诺贝尔奖与遗传学
- 格式:ppt
- 大小:1.83 MB
- 文档页数:68


从历年诺贝尔奖看⽣物学科(1985-2019)诺贝尔奖是我们中国⼈的梦想。
中国已获得两个诺贝尔奖,第⼀个是2012莫⾔的诺贝尔⽂学奖,第⼆个是2015屠呦呦的诺贝尔⽣理或医学奖。
今天,带⼤家⼀起了解⼀下⽣物领域诺贝尔奖的获奖情况。
⽣命科学的研究领域⾮常⼴泛,有⽣理学、遗传学、⽣物化学、细胞⽣物学、分⼦⽣物学等等。
让我们⼀起来了解诺贝尔奖获得者的⼯作,从⽽更好地理解这个学科。
细胞⽣物学有 1/3 以上的获奖项⽬与细胞⽣物学研究有关,所以你懂的。
那么细胞⽣物学主要研究哪些内容呢?概括地说,细胞⽣物学是研究细胞内部结构和功能的学科。
这个有点抽象,直⽩点说,⾸先要发现各种结构和功能各异的蛋⽩质、DNA、RNA、糖类、脂类化合物等。
然后研究这些⽣命分⼦在细胞内外是如何组织起来和相互作⽤的。
这些分⼦位于哪些区域,是线粒体,还是核糖体、溶酶体,哪些分⼦和哪些分⼦结合或靠近等等。
可能你会说都知道了⼜有卵⽤。
那还真是挺有⽤的,⽐如新药研发。
药物都必须作⽤于细胞活动的特定环节,假如这个药物结构特别,没法进⼊,那就必须和细胞表⾯的特定受体结合,⽐如 G 蛋⽩偶联受体,从⽽发挥药效。
●诺奖获奖项⽬1985 年:在胆固醇代谢的调控⽅⾯的发现。
1986 年:发现⽣长因⼦。
1989 年:发现逆转录病毒致癌基因的细胞来源。
1991 年:发现细胞中单离⼦通道的功能。
1992 年:发现可逆的蛋⽩质磷酸化作⽤是⼀种⽣物调节机制。
1994 年:发现 G 蛋⽩及其在细胞中的信号转导作⽤。
1999 年:发现蛋⽩质具有内在信号以控制其在细胞内的传递和定位。
1998 年:发现在⼼⾎管系统中起信号分⼦作⽤的⼀氧化氮。
2001 年:发现细胞周期的关键调节因⼦。
2009 年:发现端粒和端粒酶如何保护染⾊体。
2012 年:发现成熟细胞可被重写成多功能细胞。
2013 年:发现细胞重要运输系统—囊泡传输系统的奥秘。
2016 年:细胞⾃噬研究。
神经⽣物学神经⽣物学是当今⽣命科学领域最具活性的学科之⼀,有⼈称之为 21 世纪的明星学科。

2009年诺贝尔生理学或医学奖引言2009年,诺贝尔生理学或医学奖揭晓了由三位科学家共同获得的荣誉。
他们通过对细胞生物学和遗传调控的研究,做出了重要的贡献,为人类健康和医学领域的发展带来了突破性的进展。
本文将对这三位诺贝尔奖获得者及其研究成果进行介绍和分析。
诺贝尔奖获得者2009年诺贝尔生理学或医学奖由伊丽莎白·布莱克本、卡罗尔·格雷德尔和杰克·沙泌尔共同获得。
他们的研究突破了细胞生物学和分子遗传学的重要难题,为后续研究和治疗疾病提供了重要的理论基础。
研究成果端粒酶逆转录酶的发现和功能伊丽莎白·布莱克本和卡罗尔·格雷德尔的工作主要集中在细胞端粒酶逆转录酶(telomerase)的研究上。
端粒酶逆转录酶是一种能够延长染色体末端的酶,它在细胞分裂过程中起着关键的作用。
在布莱克本和格雷德尔的研究中,他们发现了端粒酶逆转录酶的存在,并揭示了它与细胞衰老和癌症发展之间的关系。
通过对细胞中端粒酶逆转录酶的活性进行研究,布莱克本和格雷德尔发现了一种叫做“端粒”的结构。
端粒位于染色体末端,能够保护染色体免受损伤和衰老。
他们的发现为后续研究提供了重要的线索,帮助科学家们更好地理解染色体的稳定性和细胞衰老的机制。
RNA干扰的发现与应用杰克·沙泌尔的工作则集中在RNA干扰(RNA interference)的研究上。
RNA干扰是一种基因调控的机制,通过介导特定RNA分子的降解或抑制,来控制靶基因的表达。
沙泌尔的研究发现了一种叫做“小干扰RNA”的分子,它们能够干扰靶基因的转录或翻译过程。
这项发现不仅揭示了RNA干扰机制的存在,还为科学家们开辟了一条新的基因治疗途径。
利用小干扰RNA可以有效地靶向控制基因表达,为治疗疾病提供了新的思路和方法。
科学意义和应用前景这三位诺贝尔奖获得者的研究成果为细胞生物学和遗传调控领域带来了重大的突破,对生命科学的发展产生了深远影响。

发现了调控细胞周期的关键物质利兰·哈特韦尔Leland H. Hartwell美国哈钦森癌症研究中心1939年—蒂莫西·亨特Tim Hunt英国英国帝国癌症研究基金会1943年—保罗·纳斯Sir Paul M. Nurse英国英国帝国癌症研究基金会1949年—所有生物体都由通过分裂而增殖的细胞构成。
一个成年人大约拥有100万亿个细胞,而这些细胞都源于一个受精卵细胞。
同时,成年人机体中大量的细胞还通过不断的分裂产生新细胞,以取代那些死亡细胞。
细胞必须长大到一定的程度,复制染色体,并把染色体准确地分给两个子细胞,然后细胞才能分裂。
这些不同的进程成为细胞周期。
荣获2001年诺贝尔生理学或医学奖的科学家做出了有关细胞周期的重要发现。
他们识别出了所有真核生物中调节细胞周期的关键分子,真核生物包括酵母菌、植物、动物和人。
这些基础的发现对细胞生长的所有方面都具有巨大的影响。
细胞周期控制的缺陷会导致肿瘤细胞中的某种染色体改变。
这些发现能让我们在今后很长的时间内创造治疗癌症的新方法。
哈特韦尔因为发现了控制细胞周期的一类特异基因而受奖。
其中一个叫“启动器”的基因对控制每个细胞周期的初始阶段具有主要作用。
哈特韦尔还引入了一个概念“检验点”,对于理解细胞周期很有帮助。
纳斯用遗传学和分子学方法,识别克隆并描绘了细胞周期的一个关键调节物质CDK。
他发现CDK的功能在进化中被很好的保存了下来。
CDK是通过对其他蛋白质的化学修饰来驱动细胞周期的。
亨特的贡献是发现了细胞周期蛋白(cyclin)——调节CDK功能的蛋白质。
他发现细胞周期蛋白在每次细胞分裂中都周期性地降解,该机制被证明对控制细胞周期全程的重要性。
发现了“器官发育和细胞程序性死亡”的遗传调控机制悉尼·布雷内Sydney Brenner英国美国伯克利分子科学研究所1927年—罗伯特·霍维茨H. Robert Horvitz美国美国麻省理工学院1947年—约翰·苏尔斯顿John E. Sulston英国英国剑桥桑格中心1942年—英国科学家悉尼·布雷内,选择线虫作为新颖的实验生物模型,这种独特的方法使得基因分析能够和细胞的分裂、分化,以及器官的发育联系起来,并且能够通过显微镜追踪这一系列过程。

2023年诺贝尔生理学或医学奖相关考点【标题】2023年诺贝尔生理学或医学奖:解析与考察【引言】自从1901年创立以来,诺贝尔奖一直是科学界最崇高的荣誉之一。
其中,生理学或医学奖因其对人类健康和疾病研究的突出贡献而备受关注。
在即将到来的2023年,诺贝尔生理学或医学奖必将再度揭晓,本文将对其相关考点进行探究和解析,为读者透彻理解这一科学殿堂打下坚实基础。
【1. 病理学研究——窥见人类疾病本质】1.1 亚太地区的肺癌问题肺癌作为亚太地区最常见的癌症之一,给该地区的人口健康带来巨大困扰。
为了解决肺癌问题,诺贝尔奖评委有望关注某些具有突破性的研究,如检测与筛查新方法、肿瘤免疫学的进展以及靶向治疗等。
1.2 神经退行性疾病的治疗突破随着人类寿命的延长,神经退行性疾病如阿尔茨海默病和帕金森病等的发病率呈上升趋势。
针对这些疾病,诺贝尔奖对于解析病理过程和探索新的治疗方法将产生浓厚兴趣,可能关注具有革命性进展的研究,如光遗传学、脑机接口技术等。
【2. 基因组学和遗传学领域的突破】2.1 Crispr-Cas9技术的革命性应用近年来,基因组编辑技术Crispr-Cas9以其高效精准的特点,引起了科学界的巨大关注。
该技术的应用涉及基因突变疾病的治疗、农作物改良等诸多领域。
诺贝尔评委可能会将目光聚焦在与Crispr-Cas9相关的研究上,如改进技术、扩大应用范围等。
2.2 基因变异与复杂疾病的关联复杂疾病的发生往往与基因的变异相关。
诺贝尔奖评委可能将关注点放在基因组学和遗传学的交叉领域,即寻找复杂疾病发病机制的关键基因、探索基因之间的相互作用等。
【3. 免疫学与抗病机制研究】3.1 免疫检查点抑制剂的革命性突破免疫检查点抑制剂(PD-1/PD-L1)是近年来肿瘤治疗的一大突破,其通过激活患者自身免疫系统来攻击癌细胞。
鉴于免疫治疗的巨大潜力,诺贝尔奖评委可能会关注该领域的研究并认可对免疫检查点抑制剂疗法的突破性贡献。


诺贝尔奖Nobel Prize 的创新思维诺贝尔奖Nobel Prize 包括自然界的三大科学奖:物理学奖、化学奖、生理学或医学奖。
我们举基因研究获奖为例,探讨科学研究中的创新思维。
宗所周知因研究基因而获得诺贝尔奖的多达50多人,(gene) 概念最早由W. L. Johannsen 于1909 年提出,是DNA 分子中能够表达和产生基因产物的区段,其本质是核酸。
Kossel 是最早研究核酸而获得诺贝尔奖的科学家,因在确定核酸的化学特性和化学组成等方面做出重大贡献而获1910 年诺贝尔生理学奖。
1953 年Watson 和Crick 在Nature 发表关于DNA 双螺旋结构模型,标志着现代分子生物学的诞生。
自此因研究基因而获得诺贝尔奖的科学家明显增多。
分析这些科学家的成功因素,我们可以从中得到一些有益的启示。
一.研究载体很重要:科学研究材料的选择是十分重要的因素.从获得诺贝尔奖的科学家来看,选择的实验材料是十分重要的因素。
果蝇具有一些不同于其他生物的优点:生活周期短,繁殖快,一年可以繁殖30 代,雌性与雄性个体区分比较明显,并且每个细胞中只含有4 对染色体,这些优点使果蝇适合于做遗传学研究,先后有5 位科学家以果蝇为实验材料而获诺贝尔奖。
链孢霉是一种丝状真菌,菌丝体为单倍体。
Beadle 等最初也是以果蝇为实验材料进行遗传学方面的研究,但后来意识到果蝇对于研究基因与代谢途径并不是一个好的模型,于是就和Tatum 合作,选择了链孢霉作为实验材料。
Syd2ney Brenner 决定从分子生物学转而研究神经系统的发育时,同样面临模式动物的选择的问题。
他当时已经有了明确的标准:周期短,可以在短时间得到大量的突变体;繁殖方式简单,以便利遗传操作;个体足够小,使得可以在显微镜下对每一个细胞进行观察。
于是他找到了线虫,并用EMS 突变的方法分离了一部分的突变体,主要是形态异常和运动不协调的突变体。
在所有多细胞模式动物中,线虫最大的优势是可以很方便的观察到每一个细胞的分裂及其命运、神经细胞之间的连接。

细胞生物学1987年诺贝尔生理学或医学奖“抗体多样性的遗传学原理”获奖者:利根川进the genetic principle for generation ofantibody diversitySusumu TonegawaThe Nobel Prize in Physiology or Medicine 1987"for his discovery of the genetic principle for generation of antibody diversity"Massachusetts Institute of Technology (MIT)SummaryMan is surrounded by viruses, bacteria and other microorganisms which constitute a threat to life and health. When these contagious agents enter the body they are recognized and attacked by the immune defence. Important tools in the recognition of this large variety of intruders are the antibodies. They are produced by white blood cells called B lymphocytes. The parts of the microorganisms against which antibodies react are called antigens. The number of different antigens that the body may encounter is enormous. We are dealing with hundreds of millions of substances, all of them with their specific structure. Strangely enough our immune defense have at hand antibodies which can identify all these molecules and start to counter attack - that is, hundreds of millions of different antibodies which are ready in the body already in advance before they have seen the antigen against which they can react!This fabulous capacity to vary of antibodies is known since a couple of decades. The genetic background allowing this variation has, however, been an unsolved puzzle. The structure of the antibodies is determined by genes but as the human genome only contains about 100 000 genes it seemed unreasonable that they could allow the production of maybe a billion different antibodies.The man who explained this mystery is the Japanese Scientist Susumu Tonegawa. In a pioneering study published in 1976 Tonegawa could through a series of ingenious experiments show how parts of the genome of the cell (DNA) is redistributed under its differentiation from an embryonic cell to an antibody producing B lymphocyte. During the following two years Tonegawa completely dominated this area of research. He could in increasingly greater detail clarify how those parts of the genome which gives rise to antibody are moved around in order to allow each B lymphocyte to produce its own unique antibody. Tonegawas discoveries have increased our knowledge about structure of our immune defense. They also open up possibilities to increase the immune response against pathogenic microorganism through vaccination - and also to improve inhibition of unwanted immune reactions.The antibody, a molecule with many facesThe antibody is a protein where the building stones - amino acids normally form four chains. Two of these chains (polypeptides) are longand identical. The other two are short and are likewise identical. Together the four polypeptide chains form a Y-like symmetric molecule (Figure 1). Figure 1. A picture of an antibody molecule with two long (T) and two short (L) polypeptide chains which are kept together by sulphur bridges (-S-S). The variable parts of a long chain (V,D, and J) and a light chain (Y and J) together form the antigen binding area of the antibody.In man there are five different types of long chains which have been given letters M, D, G, A and E. The naming of the long chains forms the bases for the names of the five so called immunoglobulin classes: IgM, IgD, IgG and so on. The short chains are of two types: kappa or lambda. Each antibody molecule has - regardless of class - either two kappa or two lambda chains.Towards the base of the Y there is a constant part where the sequence of amino acids is the same in all antibodies belonging to the same class. In the outer ends of the two arms of the Y, however, there exist a significant variation in the amino acid sequence when comparing different antibodies. In this variable part there are three areas where variation is very large. These areas constitute the walls in a "pocket" where the foreign substance, the antigen, will fit and can bind. Y ou can make the analogy of an antibody molecule with a lobster where the claws of the lobster correspond to the antigen binding parts of the antibody.Through its Y-form the antibody accordingly is endow two identicalantigen binding areas. These areas have a more or less good fit to a particular antigen. The better the fit the harder to grip of the antigen and the more efficient the defense. As we are continuously confronted within an enormous variety of antigens we also have to have a large number of molecules there the variable parts do fit to different antigens.The constant part of the antibody does also contain important biological functions. After the binding of the antibody to an antigen on the surface on for example a virus (Figure 2) the antibody molecule is changed in such a way that its constant part will activate important parts of the immune defense. Among these is the complement system which can directly make holes in bacteria and other microorganism and which also attract white blood cells such as macrophages ("big eaters") and granulocytes to the battle ground.Figure 2. A polio virus particle is attacked by four IgG antibodies. Through this attack the infectious capacity of the virus is destroyed. It is mainly through this mechanism that polio vaccine is functioning.The richness of variety, an equation which didn't add upAntibodies are produced by a special kind of white blood cells which are called B lymphocytes and which in an adult human being amounts to one million million cells (1012). As a single B lymphocyte only can produce its own unique antibody the number of different antibodies in an individual can theoretically not exceed that of the number of Blymphocytes.The information how antibodies should be constructed lies in the genome of the B lymphocytes. One hypothesis suggested, that in this genome there exist one gene responsible for each type of polypeptide chain in the antibody. But here the problem was that the immune defense contains hundreds of thousands times more different antibody types than there are total number of genes in the B cells. The equation simply didn't add up and the hypothesis had to be abandoned. It was replaced by a second one which explained the almost limitless capacity of variation in antibodies as a result of some changes in the DNA of the B cell during the development of the individual.Susumu Tonegawa was the one who finally answered the question how the gene material in B cells could suffice to create the structures of a seemingly endless number of different antibodies. In 1976 he could in a convincing and elegant manner show how different immunoglobulin genes which were far apart in the embryonic cell in the B lymphocyte had been moved in closer contact. Under development from the germ cells (the sperm and egg cell) to an antibody producing B lymphocyte the genes forming the immunoglobulins had accordingly been redistributed. In subsequent experiment Tonegawa could clarify how different pieces of the genome were moved around, recombined and even could be "lost" to finally give rise to the DNA which is found in the mature B lymphocyte.In the human the genes for the long chains are present on chromosome 14, for the kappa chains on chromosome 2 and for the lambda chains on chromosome 22. Thanks to Tonegawa's pioneering work we now know how many immunoglobulin genes there are in man, how they are put together and how they can give rise to this high number of different antibodies.Figure 3. Redistribution of immunoglobulin genes for the long chain during the development from an embryonic cell (top) to an antibody producing B lymphocyte (bottom). Genes from each group V, D and J are brought together in the final form for functioning gene for the variable part of the long chain of an antibody molecule.Economy through wasteToday we know that three groups of genes participate in the creation of the variable part of the long chain, that is the part which together with the variable part of the short chain is specific for each antibody. These genes have the names V, D and J (Figure 3). The short chain has V and J genes. In man the number of different Y genes for the long chains are around 200 to which should be added about 20 D genes and 4 J genes. When the functioning gene of an antibody is to be created a single V, D and J gene are drawn at random from the three groups of genes. The process can be compared to a numbers lottery (Figure 4). 200 x 20 x 4 will give rise to 16 000 different variable parts.Figure 4. A registration sign for a car with its unique registration number produced through lottery can illustrate the process which leads to the creation of a unique antibody molecule. This registration number stands for Susumu Tonegawa, the 144th Nobel Laureate in Physiology or Medicine.V, D and J are put together in an irregular manner which will further enhance the richness of variation. And as the V and D genes often are different when inherited from our father and mother this will mean that already here possibility has been created for something like five million different forms of the variable part of the long chain. On top of this the light chain contributes with more than 10 000 variants. The final sum will be many billions possibilities of variation.We are accordingly well prepared for an encounter with any possible antigen. It is likely that normally only a minor part of the antibody variance will ever be put into usage. The immune system is extremely economic when using the DNA of the individual. At the same time a large number of lymphocytes are produced and only a few of these will ever have to participate in the immune defense of the body. The economy in the usage of DNA is thus combined with a seeming waste of cells. This is, however, necessary to maintain the high state of alertness which is required against possible new infections.The discoveries of Tonegawa explain the genetic background allowingthe enormous richness of variation amongst antibodies. Beyond deeper knowledge of the basic structure of the immune system these discoveries will have importance in improving immunological therapy of different kinds, such as for instance the enforcement of vaccinations and inhibition of reactions during transplantation. Another area of importance are those diseases where the immune defense of the individual now attack the bodies own tissues, the so called autoimmune diseases.翻译抗体多样性的遗传学原理1987年利根川进因“发现抗体多样性的遗传学原理”而获1987年诺贝尔生理学或医学奖麻省理工学院摘要人类周围环境中的病毒、细菌和其他微生物对人类的生命和健康构成威胁。
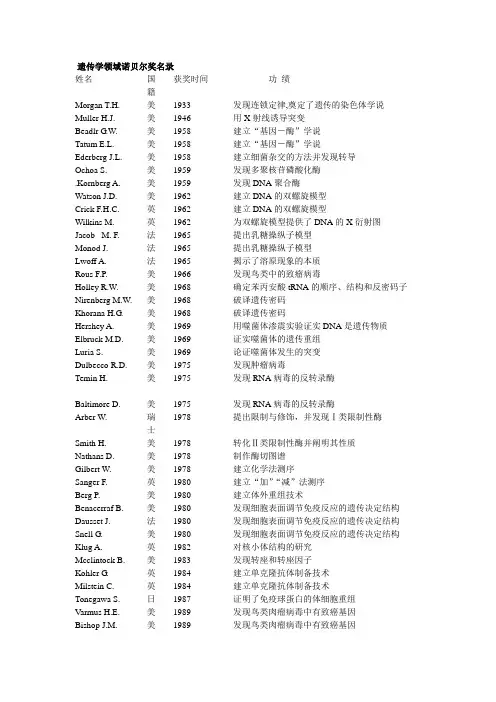
遗传学领域诺贝尔奖名录姓名国籍获奖时间功绩Morgan T.H. 美1933 发现连锁定律,奠定了遗传的染色体学说Muller H.J. 美1946 用X射线诱导突变Beadlr G.W. 美1958 建立“基因-酶”学说Tatum E.L. 美1958 建立“基因-酶”学说Ederberg J.L. Ochoa S..Kornberg A. 美美美195819591959建立细菌杂交的方法并发现转导发现多聚核苷磷酸化酶发现DNA聚合酶Watson J.D. 美1962 建立DNA的双螺旋模型Crick F.H.C. 英1962 建立DNA的双螺旋模型Wilkins M. 英1962 为双螺旋模型提供了DNA的X衍射图Jacob M. F. 法1965 提出乳糖操纵子模型Monod J. 法1965 提出乳糖操纵子模型Lwoff A. 法1965 揭示了溶原现象的本质Rous F.P. 美1966 发现鸟类中的致瘤病毒Holley R.W. 美1968 确定苯丙安酸tRNA的顺序、结构和反密码子Nirenberg M.W. 美1968 破译遗传密码Khorana H.G. 美1968 破译遗传密码Hershey A. 美1969 用噬菌体渗震实验证实DNA是遗传物质Elbruck M.D. 美1969 证实噬菌体的遗传重组Luria S. 美1969 论证噬菌体发生的突变Dulbecco R.D. 美1975 发现肿瘤病毒Temin H. 美1975 发现RNA病毒的反转录酶Baltimore D. 美1975 发现RNA病毒的反转录酶Arber W. 瑞士1978 提出限制与修饰,并发现Ⅰ类限制性酶Smith H. 美1978 转化Ⅱ类限制性酶并阐明其性质Nathans D. 美1978 制作酶切图谱Gilbert W. 美1978 建立化学法测序Sanger F. 英1980 建立“加”“减”法测序Berg P. 美1980 建立体外重组技术Benacerraf B. 美1980 发现细胞表面调节免疫反应的遗传决定结构Dausset J. 法1980 发现细胞表面调节免疫反应的遗传决定结构Snell G. 美1980 发现细胞表面调节免疫反应的遗传决定结构Klug A. 英1982 对核小体结构的研究Mcclintock B. 美1983 发现转座和转座因子Kohler G. 英1984 建立单克隆抗体制备技术Milstein C. 英1984 建立单克隆抗体制备技术Tonegawa S. 日1987 证明了免疫球蛋白的体细胞重组Varmus H.E. 美1989 发现鸟类肉瘤病毒中有致癌基因Bishop J.M. 美1989 发现鸟类肉瘤病毒中有致癌基因Cech T.R. 美1989 发现RNA的自体拼接Atman S. 美1989 发现RNA的自体拼接Mullis K. 美1993 建立PCR技术Smith M. 美1993 建立定点突变技术Sharp P.A. 加1993 发现断裂基因Roberts R.J. 美1993 发现断裂基因Gilman A. 美1994 发现细胞信息传递中的G蛋白Rodbell M. 美1994 发现细胞信息传递中的G蛋白Christine N-V. 德1995 研究了果蝇的早期发育Edward B.L. 美1995 研究了果蝇的早期发育W.Eric F. 美1995 研究了果蝇的早期发育Prusiner S.B. 美1997 朊蛋白构象的研究Boyer P.D. 美1997 三磷酸腺苷的形成过程Wolker J.E. 英1997 三磷酸腺苷的形成过程Furchgott R. F. 美1998 发现氧化氮在心血管系统中是作为一种信号分子Ignarro L.J. 美1998 发现氧化氮在心血管系统中是作为一种信号分子Murad F 美1998 发现氧化氮在心血管系统中是作为一种信号分子Blobel G 美1999 Blobel G和Bernhard Dobberstein 提出信号假说Harwell L 美2001 70年代初他发现了大量控制细胞周期的基因Nurse P 英2001 1990他发现了细胞周期蛋白依赖激酶(CDK) Hunter T 英2001 90年代初他发现了调节CDK功能的细胞周期蛋白。

论述遗传学诞生到遗传密码破译这一时期里具有重大意义的遗传学研究成果及其特点与意义1865年2月8日孟德尔根据他8年的植物杂交试验结果,在当地的科学协会上宣读了一篇题为《植物杂交实验》的论文。
但这一伟大发现被埋没了35年后才受到人们重视。
1900年遗传学诞生了。
遗传学是生物科学领域中发展最快的一门学科,几乎所有生物学科都与遗传学形成交叉学科,可见遗传学的重要性。
要想了解遗传学,就得先了解它的历史。
遗传学诞生到遗传密码破译这一时期有许多遗传学研究成果,它们对遗传学的发展有着重大的意义。
根据研究的特点,现代遗传学的发展大致可分为三个时期。
一、细胞遗传学时期(约1910-1940)1、确立了遗传的染色体学说1910年摩尔根创立了连锁定律并证明了基因在染色体上以直线方式排列,并提出了遗传的染色体理论。
这一成果还获得了1933年的诺贝尔奖。
这是一个伟大的结论,它指出了遗传的染色体学说不再是空洞抽象的概念,为遗传基因找到了物质基础;同时,它指出了某一遗传基因是在某一染色体上,为人们探索生物遗传机理开拓出了一条新路。
他阐述的基因的连锁和互换规律,解开了生物变异之迷,弥补了达尔文进化论的不足,为人们杂交育种指明了方向,为预防遗传性疾病提供了理论。
二、微生物遗传及生化遗传学时期(1941-1960)1、一个基因一个酶假说的提出G.W.Beadle和E.L.Tatum在1941年发表了链孢霉中生化反应遗传控制的研究;进而使应用各种生化突变型对基因作用的研究有了发展。
Beadle在1945年总结了这些结果,提出了一个基因一个酶的假说,认为一个基因仅仅参与一个酶的生成,并决定该酶的特异性和影响表型。
随着酶学、蛋白质化学的进展、遗传学方法的进步,进一步弄清楚了基因与酶的关系是建立在基因与多肽链严密对应的关系基础上的,表示这种对应关系的学说就是一个基因一条多肽链假说。
这一假说获得了1958年的诺贝尔奖。
这一假说为遗传物质的化学本质及基因的功能奠定了初步的理论基础。
基于诺贝尔奖情境的遗传学课程思政建设①朱慧贞,王萍,杨小芳,吴希恩,王鹏(定西职业技术学院,甘肃定西743099)一、诺贝尔奖的内涵及育人精神1895年,著名的瑞典化学家阿尔弗雷德·贝恩哈德·诺贝尔立下遗嘱,将他的遗产设立奖励基金,每年所得的利息作为奖金,颁发给在物理、化学、生理学或医学、文学及和平领域对世界有重大贡献的人。
诺贝尔物理学奖、化学奖、生理学或医学奖被称为自然科学领域的诺贝尔三大奖项。
高职学生政治思想观念比较薄弱,科学精神及人文素养普遍不足。
高职遗传学教学不仅要使学生掌握遗传学的基本知识,更重要的是让学生领悟科学精神。
科学精神体现着科学研究者的知识、思想、认知。
科学精神具体表现为实事求是、敢于质疑、包容开放、无私奉献、严谨求真、追求真理、批判创新、不断进取等。
科学精神的核心是求真、创新、奉献,其中求真是科学精神的基础,创新是科学精神的灵魂,奉献是科学精神的外在表现。
一份诺贝尔奖的获得就是一次完整的科学探索历程,基于真实的诺贝尔奖素材进行高职遗传学教学,是培养学生科学精神的良好途径。
与此同时,诺贝尔奖素材中的科学探究历程,是科学家协作共进、果敢无私、勇于奉献的真实写照,所以,诺贝尔奖也是传播人文精神的良好素材。
总之,诺贝尔奖是高职遗传学弘扬科学精神及人文精神的良好材料,对于当下高职学生有至关重要的德育意义。
二、诺贝尔奖融入高职课程的研究现状在“中国知网”官网以诺贝尔奖为主题进行搜索,与高等教育相关的文献共产生26篇,采用Citespace 文献可视化软件进分析,共现图谱如图1所示。
其中筛选出诺贝尔奖与高校思政建设相结合的文献共4篇,分别是基于诺贝尔奖案例的细胞生物学、有机合成化学、病原微生物学和医学等学科的思政建设,高职遗传学课程思政建设中融入诺贝尔奖案例的研究目前没有检索到相关文献。
图12022年以来诺贝尔奖融入高职课程思政研究现状的知识共现图谱三、诺贝尔奖融入遗传学课程思政的可行性本文挖掘高职遗传学中诺贝尔奖素材的育人元素,完善高职遗传学课程思政内容体系,对申顺先主编的《遗传学》第四版中有关诺贝尔奖的素材进行梳理,从与高职遗传学的关联内容、获奖人、获奖年限、获奖成就及学科思政教学目标几个方面进行归纳整理,完①基金项目:本文系2022—2024年度校本教科研项目“中职《遗传学》多元化情境教学的探索与实践”(课题立项号:LTNX [2022]011)的阶段性研究成果。
生物化学诺贝尔奖获得者介绍生物化学诺贝尔奖是诺贝尔奖其中的一个分支,奖励在生物化学领域做出杰出贡献的人。
以下是几位生物化学诺贝尔奖获得者的介绍: 1. 弗雷德里克·桑格(Frederick Sanger)弗雷德里克·桑格是英国生物化学家,曾两次获得诺贝尔奖,分别是在1958年和1980年,主要贡献是在蛋白质结构和DNA测序方面。
他发明了一种新的蛋白质分离方法,并且发现了一些蛋白质的结构。
同时,他也是DNA测序技术的先驱者之一,提出了一种基于化学方法的DNA测序技术,为后来的DNA测序技术奠定了基础。
2. 詹姆斯·沃森(James Watson)和弗朗西斯·克里克(Francis Crick)詹姆斯·沃森和弗朗西斯·克里克是美国和英国的生物化学家,于1962年获得诺贝尔生理学或医学奖,主要贡献是解析了DNA的结构。
他们通过对X射线衍射图像的分析和推理,提出了DNA双螺旋结构的模型,并且证明了这个模型的正确性,这对后来的DNA研究和基因工程都有着重要的意义。
3. 埃德蒙·费希尔和阿尔弗雷德·赫希(Arthur Kornberg)埃德蒙·费希尔和阿尔弗雷德·赫希都是美国的生物化学家,在1959年共同获得诺贝尔生理学或医学奖,主要贡献是在核酸代谢方面。
他们发现了DNA在细胞内的合成机制,揭示了生物体内核酸代谢的重要环节。
这对于后来的基因工程和遗传学研究都有着重要的启示作用。
4. 汤姆·斯特耐特(Tom Cech)和西德尼·阿尔特曼(Sidney Altman)汤姆·斯特耐特和西德尼·阿尔特曼是美国的生物化学家,在1989年共同获得诺贝尔化学奖,主要贡献是在RNA催化方面。
他们发现了RNA分子能够具有催化活性,从而证明了RNA不仅是基因表达的中间媒介,而且也能够在细胞内发挥催化作用,这项发现对RNA研究和生物技术的发展都有着重要的影响。
探索篇誗课题荟萃基于诺贝尔奖视角培育学生生物学核心素养的实践研究朱慧贞郎小霞包鹏科(甘肃省定西市岷县第三中学,甘肃定西)一、诺贝尔奖与高中生物学学科的联系2017年颁布的《考试大纲》对“获取信息的能力”方面进行了调整,将“生物学重要事件”调整为“与生命科学相关的突出成就及热点问题”。
诺贝尔生理学或医学奖、化学奖的研究历程往往离不开生物学学科的研究内容、方法及理论。
经统计100年以来的诺贝尔奖成就与人教版高中生物学教材中的关联章节达到了28个,其中必修部分22个,选修部分6个。
本文对人教版高中生物学必修2“遗传与进化”中与诺贝尔奖关联的内容进行了归纳与整合。
二、基于诺贝尔奖视角培育学生生物学学科素养的可行性分析《普通高中生物学课程标准(2017年版)》指出生物学学科的核心素养由生命观念、科学思维、科学探究以及社会责任四部分构成。
任何一个诺贝尔奖成就都散发着科学工作者科学思维的火花,都是其科学探究过程的浓缩,这个过程是在遵循自然规律、符合生命观念的基础上不断进行的探索和尝试。
诺贝尔奖在高中生物学教学中的引入价值不仅在于其科学素养,还有其丰富的人文价值。
探索过程不可能一蹴而就、一帆风顺,大多是一个长期的、枯燥的、不断否定又不断被修正完善的过程。
科学家凭着对科学研究的无限热忱,对生命现象的无限好奇,冲淡了科学研究本身的枯燥与艰难。
其中不乏有趣的事例,摩尔根团队为了让果蝇发生变异,煞费苦心,甚至陪着果蝇不睡觉,一睡觉就摇瓶子,熬了两年也没看到一只突变的果蝇;也有踏破铁鞋无觅处,却有玛丽·亨特得来全不费工夫的惊喜;有富兰克林穷其一生,却未能分享这份殊荣的遗憾;也有屠呦呦以身试药,索克(发明了小儿麻痹症灭活疫苗)在自己三个年幼的孩子身上试药,这些看似疯狂的举动,实则是科学研究的必然选择,是为了大我牺牲小我的无私奉献精神。
挖掘诺贝尔奖里的人文情怀,涵养学生情操,历练其深厚的家国意识及社会担当,才能更好地追求科学精神。
诺贝尔奖与生物学的发展一、诺贝尔化学奖与生物化学的发展——生物化学是研究生命的物质基础和阐明生命过程中化学变化规律的一门科学。
科学家深入到生命体的深层结构,探明构成有机体的蛋白质(包括酶)与带有遗传信息的核酸的组成、结构以及它们在生命过程中的代谢作用。
现在,科学家们已可以从分子的水平上研究和解释生命现象。
毕希纳 (1860~1917) 德国生物化学家在发酵罐内,酶使麦芽等发酵,生产出啤酒1897年发现引起发酵的物质是酶,从而把酵母细胞的生命活力与酶的化学作用联系起来,建立了酶化学。
于1907年获奖。
萨姆纳 (1887~1955) 诺思罗普 (1891~1987) 显微镜下的胰蛋白酶美国生物化学家美国生物化学家萨姆纳1926年首次提纯了酶,诺斯罗普1929年分离和提纯了胃蛋白酶、胰蛋白酶、胰凝乳蛋白酶等,他们证明了酶是一种具有催化作用的蛋白质。
于1946年获奖。
托德 (1907~1997) 酶是由数千个原子组成的非常复杂的化学物质。
英国生物化学家图为一个溶菌酶分子的模型。
首先发现并合成了核苷酸单体,证实其具有遗传特性,他还发现了核苷酸辅酶的结构。
于 1957 年获奖。
他的研究为揭开生命起源之谜开辟了道路。
康福思(1917~)澳大利亚裔英国化学家60年代证明酶是一种催化效能很高的生物催化剂,某一种酶只能对某一类化学反应起催化作用,于1975年获奖。
他为发展立体化学和阐明生物体内许多复杂的化学变化作出了重要贡献。
斯科 (1918~ ) 沃克 (1941~ ) 博耶(1918~ ) 丹麦生物化学家英国化学家美国生物化学家1957 年斯科发现了钠+、钾+-腺苷三磷酸酶; 1964至1981年博耶、沃克先后发现并阐明了腺苷三磷酸酶合成的基本酶学机制。
这一成果发现了人体细胞内负责贮藏和转输能量的“离子传输酶”,从而揭开生命过程中能量转换的奥秘。
三人于1997年获奖。
蛋白质是构成生物体的基本物质。
美国化学家鲍林40年代中期以图为电子显微镜下的蛋白质。