化工英文文献翻译
- 格式:doc
- 大小:38.50 KB
- 文档页数:5

化工学科英语作文模板英文回答:Chemical Engineering: A Multidisciplinary Field with Wide-Ranging Applications。
Chemical engineering is a branch of engineering that deals with the application of science and mathematics to the design, construction, and operation of chemical plants and processes. The discipline encompasses a wide range of topics, including thermodynamics, fluid mechanics, heat and mass transfer, process control, and chemical reaction engineering.Chemical engineers work in a variety of settings, including chemical plants, pharmaceutical companies, food processing facilities, and environmental protection agencies. They are responsible for designing and operating processes that produce a wide range of products, including chemicals, pharmaceuticals, plastics, and fuels. They alsowork to develop and improve processes that are more efficient, less polluting, and safer.Chemical engineering is a rapidly growing field, as the demand for chemicals and other products continues to increase. In addition, the need for sustainable and environmentally friendly processes is driving the development of new technologies and processes in the field.Key Features of Chemical Engineering。

化工专业英语Volatile:挥发性的 Semipermeable membrane:半透膜 immiscible:不相混的 Debit:把….记入借方 Credit:记入贷方Electrical potential:电势 Leaching:浸提 Extraction:萃取 Direct current:直流 Instantaneous:瞬间的 Successive:连续的 Collision:碰撞 Impeller:叶轮 Wavelet analysis:微元分析 Entrainment:夹带 Breakage:破坏Attrition:磨损 Indispensable:不可缺少的 Trajectory:轨道 Acrylic:丙烯酸 Baffle:挡板 Ruffle:滋扰 Discharge:释放 circulation flow:环流attrition:磨损 nucleation:成核 Catalytic:催化 frequency:频率shutter:快门 inertia:惯性 Pitched:倾斜的 histogram:柱状图breakdown:破坏 Unit 14 Distillation Dumped or ordered packings:乱堆或整齐堆放填料 Plate:板 Tray:塔盘 Hold-down and support plates:固定和支撑板 Fraction:馏分 Cascading:成瀑布落下,分多级进行 Reboiler:再沸器Overhead condenser: 塔顶冷凝器 Reflux:回流 Distillate:馏出物Countercurrent:逆流 Relative volatility:相对挥发度 Rectifying section:精馏段 Stripping section:提留段 Sidestream:侧线馏分 Circumvent:回避Hypothetical:假设的 Equilibrium-stage:平衡级(理论板) Tray efficiency:塔板效率 The number of hypothetical equilibrium stages required is then converted to a number actual trays by means of tray efficiencies, which describe the extent to which the performance of actual contact tray duplicates the performance of an equilibrium stage 然后理论塔板数通过塔板效率被转换成实际塔板数;塔板效率是实际塔板表现和理论塔板表现的比值。

3 化工英语文献3.1化工英语文献的结构Title, (Author names, Affiliation),Abstract ,(Keywords),Introduction,Experimental,Results, Discussions (Results and discussions),Conclusions,Acknowledgements,References3.2 英语文献的检索Elsevier—science directSpringerlinkWiley interscience3.3 中英文摘要1、定义以提供文献内容梗概为目的,不加评论和补充解释,简明、准切地记叙文献重要内容的短文。
好的摘要对于增加论文的被检索和引用的机会、吸引读者、扩大影响起着不可忽视的作用。
2、摘要的类型和基本内容类型:根据内容的不同,摘要分为三大类:报道性摘要、指示性摘要和报道-指示性摘要。
1)报道性摘要(informative abstract)。
也称信息性摘要或资料性摘要。
其特点是全面、简要地概括论文的目的、方法、主要数据和结论。
通常,这种摘要可部分地取代阅读全文。
2)指示性摘要(indicative abstract)。
也称说明性摘要、描述性摘要(descriptive abstract)或论点摘要(topic abstract)。
一般只用二、三句话概括论文的主题,而不涉及论据和结论,多用于综述、会议报道等。
帮助读者决定是否需要阅读全文。
3)报道-指示性摘要(informative- indicative abstract)。
以报道性摘要的形式表述一次文献中信息总价值较高的部分,以指示性摘要的形式表述其余部分。
传统的摘要多为一般式,在内容上大致包括引言(introduction)、材料和方法(materials and methods)、结果(results)和讨论(discussion)。
即IMRAD3、EI对摘要的要求《EI》中国信息部要求信息性文摘(Information Abstract)应该用简洁、明确的语言(约300汉字,150 英文字)将论文的“目的(Purposes)”,主要的研究“过程(Procedures)”及所采用的“方法(Methods)”,由此得到的主要“结果(Results)”和得出的重要“结论(Conclusions)”表达清楚。

Foreign material:Chemical Industry1.Origins of the Chemical IndustryAlthough the use of chemicals dates back to the ancient civilizations, the evolution of what we know as the modern chemical industry started much more recently. It may be considered to have begun during the Industrial Revolution, about 1800, and developed to provide chemicals roe use by other industries. Examples are alkali for soapmaking, bleaching powder for cotton, and silica and sodium carbonate for glassmaking. It will be noted that these are all inorganic chemicals. The organic chemicals industry started in the 1860s with the exploitation of William Henry Perkin’s discovery if the first synthetic dyestuff—mauve. At the start of the twentieth century the emphasis on research on the applied aspects of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having 75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia. The later required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time. The experience gained with this was to stand Germany in good stead, particularly with the rapidly increased demand for nitrogen-based compounds (ammonium salts for fertilizers and nitric acid for explosives manufacture) with the outbreak of world warⅠin 1914. This initiated profound changes which continued during the inter-war years (1918-1939).Since 1940 the chemical industry has grown at a remarkable rate, although this has slowed significantly in recent years. The lion’s share of this growth has been in the organic chemicals sector due to the development and growth of the petrochemicals area since 1950s. The explosives growth in petrochemicals in the 1960s and 1970s was largely due to the enormous increase in demand for synthetic polymers such as polyethylene, polypropylene, nylon, polyesters and epoxy resins.The chemical industry today is a very diverse sector of manufacturing industry, within which it plays a central role. It makes thousands of different chemicals whichthe general public only usually encounter as end or consumer products. These products are purchased because they have the required properties which make them suitable for some particular application, e.g. a non-stick coating for pans or a weedkiller. Thus chemicals are ultimately sold for the effects that they produce.2. Definition of the Chemical IndustryAt the turn of the century there would have been little difficulty in defining what constituted the chemical industry since only a very limited range of products was manufactured and these were clearly chemicals, e.g., alkali, sulphuric acid. At present, however, many intermediates to products produced, from raw materials like crude oil through (in some cases) many intermediates to products which may be used directly as consumer goods, or readily converted into them. The difficulty cones in deciding at which point in this sequence the particular operation ceases to be part of the chemical industry’s sphere of activities. To consider a specific example to illustrate this dilemma, emulsion paints may contain poly (vinyl chloride) / poly (vinyl acetate). Clearly, synthesis of vinyl chloride (or acetate) and its polymerization are chemical activities. However, if formulation and mixing of the paint, including the polymer, is carried out by a branch of the multinational chemical company which manufactured the ingredients, is this still part of the chemical industry of does it mow belong in the decorating industry?It is therefore apparent that, because of its diversity of operations and close links in many areas with other industries, there is no simple definition of the chemical industry. Instead each official body which collects and publishes statistics on manufacturing industry will have its definition as to which operations are classified as the chemical industry. It is important to bear this in mind when comparing statistical information which is derived from several sources.3. The Need for Chemical IndustryThe chemical industry is concerned with converting raw materials, such as crude oil, firstly into chemical intermediates and then into a tremendous variety of other chemicals. These are then used to produce consumer products, which make our livesmore comfortable or, in some cases such as pharmaceutical produces, help to maintain our well-being or even life itself. At each stage of these operations value is added to the produce and provided this added exceeds the raw material plus processing costs then a profit will be made on the operation. It is the aim of chemical industry to achieve this.It may seem strange in textbook this one to pose the question “do we need a chemical industry?” However trying to answer this question will provide(ⅰ) an indication of the range of the chemical industry’s activities, (ⅱ) its influence on our lives in everyday terms, and (ⅲ) how great is society’s need for a chemical industry. Our approach in answering the question will be to consider the industry’s co ntribution to meeting and satisfying our major needs. What are these? Clearly food (and drink) and health are paramount. Other which we shall consider in their turn are clothing and (briefly) shelter, leisure and transport.(1)Food. The chemical industry makes a major contribution to food production in at least three ways. Firstly, by making available large quantities of artificial fertilizers which are used to replace the elements (mainly nitrogen, phosphorus and potassium) which are removed as nutrients by the growing crops during modern intensive farming. Secondly, by manufacturing crop protection chemicals, i.e., pesticides, which markedly reduce the proportion of the crops consumed by pests. Thirdly, by producing veterinary products which protect livestock from disease or cure their infections.(2)Health. We are all aware of the major contribution which the pharmaceutical sector of the industry has made to help keep us all healthy, e.g. by curing bacterial infections with antibiotics, and even extending life itself, e.g. ß–blockers to lower blood pressure.(3)Clothing. The improvement in properties of modern synthetic fibers over the traditional clothing materials (e.g. cotton and wool) has been quite remarkable. Thus shirts, dresses and suits made from polyesters like Terylene and polyamides like Nylon are crease-resistant, machine-washable, and drip-dry or non-iron. They are also cheaper than natural materials.Parallel developments in the discovery of modern synthetic dyes and the technology to “bond” th em to the fiber has resulted in a tremendous increase in the variety of colors available to the fashion designer. Indeed they now span almost every color and hue of the visible spectrum. Indeed if a suitable shade is not available, structural modification of an existing dye to achieve this canreadily be carried out, provided there is a satisfactory market for the product.Other major advances in this sphere have been in color-fastness, i.e., resistance to the dye being washed out when the garment is cleaned.(4)Shelter, leisure and transport. In terms of shelter the contribution of modern synthetic polymers has been substantial. Plastics are tending to replace traditional building materials like wood because they are lighter, maintenance-free (i.e. they are resistant to weathering and do not need painting). Other polymers, e.g. urea-formaldehyde and polyurethanes, are important insulating materials f or reducing heat losses and hence reducing energy usage.Plastics and polymers have made a considerable impact on leisure activities with applications ranging from all-weather artificial surfaces for athletic tracks, football pitches and tennis courts to nylon strings for racquets and items like golf balls and footballs made entirely from synthetic materials.Like wise the chemical industry’s contribution to transport over the years has led to major improvements. Thus development of improved additives like anti-oxidants and viscosity index improves for engine oil has enabled routine servicing intervals to increase from 3000 to 6000 to 12000 miles. Research and development work has also resulted in improved lubricating oils and greases, and better brake fluids. Yet again the contribution of polymers and plastics has been very striking with the proportion of the total automobile derived from these materials—dashboard, steering wheel, seat padding and covering etc.—now exceeding 40%.So it is quite apparent even from a brief look at the chemical industry’s contribution to meeting our major needs that life in the world would be very different without the products of the industry. Indeed the level of a country’s development may be judged by the production level and sophistication of its chemical industry4. Research and Development (R&D) in Chemical IndustriesOne of the main reasons for the rapid growth of the chemical industry in the developed world has been its great commitment to, and investment in research and development (R&D). A typical figure is 5% of sales income, with this figure being almost doubled for the most research intensive sector, pharmaceuticals. It is important to emphasize that we are quoting percentages here not of profits but of sales income, i.e. the total money received, which has to pay for raw materials, overheads, staff salaries, etc. as well. In the past this tremendous investment has paid off well, leading to many useful and valuable products being introduced to the market. Examplesinclude synthetic polymers like nylons and polyesters, and drugs and pesticides. Although the number of new products introduced to the market has declined significantly in recent years, and in times of recession the research department is usually one of the first to suffer cutbacks, the commitment to R&D remains at a very high level.The chemical industry is a very high technology industry which takes full advantage of the latest advances in electronics and engineering. Computers are very widely used for all sorts of applications, from automatic control of chemical plants, to molecular modeling of structures of new compounds, to the control of analytical instruments in the laboratory.Individual manufacturing plants have capacities ranging from just a few tones per year in the fine chemicals area to the real giants in the fertilizer and petrochemical sectors which range up to 500,000 tonnes. The latter requires enormous capital investment, since a single plant of this size can now cost $520 million! This, coupled with the widespread use of automatic control equipment, helps to explain why the chemical industry is capital-rather than labor-intensive.The major chemical companies are truly multinational and operate their sales and marketing activities in most of the countries of the world, and they also have manufacturing units in a number of countries. This international outlook for operations, or globalization, is a growing trend within the chemical industry, with companies expanding their activities either by erecting manufacturing units in other countries or by taking over companies which are already operating there.化学工业1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。

合成新型硅改性聚酰亚胺Ewa Biaáecka-Florjanzyk, Andrzej Orzeszko农业大学,中国科学院化学研究所,ul.Nowoursynowska 159C ,02-787华沙,波兰电子邮件:orzeszkoa@delta.sggw.waw.pl收稿日期:2001年5月27日/修订版:2002年5月31日/接受日期:2002年6月10日总结从系列新聚(聚硅氧烷酰亚胺),N,N - dialkenylimides和1,1,3,3 - 四甲基通过polyhydrosilylation合成。
得到的聚合物从对苯二酚双[(N-allylphthalimide )-4 -羧酸]表明液晶属性。
其液晶相范围在107- 197 °C。
介绍聚酰亚胺和聚醯亚胺被称为耐高温材料和良好的透明度(1,2)。
不幸的是,这些聚合物是非常难处理的,它们溶于多数有机溶剂。
聚酰亚胺目前的处理问题和相当大的努力仍在致力于改善这些性能。
一些方法已被用来克服这个难题,同时保持它们高的热稳定。
这些措施包括插入柔性基团如乙烯或甲基链(3,4)。
此外,使用单体或大的悬挂的基团杂在主链来提高高分子的溶解度和处理。
另一方面,含硅聚合物是众所周知的可溶性物质。
他们还具有热塑性,并表现出良好的附着力。
把柔性硅基连接刚性的酰亚胺单元显著提高聚酰亚胺的加工性能。
聚(硅氧烷酰亚胺),PSIS,通常是通过缩合合成的含硅单体(1,5-7),即二胺或二酐。
我们建议另一个可能性,典型的含硅聚合反应,polyhydrosilylation酰亚胺双端烯烃组。
硅氢加成反应,即增加一个硅烷烯,在有机硅化学中是一个重要的反应。
它可以进行热或在各种催化剂中使用,例如hexachloroplatinic酸,二氯(内双环戊二烯)铂金或铂金四甲基divinyldisiloxane(PTDD)(9-12)。
在本研究中,我们合成了一系列新的聚酰亚胺,下面给出的一般公式进行酰亚胺基团和1,1,3,3-tetramethyldisiloxane单元。
对利用三元添置中子衍射得到的纳米级FeCo合金远程有序关系的探究1.简介由于具有非常高的饱和磁化强度和居里温度,FeCo合金在工业上是一种重要的工程材料。
这些合金在软磁材料的应用中发挥了重要作用,例如发电机和电动机。
进一步应用的例子是变压器磁芯,磁驱动传动器,高场磁体的磁极以及电磁阀。
在工业的大部分应用当中,面临的挑战是在保持磁性能的同时,如何提高FeCo合金的拉伸强度和韧性。
曾经尝试过的方法有改变合金设计(比如加入一些镍,钒,铌,钽,铬,钼三元金属)、进行退火处理或是采用先进的变形处理。
然而,在现代应用中,要求有更好的力学、磁学性能。
近几年来,由于对现代发电机和配电设备需求的增加,科学家在FeCo合金方面的研究兴趣与日俱增。
特别是在极端环境下,对电气应用的要求非常严格。
另一方面,针对FeCo合金的结构和物理性能,尤其是针对它的纳米结构系统提出了有趣的问题。
得益于低钴FeCo合金(钴占到质量分数的17%到35%)的发展,在满足所需的磁性能的同时,合金的成本才得以降低。
此外,较低的Co含量能够提高合金的延展性和韧性。
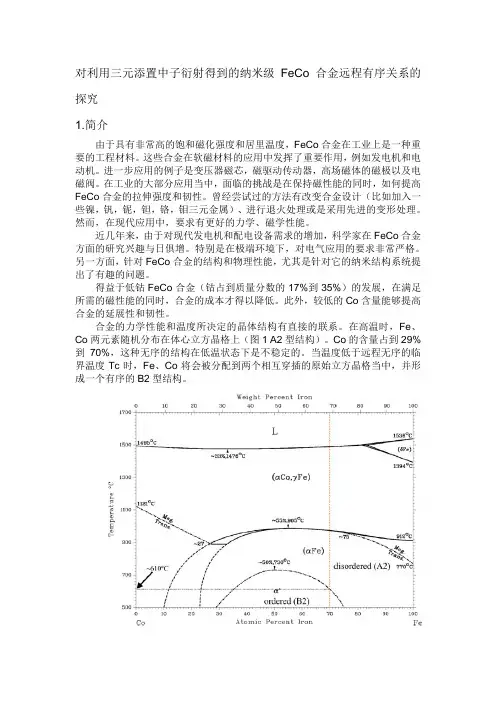
合金的力学性能和温度所决定的晶体结构有直接的联系。
在高温时,Fe、Co两元素随机分布在体心立方晶格上(图1 A2型结构)。
Co的含量占到29%到70%,这种无序的结构在低温状态下是不稳定的。
当温度低于远程无序的临界温度Tc时,Fe、Co将会被分配到两个相互穿插的原始立方晶格当中,并形成一个有序的B2型结构。
图1 二元合金FeCo的相图。
所讨论的三元合金的区域示意图。
B2型结构的合金有一些典型的特点。
比如说,“反结构”和“三点”机制产生的点缺陷能够导致晶格空位。
无序的B2型结构合金表现出波浪滑移,而局部无序型合金表现出平面滑移。
有序—无序之间的转变影响了FeCo合金的力学性能,比如合金韧性的改变、更脆的无序相、有序相等。
另外,磁性影响了结构的稳定性,造就了FeCo合金的有序性。
比如,在富铁FeCo合金中,磁有序稳定了体心立方结构,也稳定了来源于铁磁性B2相的有序性。

Preparation and characterization of carbon-coated LiFePO 4cathode materials for lithium-ion batteries with resorcinol –formaldehyde polymer as carbon precursorYachao Lan,Xiaodong Wang ⁎,Jingwei Zhang,Jiwei Zhang,Zhishen Wu,Zhijun Zhang ⁎Key Laboratory for Special Functional Materials,Henan University,Kaifeng 475004,Chinaa b s t r a c ta r t i c l e i n f o Article history:Received 8February 2011Received in revised form 26May 2011Accepted 3June 2011Available online 12June 2011Keywords:Lithium iron phosphateResorcinol –formaldehyde polymer Lithium-ion batteryLiFePO 4/C composites were synthesized by two methods using home-made amorphous nano-FePO 4as the iron precursor and soluble starch,sucrose,citric acid,and resorcinol –formaldehyde (RF)polymer as four carbon precursors,respectively.The crystalline structures,morphologies,compositions,electrochemical performances of the prepared powders were investigated with XRD,TEM,Raman,and cyclic voltammogram method.The results showed that employing soluble starch and sucrose as the carbon precursors resulted in a de ficient carbon coating on the surface of LiFePO 4particle,but employing citric acid and RF polymer as the carbon precursors realized a uniform carbon coating on the surface of LiFePO 4particle,and the corresponding thicknesses of the uniform carbon films are 2.5nm and 4.5nm,respectively.When RF polymer was used as the carbon precursor,the material showed the highest initial discharge capacity (138.4mAh g −1at 0.2C at room temperature)and the best rate performance among the four materials.©2011Elsevier B.V.All rights reserved.1.IntroductionLiFePO 4is an attractive cathode material for lithium-ion batteries because of its high theoretical capacity of 170mAh g −1,environ-mental benign,and high thermal stability.However,its poor electric conductivity of less than 10−13S cm −1limits its battery performance [1],such as the dramatic decrease in power at a high current density,which is the main drawback to commercial use.To overcome the low electric conductivity of LiFePO 4,many effective approaches have been introduced,including metal substitution [2–5],metal powder com-pounding [6],and conductive carbon coating [7–15].Among them,the preparation of LiFePO 4/carbon composite (LiFePO 4/C)is one of the attractive ways to improve the electric conductivity of the final product by forming a good conduction path.Furthermore,carbon can be also used as a reductant,which can reduce Fe 3+ions to Fe 2+ions.It should be noted that many studies involving the synthesis of nano-sized LiFePO 4employ Fe 2+salts as precursors [3,16–20],such as FeC 2O 4·2H 2O and (CH 3COO)2Fe,which are expensive.Therefore,it is necessary to use cheap materials and a convenient method.Here,we report the synthesis,characterization and electrochemical test of LiFePO 4/C composites prepared by two methods using home-made amorphous nano-FePO 4as the iron precursor and various organics as carbon precursors.The two methods using FePO 4as starting material are cheap and environmentally benign for the production of LiFePO 4material.Particularly,we present a novel method to synthesize a uniformcarbon film coated LiFePO 4cathode materials.This method involved an in situ reaction of resorcinol and formaldehyde on the surface of amorphous FePO 4.At room temperature,electrochemical tests showed that this material exhibited an initial discharge capacity of 138.4mAh g −1at 0.2C and a good cycling property at 0.5and 1.0C rate,respectively.2.Experimental2.1.Preparation of amorphous nano-FePO 4Amorphous nano-FePO 4was prepared by spontaneous precipita-tion from aqueous solutions.An equimolar solution of H 3PO 4was added to a solution of Fe(NO 3)3·9H 2O at 60°C under stirring and given amounts of PEG-400as surfactant.Then ammonia water (NH 3·H 2O)was slowly added to the mixed solution under vigorous stirring and a milk-white precipitate formed immediately.The pH of the solution was kept at 2.0.The precipitate was filtered and washed several times with distilled water.After drying in vacuum oven at 120°C for 12h,yellowish-white amorphous FePO 4was obtained.2.2.Preparation of LiFePO 4/CTwo methods were used to prepare the LiFePO 4/C composites in this study.2.2.1.Method oneA rheological phase method [21]was employed to synthesize LiFePO 4/C composite.Stoichiometric amount of amorphous FePO 4,LiOH·H 2O were used as the starting materials.The carbon precursorsPowder Technology 212(2011)327–331⁎Corresponding authors.Tel./fax:+863783881358.E-mail address:donguser@ (X.Wang).0032-5910/$–see front matter ©2011Elsevier B.V.All rights reserved.doi:10.1016/j.powtec.2011.06.005Contents lists available at ScienceDirectPowder Technologyj o u r n a l h o me p a g e :w w w.e l sev i e r.c o m /l oc a t e /pow t e care soluble starch(50.0g/1mol LiOH·H2O),sucrose(35.0g/1mol LiOH·H2O),citric acid monohydrate(21.0g/1mol LiOH·H2O),respec-tively.These carbon precursors were respectively solved in an appropri-ate amount of distilled water under stirring and heating.Then the amorphous FePO4and LiOH·H2O were added under vigorous stirring. Subsequently,the mixtures were respectively dried in an oven at120°C for6h,heated at350°C for1h in argonflow,treated at750°C for12h in argonflow,and ground.Finally,the LiFePO4/C composites were obtained and were denoted as sample A,sample B and sample C,respectively. 2.2.2.Method twoIn a typical synthesis,0.10g of CTAB was solved in30ml of distilled water solution under continuous stirring.Subsequently,1.52g FePO4·3H2O,0.055g resorcinol(R)and0.10ml formaldehyde(F)were successively added.When the temperature of water bath was up to85°C,LiOH·H2O was added.The mixture was kept stirred up in the dark for2h,dried in an oven at120°C for6h,heated at 350°C for1h in argonflow,treated at750°C for12h in argonflow, andfinally ground to obtain the LiFePO4/C composites(denoted as sample D).These four samples and their corresponding parameters are listed in Table1.The carbon contents of the samples were calculated by the loss on ignition of the four LiFePO4/C composites in air.2.3.CharacterizationThermogravimetric(TG)and differential thermal analysis(DTA) analyses were conducted with an EXSTAR6000thermal analysis system at a heating rate of10°C min−1.The powder X-ray diffraction (XRD,X'Pert Pro MPD,Philips)using Cu Ka radiation was employed to identify the crystalline phase of the prepared materials.Raman spectrum was recorded on a Renishaw RM-1000Microscopic Raman spectrometer with457.5nm excitation requiring a10mW power at room temperature.Low-magnification and high-magnification TEM images were taken on a JEM-2010transmission electron microscope (using an accelerating voltage of200kV).Electrodes were fabricated from a mixture of prepared carbon-coated LiFePO4powders(80wt.%),carbon black(12wt.%),and polyvinylidenefluoride in N-methylpyrrolidinon(8wt.%).The slurry was spread onto Al foil and dried in vacuum at120°C for12h.The carbon-coated LiFePO4loading was2mg cm−2in the experimental cells.The cells were assembled in an argon-atmosphere-filled glove box.The electrolyte was1M LiPF6in a mixture of ethylene carbonate (EC)and dimethyl carbonate(DMC)(1:1volume).The cells were galvanostatically charged and discharged at a voltage range of2.5–4.2V with LAND battery testing system at room temperature.Cyclic voltammograms were run on an IM6impedance and electrochemicalmeasurement system(Zahner,Germany)at a scan rate of0.1mV s−1 between2.5and4.0V.3.Results and discussionThe TEM images of the amorphous nano-FePO4were shown in Fig.1.The morphology of the as-prepared FePO4is an irregular particle with an average diameter of30nm.Most of the particles connected to each other because of their high surface energy which results from their small sizes.Fig.2a shows the TG/DTA curves of the FePO4·3H2O powder with a heating rate of10°C/min from room temperature to850°C in air.On the DTA curve near150°C,there is a very strong endothermic peak, associating with the sharp weight loss on the TG curve,which is related to the quick dehydration of FePO4·3H2O.During150–550°C, 26.3%weight loss on the TG curve indicates the slow elimination of residual H2O in FePO4·3H2O,exactly corresponding to the loss of crystalline water of FePO4·3H2O.And one exothermic peak is displayed at a higher temperature of590°C,which is not accompa-nied by appreciable weight loss in the TG curve,indicating the transformation of the amorphous FePO4to hexagonal FePO4crystal. The XRD patterns of FePO4·3H2O before and after calcination have been investigated in Fig.2b.As illustrated in pattern A,it can be seen that there is no evidence of diffraction peaks before calcination, indicating the synthesized FePO4·3H2O is just amorphous.While for the calcinated FePO4·3H2O at600°C for6h in air,it exhibits strong and narrow peaks revealing a well-crystallized material in pattern B. All of the diffraction peaks of the prepared FePO4are indexed to a single-phase hexagonal structure with a P3121space group and without any impurities,which is in good agreement with the standard card(JCPDS card no:72–2124).Table1Carbon precursors and residual carbon content of samples A,B,C and D.Samples A B C DCarbon precursor Starch Sucrose Citric acid RF polymer Final carbon content(wt.%) 5.48.5 4.35.1Fig.1.TEM images of the prepared amorphous nano-FePO4.n et al./Powder Technology212(2011)327–331The XRD diffraction patterns of LiFePO 4/C powders prepared with different carbon precursors were shown in Fig.3.All peaks can be indexed as a single phase with an ordered olivine structure indexed to the orthorhombic space group,Pnmb (JCPDS card no.83–2092).The obtained lattice parameters are sample A:a=10.2956Å,b=6.0367Å,and c =4.7001Å,sample B:a =10.1992Å,b =6.0483Å,andc=4.6971Å,sample C:a=10.2472Å,b=6.0208Å,and c=4.6882Åand sample D:a=10.3372Å,b=5.9993Å,and c=4.6932Å,respec-tively.There is no evidence of diffraction peaks for carbon,though some amorphous masses and films attached to the LiFePO 4particles were observed from TEM images (see Fig.4).This indicates the carbon contents are very low.Morphologies of these LiFePO 4/C composites were shown in Fig.4.It is obvious that the samples show different carbon distribution on LiFePO 4particle surface.From Fig.4a,c,e and g,we observed that the samples were composed of agglomerated particles whose sizes range from 50to 300nm.From Fig.4b and d,there is not enough carbon coating to spread throughout the substrate particles.In contrast to sample A and sample B,there are uniform carbon thin films on the grain surfaces of sample C and sample D,and the thickness of the carbon films are about 2.5nm (Fig.4f)and 4.5nm (Fig.4h),respectively.The reason may lie in that different carbon precursors have different adsorbabilities on the surface of FePO 4·3H 2O particles,resulting in different carbon distribution on the surface of LiFePO 4particle after the post treatment.Soluble starch and sucrose possess plentiful hydroxyl groups,by which soluble starch and sucrose molecules could probably weakly adsorb on the surface of FePO 4·3-H 2O particles in the hydrogen bonding.In the post treatment process,part of soluble starch and sucrose molecules desorbed from the surface of FePO 4·3H 2O particles,resulting in the de ficient carbon coating.But citric acid possesses carboxyl groups,which may be partially esteri fied by hydroxyl groups on the FePO 4·3H 2O particles,forming a tight connection.This results in more complete carbon coating after the post treatment.For sample D,we suppose that,in the present synthetic system,the surfactant CTAB may con fine the resorcinol –formaldehyde (RF)polymer molecules and FePO 4·3H 2O particles in plenty of tiny spaces,so the RF polymer molecules were tightly attached to FePO 4·3H 2O particles.After the post treatment,the RF polymer was transformed into the carbon film which tightly stuck on the surface of LiFePO 4particle.In addition,from the HRTEM image of sample D (shown in Fig.4h),the d-spacing of 0.294nm corresponds to the (211)plane of LiFePO 4.As an important aid investigating the structure of the carbon,the Raman measurement was adopted,and the results were shown in Fig.5.Every Raman spectrum consists of a small band at 940cm −1,which corresponds to the symmetric PO 4stretching vibration in LiFePO 4.The intense broad bands at 1350and 1590cm −1can be attributed to the characteristic Raman spectra of carbon.The bands at 1590cm −1mainly correspond to graphitized structured carbon of G band,while that at 1350cm −1corresponds to disordered structured carbon of D band [22,23].The graphitized carbon contains sp 2hybrid bonding,which is positively correlated with the electronic conduc-tivity of carbon,and the disordered carbon mainly corresponds to sp 3hybrid bonding.As shown in Fig.5,the integrated intensity ratios of sp 2/sp 3of the LiFePO 4/C composites synthesized with different carbon precursors are 0.865(curve A),0.857(curve B),0.856(curve C)and 0.860(curve D),respectively.So the similar sp 2/sp 3ratios of the four samples give us few clues to explain the difference in their electrochemical performances.Fig.6shows the cycling performance curves of all the samples at different rates.As shown in Fig.6,the initial discharge capacities of sample A,sample B,sample C and sample D at room temperature at 0.2C rate are 110.4,118.8,137.7and 138.4mAh g −1,respectively.The capacity of sample D gradually increases in the initial cycles.This may be due to the incomplete dispersion of the electrolyte into the electrode material at the beginning.Moreover,the capacity of sample D is highest among the four samples at 0.5C and 1.0C,indicating that method two is better than method one.The lower capacities of sample A and sample B must be due to the incomplete carbon coating on the LiFePO 4particles.The higher capacity of sample D than that of sample C may be attributed to the thicker carbon film of sample D keeping the crystal structure of LiFePO 4morestable.Fig.2.(a)TG/DTA curves of the FePO 4·3H 2O.(b)XRD patterns of the FePO 4samples before (A)and after (B)calcination inair.Fig. 3.XRD patterns of LiFePO 4/C composites synthesized with different carbon precursors.329n et al./Powder Technology 212(2011)327–331In order to further understand the electrochemical properties of the four samples,the cyclic voltammogram (CV)curves were performed at a scan rate of 0.1mV s −1at room temperature (as shown in Fig.7).Each of the CV curves consists of an oxidation peak and a reduction peak,corresponding to the charge reaction and discharge reaction of the Fe 2+/Fe 3+redox couple.In the CV pro files of the LiFePO 4cathode material,the smaller voltage difference between the charge and discharge plateaus and the higher peak current means better electrode reaction kinetics,and consequently better rate performance.Sample A and sample B electrodes have broad peaks in CV curves.In contrast,sample C and sample D electrodes demonstrate sharp redox peaks,which indicate an improvement in the kinetics of the lithium intercalation/de-intercalation at the electrode/electrolyte interface.The voltage difference of sample D is smaller than that of sample C,so sample D demonstrates a better rate performance.4.ConclusionsLiFePO 4/C composites were synthesized by two methods using home-made amorphous nano-FePO 4as the iron precursor and various organics as carbon precursors.It was found that employing soluble starch and sucrose as the carbon precursors resulted in a de ficient carbon coating on the surface of LiFePO 4particle,but employing citric acid and RF polymer as the carbon precursors realized a uniform carbon coating on the surface of LiFePO 4particle.Particularly,when RF polymer was used as the carbon precursor,the carbon film is thicker,and the material showed the highest initial discharge capacity (138.4mAh g −1at 0.2C at room temperature)and the best rate performance among the four materials.The intensities of redox peak and the voltage differences in the CV curves of the four samples are consistent with their rateperformance.Fig.4.TEM images of synthesized LiFePO 4/C composite synthesized with different carbon precursors.(a)and (b)sample A,(c)and (d)sample B,(e)and (f)sample C,(g)and (h)sampleD.Fig. 5.Raman shift of LiFePO 4/C composites synthesized with different carbonprecursors.Fig.6.The cycling performance curves of the samples with different carbon precursors at various discharge rates.n et al./Powder Technology 212(2011)327–331References[1] A.K.Padhi,K.S.Nanjundaswamy,J.B.Goodenough,Phospho-olivines as positive-electrode materials for rechargeable lithium batteries,J.Electrochem.Soc.144(1997)1188–1194.[2]T.Nakamura,Y.Miwa,M.Tabuchi,Y.Yamada,Structural and surfacemodi fications of LiFePO 4olivine particles and their electrochemical properties,J.Electrochem.Soc.153(2006)A1108–A1114.[3]S.-Y.Chung,J.T.Bloking,Y.-M.Chiang,Electronically conductive phospho-olivinesas lithium storage electrodes,Nat.Mater.1(2002)123–128.[4]P.S.Herle,B.Ellis,N.Coombs,L.F.Nazar,Nano-network electronic conduction iniron and nickel olivine phosphates,Nat.Mater.3(2004)147–152.[5]G.X.Wang,S.Bewlay,S.A.Needham,H.K.Liu,R.S.Liu,V.A.Drozd,J.-F.Lee,J.M.Chend,Synthesis and characterization of LiFePO 4and LiTi 0.01Fe 0.99PO 4cathode materials,J.Electrochem.Soc.153(2006)A25–A31.[6] F.Croce,A.D'Epifanio,J.Hasson,A.Duptula,T.Olczac,B.Scrosati,A novel conceptfor the synthesis of an improved LiFePO 4lithium battery cathode,Electrochem.Solid-State Lett.5(2002)A47–A50.[7]H.Huang,S.C.Yin,L.F.Nazar,Approaching theoretical capacity of LiFePO 4at roomtemperature at high rates,Electrochem.Solid-State Lett.4(2001)A170–A172.[8]M.Herstedt,M.Stjerndahl,A.Nyten,T.Gustafsson,H.Rensmo,H.Siegbahn,N.Ravert,M.Armand,J.O.Thomas,K.Edstroem,Surface chemistry of carbon-treated LiFePO 4particles for Li-ion battery cathodes studied by PES,Electrochem.Solid-State Lett.6(2003)A202–A206.[9]M.M.Doeff,Y.Hu,F.McLarnon,R.Kostecki,Effect of surface carbon structure onthe electrochemical performance of LiFePO 4,Electrochem.Solid-State Lett.6(2003)A207–A209.[10]Y.Hu,M.M.Doeff,R.Kostecki,R.Finones,Electrochemical performance of sol –gelsynthesized LiFePO 4in lithium batteries,J.Electrochem.Soc.151(2004)A1279–A1285.[11]X.Z.Liao,Z.Ma,L.Wang,X.Zhang,Y.Jiang,Y.He,A novel synthesis route forLiFePO 4/C cathode materials for lithium-ion batteries,Electrochem.Solid-State Lett.7(2004)A522–A525.[12] C.H.Mi,X.B.Zhao,G.S.Cao,J.P.Tu,In situ synthesis and properties of carbon-coated LiFePO 4as Li-ion battery cathodes,J.Electrochem.Soc.152(2005)A483–A487.[13]R.Dominko,M.Bele,M.Gaberscek,M.Remskar,D.Hanzel,S.Pejovnik,J.Jamnik,Impact of the carbon coating thickness on the electrochemical performance of LiFePO 4/C composites,J.Electrochem.Soc.152(2005)A607-A607.[14]K.Zaghib,J.Shim,A.Guer fi,P.Charest,K.A.Striebel,Effect of carbon source asadditives in LiFePO 4as positive electrode for lithium-ion batteries,Electrochem.Solid-State Lett.8(2005)A207–A210.[15]K.S.Park,J.T.Son,H.T.Chung,S.J.Kim,C.H.Lee,K.T.Kang,H.G.Kim,Surfacemodi fication by silver coating for improving electrochemical properties of LiFePO 4,Solid State Commun.129(2004)311–314.[16] A.Yamada,S.C.Chung,K.Hinokuma,Optimized LiFePO 4for lithium batterycathodes,J.Electrochem.Soc.148(2001)A224–A229.[17]N.Meethong,H.-Y.Shadow Huang,W.C.Carter,Y.-M.Chiang,Size-dependentlithium miscibility gap in nanoscale Li 1−x FePO 4,Electrochem.Solid-State Lett.10(2007)A134–A138.[18] C.Delacourt,P.Poizot,S.Levasseur,C.Masquelier,Size effects on carbon-freeLiFePO 4powders,Electrochem.Solid-State Lett.9(2006)A352–A355.[19] D.Choi,P.N.Kumta,Surfactant based sol –gel approach to nanostructured LiFePO 4for high rate Li-ion batteries,J.Power Sources 163(2007)1064–1069.[20]K.Zaghib,A.Mauger,F.Gendron,C.M.Julien,Surface effects on the physical andelectrochemical properties of thin LiFePO 4particles,Chem.Mater.20(2008)462–469.[21]Y.H.Huang,H.B.Ren,S.Y.Yin,Y.H.Wang,Z.H.Peng,Y.H.Zhou,Synthesis ofLiFePO 4/C composite with high-rate performance by starch sol assisted rheolog-ical phase method,J.Power Sources 195(2010)610–613.[22]M.M.Doeff,J.D.Wilcox,R.Kostecki,u,Optimization of carbon coatings onLiFePO 4,J.Power Sources 163(2006)180–184.[23]G.L.Yang,A.F.Jalbout,Y.Xu,H.Y.Yu,X.G.He,H.M.Xie,R.S.Wang,Effect ofpolyacenic semiconductors on the performance of olivine LiFePO 4,Electrochem.Solid-State Lett.11(2008)A125–A128.Fig.7.The CV pro files of the different samples at the scan rate of 0.1mV s −1.331n et al./Powder Technology 212(2011)327–331。

介绍化工专业的认识英文作文English: Chemical engineering is a specialized field of study that combines principles of chemistry, physics, biology, and mathematics to design and operate industrial processes that convert raw materials into useful products. Students studying chemical engineering gain a deep understanding of chemical reactions, process design, thermodynamics, and fluid mechanics, among other technical subjects. The field of chemical engineering is incredibly diverse, with applications in industries such as pharmaceuticals, petroleum refining, food and beverage, environmental protection, and materials manufacturing. Working in chemical engineering requires problem-solving skills, attention to detail, creativity, and the ability to work collaboratively in multidisciplinary teams to tackle complex challenges. Graduates with a degree in chemical engineering have a wide range of career opportunities available to them, including roles in research and development, process engineering, production management, quality control, and environmental engineering.中文翻译: 化工工程是一门专业研究领域,结合了化学、物理、生物学和数学原理,用于设计和运行将原材料转化为有用产品的工业过程。

什么是化工英文作文英文:Chemical engineering is a branch of engineering that deals with the design, development, and operation of chemical processes and equipment. It involves the use of chemistry, physics, mathematics, and economics to solve problems related to the production and use of chemicals, fuels, drugs, food, and other products. Chemical engineers work in a wide range of industries, including oil and gas, pharmaceuticals, food and beverage, plastics, and environmental engineering.As a chemical engineer, I have been involved in the development of new processes for producing chemicals and materials. For example, I worked on a project to develop a new method for synthesizing a polymer that is used in medical devices. This involved designing and building a new reactor system, optimizing reaction conditions, and testing the product to ensure that it met the requiredspecifications.Another aspect of chemical engineering is process optimization. This involves analyzing existing processes to identify inefficiencies and areas for improvement. For example, I worked on a project to optimize a production process for a specialty chemical. We were able to reducethe cycle time, increase yield, and improve product quality, which resulted in significant cost savings for the company.中文:化学工程是一种工程学科,涉及化学过程和设备的设计、开发和操作。


毕业设计论文化学系毕业论文外文文献翻译中英文英文文献及翻译A chemical compound that is contained in the hands of the problemsfor exampleCatalytic asymmetric carbon-carbon bond formation is one of the most active research areas in organic synthesis In this field the application of chiral ligands in enantioselective addition of diethylzinc to aldehydes has attracted much attention lots of ligands such as chiral amino alcohols amino thiols piperazines quaternary ammonium salts 12-diols oxazaborolidines and transition metal complex with chiral ligands have been empolyed in the asymmetric addition of diethylzinc to aldehydes In this dissertation we report some new chiral ligands and their application in enantioselective addition of diethylzinc to aldehydes1 Synthesis and application of chiral ligands containing sulfur atomSeveral a-hydroxy acids were prepared using the literature method with modifications from the corresponding amino acids valine leucine and phenylalanine Improved yields were obtained by slowly simultaneous addition of three fold excess of sodium nitrite and 1 tnolL H2SO4 In the preparation of a-hydroxy acid methyl esters from a-hydroxy acids following the procedure described by Vigneron a low yield 45 was obtained It was found that much better results yield 82 couldbe obtained by esterifying a-hydroxy acids with methanol-thionyl chlorideThe first attempt to convert S -2-hydroxy-3-methylbutanoic acid methyl ester to the corresponding R-11-diphenyl-2-mercapto-3-methyl-l-butanol is as the following S-2-Hydroxy-3-methylbutanoic acid methyl ester was treated with excess of phenylmagnesium bromide to give S -11-diphenyl-3-methyl-12-butanediol which was then mesylated to obtain S -11-diphenyl-3-methyl-2-methanesulfonyloxy -l-butanol Unfortunately conversion of S-11-diphenyl-3-methyl-2- methanesulfonyloxy -l-butanol to the corresponding thioester by reacting with potassium thioacetate under Sn2 reaction conditions can be achieved neither in DMF at 20-60 nor in refluxing toluene in the presence of 18-crown-6 as catalyst When S -1ll-diphenyl-3-methyl-2- methane sulfonyloxy -l-butanol was refluxed with thioacetic acid in pyridine an optical active epoxide R-22-diphenyl -3-isopropyloxirane was obtained Then we tried to convert S -11-diphenyl-3-methyl-l2-butanediol to the thioester by reacting with PPh3 DEAD and thioacetic acid the Mitsunobu reaction but we failed either probably due to the steric hindrance around the reaction centerThe actually successful synthesis is as described below a-hydroxy acid methyl esters was mesylated and treated with KSCOCH3 in DMF to give thioester this was than treated with phenyl magnesium bromide to gave the target compound B-mercaptoalcohols The enantiomeric excesses ofp-mercaptoalcohols can be determined by 1H NMR as their S -mandeloyl derivatives S -2-amino-3-phenylpropane-l-thiol hydrochloride was synthesized from L-Phenylalanine L-Phenylalanine was reduced to the amino alcohol S -2-amino-3-phenylpropanol Protection of the amino group using tert-butyl pyrocarbonate gave S -2-tert-butoxycarbonylamino-3-phenylpropane-l-ol which was then O-mesylated to give S -2-tert-butoxycarbonylamino-3-phenylpropyl methanesulfonate The mesylate was treated with potassium thioacetate in DMF to give l-acetylthio-2-tert-butoxycarbonylamino-3-phenylpropane The acetyl group was then removed by treating with ammonia in alcohol to gave S -2-tert-butoxycarbonylamino-3-phenyl-propane-l-thiol which was then deprotected with hydrochloric acid to give the desired S-2-amino-3-phenylpropane-1-thiol hydrochlorideThe enantioselective addition of diethylzinc to aldehydes promoted by these sulfur containing chiral ligands produce secondary alcohols in 65-79 Synthesis and application of chiral aminophenolsThree substituted prolinols were prepared from the naturally-occurring L-proline using reported method with modifications And the chiral aminophenols were obtained by heating these prolinols with excess of salicylaldehyde in benzene at refluxThe results of enantioselective adBelow us an illustration forexampleN-Heterocyclic carbenes and L-Azetidine-2-carboxylicacidN-Heterocyclic carbenesN-Heterocyclic carbenes have becomeuniversal ligands in organometallic and inorganic coordination chemistry They not only bind to any transition metal with low or high oxidation states but also to main group elements such as beryllium sulfur and iodine Because of their specific coordination chemistry N-heterocyclic carbenes both stabilize and activate metal centers in quite different key catalytic steps of organic syntheses for example C-H activation C-C C-H C-O and C-N bond formation There is now ample evidence that in the new generation of organometallic catalysts the established ligand class of organophosphanes will be supplemented and in part replaced byN-heterocyclic carbenes Over the past few years this chemistry has become the field of vivid scientific competition and yielded previously unexpected successes in key areas of homogeneous catalysis From the work in numerous academic laboratories and in industry a revolutionary turningpoint in oraganometallic catalysis is emergingIn this thesis Palladium Ⅱ acetate and NN"-bis- 26-diisopropylphenyl dihydro- imidazolium chloride 1 2 mol were used to catalyze the carbonylative coupling of aryl diazonium tetrafluoroborate salts and aryl boronic acids to form aryl ketones Optimal conditions include carbon monoxide 1 atm in 14-dioxane at 100℃ for 5 h Yields for unsymmetrical aryl ketones ranged from 76 to 90 for isolated materials with only minor amounts of biaryl coupling product observed 2-12 THF as solvent gave mixtures of products 14-Dioxane proved to be the superior solvent giving higher yieldsof ketone product together with less biphenyl formation At room temperature and at 0℃ with 1 atm CO biphenyl became the major product Electron-rich diazonium ion substrates gave a reduced yield with increased production of biaryl product Electron-deficient diazonium ions were even better forming ketones in higher yields with less biaryl by-product formed 2-Naphthyldiazonium salt also proved to be an effective substrate givingketones in the excellent range Base on above palladium NHC catalysts aryl diazonium tetrafluoroborates have been coupled with arylboron compounds carbon monoxide and ammonia to give aryl amides in high yields A saturated yV-heterocyclic carbene NHC ligand H2lPr 1 was used with palladium II acetate to give the active catalyst The optimal conditions with 2mol palladium-NHC catalyst were applied with various organoboron compounds and three aryl diazonium tetrafluoroborates to give numerous aryl amides in high yield using pressurized CO in a THF solution saturated with ammonia Factors that affect the distribution of the reaction products have been identified and a mechanism is proposed for this novel four-component coupling reactionNHC-metal complexes are commonly formed from an imidazolium salt using strong base Deprotonation occurs at C2 to give a stable carbene that adds to form a a-complex with the metal Crystals were obtained from the reaction of imidazolium chloride with sodium t- butoxide Nal and palladium II acetate giving a dimeric palladium II iodide NHC complex The structure adopts a flat 4-memberedring u2 -bridged arrangement as seen in a related dehydro NHC complex formed with base We were pleased to find that chloride treated with palladium II acetate without adding base or halide in THF also produced suitable crystals for X-ray anaysis In contrast to the diiodide the palladium-carbenes are now twisted out of plane adopting a non-planar 4-ring core The borylation of aryldiazonium tetrafluoroborates with bis pinacolatoborane was optimized using various NHC ligand complexes formed in situ without adding base NN"-Bis 26-diisopropylphenyl-45-dihydroimidazolium 1 used with palladium acetate in THF proved optimal giving borylated product in 79 isolated yield without forming of bi-aryl side product With K2CO3 and ligand 1 a significant amount of biaryl product 24 was again seen The characterization of the palladium chloride complex by X-ray chrastallography deL-Azetidine-2-carboxylic acidL-Azetidine-2-carboxylic acid also named S -Azetidine-2-carboxylic acid commonly named L-Aze was first isolated in 1955 by Fowden from Convallaria majalis and was the first known example of naturally occurring azetidine As a constrained amino acid S -Azetidine-2-carboxylic acid has found many applications in the modification of peptides conformations and in the area of asymmetric synthesis which include its use in the asymmetric reduction of ketones Michael additions cyclopropanations and Diels-Alder reactions In this dissertation five ways for synthesize S-Azetidine-2-carboxylic acid were studied After comparing all methods theway using L-Aspartic acid as original material for synthesize S-Azetidine-2-carboxylic acid was considered more feasible All mechanisms of the way"s reaction have also been studied At last the application and foreground of S -Azetidine-2-carboxylic acid were viewed The structures of the synthetic products were characterized by ThermalGravity-Differential Thermal Analysis TG-DTA Infrared Spectroscopy IR Mass Spectra MS and 1H Nuclear Magnetic Resonance 1H-NMR Results showed that the structures and performances of the products conformed to the anticipation the yield of each reaction was more than 70 These can conclude that the way using L-Aspartie acid as original material for synthesize S -Azetidine-2-carboxylic acid is practical and effective杂环化合物生成中包含手性等问题如催化形成不对称碳碳键在有机合成中是一个非常活跃的领域在这个领域中利用手性配体诱导的二乙基锌和醛的不对称加成引起化学家的广泛关注许多手性配体如手性氨基醇手性氨基硫醇手性哌嗪手性四季铵盐手性二醇手性恶唑硼烷和过渡金属与手性配体的配合物等被应用于二乙基锌对醛的不对称加成中在本论文中我们报道了一些新型的手性配体的合成及它们应用于二乙基锌对醛的不对称加成的结果1含硫手性配体的合成和应用首先从氨基酸缬氨酸亮氨酸苯丙氨酸出发按照文献合成α-羟基酸并发现用三倍量的亚硝酸钠和稀硫酸同时滴加进行反应能适当提高反应的产率而根据Vigneron等人报道的的方法用浓盐酸催化从α-羟基酸合成α-羟基酸甲酯时只能获得较低的产率改用甲醇-二氯亚砜的酯化方法时能提高该步骤的产率从 S -3-甲基-2-羟基丁酸甲酯合成 R -3-甲基-11-二苯基-2-巯基-1-丁醇经过了以下的尝试 S -3-甲基-2-羟基丁酸甲酯和过量的格氏试剂反应得到 S -3-甲基-11-二苯基-12-丁二醇进行甲磺酰化时位阻较小的羟基被磺酰化生成 S -3-甲基-11-二苯基-2- 甲磺酰氧基 -1-丁醇但无论将 S -3-甲基-11-二苯基-2- 甲磺酰氧基 -1-丁醇和硫代乙酸钾在DMF中反应 20~60℃还是在甲苯中加入18-冠-6作为催化剂加热回流都不能得到目标产物当其与硫代乙酸在吡啶中回流时得到的不是目标产物而是手性环氧化合物 R -3-异丙基-22-二苯基氧杂环丙烷从化合物 S -3-甲基-11-二苯基-12-丁二醇通过Mitsunobu反应合成硫代酯也未获得成功这可能是由于在反应中心处的位阻较大造成的几奥斯塑手村犯体的合成裁其在不对称奋成中肠左用摘要成功合成疏基醇的合成路是将a-轻基酸甲酷甲磺酞化得到相应的磺酞化产物并进行与硫代乙酸钾的亲核取代反应得到硫酷进行格氏反应后得到目标分子p一疏基醇用p一疏基醇与 R 义一一甲氧基苯乙酞氯生成的非对映体经H侧NM吸测试其甲氧基峰面积的积分求得其ee值 3一苯基一氨基丙硫醇盐酸盐从苯丙氨酸合成斗3一苯基一氨基丙醇由L一苯丙氨酸还原制备氨基保护后得到习一3一苯基一2一叔丁氧拨基氨基一1一丙醇甲磺酞化后得到习一3一苯基一2一叔丁氧拨基氨基一1一丙醇甲磺酸酷用硫代乙酸钾取代后得匀一3-苯基一2一叔丁氧拨基氨基一1一丙硫醇乙酸酷氨解得习一3一苯基一2一叔丁氧拨基氨基一1一丙硫醇用盐酸脱保护后得到目标产物扔3一苯基屯一氨基丙硫醇盐酸盐手性含硫配体诱导下的二乙基锌与醛的加成所得产物的产率为65一79值为O井92手性氨基酚的合成和应用首先从天然的L一脯氨酸从文献报道的步骤合成了三种脯氨醇这些手性氨基醇与水杨醛在苯中回流反应得到手性氨基酚手性氨基酚配体诱导下的二乙基锌与醛的加成所得产物的产率为45一98值为0一90手性二茂铁甲基氨基醇的合成和应用首先从天然氨基酸绿氨酸亮氨酸苯丙氨酸和脯氨酸合成相应的氨基醇这些氨基醇与二茂铁甲醛反应生成的NO一缩醛经硼氢化钠还原得到手性二茂铁甲基氨基醇手性二茂铁甲基氨基醇配体诱导下的二乙基锌与醛的加成所得产物的产率为66一97下面我们举例说明一下例如含氮杂环卡宾和L-氮杂环丁烷-2-羧酸含氮杂环卡宾含氮杂环卡宾已广泛应用于有机金属化学和无机配合物化学领域中它们不仅可以很好地与任何氧化态的过渡金属络合还可以与主族元素铍硫等形成配合物由于含氮杂环卡宾不但使金属中心稳定而且还可以活化此金属中心使其在有机合成中例如C-H键的活化C-CC-HC-O和C-N键形成反应中有着十分重要的催化效能现有的证据充分表明在新一代有机金属催化剂中含氮杂环卡宾不但对有机膦类配体有良好的互补作用而且在有些方面取代有机膦配体成为主角近年来含氮杂环卡宾及其配合物已成为非常活跃的研究领域在均相催化这一重要学科中取得了难以想象的成功所以含氮杂环卡宾在均相有机金属催化领域的研究工作很有必要深入地进行下去本文研究了乙酸钯和NN双 26-二异丙基苯基 -45-二氢咪唑氯化物1作为催化剂催化芳基四氟硼酸重氮盐与芳基硼酸的羰基化反应合成了一系列二芳基酮并对反应条件进行了优化使反应在常温常压下进行一个大气压的一氧化碳14-二氧杂环己烷作溶剂100℃反应5h 不同芳基酮的收率达7690仅有微量的联芳烃付产物 212 反应选择性良好当采用四氢呋喃或甲苯作溶剂时得到含较多副产物的混合物由此可以证明14-二氧杂环己烷是该反应最适宜的溶剂在室温或0℃与一个大气压的一氧化碳反应联芳烃变成主产物含供电子取代基的芳基重氮盐常常给出较低收率的二芳基酮而含吸电子取代基的芳基重氮盐却给出更高收率的二芳基酮及较少量的联芳烃付产物实验证明2-萘基重氮盐具有很好的反应活性和选择性总是得到优异的反应结果在此基础上由不同的芳基四氟硼酸重氮盐与芳基硼酸一氧化碳和氨气协同作用以上述含氮杂环卡宾作配体与乙酸钯生成的高活性含氮杂环卡宾钯催化剂催化较高收率地得到了芳基酰胺优化的反应条件是使用2mol的钯-H_2IPr 1五个大气压的一氧化碳以氨气饱和的四氢呋喃作溶剂由不同的有机硼化合物与三种芳基重氮盐的四组份偶联反应同时不仅对生成的多种产物进行了定 L-氮杂环丁烷-2-羧酸L-氮杂环丁烷-2-羧酸又称 S -氮杂环丁烷-2-羧酸简称为L-Aze1955年由Fowden从植物铃兰 Convallaria majalis 中分离得到成为第一个被证实的植物中天然存在的氮杂环丁烷结构作为一种非典型的氨基酸已经发现 S -氮杂环丁烷-2-羧酸可广泛用于对多肽结构的修饰以及诸如不对称的羰基还原Michael 加成环丙烷化和Diels-Alder反应等不对称合成中的多个领域本文通过对 S -氮杂环丁烷-2-羧酸合成路线的研究综述了五种可行的合成路线及方法通过比较选用以L-天冬氨酸为初始原料合成 S -氮杂环丁烷-2-羧酸的路线即通过酯化反应活泼氢保护格氏反应内酰胺化反应还原反应氨基保护氧化反应脱保护等反应来合成 S -氮杂环丁烷-2-羧酸分析了每步反应的机理并对 S -氮杂环丁烷-2-羧酸的应用及前景给予展望通过热分析红外质谱核磁等分析手段对合成的化合物的结构进行表征结果表明所得的产物符合目标产物所合成的化合物的结构性能指标与设计的目标要求一致每步反应的收率都在70%以上可以判定以L-天冬氨酸为初始原料合成 S -氮杂环丁烷的路线方案切实可行。
Preparation and characterization of Ag-TiO2 hybrid clusters powders[1](Ag-TiO2混合团簇粉末的制备和表征)Abstract:液相电弧放电法被用于制备纳米Ag-TiO2复合超细粉末。
XRD和TEM图表明颗粒呈葫芦状形态,分布狭窄。
我们讨论了实验条件对产品的影响,比较了这种方法制备的粉末和其他γ射线辐照法制备的粉末。
Introduction:材料合成技术,提高了研究特定电子和光学特性的能力。
这也导致了设备和不同效应的快速发展,如集成光学型偏振器[1]和量子霍耳效应。
所需的长度尺度对于这些结构的控制是在纳米级别的[ 2 ]。
科学家面临的一个新的挑战是半导体量子点的生长,它具有新的光学响应,引起了对其基础物理方面和三阶非线性光致发光的应用等的研究兴趣。
这方面的一个例子是Ag-TiO2复合材料通过胶体方法合成[ 3 ]或由γ射线辐照法合成[ 4 ]。
对比其他制备超细金属颗粒的方法,γ射线辐照法能在室温的环境压力下产生粉末。
在这封信中,我们开发了一种新的方法,即液相电弧放电法,用以制备纳米复合材料,当它经水热处理可以得到纳米级别的超细粉。
Preparation and photocatalytic activity of immobilized composite photocatalyst (titania nanoparticle/activated carbon)[2]固定化复合光催化剂(TiO2纳米颗粒/活性炭)的制备和光催化活性研究Abstract:制备了一种固定化复合光催化剂——TiO2纳米颗粒/活性炭(AC),并研究了它在降解纺织染料的光催化活性。
AC通过油菜籽壳制备。
碱性红18(BR18)和碱性红46(BR46)被用来作为模型染料。
并采用了傅里叶变换红外(FTIR),波长色散X射线光谱(WDX),扫描电子显微镜(SEM),紫外可见分光光度法,化学需氧量(COD)和离子色谱(IC)分析。
化工术语中英文对照在化工领域中,有许多术语用来描述不同的化学过程、化学物质和实验设备。
这些术语通常使用中文和英文两种语言来表达,因此熟悉化工术语的中英文对照是非常重要的。
本文将介绍一些常见的化工术语,并提供它们的中英文对照。
1. 化学过程术语1.1 氧化(Oxidation)•中文:氧化•英文:Oxidation1.2 还原(Reduction)•中文:还原•英文:Reduction1.3 反应(Reaction)•中文:反应•英文:Reaction1.4 反应速率(Reaction Rate)•中文:反应速率•英文:Reaction Rate1.5 氧化反应(Oxidation Reaction)•中文:氧化反应•英文:Oxidation Reaction1.6 还原反应(Reduction Reaction)•中文:还原反应•英文:Reduction Reaction1.7 酸碱中和反应(Neutralization Reaction)•中文:酸碱中和反应•英文:Neutralization Reaction 2. 化学物质术语2.1 水(Water)•中文:水•英文:Water2.2 盐(Salt)•中文:盐•英文:Salt2.3 酸(Acid)•中文:酸•英文:Acid 2.4 碱(Alkali)•中文:碱•英文:Alkali 2.5 溶液(Solution)•中文:溶液•英文:Solution2.6 离子(Ion)•中文:离子•英文:Ion2.7 分子(Molecule)•中文:分子•英文:Molecule 2.8 化合物(Compound)•中文:化合物•英文:Compound3. 实验设备术语3.1 烧杯(Beaker)•中文:烧杯•英文:Beaker3.2 镊子(Tweezers)•中文:镊子•英文:Tweezers3.3 温度计(Thermometer)•中文:温度计•英文:Thermometer 3.4 试管(Test Tube)•中文:试管•英文:Test Tube3.5 漏斗(Funnel)•中文:漏斗•英文:Funnel3.6 量筒(Graduated Cylinder)•中文:量筒•英文:Graduated Cylinder 3.7 手套(Gloves)•中文:手套•英文:Gloves3.8 磁力搅拌器(Magnetic Stirrer)•中文:磁力搅拌器•英文:Magnetic Stirrer结论掌握化工术语的中英文对照对于理解化工过程、交流和学习非常重要。
由从粉煤灰中制取的硫酸铝盐合成氧化铝姓名:曹巍巍学号:2011200387 学院:化工学院摘要:由粉煤灰制得的NH4Al(SO4)2和氨在水中适宜的PH环境下可制得高纯度氧化铝(>99.9%)。
用XRD、TG/DTA、SEM和激光分散技术来检测热源(微波对硫酸铝铵分解和氧化铝性质的影响。
和传统的加热方法相比,微波加热来分解硫酸铝铵以制得更高比表面积的α-Al2O3粉末。
关键词:硫酸铝盐、粉煤灰、微波加热1、简介NH4Al(SO4)2是一种制备高纯度的活性氧化铝的潜在原料[1-3],硫酸铝盐通常是一种从含铝原材料中生产氧化铝的中间材料[4-5]。
硫酸铝盐的分解和生成物向氧化铝的转化在相关文献中已有报告[1-3,6],但总体上结果还有一些分歧。
很多社会学家和相关的科学家对回收工业废物来制取高价值的材料很感兴趣。
在应用热点的地区,从大量的由煤燃烧产生的粉煤灰中进行资源回收是一个制得关注的问题。
粉煤灰由主要含有SiO2、Al2O3的无机粒子组成,这些粒子通常以煤胞的形式存在。
Al2O3是一种普遍使用的高性能原料,从粉煤灰中合成Al2O3具有很大的商业利益。
粉煤灰含有大量的杂质,如Fe2O3、Na2O、K2O、P2O5、MaO、MgO、CaO 和SiO2[7],这样纯粹的用酸或碱来直接提取纯净的氧化铝是很困难的。
一种从粉煤灰中获取高纯氧化铝的方法就是把硫酸铝盐作为中间产物来制。
将硫酸铝盐作为中间产物的优点就使通过直截了当的可溶沉淀物的过程来提高纯度。
和传统的加热过程相比,微波加热有它自身的优点,它具有选择性、直接性、内部性、控制性。
所以,微波在材料制备中广泛地应用[8-10]。
用酸可加速有机材料和无机材料的溶解过程,这是由于微波产生的内部热量引起的[11,12]。
目前工作的目的就是促进回收粉煤灰及生产高纯度氧化铝。
微波加热的影响和由粉煤灰制得的Al的热效应已经进入研究,氧化铝也已表征。
2、实验过程粉煤灰原片包含了23.29% Al2O3的和53.83%的SiO2,,它包含了大量的硅酸盐矿物质,它是一种混合的鳞片象和近球面形颗粒,比表面积为3.82m2/g,凝聚的大小为42.1μm(<90%).粉煤灰在600℃下在空气中焙烧2小时以出去残留的碳,随后在乙醇中球磨24小时,经过旋转真空蒸发后,干燥的粉末研磨到可通过200目的筛。
译文: 美国化学学会杂志在无溶剂条件下钯催化C-H 活化的末端烯烃的烯丙基及高烯丙基碳的二胺化合成杜海峰、袁伟成、赵保国、史一安美国科罗拉多州立大学化学系,科罗拉多州柯林斯堡市80523,在2007年3月23日收稿;E-mail: yian@二胺类烯烃提供了一个有效地的合成邻二胺的方法,其中包含各种生物活性化合物的重要功能基团合成并广泛应用在不对称合成手性化学元件1。
若干金属介导2,3和催化的二胺类物质合成反应已开发4-6。
最近,我们报道了共轭二烯、三烯可以用氮代二叔丁基二氮杂环丙酮(2)7作为氮源以Pd-(PPh 3)4作催化剂有效的在高立体选择性下进行二胺化合成(如图1)8。
在此我们希望报道末端烯烃可以在烯丙基和等位碳进行二胺化合成(如图2)。
使末端烯烃在先前的二胺化条件[量浓度10%的Pd(PPh 3)4在氘代苯中65℃] (方案1)下得到没有二胺化的烯烃,相反却观察到少量在烯丙和高烯丙碳的二胺化产物。
在多次实验后,才发现这二胺化过程可以通过缓慢加入氮代二叔丁基二氮杂环丙酮(2)在无溶剂条件下进一步完善。
例如,用4-苯基-1-丁烯和物质量浓度5%的Pd-(PPh 3)4和2(2.75当量,缓慢加入)在65℃反应7小时得到纯的二胺化产物(5a ),产率90%(表1,项目1)。
这种二胺化反应可以推广到各种末端烯烃,包括单取代(表1,项目 2-6)和1,1 - 二取代烯烃(表1,项目7-11)。
在所有这些情况下,反应发生在烯丙基和高烯丙位碳上且基本上只得到一个立体异构体9,10。
二胺化产物5可以用三氟乙酸和浓盐酸脱保护而得到游离的二胺。
(如图3) 双二胺化反应也可发生在基链含有末端双键的物质上,例如用1,9-癸二烯和量浓度10%的Pd(PPh 3)4和2反应12小时形成双二胺化的混合物(内消旋/DL ), 8a 和 8b ,产率61%(如图4)8a 的X 射线结构如图形1,它显示第一步产生的二胺化产物对第二步产生的二胺化产物的立体化学构型影响不大,然而当用1,7 - 辛二烯进行反应得到单一的非对映体二胺化产物11,产率47%(如图5)。
介绍化工专业的英语作文简短英文回答:Chemical Engineering is a branch of engineering that deals with the application of scientific and mathematical principles to the production of chemicals, fuels, pharmaceuticals, food, and other products. Chemical engineers design and operate chemical plants and processes, and they work to improve the efficiency and safety of these processes.Chemical engineering is a challenging and rewarding field that offers a wide range of opportunities for employment. Chemical engineers can work in a variety of industries, including the chemical, pharmaceutical, food, and energy industries. They can also work in government agencies and research institutions.Chemical engineering is a rapidly growing field, and there is a high demand for qualified chemical engineers.The median annual salary for chemical engineers is $105,590, and the job outlook is expected to grow by 5% over the next ten years.If you are interested in a career in chemical engineering, there are several steps you can take toprepare yourself. First, you should earn a bachelor'sdegree in chemical engineering from an accredited program. Once you have a bachelor's degree, you may choose to earn a master's degree or doctorate in chemical engineering. You should also gain experience through internships and co-ops, and you should join professional organizations such as the American Institute of Chemical Engineers (AIChE).中文回答:化学工程是工程的一个分支,它涉及科学和数学原理在化学品、燃料、药品、食品和其他产品的生产中的应用。
介绍化工产品英语作文英文回答:Chemical products are essential in our daily lives. For example, one of the most common chemical products is detergent. It is used for cleaning dishes, clothes, and even surfaces in our homes. Another example is plastic, which is used in so many different products, such as water bottles, food containers, and even toys.Chemical products are also used in various industries. For instance, in the agriculture industry, fertilizers are a type of chemical product that helps plants grow and thrive. In the automotive industry, chemicals are used in the production of car parts and components.中文回答:化工产品在我们的日常生活中是不可或缺的。
比如,最常见的化工产品之一是洗涤剂。
它用于清洁餐具、衣物,甚至家庭表面。
另一个例子是塑料,它被用在很多不同的产品中,比如水瓶、食品容器,甚至玩具中。
化工产品也被应用于各个行业。
比如,在农业行业,化肥是一种帮助植物生长茁壮的化工产品。
在汽车行业,化工产品被用于汽车零部件的生产。
Heavy Oil Development Technology of Liaohe Oilfield Han Yun(Scientific Research Information Department Exploration&Development Research Institute,Liaohe Oilfield Company)Liaohe Oilfield,the largest heavy oil production base in China,features in various reservoir types,deep burial,and wide range of crude oil viscosity.For many years,a series of technologies have been developed for different oil products and reservoir types of the oilfield,of which water flooding,foam slug drive,steam stimulation,steam drive,and SAGD are the main technologies. After continuous improvement,they have been further developed and played an important role in the development of heavy oil in the oilfield.Liaohe Oilfield is abundant in heavy oil resources,46%of the total proved reserves of Liaohe Oilfield Company. Horizontally the resources concentrates in the West Depression and the southern plunging belt of the Central Uplift in Liaohe Rift. Vertically,it is mainly distributed in Paleocene Shahejie Formation(ES). The distinctive geological feature of Liaohe 0ilfield is manifested in three aspects:first,the heavy oil reservoirs are deeply buried and 80%of them are buried more than 900m deep;second,the heavy oil viscosity ranges widely.For most of the reservoirs.the dead oil viscosity ranges in 100~100000mPa·s with the maximum 650000mPa·s.Third the reservoir types are various with complicated oil—water relationship,most of the reservoirs are edge water and bosom water reservoirs and there are also edge water reservoirs,top water reservoirs and bosom water reservoirs.For more than 20 years of development,Liaohe Oilfield has developed series of heavy oil development technologies for different oil products and different types of reservoirs,such as water flooding, foam slug drive,steam stimulation steam drive and SAGD.The most difficult issues have been overcome in the development of the superheavy oil in deeper formation.which has maintained the annual heavy oil output at 8 million tons for many years in Liaohe Oilfield.Water flooding development technology for conventional heavy oil-type 1Based on heavy oil classification,the conventional heavy oil.type I refers to the heavy oil with viscosity ranging in 50~100mPa·s,taking up about 20%of the proved oil reserves of the oilfield.The heavy oil reservoirs of this type are buried ranging from 1 500m to 2400m deep and are capable of flowing.Therefore,natural energy is utilized for conventional development and then water flooding technology is used.For example,the reservoir of $32 oil unit of Block Leng-43 is buried 1 650~l 940m deep with the average oil zone 87.7m thick and the oil viscosity in situ 58mPa·s.In 1992,the 141 m spacing square well pattern was adopted to develop the kind of reservoirs by utilizing natural energy and two sets of oil production zones.In 2004,water flooding technology was applied. Currently,the degree of reserve recovery reaches 14%.the annual oil recovery rate is 1%,and the ultimate recovery ratio is predicted to be as high as 22%.Technology of foam slug and for conventional heavy oil-type steam flooding for conventional heavy oil-type 2 The upper limit of viscosity for the conventional heavy oil—type II is l 0000mPa·s(the dead oil viscosity in situ).This kind of heavy oil is the dominant type of heavy oil in Liaohe Oilfield,accounting for 60%of total proved reserves.The reservoir of such heavy oil is buffed 800-1 600m deep in genera1. At initial development stage, steam stimulation was carried out to develop this kind of reservoirs.In the higher cycles of steam flooding,the reservoirs with the heavy oil viscosity close to the lower limit of this kind of heavy oil are conversed into the steam drive development.Pilot tests of foam slug and steam flooding have conducted inBlock Jin-90 and Block Qi-40 successfully,and they will be applied to the whole blocks in near future.The recovery factor is forecast to be up to 50%~60%.Steam stimulation technology for special heavy oilThe special heavy oil refers to the heavy oil with the viscosity ranging in 10000~50000mPa·s in situ,which takes up 10% of proved oil reserves of the oilfield. The reservoir of the kind of oil is buried ranging from 1400m to 1800m. Steam simulation technology is often applied to develop such reservoirs. However, technology of steam drive or SAGD are also under research and experiments for reservoirs of good quality.SAGD technology for super heavy oilThe super heavy oil reservoir refers to the heavy oil with dead oil viscosity in situ over 50000mPa·s, which accounts for 10% of proved reserves of the oilfield. Due to its extremely high viscosity, it is just developed for few years. For the massive super heavy oil reservoirs, SAGD can be applied in the late stage of steam stimulation. At present, good intermediate results have been obtained in SAGD pilot test in Block Du-84 of Guantao Formation, showing good prospect for application. They have been applied in the whole block and the ultimate recovery factor is predicted to achieve 55%.ConclusionVarious technology should be applied to develop different types of heavy oil reservoirs. Besides the technologies mentioned above, the technologies of combustion in situ, flue gas drive, and steam foam drive are also under research currently. Therefore, various development technologies will be increasingly improved with the heavy oil development of Liaohe Oilfield.References[1] Wang Xu. 2006. Heavy Oil Development Technologies and Discussion on the ResearchOrientation in the Next Step. Petroleum Exploration and Development [J],33(4):484~490[2] Liu Junrong. The Paper Collection of Liaohe Oilfield Development Seminar [C]:Beijing, Petroleum Industry Press, 2002[3] Zeng Yuqiang. 2006. Heavy Oil steam Stimulation Review. Special Oil&Gas Reservoir[J], 13(6):5~9辽河油田的重油开发技术韩云(科研信息部门勘探和开发研究所、辽河油田公司)辽河油田,在中国最大的重油生产基地,在不同的储层类型,具有埋藏深,与原油粘度范围宽。