国际化学奥林匹克竞赛-2002年第34届ICHO预备试题答案英文1-292
- 格式:pdf
- 大小:288.26 KB
- 文档页数:15

第34届中国化学奥林匹克(初赛)试题第1题(8分)1-1 画出小分子C3F4的Lewis结构,标出三个碳原子成键时所采用的杂化轨道。
1-2现有铝镁合金样品一份,称取2.56 g样品溶于HCl,产生2.80 L H2 (101.325 kPa, 0℃),通过计算,确定此合金组成。
1-3构筑可循环再生的聚合物材料是解决目前白色污染的有效途径之一。
1-3-1通常单官能度的单体无法参与聚合反应中的链增长,但单体A可以与B发生反应形成聚合物。
画出该聚合物的结构式。
1-3-2单体A、C、D按物质的量比例6 : 3 : 2进行聚合,得到的聚合物不能进行热加工和循环利用;但若在共聚合时加入一定量(~10%)的B,得到的聚合物又具备了可热加工和循环利用的性能。
简述原因。
第2题(10分)书写反应方程式(提示:要求系数为最简整数比;2-1和2-2中自然条件复杂,合理选择即可)。
2-1关于地球的演化,目前的主要观点是,原始地球上没有氧气,在无氧或氧气含量很低时,原核生物可利用自然存在的有机质(用CH2O表示)和某些无机物反应获得能量。
例如,SO42-和MnO2可分别作为上述过程的氧化剂,前者生成黄色浑浊物,二者均放出无色无味气体。
2-1-1 写出SO42-参与的释能反应的离子方程式。
2-1-2 写出MnO2参与的释能反应的离子方程式。
2-2 约27亿年前光合作用开始发生,随着这一过程的进行,地球上氧气不断积累,经过漫长的演变终达到如今的含量。
氧气的形成和积累,导致地球上自然物质的分布发生变化。
2-2-1 溶解在海洋中的Fe2+被氧化转变成针铁矿α-FeO(OH)而沉积,写出离子方程式。
2-2-2与铁的沉积不同,陆地上的MoS2被氧化而导致钼溶于海洋且析出单质硫,写出离子方程式。
2-32020年8月4日晚,贝鲁特港口发生了举世震惊的大爆炸,现场升起红棕色蘑菇云,破坏力巨大。
目前认定大爆炸为该港口存储的约2750吨硝酸铵的分解。

国际化学奥林匹克竞赛基本要求说明:☆化学论题的分类(下表各知识点后面的数字,前者是它归属的组别,后者是知识点序号)·组1:这些论题是绝大多数中学化学大纲里有的。
·组2:这些论题是许多中学化学大纲里有的;若大纲里没有,预期各国应向参赛学生介绍。
·组3:这些论题是大多数中学化学大纲里没有的。
☆主办国出的预备题不必涉及第一和二组论题,但对第二组论题应开具细目。
所有竞赛将涉及到的第三组论题必须包括在预备题中。
译注:☆1:本大纲制订的基础是对参赛队领队的大量问卷调查与综合分析,制订工作历时8年,三度修订。
通读本大纲可得出如下认识:本大纲是采取举例的方式来明确知识点的层次,重在体现知识点的不同层次,不能说大纲里没有列入的知识点就不属于竞赛内容。
也可以有如下认识:本大纲的一级知识水平大致与Nuffield O-水平吻合,二级知识大致与Nuffield A-水平吻合,三级知识大致为大学本科低年级知识水平。
☆2:知识点的分级旨在明确竞赛前参赛选手需要记忆多少知识作为解题的基础,不等于在竞赛试题中不得出现超纲的供参赛选手现学现用的化学知识信息以及考核参赛选手把握新知识内涵的能力(学习能力)与应用新知识的能力(应用能力)以及创造性思维的能力。
☆3:本大纲是98年10月在斯洛伐克首都布拉津斯拉伐工作会议上修订的。
在该次工作会议上与会代表用6个单元时间逐字逐句地对原在华沙会议制订的大纲进行分组审核,提出修改意见,然后又用1个单元时间进行讨论,最后由指导委员会主席携回加工定稿。
第31、32届IChO预备题均将此大纲作为附件,预备题的编制以此大纲为依据。
☆4:全国化学竞赛基本要求应根据此大纲作出相应调整。
全国初赛应与本大纲一级知识水平大致吻合,决赛(冬令营)应于本大纲一、二级知识水平大致吻合,对国家集训队进行的讲座与国家队四名选手的选拔赛应依据本大纲一、二级知识和预备题中明确的三级知识,属于本大纲三级知识而预备题未涉及的不应作为国家集训队选手已经掌握的(记忆了的以及能够熟练应用的)知识来考核。


第三十四届国际化学奥林匹克竞赛第一题 I -- 生命化学生命与化学密切相关. 了解和监控生命过程受到了化学家的极大重视.I-1 生命中的氧气分数: 6 分氧气对我们每个人的生命是至关重要的。
O 2通过肺进入我们的身体, 然后由血液传输到我们身体的各个组织。
在体内,通过糖的氧化提供能量, 其反应式如下:C 6H 12O 6 + 6 O 26 CO 2 + 6 H 2O在这个反应中, 每消耗1摩尔O 2将产生400 kJ 能量。
在血液传输过程中, 血红蛋白(Hb)中的每4个亚铁血红素基团(Hm) 与一个O 2结合。
游离亚铁血红素是由一个亚铁离子Fe 2+ 与二价卟啉负离子(porphyrin 2-)中的四个氮原子配位组成的。
O 2能与亚铁离子Fe 2+的配位点键合配位形成一个配合物 Hm ⋅O 2(反应式2)。
一氧化碳也能与亚铁离子形成配合物Hm ⋅CO(反应式1)。
CO 有毒的原因在于它与亚铁血红素的结合能力远远强于O 2。
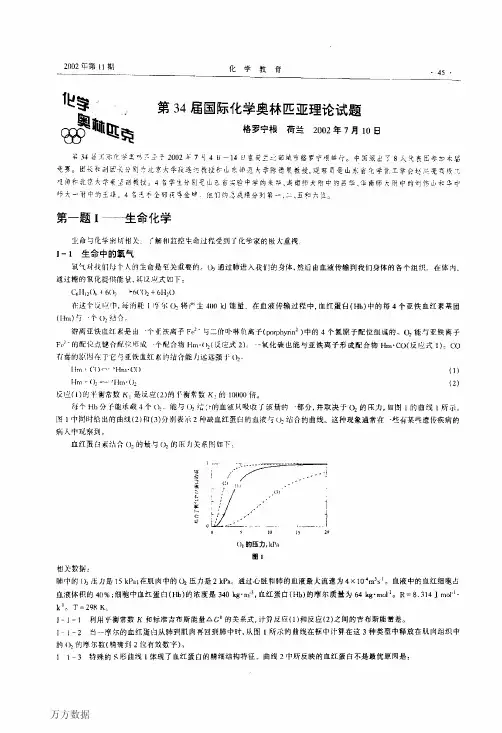
Hm + COHm .CO (1)Hm + O 2Hm .O 2(2)反应(1)的平衡常数 K 1比反应(2)的平衡常数 K 2 大10000倍。
每个Hb 分子能承载4个O 2。
能与O 2结合的血液只吸收了该量的一部分, 并取决于O 2的压力, 如图1的曲线1所示。
图1中同时给出的 曲线(2)和(3)分别表示二种缺血红蛋白的血液与O 2结合的曲线。
这种现象通常在一些有某些遗传疾病的病人中观察到。
血红蛋白素结合O 2的量与O 2的压力关系图如下:O 2的压力, kPa图1相关数据:肺中的O 2压力是15kPa; 在肌肉中的O 2压力是 2 kPa 。
通过心脏和肺的血液最大流速为 4 ⨯ 10-4 m 3s -1。
血液中的血红细胞占血液体积的40%;细胞中血红蛋白(Hb)的浓度是340 kg m -3,血红蛋白(Hb)的摩尔质量为64 kg mol -1。
R = 8.314 J mol -1 k -1。

第34届中国化学奥林匹克(初赛)试题第1题(8分)1-1 画出小分子C3F4的Lewis结构,标出三个碳原子成键时所采用的杂化轨道。
1-2现有铝镁合金样品一份,称取2.56 g样品溶于HCl,产生2.80 L H2 (101.325 kPa, 0℃),通过计算,确定此合金组成。
1-3构筑可循环再生的聚合物材料是解决目前白色污染的有效途径之一。
1-3-1通常单官能度的单体无法参与聚合反应中的链增长,但单体A可以与B发生反应形成聚合物。
画出该聚合物的结构式。
1-3-2单体A、C、D按物质的量比例6 : 3 : 2进行聚合,得到的聚合物不能进行热加工和循环利用;但若在共聚合时加入一定量(~10%)的B,得到的聚合物又具备了可热加工和循环利用的性能。
简述原因。
第2题(10分)书写反应方程式(提示:要求系数为最简整数比;2-1和2-2中自然条件复杂,合理选择即可)。
2-1关于地球的演化,目前的主要观点是,原始地球上没有氧气,在无氧或氧气含量很低时,原核生物可利用自然存在的有机质(用CH2O表示)和某些无机物反应获得能量。
例如,SO42-和MnO2可分别作为上述过程的氧化剂,前者生成黄色浑浊物,二者均放出无色无味气体。
2-1-1 写出SO42-参与的释能反应的离子方程式。
2-1-2 写出MnO2参与的释能反应的离子方程式。
2-2 约27亿年前光合作用开始发生,随着这一过程的进行,地球上氧气不断积累,经过漫长的演变终达到如今的含量。
氧气的形成和积累,导致地球上自然物质的分布发生变化。
2-2-1 溶解在海洋中的Fe2+被氧化转变成针铁矿α-FeO(OH)而沉积,写出离子方程式。
2-2-2与铁的沉积不同,陆地上的MoS2被氧化而导致钼溶于海洋且析出单质硫,写出离子方程式。
2-32020年8月4日晚,贝鲁特港口发生了举世震惊的大爆炸,现场升起红棕色蘑菇云,破坏力巨大。
目前认定大爆炸为该港口存储的约2750吨硝酸铵的分解。

2024 化学奥林匹克竞赛试题一、试题有一化学反应 A + B → C,在一定温度下,当A 的浓度为0.5mol/L,B 的浓度为1mol/L 时,反应速率为0.2mol/(L·s)。
若将 A 的浓度增大到1mol/L,B 的浓度不变,此时反应速率变为多少?解析根据反应速率方程v = k[A]^m[B]^n,设该反应中 A 的反应级数为m,B 的反应级数为n。
1. 首先求反应级数:-当 A 的浓度为0.5mol/L,B 的浓度为1mol/L 时,反应速率v1 = 0.2mol/(L·s),可得方程①:0.2 = k×0.5^m×1^n。
-当A 的浓度增大到1mol/L,B 的浓度不变时,设此时反应速率为v2,可得方程①:v2 = k×1^m×1^n。
-用方程①除以方程①可得:v2/0.2 = (k×1^m×1^n)/(k×0.5^m×1^n),化简得v2/0.2 = 2^m。
-由于只改变了A 的浓度,B 的浓度不变,且反应速率变为原来的倍数只与A 的浓度变化有关,所以可以通过设特殊值来确定m 的值。
-假设m = 1,则v2/0.2 = 2,解得v2 = 0.4mol/(L·s)。
-假设m = 2,则v2/0.2 = 4,解得v2 = 0.8mol/(L·s)。
-假设m = 3,则v2/0.2 = 8,解得v2 = 1.6mol/(L·s)等,依次类推,可通过给出的选项来确定m 的值,进而确定反应速率v2。
二、试题已知在25①时,水的离子积常数Kw = 1×10^(-14)。
在该温度下,某溶液的pH = 3,求该溶液中氢氧根离子的浓度。
解析1. 因为pH = -lg[H①],已知pH = 3,则[H①]=1×10^(-3)mol/L。
2. 又因为在任何水溶液中,Kw = [H①][OH①]。
第34届中国化学奥林匹克(初赛)试题、标准答案及评分细则
第1题(8分)
1-1画出小分子C3F4的Lewis结构,标出三个碳原子成键时所采用的杂化轨道。
1-2现有铝镁合金样品一份,称取2.56g样品溶于HCl,产生2.80 L H2 (101.325 kPa,0 ˚C),通过计算,确定此合金组成。
1-3 构筑可循环再生的聚合物材料是解决目前白色污染的有效途径之一。
1-3-1通常单官能度的单体无法参与聚合反应中的链增长,但单体A可以与B发生反应形成聚合物。
画出该聚合物的结构式。
1-3-2单体A、C、D按物质的量比例6:3:2进行共聚合,得到的聚合物不能进行热加工和循环利用;但若在共聚合时加入一定量(~10%)的B,得到的聚合物又具备了可热加工和循环利用的性能。
简述原因。
1。



SYLLABUS OF THEINTERNATIONAL CHEMISTRY OLYMPIADPart I Theoretical partLevel 1: These topics are included in the overwhelming majority of secondary school chemistry programs and need not be mentioned in the preparatory problems.Level 2: These topics are included in a substantial number of secondary school programs and maybe used without exemplification in the preparatory problems. Level 3: These topics are not included in the majority of secondary school programs and can only be used in the competition if examples are given in the preparatory problems.1. The atom1.1.Introduction1.1.1. Counting of nucleons 11.1.2. Isotopes 1 1.2.The hydrogen atom1.2.1. Concept of energy levels 11.2.2. Shape of s-orbitals 11.2.3. Shape and orientation of p-orbitals 11.2.4. Shape and orientation of d-orbitals 31.2.5. Understanding the simplest Schrodinger equation 31.2.6. Square of the wave function and probability 31.2.7. Quantum numbers (n, l, m l) 3 1.3.Radioactivity1.3.1. Types of radioactivity 11.3.2. Radioactive decay 11.3.3. Nuclear reactions 22. Chemical bonding2.1.VSEPR – Simple molecular structures with2.1.1. no more than four electron pairs about central atom 12.1.2. with central atom exceeding the “octet rule” 3 2.2.Delocalization and resonance 3 2.3.Hybrid orbital theory 3 2.4.Molecular orbital theory2.4.1. molecular orbital diagram (H2 molecule) 32.4.2. molecular orbital diagram (N2 and O2 molecules) 32.4.3. bond orders in O2, O2–, O2+ 32.4.4. unpaired electrons and paramagnetism 33. Chemical calculations3.1.1. Balancing equations 13.1.2. Stoichiometric calculations 13.1.3. Mass and volume relations (including density) 13.1.4. Empirical formula 13.1.5. Avoga dro’s number 13.1.6. Concentration calculations 14. Periodic trends4.1.Electron configuration4.1.1. Pauli exclusion principle 14.1.2. Hund’s Rule 14.1.3. Main group elements 14.1.4. Transition metal elements 14.1.5. Lanthanide and actinide metals 3 4.2.Electronegativity 1 4.3.Electron affinity 2 4.4.First ionization energy 1 4.5.Atomic size 1 4.6.Ion size 14.7.Highest oxidation number 15. Inorganic Chemistry5.1.Introduction5.1.1. Trends in physical properties of elements (Main groups)5.1.1.1. melting point 15.1.1.2. boiling point 15.1.1.3. metal character 15.1.1.4. magnetic properties 35.1.1.5. electrical conductivity 25.1.2. Oxidation number 15.1.3. Nomenclature5.1.3.1. main group compounds 15.1.3.2. transition metal compounds 15.1.3.3. simple metal complexes 3 5.2.Groups 1 and 25.2.1. Trend in reactivity of (heavy elements more reactive) 15.2.2. Products of reaction with5.2.2.1. water 15.2.2.2. halogens 15.2.2.3. oxygen 25.2.3. Basicity of oxides 15.2.4. Properties of hydrides 35.2.5. Other compounds, properties and oxidation states 3 5.3.Groups 13 – 18 and Hydrogen5.3.1. Binary molecular compounds of hydrogen5.3.1.1. Formulae 15.3.1.2. Acid-base properties of CH4, NH3, H2O, H2S 15.3.1.3. Other properties 35.3.2. P block elementsGroup 13 (Boron group)15.3.2.1 The oxidation state of boron and aluminium in theiroxides and chlorides is +35.3.2.2. The acid-base properties of aluminium2oxide/hydroxide5.3.2.3. Reaction of boron(III) oxide with water 35.3.2.4. Reaction of boron(III) chloride with water 35.3.2.5. Other compounds, properties and oxidation states 35.3.3. Group 14 (Carbon group)5.3.3.1. The oxidation state of Si in its chloride and oxide is1+425.3.3.2. The +2 and +4 oxidation states of carbon, tin andlead, the acid-base and redox properties of theoxides and chlorides5.3.3.3. Other compounds, properties and oxidation states 35.3.4. Group 15 (Nitrogen group)25.3.4.1. Phosphorus(+5) oxide and chloride, and theirreaction with water5.3.4.2. Phosphorus(+3) oxide and chloride, and their2reaction with water5.3.4.3. Oxides of nitrogena. Reaction of NO to form NO2 1b. Dimerization of NO2 1c. Reaction of NO2 with water 15.3.4.4. Redox properties ofa. HNO3 and nitrates 1b. HNO2 and NH2NH2 35.3.4.5. Bi(+5) and Bi(+3) 35.3.4.6. Other compounds, properties and oxidation states 35.3.5. Group 16 (Oxygen group)15.3.5.1. The +4 and +6 oxidation states of sulfur, reactionof their oxides with water, properties of their acids5.3.5.2. Reaction of thiosulfate anion with I2 35.3.5.3. Other compounds, properties and oxidation states 35.3.6. Group 17 (Halogens)15.3.6.1. Reactivity and oxidant strength decreases from F2to I25.3.6.2. Acid-base properties of the hydrogen halides 115.3.6.3. The oxidation state of fluorine in its compounds is–15.3.6.4. The –1, +1, +3, +5, +7 oxidation states of chlorine 15.3.6.5. Mononuclear oxoanions of chlorine 25.3.6.6. Reactions of halogens with water 35.3.6.7. Reaction of Cl2O and Cl2O7 with water 35.3.6.8. Other compounds, properties and oxidation states 35.3.7. Group 18 (Rare gases) 3 5.4. Transition elements15.4.1. Common oxidation states of common transition metals:Cr(+2), Cr(+3) Mn(+2), Mn(+4), Mn(+7) Ag(+1)Fe(+2), Fe(+3) Co(+2) Zn(+2)Hg(+1), Hg(+2) Cu(+1), Cu(+2) Ni(+2)5.4.2. Colours of ions listed above in aqueous solution 25.4.3. Insolubility of Ag, Hg and Cu in HCl 25.4.4. M2+ arising by dissolution of the other metals in HCl 25.4.5. Cr(OH)3 and Zn(OH)2 are amphoteric and the other +22 oxides/hydroxides of the metals listed above are basic5.4.6. MnO4– and Cr2O72– are strong oxidants in acid solution 15.4.7. pH dependence of products of MnO4– acting as oxidant 25.4.8. Interconversion between CrO42– and Cr2O72– 35.4.9. Other compounds, properties and oxidation states 3 nthanides and actinides 3 5.6.Coordination chemistry including stereochemistry5.6.1. Definition of coordination number 115.6.2. Writing equations for complexation reactions given allformulae5.6.3. Formulae of common complex ions5.6.3.1. Ag(NH3)2+ 15.6.3.2. Ag(S2O3)23– 35.6.3.3. FeSCN2+ 35.6.3.4. Cu(NH3)42+ 15.6.3.5. Other complex ions 35.6.4. (6.5) Ligand field theory (e g and t2g terms, high and low spin) 35.6.5. Stereochemistry5.6.5.1. (6.7) cis and trans 35.6.5.2. enantiomers 3 5.7.Selected industrial processes5.7.1. Preparation of H2SO4 15.7.2. Preparation of NH3 15.7.3. Preparation of Na2CO3 25.7.4. Preparation of Cl2 and NaOH 25.7.5. Preparation of HNO3 26. Physical chemistry6.1.Gases6.1.1. Ideal gas law 16.1.2. van der Waal’s gas law 36.1.3. definition of partial pressure 26.1.4. Dalton’s Law 3 6.2. Thermodynamics6.2.1. First Law6.2.1.1. Concept of system and surroundings 26.2.1.2. Energy, heat and work 26.2.2. Enthalpy6.2.2.1. Relationship between internal energy and enthalpy 36.2.2.2. Definition of heat capacity 26.2.2.3. Difference between C p and C v (ideal gas only) 36.2.2.4. Enthalpy is a state prop erty (Hess’s Law) 26.2.2.5. Born-Haber cycle for ionic compounds 36.2.2.6. Use of standard formation enthalpies 26.2.2.7. Enthalpies of solution and solvation 36.2.2.8. Bond enthalpies (definition and use) 26.2.3. Second Law (Entropy and Free Energy)6.2.3.1. Entropy definition (d q / T) 36.2.3.2. Entropy and disorder 36.2.3.3. Entropy definition (S = k ln W) 36.2.3.4. Gibbs energy definition (∆G = ∆H –T∆S) 36.2.3.5. Using ∆G to predict direction of natural change 36.2.3.6. Relationship between ∆G︒ and equilibrium constant K 3 6.3.Equilibrium6.3.1. Acid-base6.3.1.1. Arrhenius definitions of acids and bases 16.3.1.2. Bronsted-Lowry definitions 16.3.1.3. Conjugate acids and bases 16.3.1.4. pH definition 16.3.1.5. K w definition 16.3.1.6. K a and K b as a measure of acid and base strength 16.3.1.7. Acidity or basicity of ions 16.3.1.8. Calculation of pH from p K a(weak acid) 16.3.1.9. Calculation of pH of a simple buffer solution 26.3.2. Gas phase6.3.2.1. Equilibrium constant in partial pressures 36.3.2.2. Relating K p and K c 36.3.3. Solubility6.3.3.1. Solubility constant (product) definition (K sp) 26.3.3.2. Calculation of solubility in water from K sp 26.3.4. Compleximetric6.3.4.1. Complex formation constant (definition) 36.3.4.2. Problems involving compleximetric equilibria 36.3.4.3. Lewis acids and bases 36.3.4.4. Hard and soft Lewis acids and bases 36.3.5. Phase6.3.5.1. Temperature dependence of vapour pressure 36.3.5.2. Clausius-Clapeyron equation 36.3.5.3. Single component phase diagramsa. triple point 3b. critical point 36.3.5.4. liquid-vapour systema. ideal and nonideal systems 3b. diagram 3c. use in fractional distillation 36.3.5.5. Henry’s Law 36.3.5.6. Raoult’s Law 36.3.5.7. Deviation from Raoult’s Law 36.3.5.8. Boiling point elevation 36.3.5.9. Freezing point depression 36.3.5.10. Osmotic pressure 36.3.5.11. Partition coefficient 36.3.5.12. Solvent extraction 36.3.6. Multiple6.3.6.1. Calculation of pH for multiprotic acids 36.3.6.2. Calculation of pH for weak acid mixtures 3 6.4.Electrochemistry6.4.1. Electromotive force (definition) 16.4.2. First kind electrodes 16.4.3. Standard electrode potential 16.4.4. Nernst equation 36.4.5. Second kind electrodes 36.4.6. Relationship between G and electromotive force 37. Chemical kinetics (Homogeneous reactions)7.1.Introduction7.1.1. Factors affecting reaction rate 17.1.2. Reaction coordinates and the basic idea of a transition state 1 7.2.Rate law7.2.1. Differential rate law 27.2.2. Concept of reaction order 27.2.3. Rate constant definition 27.2.4. First order reactions7.2.4.1. Dependence of concentration on time 37.2.4.2. Concept of half life 37.2.4.3. Relationship between half life and rate constant 37.2.4.4. Calculation of first order rate constant froma. differential rate law 3b. integrated rate law 37.2.4.5. Rate constant for second and third order reactions 3 7.3.Reaction mechanisms7.3.1. Concept of molecularity 37.3.2. Rate-determining step 37.3.3. Basic concepts of collision theory 37.3.4. Opposing parallel and consecutive reactions 37.3.5. Arrhenius’s law 37.3.5.1. Definition of activation energy 37.3.5.2. Calculation of activation energy 38. Spectroscopy8.1.UV/visible8.1.1. Identification of aromatic compound 38.1.2. Identification of chromophore 38.1.3. Dyes: colour vs structure 38.1.4. Beer’s Law 3 8.2.Infrared8.2.1. Interpretation using a table of frequencies 38.2.2. Recognition of hydrogen bonds 3 8.3.x-Ray8.3.1. Bragg’s Law 38.3.2. Concept of8.3.2.1. coordination number 38.3.2.2. unit cell 38.3.3. Solid structures8.3.3.1. NaCl 38.3.3.2. CsCl 38.3.3.3. metals 3 8.4.NMR8.4.1. General Concepts8.4.1.1. chemical shift 38.4.1.2. spin-spin coupling and coupling constants 38.4.1.3. integration 38.4.2. Interpretation of a simple 1H spectrum (like ethanol) 38.4.3. Identification of o- and p-disubstituted benzene 338.4.4. Interpretation of simple spectra of 13C (proton decoupled) andother 1/2 spin nuclei8.5.Mass spectrometry8.5.1.1. Recognition of molecular ion 38.5.1.2. Recognition of fragments with the help of a table 38.5.1.3. Recognition of typical isotope distribution 39. Organic Chemistry9.1.Introduction9.1.1. (3.1.1) Alkane naming (IUPAC) 19.1.2. Trends in boiling points of9.1.2.1. (3.1.3) alkanes with structure 19.1.2.2. (3.7.1) alcohols vs ethers due to hydrogen-bonding 19.1.3. (3.3.1, 3.4.1) Geometry at singly, doubly, and triply bonded1 carbon9.1.4. Identification of common functional groups 19.1.5. Isomerism of alkenes9.1.5.1. cis-trans 19.1.5.2. E/Z 39.1.6. Enantiomers9.1.6.1. Optical activity 29.1.6.2. R/S nomenclature 3 9.2.Reactivity9.2.1. Alkanes9.2.1.1. reaction with halogensa. products 1b. free radical mechanism (initiation, termination) 29.2.1.2. Cycloalkanesa. names 2b. Strain in small rings 3c. chair/boat conformations of cyclohexane 39.2.2. Alkenes9.2.2.1. Products from Br2, HBr and H2O/H+ 19.2.2.2. Markownikoff’s rule 29.2.2.3. Mechanism involving carbocation intermediates 39.2.2.4. Relative stability of carbocations 39.2.2.5. 1,4 addition to dienes 3 9.2.3. Alkynes9.2.3.1. Acidity relative to alkenes 39.2.3.2. Differences in chemical properties from alkenes 2 9.2.4. Benzene9.2.4.1. formula 19.2.4.2. stabilization by resonance 19.2.4.3. electrophilic substitution (nitration, halogenation)a. directing effect of first substituent 3b. effect of first substituent on reactivity 3c. explanation of substituent effects 39.2.5. Halogen compounds9.2.5.1. Nomenclature of monofunctional 19.2.5.2. Substitution reactionsa. giving alcohols 3b. in which halogen is exchanged 3c. reactivityi. primary vs secondary vs tertiary 3ii. aliphatic vs aromatic 3d. S N1 and S N2 mechanisms 39.2.5.3. Elimination reactions 29.2.5.4. Competition of elimination and substitution 2 9.2.6. Alcohols9.2.6.1. Nomenclature of monofunctional 19.2.6.2. Comparison of acidity of alcohols and phenols 29.2.6.3. Dehydration to alkenes 19.2.6.4. Esters with inorganic acid 29.2.6.5. Oxidation reactions 1 9.2.7. Aldehydes and ketones9.2.7.1. Nomenclature of monofunctional 19.2.7.2. Oxidation of aldehydes 19.2.7.3. Reduction to alcohols (LiAlH4, NaBH4) 39.2.7.4. Keto/enol tautomerism 39.2.7.5. Nucleophilic addition reactions witha. HCN 3b. RNH2 (R = alkyl, HO, NH2) 3c. enolate anions (aldol condensation) 3d. alcohols to form acetals/ketals 3e. Grignard reagents 39.2.8. Carboxylic acids and their derivatives29.2.8.1. Nomenclature of carboxylic acids and theirderivatives (esters, acid halides, amides)9.2.8.2. Acidity strength related to inductive effects 39.2.8.3. Preparation of carboxylic acids by hydrolysis ofa. esters (including soaps) 1b. amides 2c. nitriles 39.2.8.4. Reaction of carboxylic acidsa. with alcohols to form esters 1b. to form acid chlorides 3c. to form anhydrides 39.2.8.5. Reaction of acid chlorides to form amides 39.2.8.6. Mechanism of esterification 39.2.8.7. Multifunctional acids (hydroxyacids, ketoacids) 39.2.8.8. Polycarboxylic acids 39.2.9. Amines9.2.9.1. Nomenclaturea. simple amines 1b. recognition of primary, secondary, tertiary 19.2.9.2. Basicitya. As a property of an amine 1b. Comparison of basicity of aliphatic and aromatic 3c. Comparison of basicity of amines and amides 3d. Preparation of aminesi. from halides 3ii. from aromatic nitro compounds 3iii. from amides (by hydrolysis) 39.2.9.3. Diazotizationa. of aliphatic amines 3b. of aromatic amines 310. Polymers10.1Synthetic.10.1.1. Addition polymers10.1.1.1. polystyrene 210.1.1.2. polyethene 110.1.1.3. chain mechanism of formation 210.1.2. Condensation polymers10.1.2.1. polyesters 210.1.2.2. polyamides 210.1.3. Silicones 310.1.4. Concept of cross-linking and its affect on properties 3 10.2.Natural10.2.1. Silicates 310.2.2. Rubber 311. Biochemistry11.1.Carbohydrates11.1.1. Glucose and fructose11.1.1.1. chain formulae 111.1.1.2. Fischer projections 211.1.1.3. Haworth formulae 311.1.2. Difference between starch and cellulose 211.1.3. Difference between α- and β- D glucose 2 11.2.Fats11.2.1. Structure of fats in relationship to properties 211.2.2. Formula of glycerol 1 11.3.Nitrogen-containing Compounds of Biological Importance11.3.1. Amino acids11.3.1.1. Ionic structure 111.3.1.2. Isoelectric point 3211.3.1.3. 20 amino acids (classification with structuresprovided)11.3.1.4. Separation by electrophoresis 311.3.1.5. The peptide linkage 111.3.2. Proteins11.3.2.1. Primary structure 111.3.2.2. –S-S- bridges 311.3.2.3. Sequence analysis 311.3.2.4. Secondary structure 311.3.2.5. Details of α-helix structure 311.3.2.6. Tertiary structure 3211.3.2.7. Denaturation (change in pH, temperature,metals, ethanol)11.3.3. Nuclei Acids and Protein Synthesis11.3.3.1. Pyrimidine and purine 311.3.3.2. Nucleosides and nucleotides 311.3.3.3. Formulae of pyrimidine and purine bases 311.3.3.4. Difference between ribose and 2-deoxyribose 311.3.3.5. Base combination CG and AT (hydrogen-bonding) 311.3.3.6. Difference between DNA and RNA 311.3.3.7. Difference between mRNA and tRNA 311.4.Enzymes11.4.1.1. General properties, active centers 311.4.1.2. Nomenclature, kinetics, coenzymes, function of ATP 312. Analytical chemistry12.1.Titrations12.1.1. acid-base12.1.1.1. Titration curve; pH (strong and weak acid) 212.1.1.2. Choice of indicators for acidimetry 212.1.2. Redox titration 3 12.2.Qualitative analysis12.2.1. Ions (Inorganic)12.2.1.1. Identification of Ag+, Ba2+, Cl–, SO42– 212.2.1.2. Identification of other anions and cations 312.2.2. Organic functional groups12.2.2.1. Lucas reagent (1-, 2-, 3-alcohols) 312.2.2.2. Iodoform reaction 3312.2.2.3. Identification of primary, secondary, tertiary,quarternary amines in the laboratory12.3.Chromatographic methods of separation 3Part I Experimental partLevel 1: is assigned to the basic experimental activities which are supposed to be mastered by competitors very wellLevel 2: is assigned to the activities which are parts of school experimental exercises in developed countries and the authors of IChO tasks mayincorporate them into the tasks without being bounded to mention it inadvanceLevel 3: is assigned to such activities which are not in the chemistry syllabus in the majority of participating countries and the authors are obliged to mentionthem in the set of preparatory tasksIf the organizer wants to apply a technique which is not mentioned in the above syllabus, this technique is set to level 3 automatically.1. Synthesis of inorganic and organic compounds1.1. Heating with burners and hotplates 1 1.2. Heating of liquids 11.3. Handling the work with inflammable substances and materials 1 1.4. Measuring of masses (analytical balance) 11 1.5. Measuring of volumes of liquids (measuring cylinder, pipette,burette)1.6. Preparation of solutions from a solid compound and solvent 1 1.7. Mixing and dilution of solutions 1 1.8. Mixing and stirring of liquids 1 1.9. Using mixer and magnetic stirrer 2 1.10. Using a dropping funnel 1 1.11. Syntheses in flat bottom vessels – general principles 1 1.12. Syntheses in round bottom vessels – general principles 1 1.13 Syntheses in a closed apparatus – general principles 1 1.14. Using microscale equipment for synthesis 3 1.15. Apparatus for heating of reaction mixture under reflux 2 1.16. Apparatus for distillation of liquids at normal pressure 2 1.17. Apparatus for distillation of liquids at reduced pressure 2 1.18. Apparatus for steam distillation 3 1.19. Filtration through flat paper filter 1 1.20. Filtration through a folded paper filter 1 1.21. Handling a water vacuum pump 1 1.22. Filtration through a Büchner funnel 1 1.23. Suction through a glass filter 1 1.24. Washing of precipitates by decantation 1 1.25. Washing of precipitates on a filter 2 1.26. Drying of precipitates on a filter with appropriate solvents 2 1.27. Recrystallization of substances from aqueous solution 1 1.28. Recrystallization of substances from a known organic solvent 2 1.29. Practical choice of an appropriate solvent for recrystallization of a3 substance1.30. Drying of substances in a drying box 2 1.31. Drying of substances in a desiccator 2 1.32. Connecting and using of a gas washing bottle 21.33. Extraction with an inmiscible solvent 12. Identification of inorganic and organic compounds:general principles2.1. Test-tube reactions 1 2.2. Technique of reactions performed in a dot dish and on a filter paper 12 2.3. Group reactions of some cations and anions specified by theorganizer2 2.4. Selective reactions of some cations and anions specified by theorganizer2.5. Specific reactions of some cations and anions specified by the organizer 32 2.6. Identification of elements by flame coloration (using a platinumwire/MgO rod, Co-glass)2.7. Using a hand spectroscope/Bunsen spectroscope 3 2.8. Melting point determination with Kofler or similar type of apparatus 3 2.9. Qualitative evidence of basic functional groups of organic2 substances specified by the organizer3 2.10. Exploitation of some specific reactions for identification of organiccompounds (specified by the organizer)3. Determination of some inorganic and organic compounds:general principles3.1. Quantitative determinations using precipitation reactions 2 3.2. Igniting of a precipitate in a crucible 1 3.3. Quantitative volumetric determinations 1 3.4. Rules at titrating 1 3.5. Use of a pipetting ball 1 3.6. Preparation of a standard solution 2 3.7. Alkalimetric and acidimetric determinations 2 3.8. Color transitions of indicators at alkalimetric and acidimetric2 determinations3.9. Direct and indirect determinations (back titration) 3 3.10. Manganometric determinations 3 3.11. Iodometric determinations 3 3.12. Other types of determinations on basis of redox reactions 3 3.13. Complexometric determinations 3 3.14. Color transitions of solutions at complexometric determinations 3 3.15. Volumetric determinations on basis of precipitation reactions 33.16. Thermometric titration 34. Special measurements and procedures4.1. Measuring with a pH-meter 2 4.2. Chromatography on thin layers 3 4.3. Column chromatography 3 4.4. Separation on ion exchanger 3 4.5. Measuring of UV-VIS absorbances with a spectral photometer 34.6. Performing of conductivity measurements 35. Evaluation of results5.1. Estimation of experimental errors (significant figures, plots scales) 1。
第34届中国化学奥林匹克(初赛)模拟考试卷-1•竞赛时间3 小时。
迟到超过半小时者不能进考场。
开始考试后1小时内不得离场。
时间到,把试卷与答卷背面朝上放在桌面上,待监考人员允许方可离开考场。
•姓名、报名号和所属学校必须写在首页左侧指定位置,写在其他位置者按废卷处理。
•所有解答必须写在卷面指定位置,写于其他位置无效。
•凡要求计算或推演的,须给出过程,无过程即使结果正确也不得分。
•用铅笔解答的部分(包括作图)无效。
•禁用涂改液和修正带,也不得用纸粘贴。
否则,整个答卷无效。
•附草稿纸一张,不得将任何纸张带入考场。
•允许使用非编程计算器以及直尺等文具。
•写有任何与试题内容无关的文字的答卷无效。
气体常数R = 8.314 J·mol·K;阿伏伽德罗常数N A = 6.022×10 mol;法拉第常数F = 9.65×104 C·mol-1;标准大气压:100 kPa;h=6.625⨯10-34 J⋅s; 1 eV=1.602⨯10-19 J。
第1题(10分)A、B、C、D是含氮中性钠盐,E、F、G、H是中性含磷钠盐。
D和H分别是I和J酸的盐;这些酸是白色固体,加热容易分解;I加热直接分解为K和水;J的热分解经过二个阶段:先1-1A与CO2能激烈作用转化为B,写出化学反应方程式(方程1);写出B 受热生成C的化学反应方程式(方程2);1-2 画出D、E、F、G、H物质阴离子的结构。
1-3 写出I和J热裂解的反应方程式。
1-4 画出K的Lewis结构图;写出与I形成相同热分解产物的其它化合物(2个)并写出它们的热分解反应方程式。
第2题(22分)2-1(13分)本题中的制备实验都在惰性气氛下进行,HCl 和(NH4)2C2O4溶液预先除氧。
仔细磨细的0.235 g 黑色粉末A溶于稍微加热的2.96 mL 10% HCl中(密度为1.048 g/ml),形成B盐的浅绿色溶液;盐B浅绿色溶液与3.676 g Co(NO3)2∙6H2O(预先溶在15 mL H2O中)混合,加入稍稍过量的(NH4)2C2O4溶液,生成3.068 g黄橙色沉淀C。
第34届中国化学奥林匹克(初赛)试题和参考答案第1题(8分)1-1画出小分子C3F4的Lewis结构,标出三个碳原子成键时所采用的杂化轨道。
1-2 现有铝镁合金样品一份,称取2.56 g样品溶于HCl,产生2.80 L H2(101.325 kPa,0 ℃),通过计算,确定此合金组成。
1-3构筑可循环再生的聚合物材料是解决目前白色污染的有效途径之一。
1-3-1通常单官能度的单体无法参与聚合反应中的链增长,但单体A可以与B发生反应形成聚合物,画出该聚合物的结构式。
1-3-2单体A、C、D按物质的量比例6:3:2进行共聚合,得到的聚合物不能进行热加工和循环利用。
但若在共聚合时加入一定量(~10%)的B,得到的聚合物又具备了可热加工和循环利用的性能。
简述原因。
第2题(10分) 书写反应方程式(提示:要求系数为最简整数比;2-1和2-2中自然条件复杂,合理选择即可)2-1关于地球的演化,目前主要的观点是,原始地球上没有氧气,在无氧或氧气含量很低时,原核生物可利用自然存在的有机质(用CH2O表示)和某些无机物反应获得能量,例如,SO42-和MnO2可分别作为上述过程的氧化剂,前者生成黄色浑浊物,二者均放出无色无味气体。
2-1-1写出SO42-参与的释能反应的离子方程式2-1-2写出MnO2参与的释能反应的离子方程式2-2约27亿年前光合作用开始发生,随着这一过程的进行,地球上氧气不断积累,经过漫长的演变重达到如今的含量。
氧气的形成和积累,导致地球上自然物质的分布发生变化。
2-2-1溶解在海洋中的Fe2+被氧化转变成针铁矿α-FeO(OH)而沉积,写出离子方程式。
2-2-2与铁的沉积不同,陆地上的MoS2被氧化而导致Mo溶于海洋并且析出单质硫,写出离子方程式。
2-3 2020年8月4日晚,贝鲁特港口发生了举世震惊的大爆炸,现场升起红棕色蘑菇云,破坏力巨大。
目前认定大爆炸为该港口存储的约2750吨硝酸铵的分解。
第 34 届中国化学奥林匹克(初赛)选拔赛 2020 年江苏省高中学生化学奥林匹克复赛试题第 1 题 不定项选择题(每题有 1-2个正确选项,每题 3 分,共 30 分) 1-1 垃圾分类是生态文明的进步,也是每个人都能参与的环境保护方式。
下列关 于垃圾处理的说法不正确的是 A.被污染的餐巾纸属于可回收垃圾 B.废蓄电池属于有害垃圾 C.厨余垃圾经发酵可制成肥料 D.废医用塑料可回收制造餐具1-2 用化学用语表示242SiO 4HF SiF 2H O +=↑+中的有关微粒,其中正确的是A.F-的结构示意图B.水分子的比例模型C.SiF4的电子式D.中子数为15的Si 原子2914Si1-3 用下列实验装置进行有关实验,能达到实验目的的是A.用甲装置制取少量 SO 2B.用乙装置蒸发 AlCl 3溶液制取 AlCl 3晶体C.用装置丙分离饱和碳酸钠和乙酸乙酯的混合液D.用装置丁除去 Cl 2中混有的少量 HCl 气体 1-4 下列有关物质的性质和用途具有对应关系的是A.活性炭具有还原性,可用作制糖业的脱色剂B.晶体硅的熔点高、硬度大,可用作制造半导体材料C.二氧化氯具有强氧化性,可用作饮用水消毒D.氯化铁溶液具有酸性,可用作刻蚀铜电路板1-5 短周期主族元素W、R、X、Y、Z 的原子序数依次增大,Y 原子半径在短周期元素中最大,W 和Y 同主族,X 与Z 同主族,R 原子最外层电子数比内层电子数多3,W、Y 原子的电子数总和与X、Z 原子的电子数总和之比为1:2。
下列说法正确的是A.原子半径r(X)>r(R)>r(W)B.X 与Y 只能形成一种化合物C.X 的简单气态氢化物的热稳定性比Z 的弱D.由W、R、X 三种元素组成的化合物可以是酸、碱或者盐1-6 下列实验中实验操作和现象、以及得出的结论均正确的是1-7 一种2-甲基色酮内酯(Y)可通过下列反应合成。
下列说法正确的是A.1molX 与浓溴水反应最多能与3molBr2加成B.X、Y 分子中所有碳原子均处于同一平面C.一定条件下,X 可以发生加成、缩聚、消去、氧化反应D.Y 与H2完全加成,每个产物分子中含有6个手性碳原子1-8 南京大学教授提出了一种高效的固碳新技术(如图)。