = charge.This format is used for example in the NBS Tables NBS 82.5.The Collect to Sheet option will collect several tables on the same spreadsheet.6.Select the Show Transitions option if you also want to see the data at the phasetransformation temperatures, such as crystal structure changes and melting.7.Select the Criss-Cobble option if you want a Criss-Cobble extrapolation for theheat capacity of aqueous species, see Chapter 21.4.8.Press Calculate (or Enter) to get the results on the screen.9.Press Print to print the results, see Fig. 2. Note that you can collect several sets ofresults in the same sheet if you have selected the Collect to Sheet option in Fig. 1.You can clear the whole sheet by pressing Clear.10.Press Copy All to get all the results into the Clipboard, then you can easily paste theresults to other Windows applications, for example, to MS-Excel, see Fig. 2. UsingCopy it is possible to copy and paste contents of individual cells to otherapplications.11.If you want to save the formula and results in an ASCII-file press Save, see Fig. 2.You can read these files back to the Reaction module using File Open, see Fig. 1.Note that Save saves all the selections in Fig. 1, so you can return these using FileOpen. The File Open HSC 2 File button reads only old HSC 2.0 files which returnonly formula, but not the selections nor temperature range.Note: 1.You can easily check the basic data from the database, which has been used in the reaction module calculations. In Fig. 1 select the formula in the Reaction Equationbox and press Peep Database. The same procedure can be found in Fig. 2 bypressing the right mouse button or selecting Edit from the menu.2.The table in Fig. 2 has some formatting and Copy - Paste functions as do othertables in HSC Chemistry. These features help to create a good printed copy of theresults for various purposes.3.HSC searches for the species data first from the Own database (OwnDB5.HSC). Ifit does not find a species there, it will search from the Main database(MainDB5.HSC). Therefore HSC always uses data in the Own database if the samespecies exists in both Own and Main databases.4.If you have selected Delta-format for the results, HSC will also search for data forthe necessary elements and calculate the formation functions of enthalpy, entropyand Gibbs energy. Usually the original experimental data is in this format: however,sometimes the comparison of data in this format may be more difficult because thedata sources often use different data for elements.5.HSC will make a Criss-Cobble extrapolation for the heat capacity of aqueousspecies at elevated temperatures (> 25 C°) if the Criss-Cobble option is selected.The extrapolation is not done if A and B of the heat capacity coefficients A, B, Cand D exist in the HSC Chemistry databases. The extrapolation error increasesrapidly at higher temperatures. More information on extrapolation is given inChapter 28.4.6.For aqueous species it is recommended to set:Lower temperature = 25 °CUpper temperature = 300 °CStep = 25 °C10.2 One Chemical Substance ResultsAfter pressing the Calculate button in the previous screen, Fig. 1, you will arrive in theresults window, Fig. 2. From this results screen you can save and print the results:1.Press Save if you wish to recalculate the results later. The Save button will alsosave the settings used in Fig. 1. You can read these files back to HSC using the FileOpen button, see Fig. 1.2.Press Print if you want a paper copy. If you have selected the Collect to Sheetoption in Fig. 1, then all results will be collected on the same sheet and can beprinted together. Clear will clear the results sheet.3.Press Copy to get the results of selected cells into the Clipboard, then you caneasily paste the results, for example, to MS -Excel, see Fig. 2.4.Press Copy All to get all the results of the sheet into the Clipboard, then you caneasily paste the results, for example, to MS -Excel, see Fig. 2.5.Press OK to return to the previous Window.10.3 Reaction EquationsFig. 3. Input data for Reaction Equation calculations.Fig. 4. Results of Reaction Equation calculations.You can write almost any kind of reaction equation into the Reaction Equation box ofHSC, Fig. 3. Here are some examples of valid equation syntax:2Cu + S = Cu2SH2O = H(+a) + OH(-a)H2(g) = 2H(+a) + 2e-H2O = 1/2O2(g) + 2H(+a) + 2e-Ag = Ag(+a) + e-3NO2(-a) + 2H(+a) = 2NO(g) + H2O + NO3(-a)2Al(+3a) + 3S(-2a) + 6H2O = 2Al(OH)3 + 3H2S(g)Write the reaction equation into the box, see Fig. 3. If you have not given the stoichiometric coefficients for the species, you can press Balance Equation for solving unknown coefficients. The balance button solves the coefficients on the basis of element balance equations. Therefore it cannot solve unknown coefficients if their number is larger than the number of elements in the corresponding reaction.On the right side of the button you may give a multiplier, which will be used to multiply all the coefficients in the reaction equation. The default value is 1, which means that the smallest stoichiometric coefficient in the reaction equation is 1.You can continue in the same way as in the One Chemical Formula option Chapter 10.1. Note that the Delta Format and Show Transitions options have no effect on the results, because the enthalpy and Gibbs energy of a reaction are in the Delta format by definition.HSC calculates the stoichiometry of the reaction given by the user, and points out errors if the element balance is incorrect.The example in Fig. 3 refers to the Mond-process for refining impure nickel. In the process raw impure nickel is first treated with CO gas at 60 °C to evaporate the nickel as a carbonyl gas. In the second stage, the temperature of the gas is increased to 200 °C to decompose the nickel carbonyl gas into pure metallic nickel and CO. This process works because the equilibrium of this reaction is on the right side (Equilibrium constant K > 1) at lower temperatures and on the left side (K < 1) at higher temperatures. The reaction is exothermic (DH is negative) at all temperatures.Vapor pressures p can be calculated by writing the reaction equation for the vaporization reaction concerned. For example, for pure magnesium the equilibrium is Mg = Mg(g), Fig.5. The activity a of pure magnesium is 1 and thus the vapor pressure in bar is equal to the equilibrium constant according to Eq. (10) in Chapter 8. Introduction and Eq. (1). See also Fig.6.K = p Mg / a Mg = p Mg[1]If a substance vaporizes into several polymers, all of them must be taken into account. The total vapor pressure is then the sum of all the individual partial pressures, if the gas phase behaves ideally.You can also calculate more complicated reactions. First write the reaction as shown in Fig. 7, then press Balance for the coefficients, see Fig. 8 and finally press Calculate for the results, see Fig. 9. Note that for aqueous ionic reactions HSC also calculates the electrode potential versus Standard Hydrogen Electrode if electron (e-) is used in the formula.You can calculate mass balances in moles, grams and liters for any reaction. The species does not need to exist in the HSC databases.Fig. 5. Input data for Reaction Equation calculations.Fig. 6. The equilibrium constant K is equal to the vapor pressure in atm according to Equation (1) if the activity of magnesium is 1. The boiling point of magnesium is about 1100 °C beyond which its vapor pressure exceeds 1 atm.Fig. 7. Write the reaction equation without stoichiometric coefficients and press Balance Equation.Fig. 8. Press Calculate to display the results.Fig. 9. Results for an aqueous ionic reaction.The data used to calculate the results can be displayed by selecting one substance in theReaction Equation box, see Fig. 3 and pressing Peep Database. The same procedure canbe found in the Results window, see Fig. 4, by pressing the right mouse button. Thedatabase content is shown in Fig. 10. Insert is available for inserting a selected formulainto the Reaction Equation box. Remove will remove a selected formula from this samebox. Note that the selections and editing in Fig. 10 do not have any effect on the HSCdatabases.Fig. 10. The database window.10.4 Reaction Equations ResultsThe operation of the buttons in Fig. 3 are described in the previous chapter. The meaningof the results can be summarized as the following:1.If the equilibrium constant K is < 1 (or log(K) < 0) the reaction goes to the left.2.If the equilibrium constant K is > 1 (or log(K) > 0) the reaction goes to the right.3.Negative Enthalpy H of the reaction means that the reaction is exothermic, i.e. heatis released, Equation 7 in 8. Introduction.4.Positive Enthalpy H of the reaction means that the reaction is endothermic, i.e. heatis needed, Equation 7 in 8. Introduction.5.Delta Format has no effect on the results of reaction equations.6.In ionic reactions POTENTIAL E yields the electrochemical potential (in Volts)versus the Standard Hydrogen Electrode.7.Equilibrium constant K is calculated using Equation 11 in 8. Introduction.。